Abstract
Inhibitors of apoptosis (IAPs) inhibit caspases, thereby preventing proteolysis of apoptotic substrates. IAPs occlude the active sites of caspases to which they are bound1-3 and can function as ubiquitin ligases. IAPs are also reported to ubiquitinate themselves and caspases4,5. Several proteins induce apoptosis, at least in part, by binding and inhibiting IAPs. Among these are the Drosophila melanogaster proteins Reaper (Rpr), Grim, and HID, and the mammalian proteins Smac/Diablo and Omi/HtrA2, all of which share a conserved amino-terminal IAP-binding motif6-14. We report here that Rpr not only inhibits IAP function, but also greatly decreases IAP abundance. This decrease in IAP levels results from a combination of increased IAP degradation and a previously unrecognized ability of Rpr to repress total protein translation. Rpr-stimulated IAP degradation required both IAP ubiquitin ligase activity and an unblocked Rpr N terminus. In contrast, Rpr lacking a free N terminus still inhibited protein translation. As the abundance of short-lived proteins are severely affected after translational inhibition, the coordinated dampening of protein synthesis and the ubiquitin-mediated destruction of IAPs can effectively reduce IAP levels to lower the threshold for apoptosis.
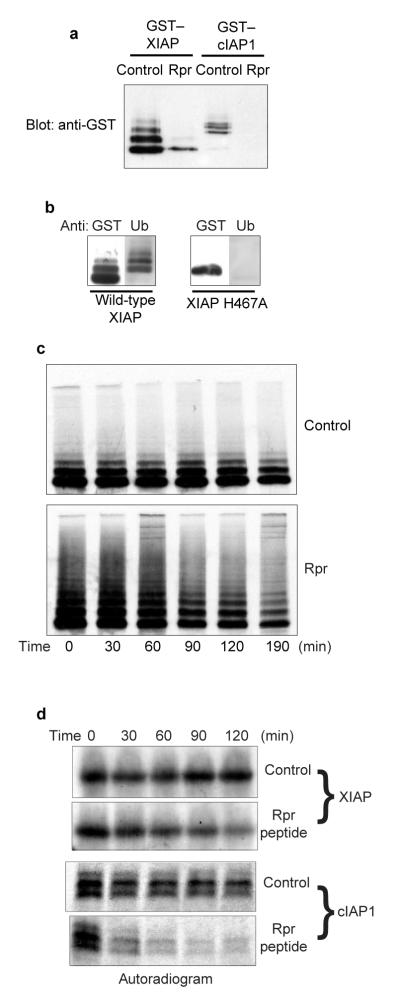
To evaluate the effects of Rpr on the function of IAPs, we cotransfected human 293T cells with untagged Rpr and human members of the IAP family: XIAP and cIAP1. In the presence of Rpr, IAP steady-state levels were much lower than in the presence of vector alone, suggesting that Rpr was preventing XIAP and cIAP1 protein accumulation (Fig. 1a). Similar results were obtained in fly embryos, where overexpression of Rpr resulted in barely detectable levels of DIAP1 (B. Hay, personal communication). Note that ‘laddered’ forms of XIAP, indicative of ubiquitination, were recognized by anti-ubiquitin antibody (Fig. 1b), consistent with previous reports of IAP auto-ubiquitination4,5.
Figure 1. Rpr stimulates IAP auto-ubiquitination and destruction.
a, HEK 293T cells were transfected with GST—XIAP or GST—cIAP1 and either Rpr or vector control, in the presence of zVAD-fmk. Following precipitation with glutathione—Sepharose, GST fusion protein levels were analysed by immunoblotting with an anti-GST polyclonal antibody. b, HEK 293T cells were transfected with either wild-type ubiquitin ligase or an XIAPH467A mutant and precipitated with glutathione—Sepharose. Precipitates were immunoblotted with an antibody against GST or ubiquitin. c, HEK 293T cells were transfected with GST—XIAP and either Rpr or vector control. Cells were then radiolabelled in a pulse-chase experiment. The resulting radiolabelled GST fusion proteins were analysed by autoradiography. d, Radiolabelled IAPs were incubated in crude Xenopus egg extracts, in the presence of either Rpr peptide or vehicle control. The resulting radiolabelled protein levels were analysed by autoradiography. Equal loading was verified by Coomassie blue staining of gels (data not shown).
We therefore hypothesized that Rpr might stimulate IAP ubiquitination and degradation. To determine whether Rpr affects IAP half-life, we performed pulse-chase analyses on cells cotransfected with XIAP and either Rpr or vector alone. Cotransfection with Rpr significantly affected XIAP stability (Fig. 1c; see also Fig. 3b). Moreover, Rpr greatly increased the appearance of laddered XIAP species. This change in IAP stability was not a consequence of Rpr-induced apoptosis, as the pulse-chase experiments were performed in the presence of the broad-spectrum caspase inhibitor zVAD-fmk.
