Summary
Subiculum, which is the primary efferent pathway of hippocampus, participates in memory for spatial tasks, relapse to drug abuse, and temporal lobe seizures. Subicular pyramidal neurons exhibit low-threshold burst firing driven by a spike after-depolarization. Here we report that burst firing can be regulated by stimulation of afferents projections to subiculum. Unlike synaptic plasticity in these and other neurons, burst plasticity did not require synaptic depolarization, activation of AMPA or NMDA receptors, or action potential firing. Rather, enhancement of burst firing required synergistic activation of group I, subtype 1 metabotropic glutamate receptors (mGluRs) and muscarinic acetylcholine receptors (mAChR). When either of these receptors was blocked, a suppression of bursting was revealed, which in turn was blocked by antagonists of group I, subtype 5 mGluRs. These results indicate that the output of subiculum can be strongly and bidirectionally regulated by activation of glutamatergic inputs within the hippocampus and cholinergic afferents from the medial septum.
Keywords: hippocampus, intrinsic plasticity, patch-clamp
Introduction
Synaptic plasticity is a leading candidate for the cellular mechanism underlying learning and memory (Martin et al., 2000), but a role for non-synaptic plasticity has also been suggested (Daoudal and Debanne, 2003; Zhang and Linden, 2003). Non-synaptic plasticity generally involves the regulation of extra-synaptically localized ligand- or voltage-gated conductances and, compared to synaptic plasticity, represents a more global change in the excitability of a neuron. Unlike synaptic plasticity, the conditions required to induce non-synaptic plasticity are relatively poorly understood. An important issue in this regard is whether the requirements for non-synaptic plasticity parallel those of synaptic plasticity or differ substantially. Resolving this issue will help to determine whether synaptic and non-synaptic plasticity are likely to occur in concert or under separate conditions.
Much of the work on synaptic plasticity has been performed in the hippocampus, an area well known for its role in spatial memory tasks in rodents and declarative memory in humans. A functionally important subregion is subiculum, because it serves as the major output pathway of hippocampus. Subicular efferents target a variety of cortical and subcortical areas, including prefrontal cortex (Jay and Witter, 1991), nucleus accumbens (Lopes da Silva et al., 1984), and hypothalamus (Kishi et al., 2000). This divergent output makes subiculum an integral component in networks underlying diverse functions and behaviors, such as regulation of the hypothalamic-pituitary axis (O’Mara, 2005) and memory for spatial tasks (O’Mara et al., 2001). Additionally, dysregulation of subicular function has been implicated in pathological conditions such as epilepsy (Cohen et al., 2002; Harris and Stewart, 2001) and drug addiction (Cooper et al., 2003; Robbins and Everitt, 2002; Sun and Rebec, 2003).
The majority of pyramidal neurons in subiculum respond to brief depolarization just above threshold with a high frequency cluster (> 100 Hz) of 2–3 action potentials (a burst). In vitro, burst firing does not require strong correlated synaptic input (Staff et al., 2000), but rather depends on activation of voltage-gated Ca2+ conductances by a Na+-dependent action potential. The resulting Ca2+ tail current, largely mediated by R-type channels, leads to an after-depolarization (ADP) that can drive burst firing. The ADP, as well as burst firing, can be limited by other conductances, including slow Ca2+-activated K+ currents (Jung et al., 2001; Staff et al., 2000). Because intrinsic conductances determine this pattern of neuronal output, their modulation can result in robust and distinct changes in burst firing, which therefore provides a good model system for the study of non-synaptic plasticity.
We used whole-cell current-clamp recordings to examine whether synaptic and non-synaptic properties of subicular pyramidal cells can be regulated in an activity-dependent manner. We describe a novel form of bidirectional plasticity, independent of synaptic plasticity, that resulted in altered levels of burst firing in these neurons (burst plasticity). The direction of this change depended on the receptor types activated during the induction stimulus. Enhancement of burst firing did not require synaptic depolarization, activation of AMPA or NMDA receptors, or action potential firing, but rather depended on synergistic activation of group I, subtype 1 mGluR (mGluR1) and mAChR. When the enhancement of burst firing was blocked, a separate process led to suppression of burst firing, mediated by synaptic activation of group I, subtype 5 mGluR (mGluR5). These results support the idea that, separate from synaptic changes, distinct mechanisms can lead to alterations in intrinsic conductances that significantly alter neuronal integration and output.
Results
Theta-burst stimulation induces synaptic and non-synaptic plasticity in subiculum
Synaptic and non-synaptic responses were assessed using whole-cell current-clamp recordings in burst-firing pyramidal neurons of subiculum. Theta-burst stimulation (TBS; see Experimental Procedures), which resembles the activity patterns observed during hippocampus-dependent learning tasks in vivo (Buzsaki, 2005; Hasselmo, 2005), was used to induce plasticity of neuronal excitability.
Excitatory postsynaptic potentials (EPSPs) were recorded during low-frequency stimulation of afferents from CA1 and entorhinal cortex. After measuring EPSPs for a 10-minute baseline period, 3 seconds of TBS (Fig. 1B) were delivered to these same afferents. As expected based on previous work (Commins et al., 1998; O’Mara et al., 2000), TBS resulted in long-term potentiation of EPSPs under control conditions, but not when NMDA receptor blockers (50 μM D-AP5 and 20 μM MK-801) were present in the bathing medium (Sup. Fig. 1; Sup. Table 1).
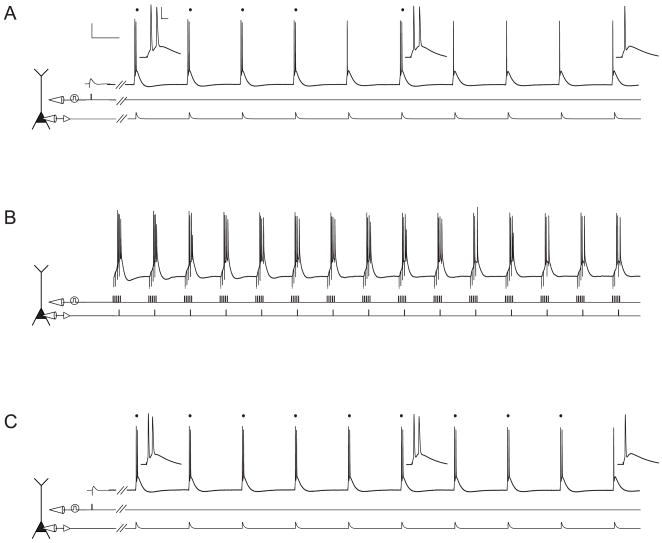
Figure 1. Experimental protocol used to study plasticity of excitability in subicular pyramidal neurons.
Scale bars in A apply to A–C and are 20 mV and 100 ms (A, C) or 150 ms (B). Inset scale bars in A apply to all insets in A and C and are 20 mV and 10 ms.
A. Voltage trace (top) from a representative subicular pyramidal neuron illustrating the response to synaptic stimulation (middle) followed by somatic current injection (bottom). Somatic current injection consisted of a train of 10 EPSC-like pulses. Hash marks indicate a 500 ms waiting period between the end of the synaptic stimulation and the beginning of the train. Dots above the voltage trace signify burst responses. Insets show a magnified view of responses to the 1st, 6th, and 10th current injections. B. Voltage trace (top) recorded during TBS (induction). TBS consisted of 5 synaptic pulses at 100 Hz (middle) paired with 1 somatic current injection (bottom), repeated at 5 Hz for 3 s. C. Voltage trace (top) from the same neuron in response to the same stimuli as in A, 30 minutes after TBS.
Additionally, neuronal output was monitored by a train of 10 brief, suprathreshold somatic current injections (see Experimental Procedures; Fig. 1A, C). Current injections at the beginning of the train elicit burst responses while those later in the train elicit single action potentials (Cooper et al., 2005). During somatic current injection, neuronal output is determined only by activation of intrinsic conductances gated by voltage and/or calcium. Therefore, a change in the number of bursts can be used as a measure of non-synaptic plasticity caused by changes in postsynaptic excitability.
Interestingly, TBS increased the number of burst responses elicited by the train of somatic current injections (Figs. 1 and 2A; see Sup. Figs. 2–4 for example traces recorded during induction). This enhancement of burst firing (non-synaptic plasticity) developed more gradually than potentiation of EPSPs (synaptic plasticity) and, unlike the synaptic plasticity, was not blocked by NMDA receptor blockers (Fig. 2B; Sup. Table 1). Furthermore, there was no correlation between the magnitude of the synaptic and non-synaptic plasticity (linear regression, R2=0.06, p=0.61; data not shown). However, both types of plasticity required TBS (induction), as neither developed over time when the TBS was not delivered (no induction; Figs. 2A, Sup. Fig. 1; Sup. Table 1).
