Abstract
Tauopathies are characterized by accumulation of filamentous tau aggregates. These aggregates can be recapitulated in transfectant M1C overproducing wild-type human brain tau 4R0N via the tetracycline off (TetOff) inducible expression mechanism. To determine the contribution of proteasomes to tau degradation and aggregation, we exposed M1C cells to epoxomicin (Epx; 2-50 nM) or MG132 (0.5 μM) on the 3rd or 4th day of a 5-day TetOff induction and demonstrated a reduction of proteasomal activity. Cultures treated with 2 nM Exp showed accumulation of full-length tau without affecting ubiquitin and β-catenin immunoblotting profiles. In contrast, cells treated with 10, 50 nM Epx or MG132 displayed changes in ubiquitin or β-catenin immunoblotting profiles and extensive tau degradation/truncation. The increase of tau degradation/truncation was accompanied with accumulation of oligomers and sarkosyl-insoluble aggregates of tau, augmented thioflavin-binding and activation of caspases and calpains. Truncated, oligomeric and sarkosyl-insoluble tau derivatives appeared with caspase-specific cleavage and their production was diminished when pretreated with a pan-caspase inhibitor. The results demonstrate (i) a dose-dependent, opposite effect of proteasome inhibition on tau processing, (ii) the participation of proteasome-dependent, ubiquitination-independent mechanisms in tau degradation and aggregation, and (iii) the promotion of tau aggregation by caspase-mediated tau degradation/truncation.
Keywords: tau degradation and aggregation, inducible transfectant, cell culture, proteasomal inhibition, caspase, calpain
Introduction
Accumulation of microtubule-associated protein tau to form filamentous inclusions in the central nervous system is a pathologic hallmark that characterizes several neurodegenerative disorders, including Alzheimer's disease (AD), progressive supranuclear palsy, corticobasal degeneration, frontotemporal dementia with parkinsonism linked to chromosome 17 and Niemann-Pick disease type C [1]. When incorporated into inclusion, tau is extensively phosphorylated as detected in humans and animal models at early stages of tauopathies [2]. According to in vitro studies, assembly of filamentous aggregates requires a critical concentration of tau and involves nucleation, oligomerization and elongation [3]. The process can be accelerated by tau mutation, removal of the carboxyl-terminus of tau or in the presence of inducers (e.g. heparin, polyunsaturated fatty acids) [4-8]. Phosphorylated tau was demonstrated to be more resistant to proteolysis and less able to bind and promote microtubule assembly [9]. In vitro studies have also reported that filamentous tau isolated from tauopathy brains or assembled in vitro are inhibitory to proteasomes. Moreover, AD brains were shown to display lower proteasome activity than that of their age-matched controls, even though their level of proteasome subunits was not affected [10, 11].
It has been documented that tau protein can be degraded in vitro by caspase [12-14], calpain [15-17], cathepsin D [18-20] and proteasomes [21-22]. Treatment of tau-expressing cells with drugs that are capable of perturbing calcium homeostasis was demonstrated to cause tau degradation [23]. However, exposing cultured cells expressing endogenous or exogenous tau to a proteasome inhibitor resulted in tau degradation [24, 25], tau accumulation [21] or failed to alter tau levels altogether [26]. In one study, lactacystin treatment of a stable tau transfectant derived from SH-SY5Y cells was shown to suppress tau degradation without accumulation of ubiquitinated tau [21]. Moreover, incubation of recombinant tau with 20S proteasomes resulted in tau degradation, suggesting a ubiquitin-independent pathway for tau processing [21]. Other studies using cell cultures that were co-transfected with tau and ubiquitin or E3 ubiquitin ligase CHIP emphasized preferential degradation of phosphorylated tau by an ubiquitin-, proteasome-dependent mechanism [27]. It was reported recently that overexpression of CHIP in rat hippocampus led to increased degradation of both phosphorylated and non- phosphorylated tau [28]. CHIP knockout mice were shown to have increased level of phosphorylated and non-phosphorylated tau [29]. Such mice were reported in one study to cause a slight increase of SDS-insoluble tau [30], but in other studies to elevate the level of phosphorylated tau without any impact on tau solubility [29]. Treatment of cell cultures overexpressing tau with a HSP90 inhibitor resulted in proteasome-mediated clearance of tau species phosphorylated at proline-directed Ser/Thr sites, but not those phosphorylated at S262/S356 [31].
Although aggregated tau derived from AD brains was shown to inhibit proteasomal activity in vitro [32], whether or not proteasomal inhibition per se plays a role in tau aggregation remains a question. Equally unknown is whether ubiquitin-independent, proteasome-dependent and ubiquitin-, proteasome-dependent pathways work in concert to maintain the level of tau in situ/in vivo. To address these issues we used transfectant M1C that overexpresses human 4R0N tau via TetOff inducible mechanism. The M1C transfectant is derived from human neuroblastoma BE(2)-M17D cells. We have previously shown that, after the TetOff induction of tau expression for 5 to 7 days, these cells accumulate a minute amount of sarkosyl-insoluble high-molecular-weight (HMW) tau aggregates and oligomeric tau of 70 kDa consisting of truncated tau species [33, 34]. A portion of the 70 kDa and nearly all of the HMW derivatives disappear to give rise to smaller species upon reduction by beta-mercaptoethanol, suggesting that formation of such tau derivatives involves at least in part disulfide-crosslink of tau fragments that are truncated at the carboxyl-terminus. We also showed that autophagic-lysosomal perturbation of M1C cells led to increased tau oligomers (70 kDa and HMW), increased thioflavin binding and aggregation of filamentous tau [35]. In the present study, we studied the impact of proteasomal inhibition on tau degradation/truncation and assembly.
Materials and Methods
Materials
Tissue culture wares were obtained from BD Biosciences (Franklin Lakes, NJ). Glass coverslips were from Bellco Glass, Inc. (Vineland, NJ). Proteasome inhibitors, MG132 and Lactacystin (0-2 μM) were from Calbiochem (San Diego, CA), and epoxomicin (Epx) was from Boston Biochem (Cambridge, MA). Pan-caspase inhibitor (Z-VAD-FMK) and fluorogenic substrate for caspase 3 (substrate II) or calpain I substrate [H-K(FAM)-EVY-GMMK (DABCYL)-OH] were purchased from Calbiochem. Fluorogenic substrate Suc-LLVY-AMC (Biomol, Plymouth Meeting, PA) was used to assay chymotrypsin (chy)-like activity. Other chemicals were from Sigma (St. Louis, MO) unless indicated otherwise.
