Abstract
The prevalence of obesity in the United States is a major health problem associated with significant morbidity, mortality and economic burden. Although obesity and drug addiction are typically considered distinct clinical entities, both diseases involve dysregulation of biogenic amine neuron systems in the brain. Thus, research efforts to develop medications for treating drug addiction can contribute insights into the pharmacotherapy for obesity. Here we review the neurochemical mechanisms of selected stimulant medications used in the treatment of obesity, as well as issues related to fenfluramine-associated cardiac valvulopathy. In particular, we discuss the evidence that cardiac valve disease involves activation of mitogenic 5-HT2B receptors by norfenfluramine, the major metabolite of fenfluramine. Advances in medication discovery suggest that novel molecular entities that target two different neurochemical mechanisms, i.e. “combination pharmacotherapy”, will yield efficacious anti-obesity medications with reduced adverse side-effects.
Introduction
Epidemiological data reveal that the prevalence of obesity is increasing in the United States 1, and obesity is associated with considerable morbidity, mortality and costs to the health care system 2. The causes of excessive weight gain are complex and involve the interplay between biological, genetic, and psychosocial factors. As aptly stated by Bray and Champagne 3, “obesity is a chronic, relapsing, stigmatized, neurochemical disease.” Diet and exercise are primary treatment approaches to reduce body weight, but for many patients the results are disappointing and patients often regain lost weight 4. Indeed, obese individuals often cycle (“yo-yo”) through periods of dieting and weight loss, followed by weight regain 5, 6. Pharmacotherapy can be a crucial component of a weight loss program. A recent study reported that pharmacotherapy combined with lifestyle modification is more effective than lifestyle modification alone 7.
Numerous publications provide excellent reviews on the medications currently in use or under investigation for the treatment of obesity 8, 9. Because obesity is a chronic relapsing disorder, many clinicians believe that long-term treatment with anorectic medications is appropriate to help patients maintain lower body weight 10, 11. Within the central nervous system (CNS), multiple neuronal mechanisms are involved in the regulation of appetite, food intake and maintenance of body weight 12. Perhaps not surprisingly, medications that target one neurochemical mechanism produce relatively small reductions in body weight (decrease of 5%-10% body weight). Weight loss of this magnitude can diminish the risk factors associated with cardiovascular morbidity and mortality 13-15, though many patients expect more substantial, cosmetically meaningful, reductions in weight (decrease of 20-25% body weight) 16. The administration of two medications that work via different neurochemical mechanisms, i.e. “combination pharmacotherapy”, is one approach to obtaining cosmetically relevant reductions in weight. The most effective example of this approach was the combination of phentermine and fenfluramine 17-21. Phentermine targets norepinephrine (NE) and dopamine (DA) neurons in the brain, whereas fenfluramine targets serotonin (5-HT) neurons. Analogous combination pharmacotherapies have proven beneficial in the treatment of various diseases including hypertension 22, diabetes 23, and possibly drug addiction. Regrettably, fenfluramine was associated with serious side-effects, including cardiac valve disease (CVD), which prompted removal of this medication from the market.
Obesity and drug addiction are distinct clinical disorders that share underlying neurocircuitry and neurochemical mechanisms 24-26. Thus, it is not surprising that efforts to develop medications for treating cocaine and methamphetamine addiction (i.e., stimulant addiction) can provide insights for similar efforts underway to treat obesity. In particular, our work has led us to advocate the development of single molecular entities that target two different neurochemical mechanisms for treating stimulant addiction 27-29. Since anorectic medications and illicit stimulants interact with biogenic amine transporters in the CNS, our efforts have focused on determining the mechanism of action of these drugs. Additionally, determining the mechanisms underlying adverse effects of anorectic medications is necessary to aid in the development of new medications devoid of serious side-effects. In this paper, the pharmacology of selected anorectic agents is reviewed. We then discuss evidence supporting the hypothesis that fenfluramine-associated CVD is caused by the agonist actions of norfenfluramine, the major metabolite of fenfluramine, at 5-HT2B receptors 30. Finally, suggestions are made with regard to the feasibility of employing combination therapies as adjuncts in the management of obesity.
Biogenic amine transporters
Many appetite suppressants are amphetamine-related stimulants, and these agents interact with biogenic amine neurons in the CNS. Neurons that synthesize, store, and release amine transmitters - NE, DA, and 5-HT - are widely distributed in the mammalian CNS. These neurons express specialized plasma membrane proteins that transport previously released transmitter molecules from the extracellular space back into the cytoplasm 31, 32. Substantial evidence has shown that there are distinct transporter proteins expressed by NE neurons (NET), DA neurons (DAT), and 5-HT neurons (SERT). These proteins belong to a superfamily of Na+/Cl- dependent transporters that share genetic, structural, and functional homologies 33, 34. Under normal circumstances, the transporter-mediated uptake of amine transmitters is the principal mechanism for inactivation of amine signaling in the brain. The biogenic amine neurotransmitters and their receptors play a critical role in the pathogenesis and treatment of a wide range of psychiatric disorders 35.
