Abstract
The cosmopolitan parasitic pathogen Toxoplasma gondii is capable of infecting essentially any warm-blooded vertebrate worldwide, including most birds and mammals, and establishes chronic infections in one-third of the globe’s human population. The success of this highly prevalent zoonosis is largely the result of its ability to propagate both sexually and clonally. Frequent genetic exchanges via sexual recombination among extant parasite lineages that mix in the definitive felid host produces new lines that emerge to expand the parasite’s host range and cause outbreaks. Highly successful lines spread clonally via carnivorism and in some cases sweep to pandemic levels. The extent to which sexual reproduction versus clonal expansion shapes Toxoplasma’s current, global population genetic structure is the central question this review will attempt to answer.
Keywords: Toxoplasma gondii, Population genetics, Virulence, Outbreaks, Sexual recombination, Clonality
1. Introduction
Toxoplasma gondii is a tissue cyst-forming coccidian parasite in the order Apicomplexa, closely related to Neospora, Hammondia, Sarcocystis and Besnoitia. It is endemic worldwide, largely because it causes life-long chronic infections in all intermediate warm-blooded vertebrate hosts and produces infectious tissue cysts that are orally transmissible to most, if not all, predators globally. The definitive felid host sheds highly infectious oocysts in its feces, and transmission to all animals can occur by ingestion of food and water contaminated with oocysts. Toxoplasmosis is generally an asymptomatic disease infecting about 2 billion people worldwide. Most seropositive individuals are unaware that they are infected because they never develop symptoms. Those who do develop disease experience sequelae such as lymphadenopathy, mild fever and muscle weakness before resolving into a relatively benign, life-long infection. At-risk populations are pregnant women (who transmit infections to their fetuses) and the immunocompromised (ie., AIDS, transplant, and cancer patients), who are susceptible to newly acquired infections or reactivation of a potentially fatal disease as immunity wanes. Occasionally, disease is severe in healthy adults resulting in loss of vision, motor and cognitive function, even fatal encephalitis. In fact, toxoplasmosis is a leading cause of foodborne-related deaths in the USA, alongside salmonella, listeria and campylobacter (Mead et al., 1999). Contributing factors thought to be associated with disease are: i) route and size of the inoculum; ii) stage of parasite causing infection (ie,. oocysts versus bradyzoites); and iii) the genotype of the parasite. This highly successful, global pathogen was only discovered 100 years ago. This review will highlight the genetic origin, evolution and transmission dynamics of new strains that emerge in nature with the potential to cause disease and dominate most other strains worldwide.
2. History
2.1. The organism
Toxoplasma gondii was first discovered in a severely ill, hamster-like rodent, the gundi (Ctenodactylus gundi) that died of infection in the laboratory of Charles Nicolle at the Pasteur Institute, Tunis. It was named for its morphology (toxo- arc or bow, plasma-life) and for the host it infected (Nicolle and Manceaux, 1908, 1909). In parallel, Splendore identified a similar coccidian parasite in a rabbit from Brazil, confirming its widespread distribution (Splendore, 1908). A child who died of disseminated disease with parasites resembling Toxoplasma in the eye identified humans as susceptible hosts (Janku, 1923). The first viable organism was isolated by Albert Sabin in 1937 from laboratory mice during a routine virus testing experiment (Sabin and Olitsky, 1937), and later from humans by inoculating infected tissues into mice (Wolf et al., 1939). Sabin speculated that the parasite was an important zoonosis when tissue cysts isolated from animals and humans were the same (Sabin, 1941). Weinman hypothesized that human infection was via consumption of livestock (such as pigs) that were known scavengers of rodents (Weinman and Chandler, 1954). The ability of tissue cysts to withstand gastric pH supported this zoonotic hypothesis (Jacobs et al., 1960). Children fed raw lamb-meat in a sanatorium in France possessed high seroprevalence rates for Toxoplasma, indicating that undercooked meat was an important source for human infection (Desmonts et al., 1965). However, high prevalence rates among Jains in India, a population of strict vegetarians, suggested that other sources likely played a role in the transmission of toxoplasmosis (ie., via consumption of contaminated vegetables or tainted drinking water) (Rawal, 1959).
2.2. The life cycle
The T. gondii life cycle and definitive host was not established until 60 years after its discovery. The role of cats and wild felids as definitive hosts had to wait until William Hutchison orally fed tissue cysts to a cat (Hutchison, 1965). A highly infectious form was identified in the feces of the cat that was both environmentally stable and remained infectious to mice for up to 12 months. He hypothesized that Toxocara cati eggs shed in felid feces served as a carrier of the infective material. This idea was later disproved by Sheffield and by Frenkel using cats that were free of Toxocara (Sheffield and Melton, 1968; Frenkel et al., 1969). In 1970, Frenkel, Dubey and Miller identified a complex life cycle for Toxoplasma. It involves sexual replication in the definitive felid host and asexual propagation in essentially any warm-blooded vertebrate, which was later shown to include marsupials and birds (Frenkel et al., 1970; Dubey and Beattie, 1988). Indeed, Toxoplasma is the “exception” among tissue dwelling coccidia; tissue cysts are orally infectious among intermediate hosts. Thus, propagation in nature is not solely dependent on sexual reproduction, and the parasite can be transmitted asexually by carnivorism (Su et al., 2003; Khan et al., 2007).
Why sexual stages develop solely in the enterocytes of the cat intestine continues to be an intriguing question (Dubey and Frenkel, 1972; Ferguson, 2002). When animals ingest oocysts or eat infected prey harbouring Toxoplasma tissue cysts, haploid zoites excyst in the intestinal lumen and invade enterocytes to establish infection. Only in felid enterocytes do they differentiate intracellularly into micro- and macrogametes. Male gametes fertilize maternal macrogametes (which possess the apicoplast and organelles) to produce a diploid zygote (Ferguson et al., 1974, 1975; Ferguson, 2002). This is important for two reasons. i) In the absence of predetermined mating types (as for example in yeast), a single parasite can effectively self-mate and be clonally amplified by cats. ii) If a cat ingests prey infected with more than one strain of the parasite, then sexual reproduction can proceed and bear progeny that are a mix of the two parental strains. The sexual cycle culminates with the production of oval-shaped oocysts 10–12 um in length that are shed into the environment. Egg-shedding typically begins 6–8 days p.i. in naïve felids, often lasts up to 8 days, and can produce in excess of 100 million oocysts (Dubey and Frenkel, 1972). In the environment, oocysts sporulate in the presence of oxygen to produce two sporocysts, each containing four highly infectious, haploid sporozoites that can persist in moist environments for months to years (Dubey et al., 1970). Oocysts are quite resistant to environmental and chemical destruction; they survive dilute bleach or chlorine, but are readily destroyed by dessication or high temperatures (Frenkel et al., 1975). These properties underscore Toxoplasma’s success in nature; transmission in all animals (herbivores and carnivores) is possible, and the parasite maintains a high prevalence in most warm-blooded vertebrates world-wide.
2.3. Transmission
The global prevalence of Toxoplasma is largely the result of its incredibly flexible life cycle. Two major routes of transmission exist in nature (sexual and asexual) and three different developmental forms (sporozoites, bradyzoites and tachyzoites) mediate colonization, dissemination and transmissibility of infection to a vast array of susceptible hosts.
Sexual transmission is mediated by sporozoites, which are encased in environmentally resistant oocysts shed in cat feces. Here, transmission occurs through ingestion of vegetables or drinking water contaminated with sporulated oocysts. These sporocysts are orally transmissible to all warm-blooded vertebrates (herbivores and carnivores) and have been linked to epidemiological human outbreaks in for example Panama, Canada, India and Brazil (Benenson et al., 1982; Bowie et al., 1997; Burnett et al., 1998; Palanisamy et al., 2006; de Moura et al., 2006). Once in the intestinal lumen of susceptible hosts, sporozoites replicate in enterocytes and myeloid cells in the lamina propria prior to differentiating into a rapidly growing, lytic tachyzoite that disseminates infection in a naïve host.