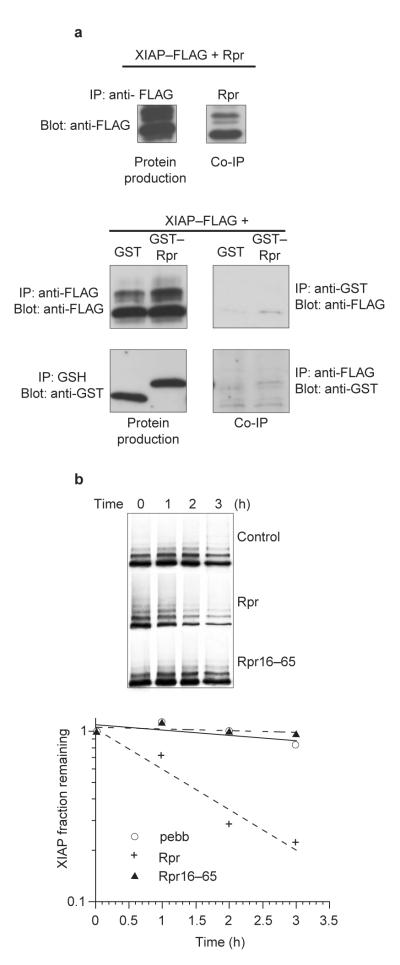
Figure 3. An unblocked Rpr N terminus is required for IAP binding and stabilization.
a, HEK 293T cells were transfected with Rpr, GST alone or GST—Rpr and FLAG-tagged XIAP protein. Proteins were precipitated with anti-FLAG or anti-Rpr antibodies and blotted with anti-FLAG. Proteins were precipitated with glutathione—Sepharose or anti-FLAG, and detected by immunoblotting with anti-GST or anti-FLAG. Although GST—Rpr and XIAP failed to coprecipitate, the untagged Rpr control clearly coprecipitated with XIAP protein. b, A pulse-chase experiment was performed as in Fig. 1c, using either wild-type Rpr (Rpr) or untagged Rpr lacking its first 15 amino acids (Rpr16-–65). The results were then quantified.
To address the effects of Rpr on IAP stability in an alternative system, we examined the half-lives of radiolabelled human IAPs added to whole-cell lysates prepared from Xenopus laevis eggs, which reconstitute both apoptotic signalling and ubiquitin-dependent proteolysis15,16. Radiolabelled, in vitro -translated cIAP1 and XIAP proteins were added to egg extracts supplemented with vehicle or with full-length, untagged Rpr, prepared by complete de novo peptide synthesis17. As in cultured cells, Rpr addition to egg extracts significantly destabilized both cIAP1 and XIAP (Fig. 1d). Similar results were obtained with a fly IAP, DIAP1 (Fig. 4c).
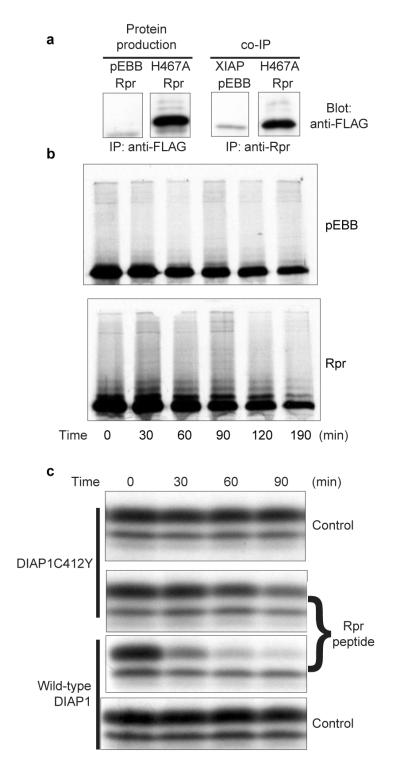
Figure 4. Rpr does not destabilize the XIAPH467A ubiquitin ligase mutant.
a, HEK 293T cells were transfected with FLAG-tagged wild-type XIAP or FLAG-tagged catalytically inactive XIAP (XIAPH467A) in conjunction with a control vector (pEBB) or untagged Rpr (Rpr). Samples were precipitated with an anti-FLAG antibody (left) or an anti-Rpr antibody (right), demonstrating a nearly quantitative association of the mutant XIAP with Rpr. b, HEK 293T cells were transfected with GST—XIAPH467A and either Rpr or vector control. Cells were then radiolabelled in a pulse-chase experiment, and the resulting radiolabelled GST—XIAPH467A proteins were analysed by autoradiography. c, Radiolabelled DIAP1 protein, either wild-type or the catalytically inactive DIAPC412Y mutant, was incubated in Xenopus egg extract lacking mitochondria, in the presence of Rpr peptide or vehicle control. The resulting radiolabelled protein levels were analysed by autoradiography.