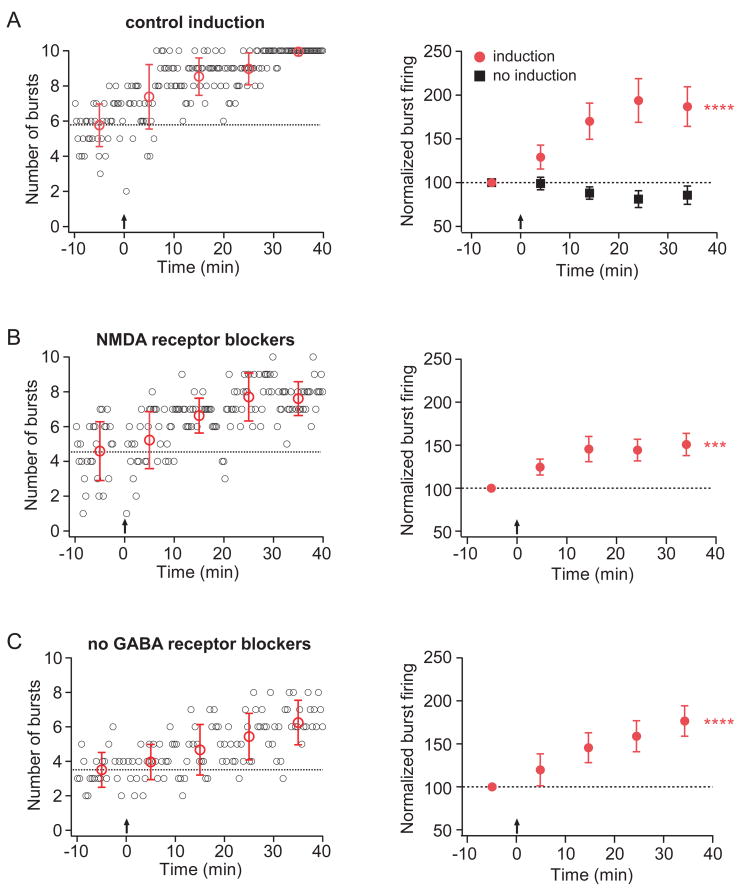
Figure 2. TBS results in an enhancement of burst firing that does not require NMDA or GABA receptor activation.
For all representative-experiment graphs (left column), small open circles (black) indicate the number of burst firing responses evoked by a train of 10 EPSC-like somatic current injections. The train was delivered every 20 seconds. Large open circles (red) represent the average number of burst firing responses per train for each 10-minute period. Error bars are ± standard deviation. For all group-data graphs (right column), filled symbols represent the average number of burst firing responses per train for each 10-minute period. Error bars are ± s.e.m. For all graphs, dotted lines indicate the average number of burst firing responses per train for the 10-minute baseline period. Arrows indicate when TBS (induction) was given. Asterisks indicate a significant effect of time, repeated measures ANOVA.
A. Representative (left) and group (right, red circles; n=10) data from experiments in which TBS was given in control conditions. Group data (right, black squares; n=9) are also shown for experiments in which no TBS was given. B. Representative (left) and group (right; n=8) data from experiments in which TBS was given in the presence of NMDA receptor blockers (50 μM D-AP5 and 20 μM MK-801) C. Representative (left) and group (right; n=8) data from experiments in which TBS was given in the absence of GABA receptor blockers.
In both the induction and no-induction groups, inhibitory neurotransmission was blocked by the inclusion of GABAA and GABAB receptor blockers (2 μM SR95531 and 3 μM CGP52432, respectively). To test whether enhancement of burst firing can be induced when inhibitory neurotransmission is intact, a more physiologically relevant condition, we delivered TBS in standard solution (no GABA receptor blockers). A comparable increase in burst firing was observed in these experiments, demonstrating that the induction of enhanced burst firing is not mediated by inhibitory neurotransmission (Fig 2C). In all subsequent experiments, we included GABAA and GABAB receptor blockers in order to isolate the effects of excitatory synaptic transmission.
Enhancement of burst firing requires synaptic activation, but not synaptic depolarization or action potential firing
In a variety of brain regions, including cortex, cerebellum, and hippocampus, synaptic and non-synaptic plasticity have been shown to require postsynaptic depolarization (Daoudal and Debanne, 2003). Physiologically, this depolarization can be achieved by action potential firing (Christie et al., 1996; Magee and Johnston, 1997), synaptic activation (Golding et al., 2002; Holthoff et al., 2004), or both. We investigated whether these sources of depolarization were necessary for the induction of enhanced burst firing by separating the induction stimulus (TBS) into its synaptic and action-potential components.
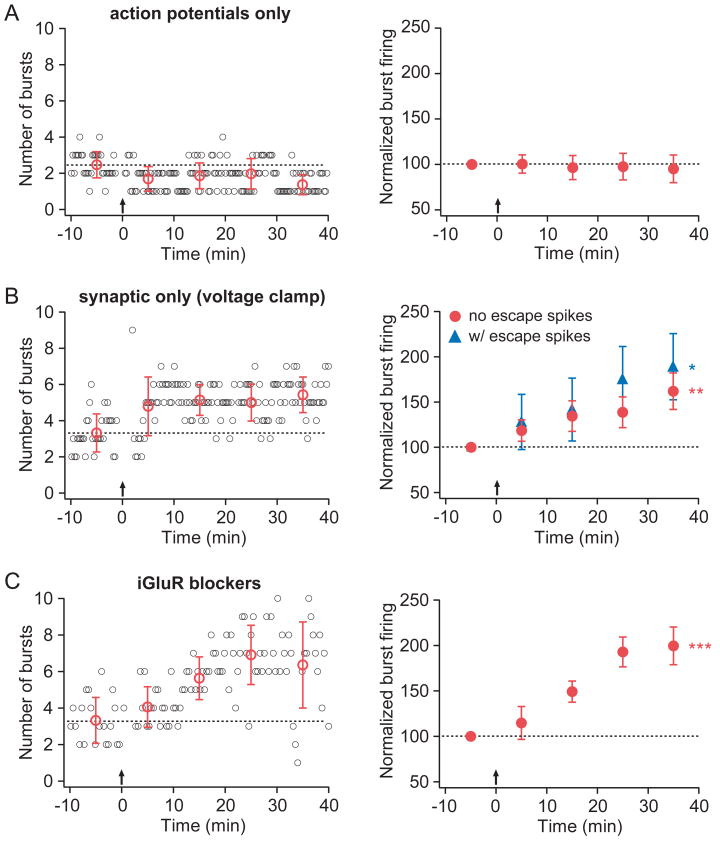
The necessity for synaptic activation was tested by somatically injecting current at 5 Hz for 3 seconds in the absence of synaptic stimulation. This action potential-only stimulus did not induce increased burst firing (Fig. 3A; Sup. Table 1), indicating a requirement beyond simple postsynaptic depolarization mediated by somatic action potential firing.
Figure 3. Synaptic stimulation alone is sufficient to induce an enhancement of burst firing.
Layout is as described for Fig. 2.
A. Representative (left) and group (right; n=18) data from experiments in which the induction stimulus consisted only of somatic current injections to evoke action potential firing. B. Representative (left) and group (right) data from experiments in which the induction stimulus consisted of synaptic stimulation during somatic voltage clamp (at −72 mV). In the group data, red circles indicate experiments in which somatic voltage clamp was effective at preventing action potential firing (n=9); blue triangles indicate experiments in which escape spikes were observed (n=4). C. Representative (left) and group (right; n=4) data from experiments in which TBS was given in the presence of ionotropic glutamate receptor (iGluR) blockers (20 μM CNQX, 50 μM D-AP5, and 20 μM MK-801).
To test the necessity for action potential firing, axonal afferents were stimulated in the theta-burst pattern (5 synaptic pulses at 100 Hz paired, repeated at 5 Hz, for 3 s) while the soma was voltage-clamped at −72 mV. Experiments were divided into two groups based on whether action potential firing was eliminated, as evidenced by the lack of visually identifiable escape spikes during the recording (no escape spikes, n=9; escape spikes, n=4). Synaptic stimulation during somatic voltage clamp resulted in enhancement of burst firing regardless of whether escapes spikes were observed (Fig. 3B). This increase was indistinguishable from that observed in the control induction group (p=0.49, two-factor repeated measures ANOVA; Sup. Table 1), demonstrating that somatic action potential firing is not necessary for the induction of burst firing enhancement.
Taken together, the results from these two experiments suggest that synaptic activation is required for induction of enhanced burst firing, while action potential firing is neither necessary nor sufficient. However, it is likely that dendritic depolarization was incompletely limited during the voltage-clamp experiments. Therefore, to determine whether dendritic depolarization is required for the induction of burst firing enhancement, experiments were performed in the presence of blockers of ionotropic glutamate receptors (iGluRs; 20 μM CNQX, 50 μM D-AP5, and 20 μM MK-801). In these experiments, the somatically recorded voltage during TBS was limited to a maximum of two millivolts (average 1.0 ± 0.4 mV; range 0.4 – 2.0 mV) and no action potentials were triggered. Despite this very limited depolarization, burst firing enhancement was induced, comparable to that observed in control conditions (Fig. 3C; Sup. Table 1).
Synergistic activation of mGluR1 and mAChR is required for enhanced burst firing
A likely explanation for the requirement of synaptic activation, but not AMPA or NMDA-receptor mediated depolarization, is that metabotropic (G-protein coupled) receptors are involved in the induction of burst firing enhancement. We tested the necessity for metabotropic receptor activation by performing experiments in the presence of antagonists for mGluRs and mAChRs.
