Abstract
It has been over one hundred years since the first reported case of dyskeratosis congenita (DC) and over twenty since the discovery of telomerase, an enzyme that adds telomeric DNA repeats to chromosome ends. Emerging evidence suggests that telomere dysfunction plays an important role in the pathogenesis of DC and other human disorders involving tissues that require rapid repair and renewal capacities. Yet we still do not fully understand how mutations in telomere maintenance genes contribute to disease development in affected individuals. In this review, we provide an up-to-date summary of the topic by discussing the results from genetic screens of patients, in vitro mutational analysis of involved molecules, and genetically engineered mouse models. While these data shed important light on the mechanisms underlying disease development, further investigation, particularly in an in vivo setting, is needed.
Keywords: Telomere, telomerase, aplastic anemia, dyskeratosis congenita, idiopathic pulmonaryfibrosis
Introduction
Human Telomerase
Telomerase is a ribonucleoprotein complex whose main function is to add six nucleotide repeats onto the ends of chromosomes utilizing its reverse transcriptase (hTERT) and its intrinsic RNA template (hTERC), as well as the associated proteins dyskerin, NOP10, NHP2, and GAR1 (Figure 1). This DNA elongation is necessary to overcome the “end-replication problem” whereby the conventional DNA polymerases cannot fully replicate linear chromosomes [1, 2]. This phenomenon, coupled with oxidative damage, and other exogenous or endogenous effects, causes our telomeres to be shortened by approximately 50-100bp per cell division. Telomere erosion limits the replicative capacity of the majority of somatic cells which do not express active telomerase [3, 4]. Cells whose telomeres shorten to a “critical length” enter a stage termed replicative senescence whereby cell division is prevented [5, 6]. Stem cells, germ cells, and certain types of somatic cells circumvent this barrier by expressing the telomerase enzyme, allowing them to maintain their telomere length and escape senescence.
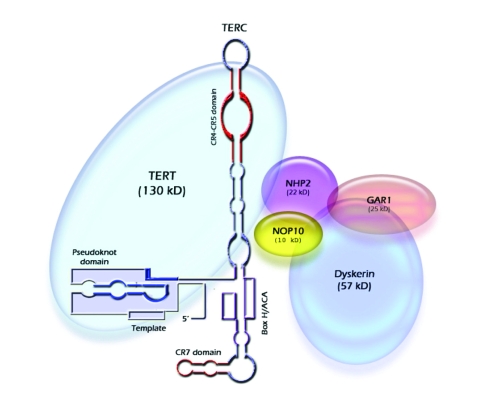
Figure 1.
Telomerase holoenzyme. Simplified illustration of the telomerase holoenzyme showing its main components: hTERT, hTERC, Dyskerin, NOP10, NHP2, and GAR1. Functional regions of the hTERC RNA (template, pseudoknot, CR4-CR5, Box H/ACA, and CR7) are indicated.
The hTERT has been extensively studied and hence several of its functional domains have been mapped [7]. The protein is defined by the catalytic domain, which contains seven reverse transcriptase motifs essential for enzymatic activity. The C terminus is short and highly divergent among different species, and its exact function is not completely clear at this point. However, one region has clearly emerged, termed the C-DAT for the C-terminal region that dissociates the activities of telomerase. Mutations in this domain generate enzymes which are catalytically active in vitro but biologically inert. In contrast, the N-terminus contains several evolutionarily conserved regions important for hTERT's cellular localization, RNA interaction, protein-protein multimerization, and enzymatic function. Functionally important regions have also been defined in the hTERC (Figure 1) [8]. Most obviously, the template region is absolutely required for the hTERT protein to reverse transcribe it into telomeric DNA repeats. In addition, the pseudoknot domain is required for telomerase activity, hTERT binding, and hTERC RNA dimerization, while the Box H/ACA domain is important for hTERC RNA processing and stability. Despite extensive work to map the aforementioned motifs, it is still relatively unclear which particular residues are absolutely required for the activity of either of the telomerase core components.
Telomere Structure and the Shelterin Complex
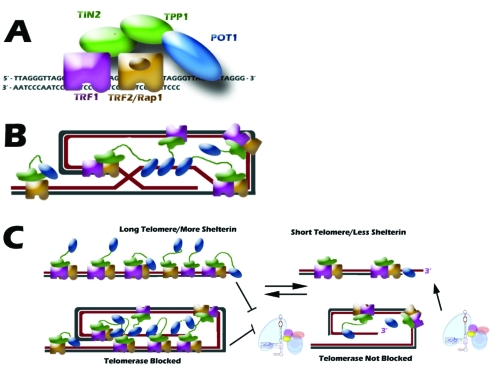
The tips of chromosome ends consist of a long strand overhang composed of the G-rich strand (TTAGGG; as opposed to the C-rich strand CCCTAA). In order to avoid being recognized as a double-strand break and corrected by DNA repair machineries, a fate quite detrimental to the cell, the single-stranded region folds back upon itself and tucks into the adjacent double-stranded telomeric region, forming a telomeric loop (t-loop) (Figure 2B; [9]). This structure is formed and protected by a collection of six proteins, termed the shelterin complex, which with the telomeric DNA repeats compose the entire nucleoprotein structure commonly referred to as telomeres. The shelterin complex is formed by the double-stranded DNA binding proteins, TRF1 and TRF2; a binding partner of TRF2, RAP1; a single-stranded DNA binding protein POT1; and the two bridging proteins, TIN2 and TPP1 (Figure 2A; [9]). Not only do these proteins function in protecting the chromosome end, they also function in telomere length regulation. Telomere length is maintained within a strict range throughout cell division, suggesting a negative feedback loop involving the shelterin complex. Due to the exquisite specificity of these DNA binding proteins, the amount of shelterin protein bound to telomeres is roughly proportional to their length (Figure 2C; [9]). Thus, a long telomere would have a greater ability to inhibit telomerase activity, while a short telomere, with less bound protein, would be more accessible to telomerase elongation.
Figure 2.
Shelterin Complex. A. Six components of the telomere-binding complex, shelterin, and their DNA and protein binding abilities. B. A schematic of the shelterin complex bound to the telomere t-loop structure. C. A proposed model for how shelterin can function to control telomere length in cis. Long telomeres allow for more shelterin binding, which can block access of telomerase to the telomere end. In contrast, a short telomere with less shelterin bound is preferentially elongated by telomerase.