Antibodies
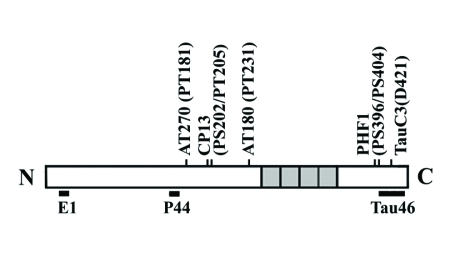
The location of epitopes recognized by different anti-tau antibodies used in the present study is illustrated in Figure 1. These antibodies are well characterized and used in a number of previous studies (14, 33, 36, 37). Monoclonal antibodies to β-catenin (9G10), spectrin, calpastatin and glyceraldehydes 3-phosphate dehydrogenase (GAPDH) were obtained from BD Biosciences, Chemicon (Temecula, CA), Sigma, and Covance Research Products, Inc. (Berkeley, CA), respectively. Polyclonal antibodies to cleaved-caspase3, caspase 3 or calpain I were form Cell Signaling (Danvers, MA). Antibodies were used at the following dilutions: P44 (1:5,000), E1 (1:2,500), Tau 46 (1:5,000), PHF-1 (1:200), CP13 (1:200), AT180 (1:200), AT270 (1:400), anti-β-catenin (1:500), anti-spectrin (1:1,000), anti-cleaved caspase3 (1:500), anti-caspase 3 (1:500), anti-calpastatin (1:250), anti-calpain I (1:250) and anti-GAPDH (1:5,000).
Figure 1.
Schematic representation of 4R0N tau and epitope map for different tau antibodies.
Cell Culture
Transfectant M1C derived from human neuroblastoma BE(2)-M17D cells [33] expresses wild-type human tau isoform 4R0N via TetOff inducible expression mechanism and accumulates filamentous tau after chloroquine treatment [35]. M1C cells were seeded at 2 × 106 cells/plate in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, G418 (400 μg/ml; Life Technologies, Gaithersburg, MD), Tet (2 μg/ml), puromycin (1 μg/ml), zeocin (100 μg/ml) and hygromycin (100 μg/ml). Twenty four hours later, tau expression was elicited by replacing the spent medium with fresh medium containing 1 ng/ml Tet. Replica cultures were exposed to proteasomal inhibitors [epoxomicin (Epx), MG132] or staurosporine (Sigma) on days 3 or 4 of a 5-day TetOff induction. Cells were harvested at the end of such tau induction. Others were treated with Z-VAD-FMK (20 μM, Calbiochem) 3 hrs before Epx incubation.
Fractionation of Cell Lysates
M1C cells were harvested and homogenized in Tris buffer containing protease and phosphatase inhibitors (30 mM β-glycerophosphate, 30 mM sodium fluoride, 1mM phenylmethylsulfonyl fluoride, 1mM EDTA and 1mM EGTA). The homogenate was centrifuged at 180g for 15 min to derive cell lysate. A portion of the lysate was further fractionated based on its solubility in Tris buffer or 2% sarkosyl to generate SN1, SN2 and S/P fractions as reported previously [33, 36]. The SN1 fraction is supernatant derived from centrifugation of lysate at 150,000g for 15 min at 4°C. The pellet was re-suspended in a buffer containing 0.8 M NaCl, 10% sucrose, 10 mM Tris/HCl, pH 7.4, 1 mM EGTA, 1 mM PMSF and 1% sarkosyl and centrifuged at 150,000g for 15 min to yield supernatant (SN2) and sarkosyl-insoluble (S/P) pellet. All of the supernatants or the sarkosyl-insoluble pellet re-suspended in Tris buffer were used immediately for immunoblotting and enzymatic assay or stored at −80°C.
Western Blotting
Cell lysates or fractionated preparations were mixed with Laemmli sample buffer with or without 1% β-mercaptoethanol (βME). Protein concentration of cell lysates was determined using the Bradford assay. Samples containing the same amount of protein (8-12 μg, corresponding to 0.8-1.2 × 105 cells per lane), were resolved by 10% SDS-polyacrylamide gel electrophoresis and subsequently transferred onto nitrocellulose membrane for immunoblotting using antibodies to tau or other proteins. The intensity of immunoreactivity was quantified by densitometry and analyzed using the MCID software (Imaging Research Inc., Ontario, Canada).
Proteasome Activity Assay
We used cell lysates containing 50 μg protein to assay proteasome activity. Samples in a reaction buffer (50 μl/sample) containing, 50 mM HEPES (pH 7.5), 5 mM MgCl2, 150 mM NaCl, 20% glycerol, 5 mM sodium pyrophosphate, 30 mM β-glycerophosphate, 30 mM sodium fluoride, and a cocktail of protease inhibitors (Sigma) were placed in a 96 well plate. They were mixed with 100 μM Suc-LLVY-AMC fluorogenic substrate for chy-like activity. The reaction mixtures were incubated at 37°C for 60 min in the dark and the fluorescence signals were measured using excitation/emission wavelengths at 360 nm/460 nm and 335 nm/410 nm to assess chy-/try-like activity in a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA). Enzyme specific signals were obtained by subtraction of signals derived from lysates mixed with proteasomal inhibitor.
Thioflavin S-binding Assay
The sarkosyl-insoluble preparations were re-suspended in Tris buffer containing 10 μM thioflavin S for 20 min and the fluorescence signal was measured with a Cary Eclipse fluorospectrophotometer (Varian, Walnut Creek, CA) using excitation at 440 nm and emission at 460-600 nm as described previously [33, 35]. All measurements were performed in triplicates from three experiments.
Results
Dose-dependent Impacts of Proteasome Inhibition on tau Processing
We have previously demonstrated that, after 5 days of TetOff induction, M1C cells accumulate derivatives of 4R0N tau that are not found in their non-induced counterparts [33-35, 38]. Without TetOff induction the level of endogenous tau in M1C cells is not detectable by Western blotting at the sample loading we used.