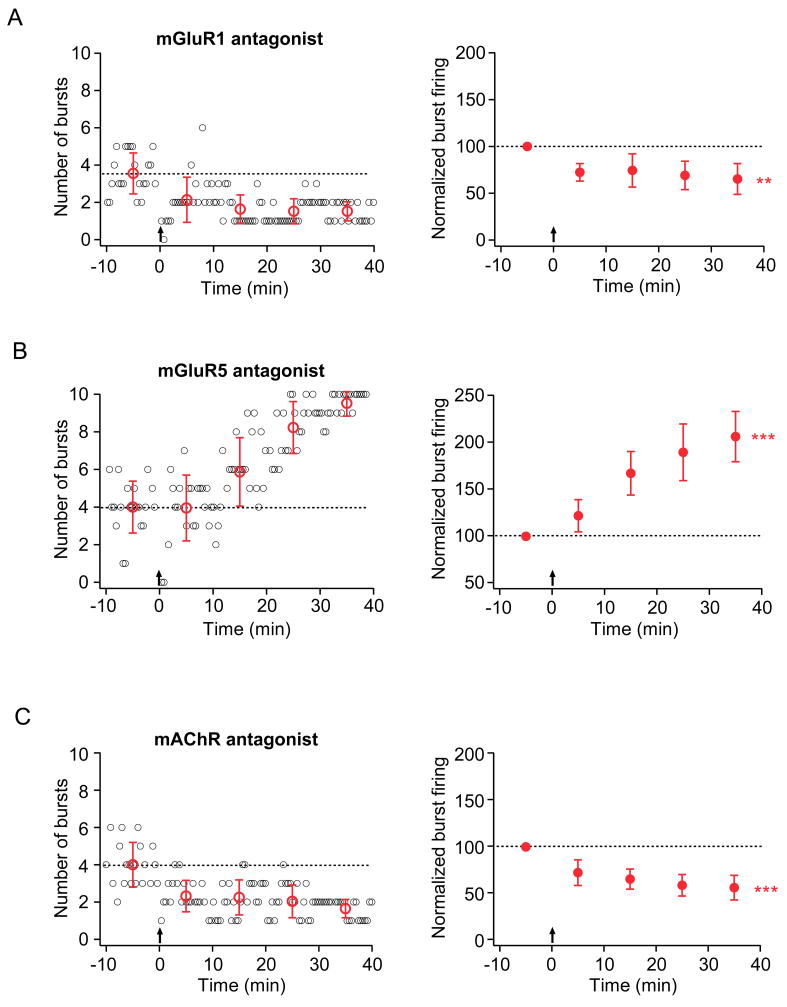
Application of an mGluR1 antagonist (LY367385, 25 μM) blocked the TBS-induced increase in burst firing and instead revealed a significant decrease in burst firing (Fig. 4A; Sup. Table 1). In contrast, an mGluR5 antagonist (MPEP, 10 μM) did not block the synaptically induced enhancement of burst firing (Fig. 4B; Sup. Table 1). A general mAChR antagonist (atropine, 10 μM) blocked the enhancement but revealed a suppression of burst firing (Fig. 4C; Sup. Table 1). Together, these results suggest burst firing is bidirectionally regulated via two competing processes: synaptic activation of mGluR1 and mAChR is required to induce an increase in burst firing, while mGluR5 activation may be involved in mediating a decrease in burst firing.
Figure 4. Activation of metabotropic glutamate or acetylcholine receptors results in differential induction of burst plasticity.
Layout is as described for Fig. 2.
A. Representative (left) and group (right; n=9) data from experiments in which TBS was given in the presence of a specific mGluR1 antagonist (25 μM LY367385). B. Representative (left) and group (right; n=5) data from experiments in which TBS was given in the presence of a specific mGluR5 antagonist (10μM MPEP). C. Representative (left) and group (right; n=6) data from experiments in which TBS was given in the presence of an mAChR antagonist (10 μM atropine).
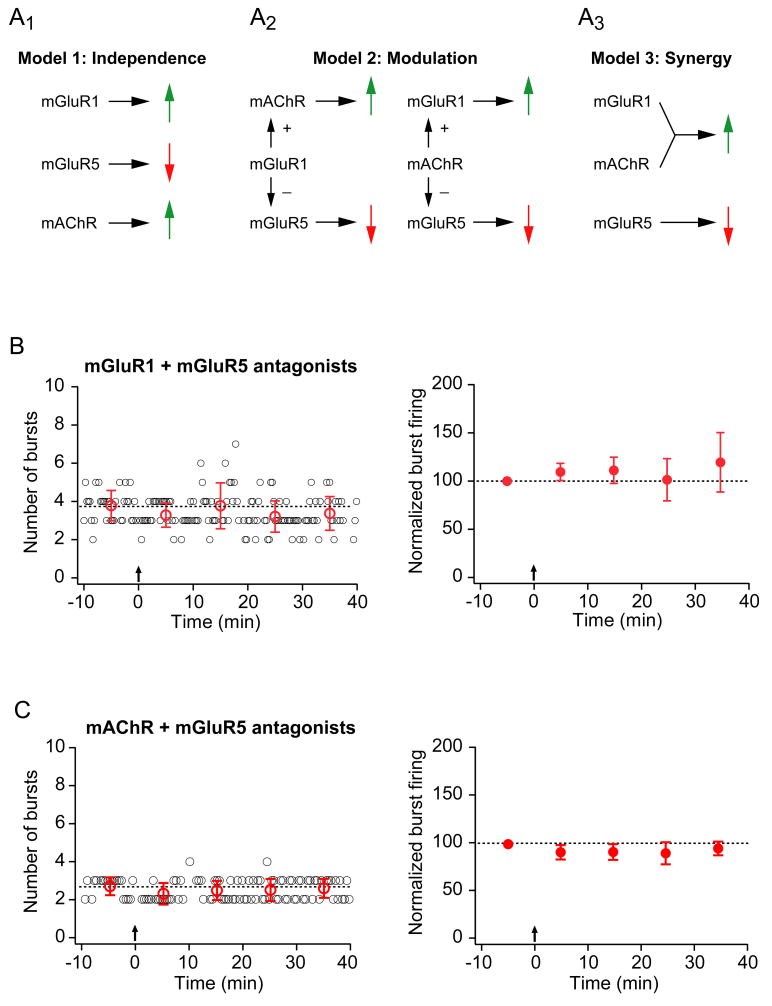
Three models could explain the results of our experiments with mGluR1, mGluR5, and mAChR antagonists (Fig. 5A). In the first model, the actions of the three receptor types are independent, with the mGluR5-mediated decrease dominating when either the mGluR1 or the mAChR-mediated enhancement is blocked. In the second model, either mAChR or mGluR1 exerts a modulatory effect on the activity of the other receptors, influencing the magnitude of the observed change in burst firing. In the third model, mGluR1 and mAChR act synergistically to produce an enhancement of burst firing that dominates an mGluR5-mediated decrease. To distinguish between these models, we tested the effects of combinations of antagonists for these receptors.
Figure 5. Synergistic activation of mGluR1 and mAChRs is required for enhancement of burst firing while activation of mGluR5 mediates suppression of burst firing.
A. Three models for the effects of metabotropic receptor activation on burst firing. A1. The first model that accounts for the effects of mGluR1, mGluR5, and mAChR on the induction of burst plasticity is based on the results of pharmacological experiments with metabotropic receptor antagonists (see Fig. 4). In Model 1, each receptor has an independent effect on burst firing: mGluR1 is necessary for an increase (because when blocked, a suppression of burst firing is observed), mGluR5 is necessary for a decrease (because when blocked, an enhancement of burst firing is observed), and mAChRs are necessary for an increase (because when blocked, a suppression of burst firing is observed). A prediction of Model 1 is that when both mGluR1 and mGluR5 are blocked, an increase in burst firing would be observed, mediated by mAChR activation alone. However, under these conditions (see panel B below) cells displayed no change in burst firing, demonstrating that this model does not account for the experimental results. A2. Model 2 proposes that mAChR or mGluR1 has a modulatory effect on the other two receptors, enhancing an mGluR1 (or mAChR) -mediated increase or inhibiting an mGluR5-mediated decrease in burst firing (or both). A prediction of Model 2 is that when mGluR5 is blocked with either mGluR1 or mAChR, an increase in burst firing would be observed due to the action of mAChR or mGluR1 alone. However, no change in burst firing was observed in these conditions (see panels B and C), demonstrating that this model can not account for the observed plasticity. A3. A third possiblitiy is that both mGluR1 and mAChR must be activated together to induce an enhancement of burst firing (and mGluR5 activation alone leads to a suppression). Model 3 predicts that when mGluR5 is blocked in combination with either mGluR1 or mAChR, no increase in burst firing should be observed. These predictions are consistent with the observed results. B. Representative (left) and group (right; n=8) data from experiments in which TBS was given in the presence of a specific mGluR1 antagonist (LY367385, 25 μM) and a specific mGluR5 antagonist (MPEP, 10 μM). For the representative-experiment graph, small open circles (black) indicate the number of burst firing responses evoked by a train of 10 EPSC-like somatic current injections. The train was delivered every 20 seconds. Large open circles (red) represent the average number of burst firing responses per train for each 10-minute period. Error bars are ± standard deviation. For the group-data graph, filled symbols represent the average number of burst firing responses per train for each 10-minute period. Error bars are ± s.e.m. For both graphs, dotted lines indicate the average number of burst firing responses per train for the 10-minute baseline period. Arrows indicate when TBS (induction) was given. C. Representative (left) and group (right; n=6) data from experiments in which TBS was given in the presence of an mAChR antagonist (atropine, 10 μM) and a specific mGluR5 antagonist (MPEP, 10 μM). Layout is as described in B.