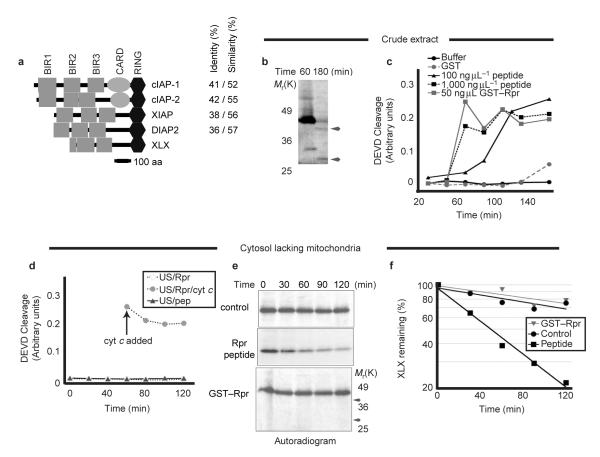
To extend these findings, we isolated a Xenopus XIAP homologue, XLX. Domain analysis of XLX revealed two complete and one partial N-terminal baculovirus inhibitory repeat (BIR) domain, and a carboxy-terminal RING domain (Fig. 2a). In common with XIAP, XLX lacks the caspase activation recruitment domain (CARD) found in cIAP1 and cIAP2 (ref. 18). Despite truncation of BIR domain 1 in our clone, we believe XLX to be full-length, as the cDNA isolated contains three in-frame stop codons within the 5′-untranslated region (UTR) preceding the start methionine.
Figure 2. XLX, a X. laevis XIAP homologue, is destabilized by Rpr peptide, but not by GST—Rpr.
a, A domain map drawn to the scale of XLX with three human IAPs: cIAP1, cIAP2, and XIAP, and D. melanogaster DIAP2 is shown. Grey boxes represent BIR domains, ovals represent caspase recruitment domains (CARD), and hexagons represent C-terminal RING domains. The percentage identity and similarity between residues of each protein to XLX are indicated. BLAST alignment of XLX to proteins in the non-redundant public database yielded chicken and human XIAP as the most similar clones. XLX has a similar domain structure to XIAP and DIAP2. b, GST—Rpr and radiolabelled XLX were added to crude Xenopus egg extract. Samples were resolved by SDS—PAGE and autoradiography at the indicated times. Arrowheads denote the ∼40K and 30K XLX cleavage products. Molecular weight markers are shown for reference with e below. c, GST—Rpr, GST, Rpr peptide or peptide vehicle were added to crude Xenopus egg extract. Caspase activity was monitored by cleavage of the colorimetric peptide substrate DEVD—pNA. d, GST—Rpr (Rpr) or Rpr peptide (Pep) were added to Xenopus egg extract depleted of mitochondria by centrifugation (US). At 60 min, human cytochrome c was added to an aliquot of the extract containing GST—Rpr (arrow). Caspase activity was monitored as in c above. e, Rpr peptide, peptide vehicle (control) or GST—Rpr were added together with radiolabelled XLX into Xenopus egg extract depleted of mitochondria. Samples were taken at the indicated times and XLX protein levels were analysed by SDS—PAGE and autoradiography. Molecular weight markers are shown for comparison with b and arrows indicate the approximate position of expected XLX cleavage fragments (which are absent). Equal loading was verified by Coomassie blue staining of gels (data not shown). f, The autoradiogram in e was quantified using a phosphorimager.
Because IAPs can be caspase substrates (Fig. 2b), the disappearance of IAPs in our extracts might have been caused, at least in part, by caspase-mediated cleavage19,20. In fact, glutathione S-transferase (GST)—Rpr induces mitochondrial cytochrome c release, thereby activating caspases in the extract21. Similarly, addition of Rpr peptide to crude Xenopus egg extracts triggered caspase activation, although at the concentration used in our IAP experiments (100 ng μl-1), caspase activation was relatively delayed (Fig. 2c). However, as reported for GST—Rpr21, the Rpr peptide could not induce caspase activation in egg cytosol lacking mitochondria (Fig. 2d; note caspase activation by cytochrome c addition to the same extract). Nevertheless, in these cytosolic extracts, the Rpr peptide significantly accelerated the destruction of XLX (Fig. 2e,f). XLX cleavage fragments were absent in these extracts (Fig. 2e, arrowheads) and in crude extracts incubated with zVAD-fmk (data not shown). Therefore, although caspases can cleave XLX, they are not essential for Rpr-accelerated IAP destruction. In contrast to the Rpr peptide, GST—Rpr (whose IAP-binding N terminus is shielded by its GST tag), failed to accelerate XLX destruction (Fig. 2e,f). These data suggest that Rpr-stimulated degradation of IAPs can occur independently of caspase activation, and that this effect requires the N terminus of Rpr to be unblocked. Consistent with the hypothesis that Rpr requires a free N terminus to promote IAP degradation, GST—Rpr and XIAP did not co-precipitate (Fig. 3a, right). In addition, an untagged Rpr lacking amino acids 1–15 (Rpr16-65) could not promote IAP degradation, further demonstrating that the extreme N terminus of Rpr is required to shorten IAP half-life (Fig. 3b).
