Abstract
Purpose
To compare the dosimetric differences of various online IGRT strategies and predict potential benefits of online re-optimization techniques in prostate cancer radiation treatments.
Material and methods
Nine prostate patients were recruited in this study. Each patient has 1 treatment planning CT images and 10 treatment day CT images. Five different online IGRT strategies were evaluated which include 3D conformal with bone alignment, 3D conformal re-planning via aperture changes, intensity modulated radiation treatment (IMRT) with bone alignment, IMRT with target alignment and IMRT daily re-optimization. Treatment planning and virtual treatment delivery were performed. The delivered doses were obtained using in-house deformable dose mapping software. The results were analyzed using equivalent uniform dose (EUD).
Results
With the same margin, rectum and bladder doses in IMRT plans were about 10% and 5% less than those in CRT plans respectively. Rectum and bladder doses were reduced as much as 20% if motion margin is reduced by 1cm. IMRT is more sensitive to organ motion. Large discrepancies of bladder and rectum doses were observed compared to the actual delivered dose with treatment plan predication. The therapeutic ratio can be improved by 14% and 25% for rectum and bladder respectively if IMRT online re-planning is employed compared to IMRT bone alignment approach. The improvement of target alignment approach is similar with 11% and 21% dose reduction to rectum and bladder respectively. However, undosing in seminal vesicles was observed on certain patients.
Conclusions
Online treatment plan re-optimization may significantly improve therapeutic ratio in prostate cancer treatments mostly due to the reduction of PTV margin. However for low risk patient with only prostate involved, online target alignment IMRT treatment would achieve similar results as online re-planning. For all IGRT approaches, the delivered organ-at-risk doses may be significantly different from treatment planning prediction.
Introduction
The purpose of treatment-room imaging technologies has been to accurately position the patient for daily treatments. This increased accuracy justifies a smaller clinical target volume to planning target volume (CTV-PTV) margin [1], decreasing the consequent collateral damage to normal tissues. While treatment-room imaging methods are certainly a step forward for radiation oncology, the efficacy of these image-guided treatments depends on a treatment plan optimized using a single snapshot of the patient anatomy, typically via the simulation computed tomography (CT). This planning method assumes that the shape and position of the target do not change from day to day. This assumption is often violated due to setup variation and daily anatomic position changes. As image guidance technologies begin to provide a level of information during each treatment rivaling that available during treatment planning, new strategies should be adopted to ensure that the best treatments are delivered to the greatest number of patients.
Perhaps the key issue in image guidance is how the information is used to modify treatment [2,3]. We classify the online image guided radiation treatments (IGRT) into three different precision levels: (1) bony alignment, (2) target alignment, and (3) re-planning.
Bony alignment
Of the three IGRT strategies, bony alignment has the lowest requirement for online image quality, as projection radiographic image quality allows visibility of high contrast bony landmarks but not soft tissues. This method can be implemented by using electronic portal imaging devices (EPIDs) [4]. In clinical treatment, the 2-D megavoltage (MV) images can be registered to a digitally reconstructed radiograph (DRR) or to treatment planning CT via 2D-3D registration [5]. Depending on the definition of registration volume and image quality, volumetric online images from kilovoltage cone beam CT (kV-CBCT) and MV-CBCT may also be registered at bony structures due to the high contrast to surrounding soft tissues [6]. Most current IGRT treatments correct only the three translational components of rigid-body motion. Six-degree-of-freedom corrections can be achieved with a robotic couch (Hexpod™, Medical Intelligence GmbH, Schwabmünchen, Germany) [7] or by rotating the couch, collimator and gantry altogether [8]. In the treatment simulation study, we registered the daily treatment images to the planning CT images at the pelvic bones with six degrees of freedom to simulate bony alignment approach.
Target alignment
Aligning directly to the target requires better image quality. The boundary of the low contrast (soft tissue) treatment target needs to be identified. For prostate treatments in particular, it may be difficult to align the target in IGRT treatment using CBCT due to its inferior image quality. Another approach to align at the target is to use implanted fiducial markers, which are visible through portal imaging [9]. The basic application of this technology would be to identify the target center of mass based on the average position of three markers. In our clinical experience however, we found that the target motion may include large rotational components. It is unrealistic to fully correct the rotation [10]. Thus, in the simulation study, we only correct the translation component by translating to the center-of-mass.
Re-planning
If the target and organs-at-risk (OARs) can be delineated on online volumetric images, it is possible to generate a new plan in each treatment day [11]. For 3D conformal radiation treatment (3D-CRT) plans, the beam aperture can be changed according to the shape of the target seen in the beam’s-eye-view (BEV) [12]. For intensity modulated radiation therapy (IMRT) plans, the beamlet weight can be re-optimized on a daily basis to minimize the dose to the OAR while maintaining the target dose [13,14]. It is reasonable to use rectum and bladder walls during inverse planning of prostate cancer treatments, so long as the intra-fraction motion can be limited to a negligible level. With these biologically relevant inverse planning objectives, the dose to the OAR may possibly be further reduced.
Re-planning theoretically provides the highest precision and does not need specialized hardware such as the robotic couch. However, online re-planning requires superior online image quality, as well as fast and robust algorithms to perform automatic region-of-interest (ROI) delineation [15], dose calculation, and beamlet weight optimization. We are actively developing the techniques to facilitate online re-planning. In this study, we performed both 3D-CRT and IMRT treatment planning and treatment simulation studies for several online IGRT strategies. The purpose of this study is to investigate the potential benefits of the re-planning approach in prostate cancer treatments, and to characterize the limits of the other IGRT methods.
Methods and Materials
A. Treatment Planning
Image data from nine prostate cancer patients treated at William Beaumont Hospital in Royal Oak, Michiga, USA were used in this study. The nine patients were randomly selected from our patient database. We assume the magnitude of organ motions of these randomly selected patients represent typical prostate population. Treatment planning CT images were acquired with a conventional helical CT scanner. There was no bowel preparatory instruction or procedure for any simulation or treatment day images. For each of these images, contours of prostate, seminal vesicles (SV), rectum (volume and wall), and bladder (volume and wall) were hand-drawn by a single observer. Based upon the contours, treatment plans were generated using the Pinnacle™ treatment planning system (TPS) (Philips Radiation Oncology System, Madison, Wisconsin). The patients were planned as if they had intermediate or high risk disease, and therefore the CTV included both prostate and seminal vesicles. The average prostate volume was 40.7 cm3 (range: 22.5 – 54.4 cm3). OARs were limited to bladder and rectum. The average rectum volume was 120.5 cm3 (range: 45.0 – 189.5 cm3).
The prostate shows significant movement relative to the pelvic bones [16]. Thus, a uniform 1-cm CTV-PTV margin is used to compensate for inter-faction organ motion in both 3D-CRT and IMRT treatment plans which are intended for use with the bony alignment treatment method. Results from these treatments will demonstrate the adequacy of a widely practiced IGRT method.
Since the target alignment treatment method can account for translational interfraction motion, the motion margin can be greatly reduced. In the 3D-CRT re-planning and IMRT re-planning methods, all interfraction motions are expected to be compensated, and so there is no motion margin component in the PTV for these methods. This will also give us a feeling about the dosimetric robustness of various IGRT methods to the target translations, deformations, and rotations involved. Intrafraction motion is neglected in this study.
An arbitrary dose of 91.0 Gy was prescribed to the PTV, though results will focus on relative dose values, scalable to any prescription. All treatment plans used five coplanar beams at 0°, 81°, 144°, 216°, and 279°. DVH-based dose-volume objectives were chosen to minimize bladder and rectum doses while maintaining PTV dose uniformity during inverse planning.
The IGRT approaches and the corresponding treatment planning methods are listed in Table 1. Five 3D-CRT and IMRT treatment planning methods formed the bases for the online IGRT treatments:
CRT 1-cm: 3D-conformal radiotherapy to 95% of the isocenter with 1-cm CTV-to-PTV margin and 6-mm PTV-to-block penumbra. This plan was used for CRT bony alignment IGRT approach (CRT bone)
CRT 0-cm: 3D-CRT plan to 95% of the isocenter with 0-cm CTV-to-PTV margin and 6- mm PTV-to-block penumbra. This plan was used for CRT re-planning via aperture change approach (CRT Re-plan).
IMRT 1-cm: Inverse-planned IMRT with 1-cm CTV-to-PTV margins and OAR-volume as optimization objectives. This was used for IMRT bone alignment treatments (IMRT Bone).
