Abstract
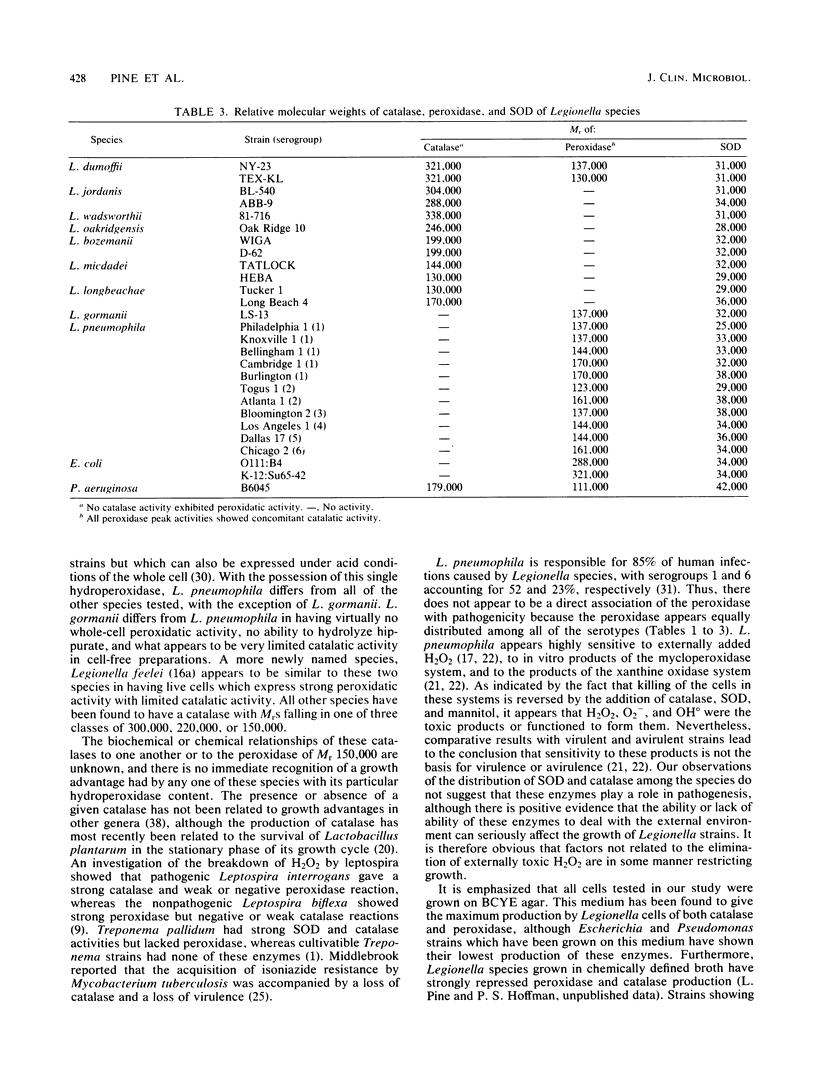
We examined 40 strains of Legionella for reduced-oxygen scavenging enzymes. Using a simple reaction chamber with a Swinney filter for the Beers and Sizer assay, we determined the catalase activity of live cells grown on buffered charcoal-yeast extract agar. For 29 strains of Legionella pneumophila, the apparent first-order rate constants for catalase ranged from 0.000 to 0.005. Similarly, low values ranging from 0.001 to 0.005 were observed for Legionella wadsworthii, Legionella oakridgensis, and Legionella gormanii. High catalase activities were found for Legionella jordanis, Legionella longbeachae, Legionella micdadei, and Legionella bozemanii, with first-order rate constant values of 0.010 to 0.035. Cell-free extracts were analyzed for catalase, peroxidase, and superoxide dismutase. Cell-free extracts of all strains had superoxide dismutase levels ranging from 8.2 to 30.5 U per mg of protein. The species could be characterized by their catalase and peroxidase since L. pneumophila and L. gormanii had only peroxidase (relative molecular weight [Mr], 150,000); L. dumoffii had a peroxidase (Mr, 150,000) plus a catalase (Mr, 174,000); and all remaining species had catalase only (Mr, 300,000, 220,000, or 150,000).