We found that blocking mGluR5 along with either mGluR1 or mAChR resulted in neither an increase nor a decrease in burst firing (Fig. 5B, C). Theses experiments rule out both the independent-action model and the modulation model, as both models predict that either mGluR1 or mAChR, acting on its own, should result in enhanced burst firing when the mGluR5-mediated decrease is blocked. The only model consistent with these experimental results is the one in which both mGluR1 and mAChR must be activated to produce a synergistic effect leading to enhancement of burst firing.
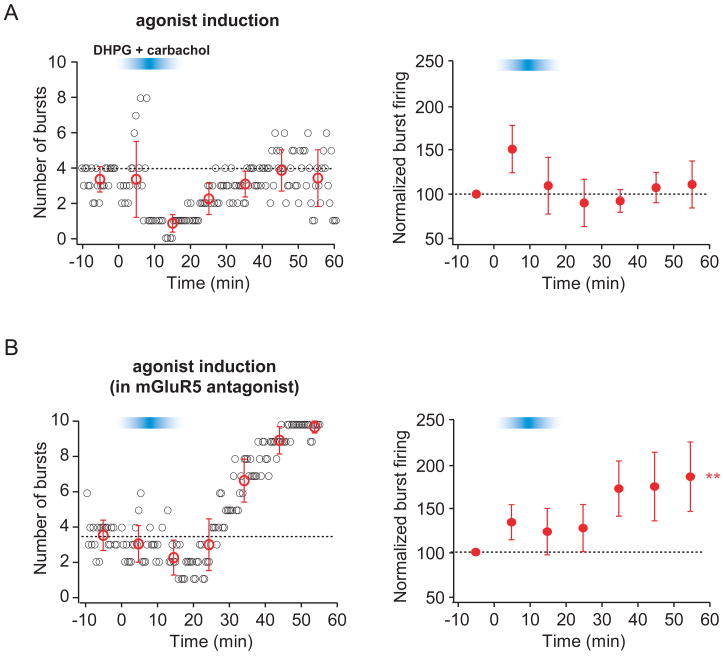
To test whether mGluR1 and mAChR activation are sufficient to induce an increase in burst firing, we bath applied agonists for these receptors instead of using TBS as the induction stimulus (Fig. 6). Because subtype-specific agonists for mGluR1 and mGluR5 do not exist, we used the general group I mGluR agonist DHPG (2 μM) in addition to the general mAChR agonist carbachol (2 μM). A transient increase in the ADP was observed during agonist application, which served as a positive control (Sup. Fig. 4). After washout of the agonists, no lasting change in burst firing was observed (Fig. 6A). DHPG activates both mGluR1 and mGluR5; therefore, this result may reflect the competition between an mGluR1/mAChR-mediated increase and an mGluR5-mediated decrease. We therefore applied DHPG and carbachol in the presence of the mGluR5 antagonist MPEP (10 μM), which resulted in a lasting increase in burst firing (Fig. 6B), consistent with a model in which synergistic activation of mGluR1 and mAChRs is sufficient to induce an enhancement of burst firing.
Figure 6. Activation of mGluR1 and mAChR are sufficient to induce an enhancement of burst firing.
Layout is as described for Fig. 2. The blue bar indicates the time during which the group I mGluR agonist (DHPG, 2 μM) and mAChR agonist (carbachol, 2 μM) were washed into and out of the bath.
A. Representative (left) and group (right; n=6) data from experiments in which bath application of agonists (DHPG + carbachol) was used as the induction stimulus. B. Representative (left) and group (right; n=6) data from experiments in which bath application of agonists (DHPG + carbachol) in the presence of the mGluR5 antagonist (MPEP, 10 μM) was used as the induction stimulus. Note that MPEP was present before, during, and after wash in and wash out of agonists.
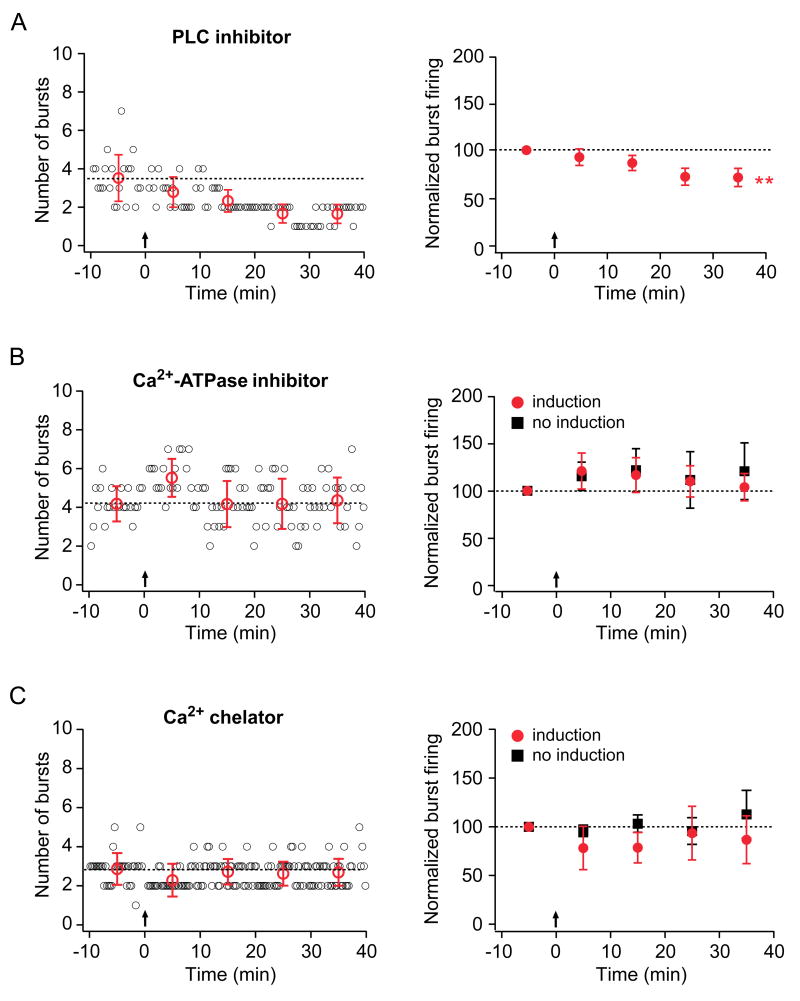
Group I mGluRs and mAChRs are members of the G-protein-coupled receptor superfamily and both couple to phospholipase C (PLC) activation via the stimulatory subunit Gqα. To test the involvement of PLC in an intracellular signaling cascade leading to the induction of burst firing enhancement, a PLC inhibitor (U-73122, 25 μM) was bath-applied to the slice. Under this condition, the increase in burst firing was blocked, and a suppression of burst firing was revealed (Fig. 7A), suggesting that mGluR1 and/or mAChRs act via a PLC-dependent pathway to result in burst firing enhancement. Furthermore, these data argue that PLC activation is not required for the mGluR5-mediated suppression of burst firing.
Figure 7. Enhancement of burst firing requires PLC activation, release of Ca2+ from internal stores, and an increase in intracellular Ca2+ concentration.
Layout is as described for Fig. 2.
A. Representative (left) and group (right; n=8) data from experiments in which TBS was given in the presence of a PLC inhibitor (U-73122, 25 μM). B. Representative (left) and group (right) data from experiments in which the internal recording solution contained the Ca2+-ATPase inhibitor thapsigargin (2 μM). In the group data, red circles indicate experiments in which TBS was given (n=6). Group data are also shown for experiments in which no TBS was given (right, black squares; n=6). C. Representative (left) and group (right) data from experiments in which the internal recording solution contained the Ca2+ chelator (BAPTA, 10 mM). In the group data, red circles indicate experiments in which TBS was given (n=5). Group data are also shown for experiments in which no TBS was given (right, black squares; n=5).
PLC catalyzes the breakdown of phosphotidylinositol 4,5-bisphosphate (PIP2) in the cellular membrane into two reaction products: diacylglycerol (DAG), which remains membrane bound, and inositol-1,4,5-triphosphate (IP3), which diffuses through the cytosol. IP3 activates IP3 receptors on the endoplasmic reticulum, causing release of Ca2+ from internal stores. To test the requirement of this Ca2+ release in the induction of burst firing enhancement, we depleted internal stores by including a Ca2+-ATPase inhibitor (thapsigargin, 2 μM) in the internal recording solution. When TBS was applied in the presence of the Ca2+-ATPase inhibitor, no increase in burst firing was induced (Fig. 7B, red circles). As a control, we recorded burst firing in the absence of TBS and observed no time-dependent effects of the Ca2+-ATPase inhibitor on burst firing (Fig. 7B, black squares). This demonstrates that stimulus-evoked release of Ca2+ from internal stores is required for the induction of burst firing enhancement.