IMRT 0-cm volume: Inverse-planned IMRT with 0-cm CTV-to-PTV margins and OAR volumes as objectives. This method was used for both IMRT target alignment (IMRT Target) and IMRT re-planning with OAR volumes as constraints approach (IMRT Volume).
IMRT 0-cm wall: Inverse-planned IMRT with 0-cm CTV-to-PTV margins and OAR walls in optimization objectives. This method was used for IMRT re-planning with OAR walls as constraints approach (IMRT Wall).
Table 1.
A summary of IGRT approaches simulated in this study. The information of a row from left to right describes how a planning method relates to an IGRT treatment method.
| Planning Method | CTV-PTV Margin | Organs at Risk | Image Guidance | Treatment Name |
|---|---|---|---|---|
| CRT 1cm | 1 cm | n/a | Bony Alignment | CRT Bone |
| CRT 0cm | 0 cm | n/a | Block shaped to daily PTV | CRT Re-plan |
| IMRT 1cm | 1 cm | bladder & rectum volumes | Bony Alignment | IMRT Bone |
| IMRT 0cm volume | 0 cm | bladder & rectum volumes | Isocenter shifted to daily PTV center of mass | IMRT Target |
| IMRT 0cm volume | 0 cm | bladder & rectum volumes | Complete daily IMRT re-optimization | IMRT Re-plan Volume |
| IMRT 0cm wall | 0 cm | bladder & rectum walls | Complete daily IMRT re-optimization | IMRT Re-plan Wall |
B. Virtual Treatment Simulations
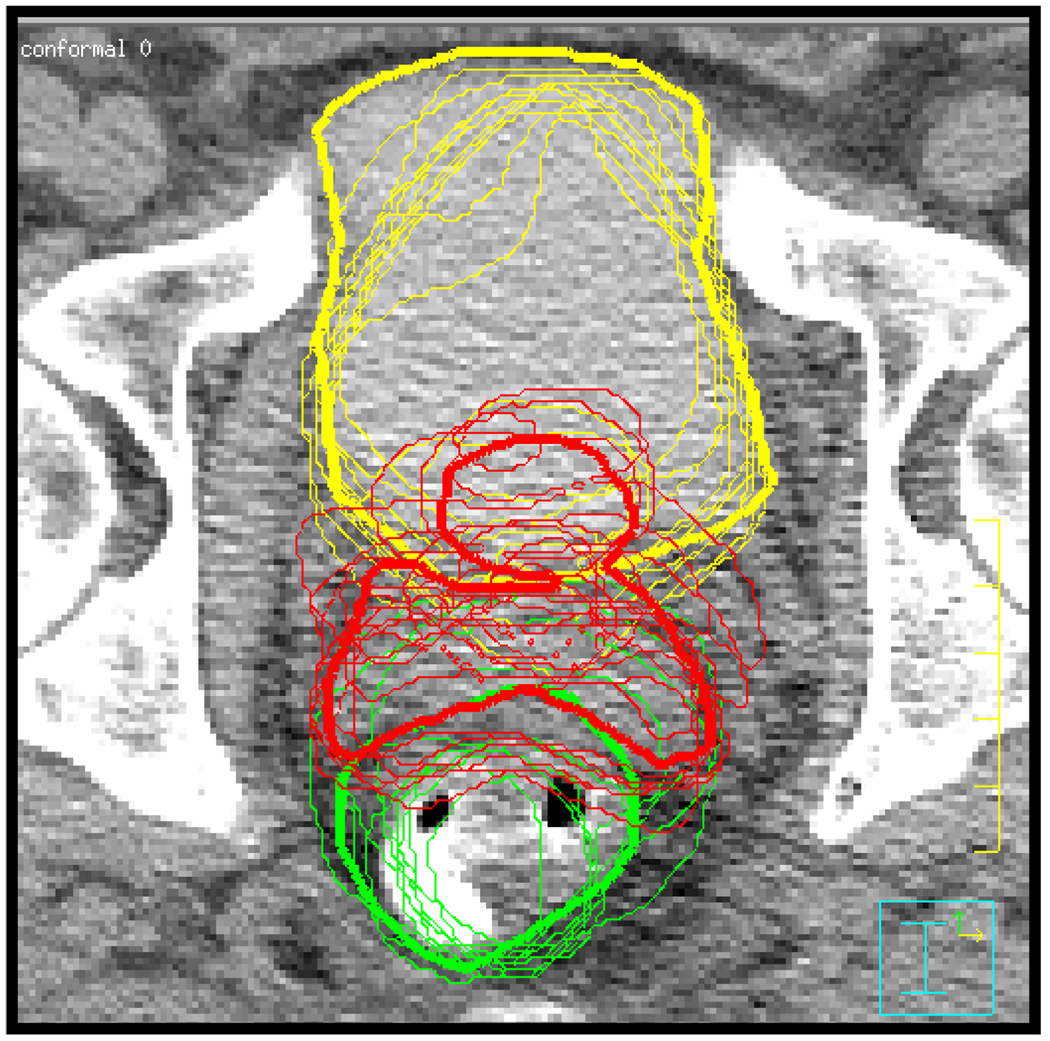
In addition to treatment planning CT images, 10 treatment day helical CTs were also acquired for each patient. The treatment day images were acquired during the 6–8 week treatment courses with intervals of 3–5 days. The 10 treatment day images were used to simulate 10 treatment fractions for all online IGRT techniques. The daily images were registered to the planning image using bony anatomy in Pinnacle. The ROIs were manually delineated on all daily images after bony registration. As an indication of the range of interfraction motion, Figure 1 shows a treatment planning image with contours from bone registered daily images overlaid.
Figure 1.
Planning CT showing bladder (yellow), CTV (red), and rectum (green) contours. Thick lines define contours from the planning day. Thin lines show the positions of the organs on different treatment days. Contours were generated after registration of bony anatomy.
Ideally, treating a patient positioned by bony landmarks can be simulated by calculating dose on the daily images. However, due to technical issues with Pinnacle, dose calculation on daily images was not possible for the images where non-zero rotational components exist in registration parameters. Previous studies have shown that replacing a daily CT with the planning CT introduces minimal dose calculation error [17]. Using daily images which were registered to planning image without rotation, we were able to determine that calculating daily dose on the planning image introduces an EUD error in the studied ROIs of less than 1%. Thus in this study, daily treatment dose was calculated using voxel densities from the planning CT, as this method introduces negligible error. Calculating DVHs from dose to daily contours will accurately represent all daily motions, as the rotation present in registered daily images will be preserved in the contour shape.
Retrospective virtual treatment simulations were performed in order to compare different online IGRT treatment methods. Compared to interfraction motion, intra-fraction motion is relatively small [16]. In this study, we only focused on interfraction motion, while intra-fraction motion was neglected. Treatment methods were simulated that were associated with different levels of image guidance. The relationship between planning methods and treatment methods is summarized in Table 1.
Bony alignment
This method can be implemented by using electronic portal imaging devices (EPIDs) [4]. In clinical treatment, the 2-D megavoltage (MV) images can be registered to a digitally reconstructed radiograph (DRR) or to treatment planning CT via 2D-3D registration [5]. Depending on the definition of registration volume and image quality, volumetric online images from kilovoltage cone beam CT (kV-CBCT) and MV-CBCT may also be registered at bony structures due to the high contrast to surrounding soft tissues. As a result, bony alignment has the lowest requirement for online image quality of the three evaluated IGRT strategies. Most current IGRT treatments correct only three translational components of rigid-body motion. Six-degree-of-freedom corrections can be achieved with a robotic couch (Hexpod™, Medical Intelligence GmbH, Schwabmünchen, Germany) [18] or by rotating the couch, collimator and gantry altogether. Bony alignment was simulated by registering the daily treatment images to the planning CT images at the pelvic bones with six degrees of freedom. Doses for CRT Bone and IMRT Bone treatments were calculated using the unmodified CRT 1-cm and IMRT 1-cm plans. This simulates the position correction available from standard imaging systems combined with the robotic couch.
