Abstract
Memory and learning in animals are mediated by neurotransmitters that are released from vesicles clustered at the synapse. As a synapse is used more frequently, its neurotransmission efficiency increases, partly because of increased vesicle clustering in the presynaptic neuron. Vesicle clustering has been believed to result primarily from biochemical signaling processes that require the connectivity of the presynaptic terminal with the cell body, the central nervous system, and the postsynaptic cell. Our in vivo experiments on the embryonic Drosophila nervous system show that vesicle clustering at the neuromuscular presynaptic terminal depends on mechanical tension within the axons. Vesicle clustering vanishes upon severing the axon from the cell body, but is restored when mechanical tension is applied to the severed end of the axon. Clustering increases when intact axons are stretched mechanically by pulling the postsynaptic muscle. Using micro mechanical force sensors, we find that embryonic axons that have formed neuromuscular junctions maintain a rest tension of ≈1 nanonewton. If the rest tension is perturbed mechanically, axons restore the rest tension either by relaxing or by contracting over a period of ≈15 min. Our results suggest that neuromuscular synapses employ mechanical tension as a signal to modulate vesicle accumulation and synaptic plasticity.
Keywords: MEMS, neuron, synapse, synaptic vesicle
The accumulation of neurotransmitter containing vesicles at the presynaptic terminal is essential for neural communication. On the arrival of an action potential at the terminal, neurotransmitters are released through exocytosis of the vesicles. The transmitters excite the postsynaptic terminal in a millisecond time frame (1). The amount of neurotransmitter release for a given action potential depends on several factors including how frequently the synapse has been used. This usage dependent plasticity is believed to be the basis of memory and learning (2). Despite a wealth of knowledge on the molecular components of the synapse (3, 4) and the modulation of the postsynaptic machinery during long-term potentiation and depression (5–7), the mechanism of vesicle accumulation at the presynaptic terminal and regulation of neurotransmission in a usage-dependent manner remains unclear (8). There is increasing experimental evidence suggesting that the mechanical microenvironment has a significant influence on a variety of cell functions including gene expression, cell growth and morphology, cytoskeletal organization, and apoptosis (9–13). Our in vivo experiments reveal that mechanical tension in axons plays a key role in vesicle accumulation.
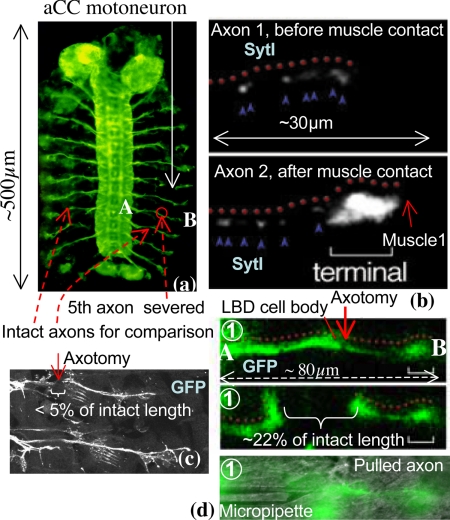
We examine the neuromuscular synapse within live embryos of Drosophila melanogaster (14) (Fig. 1A). In Drosophila, an aCC (anterior corner cell) pioneer motoneuron extends a single axon and invariably innervates its target, muscle1. A pair of aCC motoneurons occurs in every segment of the embryo, and their development is highly stereotyped. The first contact between the aCC axon and muscle1 occurs at hour 14 of embryogenesis, after which synaptogenesis progresses through a stereotyped sequence. Shortly after the first contact, the integral synaptic vesicle protein Synaptotagmin-I (SytI), essential for neurotransmission, begins to accumulate at the presynaptic terminal (Fig. 1B). SytI protein forms a complex with distinct molecules on the membrane of the neurotransmitter vesicles well before they are released (15). During the first 2 h of synaptogenesis, glutamate receptors begin to accumulate on the postsynaptic surface. Electrophysiological measurements on embryos (16, 17) show low background activity at the synapse, as well as a larger response to applied electrical fields. The background activity is because of a random release of vesicles in small quantities, whereas the stronger activity is because of coordinated vesicle release stimulated by the applied field. In this paper, we focus on the aCC axon terminal after its synaptic formation.
Fig. 1.
