Abstract
Reaction of the Rieske cluster model complex (Et4N)2[(N2CHPh)Fe2S2(S2-o-xyl)] (N2CHPh = dianion of 2,2′-(phenylmethylene)bis(3-methylindole); S2-o-xyl = dianion of 1,2-phenylenedimethanethiol) with nitric oxide results in disassembly of the iron-sulfur core and formation of {Fe(NO)2}9 dinitrosyliron complexes. Isolation and characterization of these DNICs, including the new compound, (Et4N)[(N2CHPh)Fe(NO)2], demonstrates a homology between the synthetic Riekse cluster and purely thiolate bound Fe2S2 clusters in reactions involving NO. To model the nitrogen rich environment of Rieske cluster-derived dinitroysliron species, a new type of neutral {Fe(NO)2}9 DNIC was prepared containing a β-diketiminate ligand. One-electron reduction of this compound affords the isolable {Fe(NO)2}10 DNIC. These compounds represent a rare example of structurally analogous DNIC redox partners.
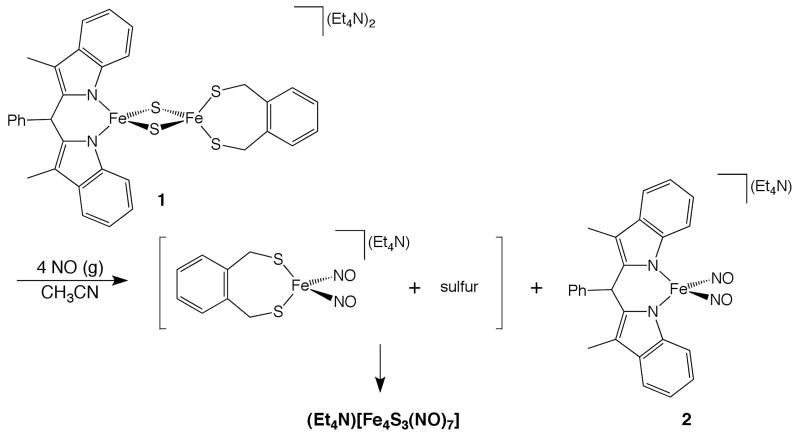
Iron-sulfur clusters are among the most ubiquitous cofactors in biology. The diversity of structures encountered in iron-sulfur proteins reflects the many functions nature employs for these versatile units, from electron transfer to substrate binding and activation.1 Of the many variations of iron-sulfur clusters, only a select few have ligands other than cysteine. Among these, the best-documented examples are Rieske-type Fe2S2 clusters2 that contain a pair of histidine residues ligating one iron atom.3,4 Recently, the first synthetic analog of a Rieske cluster was reported (1, Scheme 1).5 We were interested to explore the nitric oxide reactivity of such a compound as part of ongoing investigations into the reactivity of non-heme iron with NO. Moreover, there is very little information about the interaction of Rieske proteins with NO,6 which contrasts with the large body of literature regarding nitric oxide reactivity with purely cysteine-bound iron-sulfur clusters.7 Herein we report the results of our studies of NO reactions with 1 (Scheme 1) and introduce related N-bound dinitrosyliron complexes (DNICs) containing β-diketiminate ligands.
Scheme 1.
Reaction of the Rieske-type cluster with NO.
Reaction of Rieske model complex 1 with four equiv of nitric oxide in acetonitrile solution results in the appearance of two new nitrosyliron species after workup as judged by IR spectroscopy. The first displays νNO bands at 1795, 1743 and 1705 cm-1 (THF solution) consistent with formation of Roussin's black salt (RBS), [Fe4S3(NO)7]-. 8 We demonstrated previously that RBS can arise from reaction of NO with an Fe2S2 cluster containing (S2-o-xyl)2- ligands via the intermediacy of the thiolate bound DNIC.9 This DNIC can be observed by IR spectroscopy in reactions of 1 with Ph3CSNO in benzonitrile. After addition of 1 to 2 equiv of nitrosothiol, νNO bands at 1739 and 1679 cm-1 appear, consistent with formation of [(S2-o-xyl)Fe(NO)2]-. Upon addition of further equiv of Ph3CSNO, these IR bands diminish, giving rise to those of RBS.