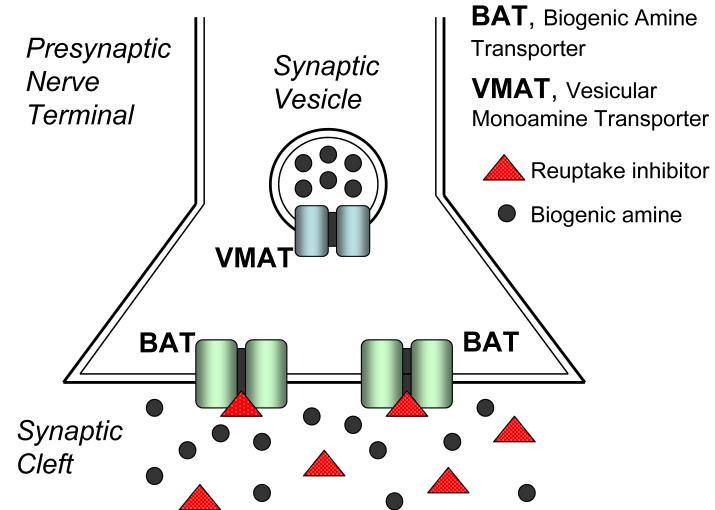
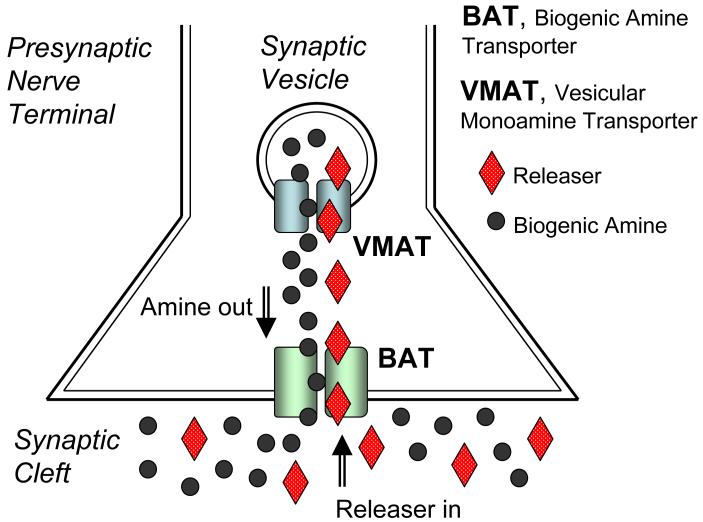
In general, drugs that target transporter proteins can be divided into two classes based on their precise mechanism of action: reuptake inhibitors and substrate-type releasers. Reuptake inhibitors bind to transporter proteins, but are not themselves transported. As depicted in Figure 1, these drugs elevate extracellular transmitter concentrations by blocking transporter-mediated recapture of transmitter molecules from the synapse. Substrate-type releasers bind to transporter proteins, and these drugs are subsequently transported into the cytoplasm of nerve terminals. Figure 2 illustrates that releasers, often referred to as substrates, elevate extracellular transmitter concentrations by a two-pronged mechanism: (1) they promote efflux of transmitter by reversing the normal direction of transporter activity, and (2) they increase cytoplasmic levels of transmitter by disrupting storage of transmitters in vesicles via interactions with vesicular monoamine transporters (VMAT) 36, 37. The exact mechanism underlying transporter-mediated release is complex and a topic of intensive investigation 38-40. Because substrate-type releasing agents must be transported into nerve terminals to promote transmitter release, reuptake inhibitors can block the effects of releasers.
Figure 1.
Mechanism of biogenic amine transporter (BAT) reuptake inhibitors. Reuptake inhibitors increase synaptic transmitter concentrations by binding to BAT proteins and blocking the recapture of previously released biogenic amine transmitters.
Figure 2.
Mechanism of biogenic amine transporter (BAT) substrate-type releasers. Releasers increase synaptic transmitter concentrations by a two pronged mechanism: 1) they promote biogenic amine efflux out of the cell by reversing the normal direction of transporter activity, and 2) they disrupt the storage of biogenic amines in vesicles by interacting with vesicular monoamine transporters (VMAT). The disruption of biogenic amine storage increases the cytoplasmic concentrations of amines available for release.
Basic pharmacology of anorectics
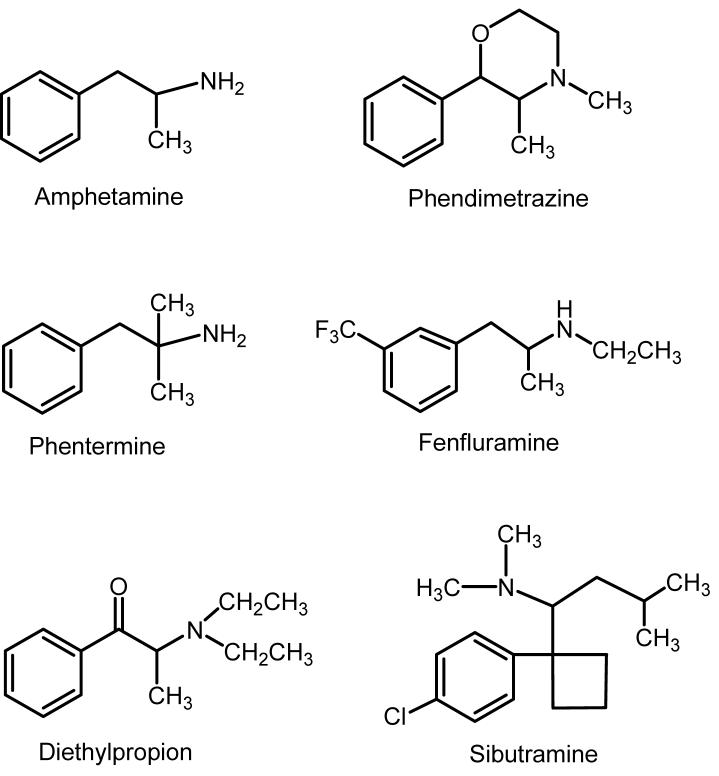
Most appetite suppressants that are currently available, or were prescribed in the past, interact with biogenic amine transporters as reuptake inhibitors or substrates. Figure 3 shows the chemical structures of commonly prescribed anorectics. With the exception of sibutramine, these medications are substituted phenylethylamine analogs (i.e. related to amphetamine). Table 1 summarizes the interaction of these anorectics with NET, DAT and SERT. The prototypical anorectic agent (+)-amphetamine 41 potently releases NE (EC50 = 7 nM) and DA (EC50 = 24.8 nM) from rat brain synaptosomes. In contrast, (+)-amphetamine has much weaker effects on 5-HT release (EC50 = 1765 nM). Consistent with the in vitro data, administration of anorectic doses of (+)-amphetamine markedly increases extracellular DA levels in rat brain while having minimal effects on extracellular 5-HT 42. Phentermine is one of the more widely prescribed appetite suppressant medications. The data in Table 1 reveal that phentermine is a substrate at NET, DAT and SERT, with its most potent action being NE release (EC50 = 39 nM). Similar to (+)-amphetamine, administration of anorectic doses of phentermine evokes DA release in the brain with lesser effects on 5-HT release 43. Ephedrine has a profile of transporter activity that is similar to phentermine.
Figure 3.