Synapse between aCC motoneuron and muscle1. (A) Embryonic nervous system of Drosophila (GFP) with aCC motoneurons. The figure also shows the axon that is severed in this study and the axons that are used for comparison. (B) Immunocytochemistry of the aCC axon with Synaptotagmin-I antibodies. Before the aCC contacts muscle1 (hour 13), Synaptotagmin-I is detected (blue arrowheads) along its axon (red dots). Soon after their initial contact (hour 14), Synaptotagmin-I (blue arrowheads) starts to exhibit distinct patterns of accumulation at the aCC axon terminal (bracket). The left end of the figure is the location of the LBD (lateral bipolar dendrite) cell and the location of the axotomy. (C) Axotomy of an axon before synaptogenesis. Here, the gap between the severed ends of the axon is small, <5% of the original length of the axon. (D) Axotomy of a typical axon AB (see A) after synaptogenesis. Now the gap between the cut ends is large, ≈22% of the original axon's length. The slack axon is pulled by a micropipette by holding the severed end with suction and moving the pipette with a stage. The motion is just enough to straighten the slack axon.
Experiments and Results
Axotomy Before Synaptogenesis Results in the Loss of Presynaptic Vesicle Clustering.
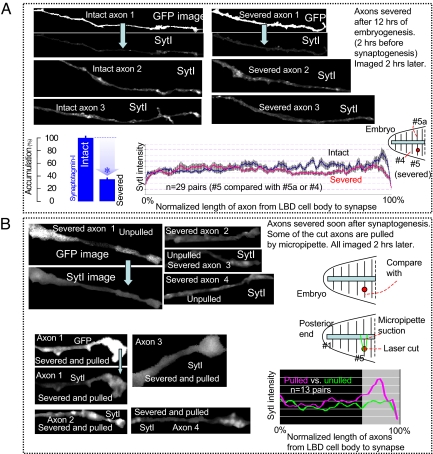
Using a laser, we severed the axon of 1 aCC motoneuron in the fifth segment (Figs. 1 and 2) from the posterior end of a wild-type embryo after ≈12 h of embryogenesis. At this stage of development, the axon is still growing toward its target muscle (Fig. 1C shows a typical axotomy). Thus, we physically and chemically isolated the advancing end of the axon (growth cone) from its cell body in the CNS (central nervous system). Two hours after the axotomy, we fillet dissected the embryo and stained it for SytI expression. We then compared the severed axon with its intact neighbor (Fig. 2A). We found that the growth cone of the severed axon reached its target muscle, just as the growth cone of the neighboring intact axon did. However, the axotomy decreased Synaptotagmin-I accumulation at the nascent synaptic site (Fig. 2A shows data from 29 embryos). The neighboring axon remains unaffected by the axotomy showing normal accumulation of SytI at the terminal. We note that the severed axon, in contrast to its intact neighbor, does not sustain any tension even after finding its target muscle. This suggests that contacting synaptic partners is not sufficient for presynaptic protein accumulation, and that the tension might be important.
Fig. 2.
Force-modulated presynaptic assembly in axons severed by axotomy. (A) SytI comparison between severed and intact axons before synaptogenesis. We take an embryo after ≈12 h of embryogenesis (2 h before axon-muscle contact). We severed the fifth right-side axon from the posterior end of the embryo. After 2 h, we dissected the embryo and stained for SytI. We compared this severed axon with the intact fifth left axon (#5a) or the fourth right axon of the same embryo depending on the quality of imaging. The figure shows 6 axons, 3 of which were severed, the rest were left intact. The GFP images for 2 axons are shown to identify their shapes. The rest of the images show SytI along the length and at the terminal. The left end of the figure is the location of the LBD (lateral bipolar dendrite) cell and the location of the axotomy (see Fig. 1). The length of the axons shown is ≈30–40 μm. We found that severed axons find their muscle targets just as their intact neighbors. However, the axotomy decreases SytI accumulation at the aCC axon terminal. The bar chart and the plot compare 29 pairs of severed and intact axons from 29 embryos. The plot shows the average vesicle density (29 severed and 29 intact axons) along the length of the axon up to the terminal. The x axis shows the normalized lengths of the axons from the LBD (left end) to the terminal (right end) (see also Image analysis in SI Text). The variability among the axons is shown by the standard deviation bars. (B) SytI comparison between the severed axons after synaptogenesis, 1 pulled, the other unpulled. We take 2 embryos at the same stage of development, within ≈30 min of synaptogenesis (∼hr 14 of embryogenesis). The pair of embryos is placed in the same solution and on the same microscope slide. In each embryo, we severed the fifth axon from the posterior end on the right side of the embryo, pulled the cut axon of 1 of the embryos using a micropipette within 10 min of severing, leaving the other unpulled. The pull was held for 2 h, after which both of the embryos were fixed and stained for SytI. Thirteen such pairs were imaged. Eight of such axon samples are shown with SytI. The GFP images for 2 axons are shown to identify their shapes. Restoring the tension at the nascent synapse allows Synaptotagmin-I to accumulate at the terminal (data from 13 pairs).