Target alignment
Aligning directly to the target requires better image quality. The boundary of the low contrast (soft tissue) treatment target needs to be identified. For prostate treatments in particular, it may be difficult to align the target in IGRT treatment using CBCT due its inferior image quality. Another approach is to use a portal imager to visualize implanted fiducial markers [9]. One application of this technology that is possible with CBCT would be to identify the target based on the average 3D position of three markers. In our clinical experience however, we found that the target motion may include large rotational components. With the standard couch, it is unrealistic to correct the rotation without modifying the treatment plan. Furthermore, the maximum rotation of the Hexpod™ robotic couch is about 2.5–3.0 degrees, and so some large rotations cannot be corrected in this fashion. In this study, we simulated an image guided treatment available to many institutions by only translating the isocenter to the daily target’s center-of-mass. After moving the isocenter, daily doses were calculated using the original IMRT 0cm Volume plans. This approximates the use of implanted markers based upon the assumption that marker position accurately reflects target position. For the purposes of this study, this treatment method was labeled IMRT Target.
Re-planning
If the target and organs-at-risk (OARs) can be delineated from online volumetric images, it is possible to generate a new plan in each treatment day. Re-planning with daily anatomy should theoretically be the most optimal IGRT approach available using standard teletherapy delivery technology. However, this method requires superior image quality, as well as fast and robust algorithms to perform automatic region-of-interest (ROI) delineation [15], dose calculation, and treatment plan optimization. We are actively developing the techniques to facilitate online re-planning.
The purpose of this study is to predict the potential benefits of the re-planning approach in prostate cancer treatments in comparison to other IGRT methods. With this approach, a new plan was generated from each daily treatment CT image. Conformal and intensity modulated techniques were investigated.
The CRT Re-plan treatments started from the CRT 0-cm plan. On each treatment day, beam apertures were changed to the daily shape of the target in the beam’s-eye view (BEV) [12], but the monitor units (MUs) delivered by each beam were kept the same as in the original treatment plan. This makes the re-planning process much simpler from a computational standpoint, as dose re-calculation is unnecessary.
For intensity modulated radiation therapy (IMRT) plans, the beamlet weight can be re-optimized on a daily basis to minimize the dose to the OAR while maintaining the target dose [14]. So long as the intra-fraction motion can be limited to a negligible level, it is reasonable to use rectum and bladder walls during inverse planning of prostate cancer treatments,. With these biologically relevant inverse planning objectives, the dose to the OAR may possibly be further reduced.
The IMRT Re-plan Volume and IMRT Re-plan Wall treatments involve complete re-planning on each treatment day. Moving beyond the CRT Re-plan method to these treatments requires overcoming the technical challenges of online dose calculation and beamlet weight optimization. To simulate this process, daily doses were calculated after daily re-planning using the IMRT 0cm Volume and IMRT 0cm Wall planning methods.
C. Dose accumulation
All daily doses were exported to an in-house dose accumulation software utility [19]. In this utility, a finite element (FE)–based deformable registration algorithm was used to obtain voxel matching information between daily and planning CT images. FE models were created using ROI contours from planning CT images. The daily contours were used as boundary conditions to limit the organ deformation. The deformation state with minimum internal elastic energy was obtained. The voxel displacements were obtained by interpolating the FE results to the CT voxel grid.
With voxel displacements, dose accumulation is straightforward. The accumulated dose D at position x⃗, can be obtained by
| (1) |
where N the total number of fractions, dj the daily dose from the treatment fraction j, and u⃗ is the daily displacement of the FE sub-volume from its original position x⃗.
D. Evaluation Methods
Dose from treatment planning and simulated delivery was evaluated using the equivalent uniform dose (EUD), as in Equation 2, where V is the reference volume, and D(v) is the dose to the sub-volume v.
| (2) |
As in previous studies [20], the volume parameter for prostate and seminal vesicles was chosen as a=−7. With regard to normal tissues, values were chosen as a=2 for bladder, and a=8.33 for rectum.
Results
A. Comparisons of treatment planning results
This study included five different treatment planning methods. 3D-CRT and IMRT were used in treatment planning and different treatment planning methods used different CTV-PTV margins (0-cm and 1-cm). The EUDs of the OARs (rectum and bladder) were calculated and compared to the prostate EUD. Unless otherwise noted, percentage values discussed in the following treatment results are based upon the target (prostate) EUD dose in the respective treatment plan.
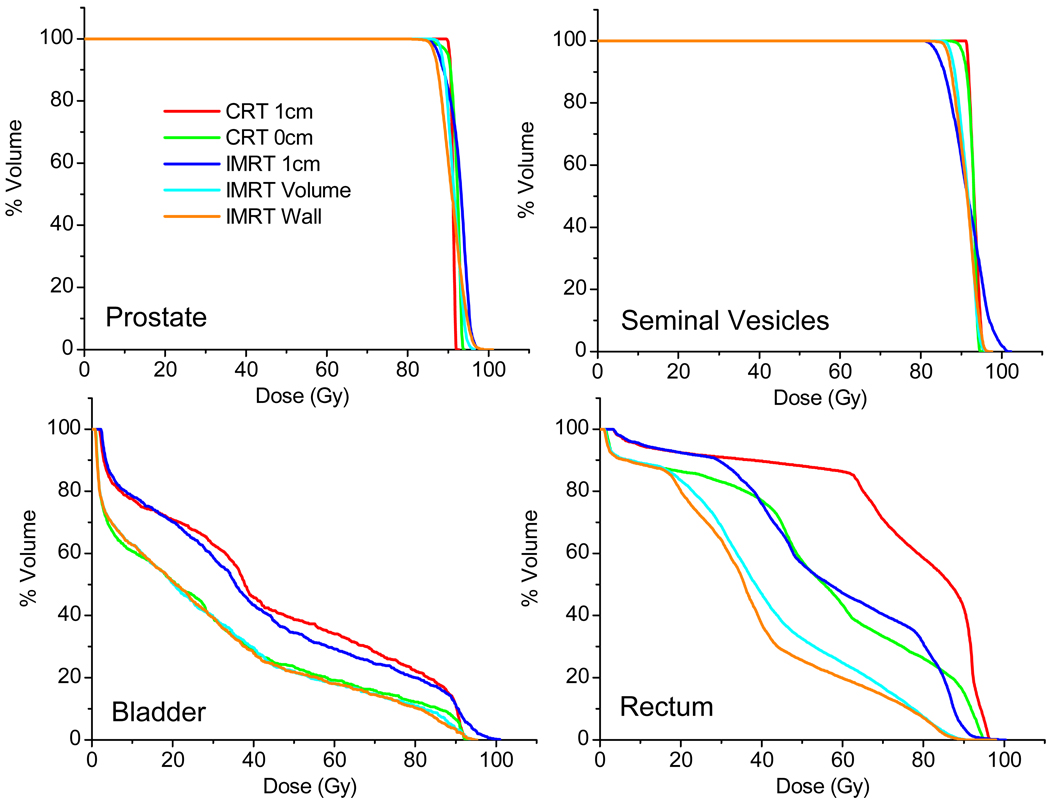
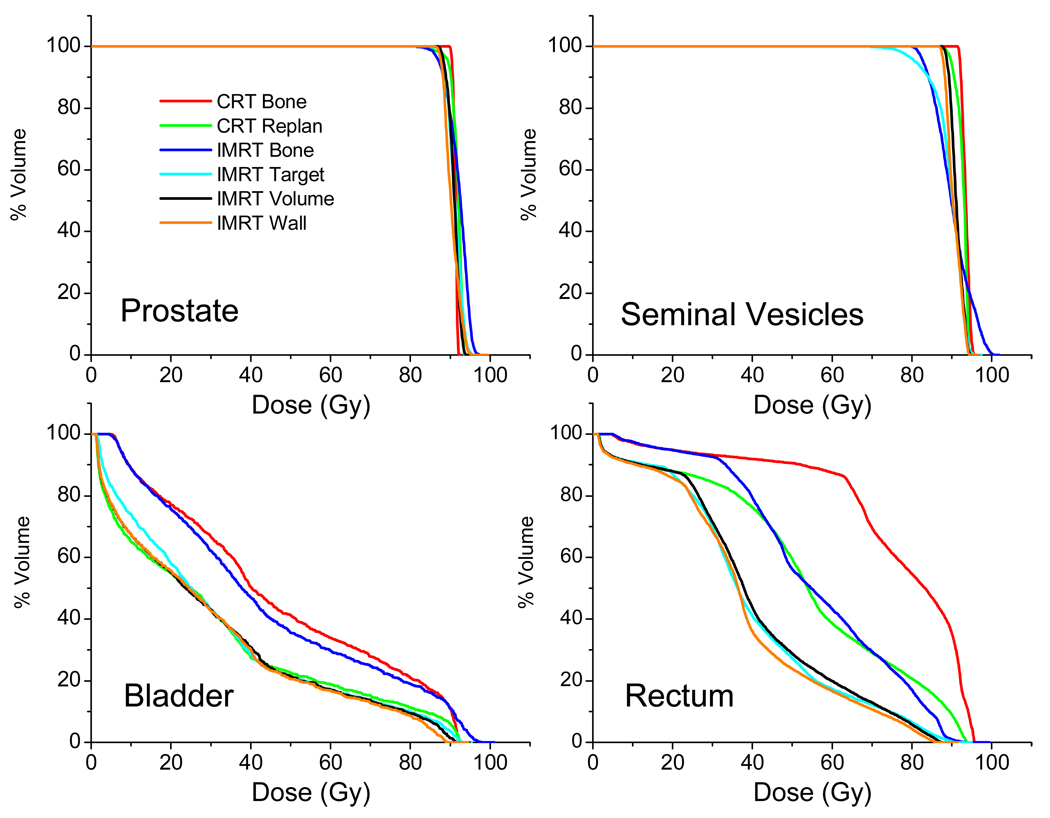
In order to provide a complete description of results from one randomly selected patient, Figure 2 shows the dose-volume histograms (DVHs) of one patient (#9) from the five planning methods studied. It can be seen that IMRT plans have less homogeneous target dose. Bladder dose appears to be similar for both plans with 1cm margins. IMRT significantly reduces rectum dose. Rectum dose is similar for the CRT 0cm method and IMRT 1cm method even though the later one has 1cm interfraction margin.