Chemical structures of commonly prescribed anorectic medications. With the exception of sibutramine, these medications are substituted phenylethylamine analogs, related to amphetamine. Fenfluramines are no longer available for clinical use.
Table 1.
Interaction of Selected Anorectic Agents with the Biogenic Amine Transporters
| Test Drug | Release NETa EC50 (nM ± SD) | NE Uptake Ki (nM ± SD) | Release DATa EC50 (nM ± SD) | DA Uptake Ki (nM ± SD) | Release SERT EC50 (nM ± SD) | 5-HT Uptake Ki (nM ± SD) |
|---|---|---|---|---|---|---|
| (+)-Amphetamine | 7.1 ± 0.9 | 24.8 ± 3.5 | 1765 ± 94 | |||
| Phentermine | 39.4 ± 6.6 | 262 ± 21 | 3511 ± 253 | |||
| (-)-Ephedrine | 43.1±4.0 | 236±9 | >10,000 | >50,000 | ||
| (+)-Ephedrine | 218±14 | 2104±68 | inactive | |||
| Diethylpropion | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| N-Ethylaminopropiophenone | 99.3 ± 6.6 | 1014 ± 80 | 2118 ± 98 | |||
| Phendimetrazine | 8300 ± 445 | >10,000 | 19,000±537 | >10,000 | >100,000 | >100,000 |
| (±)-Phenmetrazine | 50.4±5.4 | 131±11 | 7765±610 | |||
| (+)-Fenfluramine | 302 ± 20 | 22000±1100 | 51.7 ± 6.1 | |||
| (-)-Fenfluramine | 7187±559 | >10,000 | >20,000 | 147±19 | ||
| (±)-Fenfluramine | 739 ± 57 | 23700±1300 | 79.3 ± 11.5 | |||
| (±)-Norfenfluramine | 168±17 | 1925±295 | 104±5 | |||
| (+)-Norfenfluramine | 72.7±5.4 | 924±112 | 59.3±2.4 | |||
| (-)-Norfenfluramine | 474±40 | 19194±1048 | 287±14 | |||
| (R,S)-Sibutramineb | 350 | 1200 | 2800 | |||
| (R)-Desmethylsibutramineb | 4 | 12 | 44 | |||
| (S)-Desmethylsibutramineb | 870 | 180 | 9200 | |||
| (R)-Didesmethylsibutramineb | 13 | 8.9 | 140 | |||
| (s)-Didesmethylsibutramineb | 62 | 12 | 4300 |
Studies conducted in baboons indicate that phentermine and ephedrine may not release DA at clinically-relevant doses 44. In one study, high (3 mg/kg) intravenous doses of phentermine and ephedrine increased plasma NE levels, but did not suppress plasma prolactin or release central DA, as detected by displacement of [11C]raclopride binding. By contrast, intravenous (+)-amphetamine suppressed plasma prolactin and released central DA, in addition to increasing plasma NE concentrations. The possibility that phentermine and ephedrine do not release DA in humans could explain why these medications have lower abuse liabilities than (+)-amphetamine. It will be of interest to verify these results in controlled clinical investigations.
Diethylpropion and phendimetrazine display minimal interactions with monoamine transporters in vitro, even though these drugs produce psychomotor stimulation when administered in vivo. For example, phendimetrazine and diethylpropion are self-administered by animals 45, 46, and both drugs exhibit discriminative stimulus properties that generalize to cocaine 47, 48. The available data suggests that diethylpropion and phendimetrazine are “prodrugs” which are converted to bioactive metabolites upon systemic administration. In the case of diethylpropion, the N-deethylated metabolite N-ethylaminopropiophenone appears to be the bioactive metabolite since this compound potently releases NE (EC50 = 99.3 nM) with less potent effects on 5-HT release (EC50 = 2118 nM). Interestingly, N-ethylaminopropiophenone is not a DAT substrate, but instead blocks DA reuptake (EC50 = 1014 nM) 49. In the case of phendimetrazine, the N-demethylated metabolite phenmetrazine potently releases NE and DA but not 5-HT.
(±)-Fenfluramine (Pondimin™) and its more potent stereoisomer, (+)-fenfluramine (dexfenfluramine, Redux™), are anorectic agents that were widely prescribed until their removal from the market due to the occurrence of CVD 50. Historical evidence established that fenfluramines stimulate 5-HT transmission in the CNS by increasing synaptic levels of 5-HT 51. More recent data indicate that fenfluramines and their major metabolites, norfenfluramines, increase synaptic 5-HT by acting as substrates for SERT (for review see 52). In vivo microdialysis studies confirm that 5-HT release evoked by (±)-fenfluramine or (+)-fenfluramine is antagonized by pretreatment with the SERT inhibitor fluoxetine (Prozac™) 53, 54.
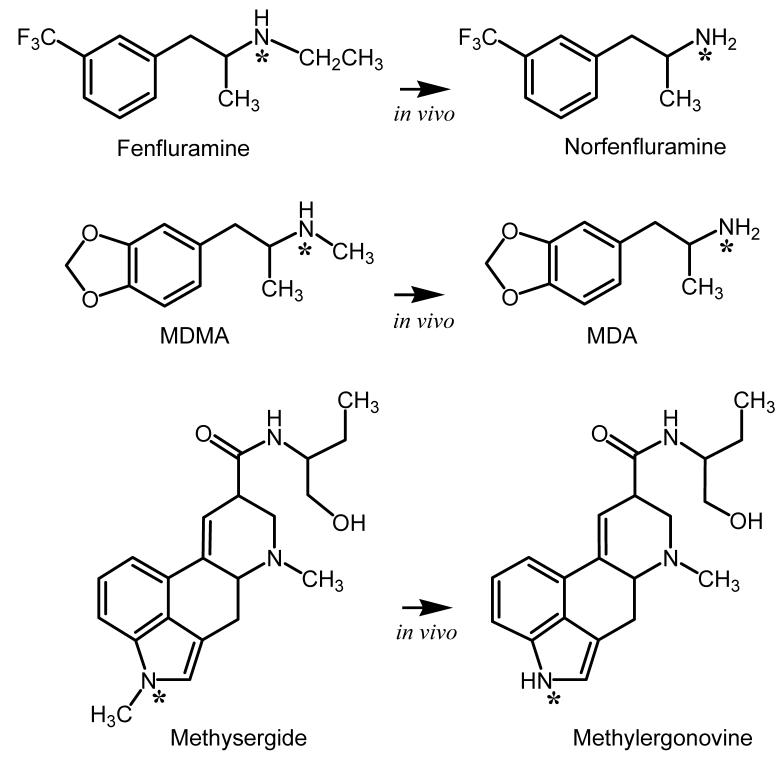
Pharmacokinetic investigations show that stereoisomers of (±)-fenfluramine are N-de-ethylated by liver enzymes to yield the metabolites, (+)- and (-)-norfenfluramine (see Figure 4) 55. In humans and animals treated with systemically administered (±)-fenfluramine, circulating concentrations of (+)-and (-)-norfenfluramine are similar to or greater than concentrations of fenfluramine itself 55, 56. Moreover, the stereoisomers of (±)-fenfluramine and (±) norfenfluramine cross the blood-brain barrier to accumulate in the brain. Thus, peripheral administration of (±)-fenfluramine gives rise to four pharmacological agents with potential neurobiological activity. With few exceptions 57-59, investigations examining the neuropharmacology of fenfluramines and norfenfluramines have focused on the 5-HT effects of these drugs (for review see 52).