A Mechanical Pull on Post-Synaptogenesis Severed Axon Restores Vesicle Clustering.
In a separate experiment, we took a pair of embryos at similar stages of development (≈14 h of embryogenesis), placed them on the same glass substrate, dissected them, and severed an axon in each (Figs. 1 and 2B). Thus, we isolate the synaptic terminals from CNS and their cell bodies both chemically and physically. Within 10 min, we held and pulled the severed end of 1 of the axons with a micropipette (Fig. 1D). The pipette was moved such that the axon just became straight. The pull was held for 120 min. The other axon was left in a state of 0 tension. We then fixed and stained both of the embryos for SytI (Fig. 2B). We found that SytI does not cluster in the presynaptic terminal of the severed and unpulled nascent synapse, but does cluster in the pulled one (Fig. 2B, data from 13 pairs). Thus, mechanical tension alone restores neurotransmitter vesicle accumulation at the terminals without any signaling from the cell body or the CNS. The results also highlight the local autonomy of the force modulated presynaptic adjustment.
In Vivo Measurements Show Axons in Wild-Type Embryos Have a Rest Tension of 1 nN.
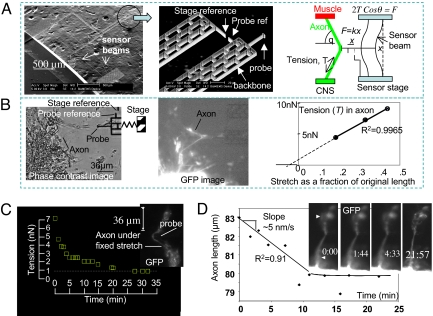
Because mechanical tension restores vesicle accumulation at the presynaptic terminal, we hypothesize that axons must be under a rest tension to sustain the accumulation. We measured tension in the axon using a micro mechanical probe attached to a force sensor with a spring constant, k = 3.5 nN/μm (Fig. 3A) (18), calibrated by an atomic force microscope (AFM). The sensor was microfabricated from a single crystal of silicon. The force was measured by 2 micromechanical beams. When a force is applied on the probe, the beams deform by x, as shown in Fig. 3A, giving a force F = kx. The deformation was measured optically using a reference probe (Fig. 3A) and subsequent image analysis with 0.1-μm resolution using an autocorrelation technique. This results in a force resolution of 0.3 ± 0.03 nN (see Materials and Methods section). The imaging time frame was <1 s (phase contrast mode), which sets the time resolution in the experiments. The probe was brought into contact with the axon of a dissected embryo (after ≈16 h of synaptogenesis) for ≈10 min to form a nonspecific adhesion with Silicon. Note that silicon forms a thin layer of SiO2 on the surface when exposed to air. Thus, the nonspecific adhesion was formed between the axon and the SiO2. The sensor was then moved by a piezo stage to stretch the axon. The tension in the axon was evaluated from the force balance. The corresponding stretch of the axon was calculated from geometry. The stretch-tension data (Fig. 3A), obtained within 2.5 min, shows a linear behavior. Extrapolation of the force-stretch line to a 0 stretch gives a rest tension of ≈1 nN (Fig. 3B). Extrapolating the force-stretch line to 0 force gives an intrinsic stretch of ≈5% of the total length (∼80 μm) of the axon. Therefore, an axotomy should lead to a retraction of the 2 cut ends of the axon, creating a gap of ≈5% of the total length of the axon. Our axotomy experiments on axons after synaptogenesis reveal that the gap is ≈15%–30% (many samples visualized, 4 recorded, Fig. 1 shows an example). Furthermore, the gap forms in a very short period (<0.5 s). Together, these observations provide conclusive proof that the axons are under tension. On the other hand, an axotomy before synaptogenesis results in a much smaller gap (<5% of intact length, Fig. 1C), and furthermore, the gap forms slowly over a period of several seconds. This suggests that there is little or no tension in the axons before synaptogenesis, possibly because of a lack of strong anchoring points (such as the muscle target) and that axons develop tension after the formation of the neuro-muscular junction. This is consistent with the observation that in vitro cultured neurons generate forces on their artificial substrates (19), which may stabilize 1 set of axon branches and retract other axon collaterals (20).