Telomerase/Telomere and Human Diseases
Bone marrow failure syndromes (BMFS) represent a diverse group of diseases with similar presentations, including dyskeratosis congenita, aplastic anemia, myelodysplatic syndromes (MDS), and others [10]. Overlapping symptoms and lack of concrete disease characterization make early diagnosis extremely difficult, especially when the few definitive phenotypes do not usually manifest until later in the disease progression. The prognosis for affected individuals can be bleak as the most prominent treatment is bone marrow transplant and frequently matching bone marrow donors are difficult to find. The fact that patients with BMFS have shortened telomeres led us and other researchers to screen these patients for mutations in telomerase and some protein components of the shelterin complex. These efforts yielded mutations in hTERT (Figure 3; [11-24]), hTERC (Figure 4; [20, 22, 25-43]), the telomerase-associated proteins dyskerin [44-57], NOP10 [58], and NHP2 [59], and the shelterin components TRF1 [60], TRF2 [60], and TIN2 (Figure 5; [61-64]). These findings support the hypothesis that dysfunctional telomeres due to mutations in telomere maintenance genes lead to exhaustion of the stem cell compartment and hence to various defects in cell types with a high turnover rate such as the hematopoietic system. In addition to hematopoietic malignancies, mutations in these components can also contribute to idiopathic pulmonary, liver, and heart fibroses [12-14, 19, 41]. Why mutations in the same proteins can be found in diseases with similar yet different phenotypes is unclear. It is likely that other factors (exogenous and/or endogenous) might be involved in the pathogenesis of these diseases, but the influence of telomere length regulation on cell proliferation cannot be discounted. Thus, a more thorough study of these molecules, their functions, and their regulation is necessary in order to fully understand them and to possibly allow more targeted therapies for these ailments.
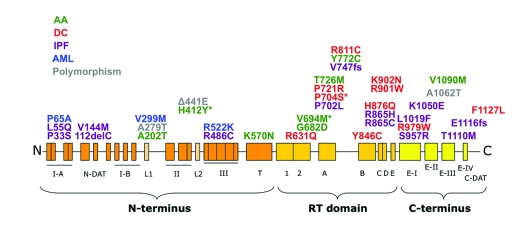
Figure 3.
Natural hTERT mutations. All exonic sequence changes identified in patients and/or controls are shown. Mutations are color-coded according to the disease in which they were first identified. Those denoted with an asterisk (*) have been found in multiple telomere dysfunction disorders (H412Y: AA, AML; V694M: AA, IPF; P704S: DC, IPF). AA: aplastic anemia; DC: dyskeratosis congenita; IPF: idiopathic pulmonary fibrosis; AML: acute myeloid leukemia.
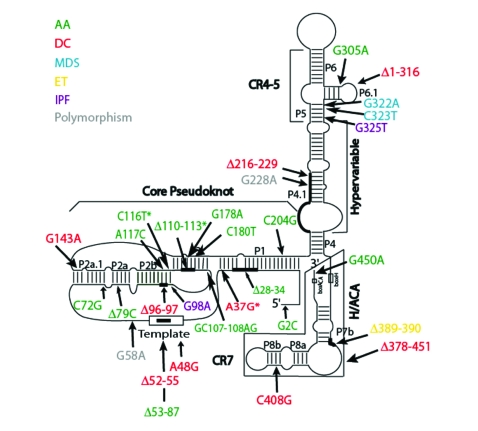
Figure 4.
Natural hTERC mutations. All naturally-occurring sequence changes in the hTERC coding region are shown. Mutations are color-coded according to the disease in which they were first identified. Those denoted with an asterisk (*) have been found in multiple telomere dysfunction disorders (A37G: DC, IPF; Δ110-113: AA, MDS; C116T: AA, MDS). AA: aplastic anemia; DC: dyskeratosis congenita; MDS: myelodysplastic syndromes; ET: essential thrombocythemia; IPF: idiopathic pulmonary fibrosis.
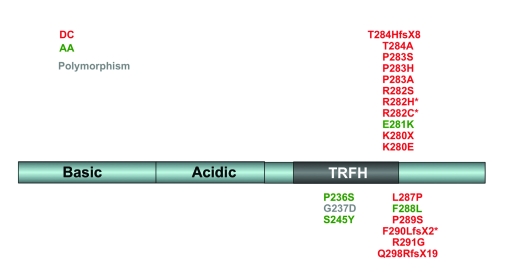
Figure 5.
Natural TIN2 mutations. All known sequence changes in the TIN2 coding region are shown. Acidic and basic domains are denoted based on the amino acid content in these regions. The TRFH domain is the general region that has been shown to interact with TRF1. Mutations are color-coded according to the disease in which they were first identified. Those denoted with an asterisk (*) have been found in multiple telomere dysfunction disorders (R282C: DC, AA; R282H: DC, AA; F290LfsX2: DC, AA). DC: dyskeratosis congenita; AA: aplastic anemia.
Telomerase Mutations in Human Blood Disorders
Dyskeratosis Congenita
Dyskeratosis congenital (DC) is a rare inherited disorder characterized by a triad of clinical symptoms: mucosal leukoplakia, nail dystrophy, and abnormal skin pigmentation [65]. The majority of the cases (>80%) occur in children, who experience BMFS and the aforementioned physical anomalies generally by the age of 10. Other symptoms indicative of premature aging, including pulmonary diseases, dental abnormalities, esophagostenosis, and alopecia, are often associated with the disease in 15-25% of the cases. Solid tumors of the gastrointestinal tract, nasopharynx and skin, and hematopoietic malignancies (e.g., MDS, Hodgkin lymphoma and acute myelogenous leukemias) have also been observed in some DC patients [66]. Since this disease affects rapidly renewing tissues, it has been speculated that DC is a telomerase disease in all three different patterns of inheritance: X-linked recessive, autosomal dominant, and autosomal recessive. In support of this theory, most DC patients have short telomeres [67, 68] and carry mutations in the three main components of the telomerase holoenzyme complex: dyskerin, hTERT (protein), and hTERC (RNA) [69].
The X-linked form of DC is the most severe and is caused by mutations in the DKC1 gene on chromosome Xq28. DKC1 encodes dyskerin, a 514 amino acid, nucleolar protein in the H/ACA family, which is highly conserved throughout evolution. As is the case with other H/ACA proteins, dyskerin is predicted to function in ribosomal RNA processing, in addition to its predicted role in the biogenesis of the telomerase holoenzyme. Most DKC1 mutations are missense mutations and include a 3' deletion, suggesting that both frameshift and null mutations are incompatible with life [44-47, 49-53, 55, 57]. Indeed, a DKC1-null mouse model is embryonically lethal [70]. In humans, one mutation (A353V) is seen quite frequently in both X-linked DC and a more severe form of the disease, the Hoyeraal-Hreidarsson (HH) syndrome (see below), and accounts for approximately 30% of all X-linked DC cases. A number of intronic mutations have also been found, in addition to a promoter mutation (−141C→G), which destroys an Sp1 binding site. Female carriers of DKC1 mutations show skewed X inactivation patterns due to the fact that cells expressing the normal allele have an inherent growth advantage. Yet, it remains to be determined the extent to which each of dyskerin's functions (rRNA processing or telomere maintenance) contributes to the X-linked DC phenotype.