Asexual transmission is via ingestion of bradyzoite tissue cysts when carnivores eat raw or under-cooked meat from Toxoplasma-infected prey. Bradyzoites are also highly infectious, establish chronic infections in long-lived cells and persist for the life of the host, apparently impervious to host immunity (Frenkel, 1973). The ability of the parasite to readily interconvert between bradyzoites and tachyzoites in intermediate hosts is thus critical for the success of this asexual phase. Tachyzoites induce a strong immune response that specifically targets the proliferating zoite for destruction. Toxoplasma survives sterilizing immunity by differentiating into bradyzoites and forming tissue cysts. Only the bradyzoites are transmissible, but rapid tachyzoite proliferation is required in order to disseminate and amplify enough parasites to ensure sufficient bradyzoites prior to the induction of host immunity (McLeod et al., 1988). Thus bradyzoite-tachyzoite interconversion serves to regulate the parasite’s pathogenesis, its long-term persistence and its transmissibility.
The extent to which either cycle contributes to the successful propagation of the parasite in nature is enigmatic. Answers to this question will need to wait until reagents are developed that are capable of detecting the stage of parasite that initiates infection (ie., sporozoite versus bradyzoite). Recent work has shown however, that host-specific factors likely exist, rendering some species more susceptible to infection, dependent on the infective stage ingested. For example, rodents and swine are highly susceptible to infection via the sexual cycle; oral inoculation of a single oocyst is sufficient (Dubey et al., 1996b), whereas mice are relatively more resistant and often require doses in excess of 100 bradyzoites before infection will take (Dubey, 2001). In complete contrast, cats can shed hundreds to millions of oocysts after ingestion of only a single bradyzoite (Dubey, 2001). Whereas cats are more resistant to infection via oocysts (>100 oocysts are required to initiate infection), not all cats infected with oocysts go on to shed oocysts (Dubey, 1996a). Hence, the infectivity of different animals by either sporozoites or bradyzoites may play a contributing, or even crucial role, in transmission dynamics, the prevalence and population genetic structure of Toxoplasma for any given ecological niche and geographical location.
3. Population genetics
3.1. Genetic diversity
The majority of coccidian parasites are host-specific. They possess strictly heteroxenous (two-host) life cycles that cycle between an intermediate host that produces infective tissue cysts and a definitive host that produces oocysts via a sexual cycle. In these obligatory two-host life cycles, ingestion of tissue cysts and/or oocysts by an inappropriate carnivorous host does not support asexual growth, nor does it support the production of tissue cysts that are infective to definitive hosts. Hence, speciation is common because the parasites only produce infective tissue cysts in a highly restricted number of intermediate hosts that serve as prey for the definitive predator host.
Toxoplasma is the exception. It is the generalist among tissue-cyst forming coccidia. The life cycle can be maintained by scavenging among carnivores (asexual expansion) or by sexual recombination in the definitive felid host (sexual expansion). For a parasite with few geographical or host barriers, and possessing a highly fecund sexual cycle in cats, the genetic diversity among isolates was expected to be substantial. This is certainly the case for the vast majority of isolates so far collected from sylvatic cycles throughout the world (ie., transmission among wild animals) and specifically North and South America, where much of the work has been carried out. But this is not the rule for isolates circulating in domestic cycles. These latter isolates, principally from symptomatic humans and their domestic animals in the USA and Europe, are genetically restricted to only several discrete lineages that are highly clonal in nature. The simplest explanation for the evolution of this type of population genetic structure is one that relies on the flexibility of this parasite’s complex life cycle (see below).
3.2. Domestic cycle
Toxoplasma can be clonally propagated either i) asexually because tissue cysts are orally infectious (Su et al., 2003), or ii) sexually within feline species via self-fertilization (or inbreeding), a process that yields genetically identical progeny that are clones of the infecting parent strain (Sibley and Boothroyd, 1992; Sibley et al., 1992). These properties establish that transmission of Toxoplasma need not be entirely dependent on sexual reproduction between two different parasite strains. Early studies using multi-locus isoenzyme (Darde et al., 1988, 1992) and PCR–restriction fragment length polymorphism (PCR-RFLP) analyses (Sibley et al., 1992; Howe and Sibley, 1995) applied against a large number of isolates infecting symptomatic humans and livestock of mainly European and North American origin revealed remarkably little variation across different genetic loci and extensive linkage disequilibrium among strains. These data suggested that Toxoplasma possesses a strikingly clonal population genetic structure composed of just three biologically distinct lineages designated Types I, II and III (Darde, 1996; Sibley and Howe, 1996). Type I strains are acutely virulent in mice and grouped together as monophyletic using a highly polymorphic BS microsatellite marker (Sibley and Boothroyd, 1992). The majority of mouse non-virulent strains grouped into the other two clonal lineages: Type II strains dominate and cause the majority of human infections (at least in Europe) (Howe et al., 1997; Ajzenberg et al., 2002, 2005; Morisset et al., 2008) and Type III strains have been found infecting both humans and livestock (Howe and Sibley, 1995). A clonal population structure was entirely unexpected, especially since cats are both highly prevalent and widely distributed in these two environments. To explain the clonality found among isolates occupying the domestic cycle, parasite propagation was proposed to be largely asexual either via oral transmission of bradzyzoite cysts or by inbreeding (Sibley and Boothroyd, 1992; Darde, 1996). The idea for inbreeding was principally because few prey had been identified superinfected with more than one parasite strain (Ferguson, 2002). Furthermore, inbreeding in Plasmodium falciparum, a related Apicomplexan parasite with an obligate sexual phase in its insect mosquito vector, was also known to occur despite the presence of mixed infections in high-transmission endemic sites that should have promoted out-crossing (Razakandrainibe et al., 2005). However, this is not a likely explanation for the apparent clonality found among Toxoplasma isolates in the domestic cycle. Experimental laboratory crosses have shown that, unlike Plasmodium, no obvious barriers for meiotic recombination exist in Toxoplasma, and out-crossing is actually favoured (Pfefferkorn and Kasper, 1983; Pfefferkorn et al., 1983; Sibley et al., 1992; Su et al., 2002). This is reflected by the presence of recombinant strains in nature (Grigg and Suzuki, 2003; Ajzenberg et al., 2004). Furthermore, mixed genotype infections are readily induced experimentally (Reikvam and Lorentzen-Styr, 1976; Araujo et al., 1997; Dao et al., 2001) and mixed strain infections are increasingly being identified in prey indigenous to geographic sites where felid hosts drive the sylvatic cycle (Ajzenberg et al., 2002; Su et al., 2006; Dubey, 2008a, 2008c).
More recent data has suggested an alternative explanation; one that invokes meiotic recombination as an effective force driving the emergence and predominance of Type I, II and III lineages in the domestic cycle. Extensive multi-locus DNA sequencing has identified just two major allelic types that segregate randomly for each locus among the three clonotypes (www.toxodb.org; Grigg et al., 2001a, 2001b). Moreover, the existence of less common, naturally occurring “recombinant” genotypes that possess different inheritance patterns for the two allelic types across loci clearly show that clonotypes I, II, and III are the dominant progeny (at least in terms of shear numbers) from a genetic out-crossing between two distinct founders (Grigg et al., 2001a). Coupling extensive, genome-wide bi-allelism, with a near total lack of “within-lineage” single nucleotide polymorphisms (SNPs) at neutral loci (ie., introns, that are not under selection pressure for diversification), genetic drift calculations date the archetypal lineages to recently-derived meiotic recombinants from a sexual cross (Grigg et al., 2001a; Su et al., 2003). Based on neutral mutation rates calculated for other pathogens, Su et al. (2003) postulated the three archetypes emerged sometime within the past 10,000 years. Whether the timing of the cross and/or clonal expansion turns out to be 100, 1000, or 10,000 years will likely require a Toxoplasma fossil and exhaustive genome-wide comparative analyses to produce more accurate genetic drift calculations. Remarkably little variation among archetypes has likewise been identified among highly polymorphic microsatellite loci, supporting the recent clonal expansion interpretation (Blackston et al., 2001; Ajzenberg et al., 2002; Lehmann et al., 2006). In aggregate, the data show that sexual reproduction has produced three parasite lines that represent recent clonal rockets in the domestic cycle.