Figure 2.
Comparison of dose from five treatment planning methods for Patient #9, described by dose-volume histograms.
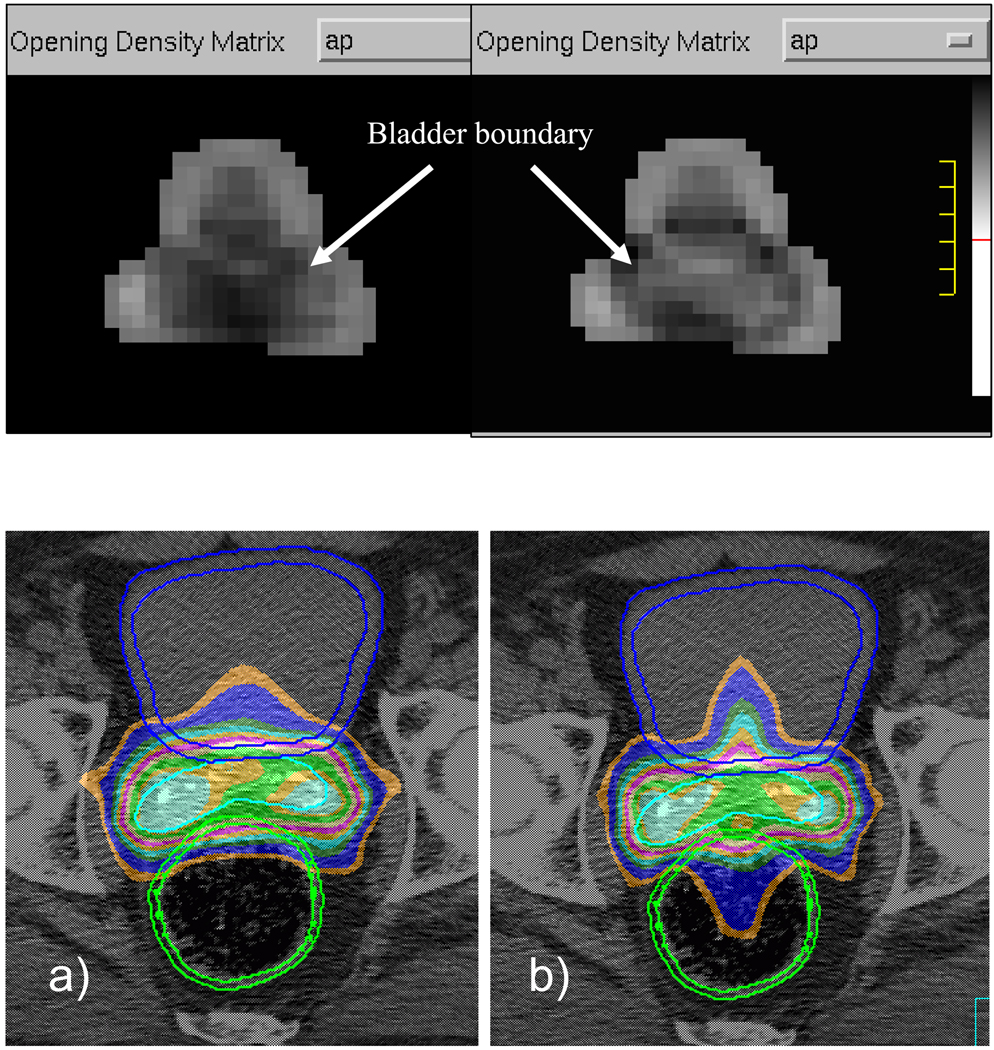
The planned bladder and rectum EUDs for all patients are summarized in Table 2. For the same patient, OAR doses by all treatment planning methods were also normalized to IMRT 1-cm plan. When rectum and bladder walls were used in IMRT optimization, the shapes of the dose distributions look different as compared to using the volumes. The top panels of Figure 3 show the fluence maps of the anterior-posterior (AP) beam and isodose distributions from IMRT 0cm Volume and IMRT 0cm Wall plans. In the volume-based plan, fluence is decreasing where the beam passes a greater distance through the whole organ, while intensity in the wall-based plan is decreased only in a ring where pathlength through the bladder wall is greater. The isodose lines form a “W” shape as they first retract along the walls, then extend into the center of bladder and rectum in IMRT Wall plan. On the contrary, the IMRT Volume plan has lower weightings for the beamlets through the center of the bladder, with isodose lines extending a minimum distance into any part of the sensitive organs. Despite this obvious qualitative difference, the improvement of the bladder and rectum EUD dose (as a percent of prostate dose) from IMRT Volume to IMRT Wall plans is barely noticeable at 1.01 +/− 0.97% and 1.71 +/− 1.53%, respectively.
Table 2.
Comparison of planned rectum and bladder EUD doses from various treatment planning methods. The values are expressed as percentages of target (prostate) EUD dose.
| Rectum | |||||
|---|---|---|---|---|---|
| Patient # | CRT 1-cm | CRT 0-cm | IMRT 1-cm | IMRT volume | IMRT wall |
| 1 | 95.0% | 84.3% | 82.3% | 67.8% | 66.7% |
| 2 | 88.9% | 82.5% | 85.9% | 74.4% | 75.4% |
| 3 | 88.5% | 80.5% | 88.1% | 74.1% | 70.2% |
| 4 | 92.5% | 84.6% | 82.9% | 77.4% | 74.2% |
| 5 | 89.9% | 80.3% | 82.1% | 72.6% | 70.7% |
| 6 | 93.6% | 83.6% | 83.3% | 70.7% | 70.1% |
| 7 | 89.4% | 77.9% | 85.2% | 70.2% | 68.4% |
| 8 | 92.3% | 80.1% | 85.7% | 71.1% | 68.0% |
| 9 | 94.7% | 84.6% | 82.8% | 72.0% | 71.2% |
| Mean | 91.6% | 82.1% | 84.3% | 72.3% | 70.5% |
| Bladder | |||||
| Patient # | CRT 1-cm | CRT 0-cm | IMRT 1-cm | IMRT volume | IMRT wall |
| 1 | 78.6% | 63.6% | 67.7% | 56.2% | 54.0% |
| 2 | 76.2% | 59.6% | 67.3% | 52.4% | 52.8% |
| 3 | 50.9% | 40.1% | 52.5% | 40.8% | 39.7% |
| 4 | 63.3% | 49.4% | 58.1% | 48.1% | 48.3% |
| 5 | 70.5% | 53.0% | 64.7% | 51.6% | 50.3% |
| 6 | 83.5% | 66.8% | 73.2% | 59.5% | 57.4% |
| 7 | 60.8% | 47.3% | 58.7% | 47.5% | 45.8% |
| 8 | 64.9% | 48.3% | 60.0% | 46.9% | 45.7% |
| 9 | 59.3% | 45.9% | 56.4% | 45.0% | 44.9% |
| Mean | 67.6% | 52.7% | 62.1% | 49.8% | 48.8% |
Figure 3.