Now considered a more severe allelic form of X-linked DC, the Hoyeraal-Hreidarsson (HH) syndrome is a multisystemic disorder characterized by mental retardation, microcephaly, intrauterine growth retardation, and aplastic anemia [71]. More recently, progressive combined immune deficiency has come to be regarded as another common symptom in this disease [72]. Missense mutations in dyskerin found in HH families segregate with the disease [48, 54, 56]. However, as of yet, there is no explanation for why different mutations in the same protein can cause such diverse phenotypes. The situation is further complicated by the identification of a female HH patient from a consanguineous marriage of asymptomatic parents who carries a homozygous mutation in hTERT [17]. However, it is quite possible that is in fact a very severe case of autosomal dominant DC due to the inheritance of moderately shortened telomeres from both parents.
The autosomal dominant form of DC (AD-DC) is much less severe and less common than the X-linked form. Like aplastic anemia (see below), mutations in hTERT and hTERC, as well as the telomere binding protein TIN2 have been associated with AD-DC (Figures 3, 4, and 5). As the vast majority of these mutations are heterozygous, it is possible that they could function as either haploinsufficient, with a single copy of the normal telomerase component being insufficient to maintain telomere length, or dominant negative, with the mutant telomerase component negatively affecting the wild-type copy. To determine which of these is the case, researchers transfect cells with both a wild-type and a mutant copy of the given telomerase component and perform the telomere-repeats amplification assay (or TRAP) in order to detect for the effect of the mutated copy on normal telomerase enzymatic function. If the mechanism is haploinsufficiency, the TRAP activity of the doubly-transfected cells will either only be slightly reduced or be the same as the activity of cells transfected with a single wild-type copy. In contrast, cells transfected with a wild-type copy and a dominant negative mutant would exhibit a complete abolishment of TRAP activity. While most of the mutations have been found to exert their effect by a haploinsufficiency mechanism, 2 mutations in the RNA component located in the template region (Δ52-55 and A48G) seem to act as dominant-negatives [22]. It remains to be seen, however, whether this is truly the case in vivo. In fact, in a different system recently developed by Errington et al, these mutations do not show the same dominant negative effect [73]. Interestingly, disease anticipation has been observed in families with AD-DC [38], a phenomenon whose mechanism has thus far always involved a genetic change, such as the expansion of triplet repeats in severe neurological disorders [74]. In AD-DC families, the genetic lesion does not change, yet the onset of disease features occurs, on average, 20 years earlier in the children than in their parents. Telomere length appears to play a role in this accelerated presentation as telomeres were significantly shorter in the second generation of affected families as compared to normal families. This trend is echoed in TERC knockout mice, where clinical features of telomere shortening and DC do not develop until the fourth generation, with sixth generation mice becoming infertile [75-78].
The causal gene for the autosomal recessive form of DC remains elusive. A recent study by Walne et al aimed at uncovering the genetic basis for the disease concluded that there is no single locus responsible [58]. Nevertheless, a homozygous mutation in the NOP10 protein was found in all 3 affected members of a single family and is predicted to alter protein structure and may affect endogenous hTERC RNA levels as NOP10 is a telomerase-associated protein that is predicted to aid in hTERC processing and assembly. This mutation (R34W) in NOP10 appears to segregate with the disease as unaffected family members are heterozygous and both patients and unaffected carriers do in fact have significantly shorter telomeres than controls. However, this mutation was not identified in any of the other 15 families screened, suggesting that it may be a very rare genetic risk factor of this form of the disease.
Aplastic Anemia
Aplastic anemia (AA) is a rare but serious bone marrow disorder, characterized by hypocellular bone marrow and low blood cell counts [79]. As patient leukocytes have significantly shorter telomeres than age-matched controls, we and other researchers have screened AA patients for mutations in telomerase components. Heterozygous mutations have been found in both the protein and RNA components of telomerase (Figures 3 and 4) as well as the telomere-binding proteins TRF1, TRF2 [60], and TIN2 (Figure 5; [62, 63]). It appears that the AA-associated RNA mutations tend to cluster in the conserved pseudoknot region, which is required for telomerase enzymatic activity and hTERT binding. All hTERC mutations identified in AA patients that have been examined thus far function as haploinsufficient, as opposed to dominant negative, at least in vitro. However, as patients with telomerase mutations present with highly varying symptoms, it remains to be seen if mutations at specific residues can explain the differing degrees of severity or if there are some other genetic or environmental factors at play. It has also been suggested that some cases of AA may be classified as cryptic and atypical form of DC as they develop slowly over time and do not show the characteristic triads of physical anomalies as frequently observed in X-linked cases. Recently, Calado et al identified a mutation in the SBDS gene, the causative gene for another bone marrow failure syndrome, Shwachman-Diamond Syndrome, in some AA patients [80]. The significance of this mutation in AA has yet to be determined.
Myelodysplastic Syndromes
Myelodysplatic syndromes (MDS) encompass a group of diseases caused by abnormal blood-forming cells, such that the bone marrow cannot effectively produce blood cells, resulting in low blood cell counts. MDS is a clonal disease, meaning that the abnormal cell population arises from a single, abnormal cell. As such, some consider MDS a form of cancer, and, in fact, about 30% of MDS cases progress into acute myeloid leukemia (AML). Despite the bone marrow defects, mutations in telomerase components are extremely rare, with no mutations in hTERT or dyskerin having been identified to date. Only 4 isolated hTERC mutations have been reported (Figure 4; [29, 31, 33, 43]), in addition to two promoter region mutations, one of which is located in a putative Sp1 binding site [33, 42]. The significance of these mutations in the disease pathogenesis is unclear, however.
Acute Myeloid Leukemia
Acute myeloid leukemia (AML) is a heterogeneous disorder of hematopoietic progenitor cells, causing abnormal proliferation and differentiation, and can evolve from AA and MDS [81, 82]. In addition, a predisposition to developing cancer, including AML, is a characteristic of DC patients [82]. As genomic instability has been shown to be important for the development of the disease, Calado et al examined three cohorts of AML patients who show no physical signs of DC for sequence variation in the hTERT and hTERC genes [24, 81]. They identified three novel missense mutations in hTERT (Figure 3), and, while the V299M sequence change did not seem to affect telomerase enzymatic activity when tested by the TRAP assay, both the P65A and R522K mutations conferred dramatic defects.
Surprisingly, they also identified three AML patients who are homozygous for sequence changes previously identified in a heterozygous state in AA patients and controls (A1062T and del441E) [23]. Thus, it appears that hTERT gene variants have low penetrance and are carried in patients with a wide variety of disorders. This phenomenon can be explained if short telomeres, as opposed to mutation status of telomerase, mediate disease pathogenesis, a hypothesis consistent with the fact that the median age at presentation for AML is 70 [81]. In corroboration with this, abnormal karyotypes were present in 18 of the 21 patients who were carriers of hTERT mutations, suggesting that these patients' excessively short telomeres have contributed to genomic instability and their development of AML. However, this correlation still needs to be validated in vivo.