The second iron nitrosyl species obtained from the reaction shown in Scheme 1 displays νNO peaks at 1761 and 1692 cm-1 (IR, KBr). Recrystallization from DMF/Et2O and subsequent spectroscopic characterization identified a new DNIC, 2 (Scheme 1). The N-bound DNIC displays a rhombic EPR signal centered at 2.03 (Supporting Information, SI) consistent with its formulation as an S = ½ paramagnetic complex and a {Fe(NO)2}9 species in the Enemark-Feltham notation.10 Compound 2 also contains a quadrupole doublet with an isomer shift of 0.16(2) mm/s in the 57Fe Mössbauer spectrum (SI), which corresponds well with the value of 0.18(2) mm/s determined previously for (Et4N)[(FePhS)2(NO)2].9 Notably, several examples of dinitrosyliron compounds containing nitrogen donor ligands have been published previously as potential models for histidine-ligated DNICs.11 The synthesis of compound 2 represents the first verification that such a DNIC can be prepared directly from reaction of an iron-sulfur cluster with nitric oxide. Compound 2 was also independently synthesized by salt metathesis of (Et4N)[FeCl2(NO)2] with the lithium salt of the nitrogen ligand displayed in Scheme 1. Spectroscopic features of the DNIC prepared in this fashion are identical in all respects to those observed for that prepared from 1.
In order to prepare a more soluble version of Rieske cluster-derived DNICs such as 2 for more extensive studies, we turned our attention to sterically demanding β-diketiminate ligands. We reasoned that β-diketiminates would chelate the {Fe(NO)2}9 unit giving rise to stable DNICs, thereby avoiding many of the common shortcomings of thiolate ligands such as tendency toward oxidation and kinetic lability. Furthermore, β-diketiminate ligands have been employed previously to stabilize low valent iron.12 As an entry into this chemistry the iodide precursor [FeI2(NO)2]- was selected as a source of the {Fe(NO)2}9 unit.13 Reaction of the lithium salt of [(2,6-diisopropylphenyl)NC(Me)]2CH (Ar-nacnac) with (PPN)[FeI2(NO)2] (PPN+ = μ-nitridobis(triphenylphosphine cation), affords the new compound, [(Ar-nacnac)Fe(NO)2] (3), after workup. Unlike typical anionic DNICs such as 2, neutral 3 is soluble in alkane and arene solvents and may be conveniently freed from salt impurities by recrystallization from pentane. The spectroscopic features of 3 (νNO = 1761, 1709 cm-1, benzene-d6) are similar to those of 2, consistent with an {Fe(NO)2}9 formulation (SI). The solid-state structure of 3 (Figure 1) displays pseudo-tetrahedral geometry at iron, with the dinitrosyliron unit residing in a steric pocket created by the β-diketiminate ligand. It is interesting to contrast the pseudo-tetrahedral structure of 3 with the square-planar geometry of a related Fe(I) dicarbonyl complex.14 The Fe-N-O metrics in 3 are similar to those observed in previously characterized DNICs.15
Figure 1.
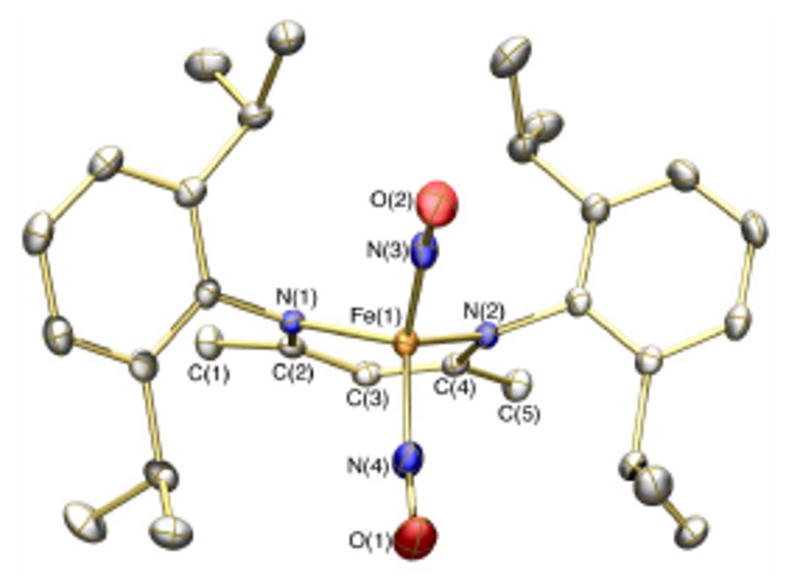
Structure of 3 displaying 50% thermal ellipsoids with hydrogen atoms omitted for clarity. Selected bond lengths (Å) and angles (deg): Fe(1)-N(3), 1.6964(18); Fe(1)-N(4), 1.6882(18); N(3)-O(2), 1.177(2); N(4)-O(1), 1.174(2); N(3)-Fe(1)-N(4), 114.27(9); Fe(1)-N(3)-O(2), 162.7(2); Fe(1)-N(4)-O(1), 170.1(2).