To determine whether IAP ubiquitin ligase activity was required for Rpr-induced IAP degradation, we cotransfected cells with Rpr and a catalytically inactive XIAP point mutant of XIAPH467A (ref. 4; see also Fig. 1b). Although Rpr bound to XIAPH467A (Fig. 4a), it failed to accelerate destruction of the mutant in a pulse-chase experiment (Fig. 4b). Rpr had similar effects on its Drosophila target, DIAP1. Again, destabilization was dependent on an intact RING domain, as the DIAP1 ubiquitin ligase mutant DIAP1C412Y was not significantly destabilized (Fig. 4c). Collectively, these data demonstrate that Rpr-stimulated IAP degradation requires that the IAP be functional as a ubiquitin ligase.
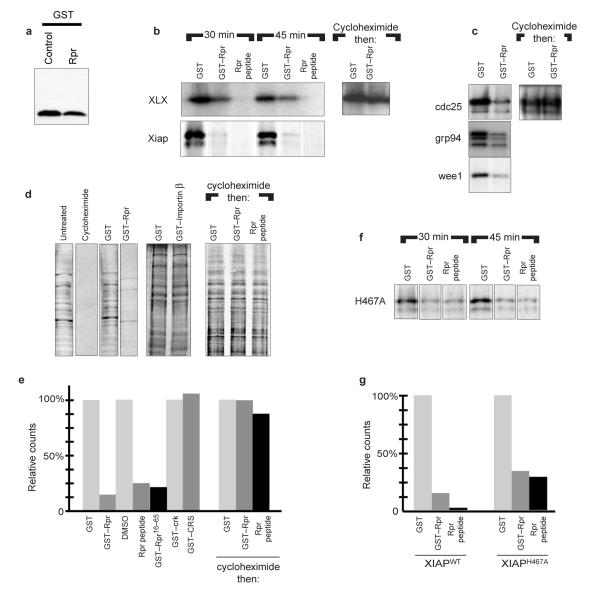
Although untagged, full-length Rpr substantially destabilized all of the wild-type IAP proteins tested, we were surprised to find that Rpr also moderately decreased steady-state levels of an unrelated protein after cotransfection of human cells (Fig. 5a, GST). Additionally, overexpression of Rpr in flies lowers the levels of a DIAP1 ubiquitin ligase mutant, implying that Rpr has effects in vivo that are independent of its effects on IAP half-life (B. Hay, personal communication). This prompted us to examine whether IAP abundance might also be affected at the level of protein production. Indeed, when we programmed reticulocyte lysates with XIAP or XLX, IAP levels were profoundly decreased by GST—Rpr and essentially eliminated by the Rpr peptide (Fig. 5b). GST, or other unrelated proteins, had no effect (Fig. 5b and data not shown). These effects on IAP levels were not caused by IAP degradation, as GST—Rpr failed to alter IAP levels when added to reticulocyte lysates, after translation had been blocked with cycloheximide (Fig. 5b).
Figure 5. Repression of translation by GST—Rpr and Rpr peptide.
a, HEK 293T cells were transfected with GST and either Rpr or vector control. Resulting GST protein levels were analysed by immunoblotting. b, XLX and human XIAP were added to rabbit reticulocyte lysates, which were supplemented with GST, GST—Rpr or Rpr peptide. Aliquots were collected at 30 and 45 min, and resolved by SDS—PAGE (left). Rabbit reticulocyte lysates were programmed with XLX and allowed to transcribe and translate for 45 min. Translation was stopped with the addition of cycloheximide and the lysates were incubated for an additional hour with GST or GST—Rpr before resolution by SDS—PAGE (right). c, Cdc25, Grp94 and Wee1 cDNAs were added to reticulocyte lysates and supplemented with GST or GST—Rpr, as above (left). The rabbit reticulocyte lysates were also allowed to transcribe and translate for 45 min, after which translation was stopped with cycloheximide (right). Samples were then incubated for an additional hour with GST or GST—Rpr, as above. d, Translationally competent, but transcriptionally inactive, Xenopus egg extracts were supplemented with 35S-Met/Cys and zVAD-fmk plus egg lysis buffer (Untreated), cycloheximide, GST, GST—Rpr, or GST—importin-β as a non-specific control (left). Translation was allowed to proceed for 45 min and products were resolved by SDS—PAGE. Egg extracts supplemented with 35S-Met/Cys and zVAD-fmk were allowed to translate for 45 min, translation was stopped with cycloheximide and extracts were incubated for an additional hour with GST, GST—Rpr or Rpr peptide (right). e, Samples prepared as in d were subjected to TCA precipitation and quantified by scintillation counting. Additionally, control proteins (GST—cyclin B1, GST—CRS, GST—Crk), peptide vehicle (DMSO), GST—Rpr16–65 or Rpr peptide were assayed in the same manner. The resulting incorporated counts were TCA precipitated and scintillation counting was performed as above. f, XIAPH467A, a mutant unable to function as a ubiquitin ligase, was added to rabbit reticulocyte lysate, which was supplemented with GST, GST—Rpr or Rpr peptide, and aliquots were collected at 30 and 45 min before resolution by SDS—PAGE. g, Phosphorimager quantification of wild-type XIAP and XIAPH467A protein levels from b and f.