Full text
PDF


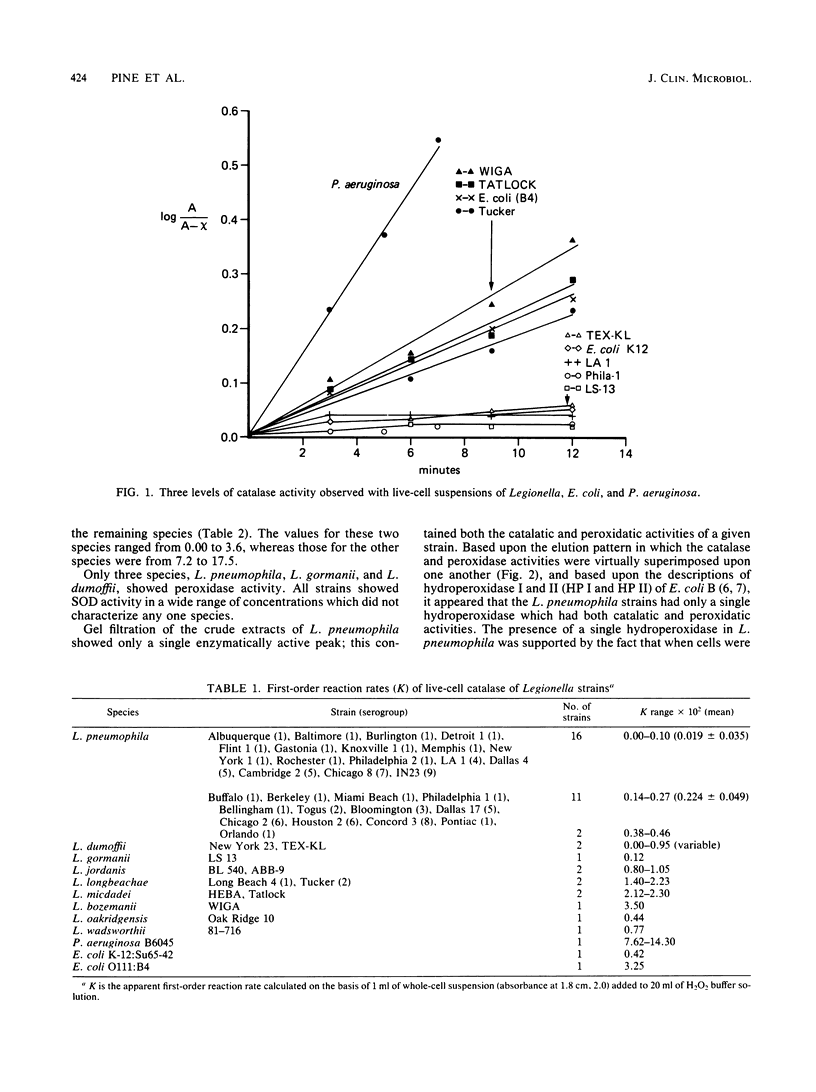

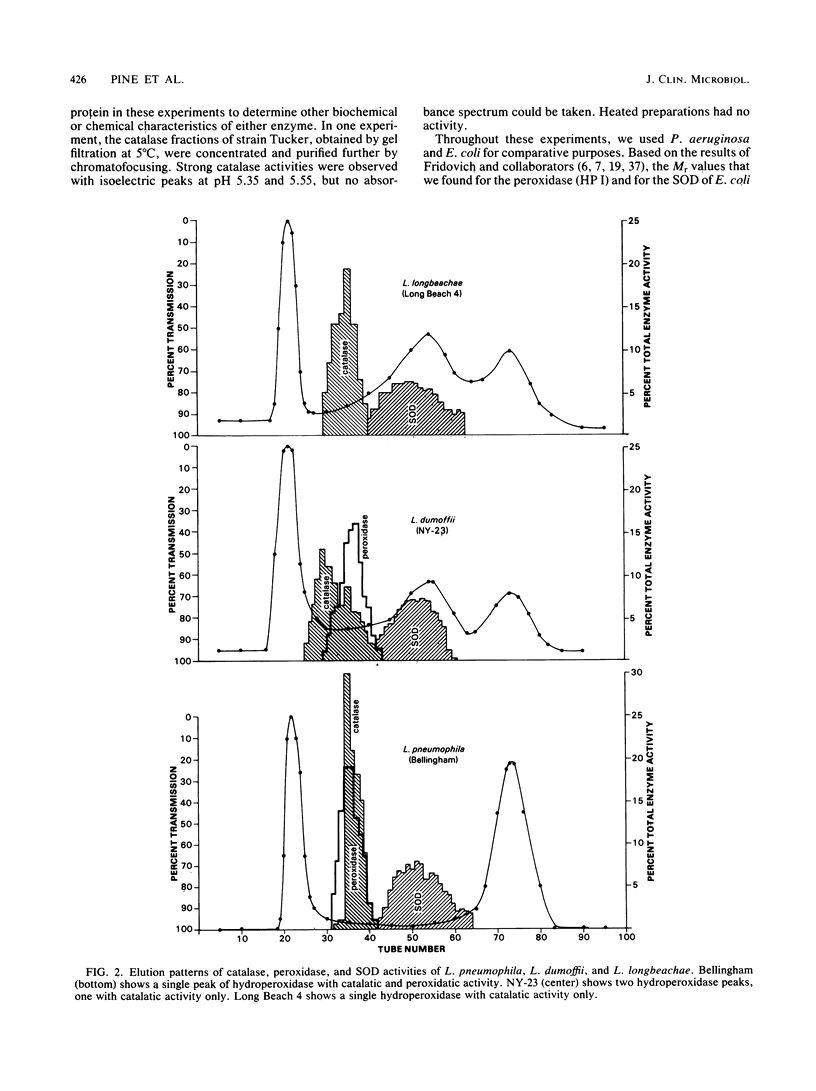
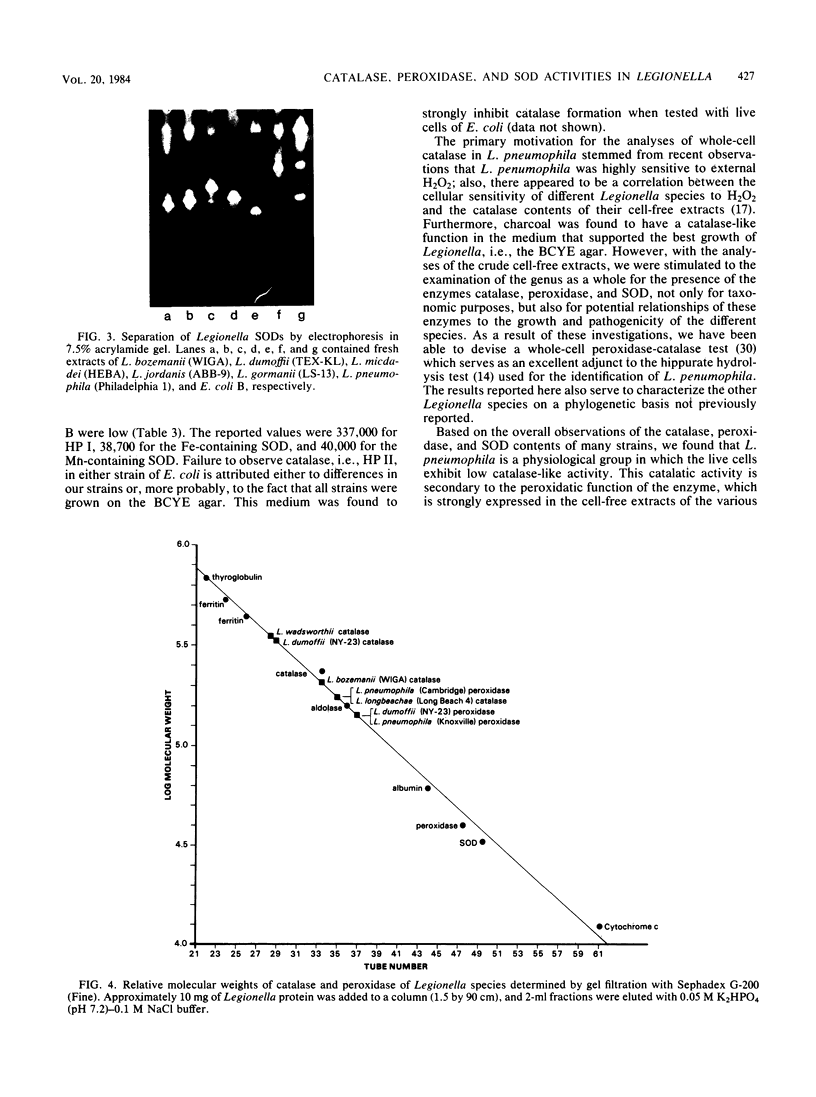

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin F. E., Barbieri J. T., Corin R. E., Grigas K. E., Cox C. D. Distribution of superoxide dismutase, catalase, and peroxidase activities among Treponema pallidum and other spirochetes. Infect Immun. 1981 Aug;33(2):372–379. doi: 10.1128/iai.33.2.372-379.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Steigerwalt A. G., McDade J. E. Classification of the Legionnaires' disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann Intern Med. 1979 Apr;90(4):656–658. doi: 10.7326/0003-4819-90-4-656. [DOI] [PubMed] [Google Scholar]
- Cherry W. B., Gorman G. W., Orrison L. H., Moss C. W., Steigerwalt A. G., Wilkinson H. W., Johnson S. E., McKinney R. M., Brenner D. J. Legionella jordanis: a new species of Legionella isolated from water and sewage. J Clin Microbiol. 1982 Feb;15(2):290–297. doi: 10.1128/jcm.15.2.290-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne A., Fridovich I. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem. 1979 May 25;254(10):4245–4252. [PubMed] [Google Scholar]
- Claiborne A., Malinowski D. P., Fridovich I. Purification and characterization of hydroperoxidase II of Escherichia coli B. J Biol Chem. 1979 Nov 25;254(22):11664–11668. [PubMed] [Google Scholar]
- Corin R. E., Boggs E., Cox C. D. Enzymatic degradation of H2O2 by Leptospira. Infect Immun. 1978 Dec;22(3):672–675. doi: 10.1128/iai.22.3.672-675.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gorman G. W., Weaver R. E., Mackel D. C., Smith H. W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978 Sep;8(3):320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Kowalski J. B., Holdeman L. V. Production and some properties of catalase and superoxide dismutase from the anaerobe Bacteroides distasonis. J Bacteriol. 1977 Mar;129(3):1298–1302. doi: 10.1128/jb.129.3.1298-1302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978 Sep 25;253(18):6445–6420. [PubMed] [Google Scholar]