The {Fe(NO)2}9 unit of most DNICs can be reduced by one electron to give the diamagnetic {Fe(NO)2}10 configuration. Typically this reduction process is quasi-reversible electrochemically. Only in rare instances can the reduced DNIC be isolated.16 With DNICs containing thiolate ligands, reduction is followed by dissociation of RS- leading to formation of the reduced Roussin red ester derivatives.17 The cyclic voltammogram of 3 (SI) displays a quasi-reversible (ipr/ipf = 0.95) one-electron reduction at -1.34 V (vs ferrocene/ferrocenium) in THF. Chemical reduction of 3 with either decamethylcobaltocene or sodium amalgam affords the reduced {Fe(NO)2}10 DNIC. Metathesis of the cobaltocenium cation for Bu4N+ produces (Bu4N)[(Ar-nacnac)Fe(NO)2] (4a) after crystallization from Et2O. Compound 4a is a stable (mp = 198 – 200 °C) diamagnetic solid as judged by sharp features in its 1 H NMR spectrum (SI). Exposure of 4a to air immediately results in regeneration of 3 as judged by IR and UV-vis spectroscopy. The νNO bands in a benzene-d6 solution of 4a appear at 1627 and 1567 cm-1, a shift of >130 cm-1 from those observed for 3. Crystals of 4a proved unsuitable for X-ray determination so the PPN+ salt, 4b, was prepared.
The solid-state structure of the anion of 4b (Figure 2) resembles closely that of 3. One considerable difference are the elongated Fe-N(nacnac) bonds in 4b, which result in a smaller displacement of the Fe atom out of the nacnac plane compared with that in 3 (0.24 vs 0.62 Å). The Fe-N(NO) bonds for 4b are also ∼0.03 to 0.04 Å shorter than those of 3 consistent with a greater degree of π-backbonding in the reduced {Fe(NO)2}10 core. This phenomenon is also manifest by the longer N-O bonds of 4b (Figs. 1 and 2).
Figure 2.
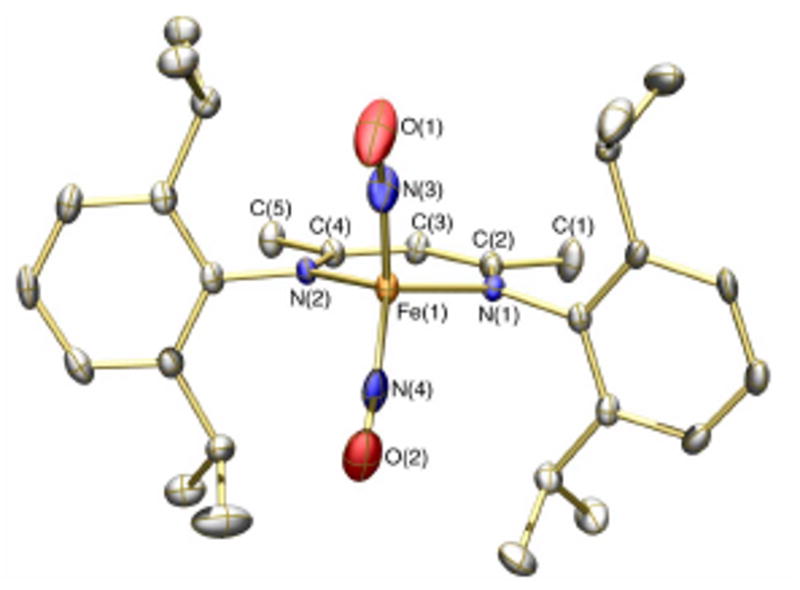
Structure of the anion of 4b displaying 50% thermal ellipsoids with hydrogen atoms omitted for clarity. Selected bond lengths (Å) and angles (deg): Fe(1)-N(3), 1.668(5); Fe(1)-N(4), 1.649(4); N(3)-O(1), 1.191(6); N(4)-O(2), 1.218(6); N(3)-Fe(1)-N(4), 109.2(2); Fe(1)-N(3)-O(1), 163.2(5); Fe(1)-N(4)-O(2), 165.1(5).
In conclusion, reaction of the cluster analog 1 with NO leads to formation of a new nitrogen containing {Fe(NO)2}9 DNIC. This reaction demonstrates that naturally occurring Rieske clusters may be similarly susceptible to disassembly by NO, as found for cysteine-bound Fe2S2 clusters. The reaction of 1 with NO (g) to form two {Fe(NO)2}9 units follows directly from previous work with synthetic clusters of the type [Fe2S2(SR)4]2- and suggests an intrinsic reactivity of the {Fe2S2}2+ core towards NO.9,18 β-Diketiminates are excellent ligands for dinitrosyliron complexes, allowing for isolation of a rare set of homologous DNIC redox partners. Preliminary studies (data not shown) with Fe(TPP)Cl (TPP = meso-tetraphenylporphine dianion) demonstrate that these DNICs display different NO transfer properties, suggesting that control of NO transfer in biology might be redox triggered. This hypothesis is currently under investigation in our laboratory.