Understanding the genetic basis for how three dominant lines can emerge is of considerable importance from both public health and economic perspectives. Boyle and colleagues asked how many crosses it took to generate the three archetypes (Boyle et al., 2006). To do this, they performed a genome-wide analysis of the pattern of SNPs that segregated among the three extant archetypes to interrogate the number of generations separating them by constructing a genealogy (or family history). They showed that most chromosomes contained a maximum of only two different SNP-type regions, and that these regions were found in contiguous segments with single transition (or “recombination”) points. The Boyle data demonstrated that only a small number (or even just one) cross(es) is sufficient to dramatically alter the natural population genetic potential of Toxoplasma. What is less clear is why these three lines emerged from the cross(es) to become dominant clonotypes of the domestic cycle.
One biological explanation proposed for this expansion is that tissue cysts from archetypal lines possess an increased capacity for oral infectivity between intermediate hosts (Su et al., 2003). Such enhanced oral transmissibility certainly provides a rationale for why some strains expanded clonally, but wider sampling showed that many less common “recombinant”, as well as many atypical strains, were likewise orally infectious, and so the trait is not tightly associated with the origin of the three clonotypes (Fux et al., 2007; Khan et al., 2007). More compelling is the recent evidence based on calculating SNP frequencies on a genome-wide level. These data show that archetypal lineages, as well as many clonal strains isolated from South America, possess a common monomorphic Chromosome Ia (Khan et al., 2006, 2007). The fixation of Chromosome Ia has been suggested by Sibley and Ajioka (2008) to contain a unique combination of alleles that confers some form of selective advantage. They hypothesized that an as yet unmapped trait facilitated the recent clonal emergence of those strains bearing a Type II-like Chromosome Ia.
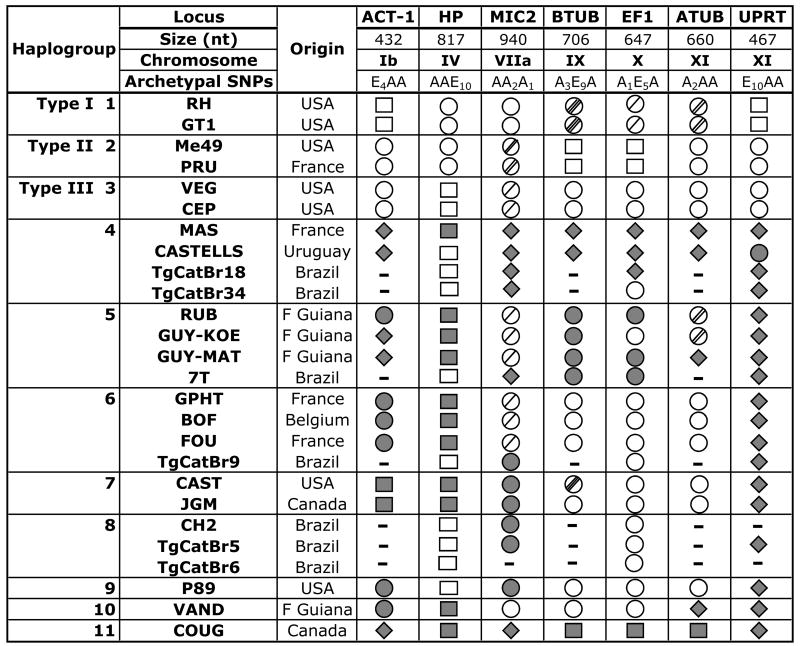
Strains other than the three dominant clonotypes have been identified in the domestic cycle. These strains possess a different mix of alleles, or entirely new alleles altogether, and represent less than 5% of strains collected from these sites (Howe and Sibley, 1995). These so-called “atypical” or “exotic” strains have also been identified in other parts of the globe (Lehmann et al., 2006). To better understand the genetic relationship among these non-archetypal strains, Khan and colleagues (2007) performed DNA sequencing against 46 strains collected world-wide using six mostly unlinked introns; genetic markers which are presumed to be selectively neutral. Their results showed that Toxoplasma possesses four distinct ancestral gene pools that resolve into 11 distinct lineages (or haplogroups), some of which show a strong geographic bias, and they concluded that sexual recombination between lineages is infrequent (Khan et al., 2007; Sibley and Ajioka, 2008). However, these results should be interpreted cautiously. It is clear that the genomic location of genetic markers used for strain-typing can have an enormous effect on the interpretation of strain relationships. When only six markers were applied against strains such as P89, TONT, P80 and SSI, they were originally thought to be recombinant progeny from the cross that gave rise to clonotypes I, II and III. But additional markers soon identified unique alleles that proved these strains had undergone additional introgressions with genetically unrelated ancestral lines (Grigg et al., 2001a; Grigg and Suzuki, 2003; Boyle et al., 2006). Hence, it is entirely possible that analyses using a small number of randomly selected loci will identify extensive linkage disequilibrium and erroneously conclude that recombination does not play a significant role in generating strain diversity. Archetypal strains I, II and III are widely accepted as genetic recombinants that possess significant linkage disequilibrium for example. When additional markers were applied against many of the same Brazilian strains analyzed by Khan and Sibley, a highly reticulated phylogenetic structure was identified that rather suggests recombination plays an important role in the diversification of strains, at least in South America (Pena et al., 2008). Furthermore, a basic tenet in molecular phylogeny is to concatenate sequences only for strains that share the same branching topology at all loci, otherwise important associations due to recombination will be effectively diminished. This is especially important when polymorphism among alleles is below 1% (Maynard Smith and Smith, 1998), as is the case with Toxoplasma introns. Indeed, reassembly of the intron sequencing data without concatenation from Khan and colleagues (2007) (www.toxohapmap.wustl.edu) shows that recombination can explain the relationship of alleles among many strains in most haplogroups. For example, in Haplogroup 7, CAST and JGM are in fact recombinants that possess unique alleles at four loci, Type III strain alleles at two loci, but are recombinant at the B-TUB locus (Fig. 1). Both CASTELLS (haplogroup 4) and RUB (haplogroup 5) are recombinants at Chromosome XI when compared to other strains within the same group. Furthermore, archetypal alleles have introgressed with novel alleles among strains in haplogroups 4, 5, 6, 7, 8, 9 and 10. The fact that many of the same recombinant genotypes have been identified in disparate geographies likewise supports the observation that clonal amplification of some lines is occurring in nature. This produces a model whereby frequent introgressions and clonal expansions are two powerful forces driving the evolution of the global population structure for T. gondii.
Fig. 1.
Allele inheritance patterns among Toxoplasma gondii strains. Multi-locus sequence analysis of strains from 11 haplogroups revealed different combinations of archetypal and unique alleles randomly inherited among seven mostly unlinked intron loci. Consensus is defined as the nucleotide sequence common to at least two of the three archetypal strains. For Archetypal single nucleotide polymorphisms (SNPs), each subscript identifies the number of unique polymorphisms relative to consensus. For archetypal strains I, II and III, only two allelic classes exist. The “A” allele is an open circle ○ and is defined as the allelic class shared by at least two of the archetypal strains. The “E” allele is an open square □. Where variation exists within the A allelic class, hatches through the circle  ; defines the number of unique nucleotide polymorphisms. Shaded circles
; defines the number of unique nucleotide polymorphisms. Shaded circles  ; and squares
; and squares  indicate a drifted archetypal allele, where polymorphism is less than 0.4% from archetypal. Shaded diamond
indicate a drifted archetypal allele, where polymorphism is less than 0.4% from archetypal. Shaded diamond  represents a unique allele with >0.4% polymorphism from the A or E alleles. “-” indicates no sequence available.
represents a unique allele with >0.4% polymorphism from the A or E alleles. “-” indicates no sequence available.