Fig. 3.
Rest tension in the axon and its self regulation. (A) Scanning electron micrograph of a micro mechanical force sensor (spring constant, k = 3.5 nN/μm) used to measure the force response of embryonic Drosophila axons. An x-y-z piezo stage held the sensor and brought the probe into contact with the axon to form nonspecific adhesion and apply stretch. The force, F = kx, on the probe was measured from the deflection, x, of the force sensing beams. The tension, T, in the axon was obtained from the force balance at the point of contact. (B) We dissected an embryo (after ≈16 h of embryogenesis) and removed the fat cells from around the fifth axon (from the posterior end of the embryo). We then used the probe to stretch the axon and measure its tension. The stretch was measured from geometry. The interval between the 2 data points was 50 s. Extrapolation of the force-stretch curve to 0 stretch point gave an estimate of the rest tension of ≈1 nN. (C) A probe pushed an axon at mid length in <1 s, and then held the stretch with time. The corresponding tension in the axon was measured as a function of time. (D) The probe was quickly released from a similarly stretched axon after its tension had relaxed to the rest value. The axon was overstretched as soon as the probe was removed. The time lapse images of the axon show that it shortened its length with time linearly with a velocity of 5 nm/s. The axon recovered its initial length in ≈10 min. R2 values of the linear fits in B and D are shown.
Force Relaxation in Axons in Response to Applied Stretch—Resetting of Tension to Rest Value.
To explore whether any force sensing mechanism exists in axons, we stretched an axon (Fig. 3C) using the force probe and held the stretch whereas the tension was monitored with time. Note that, here the stretch was induced by pushing the axon with the probe, in contrast to pulling (Fig. 3A). We assumed that pulling and pushing have similar mechanistic effect on the axonal behavior. The principle of force measurement was the same in both of the cases. However, pushing is preferred experimentally as it does not rely on adhesion between the probe and the axon. We found that tension increases instantaneously (<1 s) with applied stretch and decreases exponentially over time to a steady value of ≈1 nN, the rest tension. The time constant is ≈15 min. We then removed the probe suddenly, leaving the axon stretched. Subsequent monitoring of the axon revealed that it shortens its length by ≈3 μm and straightens itself in a period of ≈10 min (Fig. 3D). This shortening of the length is linear with respect to time, indicating a constant velocity. The change of length was measured by image analysis using Photoshop. The significance of this constant rate of shortening is discussed later.
Vesicle Clustering Increases in Response to Applied Stretch.
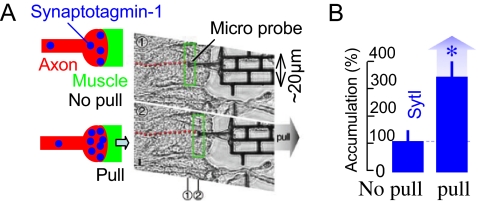
The rest tension in axons and the correlation between stretch and synaptic vesicle accumulation lead to the hypothesis that tension may be used to tune accumulation. For example, an increased stretch may enhance accumulation. We tested the hypothesis by using the force probe to directly apply force to muscle1 ≈2 h after synaptogenesis, stretching the axon (Fig. 4A). The corresponding stretch in the axon was ≈5% of its initial length. We found, in the wild-type embryos, that a single pull of muscle1 away from the aCC axon induces more than a 2-fold increase in the SytI accumulation at the axon terminal, as compared with controls (Fig. 4A, data from 6 samples). It takes ≈30 min to saturate the increase (Fig. S1), and the increased accumulation persists for at least 90 min afterward (the maximum time the axons were stretched). Whether there is any correlation between the various levels of applied stretch and the increased accumulation of vesicles remains to be seen. Note that well within 30 min of the mechanical stretch the axon relaxes its tension to the rest value (Fig. 3C). This suggests that increased vesicle clustering is a downstream event of the initial stretch and is maintained by the applied stretch in the presence of tension. Note that this time for vesicle accumulation is orders of magnitude longer than the time (ms) for increased neurotransmitter release at the motor nerve terminals in frogs because of muscle stretch (21, 22).