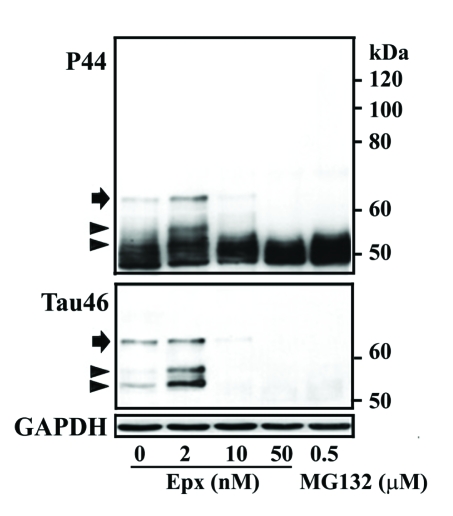
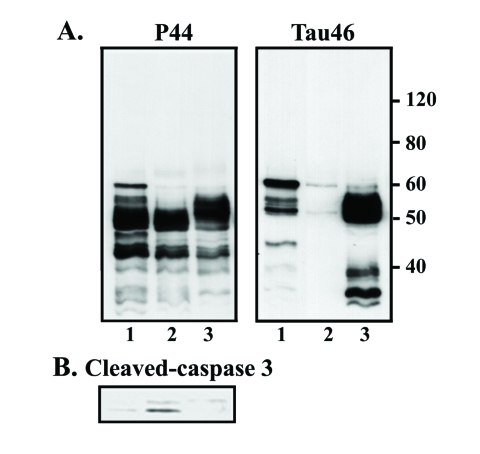
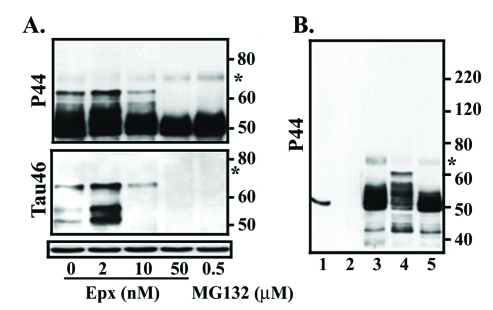
In the present study, exposure of M1C cells to 2 nM Epx during the last 3rd and 5th day of TetOff induction using 1ng/ml Tet was demonstrated to increase the level of at least 3 exogenous tau species of 52-62 kDa (Figure 1) as compared to their vehicle control (arrow and arrowhead, compare lanes 2 and 1 in Figure 2). These bands reacted with antibodies that are specific to the mid-region (P44), the carboxyl-end (Tau46) (Figure 2) and the amino terminus (E1 or Tau12, data not shown) of tau, suggesting that they are intact tau species exhibiting different states of posttranslational modifications. Tau species smaller than 52 kDa were detected in the 2 nM Epx treated and vehicle control. Because intact recombinant 4R0N human tau has an apparent molecular weight of 52 kDa, molecules smaller than 52 kDa are degraded derivatives. Quantitative analysis of P44 and Tau46 immunoreactivities indicates that, compared to the vehicle control, the level of 62 kDa tau increased to 400% and 200%, respectively, following the incubation with 2 nM Epx (Figure 2). In contrast, exposure of their sibling cultures to the same drug at 10 nM or 50 nM) led to a decrease in the level of P44-positive 52-62 kDa species but an increase in that of P44-positive ∼50 kDa tau (Figure 2). The low-molecular-weight tau (<50 kDa) displayed P44, but not Tau46 immunoreactivity, indicating that they are carboxyl-terminally truncated. A similar tau profile was also observed with replica cultures following their treatment with proteasome inhibitors MG132 (0.5 μM) (Figure 2) and lactacystin (0.5, 1 and 2 μM; data not shown).
Figure 2.
Dose-dependent impacts of proteasome inhibition on tau processing. M1C cells were subjected to a 5-day TetOff induction plus exposure to epoxomicin (Epx) at 2 or 10 nM for 2 days or at 50 nM for 1 day or to 0.5 μM MG132 for a day before such induction ended. Cultures treated with DMSO served as vehicle control (Epx 0). Cell lysates were immunoblotted with antibody P44 (top panel) and reprobed with antibody Tau46 (mid-panel). Replica blot was probed with GAPDH antibody to verify loading (bottom panel). In the 2 nM Epx-treated, tau species of 62, 54 and 52 kDa displayed P44, Tau46 and E1 (data not shown) immunoreactivities, suggesting that they are intact tau with different extents of posttranslational modification. Based on Tau46 immunoblotting, the 52-kDa (arrowhead) was the most abundant followed by 54 (arrowhead) and 62 kDa (arrow). In contrast, in vehicle-treated cells, the 62-kDa was the most abundant followed by 52 and 54 kDa species. In cultures exposed to Epx at higher concentrations or to MG132 Tau46-positive tau species were reduced or not detectable, but P44-positve tau species were observed, indicating most of these tau are carboxyl-terminally truncated.
Proteasome-dependent and Ubiquitination-independent tau Processing
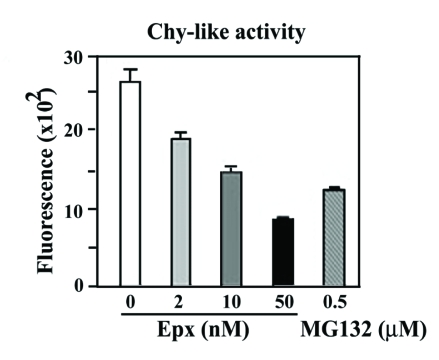
Because treating cells with 2 nM Epx gave rise to tau profile that is different from what can be obtained with higher doses (10 or 50 nM), it is important to demonstrate the impact of Epx treatment (2, 10 or 50 nM) on proteasomal activity. We used fluorogenic substrates and cell lysate prepared from the treated cultures and vehicle control for measurement of chy-like activity (Figure 3). After exposing cells to 2 nM Exp, chy-like activity was significantly reduced to 70% of that found in cells treated with the vehicle only (p<0.05., Student's t test; n=3). A further reduction in such activity was noted in the cells treated with higher concentration of Epx or MG132 (data not shown).
Figure 3.
Dose-dependent inhibition of proteasomal activity. Fluorogenic assays were used to assess chymotrypsin-like (chy-like) activity. Cultures with Epx exposure exhibited lower proteasome activity than their vehicle-treated counterparts, and the extent of reduction depended on the drug concentration. The chy-like activity of the Epx-treated (at 2 nM) was 70% of that found with its vehicle control verifying proteasomal inhibition of such drug exposure (p <0.05, Student's t test; n=3). Y axis showed fluorescence intensity in arbitrary units.