3.3. Sylvatic cycle
Recent multi-locus PCR-RFLP analyses followed by limited DNA sequencing using either microsatellite markers, a combination of microsatellites and polymorphic gene loci, or limited numbers of intronic loci (which should not be under any selection pressure for diversification) have identified a myriad of archetypal as well as highly divergent alleles that segregate differently among isolates collected from wild animals in geographically isolated parts of the world (ie, jungles of French Guiana, the Amazon basin in Brazil, the Boreal forests of Canada) (see Fig. 1). A large diversity of strains have been identified infecting humans, chickens, wild cats throughout South America (Ajzenberg et al., 2004; Dubey et al., 2006; Ferreira Ade et al., 2006; Khan et al., 2006, 2007; Lehmann et al., 2006; Su et al., 2006; Dubey, 2007; Pena et al., 2008), in wild animals in North America (Lehmann et al., 2000; Dubey et al., 2004; Dubey, 2008a, 2008b, 2008c), as well as a unique lineage of strains, called Type X, recently identified infecting Pacific coastal marine mammals including mustellids, pinnipeds, manatees and cetaceans (Cole et al., 2000; Miller et al., 2001, 2004; Dubey et al., 2003; Conrad et al., 2005; Sundar et al., 2008). Indeed, haplotypes of alleles in sylvatic strains have been shown to be inherited in discrete blocks, apparently randomly throughout different parts of the genomes in these “atypical” strains. These data can be used to explain the extensive linkage disequilibrium found by Khan and colleagues (2007) when only a few genetic markers are applied, and indicate that recombination is likely an effective force driving genetic diversity in the T. gondii sylvatic cycle. Hence, the diversity and inheritance of alleles among sylvatic strains supports a panmictic population genetic structure for Toxoplasma. Given the flexible life cycle for this parasite, it is likely that the sheer diversity of strains infecting a variety of prey in wild cycles globally (ie., jungles of South America, forests of Canada), breeds the emergence of new “successful” strains capable of punctuated clonal expansions that can “sweep” the domestic cycle.
3.4. Outbreaks
In recent years, over a dozen outbreaks of toxoplasmosis have been recorded and all produced symptomatic infections that were identified principally because of their severity (Table 1). It is likely that additional outbreaks are occurring, but they produce asymptomatic infections that go unnoticed. It is becoming increasingly apparent that disease-producing strains emerge from sylvatic cycles, and that parasite genotype likely plays a major role in determining disease severity (Boothroyd and Grigg, 2002). As just one example, Type I and “atypical” strain genotypes have been shown to be associated with recurrent and severe ocular disease in healthy adults (Glasner et al., 1992; Silveira et al., 2001; Grigg et al., 2001b).
Table 1.
Human toxoplasmosis outbreaks.
| Place of outbreak |
Route of Transmission |
Stage involved |
People exposed |
People symptomatic |
Isolate/ genotype |
Date | Symptoms | Reference |
|---|---|---|---|---|---|---|---|---|
| Alabama, USA | Cat feces contamination | Oocyst | 30 | 10 | - | 1976 | Chorioretinitis, fever, neurologic deficits | Stagno et al., 1980 |
| Atlanta, USA | Inhalation | Oocyst | 37 | 37 | - | 1977 | Febrile reaction | Teutsch, et al., 1979 |
| Panama | Water | Oocyst | 35 | 29 | - | 1979 | Fever, Lymphadenopathy, myalgia, abdominal pain, stiff neck and eye pain | Benenson et al., 1982 |
| Victoria, Canada | Water | Oocyst | 2894 – 7718 | 110 | Atypical | 1995 | Retinitis, lymphadenopathy | Bowie et al., 1997, Burnett et al., 1998 |
| Korea | Raw liver, spleen-wild pig | Bradyzoite | 3 | 3 | - | 1996 | Unilateral chorioretinitis | Choi et al., 1997 |
| Korea | Raw liver- domestic pig | Bradyzoite | 11 | 5 | - | 1996 | Lymphadenopathy | |
| USA | Raw venison | Bradyzoite | 5 | 5 | - | 2000 | Flu like symtoms with visual loss and retinits | Ross et al., 2001 |
| Santa Isabel | Water | Oocyst | 426 | 155 | Atypical | 2001 | Headache, malaise, myalgia, lymphadenitis, arthralgia | de Moura et al., 2006 |
| Turkey | Cat feces contamination | Oocyst | 1797 | 171 | - | 2002 | Lymphadenopathy, fever, myalgia, dizziness, headache | Doganci et al., 2006 |
| Suriname | Water | Oocyst | 11 | 8 | Atypical | 2003 | Fever, myalgia, impairment, congenital abortion and death | Demar et al., 2007 |
| India | Water | Oocyst | Not assessed | 213 | - | 2004 | Chorioretinitis | Palanisamy et al., 2006 |
“-” indicates no isolate recovered or strain genotyped from infected material
Outbreaks and the emergence of new pathogenic strains that circulate in sylvatic cycles often occur at the edges where human society collides with wildlife during deforestation and urbanization of previously ecologically remote or pristine environments. It is here where mammals (ie., rodents, livestock, cats and dogs), moving freely in and out of human dwellings, often in rural communities, mix with sylvatic animals (ie., wild cats, lynxes, birds) that are attracted to humans and their domestic food sources. At this interface between domestic and sylvatic cycles, new strains can be transmitted or gain access to domestic cycles and emerge as epizootics to cause debilitating outbreaks.
A water-borne outbreak in Victoria, Canada (March 1995) of acute toxoplasmosis affecting >4,000 people identified more than 100 individuals who experienced overt clinical disease (Bowie et al., 1997; Burnett et al., 1998). Investigations performed to trace the source and spread of the parasite identified a local reservoir that was epidemiologically linked with this severe toxoplasmosis outbreak (Eng et al., 1999). Transmission was the result of drinking unfiltered water from an open reservoir contaminated with oocysts from wild cougars (Aramini et al., 1998, 1999; Isaac-Renton et al., 1998). The one isolate recovered from the outbreak was Type I-like, and shown to be acutely virulent in animal models of infection (Shobab, James and Grigg, unpublished data). The Victoria outbreak was also associated with a high incidence (~21%) of toxoplasmic retinochoroiditis (Burnett et al., 1998), which is 10 times above the 2% prevalence rate for ocular toxoplasmosis among infected individuals in North America (Holland, 2003, 2004).
Three additional outbreaks of water-borne epizootics with high levels of associated eye disease highlight the importance of the intersection and transmission of sylvatic strains in domestic cycles (Table 1). In Panama, 35 USA army recruits contracted disease after drinking unfiltered water from a local pond during a routine training mission. Recruits experienced symptoms of fever, lymphadenopathy, abdominal pain, headaches and eye disease (Benenson et al., 1982). In the town of Santa Isabel do Ivai, Parana state, Brazil, an outbreak in 2001 affected at least 426 individuals and was epidemiologically linked to a contaminated cistern that served the town’s water supply. One hundred and fifty-six people were identified as having acute infections with elevated titers of Toxoplasma-specific IgM and IgG antibodies. Two isolates obtained from water filters collected at the time of the outbreak were genotypically identified as Type I-like strains and at least 10% of people infected developed eye disease (de Moura et al., 2006). In September 2004, a sudden increase in ocular toxoplasmosis cases was observed in a regional eye hospital at Coimbatore, India. Over a period of a few months, 249 cases tested positive for Toxoplasma antibodies and 178 of these cases had high titres of both IgM and IgG, indicating acute infection. A total of 213 patients had active ocular lesions. The outbreak was consistent with a point source contamination from an unfiltered municipal water supply (Palanisamy et al., 2006). These studies identify the importance of filtering water supplies at risk of environmental contamination via feral or wild cat oocyst deposition; water has also been shown to be a main source of both symptomatic and asymptomatic infection in different parts of Brazil (Bahia-Oliveira et al., 2003; Heukelbach et al., 2007).