Fig. 4.
Force-modulated presynaptic assembly in axons. (A) A micro mechanical force probe contacts the muscle near the synapse in a wild-type embryo (≈16 h after embryogenesis) after fillet dissection, and pulls it, thus stretching the axon along its length (∼ 5% of its initial length). The axon was not subject to axotomy. The stretch was held for 90 min. The embryo was then stained for SytI. (B) We found that SytI accumulation in the pulled terminals was >2-fold higher compared with that in the unpulled neighboring terminals (data from 6 pairs, 100% implies the average value for the unpulled synapse).
Discussion
We draw several conclusions from these sets of experiments. First, the correct recognition of synaptic partners is not sufficient to initiate presynaptic protein accumulation at the nascent synapse; tension in the axon is essential. This tension possibly develops during synaptogenesis. To gain insight into the mechanisms that might contribute to this tension/stretch dependence of vesicle clustering, we note the following.
Time to Vesicle Clustering.
The time required for increased vesicle clustering because of stretch is long, >30 min. However, the time required by the axon to relax its tension after it is subjected to a sudden but sustained stretch is shorter, approximately 15 min. This suggests that clustering continues after tension relaxes, and both tension and stretch are required for vesicle clustering. Can this dependence explain vesicle clustering at the synapse during development?
Vesicle Clustering During Synaptogenesis—the Role of Muscle Force.
During the early phase of synaptogenesis when the growth cone approaches the target muscle, small protrusions (myopodia) reach out from the muscle toward the cone to form an interdigitated interface (23). The muscle protrusions then retract, pulling the axon. In our studies, axotomy before synaptogenesis prevents both tension development and muscle induced stretching during synaptogenesis, because the severed end is free. After 2 h, such synapses show a lack of clustering. Similarly, axotomy soon after synaptogenesis, and staining 2 h thereafter, shows a loss of clustering, possibly because of dissemination of vesicles in the absence of tension. A resupply of tension with a micropipette soon after axotomy, and staining 2 h thereafter, shows clustering. This clustering might be because of a relocalization of dispersed vesicles induced by the tension, or vesicle dispersion might have been inhibited by the immediate restoration of tension after the axotomy.
Origin of Tension—the Role of Actin.
Tension in the axons is possibly generated by actomyosin contractile machinery, where myosin bridges parallel F-actin. In axons of Drosophila embryos, the cytoskeleton consists of actin and microtubules. Thus, F-actin may bridge and transfer forces between focal complexes at the terminal and microtubules (24). Under an acute imposed stretch, the axonal cytoskeleton deforms elastically when the tension is high (18). Here, the imposed stretch rate is assumed to be much higher than the possible rate of growth of the axon under an applied stretch (25). With the stretch sustained, F-actin possibly slides with respect to each other giving rise to a relaxation of the tension. This possibility may be realized whether the applied force exceeds the force generated by the myosin motors holding the parallel actin fibers. Increased tension may also promote actin polymerization, as will be considered later (26, 27). When the stretched axon is released with no tension left, myosin may slide F-actin in the reverse direction resulting in shrinking of the axonal length with time. The time rate of length shortening is expected to be linear in the absence of tension, because each myosin can “walk” unimpeded at a constant velocity. Fig. 3 shows that the rate of shortening is indeed linear with time. Furthermore, the rate is ≈5 nm/s, surprisingly similar to the walking rate of a single myosin V on actin (4–8 nm/s) (28). The close correspondence between the velocity of a single motor and the macroscopic velocity of axon shrinking suggests that F-actin and motors are mostly located in 1 region of the axon, possibly near the synapse. If the motors and F-actin were distributed widely along the axon, then the macroscopic rate of shrinking would be much higher, because the shrinking would be contributed to by many regions of the axon, each region shrinking at 4–8 nm/s. Indeed, it is well known that both growth cones and presynaptic terminals are highly enriched by F-actin, whereas G-actin is distributed throughout the axon maintaining a steady pool for F-actin (29). Furthermore, actin perturbing drugs inhibit synaptogenesis (30). To estimate the number of motors involved in force generation, we note that each myosin motor generates a force of ≈2 pN (31). Thus, it would take ≈500 motors to generate a rest tension of 1 nN in the axons. This actomyosin based mechanism implies that tension generation in axons and its resetting to the rest value is governed by a local passive process, as long as the average number of active motor proteins remains steady with time. How can this tension be correlated with vesicle clustering? Co-localization of F-actin and vesicles at the presynaptic terminal reported in the literature (29, 32) may provide the link to this correlation.