Figure 4.
In vivo metabolism of selected drugs to form potent 5-HT2B receptor agonists. In each case illustrated, N-demethylation or N-deethylation produces bioactive metabolites that display much greater agonist activity at 5-HT2B receptors when compared to their corresponding parent compounds.
As reported in Table 1, the most potent action of (±)-fenfluramine and its stereoisomers is to evoke [3H]5-HT release, whereas these compounds are essentially inactive in the DA release assay. (+)-Fenfluramine releases [3H]5-HT with an EC50 value of 51.7 nM whereas (-)-fenfluramine releases [3H]5-HT with an EC50 value of 147 nM, a 2.8-fold difference in potency. In the [3H]NE release assay, (+)-fenfluramine displays appreciable activity (EC50 = 302 nM) that is about 6-fold weaker than its activity in the [3H]5-HT release assay. (-)-Fenfluramine is very weak in NE release assay. Thus, (-)-fenfluramine appears more selective than (+)-fenfluramine as a [3H]5-HT releaser, but the (-)-isomer is generally less potent.
(±)-Norfenfluramine and its stereoisomers are much more potent at releasing [3H]5-HT when compared to [3H]DA. (+)-Norfenfluramine releases [3H]5-HT with an EC50 value of 59.3 nM, whereas (-)-norfenfluramine releases [3H]5-HT with an EC50 value of 287 nM. (±)-Norfenfluramine and its stereoisomers are much more potent at releasing [3H]NE and [3H]DA than fenfluramines. For example, (+)-norfenfluramine releases [3H]NE with an EC50 value of 72.7 nM, compared to (+)-fenfluramine, which releases [3H]NE with an EC50 value of 302 nM (see above). It is important to note that norfenfluramines release [3H]NE and [3H]5-HT with roughly equivalent potency. Additionally, the potency of (+)-norfenfluramine to evoke [3H]NE release is similar to the potency of phentermine, a known NE-releasing agent. Consistent with these in vitro data, in vivo microdialysis experiments showed that (+)-fenfluramine produced dose-related increases in extracellular NE in the frontal cortex of rats 59. These data indicate that noradrenergic, as well as serotonergic, mechanisms contribute to the anorectic effect of fenfluramine.
Unlike amphetamine and related compounds described above, sibutramine and its metabolites are uptake inhibitors that vary in their potency and selectivity for NET, DAT and SERT (Table 1) 60. It is notable that sibutramine itself is relatively less potent as an uptake inhibitor than its metabolites, indicating that sibutramine acts as a pro-drug. Several of the metabolites are potent DA uptake inhibitors, perhaps providing an explanation for the greater weight loss observed at higher than approved doses (30 mg per day) 15.
Fenfluramine and cardiac valvulopathy
As mentioned already, (±)-fenfluramine and (+)-fenfluramine were removed from clinical use due to the occurrence of CVD in some patients 50. A meta-analysis of available data indicates the odds ratio for fenfluramine-associated CVD is 2.0, suggesting a moderate albeit significant risk for the disease in patients taking these mediations 61. Although a serotonergic mechanism was suspected as a possible cause of CVD 62, little was known about the pathogenesis of this adverse effect in the late 1990s. In light of the established role of 5-HT as a mitogen 63, we carried out a study to determine if stereoisiomers of fenfluramine or norfenfluramine might activate mitogenic 5-HT receptors 30. Several other drugs were examined to provide both positive and negative controls. Additional positive control drugs included methysergide, its active metabolite methylergonovine 64 (see Figure 4), and ergotamine. Methysergide and ergotamine are well known to produce primarily left-sided CVD affecting the mitral valve 65, 66. Negative controls included phentermine, fluoxetine and its metabolite, norfluoxetine, which are not associated with CVD. We also included the antidepressant trazodone and its metabolite m-chlorophenylpiperazine (mCPP) as additional negative control drugs. mCPP displays agonist activity at a wide range of 5-HT receptors 67 and also is capable of releasing 5-HT via a SERT-mediated mechanism 68. The clinical use of trazodone is not associated with VHD. Our working hypothesis was that positive control drugs would share in common the ability to activate a mitogenic 5-HT receptor subtype expressed in heart valves, whereas negative control drugs would not have this effect. An initial receptorome screen 69 led to a detailed evaluation of the binding of these drugs to the 5-HT2 family of 5-HT receptors.
Table 2 reports binding data, while Table 3 reports functional effects of these compounds at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptor subtypes. Fenfluramines had low affinity for all 5-HT2 receptor subtypes. In contrast, norfenfluramines had high affinity (KI = 10-50 nM) for 5-HT2B receptors but not for 5-HT2A or 5-HT2C sites, in confirmation of other studies 70, 71. Functional studies demonstrated that norfenfluramines were full agonists at the 5-HT2B site. Ergotamine was a potent partial agonist at the 5-HT2B receptor, whereas methysergide was a low efficacy partial agonist at this site. Methylergonovine, the active metabolite of methysergide, was a high affinity partial agonist at 5-HT2B sites. Among the negative control drugs, mCPP was a partial agonist at 5-HT2B receptors with the same efficacy as methylergonovine. With the exception of the findings with mCPP, our data suggested that the receptor responsible for producing CVD was the 5-HT2B receptor. Trazodone binds with moderate affinity to the 5-HT2B receptors but functions as an antagonist. The 5-HT2B antagonist activity of trazodone probably explains why trazodone administration in humans is not associated with VHD.