Additional outbreaks have been reported and sources of infection include inhalation of aerosolized oocysts or eating undercooked meat. A 1976 outbreak involving a family in Alabama, USA was epidemiologically linked to the ingestion of soil contaminated with cat feces (Stagno et al., 1980). A 1977 outbreak in Atlanta, USA was attributed to nasopharyngeal ingestion of dust contaminated with oocysts from feral cat feces aerosolized during an equestrian event (Teutsch et al., 1979; Dubey et al., 1981). In Korea, unilateral chorioretinitis was observed in three patients after eating raw pork from a wild pig (Choi et al., 1997). A 2002 outbreak in Turkey identified 171 students living in a boarding school who developed acute toxoplasmosis with elevated levels of both IgM and IgG antibodies. The exact cause of the outbreak was not identified, but the presence of stray cats in a shelter near the dining hall was suspected (Doganci et al., 2006). Finally, in a Surinamese village near French Guiana, an outbreak of multivisceral toxoplasmosis in 11 immunocompetent patients was particularly severe and resulted in three deaths (Demar et al., 2007). The source of infection was never established, but the settlement is surrounded by jungle, and infection was likely the result of accidental ingestion of oocysts shed from sylvatic cats that roam freely at the edge of the village during the night.
As yet, no systematic study has been done to investigate the phylogeographical relationship among outbreak and endemic strains circulating in afflicted communities just prior to, during and after an outbreak. Tracking the sources, transmission dynamics and molecular origins of sylvatic strains associated with outbreaks will be important studies to perform that will ultimately help to identify the molecular basis for the emergence of parasite strains from sylvatic cycles that possess epidemic potential.
Outbreaks and the emergence of toxoplasmosis impact not just people, but also animals that share the same ecological niche. A recent outbreak of protozoal meningoencephalitis attributed to a clade of Toxoplasma strains referred collectively as Type X has been identified infecting marine mammals off the coast of California. Type X is responsible for greater than 75% of all T. gondii sea otter infections (Miller et al., 2004; Conrad et al., 2005; Sundar et al., 2008) and this protozoal disease has impacted the recovery of the threatened southern sea otter, as well as other sub-adult marine mammals including pinnipeds, cetaceans and mustelids (Dubey et al., 2004). Why Type X produces such a virulent infection in some sea otters and whether Type X emerged recently to infect marine mammals is not currently known. A major risk factor for infection of sea otters was determined previously to be coastal surface fresh water runoff, and peaks of otter mortality typically spike after seasonal storms along the California coast (Miller et al., 2002). The source and which hosts served as the reservoir of Type X infections in nature was enigmatic until the parasite was identified infecting sylvatic animals (mountain lions, bobcats and foxes) that reside in coastal watersheds adjacent to sea otter habitat (Miller et al., 2008). When Type X Toxoplasma DNA was identified in a California mussel collected from an estuary draining into sea otter habitat, it quickly became evident that marine invertebrates concentrate oocysts deposited by sylvatic felines in surface water run-off that flows from land-to-sea (Miller et al., 2008). Marine bivalves are filter feeders that represent a staple in the sea otter diet, are known to concentrate infectious T. gondii oocysts in the laboratory (Arkush et al., 2003; Lindsay et al., 2003, 2004), and likely represent one route of exposure for near-shore dwelling sea otters. Why Type X strains dominate the marine environment and cause death among sea otters is unclear, so identifying the genetic basis for their emergence and prevalence (ie., are they particularly virulent clonal progeny? Are they related to archetypal I, II and III strains?) will be important studies in order to mitigate Type X transmission and disease in marine mammals.
3.5. Sexual recombination and virulence
A pathogen’s virulence or ability to cause disease in a susceptible host is often an accurate gauge by which to measure its likely success, in terms of transmissibility, penetrance in new hosts and ecological niches, and fitness in nature. The genetic basis for virulence in Toxoplasma is apparently as complex and flexible as its life cycle. It possesses virulence genes that, regardless of the genetic background they are expressed in, appear to confer an altered biological potential on most strains, and these genes are often referred to as “intrinsic” virulence loci (Sibley and Ajioka, 2008). Certain alleles for the genes ROP18 and ROP16, for instance, possess an heritable, virulence-enhancing capacity to strains that bear them (Saeij et al., 2006, 2007; Taylor et al., 2006). What is not clear is why these three archetypal lines emerged to become the dominant clonotypes in the domestic cycle. A plausible explanation is that virulence in Toxoplasma need not be solely dependent on the inheritance of specific alleles at just a few dominant loci (Grigg and Suzuki, 2003). This is because sexual recombination, by reshuffling existing alleles between two parents that do not possess intrinsic virulence loci, is also sufficient to yield progeny with dramatically altered biological properties (Grigg, 2001a). In combination, the data above suggest that virulence in Toxoplasma is a quantitative trait involving many loci, some of which may be phenotypically epistatic in some genetic backgrounds, hence virulence will vary depending on both the dominant loci expressed (as for example ROP18), and the specific combinations of alleles at contributing loci that reassort in a cross (Grigg, 2007). In effect, variation in genetic background is important, chiefly because it confers new phenotypic potential among progeny and a spectrum of virulence that can range across several orders of magnitude. And when the right mix is achieved, fit strains emerge from genetic crosses with a potential to rapidly expand clonally and dominate domestic cycles via carnivorism, asexual propagation and selfing for extended periods of time.
Another notable feature in the population biology of Toxoplasma is the fecundity of its sexual cycle. A single cat can produce in excess of 100 million recombinant progeny each harboring a unique “mix” of parental alleles. It is this ability to profoundly alter genetic associations at a genome-wide population level in just a single cross that is likely driving the range of phenotypes observed in different animal hosts infected by this highly successful zoonosis. It is also this flexibility that allows natural selection among intermediate hosts for those clones that maximize parasite transmissibility in a variety of ecological niches and among the different species of sylvatic and domestic mammals and birds that Toxoplasma infects.
4. Conclusion
The data support the idea that Toxoplasma gondii exists as a cosmopolitan pathogen that possesses both a complex and simple population genetic structure that is punctuated by clonal “sweeps” of particularly successful lines that emerge in domestic cycles as products of meiotic recombination. Outcrossing is likely occurring in sylvatic cycles where strain diversity, natural selection, and the genetic diversity of the definitive feline host is highest. The frequency of sexual recombination in driving this emergence is a matter of debate. Whether genetic sex happens frequently or not, out-crossing between two radically different haplotypes capable of generating progeny with a punctuated biological potential for expansion, either as a localized epizootic, or with an ability to spread to endemic and pandemic levels, is more likely to happen only infrequently. Like viruses such as Influenza, it is impossible to predict when the next pandemic line will evolve, but by tracking genealogies among outbreak strains one can begin to assess the number of times genetic crosses have contributed to the emergence of successful lines through history.