Because the IAP constructs used in the reticulocyte lysates lacked native 5′-or 3′-UTR sequences, we considered it unlikely that the degradation-independent effects of Rpr were IAP-specific. Accordingly, when reticulocyte lysates were programmed with unrelated messages, the GST—Rpr protein also effectively dampened their expression (Fig. 5c). Again, this effect was not caused by protein degradation, as GST—Rpr addition did not affect levels of previously transcribed and translated proteins in reticulocyte lysates (Fig. 5c).
To assess the effects of Rpr on total protein synthesis, we added GST—Rpr to Xenopus egg extracts, which were translationally competent and transcriptionally inactive. These extracts were supplemented with 35S-Met/Cys and high levels of zVAD-fmk to prevent caspase-mediated cleavage of translation factors. Addition of GST—Rpr or Rpr peptide to Xenopus egg extracts globally suppressed protein synthesis (Fig. 5d,e). Importantly, unrelated GST fusion proteins prepared in the same manner as GST—Rpr had no such effect (Fig. 5d,e). Rpr did not reduce protein levels by accelerating general protein degradation, as co-addition of GST—Rpr or Rpr peptide and cycloheximide to extracts after 45 min of translation did not result in destruction of nascent proteins (Fig. 5d,e). These data strongly suggest that the ability of Rpr to post-translationally destabilize proteins is specific to the IAPs. Thus, Rpr can decrease generalized translation in a manner distinct from its ability to accelerate the ubiquitin-mediated destruction of extant IAPs. Unlike the effect on IAP protein stability, the Rpr effect on translation did not require a free N terminus, as GST—Rpr was effective in translational inhibition. GST—Rpr16–65, which lacks the first 15 amino acids of Rpr, also inhibited translation, confirming that the extreme N terminus of Rpr is dispensable for translational inhibition (Fig. 5e).
Although GST—Rpr was able to decrease IAP levels (Fig. 5b), the Rpr peptide was more effective in this regard. We hypothesized that the peptide might more effectively lower wild-type XIAP protein levels by simultaneously shortening XIAP half-life and inhibiting protein translation. We therefore returned to the reticulocyte lysate system to examine levels of the XIAPH467A, as this mutant is not subject to Rpr-mediated degradation. When the XIAP mutant was examined in this system, we found that GST—Rpr indeed suppressed translation of this protein, as it had with other proteins tested. However, whereas the abundance of wild-type XIAP had been more dramatically reduced by the Rpr peptide than by GST—Rpr, the abundance of the XIAP ubiquitin ligase mutant was suppressed equally by both (compare Fig. 5b and f).
Despite the robust translational inhibition by Rpr in vitro, we wanted to determine whether we could detect such effects of Rpr in intact cells. Accordingly, we injected whole Xenopus oocytes with zVAD-fmk and either rpr sense or anti-sense mRNA. After 12 h incubation to allow translation of the Rpr protein, we re-injected oocytes with 35S-methionine, incubated them for a further 4 h, lysed the oocytes and assessed the level of total protein synthesis by measurement of TCA-precipitable radioactivity. The oocytes injected with zVAD-fmk and rpr sense mRNA incorporated approximately sevenfold less counts than the anti-sense controls (∼1.1 × 105 cpm versus ∼7.9 × 105 cpm). These data demonstrate that even when synthesized de novo within an intact cell, Rpr can inhibit protein translation. Consistent with these results, cotransfection of human cells with Rpr and GST reduced GST synthesis by ∼30% in a pulse labelling experiment, despite the very low levels of Rpr produced in these cells (data not shown). Although these results were more modest than those obtained in reticulocyte lysates or oocytes, we have not been able to achieve comparable levels of Rpr in the intact tissue culture cells. However, even a moderate reduction in protein synthesis, coupled with a decrease in IAP stability, would synergize to produce an effective elimination of the IAPs.
