Abstract
The preponderance of pancreatic tumors is adenocarcinoma of the ductal type; carcinomas with multiple lineage differentiation are extremely rare. We report an unusual case of pancreatic carcinoma with combined acinar and neuroendocrine differentiation and minor ductal component with concurrent acinar-ductal metaplasia (ADM), an early lesion implicated in ductal carcinogenesis. The patient is a 56-year-old man with vague complaints of dull left upper quadrant pain with radiation across the mid-portion of his abdomen. A computer tomography scan revealed an irregular enlargement of the distal 3.2 cm of the pancreatic body. A distal pancreatectomy was then performed. Histologic examination revealed a pancreatic carcinoma with cellular features of eosinophilic granular cytoplasm and salt-pepper nuclei. The acinar differentiation of the carcinoma was confirmed by positivity on periodic acid-Schiff stain resistant to diastase digestion (dPAS), positivity for antitrypsin on immunohistochemistry (IHC), and presence of zymogen granules on electron microscopy (EM). The neuroendocrine differentiation was evident by positive synaptophysin and chromogranin stain on IHC and neuroendocrine granules on EM. The ductal component was only visible by PAS stain and immunostains for CEA and CK19A and accompanied by a number of the acinar-ductal metaplasia lesions adjacent to the main tumor. Thus, the histological, histochemical, immunohistochemical and electron-microscopic evidence all suggested that the pancreatic carcinoma underwent trilineage differentiation.
Keywords: Adenocarcinoma, pancreas, neuroendocrine differentiation, metaplasia
Introduction
The pancreas is composed of three cell types with either exocrine (acinar/ductal) or endocrine function. Pancreatic tumors are among the most aggressive neoplasms and patients often present in an advanced stage. Ductal adenocarcinoma of the exocrine pancreas comprises about 85% of all pancreatic malignancies [1]. Tumors with multiple cell lineage differentiation are rare and tumors with trilineage (ductal, acinar and neuroendocrine) differentiation are even more exceptional. These tumors are often diagnostically challenging and immunohistochemical staining and electron-microscopic analysis are essential for accurate diagnosis.
To date, to the best of our knowledge, only five cases of a pancreatic tumor with ductal, acinar and neuroendocrine differentiation have been described [2, 3, 4, 5, 6]. However, most of the cases in the literature do not have supporting evidence for trilineage differentiation by immunohistochemistry and electron microscopy. In this study, we present a case of a 56-year-old man with pancreatic carcinoma showing predominantly acinar and neuroendocrine differentiation, but containing minor ductal component accompanied by the acinar-ductal metaplasia, an early ductal lesion that are implicated in ductal carcinogenesis. The findings are confirmed by histologic evaluation, histochemical and immunohistochemical stains, and electron microscopy.
Case History
A 56-year old obese man (BMI: 40) with hypertension, diabetes type II, and prior alcohol abuse with abstinence for 20 years presented to the medical clinic complaining of sexual and urinary dysfunction. The patient, while being examined for this complaint, admitted to a three month history of left upper quadrant pain with occasional radiation across the mid-portion of his abdomen accompanied by fatigue and weight gain.
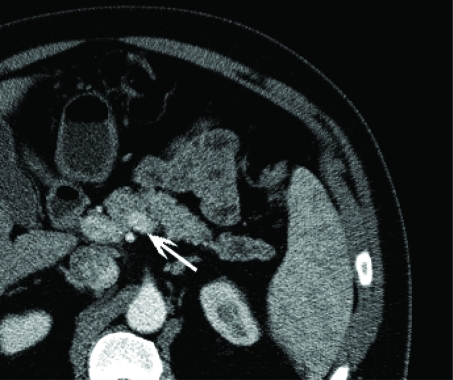
The patient underwent a computer tomography as part of a urological workup, which revealed an incidental lesion in the tail of the pancreas (Figure 1). The distal 3.2 cm segment of the pancreatic body appears irregularly enlarged measuring approximately 2.4 cm without associated attenuation and enhancement abnormalities. Distal to this segment, there is irregular dilatation of the pancreatic duct for approximately 6 cm extending all the way to the splenic hilum. The duct averages 8 mm throughout and measures approximately 1 cm in its greatest dimension. There was no evidence of abnormal masses to suggest lymphadenopathy. These findings were suggestive of an obstructive process in the distal pancreatic body. A magnetic resonance imaging and cholangiopancreatography confirmed the presence of segmental dilatation within the body and tail of the pancreas and of the main and adjacent side-branches of the pancreatic duct which extends for approximately 5 cm. No obstructing masses were visualized at the area of the main pancreatic ductal transition. There were no visualized abdominal lymphadenopathy and the surrounding structures were unremarkable. A laparoscopic distal pancreatectomy was performed and the patient was discharged without complications on postoperative day 6.