Table 2.
Ki Values of Test Drugs at 5-HT2 Receptors
| Drug | Human 5-HT2a |
Human 5-HT2B |
Human 5-HT2C |
|---|---|---|---|
| (±)-Fenfluramine | 5216 ± 423 | 4134±1281 | 3183 ± 637 |
| (+)-Fenfluramine | 11107 ± 2303 | 5099 ± 1173 | 6245 ± 874 |
| (-)-Fenfluramine | 5463 ±600 | 5713 ± 2285 | 3415 ± 922 |
| (±)-Norfenfluramine | 2316 ± 278 | 52.1±21 | 557 ± 61 |
| (+)-Norfenfluramine | 1516 ± 150 | 11.2 ± 7.3 | 324 ± 12 |
| (-)-Norfenfluramine | 3841 ± 614 | 47.8 ± 30.6 | 814 ± 98 |
| Ergotamine | 9.0 ± 1.0 | 3.0 ± 0.4 | 12 ± 1.5 |
| Methysergide | 15.0 ± 4.0 | 9.1 ± 4.9 | 1.8 ± 0.2 |
| Methylergonovine | 12.6 ± 1.0 | 0.49 ± 0.16 | 12.4 ± 1.0 |
| Fluoxetine | 299 ± 53 | 5030±1960 | 50 ± 10 |
| Norfluoxetine | 638 ± 108 | 5063±1974 | 286 ± 60 |
| Trazodone | 19.8±2.4 | 73.6 ± 36 | 402±44 |
| m-CPP | 391±47 | 3.2 ± 1.0 | 59±11 |
| 5-HT | 614±74 | 4.0 ± 1.9 | 12.2±1.3 |
| Phentermine | >10,000 | >10,000 | >10,000 |
Values are mean ± SD for n=3 experiments. Data taken from Data taken from 30.
Table 3.
Functional Activity of Test Drugs at 5-HT2 Receptors
| Drug | Human 5-HT2a Kact (nM±SD) Vmax (Percent of 5-HT±SD) |
Human 5-HT2B Kact (nM±SD) Vmax (Percent of 5-HT±SD) |
Human 5-HT2C Kact (nM±SD) Vmax (Percent of 5-HT±SD) |
|---|---|---|---|
| (±)-Fenfluramine | 4131±2448 15±4 |
ND | ND |
| (+)-Fenfluramine | >10,000 Not Done |
379 ± 120 38 ± 14 |
362 ± 109 80 ± 10 |
| (-)-Fenfluramine | 5279± 998 43 ± 7.2 |
1248 ± 430 47 ± 5 |
360 ± 155 84 ± 15% |
| (±)-Norfenfluramine | ND | ND | ND |
| (+)-Norfenfluramine | 630 ± 240 88± 9 |
18.4 ± 9 73 ± 6 |
13 ± 4 100 ± 11 |
| (-)-Norfenfluramine | 1565 ± 323 93 ± 9 |
357± 180 71 ± 15 |
18 ± 9 80 ± 17 |
| Ergotamine | 16 ± 4 75 ± 3 |
9.8 ± 3 56 +/- 3 |
5 ±3 75 ± 15 |
| Methysergide | 3.5 ±- 1.7 24 ± 3 |
150 ± 43 18 ± 4 |
2.9 ± 1.5 33 ± 3.5 |
| Methylergonovine | 1.3 ± 0.4 70 ± 7 |
0.8 ± 0.5 40 ± 3 |
2.5 ± 1.2 103 ± 7 |
| Fluoxetine | ND | ND | Antagonist Ki = 616±172 |
| Norfluoxetine | ND | ND | Antagonist Ki = 43±17 |
| Trazodone | Antagonist | Antagonist | Antagonist |
| m-CPP | 65 ± 17 55 ± 11 |
64 ± 27 43 ± 14 |
0.64 ± 0.3 79 ± 15 |
| 5-HT | 66 ± 26 100% |
2.4 ± 1.5 100 |
0.6 ± 0.18 100 |
| Phentermine | ND | ND | 1394 ± 450 66 ± 10 |
Values are mean ± SD for n=3 experiments. Data taken from Data taken from 30.
Among the receptors assayed, several lines of evidence indicated that the 5-HT2B receptor mediates the valvulopathic effects of fenfluramine: (1) 5-HT2B receptors are located on both mitral and aortic valves 70; (2) these receptors mediate mitogenisis 72; (3) norfenfluramines have high affinity and efficacy at the 5-HT2B receptor; (4) methylergonovine, the active metabolite of methysergide, is a high affinity partial agonists for the 5-HT2B receptor; (5) most of the negative control drugs (fluoxetine, norfluoxetine, phentermine) have very low affinity and lack agonist effects at this receptor. The finding that mCPP has agonist activity at 5-HT2B receptors must be reconciled with observations that trazodone is not associated with CVD. Therapeutic oral doses of trazodone generate plasma levels of mCPP from 150-550 nM 73, and these concentrations are within the range needed to activate 5-HT2B receptors. However, trazodone is a potent 5-HT2B receptor antagonist, and its plasma levels are about five-fold higher than that of mCPP 73. Thus, when trazodone is administered in vivo, antagonist actions of the drug would serve to block activation of 5-HT2B receptors by mCPP.