Top: AP beam fluences from treatment plans optimized with OAR volume (left) and OAR wall (right). Bottom: Isodose profiles from IMRT treatment plans using a) organ volumes and b) organ walls. Bladder wall, seminal vesicles, and rectum wall are contoured in dark blue, light blue, and green, respectively. Isodose levels range from 55–105% of the isocenter dose.
Selecting an altogether different planning method has a greater impact on the OAR dose than changing the particular definition of the normal tissue volume. With the same 0-cm margin, IMRT plans (IMRT Volume and IMRT Wall) reduced bladder dose by an average of only 3% compared to 3D-CRT plans (CRT-0-cm), and rectum doses were reduced by 11%. When using a 1-cm margin, there are decreases when changing from CRT to IMRT plans in rectum and bladder doses, by about 7% and 6% of target dose, respectively. The choice of an appropriate CTV-PTV margin is a more important factor that determines OAR doses. For the same planning method, the rectum dose in 0-cm margin plans is respectively 10% and 11% lower using CRT and IMRT methods than their corresponding plans with 1-cm margins. The bladder dose reductions are even larger, at 15% and 14% when a 0-cm margin is used instead of a 1-cm margin for CRT and IMRT respectively.
B. Virtual treatment simulation results
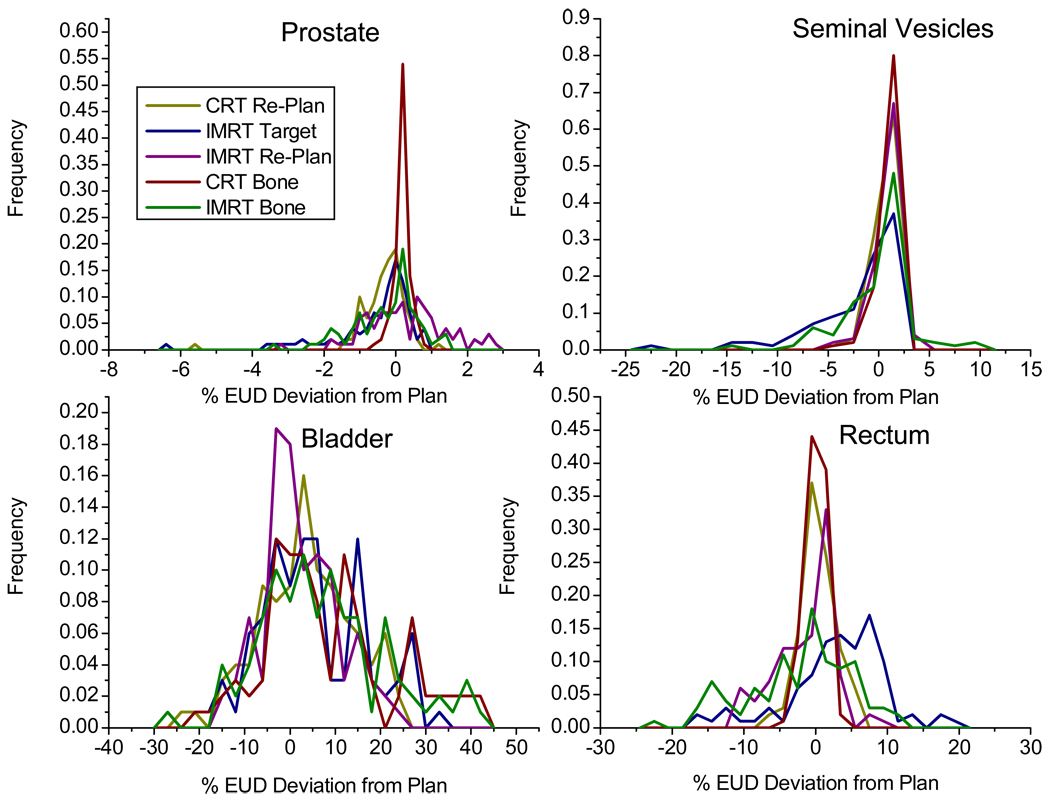
Fractional dose
The fractional doses for different online IGRT strategies were calculated using the methods described in previous sections. Histograms of differences of the individual fraction dose from the planned EUD can be seen in Figure 4 for all treatment fractions (N=90). All IGRT strategies were able to deliver consistent dose to the prostate. However, dose to seminal vesicle (as a fraction of planned seminal vesicle dose) was reduced by more than 20% in one fraction from the IMRT Target method. On this day, the prostate had moved 0.17cm to the posterior from the planning position while the seminal vesicles had moved 1.35cm in the anterior direction. There were several fractions where IMRT Bone delivered a seminal vesicle dose that was at least 5% less than planned. Bladder dose deviation appears to be similarly random for all treatment methods. In the rectum, only the two 3D-CRT methods delivered dose that was consistently within ±10% of the planned amount. But remember rectum dose is much higher in CRT plan than IMRT plan.
Figure 4.
Histogram of individual fraction deviations from planned EUD, expressed as percent of the respective organ planning dose. Total number of treatment fraction N is 90.
Accumulated dose
The fractional doses were accumulated using in-house FE-based dose accumulation software. Figure 5 shows the DVH of accumulated dose for Patient #9. Figure 6 shows the accumulated doses for all patients after ten fractions. The EUD values were normalized to the target (prostate) EUD in the treatment plan of the same patient with the same IGRT strategy. The prostates were all well covered, with only one patient’s EUD value at about 97% in the IMRT target alignment approach. Seminal vesicles, however, showed a larger difference, with the IMRT target alignment approach showing more severe underdose than other methods. The delivered EUD was only about 93% of the planned value on patient #2. Even with 1-cm CTV-PTV margin, the IMRT bone-alignment showed slight underdose on patients #2 and #9. The accumulated dose results support the conclusion derived from individual fraction doses, that IMRT is more sensitive to organ motion than 3D-CRT treatments.
Figure 5.
DVH of accumulated dose after 10 treatment fractions (patient #9).
Figure 6.
Accumulated EUD from 10 treatment fractions for different online IGRT strategies.
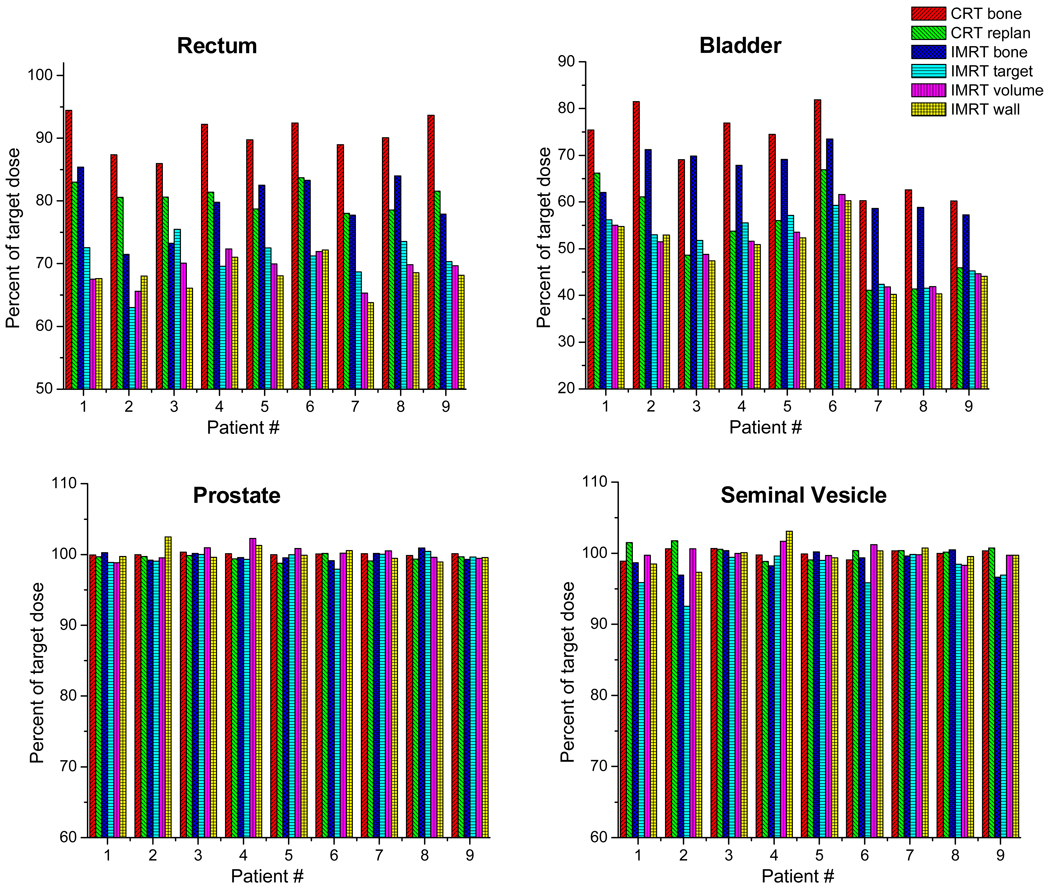
Table 3 summarizes rectum and bladder doses achieved by different online IGRT approaches. Remarkable differences in OAR doses are observed when choosing different online IGRT strategies. The CRT Bone approach delivered the highest OAR doses, with the bladder and rectum respectively on the average receiving 91% and 71% of target dose. IMRT re-planning approaches obtained the lowest bladder and rectum doses, with average values of 68–69% of target dose to rectum and 49–50% to the bladder. The IMRT target alignment approach obtained OAR doses that are similar to though slightly (1–5%) higher than the IMRT re-planning approaches on most patients. It should be noted that even though the IMRT target alignment method results in similarly low OAR dose, it did not deliver as reliable seminal vesicle dose as re-planning approaches. However, if only prostate is the target, IMRT target alignment method would be close to re-planning approach.