Paroxysmal Nocturnal Haemoglobinuria
Paroxysmal nocturnal haemoglobinuria (PNH) is a clonal blood disorder arising from a defective blood cell lacking glycosylphosphatidylinositol (GPI)-anchored proteins due to a mutation in the PIGA gene [83, 84]. This disorder is commonly associated with aplastic anemia and as such, patients have been screened for mutations in telomerase components. While no mutations in dyskerin, hTERT, or hTERC have been found, a single mutation (−99C→G) within the Sp1 binding site in the promoter region of hTERC was isolated in a PNH patient [85]. Interestingly, this mutation was also found in patients with MDS [33]. While the effect of disrupting this site has not yet been determined in vivo, its activity in vitro seems to vary depending on the exact promoter context used for the luciferase reporter assay. In the minimal promoter context (nucleotides −107 to +10), the −99C/G mutation results in an increase in luciferase activity, suggesting a repressive role for the Sp1 binding site. However, a similar experiment performed by the same group, using a longer hTERC promoter sequence (−107 to +69) and a double substitution in the same site (C-101A/C-100A), identified this site as a positive regulator of hTERC transcription. Furthermore, preliminary data from our lab suggests that the −99C/G sequence change may not confer a dramatic defect when considered in the context of a much larger promoter construct of 1457bp (unpublished data). As both of these mutations have been shown to disrupt Sp1/Sp3 binding, these results suggest that this site may act as both positive and negative regulatory element to control hTERC gene expression.
Essential Thrombocythemia
Essential thrombocythemia (ET) is a rare chronic myeloproliferative neoplasm (CMPN), usually characterized by the overproduction of platelets by megakaryocytes in the bone marrow which generally affects middle-aged to elderly individuals [86]. An ET patient was recently identified who carries an hTERC allele with a two-nucleotide deletion [29]. This mutation (Δ389-390) failed to reconstitute telomerase activity in vitro, suggesting that telomerase may play a role in the disease pathogenesis of some patients. Since this disease tends to have a later age of onset, progressive telomere shortening and resulting genomic instability could possibly contribute to the ET phenotype. We have undertaken an effort to screen 90 patients who have been clinically diagnosed with CMPN, including 43 patients with ET, and found no mutations in the hTERC gene [26]. It is possible that other genetic factors may play a role in this group of diseases with highly diverse features. Indeed, recent studies have shown that a single acquired (somatic) mutation (V617F) in the tyrosine kinase JAK2 gene seems to be strongly associated with CMPNs, found in more than half of patients with either ET or chronic idiopathic myelofibrosis and in almost all patients with polycythemia vera [87-89]. The mutation leads to constitutive activation of JAK2, which promotes cytokine hypersensitivity [90]. It may cause constitutive activation of an erythropoietin receptor (EpoR) even in the absence of stimulation by its natural ligand erythropoietin, which has been shown to induce erythocytosis in a mouse model.
Telomerase Mutations in Non-Hematological Disorders
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a progressive disorder with an autosomal dominant pattern of inheritance and variable degrees of penetrance and accounts for greater than 70% of all cases of idiopathic interstitial pneumonias (IIPs). It is characterized by symptoms of chronic cough and shortness of breath, as well as diffuse interstitial fibrosis [91]. Approximately 20% of DC patients also have some form of pulmonary disease, which can sometimes lead to permanent scarring of the lungs. It has been hypothesized that since there is an inverse relationship between caveolin-1 and TGF-β1 expression and TGF-β1 negatively regulates telomerase activity, there may be a link between a genetic predisposition and the actual molecular signaling. Wang et al has shown that patients with IPF have reduced expression of caveolin-1, providing a possible mechanism by which a change in gene expression may lead to telomere shortening in certain lung tissue progenitor cells [92]. In addition, different groups have independently isolated heterozygous mutations in both hTERT and hTERC in patients with IPF, which appear to function via haploinsufficiency [12, 14, 19, 41]. Both patients and carriers have shorter telomeres than age-matched controls. In fact, a recent paper by Alder et al shows that telomere shortening, in the presence or absence of mutations in telomerase components, may contribute to disease risk in IIP patients who have no family history [41]. Although the mutations appear to impair telomerase activity to different extents in in vitro TRAP assays, they confer a dramatic increase in susceptibility to this adult-onset and fatal disease. The significance of telomerase mutations in the development of IPF still needs to be demonstrated in vivo.
Cri du Chat Syndrome
Cri du chat syndrome (CdCS) is a disease in infants, which is characterized by a distinct cat-like cry, in addition to other physical anomalies, including microcephaly, widely spaced eyes, low set ears, a low broad nasal bridge, and palmar creases [93]. It results from loss of the distal portion of chromosome 5p, a region that encompasses the hTERT and several other genes. Indeed, fluorescence in situ hybridization (FISH) analysis on patient lymphocytes and fibroblasts showed only a single copy of hTERT, indicating that cells may be haploinsufficient for telomere maintenance [94]. The accelerated telomere shortening predicted by this hypothesis was confirmed in patient lymphocytes by a reduction in Q-FISH signal and shorter telomere restriction fragments (TRFs) as compared to those of age-matched controls. While it has been shown that patient lymphocytes and fibroblasts have only one copy of hTERT and that dermal fibroblasts have an impaired replicative capacity, it is unclear how loss of the catalytic component of telomerase could cause all the symptoms associated with CdCS. It has been proposed that accelerated telomere shortening and subsequent progenitor cell death could adversely affect normal fetal development. However, since other genes are also deleted from chromosome 5p in this disease, it remains to be seen whether hTERT is truly the causal gene or just one of many genetic factors leading to CdCS.
Mouse Models of DC
X-Linked DC
Two approaches have been taken in order to generate a mouse model of X-linked DC: (1) targeted C-terminal deletion of the Dkc1 gene utilizing the Cre-Lox system and (2) hypomorphic allele in which the wild-type Dkc1 gene is expressed at reduced levels. While null dyskerin mutations are embryonically lethal, Gu et al have constructed a mouse which carries a dyskerin mutation designed to mimic a mutation found in a family with X-linked DC [55, 70, 95]. From studies on these mice and embryonic stem (ES) cells, they have shown that cells expressing wild-type dyskerin have a growth advantage over those expressing a truncated version, a phenomenon that is telomerase-, but not telomere length, dependent. In addition, mutant ES cells exhibit an enhanced DNA damage response via the classical p53/ATM pathway and the damage foci colocalize with telomeres [95]. Interestingly, this model does not show any alterations in ribosome biogenesis nor any characteristic phenotypes of DC. On the contrary, mice expressing a hypomorphic allele of Dkc1 exhibit several phenotypes observed in DC patients, including bone marrow failure, dyskeratosis of the skin, lung abnormalities, and an increased susceptibility to cancer development [96]. Moreover, these pathological features were observed in first- and second-generation mice, suggesting that they arose independent of telomere length. Telomere shortening was not observed in these mice until generation 4 (G4), accompanied by a decrease in telomerase activity caused by decreased mouse telomerase RNA (mTERC) stability. The X-linked DC phenotypes in these mice seem to be initiated by decreased ribosomal RNA (rRNA) processing and an impairment in internal ribosomal entry site (IRES)-mediated translation [97]. While each of these models sheds important light on dyskerin's various functions in the cell, it seems most likely that a combination of the observed defects contributes to X-linked DC pathogenesis in humans. In fact, mice carrying two different mutations identified in patients exhibit varying defects in mTERC and small nucleolar RNA (snoRNA) accumulation, telomerase activity, telomere length, and rRNA processing [98]. More careful mapping of specific domains and residues necessary for each of dyskerin's cellular activities should help to shed some light on a possible mechanism underlying this disease.