- Herwaldt L. A., Gorman G. W., McGrath T., Toma S., Brake B., Hightower A. W., Jones J., Reingold A. L., Boxer P. A., Tang P. W. A new Legionella species, Legionella feeleii species nova, causes Pontiac fever in an automobile plant. Ann Intern Med. 1984 Mar;100(3):333–338. doi: 10.7326/0003-4819-100-3-333. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Pine L., Bell S. Production of superoxide and hydrogen peroxide in medium used to culture Legionella pneumophila: catalytic decomposition by charcoal. Appl Environ Microbiol. 1983 Mar;45(3):784–791. doi: 10.1128/aem.45.3.784-791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert G. A. Hippurate hydrolysis by Legionella pneumophila. J Clin Microbiol. 1981 Jan;13(1):240–242. doi: 10.1128/jcm.13.1.240-242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr D. E., Bibb W. F., Moss C. W. Isoprenoid quinones of the genus Legionella. J Clin Microbiol. 1982 Jun;15(6):1044–1048. doi: 10.1128/jcm.15.6.1044-1048.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Functional significance of manganese catalase in Lactobacillus plantarum. J Bacteriol. 1983 Aug;155(2):742–746. doi: 10.1128/jb.155.2.742-746.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lochner J. E., Friedman R. L., Bigley R. H., Iglewski B. H. Effect of oxygen-dependent antimicrobial systems on Legionella pneumophila. Infect Immun. 1983 Jan;39(1):487–489. doi: 10.1128/iai.39.1.487-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Jacobs R. F., Wilson C. B., Weaver W. M., Klebanoff S. J. Susceptibility of Legionella pneumophila to oxygen-dependent microbicidal systems. J Immunol. 1982 Nov;129(5):2192–2197. [PubMed] [Google Scholar]