Table 3.
Comparison of delivered rectum and bladder doses by different online IGRT methods. The values are expressed as percentages of target (prostate) dose.
| Rectum | ||||||
|---|---|---|---|---|---|---|
| Patient # | CRT Bone | CRT Re-plan | IMRT Bone | IMRT Target | IMRT Re-plan Volume | IMRT Re-plan Wall |
| 1 | 94.5% | 83.3% | 85.2% | 73.4% | 68.3% | 67.8% |
| 2 | 87.4% | 80.8% | 72.0% | 63.6% | 65.9% | 66.4% |
| 3 | 85.7% | 80.8% | 73.1% | 75.4% | 69.4% | 66.3% |
| 4 | 92.1% | 81.9% | 80.2% | 70.1% | 70.7% | 70.1% |
| 5 | 89.8% | 79.7% | 82.9% | 72.6% | 69.4% | 68.2% |
| 6 | 92.4% | 83.6% | 84.0% | 72.7% | 71.8% | 71.8% |
| 7 | 88.9% | 78.8% | 77.6% | 68.6% | 65.0% | 64.2% |
| 8 | 90.2% | 79.1% | 83.2% | 73.3% | 70.1% | 69.3% |
| 9 | 93.5% | 81.8% | 78.4% | 70.6% | 70.1% | 68.4% |
| Mean | 90.5% | 81.1% | 79.6% | 71.1% | 69.0% | 68.0% |
| Bladder | ||||||
| Patient # | CRT Bone | CRT Re-plan | IMRT Bone | IMRT Target | IMRT Re-plan Volume | IMRT Re-plan Wall |
| 1 | 75.5% | 66.4% | 61.9% | 56.8% | 55.7% | 54.9% |
| 2 | 81.5% | 61.3% | 71.8% | 53.5% | 51.8% | 51.7% |
| 3 | 68.9% | 48.7% | 69.7% | 51.8% | 48.3% | 47.6% |
| 4 | 76.9% | 54.1% | 68.1% | 55.9% | 50.5% | 50.3% |
| 5 | 74.5% | 56.7% | 69.5% | 57.2% | 53.1% | 52.4% |
| 6 | 81.8% | 66.8% | 74.1% | 60.6% | 61.5% | 60.0% |
| 7 | 60.3% | 41.5% | 58.6% | 42.4% | 41.7% | 40.5% |
| 8 | 62.7% | 41.7% | 58.3% | 41.4% | 42.1% | 40.8% |
| 9 | 60.2% | 46.0% | 57.7% | 45.5% | 44.9% | 44.3% |
| Mean | 71.3% | 53.7% | 65.5% | 51.7% | 49.9% | 49.2% |
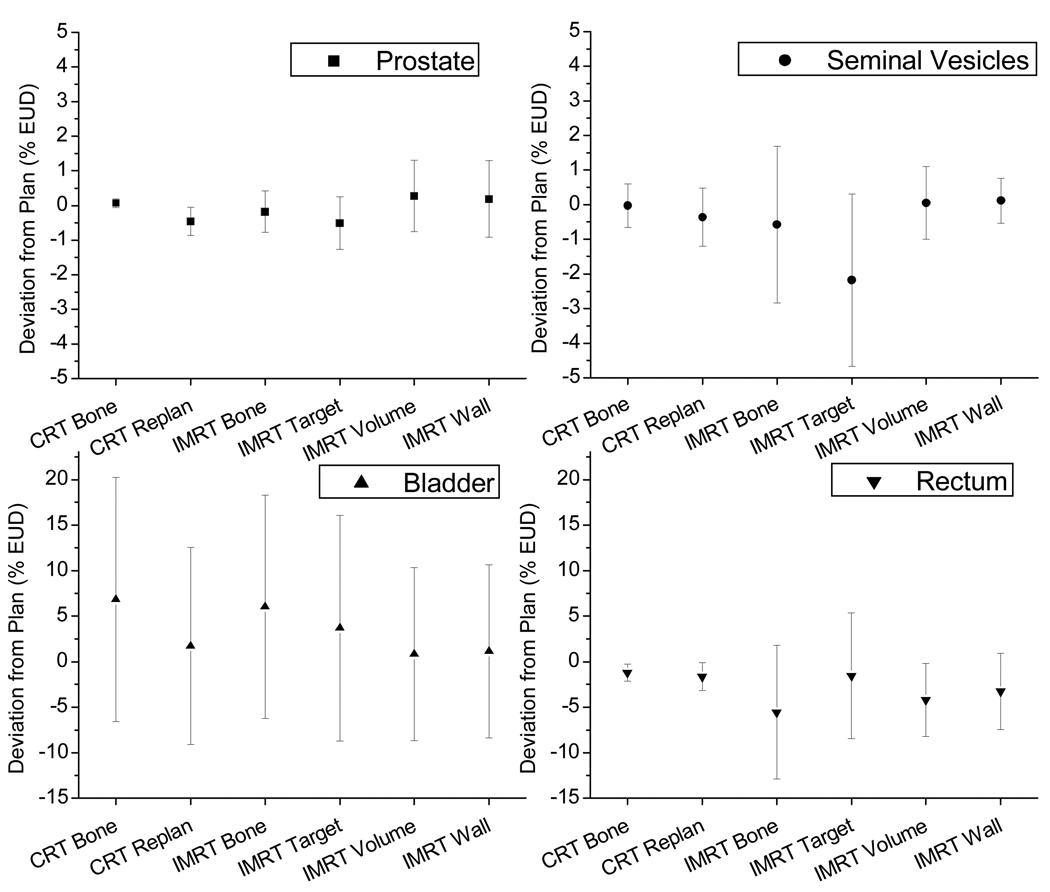
Difference from treatment plan prediction
A major purpose of treatment planning is to predict OAR doses. With an estimate of the risk of complication based on this dose, an appropriate maximum prescription dose can be determined. However, due to the organ motion, the actual delivered dose may be very different from the prediction in the treatment plan. We plotted the discrepancies between treatment plan prediction and accumulated dose after virtually simulated delivery in Figure 7. For all IGRT approaches, the deviation of prostate dose is small. Online IMRT re-planning approaches show small but noticeable target dose variations. This is due to the daily geometry changes that altered the weightings of optimization components. Thus, it may be necessary to re-scale the monitor units (MUs) based on the prostate dose in daily re-optimization. The seminal vesicles show large variations from IMRT bone and target localization approaches.
Figure 7.
Discrepancies of cumulative delivered doses from treatment plan prediction, expressed as mean ± 1 standard deviation of all patients. The columns are the mean and the error bars are the standard deviation of the difference.
The rectum doses show large plan-delivery discrepancies in all IMRT treatments. Although the mean discrepancy across all patients is close to zero, the standard deviation is larger than 5%. The standard deviation is slightly smaller in IMRT re-planning approaches, but not very significant. The 3D-CRT treatments are more consistent, as the mean of discrepancies is close to zero and standard deviation is smaller than 3%. For bladder, all online IGRT approaches show large variations from treatment plan predictions. The standard deviation of plan-delivery discrepancies is large, around 15% in most IGRT approaches, while the standard deviations for IMRT re-planning approaches are smaller, at about 10%.