Further support for the 5-HT2B receptor hypothesis of drug-induced CVD comes from studies of 3,4-methylenedioxymethamphetamine (MDMA). Setola et al. reported that MDMA and its N-demethylated metabolite, 3,4-methylenedioxyamphetamine (MDA) (see Figure 4), are 5-HT2B receptor agonists that elicit prolonged mitogenic responses in human valvular interstitial cells 74. As predicted by the in vitro findings, chronic heavy users of MDMA are reported to exhibit an increased prevalence of valvular regurgitation indicative of CVD 75. The critical role of 5-HT2B receptors in mediating CVD has been strengthened by recent data demonstrating that two ergot medications known to cause valvulopathy- carbergoline and pergolide- are potent agonists at 5-HT2B receptor sites (for review see: 76). Collectively, these findings indicate the importance of screening potential medications for agonist activity at 5-HT2B receptors 69. The recent description of lorcaserin as a potential anti-obesity medication illustrates this strategy 77. Lorcaserin displays potent 5-HT2C agonist activity that is much greater than its effects on 5-HT2B receptors. This medication is currently in phase 3 clinical trials (http://www.arenapharm.com/wt/page/lho.html) 78.
Combination therapy for obesity
As noted in the Introduction, the regulation of appetite, food intake and body weight are controlled by multiple neuronal mechanisms working in a parallel manner 12. Medications that targets one neurochemical mechanism produce a relatively small degree of weight loss, such as that observed with the cannabinoid receptor antagonist rimonabant 79. While weight loss of this magnitude can produce significant reductions in risk factors associated with cardiovascular morbidity and mortality 13-15, patients often expect more significant weight loss 16. Combination pharmacotherapy is one potential strategy for obtaining greater, cosmetically-relevant, reductions in weight; this approach is illustrated by phentermine/fenfluramine combination pharmacotherapy 17-21. As shown in Table 1, phentermine releases NE and DA from neurons whereas fenfluramines release 5-HT. By recruiting both catecholaminergic and serotonergic mechanisms, the phentermine/fenfluramine combination engenders greater effects on weight loss than either treatment administered alone.
The occurrence of CVD in patients receiving phentermine/fenfluramine treatment raised serious concerns about the use of anti-obesity medications 80, and more broadly about using combinations of medications 81. Based on the research reviewed in this article, it is now apparent that CVD associated with the use of phentermine/fenfluramine resulted from activation of 5-HT2B receptors localized to heart valves. The major culprits in stimulating these receptors are the fenfluramine metabolites, (+)- and (-)-norfenfluramine. Importantly, there is no evidence to suggest that phentermine alone increases the risk for CVD, or that co-administration of phentermine with fenfluramine somehow augments the risk posed by fenfluramine.
Having identified the likely cause of fenfluramine-associated CVD, the principle of combining phentermine with a serotonergic agent to achieve improved efficacy in the treatment of obesity remains a valid approach worthy of further exploration. The one important caveat is that serotonergic medications should not activate 5-HT2B receptors. For example, the combination of phentermine with the 5-HT precursor, L-5-hydroxytryptophan, is a feasible means of increasing synaptic DA and 5-HT. This combination would be predicted to display superior efficacy as an appetite suppressant. Another approach that we have suggested 29 is the development of single molecular entities that release DA and 5-HT 82. As long as such treatments do not activate 5-HT2B receptors they should be safe and efficacious mediations. Finally, reinforcing the fact that obesity and drug addiction are driven in part by common neurochemical mechanisms 26, it is predicted that research conducted to discover medications for treating stimulant addiction will provide clues for the development of medications to treat obesity.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse.
References
- 1.Anonymous State-specific prevalence of obesity among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2006 Sep 15;55(36):985–988. [PubMed] [Google Scholar]
- 2.Ogden CL, Yanovski SZ, Carroll MD, et al. The epidemiology of obesity. Gastroenterology. 2007 May;132(6):2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA, Champagne CM. Beyond energy balance: there is more to obesity than kilocalories. J Am Diet Assoc. 2005 May;105(5 Suppl 1):S17–23. doi: 10.1016/j.jada.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003 Jun;11(6):722–733. doi: 10.1038/oby.2003.102. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Cuzzolaro M, Mannucci E, et al. Weight cycling in treatment-seeking obese persons: data from the QUOVADIS study. Int J Obes Relat Metab Disord. 2004 Nov;28(11):1456–1462. doi: 10.1038/sj.ijo.0802741. [DOI] [PubMed] [Google Scholar]
- 6.Amigo I, Fernandez C. Effects of diets and their role in weight control. Psychol Health Med. 2007 May;12(3):321–327. doi: 10.1080/13548500600621545. [DOI] [PubMed] [Google Scholar]
- 7.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005 Nov 17;353(20):2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 8.Bray GA, Ryan DH. Drug treatment of the overweight patient. Gastroenterology. 2007 May;132(6):2239–2252. doi: 10.1053/j.gastro.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 9.Smith BM, Thomsen WJ, Grottick AJ. The potential use of selective 5-HT2C agonists in treating obesity. Expert Opin Investig Drugs. 2006 Mar;15(3):257–266. doi: 10.1517/13543784.15.3.257. [DOI] [PubMed] [Google Scholar]
- 10.Klein S. Long-term pharmacotherapy for obesity. Obes Res. 2004 Dec;12(Suppl):163S–166S. doi: 10.1038/oby.2004.283. [DOI] [PubMed] [Google Scholar]
- 11.Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol. 2006;2:357–377. doi: 10.1146/annurev.clinpsy.2.022305.095249. [DOI] [PubMed] [Google Scholar]