Link Between Tension and Vesicle Clustering—Role of Actin.
It has been reported that depolymerization of actin in Drosophila results in a dissemination of vesicles from the synapse, and that actin may serve as a scaffold for the vesicles or as a conduit for the transport of vesicles within the terminal (29, 33, 34). Thus, increased F-actin polymerization at the synapse may result in increased vesicle clustering. Synaptic stimulation is known to increase F-actin polymerization at the presynaptic junction reversibly. Actin can return to the basal state through depolymerization within a few minutes of poststimulation (35). It is known that mechanical tension/stretch or cell substrate stiffness promotes focal adhesion clustering, increased actin polymerization, and cell stiffness (26, 36–41). The release of tension through myosin II inhibition using blebbistatin leads to actin depolymerization (42). Thus, a release of tension in the axon through axotomy may depolymerize actin at the synapse, which may facilitate the dissemination of clustered vesicles. A resupply of tension may initiate actin polymerization and restore clustering. An increased stretch/tension from the resting state of the axon may induce further actin polymerization and increased clustering via mechanical trapping or interaction forces between F-actin and vesicles.
Role of Cell Adhesion Molecules.
Force transfer through actin requires an anchor, which involves cell adhesion molecules connecting the axon with the muscle. Increased tension/stretch may lead to a conformational change in these molecules leading to signal cascades downstream that may induce actin polymerization. Indeed, high stretch sensitivity of motor nerve terminals has been attributed to integrin (22). Here, both the amplitude and the frequency of neurotransmitter release increase by several fold with the application of stretch on frog muscle. Stretch sensitivity is suppressed in the presence of peptides containing an Arg-Gly-Asp sequence, which is known to be bound by integrin. However, enhancement of transmitter release occurs within a few milliseconds of the stretch application, a time scale that is shorter by a few orders of magnitude compared with the time for vesicle accumulation (30 min) because of the applied stretch on Drosophila axons observed in our study. The long time delay in vesicle clustering implicates much slower and localized processes that may involve assembly and disassembly of submembrane plaques and co-clustering of signaling molecules. These processes may lead to cascades of downstream signaling events including polymerization and depolymerization of F-actin at the presynaptic terminal (36).
Role of Ion Flux.
One possible effect of stretch on axons is the enhanced ion flux through stretch sensitive ion channels. In particular, Ca2+ influx can trigger increased actin polymerization (40, 41), force generation, regulation, and downstream signaling cascades, as well as mediate vesicle localization under the membrane from which they are released. Mechanical stimulation using low frequency, low intensity ultrasound has been shown to excite neurons in mouse brain by activating voltage-gated sodium and calcium channels (43). The elimination of tension through an axotomy may inhibit local ion exchange and result in a subsequent loss of vesicle accumulation. A resupply of tension or stretch using a micropipette may reestablish the exchange and the corresponding accumulation. In this regard, we note that an axotomy and micropipette pulling may also result in an uncontrolled ion flux and leakage of intracellular content at the cut end of the axon. However, increased accumulation at the terminals of uncut axons because of stretch applied by micro probes suggests that the local effects at the cut ends do not have significant influence on accumulation.
The above discussion leads to the following hypothesis: F-actin serves as a scaffold for synaptic vesicles at the presynaptic terminal through interaction forces or mechanical entrapment. A rest tension is necessary to maintain F-actin. The rest tension is generated and maintained by acto-myosin machinery. A lack of tension leads to depolymerization of F-actin with a subsequent dissemination of vesicles. An application of stretch increases the tension in the axon momentarily, but under a fixed stretch, the tension relaxes to the rest value. This stretch/tension history promotes F-actin polymerization and hence, increased scaffolding, which leads to increased vesicle clustering.
There is an increasing body of evidence that points to the role of the mechanical microenvironment in determining cell functionality. Our experiments reveal that the clustering of neurotransmitter vesicles may be fundamentally linked with the mechano-sensitivity of synaptic terminals. The precise molecular mechanism of this mechano-sensitivity is yet to be uncovered.