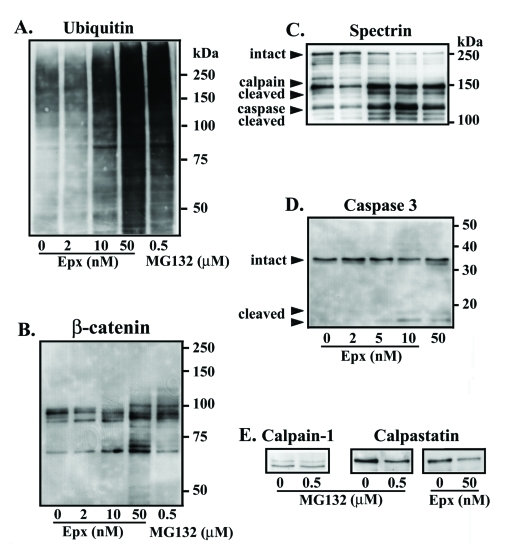
To determine if Epx treatment and the resulting decrease in proteasome activity led to accumulation of ubiquitinated proteins, we immunoblotted the cell lysates using antibodies to ubiquitin. Unlike the change in tau levels observed following 2 nM Epx treatment, no change in the ubiquitin profile was observed between cells treated with 2 nM Epx and vehicle control (Figure 4A). In contrast, a substantial increase in ubiquitin immunoreactivity was evident in samples exposed to higher concentrations of Epx (10 and 50 nM). To verify that 2 nM Epx treatment has no impact on tau ubiquitination, we performed immunoprecipitation studies. Cell lysates were incubated with anti-tau antibodies before probing the resulting immunoprecipitate with ubiquitin antibodies. There was no difference in the ubiquitin immunoprofile between cells exposed to 0 or 2 nM Epx (data not shown). Together, our results suggest that tau accumulation in the 2 nM Epx-treated cells is proteasome-dependent and ubiquitin-independent.
Figure 4.
Impact of proteasome inhibition on the integrity of various proteins. Immunoblotting of lysates derived from the TetOff induced cells, with or without the exposure to proteasome inhibitors, using antibodies to (A) ubiquitin, (B) β-catenin, (C) spectrin, (D) caspase-3, and (E) calpain I and calpastatin. Exposure to 2 nM Epx had very little impact on the profile and immunoreactivity of the aforementioned proteins studied (data for calpain I and calpastatin not shown). In contrast, exposure to more concentrated Epx (>10 nM) or 0.5 μM MG132 caused an increase in ubiquitin and cleaved caspase-3 immunoreactivities, a decrease in calpastatin immunoreactivity and altered β-catenin and spectrin profiles without affecting the level of calpain I.
Dose-dependent Effect of Proteasomal Inhibitors on Caspase and Calpain Activation
To test if caspases and/or calpain play a role in the tau degradation/truncation when the cells were treated with high concentrations of proteasome inhibitors, we examined levels of β-catenin (a substrate of caspase), spectrin (a substrate of calpain and caspase), caspase 3, calpain, and calpastatin (endogenous calpain inhibitor) by Western blot analysis (Figures 4B, C and E). There was very little difference in the expression level of these proteins in cells treated with 2 nM Epx compared to the vehicle control. In contrast, increased degradation of β-catenin (Figure 4B) and spectrin (Figure 4C) was observed in cells exposed to higher doses of Epx (10 and 50 nM) or 0.5 μM MG132. For instance, the level of intact spectrin (250 kDa) decreased, while that of spectrin degradation products increased. Based on previous studies [39-41], these changes probably reflect degradation of β-catenin by caspases and degradation of spectrin by calpains and/or caspases. This was supported by subsequent immunoblotting data in which cleaved-caspase 3 (active form) was detectable in the cells treated with Epx at 10 nM or higher concentration (Figure 4D), suggesting apoptotic activity. It is also consistent with the results from previous studies in which exposure of neurons or non-neuronal cells to a proteasomal inhibitor led to apoptosis [42-46].
Calpain immunoblotting showed no difference between the vehicle control and the Epx (50 nM)- or MG132 (0.5 μM)-treated cells (Figure 4E). However, the drug-treated clearly displayed less calpastatin immunoreactivity (Figure 4E). Since calpastatin is a substrate of caspase-3, the degradation of calpastatin may have been caused by caspase-3 activation [47]. Given that calpastatin is an endogenous inhibitor of calpain, the reduction in calpastatin indicates that calpain is likely activated in the treated cells. Together, these results suggest that tau degradation from the exposure to proteasome inhibitors (i.e. > 10 nM Epx) involves activation of calpain and/or caspases.
Caspase Activation and tau Degradation
To determine whether caspase activation plays a role in tau degradation caused by proteasome inhibition, we treated the M1C cultures to 50 nM Epx in the absence or presence of the pan-caspase inhibitor, Z-VAD-FMK. Cultures exposed to Z-VAD-FMK and Epx contained more intact tau (i.e. >52-kDa tau immunopositive for P44 and Tau46) than those treated with Epx alone (Figure 5A). Pretreatment with the caspase inhibitor also caused a reduction in the amount of cleaved-caspase 3 (Figure 5B), supporting the involvement of caspase in tau cleavage.
Figure 5.
Caspase-mediated tau degradation from proteasome inhibition. M1C cultures were induced to express tau via TetOff induction for 5 days and treated (1) with vehicle (DMSO), (2) with 50 nM Epx during the last day of induction, or (3) treated with a pan-caspase inhibitor Z-VAD-FMK (20 μM), 3 hrs before Epx (50 nM) incubation. Cell lysates were probed with (A) antibodies P44, Tau46 and (B) antibodies to cleaved caspase-3. As was illustrated in Figure 2, P44 detected in the 50 nM Epx-treated cells mostly truncated tau species that lack Tau46 immunoreactivity (lanes 2). Such loss of Tau46 immunoreactivity was, however, absent in the Exp-treated cells that were pretreated with Z-VAD-FMK (lane 3). The level of cleaved caspase-3 was higher in the Exp-treated than its vehicle control, and such change was prevented by caspase inhibition (B, compare lanes 2 and 3).