To determine whether intracellular Ca2+ elevation is required for the induction of burst firing enhancement, we included a fast Ca2+ chelator (BAPTA, 10 mM) in the internal solution. When TBS was applied in the presence of the Ca2+ chelator, no enhancement of burst firing was observed (Fig. 7C, red circles), suggesting that elevation of intracellular Ca2+ is required for induction. We also performed control experiments in which burst firing was monitored in the absence of TBS to ensure that there were no time-dependent effects of recording with the Ca2+ chelator (Fig. 7C, black squares). In addition to a lack of burst firing enhancement in the Ca2+-ATPase inhibitor and the Ca2+ chelator experiments, no decrease in burst firing was observed suggesting further that induction of burst firing suppression may depend on a rise in intracellular Ca2+ concentration, perhaps through release from internal stores.
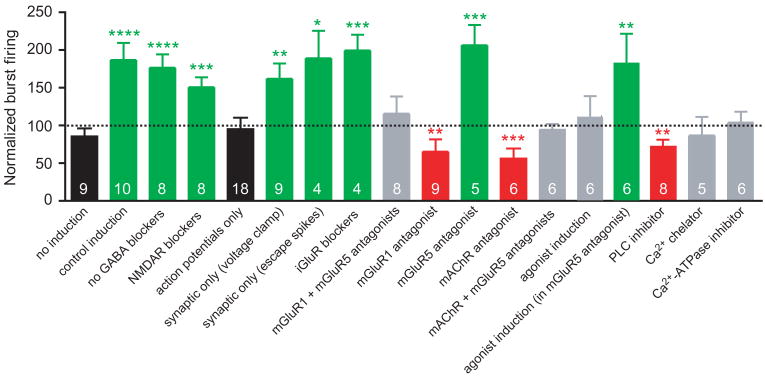
The results of all experimental manipulations are summarized in Fig. 8. Groups are color-coded according to one of four conditions: 1. Black - no synaptic activation during induction; 2. Green - synaptic activation during induction, resulting in an enhancement of burst firing; 3. Red - synaptic activation during induction, resulting in a suppression of burst firing; and 4. Gray - synaptic activation during induction, resulting in no change in burst firing.
Figure 8. Summary of burst firing plasticity under different experimental conditions.
Normalized burst firing (the average number of burst firing responses per train at 30–40 minutes post-induction as a fraction of the average number of burst firing responses per train in the 10-minute period before induction, or comparable time points in the no-induction group) is shown for each experimental condition. Bar are color-coded according to one of four conditions: 1. Black - no synaptic activation during induction, 2. Green - synaptic activation during induction, resulting in an enhancement of burst firing; 3. Red - synaptic activation during induction, resulting in a suppression of burst firing; and 4. Gray - synaptic activation during induction, resulting in no change in burst firing. Numbers at the bottom of the bars indicate n for that group. The dotted line indicates no change in the number of burst firing responses compared to the baseline period (100% of baseline).
One difficulty in understanding the mechanisms responsible for the modulation of bursting is that small changes in action potential threshold and passive membrane properties, which occur in all long-term whole-cell recordings, can contribute to changes in burst firing. We therefore carried out a detailed analysis of these factors across all of our experimental groups and found that all groups exhibited a decrease in action potential threshold and an increase in the response to a small subthreshold current injection (Sup. Fig. 5). These changes were greatest for experimental conditions that enhanced burst firing compared to conditions that produced a decrease or no change in burst firing. However, a careful analysis of these changes suggests that they account only partially for the observed plasticity (see Sup. Fig. 5 legend for details). These results suggest that the ion channels altered to produce plasticity of burst firing include those activated below threshold (thus affecting the action potential threshold and responses below it) as well as channels activated above threshold, following firing of the action potential.
Theta-burst stimulation increases neuronal excitability in response to a noisy stimulus
To determine the effect of TBS on neuronal excitability in response to an irregular stimulus, a noisy current injection (see Experimental Procedures) was used to evoke action potential firing (Sup. Fig. 6). One effect of TBS was to increase the overall number of action potentials evoked. For example, in some cases a previously subthreshold current injection subsequently reached threshold for an action potential. Bursts, which constituted the majority of events, did not increase in number but the number of action potentials per burst increased. Thus, in response to an irregular, noisy stimulus the TBS-induced increase in neuronal excitability was expressed both as a global increase in the probability of reaching threshold for action potential firing as well as an increase in the strength of burst firing. The difference in the way in which burst firing was enhanced with this stimulus compared to the train of 10 EPSC-like current injections is likely due to the nature of the stimulus used to evoke firing. EPSC-like current injections are very brief and largely over by the time of the second action potential in a burst. In contrast, during the longer “noisy” current injection, there is still positive current being injected during the burst, making additional action potentials more likely. Thus, the effect of activity-dependent plasticity of intrinsic excitability can be manifested as either an increase in burst firing or an overall increase in the number of action potentials, depending on the nature of the stimulus activating the firing.
Discussion
The results of these experiments suggest that theta-burst patterned synaptic stimulation, which mimics hippocampal activity during exploratory activity in vivo, induces a long-term change in the firing of intrinsically bursting pyramidal neurons in the subiculum. This form of plasticity is robust, with the number of bursts nearly doubling for at least tens of minutes following a three-second period of theta-burst stimulation. The enhancement of burst firing requires synaptic activation of mGluRs and mAChRs, but does not require activation of AMPA or NMDA-type glutamate receptors, synaptic depolarization, or action potential firing. When mGluR1 or mAChRs are blocked, an activity-dependent suppression of burst firing is observed, which requires activation of mGluR5. Because bursts are not synaptically driven in these experiments, but are elicited by direct somatic current injection, the observed increases and decreases in burst firing must be caused by alterations in voltage- and/or calcium-activated conductances. Therefore, these experiments demonstrate a powerful form of long-term, activity-dependent, bidirectional plasticity of intrinsic firing in pyramidal neurons of subiculum.
In vivo, the change in action potential firing resulting from this increase or decrease in excitability will depend on the nature of the synaptic input driving firing. Repeated synchronous inputs will result in more bursting, while inputs of lower amplitude and frequency are likely to result in enhanced spiking through an increase in the number of isolated spikes and more spikes occurring within bursts. A decrease in excitability is likely to occur in vivo when hippocampal activity is present in the absence of cholinergic activation via the medial septum. Thus, the septal cholinergic system may serve as a cellular switch between conditions favorable to the induction of burst-firing enhancement or suppression.
Comparison to other forms of non-synaptic plasticity
The burst plasticity we describe here differs markedly from other types of non-synaptic plasticity reported in the literature. Bliss and Lomo’s (1973) initial report of activity-dependent changes in synaptic strength also noted an increase in the amplitude of the population spike that was larger than what could be accounted for simply by the increase in EPSP amplitude. Although a reduction of feed-forward inhibition may account for some of this effect (Abraham et al., 1987; Chevaleyre and Castillo, 2003; Staff and Spruston, 2003), there is also some evidence to suggest that alterations in intrinsic excitability also contribute this increased firing probability, referred to as EPSP-to-spike (E-S) potentiation (Chavez-Noriega et al., 1990; Hess and Gustafsson, 1990; Jester et al., 1995). A recent report (Campanac and Debanne, 2008) demonstrated that changes in E-S coupling of CA1 pyramidal cells that occur in parallel with spike timing-dependent synaptic plasticity, even in the presence of GABAergic antagonists. The burst plasticity we describe here differs from E-S potentiation in two important respects: first, it is mediated solely by changes in excitability, and second, it occurs via mechanisms quite distinct from those required for the induction of LTP following the same stimulus.
Several other forms of plasticity of intrinsic excitability have been reported. In cell culture, chronic isolation of neurons from excitatory or inhibitory inputs can up- or down-regulate excitability, respectively (Turrigiano and Nelson, 2004). More rapid induction of non-synaptic plasticity has also been demonstrated. In acute cerebellar slices, high-frequency synaptic stimulation resulted in an increase in the number of action potentials elicited by a depolarizing current step (Aizenman and Linden, 2000). In hippocampal slices, direct depolarization and synaptic stimulation of CA1 pyramidal cells produce local changes in the intrinsic excitability of stimulated dendritic regions (Frick et al., 2004; van Welie et al., 2004). In one study, depolarization combined with cholinergic activation, via the agonist carbachol, induced an increase in the voltage and Ca2+ signal produced by distinct dendritic branches (Losonczy et al., 2008). Another previous study showed that an increase in excitability mediated by downregulation of the after-hyperpolarization (AHP) in CA1 pyramidal neurons requires co-activation of glutamatergic and β-adrenergic receptors (Gereau and Conn, 1994). In vivo, hippocampus-dependent trace eye-blink conditioning results in reduction of the AHP of CA1 neurons, which is permissive for learning the task (Moyer et al., 1996). Intriguingly, this effect is enhanced by upregulation of cholinergic innervation (Disterhoft and Oh, 2003). These examples illustrate that plasticity of intrinsic excitability is likely to be a widespread and functionally important phenomenon in the nervous system.
Comparison to synaptic plasticity
A number of features suggest that burst plasticity is distinct from synaptic plasticity in subiculum. First, the time course of development for burst plasticity is slower than that of synaptic plasticity. Second, synaptic plasticity is blocked by NMDA receptor blockers, but burst plasticity is not. Third, the requirement for synaptic depolarization and/or action potential firing, which have been well documented for many forms of synaptic plasticity (Golding et al., 2001; Gustafsson et al., 1987; Kelso et al., 1986), is absent for burst plasticity. Rather, the induction of enhanced burst firing requires synergistic activation of at least two metabotropic receptor types (mGluR1 and mAChRs). The plasticity induction paradigm used in these experiments, when no pharmacological manipulations are present, results in both increased synaptic strength and increased non-synaptic excitability. However, there are likely other induction protocols in vitro and behavioral states in vivo where activity-dependent synaptic and non-synaptic plasticity may interact in more complex ways to modulate subicular output. For example, hippocampal activity in the absence of medial septal activation could lead to suppression of burst firing. Thus, burst plasticity provides an additional mechanism, complementary to synaptic plasticity, by which subicular pyramidal neurons can modify their properties and influence adaptive behaviors contributing to learning and memory.