Figure 1.
An ill-defined incidental pancreatic mass (arrow) on CT.
Pathological Findings
The resection specimen consists of a tan-red distal portion of pancreas measuring 7 × 4 × 2 cm. There was a tan-white, firm, fleshy, homogeneous, well-circumscribed nodule that measures 1.2 × 1.2 × 0.5 cm and is located 0.2 cm from the proximal margin. The remainder of the pancreatic parenchyma is unremarkable. Two lymph nodes are present in the posterior and inferior soft tissue surrounding the pancreatic tissue, the largest measuring 0.2 cm in greatest dimension.
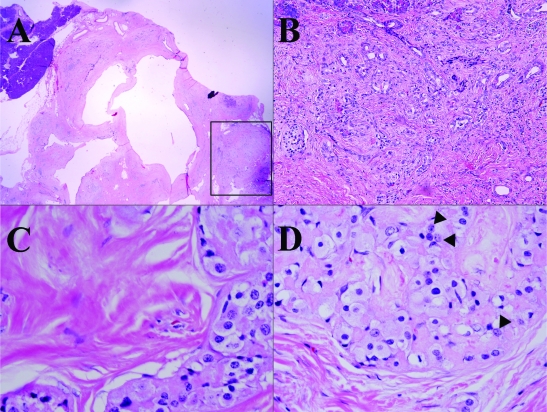
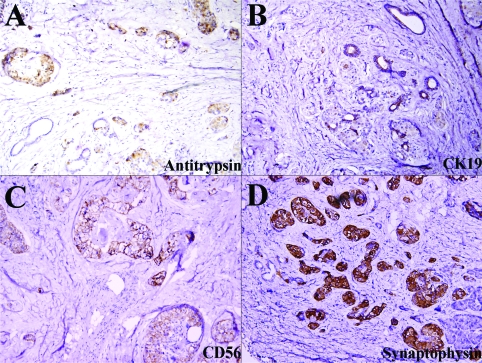
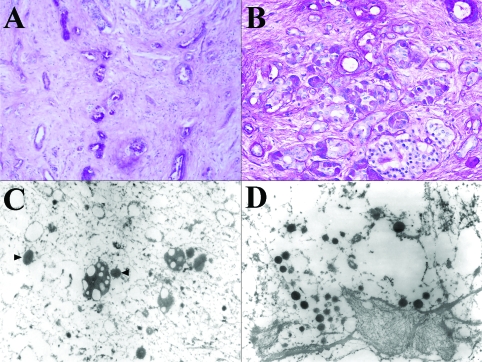
Histologically the tumor is composed of scattered carcinomatous nests and tubules (ducts) (Figure 2) in a background of dense fibrocollagenous tissue (Figure 2A). The cells have abundant eosinophilic granular cytoplasm (Figures 2C and 2D) and enlarged nuclei with a salt and pepper appearance (Figure 2D, arrow head) and occasional prominent nucleoli. The tumor cells focally invade into adjacent normal parenchyma and are intermixed with acinar-ductal metaplasia at the edge of carcinoma (Figures 2B and 4B). The tumor and metaplastic cells are stained positively by PAS (Figure 3A) and the positive stain is resistant to digestion by diastase (PAS-D) (Figure 3B) stain. The immunohistochemical staining for pancreatic enzymes, antitrypsin, highlights the acinar component in approximately 40% of the tumor (Figure 4A). Ultrastructually, electron microscopy of the acinar component reveals the tumor cells with abundant mitochondria and rough endoplasmic reticulum. There are also cells containing large electron dense zymogen granules (Figure 3C). These results indicate the acinar differentiation of the cells. The areas of acinar-ductal metaplasia and possible ductal differentiation of carcinoma are stained strongly and weakly for cytokeratin 19, respectively (Figure 4B) and carcinoembryonic antigen (data not shown) in greater about 10% of the tumor. Furthermore, IHC reveals positivity for CD56 (Figure 4C), synaptophysin (Figure 4D), chromogranin (data not shown) in greater than 25% of the tumor. The ductal, neuroendocrine and acinar components of this tumor are all distinct on morphological, histochemical, electron microscopic and immunohistochemical levels. No apparent vascular, lymphatic or perineural invasion was appreciated. The adjacent benign pancreatic tissue shows atrophic chronic pancreatitis and islet cell hyperplasia.