Differential Impacts of Proteasome Inhibition on Accumulation of Phosphorylated and Non-phosphorylated tau
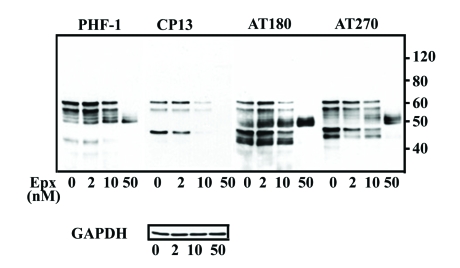
Previous studies have reported that phosphorylated tau is more resistant than its non-phosphorylated counterpart to proteolytic degradation [23], and that distinct chaperons may be involved in degrading tau phosphorylated at different sites [31]. To study the impact of proteasome inhibition on phosphorylated tau, we examined levels of phosphorylated tau in the lysates obtained from M1C cell following Epx treatment using antibodies PHF-1, CP13, AT180 and AT270 which recognize distinct phosphorylated epitopes (Figure 6). When compared to the vehicle control, the level of PHF-1 or AT180 immunoreactivity in the 2 nM Epx-treated cells increased consistently. In contrast, AT270 immunoreactivity tended to decrease and CP13 immunoreactivity was not consistently affected, suggesting that tau phosphorylated at some sites are more resistant than others to proteasomal degradation. Treatment with 10 nM Epx reduced the amount of phosphorylated 62 kDa tau, and phosphorylated tau decreased further when exposed to Epx at even higher concentrations.
Figure 6.
Impact of proteasome inhibition on processing of phosphorylated tau. Lysates of M1C cultures, with or without proteasome inhibition, were immunoblotted using phospho-tau antibodies to 6 epitopes (see Figure 1) collectively, and reprobed subsequently using GAPDH to verify sample loading. In the cultures treated with 2 nM Epx, antibodies PHF-1, and AT180 detected the 62-kDa with its intensity higher than that of the vehicle control, whereas A270 immunoreactivity appeared reduced and CP13 immunoreactivity was affected inconsistently. Fewer phospho-tau bands were present in the samples treated with 50 nM Epx, and most of them were around 50 kDa.
To determine if proteasomal inhibition preferentially causes accumulation of intact tau species that are less or not phosphorylated, we compared the relative amount of Tau46-immunoreactive 62, 54 and 52 kDa (Figure 1) tau in cultures treated with 2 nM Epx and their vehicle control. The ratio of 62, 54 and 52 kDa tau species in the vehicle control is 1:0.2:0.7 whereas that in the 2 nM Epx-treated is 1:1.9: 2.7. Such increase in the proportion of 52 and 54 kDa tau probably reflects accumulation of the less phosphorylated tau species, since electrophoretic mobility of tau is known to be retarded by phosphorylation. Our results are in agreement with previous reports in which phosphorylated tau was shown to be less susceptible to proteolysis [23].
Proteasomal Inhibition-induced tau Oligomerization and Aggregate Assembly
We showed previously that a 5-7 day TetOff induction at 1 ng/ml Tet leads to formation of high-molecular-weight aggregates via disulfide cross-linking of C-end truncated tau species, including 70-kDa tau oligomers and a small quantity of sarkosyl-insoluble tau in M1C cells [33, 35]. We also showed that increased production of 70 kDa tau oligomer is associated with increased accumulation of high-molecular-weight tau [33, 35]. However, it is uncertain whether formation of tau aggregates involves incorporation of caspase-cleaved tau fragments. We examined this issue by Western blot analysis using lysates (see Figure 7) from cultures with and without exposure to Epx and their fractionated preparations (see Figure 8).
Figure 7.
Accumulation of the 70-kDa tau from proteasome inhibition or staurosporine treatment. M1C cells, induced to express tau for 5 days via TetOff induction using 1 ng/ml Tet supplementation were exposed to Epx (50 nM) and staurosporine (100 nM) for 1 day and 12 hours, respectively, before the end of such induction or to DMSO throughout the induction. Cell lysates were probed with antibody P44, Tau 46 and GAPDH. (A) The 70-kDa species (asterisk), not readily discernible in Figure 2, appeared after prolonged exposure. They displayed P44 but not Tau46 immunoreactivity. The amount of the 70-kDa tau did not change in the M1C cells after 2 nM Epx treatment, but increased in those exposed to higher concentrations of Epx or with MG132. (B) Staurosporine treatment (lane 5) caused an increase of the 70-kDa and smaller sizes tau species as that observed in the Epx treated (lane 3). The increase of 70-kDa tau was less in the staurosporine-treated. Cells without TetOff induction were included to show the impact of induction on tau expression (lane 2) and recombinant 4R0N tau was included as a reference (lane 1).
Figure 8.
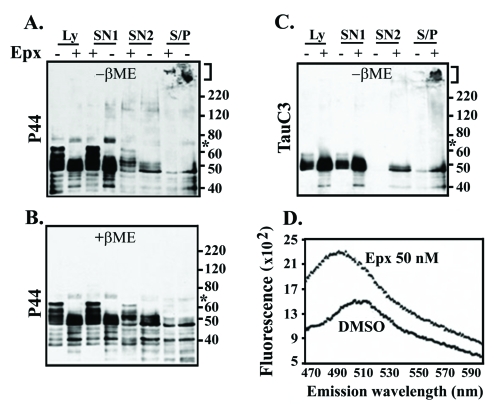
Epoxomicin exposure caused accumulation of sarkosyl-insoluble tau aggregates and increase in thioflavin binding. Lysates (Ly) from M1C cells with or without 50 nM Exp treatment were fractionated to derive SN1, SN2 and S/P fractions. A portion of each sample was analyzed by Western blotting in the presence or absence of β-mercaptoethanol (βME) using antibodies P44 (A and B), Tau C3 (C) and Tau46 (data not shown). (D) Thioflavin-S binding assay of the S/P preparations derived from Exp- or DMSO-treated cells. The 70-kDa tau (asterisk) did not react with Tau C3 or Tau 46. High-molecular-weight tau aggregates (bracket) were evident in the S/P fraction. The level of such aggregates was higher in the Epx-treated. Moreover, the presence of Tau C3-positive tau aggregates in the S/P fraction distinguishes the Epx-treated samples from their vehicle control. Samples processed under the reducing (+βME) condition lacked high-molecular-weight tau aggregates and exhibited less tau oligomers of 70∼220-kDa, when compared to their non-reduced counterparts. Enhanced thioflavin-S binding was evident in the Epx-treated but not in its vehicle control (D).