Discussion
Traditional radiation treatments strategically treat volumes greater than the clinical target tissues to the prescription dose in order to achieve a high probability of treatment success in spite of daily tumor position uncertainty. Many studies have been done to typify organ motion, and a number of CTV-to-PTV margin recipes [21,22] have been devised to make the selection of margin size a more informed decision. Schaly [2] found that normal tissue dose is not reduced with image guidance alone, but by with the combination of image guidance with smaller CTV-PTV margins. This result is supported by our study.
Interfraction motion has been recognized as the largest contributor to target position uncertainty in tumors unaffected by respiratory motion [16]. In this study, intrafraction motion was not simulated. Ghilezan et al showed that prostate intrafraction motion correlated with rectal filling [20] and the amplitude of motion increased with elapsed time. This suggested the importance of shortening treatment time. Recently study by Nijkamp et al show that the diet and laxatives can reduce prostate motion during treatment [23]. The patients in this study had no special procedures to limit rectal filling, so our results represent an upper limit of therapeutic ratio which may be lower for patients who do use methods to control rectal volume. Also, a realistic margin would compensate for other uncertainties, such as errors in contouring, image registration, and position alignment.
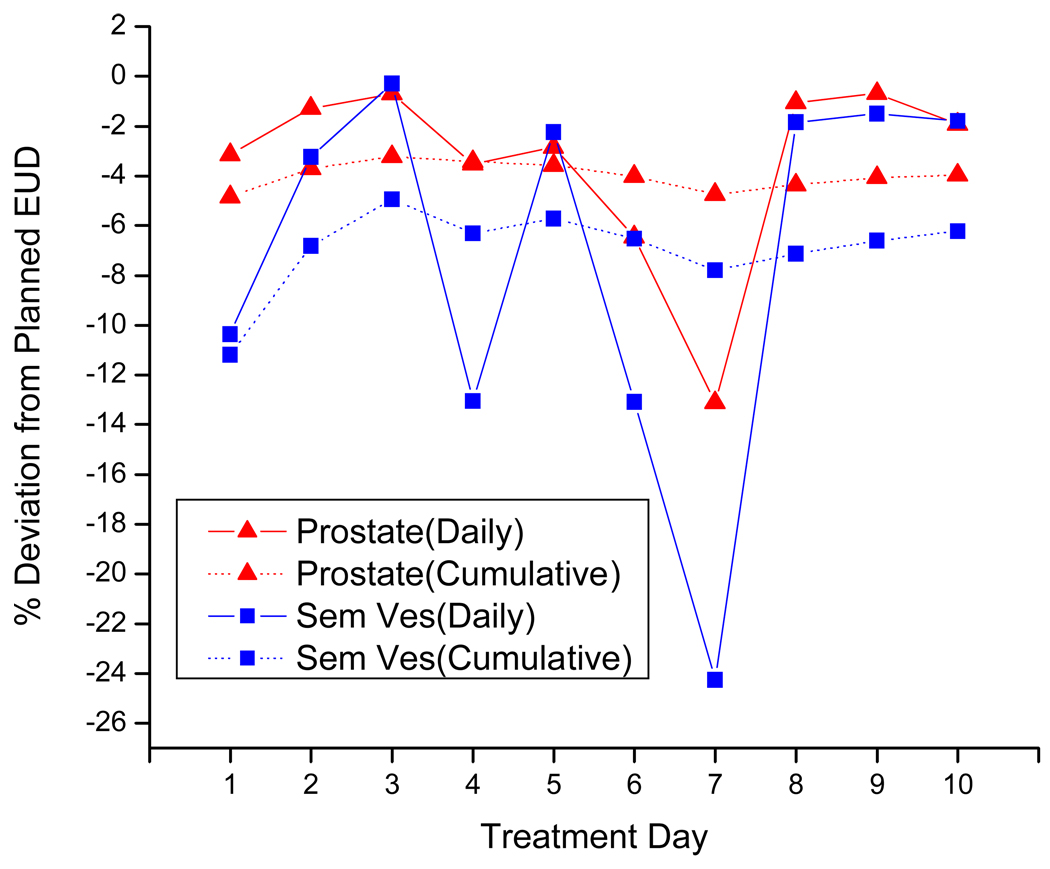
Due to limited image data sets, only 10 treatment fractions were simulated in this study. The results of this study may be directly applied to hypo-fractionated treatment. A regular fractionation prostate treatment may involve 35–40 fractions. We should be pay attention when extrapolate results from 10 fraction to more fractions. We expect the target underdosing will be reduced if the motion is mostly random and the CTV definition in treatment is close to the mean position. However, if the target motion has a trend or the CTV definition is deviated from mean position, dose coverage would not improve with more fractions. To illustrate the change of accumulated dose during treatment course, Figure 8 shows daily doses and accumulated doses of patient #6 for 10 fractions. The coverage is improved during the first three fractions and then stabilized after that.
Figure 8.
Change of accumulated dose and daily dose through 10 fractions of patient #6.
Technologies such as EPIDs, CALYPSO, CBCT, and CT-on-rails allow accurate tracking of the target on a daily basis, and their use justifies reduction of the margin size. With no margins, the IMRT Target simulations essentially treated only the CTV. While there was underdosing in the seminal vesicles, satisfactory prostate dose was achieved for most patients. This result indicates that much smaller margins are justified around the prostate than the seminal vesicles, and that when treating prostate alone by this method, the size of the motion margin can be dictated only by the extent of intra-fraction motion. For low risk patients, using implanted markers to locate the target each day can significantly reduce normal tissue dose while maintaining target coverage. For patients with high risk disease, the chance of microscopic or occult disease is increased. In the case of prostate patients, this is evidenced by extracapsular extension [24] and seminal vesicle involvement [25]. While the CTV-PTV margin has nominally accounted for target motion, its unintentional function has been to also treat these cells that are not traditionally included in the CTV. Therefore, this extremely conformal approach, which does not account for target rotations and deformations, is expected to be less successful in high risk patients.
Increasing levels of image guidance complexity provide more information with which to re-evaluate the treatment on a more frequent basis. The CRT Re-plan method using aperture change makes easy use of this information, as it simply shapes the blocks each day to the new PTV. After ten treatment fractions, the rectum dose from this method was very similar to that from the more standard IMRT Bone method. Also, Figure 7 shows that all patients received adequate seminal vesicle dose and 8 of 9 patients received lower bladder dose by the CRT re-planning method. Though normal tissue sparing is limited by the convexity of the dose distribution and the penumbra size, this treatment provides very consistent target coverage. In reality, this treatment is not feasible in the absence of robust automatic contouring technology. However, since this method requires no further optimization, it is probably the least computationally intensive online adaptation that can be performed with this image data.
The most computationally intensive adaptation would involve a complete IMRT re-optimization of the original treatment plan. Compared to the IMRT Bone treatments, target coverage was maintained for all patients while bladder and rectum doses were respectively reduced by approximately 16% and 11%. This approach requires online ROI delineation and online plan re-optimization techniques. Although challenging, it is feasible to achieve these goals in the near future. We have developed an automatic ROI delineation method for HN images [15]. In principle, the same method can also be used on prostate images, but CBCT image quality is a major obstacle. In order to perform IMRT re-planning online, high performance computing techniques can be employed to accelerate computation.
We were able to accelerate the deformable image registration speed by a factor close to the number of processing elements using a Beowulf cluster. Wu et al showed that online IMRT re-optimization and MLC leaf conversion are achievable within 2–3 minutes [13].
In this study, we employed online IMRT re-planning approaches in which bladder and rectum volumes or walls were used as optimization constraints. The resulting difference between the two approaches, IMRT Re-plan Volume and IMRT Re-plan Wall, was shown to be small. When choosing OAR walls instead of the standard volumes, reduction of doses to bladder and rectum is limited to less than 1% on average, though using this approach does not drastically increase technical complexity. Extracting bladder and rectum walls from volumes would be straightforward, so it would be reasonable to employ the IMRT-wall approach for a small but cost-free improvement. This conclusion applies specifically to online re-planning methods, as any benefit from standard planning methods is expected to be negated by interfractional tissue shape changes.
An interesting result of this study is that IMRT techniques are much more sensitive to organ motion than CRT techniques. This is because the IMRT plan is more conformal, meaning that the dose gradient surrounds the target more tightly. Even with a 1-cm margin, underdoses in SV were still observed in several patients. Thus, regardless of margin size, high precision image guidance is more important in highly conformal treatment techniques. From this result, we would expect image guidance and intensity/energy modulation to be a decisive factor in proton prostate cancer treatments, where sharp dose gradients beyond the Bragg peak combined with particle penetration depth uncertainties create opportunities for significant dosimetric consequences.