Figure 2.
Histological features of the tumor, at low (A, 20×), medium (B, 200×) and high (C and D, 400×) magnification. Granular cytoplasm (C and D) and salt-pepper nuclei (D, arrow head) are prominent at high magnification. At the edge of tumor, there is the acinar-ductal metaplasia.
Figure 4.
Immunohistochemical features of the tumor. The carcinoma is positive for antitrypsin (A), CK19 (B), CD56 (C) and synaptophysin (D).
Figure 3.
Histochemical and electron microscopic features of the tumor. The cancer cells are positive for PAS (A) and PAS-D (B) stains. EM shows zymogen (C) and neuroendocrine granules (D).
Discussion
The main differential diagnoses in this case include poorly-differentiated ductal carcinoma, acinar cell carcinoma, pancreatoblastoma, and mixed acinar-endocrine carcinoma. The diagnosis of pancreatoblastoma can essentially be ruled out due to the patient's age and morphology of the tumor. Pancreatoblastoma resembles acinar cell carcinoma except for squamoid corpuscles, which are not present in this case. In addition, pancreatoblastoma is usually partially encapsulated, often lobulated and has a mean size of 10 cm; none of these is present in this case.
Establishing the diagnosis of a mixed carcinoma requires qualitative and quantitative assessments of individual components in the tumor. In this case, initial morphological examination suggested that the tumor contains the ductal (tubular), acinar and neuroendocrine components and these components became more evident after ancillary studies. The acinar differentiation was confirmed by the production of pancreatic enzymes which were packaged in zymogen granules by the neoplastic cells as previously described [7], as well as positivity for periodic acid-Schiff (PAS), resistant to diastase digestion (dPAS) in 95% of acinar cell carcinomas [8]. The ductal component was less evident, but was supported by the presence of tubular structure on histology and positive staining for cytokeratin 19 and carcinoembryonic antigen. However, the majority of ductal component are those of acinar-ductal metaplasia. The neuroendocrine component was demonstrated by positive immunohistochemical staining for CD56, synaptophysin, and chromogranin. The tumor components were then strictly scrutinized further to assess the percentage of ductal, acinar and neuroendocrine composition. Each cell type composed greater than 25% of the tumor (acinar=40%, neuroendocrine=30%, and 30% unclassified or ductal component together with acinar-ductal metaplasia) defining this tumor as a mixed tumor with acinar, endocrine, and ductal differentiation.
The mixed or hybrid pancreatic tumors have multiple diagnostic pitfalls. The acinar component in these tumors tends to dominate, as in this case, and acinar tumors may contain focal neuroendocrine and/or ductal differentiation. Thus, one must assess the extent of the cellular components and multiple sections of tumor should be examined both histologically and immunohistochemically as was performed in this case.
The histogenesis of mixed pancreatic tumor with multilineage differentiation (acinar, neuroendocrine, and ductal) is of much debate. All three cell lineages develop from the embryonic foregut thus sharing a common origin during fetal development. Currently the leading theory suggests a possible origin from a stem or progenitor cell with the ability for multidirectional differentiation [9]. Thus, the presence of all three components in this tumor together with the other five reported cases supports a common ancestor theory.