As expected from previous studies [33, 35], the 70-kDa tau was detectable in small amount in vehicle treated cultures. The 70-kDa tau was immunopositive for P44 but not Tau46, and its detection required long exposure time for image capturing or increased sample loading (asterisks, Figure 7A is derived from Figure 2 after extended development). The amount of the 70-kDa tau was comparable between M1C cells treated with vehicle and those treated with 2 nM Epx (Figure 7A), but increased in the cells treated with higher concentrations of Epx. Since increased levels of cleaved-caspase were observed in cultures treated with >10 nM Epx (Figure 4D), this raised the possibility that truncated-tau derived from caspase cleavage may contribute to the assembly of the 70-kDa species. We tested this possibility by treating the M1C cultures with staurosporine, an inducer of apoptosis (lane 5, Figure 7B). Although the staurosporine-treated cultures contained more abundant 70-kDa tau than the vehicle control (lane 4, Figure 7B) and they displayed a tau profile resembling that of 50 nM Epx-treated cells (lane 3, Figure 7B), the 70-kDa tau was not recognized by Tau C3, an antibody specific for tau cleaved by caspase at D421(data not shown).
To study the impact of proteasome inhibition on accumulation of aggregated tau, we used M1C cultures treated with 50 nM Epx (Figure 8), since such samples showed increased level of 70 kDa tau oligomers. Lysates derived from these cells were separated into three fractions (SN1, SN2, S/P) based on their solubility in buffer or sarkosyl. Fractions from the vehicle- or Epx-treated cells were analyzed under non-reducing (−βME) or reducing (+βME) conditions and compared for their reactivity to antibodies P44 (Figure 8A and B) and Tau C3 (Figure 8B). Under non-reducing condition, more P44- and Tau C3-positive sarkosyl-insoluble (S/P) tau appeared in samples from the Epx-treated cultures than those from vehicle controls. Some of the insoluble tau species were too large to be separated by SDS-polyacrylamide gel (bracket, Figure 8). These tau aggregates and oligomeric tau species, except for the 70-kDa tau, were not observed under reducing condition (Figure 8B, +βME, P44). The 70-kDa tau was only partially sensitive to βME. The results are in agreement with previous report indicating the involvement of disulfide cross-link in oligomeric tau production [33, 35]. It is worth noting that high-molecular-weight insoluble aggregates display Tau C3 immunoreactivity, whereas the 70-kDa oligomeric tau does not (Figure 8C). The increase of the 70-kDa species as a consequence of proteasome inhibition, therefore, is likely due to the assembly tau fragments that were cleaved by proteases other than caspases. One such candidate protease is calpain I, since the cultures treated with 50 nM Epx or 0.5 μM MG132 showed calpain activation (Figure 4). Our data, however, do not rule out the possibility that caspase-cleaved tau may be degraded further by calpains or other proteases to generate fragments from which the 70-kDa oligomer is assembled.
The possibility that sarkosyl-insoluble samples contain tau with altered conformation is substantiated by their augmented thioflavin-binding, which reflects transformation to β-sheet structure. Such enhanced affinity for thioflavin S was evident in the 50 nM Epx-treated compared to their vehicle-treated controls (Figure 8D, thioflavin binding). The integrated fluorescence signals for the vehicle control and the Epx-treated samples are 1085.8 ± 168.7, and 1591.1 ± 96.3 in arbitrary unit, respectively (Student's t test, p<0.05, n=3).
Discussion
There is ample evidence indicating the ubiquitin-proteasome system is compromised in AD brains. However, it remains uncertain whether such abnormality causes accumulation of monomeric tau leading to its assembly to form aggregates or is a result of interactions between aggregated tau and proteasomes. While some cell-based studies showed that proteasomal inhibition decreases turnover of wild-type tau, others showed that it accelerated tau turnover [21, 22, 24-26]. The discrepancy could be due to variations in the type proteasome inhibitor and/or its concentration used in different studies. Moreover, these studies did not investigate whether proteasomal perturbation leads to production/accumulation of tau aggregates.
In the present studies, we treated M1C cells that produces wild-type human tau with different concentrations of proteasome inhibitor Epx and analyzed the tau profile of cell lysates. We demonstrated that exposure to 2, 10 or 50 nM Epx results in a decrease in chymotrypsin-like activity in a dose-dependent manner, ranging from 30 to 70% reduction. We also demonstrated that tau degradation in M1C cells was inhibited by the 2 nM Epx treatment and accelerated by higher dosages of Epx. Such enhanced tau degradation was accompanied with activation of caspase-3 and decrease of calpain I inhibitor (i.e. calpastatin), and this was prevented by pretreatment with a pan caspase inhibitor. In addition, Tau C3-positive tau fragments were demonstrated in the Epx (50 nM)- or staurosporine-treated cells, but not in those pretreated for caspase inhibition. The tau degradation from Exp treatment, therefore, is likely to involve caspase-3. It may also involve calpain I as suggested by the decrease of its endogenous inhibitor calpastatin. Lysosomal proteases appears to play very little role in such tau degradation, since we have previously shown that exposing the M1C cells to chloroquine results in a substantial accumulation of full-length tau [35]. It is worth noting that the level of 70 kDa tau oligomers was not affected by 2 nM Exp treatment as was demonstrated in cultures treated with chloroquine [35].
The 70-kDa tau appeared with higher abundance in cultures exposed to 50 nM Exp than that of those treated with lower doses of Epx or their vehicle control. Our analysis of fractionated preparations showed more sarkosyl-insoluble tau in the 50 nM Exp-treated cultures than that of the vehicle control or those treated with 2 nM Exp (data not shown). The results are consistent with our previous studies in which assembly of high-molecular-weight, soluble tau oligomers and insoluble tau aggregates was shown to associate with production of 70 kDa tau [33, 35].