Autosomal Dominant DC
Knock-outs of mTERC and mTERT exhibit very similar phenotypes. Neither component, although absolutely essential for telomerase enzymatic activity, is essential for embryonic development, and disease states do not manifest until later generations when telomeres have significantly shortened [75-78, 99-102]. In confirmation of this finding, Hao et al have shown that, even in the presence of telomerase activity, short telomeres can limit tissue renewal in the bone marrow, intestines, and testes [103]. In order to generate mice with sufficiently short telomeres, they backcrossed an mTERC+/− C57BL/6 mouse with a CAST/EiJ mouse, which is known to have very short telomeres, for five generations in order to generate a heterozygous generation 1 (HG1) mouse. These heterozygotes were intercrossed to obtain successive generations of mTERC+/+ (wt*), mTERC+/−(HG), and mTERC−/− (KO) animals. Several tissue renewal defects were observed in the mTERC null mice, including small intestine atrophy, hematopoietic defects, and impaired wound healing, and were found to follow the disease anticipation phenomenon observed in humans, whereby disease phenotypes appear at an earlier age in later generations due to the inheritance of shortened telomeres. Interestingly, despite the presence of telomerase activity, late generation HG and wt* mice also exhibited telomere shortening and signs of occult genetic disease. Despite these exciting findings, none of these mice exhibited the characteristic triad of features associated with DC [76, 78].
A couple other intriguing results have been obtained through studies on mTERT knock-out mice. First, Rajaraman et al conducted a study on telomere dysfunction-induced apoptosis in the intestinal crypts of late generation mTERT−/− mice [104]. In doing so, they found that gastrointestinal (GI) progenitor cells undergo apoptosis due to shortened telomeres shortly after S-phase, but before mitosis, suggesting that telomere uncapping in these cells occurs in late S-phase or in G2. This timing is consistent with the timing of telomere replication and supports a mechanism whereby disruption of telomere end structure induces apoptosis directly without the need for a fusion-bridge breaking cycle. Secondly, in addition to its roles in telomere maintenance, TERT has been proposed to perform other “extracurricular activities” in the cell (summarized in [105, 106]). Consistent with this idea, conditional induction of mTERT in mouse skin epithelium causes rapid proliferation of hair follicle stem cells independent of hTERC, suggesting that TERT may directly support the processes of differentiation and proliferation [107].
In contrast with the mTERC and mTERT knock-outs, inactivation of the telomere binding protein TIN2 results in early embryonic lethality which is not rescued by telomerase deficiency [108]. Embryos die before day 7.5 of their embryonic development, suggesting that TIN2 serves telomerase-independent roles in the cell which are absolutely required for life. Unfortunately, the exact cause of death could not be analyzed due to the rapid death of TIN2−/− ES cells in culture. The embryonic lethality of this mouse model mirrors that of TRF1- and TRF2-deficient mice [109, 110]. Further analysis of the in vivo functions of these proteins will require conditional or tissue-specific knock-outs.
By far the most successful genetically engineered model of DC is a telomere degradation mouse generated by Hockemeyer et al and Wu et al [111-114]. Interestingly, while human telomeres are protected by one single-stranded DNA binding protein POT1, mouse telomeres contain two POT1 paralogs, POT1a and POT1b [112, 114]. Lack of POT1a results in embryonic lethality, activation of the DNA damage response, and aberrant homologous recombination. In contrast, POT1b knock-out mice are viable and fertile, but exhibit an increase in C-strand degradation. Despite the independent functions of POT1a and POT1b in repressing a DNA damage signal and in regulating the structure of the telomere end, respectively, full protection of telomeres requires both factors. The most exciting finding is that POT1b-deficient mice display several distinctive features of DC patients: abnormal skin pigmentation, nail dystrophy, and bone marrow failure [111, 113]. Furthermore, these phenotypes are exacerbated by haploinsufficiency for mTERC and double knock-outs for POT1b and mTERC are embryonic lethal. These symptoms arise in the background of normal telomerase activity, strengthening the argument that DC is due to dysfunctional telomeres. It is interesting to note that whereas mutations in the shelterin components TRF1, TRF2, and TIN2 have been identified in patients with bone marrow failure syndromes, mutations in POT1 have not yet been reported.
Conclusions
Although it has been over a century since dyskeratosis congenita was first described [115] and over two decades since the discovery of telomerase [116], this disease and its specific etiology as it relates to telomere dysfunction retain their mystery. In vitro studies have been helpful in dissecting the potential roles of telomere maintenance genes in disease pathogenesis, but much of this data still needs to be validated in vivo. Unfortunately, while it would be ideal to study patient tissues, this is difficult due to the limited availability of samples and the nature of the desired cells. It is potentially problematic to obtain sufficient bone marrow or blood cells from an already hematologically compromised patient. Thus, several mouse models have been developed, both genetically and chemically (reviewed in [117]), to study the physiological effects of deficiencies in the telomere maintenance pathway. Despite the large telomere reserve of mice and the inherent differences in shelterin complex composition between mice and humans, mouse studies have not only strengthened the in vitro findings, but also shed light on some interesting new phenomena. Whether these conclusions will extend themselves to humans remains to be seen.
Acknowledgments
The authors thank Matthew Carroll for his assistance in creating figures. We apologize to investigators whose work could not be included in this article due to space constraint. This work was supported in part by grants from the American Cancer Society (RSG-06-162-01-GMC), AA&MDSIF, SERCEB (U54 AI057157), Emory CFAR (P30 AI050409), and Emory DDRDC (DK64399). K.A.C. was supported in part by a pre-doctoral training grant (T32 GM008490).