- 12.Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature. 2006 Sep 21;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 13.Moyers SB. Medications as adjunct therapy for weight loss: approved and off-label agents in use. J Am Diet Assoc. 2005 Jun;105(6):948–959. doi: 10.1016/j.jada.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005 Nov 17;353(20):2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 15.Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight loss. Obes.Res. 1999;7:189–198. doi: 10.1002/j.1550-8528.1999.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 16.Foster GD, Wadden TA, Vogt RA, et al. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol. 1997 Feb;65(1):79–85. doi: 10.1037//0022-006x.65.1.79. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub M. Long-term weight control study: conclusions. Clinical Pharmacology And Therapeutics. 1992;51:642–646. doi: 10.1038/clpt.1992.76. [DOI] [PubMed] [Google Scholar]
- 18.Dhurandhar NV, Atkinson RI. Comparison of serotonin agonists in combination with phentermine for treatment of obesity; NAASO Conference; 1996.p. 3231. [Google Scholar]
- 19.Atkinson RL, Blank RC, Schumacher D, et al. Long-term drug treatment of obesity in a private practice setting. Obes.Res. 1997;5:578–586. doi: 10.1002/j.1550-8528.1997.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 20.Ryan DH, Bray GA, Helmcke F, et al. Serial echocardiographic and clinical evaluation of valvular regurgitation before, during, and after treatment with fenfluramine or dexfenfluramine and mazindol or phentermine. Obes.Res. 1999;7:313–322. doi: 10.1002/j.1550-8528.1999.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 21.Redmon JB, Raatz SK, Kwong CA, et al. Pharmacologic induction of weight loss to treat type 2 diabetes. Diabetes.Care. 1999;22:896–903. doi: 10.2337/diacare.22.6.896. [DOI] [PubMed] [Google Scholar]
- 22.Weir MR. Risk-based classification of hypertension and the role of combination therapy. J Clin Hypertens (Greenwich) 2008 Jan;10(1 Suppl 1):4–12. doi: 10.1111/j.1524-6175.2007.08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derosa G, Sibilla S. Optimizing combination treatment in the management of type 2 diabetes. Vasc Health Risk Manag. 2007;3(5):665–671. [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman RB, Baumann MH. Neurochemical mechanisms of phentermine and fenfluramine: therapeutic and adverse effects. Drug Dev Res. 2000;51:52–65. [Google Scholar]
- 25.Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann N Y Acad Sci. 2002 Jun;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005 May;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 27.Baumann MH, Rothman RB. Serotonergic dysfunction during cocaine withdrawal: Implications for cocaine-induced depression. In: Karch SB, editor. Drug Abuse Handbook. CRC Press; Boca Raton: 1998. pp. 463–484. [Google Scholar]
- 28.Rothman RB, Baumann MH. Therapeutic potential of monoamine transporter substrates. Curr Top Med Chem. 2006;6(17):1845–1859. doi: 10.2174/156802606778249766. [DOI] [PubMed] [Google Scholar]
- 29.Rothman RB, Blough BE, Baumann MH. Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol Sci. 2006 Oct 20;27(12):612–618. doi: 10.1016/j.tips.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Rothman RB, Baumann MH, Savage JE, et al. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 31.Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 32.Masson J, Sagne C, Hamon M, et al. Neurotransmitter transporters in the central nervous system. Pharmacol Rev. 1999;51:439–464. [PubMed] [Google Scholar]
- 33.Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. [Review] J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- 34.Uhl GR, Johnson PS. Neurotransmitter transporters: three important gene families for neuronal function. J.Exp.Biol. 1994;196:229–236. doi: 10.1242/jeb.196.1.229. [DOI] [PubMed] [Google Scholar]
- 35.Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug.Alcohol.Depend. 1998;51:87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- 36.Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. [Review] Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 37.Rudnick G. Mechanisms of biogenic amine transporters. In: Reith EA, editor. Neurotransmitter Transporters: Structure, Function and Regulation. Humana Press; Totowa NJ: 1997. pp. 73–100. [Google Scholar]
- 38.Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 2005 Aug;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- 39.Sitte HH, Freissmuth M. Oligomer formation by Na+-Cl--coupled neurotransmitter transporters. Eur J Pharmacol. 2003 Oct 31;479(13):229–236. doi: 10.1016/j.ejphar.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 40.Sulzer D, Sonders MS, Poulsen NW, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005 Apr;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Craddock D. Anorectic drugs: use in general practice. Drugs. 1976;11(5):378–393. doi: 10.2165/00003495-197611050-00002. [DOI] [PubMed] [Google Scholar]
- 42.Rothman RB, Blough BE, Woolverton WL, et al. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005 Jun;313(3):1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- 43.Baumann MH, Ayestas MA, Dersch CM, et al. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Alexander M, Rothman RB, Baumann MH, et al. Noradrenergic and dopaminergic effects of (+)-amphetamine-like stimulants in the baboon Papio anubis. Synapse. 2005 May;56(2):94–99. doi: 10.1002/syn.20126. [DOI] [PubMed] [Google Scholar]
- 45.Gotestam KG, Andersson BE. Assessment of reinforcing properties of amphetamine analogues in self-administering rats. Postgrad.Med.J. 1975;51(Suppl 1):80–83. [PubMed] [Google Scholar]
- 46.Griffiths RR, Winger G, Brady JV, et al. Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacology. 1976;50:251–258. doi: 10.1007/BF00426841. [DOI] [PubMed] [Google Scholar]
- 47.Evans SM, Johanson CE. Amphetamine-like effects of anorectics and related compounds in pigeons. J.Pharmacol.Exp.Ther. 1987;241:817–825. [PubMed] [Google Scholar]
- 48.Wood DM, Emmett Oglesby MW. Substitution and cross-tolerance profiles of anorectic drugs in rats trained to detect the discriminative stimulus properties of cocaine. Psychopharmacology. 1988;95:364–368. doi: 10.1007/BF00181948. [DOI] [PubMed] [Google Scholar]
- 49.Yu H, Kim IJ, Folk JE, et al. Synthesis and pharmacological evaluation of 3-(3,4-dichlorophenyl)-1-indanamine derivatives as nonselective ligands for biogenic amine transporters. J Med Chem. 2004 May 6;47(10):2624–2634. doi: 10.1021/jm0305873. [DOI] [PubMed] [Google Scholar]
- 50.Connolly HM, McGoon MD. Obesity drugs and the heart. Curr Probl Cardiol. 1999;24:745–792. doi: 10.1016/s0146-2806(99)90013-0. [DOI] [PubMed] [Google Scholar]