In aggregate, our data suggest that Rpr eliminates IAPs by simultaneously stimulating their ubiquitin-mediated degradation and down-regulating total protein translation. This reduction in IAP levels by Rpr lowers the threshold for caspase function, thereby facilitating apoptotic progression.
Note added in proof: Several other papers in this issue also demonstrate that Reaper functions to stimulate IAP degradation23-25. Additionally, another paper in this issue supports our findings that Reaper suppresses general protein translation26.
Methods
Cell culture, transfections, immunoblotting, and pulse-chase analysis
All cell culture reagents were obtained from Gibco (Rockville, MD) unless otherwise specified. HEK 293T cells were obtained from the American Type Culture Collection (ATCC) through the Duke Cell Culture Facility, and were maintained in MEM, which was supplemented with 10% foetal bovine serum, 1 mM sodium pyruvate and 0.1 mM MEM non-essential amino acids solution. The Drosophila rpr gene was cloned into pEBB using standard methods. For the immunoblots shown, 1 × 106 cells were plated in 100-mm dishes and transfected 24 h later using the Fugene 6 reagent (Roche Molecular, Indianapolis, IN) and 10 μg of total DNA, according the manufacturer’s instructions. 24–48 h after transfection, cells were washed once in PBS, collected in lysis buffer (10 mM HEPES at pH 7.4, 50 mM potassium chloride, 2.5 mM magnesium chloride and 50 mM sucrose, plus 1× Complete protease inhibitor (Roche Molecular)) and briefly sonicated. Lysates were incubated for 10 min on ice and cleared by centrifugation at 10,000g for 10 min. Cleared lysates were then incubated with glutathione—Sepharose (Pharmacia) or the M2 anti-FLAG antibody (Sigma, St Louis, MO) and Protein G—agarose (Oncogene Research Products, Boston, MA) or K1 anti-Rpr antibody and Protein A—Sepharose (Sigma) at 4 °C for 1 h. The bead-bound material was washed three times in lysis buffer and released in 2×SDS sample buffer. This material was then separated by SDS—polyacrylamide gel electrophoresis (PAGE) and transferred to PVDF membranes by standard methods. Membranes were blocked in PBS containing 0.1% Tween-20 and 5% dry milk. For immunoblotting to detect GST fusions, rabbit antiserum to GST was used at 1:3,000 in PBS containing 0.1% Tween-20 and 2% BSA, before incubation with Protein A—horseradish peroxidase (HRP; Amersham, Sunnyvale, CA) at 1:10,000. Immunoblots to detect FLAG-tagged proteins were handled similarly using the M2 anti-FLAG antibody (1 μg μl-1) and goat anti-mouse-HRP (Jackson ImmunoResearch, West Grove, PA), whereas ubiquitin was detected using mouse anti-ubiquitin (1:100; Zymed, San Francisco, CA) and Protein A—HRP without pre-blocking the membrane. Blots were developed using Renaissance ECL reagents (NEN, Boston, MA) and exposed to Biomax ML film (Kodak, Rochester, NY). For pulse-chase analysis, 200,000 cells were plated per well in 6-well plates and transfected as above, except that a total of 1.5 μg DNA was used. 16–20 h after transfection, cells were washed once in prewarmed pulse medium (DMEM minus L-Met and L-Cys supplemented with 10% dialysed foetal bovine serum and 1 mM sodium pyruvate) and then incubated for 15 min in pulse medium to deplete Met and Cys levels. Cells were then radiolabelled for 30 min with pulse medium containing 200 μCi ml-1 of 35S-Trans label (ICN, Costa Mesa, CA). After labelling, cells were washed once with their normal culture medium and incubated in the complete medium for the chase times indicated. Radiolabelled proteins were harvested by rinsing the cells once in PBS and then lysing in 0.1% NP40, 150 mM sodium chloride, 50 mM HEPES at pH 7.4 and 1 mM EDTA, plus 1× protease inhibitors as above. Cell lysates were cleared by incubation on ice and centrifugation as above. GST—fusion proteins were captured on GSH—Sepharose and separated by SDS—PAGE as above. Gels were soaked in 1 M salicylate (Sigma) for 30 min before drying and overnight exposure to Biomax MR film (Kodak).
Cloning of XLX
A probe derived from the RING domain of human cIAP1 was generated using the Random Primed Labelling kit (Roche Molecular) and used to screen ∼500,000 clones of a λ-zap Xenopus gastrula library at low stringency. Several clones >1 kB were isolated, excised and partially sequenced. A secondary screen was performed for one of the clones isolated using oligonucleotides designed to anneal to the linker region between the BIR and RING domains. The probe was generated by PCR with radiolabelled nucleotides (oligonucleotides: 5′-GATCTTTAGAAGCCCAGAGTCCTCTCCT-3′ and 5′-GATCCTTGCTCTGAATTAGACTTGCCAC-3′). This screen failed to isolate any larger clones. The ∼1.6 kB cDNA was fully sequenced and deposited in GenBank. A BLAST alignment was performed using both the complete cDNA and the longest uninterrupted open reading frame. Domain analysis was performed using InterPro (http://www.ebi.ac.uk/interpro/).