Signal transduction mechanisms for induction of burst plasticity
The lack of a requirement for depolarization, fast synaptic neurotransmission, or action potential firing led to the hypothesis that the induction of burst plasticity depends on activation of metabotropic receptors. Indeed, mGluR1, mGluR5, and mAChRs all have roles in the induction of bidirectional burst plasticity. The data are consistent with a model in which activation of both mGluR1 and mAChR is required to enhance burst firing, while mGluR5 activation produces a decrease in burst firing. When all three receptor types are activated, the enhancement dominates the suppression, but when either mGluR1 or mAChR are blocked, the mGluR5-mediated decrease in burst firing is dominant. These results suggest that a synergistic action of mGluR1 and mAChR is required to override the effects of mGluR5 and produce an enhancement of burst firing.
Several scenarios could underlie the requirement for synergistic activation of mGluR1 and mAChRs in the induction of enhanced burst firing. One possibility is that presynaptic receptors for one transmitter may affect the release of the other. For example, activation of mGluR1 receptors on cholinergic terminals may be required to permit or promote release of acetylcholine (ACh) during induction, which leads to the enhancement of burst firing. Postsynaptically, these receptor subtypes may also interact in complex ways. For example, different metabotropic receptors have been shown to form heteromeric complexes (Enz, 2007). In particular, heteromeric interactions of adenosine or GABAB receptors with mGluR1 have been reported to regulate transmembrane currents (Ciruela et al., 2001; Tabata et al., 2007). Another possibility is that each receptor subtype is coupled to separate signaling pathways, both of which are required to induce plasticity, or that different subcellular locations of these receptor subtypes recruit signaling pathways in specific neuronal compartments. For example, the actions of mGluR1 and mAChRs have been shown to activate extracellular signal-regulated kinase (ERK) in different cellular compartments (Berkeley et al., 2001). Alternatively, activation of postsynaptic mGluR1 and mAChRs may converge on a common intracellular signaling pathway to produce a higher level of a critical second messenger. A prediction of such a mechanism is that sufficiently high levels of glutamate or acetylcholine (ACh) may produce comparable effects on burst firing, even in the absence of synergism. Thus, burst plasticity may require activation of CA1 and/or EC (leading to glutamate release in the subiculum) in addition to activation of the medial septum to stimulate release of ACh, or may be induced when one region is very strongly activated.
Activation of mGluR1 and mAChRs may be sufficient to induce burst plasticity, or may be necessary but not sufficient. Experiments in which agonist application (DHPG to activate group I mGluRs and carbachol to activate mAChRs) was substituted for TBS during induction begin to address this question. Under these conditions, an increase in burst firing was observed, provided that mGluR5 was blocked. This result is consistent with the idea that mGluR1 and mAChRs are sufficient for burst firing enhancement, but it does not rule out a role for other receptors under physiological conditions in vivo, because agonist application does not completely mimic synaptic activation. In evidence of this, when TBS was used as the induction stimulus, mGluR1, mGluR5, and mAChRs were all activated, and an increase in burst firing was observed. Following bath-applied agonists activating each of these receptors, however, no long-term changes in burst firing were observed (compare Figs. 2A and 6A). This may reflect additional requirements, besides mGluR5 activation, to cause a suppression of burst firing, or may be due to the differences in location, concentration, or duration of agonist application compared to synaptic stimulation.
It is possible that synaptic activation is required to release glutamate, but not ACh, and that basal levels of ACh are sufficient to induce burst plasticity (or, vice versa, that stimulation is required to release ACh, but that basal levels of glutamate are sufficient to induce burst plasticity). This question is difficult to address because, in hippocampal slices, an extracellular stimulating electrode is likely to recruit both glutamatergic and cholinergic release. Antagonists of either receptor block the effects of stimulated neurotransmission, but also block the effects of basal levels of the neurotransmitter, with no method available to distinguish between the two.
Activation of group I mGluRs and some mAChRs (specifically M1, M3, and M5) releases the Gqα subunit, which in turn activates PLC, producing two second messengers: DAG and IP3. These can directly activate ion channels or cause Ca2+ release from intracellular stores. Additionally, they may activate protein kinases such as protein kinase C (PKC) and extracellular signal-regulated kinase (ERK), which have been shown to play critical roles in synaptic plasticity. Other signal transduction mechanisms may also be involved since activation of mGluR1 and mGluR5, which are both coupled to Gqα and PLC, did not have equivalent roles in the induction of burst plasticity. Indeed, it is somewhat surprising that the enhancement of burst firing depends on mGluR1 activation, as immunohistological studies show no or very little staining for mGluR1 in CA1 or subiculum, while mGluR5 is abundantly expressed (Fotuhi et al., 1994; Shigemoto et al., 1997). Nevertheless, there are a number of electrophysiological studies that report mGluR1-mediated effects in CA1 pyramidal neurons that are distinct from those observed when mGluR5 is activated alone (Volk et al., 2006; Chaouloff et al., 2007).
Candidate mechanisms for the expression of burst plasticity
An important question is which conductances are altered to produce the observed changes in burst firing. Our analysis of the bursting responses before and after TBS (not shown) has yielded few clues as to the nature of the affected conductances, so further work will be required to address this question. Possible candidates that should be considered include voltage-gated Ca2+ conductances that drive the ADP following spikes (Metz et al., 2005; Su et al., 2001), voltage-gated Na+ conductances that drive spiking and may also affect the ADP (Azouz et al., 1996), and voltage- and/or Ca2+-activated K+ channels that may affect spiking, the ADP, and the slow after hyperpolarization (AHP) following bursts (Jung et al., 2001; Metz et al., 2007; Rhoades and Gross, 1994; Staff et al., 2000; Yue and Yaari, 2004). Other types of channels, such as Ca2+-activated non-specific cation channels, including members of the TRP channel family, are also possible candidates. In addition to simple up- or down-regulation of these channel types, shifts in properties such as slow inactivation of Na+ channels, which has been shown to affect repetitive burst firing in the subiculum (Cooper et al., 2005), must also be considered.
Functional significance of burst plasticity
Burst firing has been observed in a variety of brain regions and has been posited to play a number of roles. At central nervous system synapses, bursts of two action potentials increase the probability of release per event from 10–50% to over 90% (Lisman, 1997; Stevens and Wang, 1995). Therefore, upregulation of burst firing may represent a relative increase in the strength of a particularly important or salient stimulus, allowing activity to propagate more reliably through the network. Hippocampal sharp-wave bursts are associated with the transition between neocortical down- and up-states (Battaglia et al., 2004), thought to be related to the transition between quiescence and alertness, and can also drive down-to-up state transitions in the nucleus accumbens (Lape and Dani, 2004). In this context, an increase in burst firing in subiculum may be important in driving transitions between operational states of downstream target regions, particularly because subiculum is the major output of hippocampus. In addition, bursts from place cells in hippocampus provide a more accurate spatial map than all firing considered together (Muller et al., 1987). Likewise, bursts in visual cortex provide more information about the stimulus than do single action potentials (Cattaneo et al., 1981; Livingstone et al., 1996). Thus, increased burst firing may help to refine cortical maps by strongly, but selectively, activating particular neuronal connections.
Regulation of burst firing may also be important in learning and memory. Increased burst firing in presynaptic neurons increases postsynaptic responsiveness of synaptically connected cells. Increased burst firing in postsynaptic neurons contributes further dendritic and somatic depolarization. Both these changes could result in an increase in correlated activity, which may be a crucial feature contributing to Hebbian synaptic plasticity. Indeed, postsynaptic bursting has been shown to enhance long-lasting synaptic plasticity, such as long-term potentiation (Pike et al., 1999; Wittenberg and Wang, 2006). The requirement for cholinergic activation suggests that increases in burst firing (or decreases owing to local, glutamatergic activity in the absence of extrinsic cholinergic activity) could influence memory formation, consolidation, or retrieval, as cholinergic activation is well known to influence learning in vivo (Disterhoft et al., 1999; Gold, 2003; Power et al., 2003).
On the other hand, abnormal upregulation of burst firing may contribute to diseases that manifest as hyperexcitability. In acute brain slices from normal rats, seizure-like events were initiated in subiculum, and maintained even when disconnected from the CA and EC regions (Behr and Heinemann, 1996; Dreier and Heinemann, 1991). In rat models of epilepsy, this type of activity in subiculum can spread to other structures, including CA1 and the EC (Benini and Avoli, 2005; Kemppainen et al., 2002). Tissue from human patients with temporal lobe epilepsy also demonstrated spontaneous electrical activity initiated in subiculum, as well as synaptic and cellular changes associated with increased spontaneous activity (Cohen et al., 2002; Wozny et al., 2005). Therefore, activity-dependent increases in burst firing, such as those demonstrated here, although likely to contribute to the normal function of subiculum, may also increase susceptibility to seizure-like activity and influence the propagation of seizure activity to other areas.