The limited number of cases of mixed ductal, acinar and neuroendocrine carcinoma does not allow sufficient assessment of its biological behavior or prognosis. However, studies of mixed carcinoma suggest that a poor prognosis may be related to the cellular components of the tumor [10] and neuroendocrine differentiation may be related to a different prognosis [11]. Klimstra et al reported that all of these mixed acinar carcinomas are clinically aggressive, similar to pure acinar cell carcinomas [8, 12, 13]. This similarity may suggest that reports concerning acinar cell carcinoma may be extrapolated to include mixed pancreatic tumors. The small size of this tumor [1.2 cm], negative margins, lack of vascular, lymphatic or perineural invasion or metastatic disease may afford this patient a relatively better prognosis. The patient remains disease free 2 months post excision.
In summary, we report the clinicopathological features of a pancreatic carcinoma with acinar, neuroendocrine, and ductal differentiation. The cases like the reported herein often present diagnostic and clinical problems due to their rarity and unclear histological classification. Close morphological examination and extensive sectioning of the tumor are needed when suspecting a pancreatic tumor with trilineage origin. Immunohistochemical staining and electron microscopy are pivotal in making an accurate diagnosis. Better understanding of the behavior and prognosis of pancreatic tumor with trilineage origin requires evaluation of additional cases due to its rarity.
Acknowledgments
We would like to thank Mr. Nick Cassai for his assistance in electron microscopy.
References
- 1.Kumar V, Abbas A, Fausto N. In: Pathologic Basis of Disease. Seventh edition. Robbins, Cotran, editors. Philadelphia: Saunders; 2005. pp. 948–952. [Google Scholar]
- 2.Schron D, Mendelson G. Pancreatic carcinoma with duct, endocrine, and acinar differentiation. A histologic, immunohistochemical, and ultrastructural study. Cancer. 1984;54:1766–1770. doi: 10.1002/1097-0142(19841101)54:9<1766::aid-cncr2820540903>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Tanakaya K, Teramoto N, Konaga E, Takeuchi H, Yasui Y, Takeda A, Yunoki Y, Murakami I. Mixed duct-acinar-islet cell tumor of the pancreas. Report of a case. Surg Today. 2001;31:177–179. doi: 10.1007/s005950170207. [DOI] [PubMed] [Google Scholar]
- 4.Nonomura A, Kono N, Mizukami Y, Nakanuma Y, Matsubara F. Duct-Acinar-Islet cell tumor of the pancreas. Ultrastruct Pathol. 1992;16:317–329. doi: 10.3109/01913129209061361. [DOI] [PubMed] [Google Scholar]
- 5.Okada Y, Mori H, Tsutsumi A. Duct-Acinar-Islet cell tumor of the pancreas. Pathol Int. 1995;45:669–676. doi: 10.1111/j.1440-1827.1995.tb03520.x. [DOI] [PubMed] [Google Scholar]
- 6.Tadashi T, Masaharu K, Kazuo F, Yasutomo S, Yoshiyuki O. Case Report: Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductual growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 7.Klimstra D, Heffess C, Oertel J, Rosai J. Acinar cell carcinoma of the pancreas: A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815–837. doi: 10.1097/00000478-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Klimstra D. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20:S94–S112. doi: 10.1038/modpathol.3800686. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich A, Schmied B, Standop J, Schneider M, Pour P. Pancreatic cell lines: A review. Pancreas. 2002;24:111–120. doi: 10.1097/00006676-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Yantiss R, Chang H, Farraye F, Compton C, Odze R. Prevalence and prognostic significance of acinar cell differentiation in pancreatic endocrine tumors. Am J Surg Pathol. 2002;26:893–901. doi: 10.1097/00000478-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Machado M, Machado M, Perini M, Herman P, Jukemura J, Leite K, Bacchella T. Acinar cell carcinoma of the pancreas: is the absence of neuroendocrine component related to a more malignant behavior? Hepatogastroenterology. 2008;55:708–710. [PubMed] [Google Scholar]
- 12.Klimstra D, Rosai J, Heffess C. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994;18:765–778. doi: 10.1097/00000478-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Holen K, Klimstra D, Hummer A, Gonen M, Conlon K, Brennan M, Saltz L. Clinical Characteristics and Outcomes From an Institutional Series of Acinar Cell Carcinoma of the Pancreas and Related Tumors. J Clin Oncol. 2002;20:4673–4678. doi: 10.1200/JCO.2002.02.005. [DOI] [PubMed] [Google Scholar]