Supplementary Material
Acknowledgments
This work was supported by the NSF grant CHE-0611944. Z.J.T. acknowledges postodoctoral fellowship support from the NIH General Medical Sciences (1 F32 GM082031-02). The authors thank Dr. Daniela Bucella and Dr. Peter Müller for assistance with X-ray crystallography.
Footnotes
Supporting Information Available: Experimental details, additional reaction schemes, spectroscopic data, crystallographic data, refinement details, and fully labeled thermal ellipsoid diagrams for 3 and 4b, as well as the corresponding CIF files. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Beinert H, Holm RH, Münck E. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 2.Rieske JS. Biochim Biophys Acta, Rev Bioenerg. 1976;456:195–247. doi: 10.1016/0304-4173(76)90012-4. [DOI] [PubMed] [Google Scholar]
- 3.Iwata S, Saynovits M, Link TA, Michel H. Structure. 1996;4:567–579. doi: 10.1016/s0969-2126(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 4.Gurbiel RJ, Batie CJ, Sivaraja M, True AE, Fee JA, Hoffman BM, Ballou DP. Biochemistry. 1989;28:4861–4871. doi: 10.1021/bi00437a051. [DOI] [PubMed] [Google Scholar]
- 5.Ballmann J, Albers A, Demeshko S, Dechert S, Bill E, Bothe E, Ryde U, Meyer F. Angew Chem Int Ed Engl. 2008;47:9537–9541. doi: 10.1002/anie.200803418. [DOI] [PubMed] [Google Scholar]
- 6.Welter R, Yu L, Yu CA. Arch Biochem Biophys. 1996;331:9–14. doi: 10.1006/abbi.1996.0276. [DOI] [PubMed] [Google Scholar]
- 7.See for example: Foster MW, Cowan JA. J Am Chem Soc. 1999;121:4093–4100.Ding H, Demple B. Proc Natl Acad Sci USA. 2000;97:5146–5150. doi: 10.1073/pnas.97.10.5146.
- 8.D'Addario S, Demartin F, Grossi L, Iapalucci MC, Laschi F, Longoni G, Zanello P. Inorg Chem. 1993;32:1153–1160. [Google Scholar]
- 9.Harrop TC, Tonzetich ZJ, Reisner E, Lippard SJ. J Am Chem Soc. 2008;130:15602–15610. doi: 10.1021/ja8054996. [DOI] [PubMed] [Google Scholar]
- 10.Enemark JH, Feltham RD. Coord Chem Rev. 1974;13:339–406. [Google Scholar]
- 11.(a) Reginato N, McCrory CTC, Pervitsky D, Li L. J Am Chem Soc. 1999;121:10217–10218. [Google Scholar]; (b) Wang X, Sundberg EB, Li L, Kantardjieff KA, Herron SR, Lim M, Ford PC. Chem Commun. 2005:477–479. doi: 10.1039/b412086h. [DOI] [PubMed] [Google Scholar]; (c) Tsai ML, Hsieh CH, Liaw WF. Inorg Chem. 2007;46:5110–5117. doi: 10.1021/ic0702567. [DOI] [PubMed] [Google Scholar]; (d) Huang HW, Tsou CC, Kuo TS, Liaw WF. Inorg Chem. 2008;47:2196–2204. doi: 10.1021/ic702000y. [DOI] [PubMed] [Google Scholar]
- 12.Holland PL. Acc Chem Res. 2008;41:905–914. doi: 10.1021/ar700267b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connelly NG, Gardner C. J Chem Soc, Dalton Trans. 1976:1525–1527. [Google Scholar]
- 14.Sadique AR, Brennessel WW, Holland PL. Inorg Chem. 2008;47:784–786. doi: 10.1021/ic701914m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanina NA, Aldoshin SM. Russ Chem Bull. 2004;53:2428–2448. [Google Scholar]
- 16.Atkinson FL, Blackwell HE, Brown NC, Connelly NG, Crossley JG, Orpen AG, Rieger AL, Rieger PH. J Chem Soc, Dalton Trans. 1996:3491–3502. [Google Scholar]
- 17.(a) Tsou CC, Lin ZS, Lu TT, Liaw WF. J Am Chem Soc. 2008;130:17154–17160. doi: 10.1021/ja806050x. [DOI] [PubMed] [Google Scholar]; (b) Lu TT, Tsou CC, Huang HW, Hsu IJ, Chen JM, Kuo TS, Wang Y, Liaw WF. Inorg Chem. 2008;47:6040–6050. doi: 10.1021/ic800360m. [DOI] [PubMed] [Google Scholar]
- 18.Tsai FT, Chiou SJ, Tsai MC, Tsai ML, Huang HW, Chiang MH, Liaw WF. Inorg Chem. 2005;44:5872–5881. doi: 10.1021/ic0505044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.