References
- 1.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 2.Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 3.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 4.Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 5.Harley CB, Vaziri H, Counter CM, Allsopp RC. The telomere hypothesis of cellular aging. Exp Gerontol. 1992;27:375–382. doi: 10.1016/0531-5565(92)90068-b. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Autexier C, Lue NF. The Structure and Function of Telomerase Reverse Transcriptase. Ann Rev Biochem. 2006;75:493–517. doi: 10.1146/annurev.biochem.75.103004.142412. [DOI] [PubMed] [Google Scholar]
- 8.Chen JL, Greider CW. Telomerase RNA structure and function: implications for dyskeratosis congenita. Trends Biochem Sci. 2004;29:183–192. doi: 10.1016/j.tibs.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 9.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 10.Dokal I, Vulliamy T. Inherited aplastic anaemias/bone marrow failure syndromes. Blood Reviews. 2008;22:141–153. doi: 10.1016/j.blre.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci USA. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 13.Basel-Vanagaite L, Dokal I, Tamary H, Avigdor A, Garty BZ, Volkov A, Vulliamy T. Expanding the clinical phenotype of autosomal dominant dyskeratosis congenita caused by TERT mutations. Haematologica. 2008;93:943–944. doi: 10.3324/haematol.12317. [DOI] [PubMed] [Google Scholar]
- 14.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere Shortening in Familial and Sporadic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du H-Y, Pumbo E, Manley P, Field JJ, Bayliss SJ, Wilson DB, Mason PJ, Bessler M. Complex inheritance pattern of dyskeratosis congenita in two families with 2 different mutations in the telomerase reverse transcriptase gene. Blood. 2008;111:1128–1130. doi: 10.1182/blood-2007-10-120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J, Yagasaki H, Kamachi Y, Hama A, Matsumoto K, Kato K, Kudo K, Kojima S. Mutations in telomerase catalytic protein in Japanese children with aplastic anemia. Haematologica. 2006;91:656–658. [PubMed] [Google Scholar]
- 17.Marrone A, Walne A, Tamary H, Masunari Y, Kirwan M, Beswick R, Vulliamy T, Dokal I. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110:4198–4205. doi: 10.1182/blood-2006-12-062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage S, Stewart B, Weksler B, Baerlocher G, Lansdorp P, Chanock S, Alter B. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis. 2006;37:134–136. doi: 10.1016/j.bcmd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 21.Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis. 2005;34:257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Xin ZT, Beauchamp AD, Calado RT, Bradford JW, Regal JA, Shenoy A, Liang Y, Lansdorp PM, Young NS, Ly H. Functional characterization of natural telomerase mutations found in patients with hematologic disorders. Blood. 2007;109:524–532. doi: 10.1182/blood-2006-07-035089. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi H, Calado R. T., Ly H., Kajigaya S., Baerlocher G. M., Chanock S. J., Lansdorp P. M., Young N. S. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. New Engl J Med. 2005;352:1413–1483. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 24.Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, Zago MA, Lansdorp PM, Hogge D, Chanock SJ, Estey EH, Falcão RP, Young NS. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci USA. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerone M, Ward R, Londoño-Vallejo J, Autexier C. Telomerase RNA mutated in autosomal dyskeratosis congenita reconstitutes a weakly active telomerase enzyme defective in telomere elongation. Cell Cycle. 2005;4:585–589. [PubMed] [Google Scholar]
- 26.Danzy S, Su CY, Park S, Li SY, Ferraris AM, Ly H. Absence of pathogenic mutations of the human telomerase RNA gene (hTERC) in patients with chronic myeloproliferative disorders. Leukemia. 2006;20:893–894. doi: 10.1038/sj.leu.2404159. [DOI] [PubMed] [Google Scholar]
- 27.Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, Read EJ, Lansdorp PM, Young NS. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 28.Fu D, Collins K. Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol Cell. 2003;11:1361–1372. doi: 10.1016/s1097-2765(03)00196-5. [DOI] [PubMed] [Google Scholar]
- 29.Ly H, Calado RT, Allard P, Baerlocher GM, Lansdorp PM, Young NS, Parslow TG. Functional characterization of telomerase RNA variants found in patients with hematological disorders. Blood. 2005;105:2332–2339. doi: 10.1182/blood-2004-09-3659. [DOI] [PubMed] [Google Scholar]
- 30.Ly H, Schertzer M, Jastaniah W, Davis J, Yong SL, Ouyang Q, Blackburn EH, Parslow TG, Lansdorp PM. Identification and functional characterization of 2 variant alleles of the telomerase RNA template gene (TERC) in a patient with dyskeratosis congenita. Blood. 2005;106:1246–1252. doi: 10.1182/blood-2005-01-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrone A, Sokhal P, Walne A, Beswick R, Kirwan M, Killick S, Williams M, Marsh J, Vulliamy T, Dokal I. Functional characterization of novel telomerase RNA (TERC) mutations in patients with diverse clinical and pathological presentations. Haematologica. 2007;92:1013–1020. doi: 10.3324/haematol.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrone A, Stevens D, Vulliamy T, Dokal I, Mason PJ. Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood. 2004;104:3936–3942. doi: 10.1182/blood-2004-05-1829. [DOI] [PubMed] [Google Scholar]
- 33.Ortmann CA, Niemeyer CM, Wawer A, Ebell W, Baumann I, Kratz CP. TERC mutations in children with refractory cytopenia. Haematologica. 2006;91:707–708. [PubMed] [Google Scholar]
- 34.Theimer CA, Finger LD, Feigon J. YNMG tetraloop formation by a dyskeratosis congenita mutation in human telomerase RNA. RNA. 2003;9:1446–1455. doi: 10.1261/rna.5152303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theimer CA, Finger LD, Trantirek L, Feigon J. Mutations linked to dyskeratosis congenita cause changes in the structural equilibrium in telomerase RNA. Proc Natl Acad Sci USA. 2003;100:449–454. doi: 10.1073/pnas.242720799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359:2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 37.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 38.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 39.Wilson DB, Ivanovich J, Whelan A, Goodfellow PJ, Bessler M. Human telomerase RNA mutations and bone marrow failure. Lancet. 2003;361:1993–1994. doi: 10.1016/S0140-6736(03)13575-1. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 41.Alder JK, Chen JJL, Lancaster L, Danoff S, Su S-c, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field JJ, Mason PJ, An P, Kasai Y, McLellan M, Jaeger S, Barnes YJ, King AA, Bessler M, Wilson DB. Low frequency of telomerase RNA mutations among children with aplastic anemia or myelodysplastic syndrome. Pediatr Hematol Oncol. 2006;28:450–453. doi: 10.1097/01.mph.0000212952.58597.84. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi J, Ly H, Yamaguchi H, Carroll KA, Kosaka F, Sawaguchi K, Mitamura Y, Watanabe A, Gomi S, Inokuchi K, Dan K. Identification and functional characterization of novel telomerase variant alleles in Japanese patients with bone-marrow failure syndromes. Blood Cells Mol Dis. 2008;40:185–191. doi: 10.1016/j.bcmd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding Y, Zhu T, Jiang W, Yang Y, Bu D, Tu P, Zhu X, Wang B. Identification of a novel mutation and a de novo mutation in DKC1 in two Chinese pedigrees with Dyskeratosis congenita. J Invest Dermatol. 2004;123:470–473. doi: 10.1111/j.0022-202X.2004.23228.x. [DOI] [PubMed] [Google Scholar]