Materials and Methods
Fly Stocks.
Experiments described in this study used wild-type (+/+) (source: M. Yoshihara) embryos. Some wild-type embryos were made to express membrane-targeted GFP (green fluorescent protein) in either all muscles (GAL424B/UAS-gapGFP) or all neurons (elav'-GAL4/UAS-gapGFP) for visualization purposes.
Immunocytochemistry.
Rabbit anti-Synaptotagmin-I (source: Hugo Bellen, 1:1,000 dilution) and goat anti-HRP (horseradish peroxidase) (source: Jackson Laboratories, 1:1,000 dilution) antibodies were used to assess the presynaptic terminal differentiation. Fluorescently-labeled secondary antibodies were used to detect the antibodies using a Zeiss LSM510 confocal microscope. All of the confocal microscopy settings were fixed for all experiments reported. Image analysis is described in SI Text.
Staging.
Embryos of different genotypes were staged individually based on the actual time that elapsed from the egg laying, their gut morphology, and the degree of their CNS condensation.
Axotomy.
A nitrogen pulsed dye laser VSL-3377ND (Laser Science, Inc.) was used to detach growth cones in whole Drosophila embryos. The laser was aligned with a Zeiss Axioskop, which was used for targeting and visualization of nerves. The laser was focused using a planoconvex (Newport) lens and passed across a 475 DRLP dichroic mirror (Chroma) and through an attenuator (Newport) to regulate the energy passed to the animal. The dichroic mirror allowed a simultaneous visualization of GFP (480 nm) and delivery of laser energy (440 nm).
Re-Supply of Mechanical Force.
A ‘patch-clamp’ micropipette was used to grab the end of the detached axonal growth cone with minimal suction. The end was held steadily until fixing and staining. The steps are as follows.
We severed hemi-segment 4 axons of 2 undissected, identically staged, genotyped embryos, whose aCC axons had reached muscle1 within the previous 30 min (≈14 h of embryogenesis). We then dissected both of the embryos on a single miniwell covered with saline. The severed end of 1 of the axons was gripped by a micropipette needle (controlled by a micromanipulator) by suction. We then retracted the needle just to the point where all of the slack in the axon was removed. We waited 2 h, then fixed and stained the embryos for SytI in the same solutions in the same mini wells.
Micro Mechanical Force Sensor.
Such sensors have been described previously (18). The force sensor was held and manipulated by a piezoelectric actuator. It was calibrated by an AFM (atomic force microscope) as follows. The sensor probe was used to push the AFM tip. The resulting deformations of the sensor beams and the AFM cantilever were measured. The spring constant of the force sensor was calculated from these 2 deformations, and the spring constant of the AFM cantilever, which was measured by thermal method (44) with 5% precision and 10% accuracy. Thus, the measured spring constant of the sensor is expected to be accurate within 10% as well. This results in a force resolution of 0.3 ± 0.03 nN. The probe of the sensor was used to contact the axon or the muscle to apply a stretch and measure the restoring force.
Supplementary Material
Acknowledgments.
We thank Professor Mike Sheetz (Columbia University), members of the Chiba lab, and Dr. Jagannathan Rajagopalan of the Saif lab for comments on the manuscript. This work was supported by grants from National Institutes of Health/National Institute of Mental Health (A.C.), National Institutes of Health/National Institute of Neurological Disorders and Stroke NS063405–01 (to A.C. and T.S.), National Science Foundation ECS 05–24675, National Science Foundation Civil, Mechanical and Manufacturing Innovation 0800870, National Science Foundation Electrical, Communications, and Cyber Systems 0801928 (to T.S.), and a Campus Research Board Grant, University of Illinois at Urbana–Champaign (T.S.).
Footnotes
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901867106/DCSupplemental.