Acknowledgments
I would like to thank John Boothroyd, Patrick Keeling, Gary Holland, Jeff Jones, Melissa Miller, Pat Conrad, Bill Bowie, JP Dubey, David Roos and members of the Grigg laboratory for many helpful discussions; and David Sibley for access to intron sequencing data at www.toxohapmap.wustl.edu. This work was supported by the National Science Foundation and the Intramural Research Program of the NIH and NIAID. MEG is a scholar of the Canadian Institute for Advanced Research (CIFAR) Program for Integrated Microbial Biodiversity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajzenberg D, Banuls AL, Tibayrenc M, Darde ML. Microsatellite analysis of Toxoplasma gondii shows considerable polymorphism structured into two main clonal groups. International journal for parasitology. 2002;32:27–38. doi: 10.1016/s0020-7519(01)00301-0. [DOI] [PubMed] [Google Scholar]
- Ajzenberg D, Banuls AL, Su C, Dumetre A, Demar M, Carme B, Darde ML. Genetic diversity, clonality and sexuality in Toxoplasma gondii. International journal for parasitology. 2004;34:1185–1196. doi: 10.1016/j.ijpara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Ajzenberg D, Dumetre A, Darde ML. Multiplex PCR for typing strains of Toxoplasma gondii. Journal of clinical microbiology. 2005;43:1940–1943. doi: 10.1128/JCM.43.4.1940-1943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramini JJ, Stephen C, Dubey JP. Toxoplasma gondii in Vancouver Island cougars (Felis concolor vancouverensis): serology and oocyst shedding. The Journal of parasitology. 1998;84:438–440. [PubMed] [Google Scholar]
- Aramini JJ, Stephen C, Dubey JP, Engelstoft C, Schwantje H, Ribble CS. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiology and infection. 1999;122:305–315. doi: 10.1017/s0950268899002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F, Slifer T, Kim S. Chronic infection with Toxoplasma gondii does not prevent acute disease or colonization of the brain with tissue cysts following reinfection with different strains of the parasite. The Journal of parasitology. 1997;83:521–522. [PubMed] [Google Scholar]
- Arkush KD, Miller MA, Leutenegger CM, Gardner IA, Packham AE, Heckeroth AR, Tenter AM, Barr BC, Conrad PA. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis) International journal for parasitology. 2003;33:1087–1097. doi: 10.1016/s0020-7519(03)00181-4. [DOI] [PubMed] [Google Scholar]
- Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Orefice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerging infectious diseases. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. New England journal of Medicine. 1982;307:666–669. doi: 10.1056/NEJM198209093071107. [DOI] [PubMed] [Google Scholar]
- Blackston CR, Dubey JP, Dotson E, Su C, Thulliez P, Sibley D, Lehmann T. High-resolution typing of Toxoplasma gondii using microsatellite loci. The Journal of parasitology. 2001;87:1472–1475. doi: 10.1645/0022-3395(2001)087[1472:HRTOTG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Current opinion in microbiology. 2002;5:438–442. doi: 10.1016/s1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, Marion SA. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;350:173–177. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- Boyle JP, Rajasekar B, Saeij JP, Ajioka JW, Berriman M, Paulsen I, Roos DS, Sibley LD, White MW, Boothroyd JC. Just one cross appears capable of dramatically altering the population biology of a eukaryotic pathogen like Toxoplasma gondii. Proceedings of the national academy of sciences of the United States of America. 2006;103:10514–10519. doi: 10.1073/pnas.0510319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AJ, Shortt SG, Renton JI, King A, Weker D, Bowie WR. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Opthalmology. 1998;105:1032–1037. doi: 10.1016/S0161-6420(98)96004-3. [DOI] [PubMed] [Google Scholar]
- Choi WY, Nam HW, Kwak NH, Huh W, Kim YR, Kang MW, Cho SY, Dubey JP. Foodborne outbreaks of human toxoplasmosis. Journal of infectious diseases. 1997;175 doi: 10.1086/593702. [DOI] [PubMed] [Google Scholar]
- Cole RA, Lindsay DS, Howe DK, Roderick CL, Dubey JP, Thomas NJ, Baeten LA. Biological and molecular characterizations of Toxoplasma gondii strains obtained from southern sea otters (Enhydra lutris nereis) The Journal of parasitology. 2000;86:526–530. doi: 10.1645/0022-3395(2000)086[0526:BAMCOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Conrad PA, Miller MA, Kreuder C, James ER, Mazet J, Dabritz H, Jessup DA, Gulland F, Grigg ME. Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. International Journal of Parasitology. 2005;35:1155–1168. doi: 10.1016/j.ijpara.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dao A, Fortier B, Soete M, Plenat F, Dubremetz JF. Successful reinfection of chronically infected mice by a different Toxoplasma gondii genotype. International journal for parasitology. 2001;31:63–65. doi: 10.1016/s0020-7519(00)00151-x. [DOI] [PubMed] [Google Scholar]
- Darde ML, Bouteille B, Pestre-Alexandre M. Isoenzymic characterization of seven strains of Toxoplasma gondii by isoelectrofocusing in polyacrylamide gels. The American journal of tropical medicine and hygiene. 1988;39:551–558. doi: 10.4269/ajtmh.1988.39.551. [DOI] [PubMed] [Google Scholar]
- Darde ML, Bouteille B, Pestre-Alexandre M. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. The Journal of parasitology. 1992;78:786–794. [PubMed] [Google Scholar]
- Darde ML. Biodiversity in Toxoplasma gondii. Current topics in microbiology and immunology. 1996;219:27–41. doi: 10.1007/978-3-642-51014-4_3. [DOI] [PubMed] [Google Scholar]
- de Moura L, Bahia-Oliveira LM, Wada MY, Jones JL, Tuboi SH, Carmo EH, Ramalho WM, Camargo NJ, Trevisan R, Graca RM, da Silva AJ, Moura I, Dubey JP, Garrett DO. Waterborne toxoplasmosis, Brazil, from field to gene. Emerging infectious diseases. 2006;12:326–329. doi: 10.3201/eid1202.041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, Valery N, Peneau C, Daigre JL, Aznar C, Cottrelle B, Terzan L, Darde ML, Carme B. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clinical infectious diseases. 2007;45:e88–95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- Desmonts G, Couvreur J, Alison F, Baudelot J, Gerbeaux J, Lelong M. Étude épidémiologique sur la toxoplasmose: de l’influence de la cuisson des viandes de boucherie sur la fréquence de l’infection humaine. Rev Fr Études Clin Biol. 1965;10:952–958. [PubMed] [Google Scholar]
- Doganci L, Tanyuksel M, Araz ER, Besirbellioglu BA, Erdem U, Ozoguz CA, Yucel N, Ciftcioglu A. A probable outbreak of toxoplasmosis among boarding school students in Turkey. Clin microbiol infect. 2006;12:672–674. doi: 10.1111/j.1469-0691.2006.01449.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Miller NL, Frenkel JK. The Toxoplasma gondii oocyst from cat feces. Journal of experimental medicine. 1970;132:636–662. doi: 10.1084/jem.132.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Frenkel JK. Cyst-induced toxoplasmosis in cats. Journal of protozoology. 1972;19:155–177. doi: 10.1111/j.1550-7408.1972.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sharma SP, Juranek DD, Sulzer AJ, Teutsch SM. Characterization of Toxoplasma gondii isolates from an outbreak of toxoplasmosis in Atlanta, Georgia. American journal of veterinary research. 1981;42:1007–1010. [PubMed] [Google Scholar]
- Dubey JP, Beattie CP. Toxoplasmosis of animals and man. CRC Press; Boca Raton, Florida: 1988. p. 220. [Google Scholar]
- Dubey JP. Infectivity and pathogenicity of Toxoplasma gondii oocysts for cats. Journal of parasitology. 1996a;82:957–960. [PubMed] [Google Scholar]
- Dubey JP, Lunney JK, Shen SK, Kwok OCH, Ashford DA, Thulliez P. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. Journal of parasitology. 1996b;82:438–443. [PubMed] [Google Scholar]