- 45.Heiss NS, Megarbane A, Klauck SM, Kreuz FR, Makhoul E, Majewski F, Poustka A. One novel and two recurrent missense DKC1 mutations in patients with dyskeratosis congenita (DKC) Genet Couns. 2001;12:129–136. [PubMed] [Google Scholar]
- 46.Hiramatsu H, Fujii T, Kitoh T, Sawada M, Osaka M, Koami K, Irino T, Miyajima T, Ito M, Sugiyama T, Okuno T. A novel missense mutation in the DKC1 gene in a Japanese family with X-linked dyskeratosis congenita. Pediatr Hematol Oncol. 2002;19:413–419. doi: 10.1080/08880010290097170. [DOI] [PubMed] [Google Scholar]
- 47.Kanegane H, Kasahara Y, Okamura J, Hongo T, Tanaka R, Nomura K, Kojima S, Miyawaki T. Identification of DKC1 gene mutations in Japanese patients with X-linked dyskeratosis congenita. Br J Haematol. 2005;129:432–434. doi: 10.1111/j.1365-2141.2005.05473.x. [DOI] [PubMed] [Google Scholar]
- 48.Knight SW, Heiss NS, Vulliamy TJ, Aalfs CM, McMahon C, Richmond P, Jones A, Hennekam RC, Poustka A, Mason PJ, Dokal I. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br J Haematol. 1999;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- 49.Knight SW, Heiss NS, Vulliamy TJ, Greschner S, Stavrides G, Pai GS, Lestringant G, Varma N, Mason PJ, Dokal I, Poustka A. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet. 1999;65:50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knight SW, Vulliamy TJ, Morgan B, Devriendt K, Mason PJ, Dokal I. Identification of novel DKC1 mutations in patients with dyskeratosis congenita: implications for pathophysiology and diagnosis. Hum Genet. 2001;108:299–303. doi: 10.1007/s004390100494. [DOI] [PubMed] [Google Scholar]
- 51.Kraemer DM, Goebeler M. Missense mutation in a patient with X-linked dyskeratosis congenita. Haematologica. 2003;88:ECR11. [PubMed] [Google Scholar]
- 52.Lin JH, Lee JY, Tsao CJ, Chao SC. DKC1 gene mutation in a Taiwanese kindred with X-linked dyskeratosis congenita. Kaohsiung J Med Sci. 2002;18:573–577. [PubMed] [Google Scholar]
- 53.Salowsky R, Heiss N, Benner A, Wittig R, Poustka A. Basal transcription activity of the dyskeratosis congenita gene is mediated by Sp1 and Sp3 and a patient mutation in a Sp1 binding site is associated with decreased promoter activity. Gene. 2002;293:9–19. doi: 10.1016/s0378-1119(02)00725-4. [DOI] [PubMed] [Google Scholar]
- 54.Sznajer Y, Baumann C, David A, Journel H, Lacombe D, Perel Y, Blouin P, Segura JF, Cezard JP, Peuchmaur M, Vulliamy T, Dokal I, Verloes A. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal-Hreidarsson syndrome) Eur J Pediatr. 2003;162:863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- 55.Vulliamy TJ, Knight SW, Heiss NS, Smith OP, Poustka A, Dokal I, Mason PJ. Dyskeratosis congenita caused by a 3' deletion: germline and somatic mosaicism in a female carrier. Blood. 1999;94:1254–1260. [PubMed] [Google Scholar]
- 56.Yaghmai R, Kimyai-Asadi A, Rostamiani K, Heiss NS, Poustka A, Eyaid W, Bodurtha J, Nousari HC, Hamosh A, Metzenberg A. Overlap of dyskeratosis congenita with the Hoyeraal-Hreidarsson syndrome. J Pediatr. 2000;136:390–393. doi: 10.1067/mpd.2000.104295. [DOI] [PubMed] [Google Scholar]
- 57.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 58.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proceedings of the National Academy of Sciences. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savage SA, Calado RT, Xin ZT, Ly H, Young NS, Chanock SJ. Genetic variation in telomeric repeat binding factors 1 and 2 in aplastic anemia. Exp Hematol. 2006;34:664–671. doi: 10.1016/j.exphem.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a Component of the Shelterin Telomere Protection Complex, Is Mutated in Dyskeratosis Congenita. Am J Hum Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du H-Y, Mason PJ, Bessler M, Wilson DB. TINF2 mutations in children with severe aplastic anemia. Pediatr Blood Cancer. 2008;52:687. doi: 10.1002/pbc.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsangaris E, Adams SL, Yoon G, Chitayat D, Lansdorp P, Dokal I, Dror Y. Ataxia and pancytopenia caused by a mutation in TINF2. Hum Genet. 2008;124:507–513. doi: 10.1007/s00439-008-0576-7. [DOI] [PubMed] [Google Scholar]
- 65.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 66.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009 doi: 10.1182/blood-2008-12-192880. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Du H-Y, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, Chirnomas D, Shimamura A, Vlachos A, Lipton JM, Goyal RK, Goldman F, Wilson DB, Mason PJ, Bessler M. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marrone A, Walne A, Dokal I. Dyskeratosis congenita: telomerase, telomeres and anticipation. Curr Opin Genet Dev. 2005;15:249–257. doi: 10.1016/j.gde.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 70.He J, Navarrete S, Jasinski M, Vulliamy T, Dokal I, Bessler M, Mason PJ. Targeted disruption of Dkc1, the gene mutated in X-linked dyskeratosis congenita, causes embryonic lethality in mice. Oncogene. 2002;21:7740–7744. doi: 10.1038/sj.onc.1205969. [DOI] [PubMed] [Google Scholar]
- 71.Hreidarsson S, Kristjansson K, Johannesson G, Johannsson JH. A syndrome of progressive pancytopenia with microcephaly, cerebellar hypoplasia and growth failure. Acta Paediatr Scand. 1988;77:773–775. doi: 10.1111/j.1651-2227.1988.tb10751.x. [DOI] [PubMed] [Google Scholar]
- 72.Berthet F, Tuchschmid P, Boltshauser E, Seger RA. The Hoyeraal-Hreidarsson syndrome: don't forget the associated immunodeficiency. Eur J Pediatr. 1995;154:998. doi: 10.1007/BF01958649. [DOI] [PubMed] [Google Scholar]
- 73.Errington TM, Fu D, Wong JMY, Collins K. Disease-Associated Human Telomerase RNA Variants Show Loss of Function for Telomere Synthesis without Dominant-Negative Interference. Mol Cell Biol. 2008;28:6510–6520. doi: 10.1128/MCB.00777-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shastry BS. Heritable trinuclotide repeats and neurological disorders. Experientia. 1994;50:1099–1105. doi: 10.1007/BF01923467. [DOI] [PubMed] [Google Scholar]
- 75.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 76.Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 78.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 79.Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136:534–546. doi: 10.7326/0003-4819-136-7-200204020-00011. [DOI] [PubMed] [Google Scholar]