References
- 1.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw Hill; 2000. [Google Scholar]
- 2.Hebb D. Organization of behavior. New York: Wiley; 1949. [Google Scholar]
- 3.Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Sheng M. Some assembly required: The development of neuronal synapses. Nat Rev Mol Cell Biol. 2003;4:833–841. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- 5.Siegelbaum SA, Kandel ER. Learning-related synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1991;1:113–120. doi: 10.1016/0959-4388(91)90018-3. [DOI] [PubMed] [Google Scholar]
- 6.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 7.Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nat Neurosci. 1999;2:597–604. doi: 10.1038/10154. [DOI] [PubMed] [Google Scholar]
- 8.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 9.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 10.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 12.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 13.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 14.Keshishian H, Broadie K, Chiba A, Bate M. The Drosophila neuromuscular junction: A model for studying synaptic development and function. Annu Rev Neurosci. 1996;19:545–575. doi: 10.1146/annurev.ne.19.030196.002553. [DOI] [PubMed] [Google Scholar]
- 15.Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa K, Kidokoro Y. Junctional and extrajunctional glutamate receptor channels in Drosophila embryos and larvae. J Neurosci. 1995;15:7905–7915. doi: 10.1523/JNEUROSCI.15-12-07905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrus SB, DiAntonio A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr Biol. 2004;14:924–931. doi: 10.1016/j.cub.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Saif T. Reversible and repeatable linear local cell force response under large stretches. Exp Cell Res. 2005;305:42–50. doi: 10.1016/j.yexcr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Heidemann SR, Lamoureux P, Buxbaum RE. Cytomechanics of axonal development. Cell Biochem Biophys. 1995;27:135–155. doi: 10.1007/BF02738107. [DOI] [PubMed] [Google Scholar]
- 20.Anava S, Greenbaum A, Jacob EB, Hanein Y, Ayali A. The regulative role of neurite mechanical tension in network development. Biophys J. 2009;96:1661–1670. doi: 10.1016/j.bpj.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen BM, Grinnell AD. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science. 1995;269:1578–1580. doi: 10.1126/science.7667637. [DOI] [PubMed] [Google Scholar]
- 23.Ritzenthaler S, Suzuki E, Chiba A. Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat Neurosci. 2000;3:1012–1017. doi: 10.1038/79833. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich D, Sackmann E. Active mechanical stabilization of the viscoplastic intracellular space of Dictyostelia cells by microtubule–actin crosstalk. Acta Biomaterialia. 2006;2:619–631. doi: 10.1016/j.actbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Pfister BJ, Iwata A, Meaney DF, Smith DS. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshpande VS, McMeeking RM, Evans AG. A bio-chemo-mechanical model for cell contractility. Proc Natl Acad Sci USA. 2006;103:14015–14020. doi: 10.1073/pnas.0605837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parekh SH, Chaudhuri O, Theriot JA, Fletcher DA. Loading history determines the velocity of actin-network growth. Nat Cell Biol. 2005;7:1219–1223. doi: 10.1038/ncb1336. [DOI] [PubMed] [Google Scholar]
- 28.Yildiz A, et al. Myosin V walks hand-over-hand: Single fluorophore imaging with 1.5-nm localization. Science. 2003;300:2061–2065. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- 29.Dillon D, Goda Y. The actin cytoskeleton: Integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. J Neurosci. 2001;21:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DC. Movement and force produced by a single myosin head. Nature. 1995;378:132–133. doi: 10.1038/378209a0. [DOI] [PubMed] [Google Scholar]
- 32.Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 33.Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 34.Cingolani LA, Goda Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 35.Colicos MA, Collins BE, Sailor MJ, Goda Y. Remodeling of synaptic actin induced by photoconductive stimulation. Cell. 2001;107:605–616. doi: 10.1016/s0092-8674(01)00579-7. [DOI] [PubMed] [Google Scholar]
- 36.Geiger B, Bershadsky A. Exploring the neighborhood: Adhesion-coupled cell mechanosensors. Cell. 2002;110:139–142. doi: 10.1016/s0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- 37.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 38.Janmey PA, McCulloch CA. Cell mechanics: Integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 39.Besser A, Safran SA. Force-induced adsorption and anisotropic growth of focal adhesions. Biophys J. 2006;90:3469–3484. doi: 10.1529/biophysj.105.074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glogauer M, et al. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. J Cell Sci. 1997;110:11–21. doi: 10.1242/jcs.110.1.11. [DOI] [PubMed] [Google Scholar]
- 41.Glogauer M, et al. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J Biol Chem. 1998;273:1689–1698. doi: 10.1074/jbc.273.3.1689. [DOI] [PubMed] [Google Scholar]
- 42.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121:2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 43.Tyler WJ, et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matei GA, Thoreson EJ, Pratt JR, Newell DB, Bumham NA. Precision and accuracy of thermal calibration of atomic force microscopy Cantilevers. Rev Sci Instrum. 2006;77 083703. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.