Extract preparation
Preparation of crude interphase egg extracts (CS) was performed as previously described21. To fractionate the crude egg extract into cytosolic (US) and membranous components, the crude extract was centrifuged further at 200,000g for 1 h in a Beckman TLS-55 rotor using a TL-100 centrifuge. The cytosolic fraction (ultra-S or US) was removed and recentrifuged for an additional 25 min at 200,000g. These reconstituted extracts were supplemented with an energy regenerating system consisting of 2 mM ATP, 5 mg ml-1 creatine kinase, and 20 mM phosphocreatine (final concentrations).
Production of GST, GST—Rpr and Rpr peptide
GST and GST—Rpr were prepared as previously described21. Rpr was also generated as a full-length, untagged synthetic peptide by B. Kaplan (City of Hope, Beckman Research Institute). The peptide was received as a lyophilized powder, which was stored solid at 4 °C. Before use, the peptide was resuspended in dimethylsulphoxide (DMSO) at 10 mg ml-1, and then diluted to 1 mg ml-1 in egg lysis buffer (10 mM HEPES at pH 7.4, 50 mM potassium chloride, 2.5 mM magnesium chloride, 50 mM sucrose and 1 mM dithiothreitol (DTT)).
DEVD assay
Recombinant GST, GST—Rpr, Rpr peptide or peptide vehicle (10% v/v DMSO in egg lysis buffer) was added at a 1:10 dilution to CS or US extract containing energy regenerating mix (see above). At the indicated times, 3-μl aliquots were withdrawn and incubated with 90 μl of assay buffer (50 mM HEPES at pH 7.5, 100 mM sodium chloride, 0.1% CHAPS, 10 mM DTT, 1mM EDTA and 10% glycerol) containing 200 μM Ac—DEVD pNA colorimetric substrate (BioMol, Plymouth Meeting, PA). Caspase-3 activity was monitored by the measurement of absorbance at 405 nm using a LabSystems MultiSkan MS microtiter 96-well plate reader (Helsinki, Finland).
In vitro translation
XIAP ORFs were subcloned using standard techniques into pBS II-SK and pSP64T, a TNT expression vector with flanking 5′ and 3′β-globin UTR and a polyadenosine tail. To produce radioactive protein for half-life assays, Cdc 25, Grp94, Wee1, XLX, wild-type XIAP/DIAP, and XIAPH467A/DIAPC412Y templates were added at 20 ng μl-1 to rabbit reticulocyte lysate (Stratagene, La Jolla, CA) containing 1 μCi μl-1 of Trans label, 1× (minus-Cys, minus-Met) amino acid mix and other components, in accordance with the manufacturer’s protocol. For Xenopus stability assays, the reaction was stopped after 90 min and proteins were snap frozen in liquid nitrogen for later use. For translation inhibition assays, reticulocyte lysate reactions were supplemented with 100 ng μl-1 of recombinant GST or GST—Rpr proteins, or Rpr peptide. Aliquots were withdrawn at the indicated times, resolved by SDS—PAGE, quantified with a Phosphorimager (Molecular Dynamics, Sunnyvale, CA), and exposed to film. Protein degradation was assayed by allowing translation to proceed for 45 min, at which point cycloheximide was added to a final concentration of 500 ng μl-1. Subsequently, GST—Rpr, Rpr peptide or GST were added to a final concentration of 100 ng μl-1 and the mixture was incubated for 60 min at room temperature. Translated proteins were resolved by SDS—PAGE and quantified with the phosphorimager as described above.
Xenopus extract stability assay
In vitro-translated proteins were added on ice at 1:10 dilution into 100 μl of either CS or US lysate (with energy mix) that had been supplemented with 100 ng μl-1 GST, GST—Rpr, or Rpr peptide. Where indicated, zVAD-fmk (BioMol) or DMSO vehicle was also added at a final concentration of 100 μM (data not shown). Samples were shifted to room temperature or 4 °C and 20-μl aliquots were withdrawn at the indicated times, mixed with 40 μl SDS loading buffer and flash frozen in liquid nitrogen. Samples were thawed by boiling for 5 min and then assayed by SDS—PAGE before quantification on a phosphorimager and exposure to film.