Taken together, these results demonstrate a novel form of intrinsic plasticity, distinct from synaptic plasticity, in burst firing neurons of subiculum. The ability to increase or decrease burst firing in response to physiologically relevant activity patterns may represent a complementary cellular mechanism for the recognition, coding, and storage of hippocampally important stimuli.
Experimental Procedures
Animals
Male Wistar rats, aged 25–45 days, were used for all experiments. Animals were colony housed on a 12-hour light/dark cycle with free access to food and water. All animal procedures were approved by the Northwestern University Animal Care and Use Committee.
Solutions
Artificial cerebrospinal fluid (ACSF) consisted of (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, and 25 dextrose (Fisher Scientific, Pittsburgh, PA). The pH of the ACSF was 7.2–7.4 and the osmolarity was 305–320 mOsm. ACSF was always oxygenated by constant bubbling with a gas mixture of 95% O2/5% CO2. Internal recording solution consisted of: 115 K-gluconate, 20 KCl, 10 sodium phosphocreatine (Na2-Pcr), 10 HEPES, 2 MgATP, and 0.3 NaGTP with 0.10% biocytin for subsequent determination of morphology (all Sigma-Aldrich, St. Louis, MO, except KCl and HEPES, Fisher Scientific). 1 M KOH was used to pH the internal solution to 7.3–7.4. The osmolarity was 272–290 mosM.
Unless otherwise indicated, ACSF used to perfuse slices in the recording chamberincluded 2 μM SR95531, a γ-aminobutyric acid (GABA)A blocker (Sigma-Aldrich), and 3 μM CGP52432, a GABAB antagonist (Tocris-Cookson, Bristol, UK). Where noted, one of the following antagonists or combinations of antagonists (Sigma-Aldrich unless otherwise indicated) was also included in the perfusion ACSF and present for the entire duration of recording: 1. N-methyl-D-aspartate (NMDA) receptor blockers: 50 μM D-2-amino-5-phosphonopentanoate (D-AP5) and 20 μM MK-801; 2. ionotropic glutamate (iGluR) receptor blockers: 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 50 μM D-AP5, and 20 μM MK-801; 3. mGluR antagonists: 25 μM LY367385, an antagonist of mGluR1, and/or 10 μM 2-methyl-6-(phenylethynyl)-pyridine (MPEP), an antagonist of mGluR5 (both Tocris-Cookson); 4. an mAChR antagonist: 10 μM atropine (in some experiments, in combination with 10 μM MPEP); 5. a phospholipase C (PLC) inhibitor: 25 μM U-73122 (Tocris-Cookson). In some experiments, additional drugs were added to the intracellular recording solution: either 10 mM 1,2-bis(2-aminophenoxy)ethane N,N,N,N-tetraacetic acid (BAPTA; Sigma-Aldrich), a Ca2+ chelator, or 2 μM thapsigargin (Tocris-Cookson), which depletes intracellular Ca2+ stores in the endoplasmic reticulum by inhibiting Ca2+-ATPases. To allow time for the intracellular stores to be depleted, cells were exposed to thapsigargin for at least 30 minutes before the induction stimulus was given.
Slice preparation and experimental setup
Rats were anesthetized with halothane, intracardially perfused with ice-cold ACSF for less than 1 min, then decapitated and the brains rapidly removed. Transverse hippocampal slices, 300 μm thick, were made with a Vibratome 3000 (Ted Pella, Inc., Redding CA), transferred to a storage chamber, and incubated at 32–35°C for 20–30 min. Afterwards, the chamber was maintained at room temperature.
Prior to electrophysiological recordings, slices were transferred to a submerged chamber and maintained at 32–35 °C by constant perfusion of warmed ACSF, at a rate of approximately 1 mL/s. A Zeiss Axioskop (Oberkochen, Germany) equipped with differential interference contrast (DIC) optics was used in conjunction with a Hamamatsu camera system to visually identify subicular pyramidal cells. Subiculum was distinguished from bordering regions by the diffuse distribution of pyramidal cells, compared to the tightly packed pyramidal cell layer of CA1, and the lack of distinct cortical layers seen in entorhinal cortex. Recording pipettes were fabricated (Flaming/Brown Micropipette Puller, Sutter Instruments, Novato, CA) from thick-walled borosilicate capillary glass (Garner Glass Company, ID = 1.2 ± 0.05 mm, OD = 2.0 ± 0.05 mm) and filled with the K-gluconate-based internal solution to obtain a 3–5 MΩ open-tip resistance in the bath. A motorized micromanipulator (Sutter Instruments) was used to position the recording pipette and whole-cell configuration was achieved by mouth suction.
To evoke synaptic responses, an extracellular stimulating pipette, fabricated from borosilicate theta glass (Sutter Instruments) was filled with ACSF and placed 50–200 μm away from the site of the whole-cell recording on the apical dendritic side of the soma. In all cases, it is likely that CA1 and entorhinal cortex afferents were jointly recruited and contributed to the synaptic response.
Electrophysiological recordings
Whole-cell current-clamp recordings were made through via a silver chloride-coated electrode connected to an amplifier (Dagan BVC-700, Minneapolis, MN). Only cells that had a resting potential between −56 mV and −70 mV at break-in were used. Experiments were restricted to burst-firing neurons, which were defined as those that exhibited two or more action potentials with an instantaneous frequency of greater than 100 Hz in response to a just-above threshold, long (600 ms) square pulse.
Neuronal output was monitored once every 20 seconds (0.05 Hz) by using a train of 10 somatic EPSC-like (τrise = 0.2 ms, τdecay = 6 ms) current injections to evoke action potential firing (Fig. 1). The frequency (5 Hz, n=38; 7 Hz, n=43; or 10 Hz, n=17) and amplitude (800 pA – 2400 pA) of somatic current injections were set such that, for each train, 2–7 responses were bursts (while the remaining responses were single action potentials). In all cases, burst firing occurred mostly at the beginning of the train and single action potentials occurred toward the end of the train.
Synaptic strength (EPSP amplitude) and subthreshold voltage response (an index of passive membrane properties) were also monitored once every 20 seconds. The synaptic stimulus (0.2 ms square current pulse through the extracellular bipolar electrode; Axon stimulus isolator) was set to elicit EPSPs of 1–6 mV. Subthreshold responses were monitored with EPSC-like somatic current injections (8% of burst-monitoring amplitude). In some neurons, a hyperpolarizing square current injection (5% of burst-monitoring amplitude, 500 ms) was used to monitor input resistance.
In one set of experiments, a more physiologically realistic stimulus (noisy current injection) was used to evoke action potential firing. The noisy current was obtained by depolarizing the cell to just below action potential threshold and recording spontaneous membrane potential fluctuations. A scaled version of this trace was then injected back into the cell as a current wave form.
Except where noted, the induction stimulus (TBS) consisted of theta-burst-patterned synaptic activation (5 stimuli at 100 Hz) paired with somatic current injection (2 ms square current pulse at the burst-monitoring amplitude), repeated at 5 Hz for 3 seconds (Fig. 1). The induction stimulus was given approximately 30 minutes after whole-cell configuration was achieved (average: 30 ± 1 min.; range: 11 – 76 min.). There was no difference in the time of induction relative to break-in across groups (p=0.57, one-factor ANOVA). In one set of experiments, the induction stimulus consisted of 10-minute bath application of agonists (2 μM DHPG to activate group I mGluR and 2 μM carbachol to activate mAChR) rather than TBS. In some of these experiments, a specific mGluR5 antagonist (MPEP, 10 μM) was included in perfusion ACSF for the entire duration of the recording. An increase (several mV) in the size of the ADP following a burst was taken as a positive control for the presence of the agonists in the bath.
All neurons were held at membrane potentials between −63 mV and −67 mV for the duration of the recordings (except in voltage-clamp experiments when, during the induction stimulus only, cells were held at −72 mV). Cells that required more than 250 pA of current to maintain these potentials were excluded from the data set. There were no statistically significant differences in membrane potential between experimental groups over time (p=0.76, two-factor ANOVA; Sup. Table 1). Bridge balance and capacitance compensation were monitored and adjusted throughout the duration of each experiment; recordings in which the series resistance exceeded 50 MΩ were excluded. Cells were generally recorded from for a total of 50–70 minutes, but, in some cases, were held up to 100 minutes.
Data acquisition and statistical analysis
Voltage responses were filtered at 5 kHz, digitized at 50 kHz, and stored via an ITC-16 analog-to-digital converter (Instrutech, Port Washington, NY) on a Dell Dimension PC. All acquisition and analysis procedures were custom programmed in IGOR Pro (Wavemetrics, Lake Oswego, OR). Statistical analyses of group data were performed using paired, two-tailed Student’s t-tests, or one- or two-factor repeated measures ANOVA, where appropriate, with Prism software (GraphPad Software, Inc., San Diego, CA). Asterisks indicate a significant effect of time, repeated measures ANOVA: * = p<0.05, ** = p<0.01, *** = p<0.001, **** = p<0.0001. When a significant main effect was detected with ANOVA tests, Bonferroni’s post-hoc correction was applied to determine significance between pairwise comparisons. Unless stated otherwise, reported values are mean ± s.e.m. of data collected 30–40 minutes after the induction stimulus was given, or comparable time points in the agonist-induction and no-induction groups. Normalized values are plotted as a percentage of the value during the baseline.