- MIDDLEBROOK G. Isoniazid-resistance and catalase activity of tubercle bacilli; a preliminary report. Am Rev Tuberc. 1954 Mar;69(3):471–472. doi: 10.1164/art.1954.69.3.471. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Bibb W. F., Karr D. E., Guerrant G. O., Lambert M. A. Cellular fatty acid composition and ubiquinone content of Legionella feeleii sp. nov. J Clin Microbiol. 1983 Oct;18(4):917–919. doi: 10.1128/jcm.18.4.917-919.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrison L. H., Cherry W. B., Tyndall R. L., Fliermans C. B., Gough S. B., Lambert M. A., McDougal L. K., Bibb W. F., Brenner D. J. Legionella oakridgensis: unusual new species isolated from cooling tower water. Appl Environ Microbiol. 1983 Feb;45(2):536–545. doi: 10.1128/aem.45.2.536-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasculle A. W., Feeley J. C., Gibson R. J., Cordes L. G., Myerowitz R. L., Patton C. M., Gorman G. W., Carmack C. L., Ezzell J. W., Dowling J. N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980 Jun;141(6):727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Hoffman P. S., Malcolm G. B., Benson R. F., Gorman G. W. Whole-cell peroxidase test for identification of Legionella pneumophila. J Clin Microbiol. 1984 Feb;19(2):286–290. doi: 10.1128/jcm.19.2.286-290.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam G. C., Codd G. A. A rapid whole-cell assay for superoxide dismutase. Anal Biochem. 1982 Mar 15;121(1):207–212. doi: 10.1016/0003-2697(82)90577-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]
- Yoshpe-Purer Y., Henis Y. Factors affecting catalase level and sensitivity to hydrogen peroxide in Escherichia coli. Appl Environ Microbiol. 1976 Oct;32(4):465–469. doi: 10.1128/aem.32.4.465-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]
- Yousten A. A., Johnson J. L., Salin M. Oxygen metabolism of catalase-negative and catalase-positive strains of Lactobacillus plantarum. J Bacteriol. 1975 Jul;123(1):242–247. doi: 10.1128/jb.123.1.242-247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]