We have demonstrated in the present and previous studies that the 70-kDa protein is partially resistant to reducing agent β-mercaptoethanol and it contains carboxyl-terminally cleaved tau [33, 35]. Although the cleavage site(s) remains to be determined, it is evident from Tau C3 immunoblotting that caspase-cleaved tau fragments are absent in the 70-kDa protein. Tau C3-positive tau fragments, detected in the vehicle control, are present in the sarkosyl-insoluble, high-molecular-weight aggregates produced by the Exp-treated cells. This may reflect co-assembly of caspase-cleaved fragments with the 70-kDa tau or other oligomers. Alternatively, some tau aggregates may be produced from self-assembly of Tau C3-positive fragments. In this regard, previous immunocytochemical studies revealed that caspase-cleaved tau is present in AD brains and that Tau C3 labels only a subset of neurofibrillary tangles [48-50]. Moreover, a tau antibody to a conformation-dependent epitope was demonstrated to label neurofibrillary tangles differently from what conformation-independent antibodies did [49]. These studies led to a model proposed for the progression of tangle formation that predicts incorporation of Tau C3-positive fragments subsequent to conformational change of tau to acquire Alz50 epitope.
Our finding of increased amount of Tau C3-positive sarkosyl-insoluble, thioflavin positive tau in the cultures exhibiting marked proteasome inhibition is consistent with the view that proteasomal dysfunction can contribute to the assembly and accumulation of tau proteins in tauopathies, and that tau assembly can be enhanced by its truncation at the carboxyl-terminus. It has been reported that proteasomes prepared from AD brains display significantly lower level of chymotrypsin-like activity when compared to age- and postmortem-matched controls or subjects with mild cognitive impairment (MCI) [51]. On average, the chymotrypsin-like activity of AD samples is about 28% less than that of normal controls or MCI. It is worth noting that the extent of reduction found in AD preparations is comparable to that demonstrated in the M1C cultures treated with 2 nM Epx, which is about 30 % less than their vehicle control. The results of our cell-based studies, therefore, suggest that a moderate decrease in chymotrypsin activity in AD brain may impact the accumulation of intact tau more than that of fibrillogenic tau fragments. Since most studies of human brain proteasomes used subjects at the end-stages of AD, it remains unknown whether the disease progresses with a gradual decline in proteasomal activity and whether the decline is much more severe in some individuals than others. Regardless, our cell-based studies support the possibility that accumulation of tau aggregate in tauopathies can be initiated by accumulation of carboxyl-terminally truncated tau from severe dysfunction of proteasomes or by accumulation of full-length as a consequence of impaired autophagic-lysosomal pathway.
In summary, our studies demonstrate that exposures of M1C cultures to proteasome inhibitors can lead to accumulation or degradation/truncation of tau, depending on the concentration of inhibitors. It is likely that enhanced tau degradation/truncation results from caspase and calpain activation, and contributes to the augmented assembly of oligomers and aggregates containing tau cleaved at D421.
Acknowledgments
We are grateful to Drs. L.I. Binder (Northwestern University, Chicago, IL), and V. M. Lee (University of Pennsylvania, Philadelphia, PA) for their generous gift of antibodies Tau C3 and Tau46, respectively. This study was supported by the NIH grant AG17216 NIH and NS 48052, and Mayo foundation.
References
- 1.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Ann Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 2.Hutton M, Lewis J, Dickson D, Yen SH, McGowan E. Tauopathies with transgenic mice. Trends Mol Med. 2001;7:467–470. doi: 10.1016/s1471-4914(01)02123-2. [DOI] [PubMed] [Google Scholar]
- 3.Friedhoff P, von Bergen M, Mandelkow EM, Mandelkow E. Structure of tau protein and assembly into paired helical filaments. Biochim Biophys Acta. 2000;1502:122–132. doi: 10.1016/s0925-4439(00)00038-7. [DOI] [PubMed] [Google Scholar]
- 4.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DM, Binder LI. Free fatty acids stimulate the polymerization of tau and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer's disease. Am J Pathol. 1997;150:2181–2195. [PMC free article] [PubMed] [Google Scholar]
- 6.Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, Yen SH. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett. 1999;447:195–199. doi: 10.1016/s0014-5793(99)00294-x. [DOI] [PubMed] [Google Scholar]
- 7.Abraha, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer's disease. J Cell Sci. 2000;113:3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Kuret J. C-terminal truncation modulates both nucleation and extension phases of tau fibrillization. FEBS Lett. 2006;6580:211–215. doi: 10.1016/j.febslet.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 9.Billinglsey ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein; effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;3234:577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer's disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 11.Lam YA, Pickart CM, Alban A, Landon M, Jamieson C, Ramage R, Mayer RJ, Layfield R. Inhibition of the ubiquitin-proteasome system in Alzheimer's disease. Proc Natl Acad Sci USA. 2000;97:9902–9906. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 13.Fasulo L, Ugolini G, Visintin M, Bradbury A, Brancolini C, Verzillo V, Novak M, Cattaneo A. The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J Neurochem. 2000;75:624–633. doi: 10.1046/j.1471-4159.2000.0750624.x. [DOI] [PubMed] [Google Scholar]
- 14.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson GV, Jope RS, Binder LI. Proteolysis of tau by calpain. Biochem Biophys Res Com. 1989;163:1505–1511. doi: 10.1016/0006-291x(89)91150-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang LS, Gordon-Krajcer W, Ksiezak-Reding H. Tau released from paired helical filaments with formic acid or guanidine is susceptible to calpain-mediated proteolysis. J Neurochem. 1997;69:1548–1558. doi: 10.1046/j.1471-4159.1997.69041548.x. [DOI] [PubMed] [Google Scholar]
- 17.Yen S, Easson C, Nacharaju P, Hutton M, Yen SH. FTDP-17 tau mutations decrease the susceptibility of tau to calpain I digestion. FEBS Lett. 1999;461:91–95. doi: 10.1016/s0014-5793(99)01427-1. [DOI] [PubMed] [Google Scholar]