- 51.Rothman RB, Baumann M. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther. 2002 Jul;95(1):73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 52.Garattini S, Mennini T, Bendotti C, et al. Neurochemical mechanism of action of drugs which modify feeding via the serotoninergic system. Appetite. 1986;7(Suppl):15–38. doi: 10.1016/s0195-6663(86)80050-2. [DOI] [PubMed] [Google Scholar]
- 53.Gundlah C, Martin KF, Heal DJ, et al. In vivo criteria to differentiate monoamine reuptake inhibitors from releasing agents: sibutramine is a reuptake inhibitor. J.Pharmacol.Exp.Ther. 1997;283:581–591. [PubMed] [Google Scholar]
- 54.Tao R, Fray A, Aspley S, et al. Effects on serotonin in rat hypothalamus of D-fenfluramine, aminorex, phentermine and fluoxetine. Eur J Pharmacol. 2002 Jun 7;445(12):69–81. doi: 10.1016/s0014-2999(02)01751-x. [DOI] [PubMed] [Google Scholar]
- 55.Caccia S, Conforti I, Duchier J, et al. Pharmacokinetics of fenfluramine and norfenfluramine in volunteers given D- and DL-fenfluramine for 15 days. Eur.J.Clin.Pharmacol. 1985;29:221–224. doi: 10.1007/BF00547426. [DOI] [PubMed] [Google Scholar]
- 56.Marchant NC, Breen MA, Wallace D, et al. Comparative biodisposition and metabolism of 14C-(+/-)-fenfluramine in mouse, rat, dog and man. Xenobiotica. 1992 Nov;22(11):1251–1266. doi: 10.3109/00498259209053154. [DOI] [PubMed] [Google Scholar]
- 57.Pettersson E. Studies of four novel diphenylbutylpiperazinepyridyl derivatives on release and inhibition of reuptake of dopamine, serotonin and noradrenaline by rat brain in vitro. Eur.J.Pharmacol. 1995;282:131–135. doi: 10.1016/0014-2999(95)00300-a. [DOI] [PubMed] [Google Scholar]
- 58.Cozzi NV, Frescas S, Marona-Lewicka D, et al. Indan analogs of fenfluramine and norfenfluramine have reduced neurotoxic potential. Pharmacol.Biochem.Behav. 1998;59:709–715. doi: 10.1016/s0091-3057(97)00557-1. [DOI] [PubMed] [Google Scholar]
- 59.Rothman RB, Clark RD, Partilla JS, et al. (+)-fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. J Pharmacol Exp Ther. 2003 Jun;305(3):1191–1199. doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- 60.Glick SD, Haskew RE, Maisonneuve IM, et al. Enantioselective behavioral effects of sibutramine metabolites. Eur.J.Pharmacol. 2000 May 26;397(1):93–102. doi: 10.1016/s0014-2999(00)00216-8. 2000;397:93-102. [DOI] [PubMed] [Google Scholar]
- 61.Sachdev M, Miller WC, Ryan T, et al. Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies. Am Heart J. 2002 Dec;144(6):1065–1073. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- 62.Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N.Engl.J.Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 63.Seuwen K, Magnaldo I, Pouyssegur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988;335:254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- 64.Bredberg U, Eyjolfsdottir GS, Paalzow L, et al. Pharmacokinetics of methysergide and its metabolite methylergometrine in man. Eur.J.Clin.Pharmacol. 1986;30:75–77. doi: 10.1007/BF00614199. [DOI] [PubMed] [Google Scholar]
- 65.Bana DS, MacNeal PS, LeCompte PM, et al. Cardiac murmurs and endocardial fibrosis associated with methysergide therapy. Am.Heart J. 1974;88:640–655. doi: 10.1016/0002-8703(74)90251-8. [DOI] [PubMed] [Google Scholar]
- 66.Hendrikx M, Van Dorpe J, Flameng W, et al. Aortic and mitral valve disease induced by ergotamine therapy for migraine: a case report and review of the literature. Journal of Heart Valve.Disease. 1996;5:235–237. [PubMed] [Google Scholar]
- 67.Hoyer D, Clarke DE, Fozard JR, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 68.Baumann MH, Ayestas MA, Dersch CM, et al. 1-(m-Chlorophenyl)piperazine (mCPP) Dissociates In Vivo Serotonin Release from Long-Term Serotonin Depletion in Rat Brain. Neuropsychopharmacology. 2001;24:492–501. doi: 10.1016/S0893-133X(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 69.Setola V, Roth BL. Screening the receptorome reveals molecular targets responsible for drug-induced side effects: focus on ‘fen-phen’. Expert Opin Drug Metab Toxicol. 2005;1(3):377–387. doi: 10.1517/17425255.1.3.377. [DOI] [PubMed] [Google Scholar]
- 70.Fitzgerald LW, Burn TC, Brown BS, et al. Possible role of valvular serotonin 5-HT2B receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- 71.Porter RH, Benwell KR, Lamb H, et al. Functional characterization of agonists at recombinant human 5-HT2A, 5- HT2B and 5-HT2C receptors in CHO-K1 cells. Br.J.Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopez-Ilasaca M. Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem.Pharmacol. 1998;56:269–277. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 73.Otani K, Tybring G, Mihara K, et al. Correlation between steady-state plasma concentrations of mianserin and trazodone in depressed patients. Eur.J.Clin.Pharmacol. 1998;53:347–349. doi: 10.1007/s002280050391. [DOI] [PubMed] [Google Scholar]
- 74.Setola V, Hufeisen SJ, Grande-Allen KJ, et al. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) Induces Fenfluramine-Like Proliferative Actions on Human Cardiac Valvular Interstitial Cells in Vitro. Mol Pharmacol. 2003 Jun;63(6):1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- 75.Droogmans S, Cosyns B, D’Haenen H, et al. Possible Association Between 3,4-Methylenedioxymethamphetamine Abuse and Valvular Heart Disease. The American Journal of Cardiology. 2007;100(9):442–445. doi: 10.1016/j.amjcard.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 76.Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007 Jan 4;356(1):6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- 77.Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, A Novel Selective Human 5-HT2C Agonist: In Vitro and In Vivo Pharmacological Characterization. J Pharmacol Exp Ther. 2008 Feb 5; doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 78.Halford JC, Harrold JA, Boyland EJ, et al. Serotonergic drugs : effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67(1):27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 79.Curioni C, Andre C. Rimonabant for overweight or obesity. Cochrane Database Syst Rev. 2006;(4):CD006162. doi: 10.1002/14651858.CD006162.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Curfman GD. Diet Pills Redux. N Engl J Med. 1997;337:629–630. doi: 10.1056/NEJM199708283370909. [DOI] [PubMed] [Google Scholar]
- 81.Devereux RB. Appetite suppressants and valvular heart disease. N.Engl.J.Med. 1998;339:765–767. doi: 10.1056/NEJM199809103391109. [DOI] [PubMed] [Google Scholar]
- 82.Rothman RB, Blough BE, Woolverton WL, et al. Development of a rationally-designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005;313(3):1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]