Xenopus extract translation assay
In vitro translation assays using Xenopus extract were conducted by adding 1 μCi μl-1 of Trans label, 100 μM zVAD-fmk and 100 ng μl-1 of recombinant GST, GST—crk, GST—CRS (Cyclin B cytoplasmic retention sequence), GST—importin-β, GST—Rpr, GST—Rpr16–65 proteins or Rpr peptide to crude egg extract. The extent of protein translation was assayed by SDS—PAGE analysis and quantified by autoradiogram and phosphorimager, or by TCA precipitation (80 μg of extract in 20% TCA). Rpr-induced degradation was assayed as in reticulocyte lysates (above), save that in addition, total translated protein was also quantified by TCA precipitation as described.
Oocyte micro-injection and translation assay
Stage VI oocytes of X. laevis were prepared for micro-injection as described22. 25 nl of 0.4 μg μl-1 sense or antisense rpr RNA produced using the mCAP RNA capping kit (Stratagene) were injected into oocytes along with 100 μM zVAD-fmk. Rpr expression was allowed to proceed overnight, before an injection of Trans Label (25 nl of 10 μCi μl-1). 25 oocytes injected with rpr sense or antisense RNA were collected 4 and 5 h after Trans label injection. The oocytes were lysed in buffer (5 mM HEPES at pH 7.8, 88 mM sodium chloride, 1 mM potassium chloride, 1 mM magnesium sulphate, 2.5 mM NaHCO3, 0.7 mM calcium chloride and 50 ng μl-1 apropotein/leupetin/ cytochalasin B) by centrifugation at 16,000g for 15 min. Total protein translation was assayed by TCA precipitation (80 μg of oocyte extract in 20% TCA) as described.
Accession numbers
X. laevis XIAP (XLX) was submitted to GenBank and given the accession number AF468029.
ACKNOWLEDGEMENTS
We thank B. Kaplan for his generous provision of the Rpr peptide. We are grateful to B. Hay for provision of DIAP clones and for helpful discussion. We thank C. Duckett for generously providing XIAP-expression clones. We also thank B. Mayer for the Xenopus cDNA library and M. Hardwick for providing the c-IAP clone. This work was supported by a National Institutes of Health grant to S.K. (RO1 GM61919). S.K. is a Scholar of the Leukemia and Lymphoma Society. D.C.R. is a Gates Millenium Fellow. C.H. and M.O. are predoctoral fellows of the US Army Medical Research and Material Command Breast Cancer Research Program, as well as the NIH Medical Scientist Training Program.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare that they have no competing financial interests.
References
- 1.Riedl SJ, et al. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, et al. Cell. 2001;104:781–790. [PubMed] [Google Scholar]
- 3.Chai J, et al. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 5.Huang H, et al. J. Biol. Chem. 2000;275:26661–26664. doi: 10.1074/jbc.C000199200. [DOI] [PubMed] [Google Scholar]
- 6.White K, et al. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 7.Goyal L, McCall K, Agapite J, Hartwieg E, Steller H. EMBO J. 2000;19:589–597. doi: 10.1093/emboj/19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu G, et al. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 9.Wu JW, Cocina AE, Chai J, Hay BA, Shi Y. Mol. Cell. 2001;8:95–104. doi: 10.1016/s1097-2765(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 10.Verhagen AM, et al. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, et al. Mol. Cell. 2001;8:613–621. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 12.Martins LM, et al. J. Biol. Chem. 2002;277:439–444. doi: 10.1074/jbc.M109784200. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Nordstrom W, Gish B, Abrams JM. Genes Dev. 1996;10:1773–1782. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- 14.Grether ME, Abrams JM, Agapite J, White K, Steller H. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 15.Newmeyer DD, Farschon DM, Reed JC. Cell. 1994;79:353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 16.Glotzer M, Murray A, Kirschner M. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 17.Avdonin V, et al. Proc. Natl Acad. Sci. USA. 1998;95:11703–11708. doi: 10.1073/pnas.95.20.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter BW, Duckett CS. [(cited 8 August 2000)];Science STKE. 2000 doi: 10.1126/stke.2000.44.pe1. [online] http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2000/44/pe1. [DOI] [PubMed]
- 19.Clem RJ, et al. J. Biol. Chem. 2001;276:7602–7608. doi: 10.1074/jbc.M010259200. [DOI] [PubMed] [Google Scholar]
- 20.Deveraux QL, et al. EMBO J. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans EK, et al. EMBO J. 1997;16:7372–7381. doi: 10.1093/emboj/16.24.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swenson KI, Jordan JR, Beyer EC, Paul DL. Cell. 1989;57:145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- 23.Ryoo HD, et al. Nature Cell Biol. DOI:10.1038/ncb795. [Google Scholar]
- 24.Hays R, et al. Nature Cell Biol. doi: 10.1038/ncb794. DOI:10.1038/ncb794. [DOI] [PubMed] [Google Scholar]
- 25.Wing JP, et al. Nature Cell Biol. DOI:10.1038/ncb800. [Google Scholar]
- 26.Yoo SJ, et al. Nature Cell Biol. DOI:10.1038/ncb793. [Google Scholar]