- Dubey JP. Oocyst shedding by cats fed isolated bradyzoites and comparision of infectivity of bradyzoites of the VEG strain Toxoplasma gondii to cats and mice. Journal of parasitology. 2001;87:215–219. doi: 10.1645/0022-3395(2001)087[0215:OSBCFI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Zarnke R, Thomas NJ, Wong SK, Van Bonn W, Briggs M, Davis JW, Ewing R, Mense M, Kwok OC, Romand S, Thulliez P. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Veterinary parasitology. 2003;116:275–296. doi: 10.1016/s0304-4017(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Graham DH, De Young RW, Dahl E, Eberhard ML, Nace EK, Won K, Bishop H, Punkosdy G, Sreekumar C, Vianna MC, Shen SK, Kwok OC, Sumners JA, Demarais S, Humphreys JG, Lehmann T. Molecular and biologic characteristics of Toxoplasma gondii isolates from wildlife in the United States. The Journal of parasitology. 2004;90:67–71. doi: 10.1645/GE-110R. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Patitucci AN, Su C, Sundar N, Kwok OC, Shen SK. Characterization of Toxoplasma gondii isolates in free-range chickens from Chile, South America. Veterinary parasitology. 2006;140:76–82. doi: 10.1016/j.vetpar.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Hill DE, Sundar N, Velmurugan GV, Bandini LA, Kwok OCH, Pierce V, Kelly K, Dulin M, Thulliez P, Iwueke C, Su C. Endemic toxoplasmosis in pigs on a farm in Maryland: isolation and genetic characterization of Toxoplasma gondii. Journal of parasitology. 2008b;94:36–41. doi: 10.1645/GE-1312.1. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Quirk T, Pitt JA, Sundar N, Velmurugan G, Kwok OCH, Leclair D, Hill R, Su C. Isolation and genetic characterization of Toxoplasma gondii from raccoons (Procyon lotor), cats (Felis domesticus), striped skunk (Mephitis mephitis), black bear (Ursus americanus), and cougar (Puma concolor) from Canada. Journal of parasitology. 2008a;94:42–45. doi: 10.1645/GE-1349.1. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Gennari SM, Minervino AHH, Farias NAR, Ruas JL, dos Santos TRB, Cavalcante GT, Kwok OCH, Su C. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Pará state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Veterinary parasitology. 2007;143:182–188. doi: 10.1016/j.vetpar.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Velmurugan GV, Bandini LA, Kwok OCH, Majumdar D, Su C. High prevalence and abundant atypical genotypes of Toxoplasma gondii isolated from lambs destined for human consumption in the USA. International journal of parasitology. 2008c;38:999–1006. doi: 10.1016/j.ijpara.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Eng SB, Werker DH, King AS, Marion SA, Bell A, Issac-Renton JL, Irwin GS, Bowie WR. Computer-generated dot maps as an epidemiologic tool: investigating an outbreak of toxoplasmosis. Emerging infectious diseases. 1999;5:815–819. doi: 10.3201/eid0506.990613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DJ, Hutchison WM, Dunachie JF, Siim JC. Ultrastructural study of early stages of asexual multiplication and microgametogony of Toxoplasma gondii in the small intestine of the cat. Acta pathologica et microbiologica Scandinavica. 1974;82:167–181. doi: 10.1111/j.1699-0463.1974.tb02309.x. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Hutchison WM, Siim JC. The ultrastructural development of the macrogamete and formation of the oocyst wall of Toxoplasma gondii. Acta Pathol Microbiol Scand [B] 1975;83:491–505. doi: 10.1111/j.1699-0463.1975.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Ferguson DJP. Toxoplasma gondii and sex: essential or optional extra? Trends in parasitology. 2002;8:354–359. [PubMed] [Google Scholar]
- Ferreira Ade M, Vitor RW, Gazzinelli RT, Melo MN. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol. 2006;6:22–31. doi: 10.1016/j.meegid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Frenkel JK, Dubey JP, Miller NL. Toxoplasma gondii: fecal forms separated from eggs of the nematode Toxocara cati. Science (New York, NY) 1969;164:432–433. doi: 10.1126/science.164.3878.432. [DOI] [PubMed] [Google Scholar]
- Frenkel JK, Dubey JP, Miller NL. Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science (New York, NY) 1970;167:893–896. doi: 10.1126/science.167.3919.893. [DOI] [PubMed] [Google Scholar]
- Frenkel JK. Toxoplasma in and around us. BioScience. 1973;23:343–352. [Google Scholar]
- Frenkel JK, Ruiz A, Chinchilla M. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. American Journal of Tropical Medicine and Hygeine. 1975;24:439–443. doi: 10.4269/ajtmh.1975.24.439. [DOI] [PubMed] [Google Scholar]
- Fux B, Nawas J, Khan A, Gill DB, Su C, Sibley LD. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infection and immunity. 2007;75:2580–2590. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner PD, Silveira C, Kruszon-Moran D, Martins MC, Burnier Junior M, Silveira S, Camargo ME, Nussenblatt RB, Kaslow RA, Belfort Junior R. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. American journal of ophthalmology. 1992;114:136–144. doi: 10.1016/s0002-9394(14)73976-5. [DOI] [PubMed] [Google Scholar]
- Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, Boothroyd JC. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science (New York, NY) 2001a;294:161–165. doi: 10.1126/science.1061888. [DOI] [PubMed] [Google Scholar]
- Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. The Journal of infectious diseases. 2001b;184:633–639. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- Grigg ME, Suzuki Y. Sexual recombination and clonal evolution of virulence in Toxoplasma. Microbes and infection/Institut Pasteur. 2003;5:685–690. doi: 10.1016/s1286-4579(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Grigg ME. Population genetics, sex, and the emergence of clonal lines of Toxoplasma gondii. In: Ajioka JW, Soldati D, editors. Toxoplasma - Molecular and Cellular Biology. 2007. pp. 227–240. [Google Scholar]
- Heukelbach J, Meyer-Cirkel V, Moura RC, Gomide M, Queiroz JA, Saweljew P, Liesenfeld O. Waterborne toxoplasmosis, northeastern Brazil. Emerging infectious diseases. 2007;13:287–289. doi: 10.3201/eid1302.060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. American journal of ophthalmology. 2003;136:973–988. doi: 10.1016/j.ajo.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. American journal of ophthalmology. 2004;137:1–17. [PubMed] [Google Scholar]
- Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. Journal of infectious diseases. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Howe DK, Honore S, Derouin F, Sibley LD. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. Journal of clinical microbiology. 1997;35:1411–1414. doi: 10.1128/jcm.35.6.1411-1414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WM. Experimental transmission of Toxoplasma gondii. Nature. 1965;206:961–962. doi: 10.1038/206961a0. [DOI] [PubMed] [Google Scholar]
- Isaac-Renton J, Bowie WR, King A, Irwin GS, Ong CS, Fung CP, Shokeir MO, Dubey JP. Detection of Toxoplasma gondii oocysts in drinking water. Applied and environmental microbiology. 1998;64:2278–2280. doi: 10.1128/aem.64.6.2278-2280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L, Remington JS, Melton ML. The resistance of the encysted form of Toxoplasma gondii. The Journal of parasitology. 1960;46:11–21. [PubMed] [Google Scholar]
- Janku J. Pathogénèse et anatomie pathologique de la macula dans un oeil de dimension normale et dans un oeil microphtalme avec parasite dans la rétine. Casopis Lekaruv Ceskych. 1923;62:1021–1027. [Google Scholar]
- Khan A, Bohme U, Kelly KA, Adlem E, Brooks K, Simmonds M, Mungall K, Quail MA, Arrowsmith C, Chillingworth T, Churcher C, Harris D, Collins M, Fosker N, Fraser A, Hance Z, Jagels K, Moule S, Murphy L, O’Neil S, Rajandream MA, Saunders D, Seeger K, Whitehead S, Mayr T, Xuan X, Watanabe J, Suzuki Y, Wakaguri H, Sugano S, Sugimoto C, Paulsen I, Mackey AJ, Roos DS, Hall N, Berriman M, Barrell B, Sibley LD, Ajioka JW. Common inheritance of chromosome Ia associated with clonal expansion of Toxoplasma gondii. Genome research. 2006;16:1119–1125. doi: 10.1101/gr.5318106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Fux B, Su C, Dubey JP, Darde ML, Ajioka JW, Rosenthal BM, Sibley LD. Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14872–14877. doi: 10.1073/pnas.0702356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Blackston CR, Parmley SF, Remington JS, Dubey JP. Strain typing of Toxoplasma gondii: comparison of antigen-coding and housekeeping genes. The Journal of parasitology. 2000;86:960–971. doi: 10.1645/0022-3395(2000)086[0960:STOTGC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP. Globalization and the population structure of Toxoplasma gondii. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11423–11428. doi: 10.1073/pnas.0601438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS, Collins MV, Mitchell SM, Cole RA, Flick GJ, Wetch CN, Lindquist A, Dubey JP. Sporulation and survival of Toxoplasma gondii oocysts in seawater. The Journal of eukaryotic microbiology. 2003;50(Suppl):687–688. doi: 10.1111/j.1550-7408.2003.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Collins MV, Mitchell SM, Wetch CN, Rosypal AC, Flick GJ, Zajac AM, Lindquist A, Dubey JP. Survival of Toxoplasma gondii oocysts in Eastern oysters (Crassostrea virginica) The Journal of parasitology. 2004;90:1054–1057. doi: 10.1645/GE-296R. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J, Smith NH. Detecting recombination from gene trees. Molecular biology and evolution. 1998;15:590–599. doi: 10.1093/oxfordjournals.molbev.a025960. [DOI] [PubMed] [Google Scholar]