Supplementary Material
Supplemental Table 1 and Supplemental Figures 1-6.
Acknowledgments
The authors would like to thank members of the Spruston lab, Drs. Jason Hardie, Catherine Cook Kaczorowski, and Stefan Remy, for helpful discussions and critical reading of this manuscript. Preliminary work has been presented in abstract form (SfN 2006, Atlanta, GA). Support provided by NIH MH067564 (SJ Moore) and NIH NS35180, MH074866, and RR015497 (N Spruston).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Gustafsson B, Wigstrom H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J Physiol. 1987;394:367–380. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492(Pt 1):211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr J, Heinemann U. Low Mg2+ induced epileptiform activity in the subiculum before and after disconnection from rat hippocampal and entorhinal cortex slices. Neurosci Lett. 1996;205:25–28. doi: 10.1016/0304-3940(96)12360-0. [DOI] [PubMed] [Google Scholar]
- Benini R, Avoli M. Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol. 2005;566:885–900. doi: 10.1113/jphysiol.2005.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Maffei L, Morrone C. Patterns in the discharge of simple and complex visual cortical cells. Proc R Soc Lond B Biol Sci. 1981;212:279–297. doi: 10.1098/rspb.1981.0039. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Halliwell JV, Bliss TV. A decrease in firing threshold observed after induction of the EPSP-spike (E-S) component of long-term potentiation in rat hippocampal slices. Exp Brain Res. 1990;79:633–641. doi: 10.1007/BF00229331. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Christie BR, Magee JC, Johnston D. The role of dendritic action potentials and Ca2+ influx in the induction of homosynaptic long-term depression in hippocampal CA1 pyramidal neurons. Learn Mem. 1996;3:160–169. doi: 10.1101/lm.3.2-3.160. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Escriche M, Burgueno J, Angulo E, Casado V, Soloviev MM, Canela EI, Mallol J, Chan WY, Lluis C, et al. Metabotropic glutamate 1alpha and adenosine A1 receptors assemble into functionally interacting complexes. J Biol Chem. 2001;276:18345–18351. doi: 10.1074/jbc.M006960200. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Commins S, Gigg J, Anderson M, O’Mara SM. The projection from hippocampal area CA1 to the subiculum sustains long-term potentiation. Neuroreport. 1998;9:847–850. doi: 10.1097/00001756-199803300-00015. [DOI] [PubMed] [Google Scholar]
- Cooper DC, Chung S, Spruston N. Output-Mode Transitions Are Controlled by Prolonged Inactivation of Sodium Channels in Pyramidal Neurons of Subiculum. PLoS Biol. 2005;3:e175. doi: 10.1371/journal.pbio.0030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DC, Moore SJ, Staff NP, Spruston N. Psychostimulant-induced plasticity of intrinsic neuronal excitability in ventral subiculum. J Neurosci. 2003;23:9937–9946. doi: 10.1523/JNEUROSCI.23-30-09937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Kronforst-Collins M, Oh MM, Power JM, Preston AR, Weiss C. Cholinergic facilitation of trace eyeblink conditioning in aging rabbits. Life Sci. 1999;64:541–548. doi: 10.1016/s0024-3205(98)00599-2. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh M. Modulation of cholinergic transmission enhances excitability of hippocampal pyramidal neurons and ameliorates learning impairments in aging animals. Neurobiol Learn Mem. 2003;80:223–233. doi: 10.1016/j.nlm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Enz R. The trick of the tail: protein-protein interactions of metabotropic glutamate receptors. Bioessays. 2007;29:60–73. doi: 10.1002/bies.20518. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gereau RWt, Conn PJ. A cyclic AMP-dependent form of associative synaptic plasticity induced by coactivation of beta-adrenergic receptors and metabotropic glutamate receptors in rat hippocampus. J Neurosci. 1994;14:3310–3318. doi: 10.1523/JNEUROSCI.14-05-03310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Golding NL, Kath WL, Spruston N. Dichotomy of action-potential backpropagation in CA1 pyramidal neuron dendrites. J Neurophysiol. 2001;86:2998–3010. doi: 10.1152/jn.2001.86.6.2998. [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Wigstrom H, Abraham WC, Huang YY. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E, Stewart M. Propagation of synchronous epileptiform events from subiculum backward into area CA1 of rat brain slices. Brain Res. 2001;895:41–49. doi: 10.1016/s0006-8993(01)02023-6. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?--Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hess G, Gustafsson B. Changes in field excitatory postsynaptic potential shape induced by tetanization in the CA1 region of the guinea-pig hippocampal slice. Neuroscience. 1990;37:61–69. doi: 10.1016/0306-4522(90)90192-7. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Kovalchuk Y, Yuste R, Konnerth A. Single-shock LTD by local dendritic spikes in pyramidal neurons of mouse visual cortex. J Physiol. 2004;560:27–36. doi: 10.1113/jphysiol.2004.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jester JM, Campbell LW, Sejnowski TJ. Associative EPSP--spike potentiation induced by pairing orthodromic and antidromic stimulation in rat hippocampal slices. J Physiol. 1995;484(Pt 3):689–705. doi: 10.1113/jphysiol.1995.sp020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HY, Staff NP, Spruston N. Action potential bursting in subicular pyramidal neurons is driven by a calcium tail current. J Neurosci. 2001;21:3312–3321. doi: 10.1523/JNEUROSCI.21-10-03312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso SR, Ganong AH, Brown TH. Hebbian synapses in hippocampus. Proc Natl Acad Sci U S A. 1986;83:5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen S, Jolkkonen E, Pitkanen A. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus. 2002;12:735–755. doi: 10.1002/hipo.10020. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lape R, Dani JA. Complex response to afferent excitatory bursts by nucleus accumbens medium spiny projection neurons. J Neurophysiol. 2004;92:1276–1284. doi: 10.1152/jn.00066.2004. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Freeman DC, Hubel DH. Visual responses in V1 of freely viewing monkeys. Cold Spring Harb Symp Quant Biol. 1996;61:27–37. [PubMed] [Google Scholar]
- Lopes da Silva FH, Arnolds DE, Neijt HC. A functional link between the limbic cortex and ventral striatum: physiology of the subiculum accumbens pathway. Exp Brain Res. 1984;55:205–214. doi: 10.1007/BF00237271. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Metz AE, Jarsky T, Martina M, Spruston N. R-type calcium channels contribute to after depolarization and bursting in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:5763–5773. doi: 10.1523/JNEUROSCI.0624-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz AE, Spruston N, Martina M. Dendritic D-type potassium currents inhibit the spike after depolarization in rat hippocampal CA1 pyramidal neurons. J Physiol. 2007 doi: 10.1113/jphysiol.2006.127068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara SM, Commins S, Anderson M. Synaptic plasticity in the hippocampal area CA1-subiculum projection: implications for theories of memory. Hippocampus. 2000;10:447–456. doi: 10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- O’Mara SM, Commins S, Anderson M, Gigg J. The subiculum: a review of form, physiology and function. Prog Neurobiol. 2001;64:129–155. doi: 10.1016/s0301-0082(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Pike FG, Meredith RM, Olding AW, Paulsen O. Rapid report: postsynaptic bursting is essential for ‘Hebbian’ induction of associative long-term potentiation at excitatory synapses in rat hippocampus. J Physiol. 1999;518(Pt 2):571–576. doi: 10.1111/j.1469-7793.1999.0571p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80:178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Rhoades BK, Gross GW. Potassium and calcium channel dependence of bursting in cultured neuronal networks. Brain Res. 1994;643:310–318. doi: 10.1016/0006-8993(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Staff NP, Jung HY, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84:2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- Staff NP, Spruston N. Intracellular correlate of EPSP-spike potentiation in CA1 pyramidal neurons is controlled by GABAergic modulation. Hippocampus. 2003;13:801–805. doi: 10.1002/hipo.10129. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Su H, Alroy G, Kirson ED, Yaari Y. Extracellular calcium modulates persistent sodium current-dependent burst-firing in hippocampal pyramidal neurons. J Neurosci. 2001;21:4173–4182. doi: 10.1523/JNEUROSCI.21-12-04173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Kawakami D, Hashimoto K, Kassai H, Yoshida T, Hashimotodani Y, Fredholm BB, Sekino Y, Aiba A, Kano M. G protein-independent neuromodulatory action of adenosine on metabotropic glutamate signalling in mouse cerebellar Purkinje cells. J Physiol. 2007;581:693–708. doi: 10.1113/jphysiol.2007.129866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Welie I, van Hooft JA, Wadman WJ. Homeostatic scaling of neuronal excitability by synaptic modulation of somatic hyperpolarization-activated Ih channels. Proc Natl Acad Sci U S A. 2004;101:5123–5128. doi: 10.1073/pnas.0307711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GM, Wang SS. Malleability of spike-timing-dependent plasticity at the CA3-CA1 synapse. J Neurosci. 2006;26:6610–6617. doi: 10.1523/JNEUROSCI.5388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C, Knopp A, Lehmann TN, Heinemann U, Behr J. The subiculum: a potential site of ictogenesis in human temporal lobe epilepsy. Epilepsia. 2005;46(Suppl 5):17–21. doi: 10.1111/j.1528-1167.2005.01066.x. [DOI] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike after depolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 and Supplemental Figures 1-6.