- 18.Bednarski E, Lynch G. Cytosolic proteolysis of tau bycathepsin D in hippocampus following suppression of cathepsin B and L. J Neurochem. 1996;67:1846–1855. doi: 10.1046/j.1471-4159.1996.67051846.x. [DOI] [PubMed] [Google Scholar]
- 19.Kenessey A, Nacharaju P, Ko L, Yen SH. Degradation of tau by lysosomal enzyme cathepsin D: implication for Alzheimer neurofibrillary degeneration. J Neurochem. 1997;69:2026–2038. doi: 10.1046/j.1471-4159.1997.69052026.x. [DOI] [PubMed] [Google Scholar]
- 20.Bi X, Haque TS, Zhou J, Skillman AG, Lin B, Lee CE, Kuntz ID, Ellman JA, Lynch G. Novel cathepsin D inhibitors block the formation of hyperphosphorylated tau fragments in hippocampus. J Neurochem. 2000;74:1469–1477. doi: 10.1046/j.1471-4159.2000.0741469.x. [DOI] [PubMed] [Google Scholar]
- 21.David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG. Proteasomal degradation of tau protein. J Neurochem. 2002;83:176–185. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 22.Cardozo C, Michaud C. Proteasome-mediated degradation of tau proteins occurs independently of the chymotrypsin-like activity by a non-processive pathway. Arch Biochem Biophys. 2002;408:103–110. doi: 10.1016/s0003-9861(02)00493-9. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GV. Tau phosphorylation and proteolysis: insights and perspectives. J Alzheimers Dis. 2006;9:243–250. doi: 10.3233/jad-2006-9s326. [DOI] [PubMed] [Google Scholar]
- 24.Feuillette S, Blard O, Lecourtois M, Frebourg T, Campion D, Dumanchin C. Tau is not normally degraded by the proteasome. J Neurosci Res. 2005;80:400–405. doi: 10.1002/jnr.20414. [DOI] [PubMed] [Google Scholar]
- 25.Delobel P, Leroy O, Hamdane M, Sambo AV, Delacourte A, Buee L. Proteasome inhibition and Tau proteolysis: an unexpected regulation. FEBS Lett. 2005;579:1–5. doi: 10.1016/S0014-5793(05)01501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown MR, Bondada V, Keller JN, Thorpe J, Geddes JW. Proteasome or calpain inhibition does not alter cellular tau levels in neuroblastoma cells or primary neurons. J Alzheimers Dis. 2005;7:15–24. doi: 10.3233/jad-2005-7103. [DOI] [PubMed] [Google Scholar]
- 27.Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem. 2004;279:4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YJ, Xu YF, Liu XH, Li D, Yin J, Liu YH, Chen XQ, Wang JZ. Carboxyl terminus of heat-shock cognate 70-interacting protein degrades tau regardless its phosphorylation status without affecting the spatial memory of the rats. J Neural Transmission. 2008;115:483–491. doi: 10.1007/s00702-007-0857-7. [DOI] [PubMed] [Google Scholar]
- 29.Dickey CA, Yue M, Lin WL, Dickson DW, Dunmore JH, Lee WC, Zehr C, West G, Cao S, Clark AM, Caldwell GA, Caldwell KA, Eckman C, Patterson C, Hutton M, Petrucelli L. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J Neurosci. 2006;26:6985–6996. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, Takahashi R, Murata S, Tanaka K, Takashima A. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 31.Dickey CA, Dunmore J, Lu B, Wang JW, Lee WC, Kamal A, Burrows F, Eckman C, Hutton M, Petrucelli L. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. FASEBJ. 2006;20:753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- 32.Keck S, Nitsch R, Grune T, Ullrich O. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. J Neurochem. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 33.Ko L, Rush T, Sahara N, Kersh JS, Easson C, DeTure M, Lin WL, Connor YD, Yen SH. Assembly of filamentous tau aggregates in human neuronal cells. J Alzheimers Dis. 2004;6:605–622. doi: 10.3233/jad-2004-6605. [DOI] [PubMed] [Google Scholar]
- 34.Ko L, DeTure M, Sahara N, Chihab R, Vega IE, Yen SH. Recent advances in experimental modeling of the assembly of tau filaments. Biochim Biophys Acta. 2005;1739:125–139. doi: 10.1016/j.bbadis.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 36.Sahara N, Lewis J, DeTure M, McGowan E, Dickson DW, Hutton M, Yen SH. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–1508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- 37.DeTure M, Ko L, Easson C, Yen SH. Tau assembly in inducible transfectants expressing wild-type or FTDP-17 tau. Am J Pathol. 2002;161:1711–1722. doi: 10.1016/S0002-9440(10)64448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gendron TF, McCartney S, Causevic E, Ko L, Yen SH. Ethanol enhances tau accumulation in neuroblastoma cells that inducibly express tau. Neurosci Lett. 2008;443:67–71. doi: 10.1016/j.neulet.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brancolini C, Lazarevic D, Rodriguez J, Schneider C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J Cell Biol. 1997;139:759–771. doi: 10.1083/jcb.139.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanags DM, Pörn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 41.Pike BR, Flint J, Dutta S, Johnson E, Wang KK, Hayes RL. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 42.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 43.Lang-Rollin I, Vekrellis K, Wang Q, Rideout HJ, Stefanis L. Application of proteasomal inhibitors to mouse sympathetic neurons activates the intrinsic apoptotic pathway. J Neurochem. 2004;90:1511–1544. doi: 10.1111/j.1471-4159.2004.02684.x. [DOI] [PubMed] [Google Scholar]
- 44.Suh J, Lee YA, Gwag BJ. Induction and attenuation of neuronal apoptosis by proteasome inhibitors in murine cortical cell cultures. J Neurochem. 2005;95:684–694. doi: 10.1111/j.1471-4159.2005.03393.x. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Gulyas M, Hjerpe A, Dobra K. Proteasome inhibitor PSI induces apoptosis in human mesothelioma cells. Cancer Lett. 2006;232:161–169. doi: 10.1016/j.canlet.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Anan A, Baskin-Bey ES, Bronk SF, Werneburg NW, Shah VH, Gores GJ. Proteasome inhibition induces hepatic stellate cell apoptosis. Hepatology. 2006;43:335–344. doi: 10.1002/hep.21036. [DOI] [PubMed] [Google Scholar]
- 47.Rami A, Volkmann T, Agarwal R, Schooninger S, Nurnberger F, Saido TC, Winckler J. β2-adrenergic receptor responsivenss of the calpain-calpastatin system and attenuation of neuronal death in rat hippocampus after transient global ischemia. Neurosci Res. 2003;47:373–383. doi: 10.1016/j.neures.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer's disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Neurobiol Aging. 2005;26:1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer's disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 51.Cecarini V, Ding Q, Keller JN. Oxidative inactivation of the proteasome in Alzheimer's disease. Free Radical Res. 2007;41:673–680. doi: 10.1080/10715760701286159. [DOI] [PubMed] [Google Scholar]