- 80.Calado RT, Graf SA, Wilkerson KL, Kajigaya S, Ancliff PJ, Dror Y, Chanock SJ, Lansdorp PM, Young NS. Mutations in the SBDS gene in acquired aplastic anemia. Blood. 2007;110:1141–1146. doi: 10.1182/blood-2007-03-080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 82.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bessler M, Schaefer A, Keller P. Paroxysmal nocturnal hemoglobinuria: insights from recent advances in molecular biology. Transfus Med Rev. 2001;15:255–267. doi: 10.1053/tmrv.2001.26958. [DOI] [PubMed] [Google Scholar]
- 84.Kinoshita T, Inoue N. Relationship between aplastic anemia and paroxysmal nocturnal hemoglobinuria. Int J Hematol. 2002;75:117–122. doi: 10.1007/BF02982015. [DOI] [PubMed] [Google Scholar]
- 85.Keith WN, Vulliamy T, Zhao J, Ar C, Erzik C, Bilsland A, Ulku B, Marrone A, Mason PJ, Bessler M, Serakinci N, Dokal I. A mutation in a functional Sp1 binding site of the telomerase RNA gene (hTERC) promoter in a patient with Paroxysmal Nocturnal Haemoglobinuria. BMC Blood Disord. 2004;4:3. doi: 10.1186/1471-2326-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schafer AI. Molecular basis of the diagnosis and treatment of polycythemia vera and essential thrombocythemia. Blood. 2006;107:4214–4222. doi: 10.1182/blood-2005-08-3526. [DOI] [PubMed] [Google Scholar]
- 87.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 88.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 89.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D'Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 90.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 91.Gross TJ, Hunninghake GW. Idiopathic Pulmonary Fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 92.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, Choi AMK. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–2906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cerruti Mainardi P. Cri du Chat syndrome. Orphanet J Rare Dis. 2006;1:33. doi: 10.1186/1750-1172-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang A, Zheng C, Hou M, Lindvall C, Li KJ, Erlandsson F, Björkholm M, Gruber A, Blennow E, Xu D. Deletion of the telomerase reverse transcriptase gene and haploinsufficiency of telomere maintenance in Cri du chat syndrome. Am J Hum Genet. 2003;72:940–948. doi: 10.1086/374565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu B-W, Bessler M, Mason PJ. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proc Natl Acad Sci USA. 2008;105:10173–10178. doi: 10.1073/pnas.0803559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, Cordon-Cardo C, Pandolfi PP. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 97.Yoon A, Peng G, Brandenburg Y, Zollo O, Xu W, Rego E, Ruggero D. Impaired Control of IRES-Mediated Translation in X-Linked Dyskeratosis Congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 98.Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci USA. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y, Snow BE, Hande MP, Yeung D, Erdmann NJ, Wakeham A, Itie A, Siderovski DP, Lansdorp PM, Robinson MO, Harrington L. The telomerase reverse transcriptase is limiting and necessary for telomerase function in vivo. Curr Biol. 2000;10:1459–1462. doi: 10.1016/s0960-9822(00)00805-8. [DOI] [PubMed] [Google Scholar]
- 100.Yuan X, Ishibashi S, Hatakeyama S, Saito M, Nakayama J, Nikaido R, Haruyama T, Watanabe Y, Iwata H, Iida M, Sugimura H, Yamada N, Ishikawa F. Presence of telomeric G-strand tails in the telomerase catalytic subunit TERT knockout mice. Genes Cells. 1999;4:563–572. doi: 10.1046/j.1365-2443.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 101.Erdmann N, Liu Y, Harrington L. Distinct dosage requirements for the maintenance of long and short telomeres in mTert heterozygous mice. Proc Natl Acad Sci USA. 2004;101:6080–6085. doi: 10.1073/pnas.0401580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niida H, Matsumoto T, Satoh H, Shiwa M, Tokutake Y, Furuichi Y, Shinkai Y. Severe growth defect in mouse cells lacking the telomerase RNA component. Nat Genet. 1998;19:203–206. doi: 10.1038/580. [DOI] [PubMed] [Google Scholar]
- 103.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 104.Rajaraman S, Choi J, Cheung P, Beaudry V, Moore H, Artandi SE. Telomere uncapping in progenitor cells with critical telomere shortening is coupled to S-phase progression in vivo. Proc Natl Acad Sci USA. 2007;104:17747–17752. doi: 10.1073/pnas.0706485104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bollmann FM. The many faces of telomerase: emerging extratelomeric effects. Bio Essays. 2008;30:728–732. doi: 10.1002/bies.20793. [DOI] [PubMed] [Google Scholar]
- 106.Chung HK, Cheong C, Song J, Lee H-W. Extratelomeric Functions of Telomerase. Curr Mol Med. 2005;5:233–241. doi: 10.2174/1566524053586635. [DOI] [PubMed] [Google Scholar]
- 107.Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiang YJ, Kim S-H, Tessarollo L, Campisi J, Hodes RJ. Telomere-Associated Protein TIN2 Is Essential for Early Embryonic Development through a Telomerase-Independent Pathway. Mol Cell Biol. 2004;24:6631–6634. doi: 10.1128/MCB.24.15.6631-6634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karlseder J, Kachatrian L, Takai H, Mercer K, Hingorani S, Jacks T, de Lange T. Targeted deletion reveals an essential function for the telomere length regulator Trf1. Mol Cell Biol. 2003;23:6533–6541. doi: 10.1128/MCB.23.18.6533-6541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 111.He H, Wang Y, Guo X, Ramchandani S, Ma J, Shen M-F, Garcia DA, Deng Y, Multani AS, You MJ, Chang S. Pot1b deletion and telomerase haploinsufficiency in mice initiate an ATR-dependent DNA damage response and elicit phenotypes resembling dyskeratosis congenita. Mol Cell Biol. 2009;29:229–240. doi: 10.1128/MCB.01400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hockemeyer D, Daniels J-P, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 113.Hockemeyer D, Palm W, Wang RC, Couto SS, de Lange T. Engineered telomere degradation models dyskeratosis congenita. Genes Dev. 2008;22:1773–1785. doi: 10.1101/gad.1679208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu L, Multani AS, He H, Cosme-Blanco W, Deng Y, Deng JM, Bachilo O, Pathak S, Tahara H, Bailey SM, Deng Y, Behringer RR, Chang S. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126:49–62. doi: 10.1016/j.cell.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 115.Zinsser F. Atrophia cutis reticularis cum pigmentatione, dystrophia unguium et leukoplakia oris. Ikonogr Dermatol (Hyoto) 1906;5:219–223. doi: 10.1007/BF00476707. [DOI] [PubMed] [Google Scholar]
- 116.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 117.Chen J. Animal models for acquired bone marrow failure syndromes. Clin Med Res. 2005;3:102–108. doi: 10.3121/cmr.3.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]