We noticed that even in the online re-planning techniques, large discrepancies are shown between planning and accumulated bladder and rectum doses. This encourages an close-loop adaptive treatment technique with dose tracking, in which the re-planning takes account of accumulated dose up to that point in the treatment course [26,27]. Otherwise, OAR doses meet constraints in treatment planning, they are likely over the limits in actual treatments.
Conclusions
Online treatment plan re-optimization may significantly improve therapeutic ratio in prostate cancer treatments when seminal vesicle is involved mostly due to the reduction of PTV margin. However for low risk patient with only prostate involved, online target alignment IMRT treatment would achieve similar results as online re-planning. For all IGRT approaches, the delivered organ-at-risk doses may be significantly different from treatment planning prediction.
Acknowledgements
This study is partially supported by department of defense prostate cancer research program under award #W81XWH-07-0083.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Beltran C, Herman MG, Davis BJ. Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods. Int.J.Radiat.Oncol.Biol.Phys. 2008;70:289–295. doi: 10.1016/j.ijrobp.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Schaly B, Bauman GS, Song W, Battista JJ, Van DJ. Dosimetric impact of image-guided 3D conformal radiation therapy of prostate cancer. Phys.Med.Biol. 2005;50:3083–3101. doi: 10.1088/0031-9155/50/13/008. [DOI] [PubMed] [Google Scholar]
- 3.Ling CC, Yorke E, Fuks Z. From IMRT to IGRT: frontierland or neverland? Radiother.Oncol. 2006;78:119–122. doi: 10.1016/j.radonc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Herman MG. Clinical use of electronic portal imaging. Semin.Radiat.Oncol. 2005;15:157–167. doi: 10.1016/j.semradonc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Jans HS, Syme AM, Rathee S, Fallone BG. 3D interfractional patient position verification using 2D-3D registration of orthogonal images. Med.Phys. 2006;33:1420–1439. doi: 10.1118/1.2192907. [DOI] [PubMed] [Google Scholar]
- 6.Stutzel J, Oelfke U, Nill S. A quantitative image quality comparison of four different image guided radiotherapy devices. Radiother.Oncol. 2008;86:20–24. doi: 10.1016/j.radonc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Guckenberger M, Meyer J, Wilbert J, Baier K, Sauer O, Flentje M. Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther. Onkol. 2007;183:307–313. doi: 10.1007/s00066-007-1695-0. [DOI] [PubMed] [Google Scholar]
- 8.Yue NJ, Knisely JP, Song H, Nath R. A method to implement full six-degree target shift corrections for rigid body in image-guided radiotherapy. Med.Phys. 2006;33:21–31. doi: 10.1118/1.2138009. [DOI] [PubMed] [Google Scholar]
- 9.Chung PW, Haycocks T, Brown T, Cambridge Z, Kelly V, Alasti H, Jaffray DA, Catton CN. On-line aSi portal imaging of implanted fiducial markers for the reduction of interfraction error during conformal radiotherapy of prostate carcinoma. Int.J.Radiat.Oncol.Biol.Phys. 2004;60:329–334. doi: 10.1016/j.ijrobp.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Wertz H, Lohr F, Dobler B, Mai S, Welzel G, Boda-Heggemann J, Wenz F. Dosimetric consequences of a translational isocenter correction based on image guidance for intensity modulated radiotherapy (IMRT) of the prostate. Phys.Med.Biol. 2007;52:5655–5665. doi: 10.1088/0031-9155/52/18/012. [DOI] [PubMed] [Google Scholar]
- 11.Ding GX, Duggan DM, Coffey CW, Deeley M, Hallahan DE, Cmelak A, Malcolm A. A study on adaptive IMRT treatment planning using kV cone-beam CT. Radiother.Oncol. 2007;85:116–125. doi: 10.1016/j.radonc.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Castro-Pareja C, Shekhar R, Yu C. Direct aperture deformation: an interfraction image guidance strategy. Med.Phys. 2006;33:4490–4498. doi: 10.1118/1.2374675. [DOI] [PubMed] [Google Scholar]
- 13.Wu QJ, Thongphiew D, Wang Z, Mathayomchan B, Chankong V, Yoo S, Lee WR, Yin FF. On-line re-optimization of prostate IMRT plans for adaptive radiation therapy. Phys.Med.Biol. 2008;53:673–691. doi: 10.1088/0031-9155/53/3/011. [DOI] [PubMed] [Google Scholar]
- 14.Wu C, Jeraj R, Olivera GH, Mackie TR. Re-optimization in adaptive radiotherapy. Phys.Med.Biol. 2002;47:3181–3195. doi: 10.1088/0031-9155/47/17/309. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Chi Y, Meldolesi E, Yan D. Automatic delineation of on-line head-and-neck computed tomography images: toward on-line adaptive radiotherapy. Int.J.Radiat.Oncol.Biol.Phys. 2007;68:522–530. doi: 10.1016/j.ijrobp.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Langen KM, Jones DT. Organ motion and its management. Int.J.Radiat.Oncol.Biol.Phys. 2001;50:265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 17.Orton NP, Tome WA. The impact of daily shifts on prostate IMRT dose distributions. Med.Phys. 2004;31:2845–2848. doi: 10.1118/1.1784592. [DOI] [PubMed] [Google Scholar]
- 18.Guckenberger M, Meyer J, Wilbert J, Baier K, Sauer O, Flentje M. Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther. Onkol. 2007;183:307–313. doi: 10.1007/s00066-007-1695-0. [DOI] [PubMed] [Google Scholar]
- 19.Yan D, Jaffray DA, Wong JW. A model to accumulate fractionated dose in a deforming organ. Int.J.Radiat.Oncol.Biol.Phys. 1999;44:665–675. doi: 10.1016/s0360-3016(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 20.Ghilezan M, Yan D, Liang J, Jaffray D, Wong J, Martinez A. Online image-guided intensity-modulated radiotherapy for prostate cancer: How much improvement can we expect? A theoretical assessment of clinical benefits and potential dose escalation by improving precision and accuracy of radiation delivery. Int.J.Radiat.Oncol.Biol.Phys. 2004;60:1602–1610. doi: 10.1016/j.ijrobp.2004.07.709. [DOI] [PubMed] [Google Scholar]
- 21.Beltran C, Herman MG, Davis BJ. Planning Target Margin Calculations for Prostate Radiotherapy Based on Intrafraction and Interfraction Motion Using Four Localization Methods. Int.J.Radiat.Oncol.Biol.Phys. 2007 doi: 10.1016/j.ijrobp.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 22.van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. Int.J.Radiat.Oncol.Biol.Phys. 2002;52:1407–1422. doi: 10.1016/s0360-3016(01)02805-x. [DOI] [PubMed] [Google Scholar]
- 23.Nijkamp J, Pos FJ, Nuver TT, de JR, Remeijer P, Sonke JJ, Lebesque JV. Adaptive radiotherapy for prostate cancer using kilovoltage cone-beam computed tomography: first clinical results. Int.J.Radiat.Oncol.Biol.Phys. 2008;70:75–82. doi: 10.1016/j.ijrobp.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 24.Chao KK, Goldstein NS, Yan D, Vargas CE, Ghilezan MI, Korman HJ, Kernen KM, Hollander JB, Gonzalez JA, Martinez AA, Vicini FA, Kestin LL. Clinicopathologic analysis of extracapsular extension in prostate cancer: should the clinical target volume be expanded posterolaterally to account for microscopic extension? Int.J.Radiat.Oncol.Biol.Phys. 2006;65:999–1007. doi: 10.1016/j.ijrobp.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Kestin L, Goldstein N, Vicini F, Yan D, Korman H, Martinez A. Treatment of prostate cancer with radiotherapy: should the entire seminal vesicles be included in the clinical target volume? Int.J.Radiat.Oncol.Biol.Phys. 2002;54:686–697. doi: 10.1016/s0360-3016(02)03011-0. [DOI] [PubMed] [Google Scholar]
- 26.Birkner M, Yan D, Alber M, Liang J, Nusslin F. Adapting inverse planning to patient and organ geometrical variation: algorithm and implementation. Med.Phys. 2003;30:2822–2831. doi: 10.1118/1.1610751. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Liang J, Yan D. Application of dose compensation in image-guided radiotherapy of prostate cancer. Phys.Med.Biol. 2006;51:1405–1419. doi: 10.1088/0031-9155/51/6/003. [DOI] [PubMed] [Google Scholar]