- McLeod R, Frenkel JK, Estes RG, Mack DG, Eisenhauer PB, Gibori G. Subcutaneous and intestinal vaccination with tachyzoites of Toxoplasma gondii and acquisition of immunity to peroral and congenital Toxoplasma challenge. J Immunol. 1988;140:1632–1637. [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerging infectious diseases. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Crosbie PR, Sverlow K, Hanni K, Barr BC, Kock N, Murray MJ, Lowenstine LJ, Conrad PA. Isolation and characterization of Sarcocystis from brain tissue of a free-living southern sea otter (Enhydra lutris nereis) with fatal meningoencephalitis. Parasitol Res. 2001;87:252–257. doi: 10.1007/s004360000340. [DOI] [PubMed] [Google Scholar]
- Miller MA, Gardner IA, Kreuder C, Paradies DM, Worcester KR, Jessup DA, Dodd E, Harris MD, Ames JA, Packham AE, Conrad PA. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) International journal for parasitology. 2002;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Miller MA, Grigg ME, Kreuder C, James ER, Melli AC, Crosbie PR, Jessup DA, Boothroyd JC, Brownstein D, Conrad PA. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. International journal for parasitology. 2004;34:275–284. doi: 10.1016/j.ijpara.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Miller MA, Miller WA, Conrad PA, James ER, Melli AC, Leutenegger CM, Dabritz HA, Packham AE, Paradies D, Harris M, Ames J, Jessup DA, Worcester K, Grigg ME. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. International journal for parasitology. 2008;38:1319–1328. doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Morisset S, Peyron F, Lobry JR, Garweg J, Ferrandiz J, Musset K, Gomez-Marin JE, de la Torre A, Demar M, Carme B, Mercier C, Garin JF, Cesbron-Delauw MF. Serotyping of Toxoplasma gondii: striking homogeneous pattern between symptomatic and asymptomatic infections within Europe and South America. Microbes and infection/Institut Pasteur. 2008;10:742–747. doi: 10.1016/j.micinf.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Nicolle C, Manceaux L. Sur une infection à corps de Leishman (ou organismes voisins) du gondi. C R Seances Acad Sci. 1908;147:763–766. [Google Scholar]
- Nicolle C, Manceaux L. Sur un protozoaire nouveau du gond. C R Seances Acad Sci. 1909;148:369–372. [Google Scholar]
- Palanisamy M, Madhavan B, Balasundaram MB, Andavar R, Venkatapathy N. Outbreak of ocular toxoplasmosis in Coimbatore,India. Indian journal of opthalmology. 2006;54:129–131. doi: 10.4103/0301-4738.25839. [DOI] [PubMed] [Google Scholar]
- Pena HF, Gennari SM, Dubey JP, Su C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. International journal for parasitology. 2008;38:561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER, Kasper LH. Toxoplasma gondii: genetic crosses reveal phenotypic suppression of hydroxyurea resistance by fluorodeoxyuridine resistance. Experimental parasitology. 1983;55:207–218. doi: 10.1016/0014-4894(83)90015-2. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER, Schwartzman JD, Kasper LH. Toxoplasma gondii: use of mutants to study the host-parasite relationship. Ciba Foundation symposium. 1983;99:74–91. doi: 10.1002/9780470720806.ch5. [DOI] [PubMed] [Google Scholar]
- Rawal BD. Toxoplasmosis. A dye- test on sera from vegetarians and meat eaters in Bombay. Transactions of Royal Society of Tropical Medicine and Hygiene. 1959;53:61–63. doi: 10.1016/0035-9203(59)90084-7. [DOI] [PubMed] [Google Scholar]
- Razakandrainibe FG, Durand P, Koella JC, De Meeus T, Rousset F, Ayala FJ, Renaud F. Clonal” population structure of the malaria agent Plasmodium falciparum in high-infection regions. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17388–17393. doi: 10.1073/pnas.0508871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikvam A, Lorentzen-Styr AM. Virulence of different strains of Toxoplasma gondii and host response in mice. Nature. 1976;261:508–509. doi: 10.1038/261508a0. [DOI] [PubMed] [Google Scholar]
- Ross RD, Stec LA, Werner JC, Blumenkranz MS, Glazer L, Williams GA. Presumed acquired ocular toxoplasmosis in deer hunters. Retina. 2001;21:226–229. doi: 10.1097/00006982-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Sabin AB, Olitsky PK. Toxoplasma and Obligate Intracellular Parasitism. Science (New York, NY) 1937;85:336–338. doi: 10.1126/science.85.2205.336. [DOI] [PubMed] [Google Scholar]
- Sabin AB. Toxoplasmic encephalitis in children. Journal of American Medical Association. 1941;116:801–807. [Google Scholar]
- Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science (New York, NY) 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma coopts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield HG, Melton ML. The fine structure and reproduction of Toxoplasma gondii. The Journal of parasitology. 1968;54:209–226. [PubMed] [Google Scholar]
- Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- Sibley LD, LeBlanc AJ, Pfefferkorn ER, Boothroyd JC. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii. Genetics. 1992;132:1003–1015. doi: 10.1093/genetics/132.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD, Howe DK. Genetic basis of pathogenicity in toxoplasmosis. Current topics in microbiology and immunology. 1996;219:3–15. doi: 10.1007/978-3-642-51014-4_1. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Ajioka JW. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annual review of microbiology. 2008;62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- Silveira C, Belfort R, Jr, Muccioli C, Abreu MT, Martins MC, Victora C, Nussenblatt RB, Holland GN. A follow-up study of Toxoplasma gondii infection in southern Brazil. American journal of ophthalmology. 2001;131:351–354. doi: 10.1016/s0002-9394(00)00830-8. [DOI] [PubMed] [Google Scholar]
- Splendore A. Un nuovo protozoa parassita de’ conigli. incontrato nelle lesioni anatomiche d’une malattia che ricorda in molti punti il Kala-azar dell’ uomo Nota preliminare pel. Rev Soc Scient Sao Paulo. 1908;3:109–112. [Google Scholar]
- Stagno S, Dykes AC, Amos CS, Head RA, Juranek DD, Walls K. An outbreak of toxoplasmosis linked to cats. Pediatrics. 1980;65:706–712. [PubMed] [Google Scholar]
- Su C, Howe DK, Dubey JP, Ajioka JW, Sibley LD. Identification of quantitative trait loci controlling acute virulence in Toxoplasma gondii. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10753–10758. doi: 10.1073/pnas.172117099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science (New York, NY) 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- Su C, Zhang X, Dubey JP. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. International journal for parasitology. 2006;36:841–848. doi: 10.1016/j.ijpara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Sundar N, Cole RA, Thomas NJ, Majumdar D, Dubey JP, Su C. Genetic diversity among sea otter isolates of Toxoplasma gondii. Veterinary parasitology. 2008;151:125–132. doi: 10.1016/j.vetpar.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science (New York, NY) 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- Teutsch SM, Juranek DD, Sulzer A, Dubey JP, Sikes RK. Epidemic toxoplasmosis associated with infected cats. The New England journal of medicine. 1979;300:695–699. doi: 10.1056/NEJM197903293001302. [DOI] [PubMed] [Google Scholar]
- Weinman D, Chandler AH. Toxoplasmosis in swine and rodents; reciprocal oral infection and potential human hazard. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, NY) 1954;87:211–216. doi: 10.3181/00379727-87-21337. [DOI] [PubMed] [Google Scholar]
- Wolf A, Cowen D, Paige B. Human Toxoplasmosis: Occurrence in Infants as an Encephalomyelitis Verification by Transmission to Animals. Science (New York, NY) 1939;89:226–227. doi: 10.1126/science.89.2306.226. [DOI] [PubMed] [Google Scholar]