Abstract
Anaplastic lymphoma kinase-positive large B-cell lymphoma (ALK+ LBCL) represents a distinct subtype of mature B-cell neoplasms in the most recent WHO classification of hematolymphoid neoplasms. It has a characteristic immunoblastic/plasmablastic morphology, a distinct immunophenotypic profile and recurrent cytogenetic/molecular genetic abnormalities, and has been reported in both the adult and pediatric populations. With the advent of new ALK inhibitors for possible targeted therapy clinical trials, it is important to recognize this new entity, particularly in the pediatric population because the prognosis is worse than the more common ALK+ anaplastic large cell lymphoma. Though rare, awareness of its existence will avoid potential misdiagnosis and facilitate appropriate management.
Keywords: Anaplastic lymphoma kinase, ALK, diffuse large B-cell lymphoma, t(2;17), CLTC/ALK
Introduction
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase of the insulin receptor superfamily. ALK was first discovered as part of the nucleophosmin (NPM)-ALK fusion protein as a result of the t(2;5)(p23;q35) chromosomal translocation frequently seen in anaplastic large cell lymphoma, a subtype of mature T-cell neoplasms [1, 2]. The native ALK is mainly expressed in the developing central and peripheral nervous system, and is normally not expressed in hematopoietic cells [3-5]. Besides ALK-positive anaplastic large cell lymphoma, various solid tumors, including inflammatory myofibroblastic tumor and other soft tissue tumors [6-10], lung cancer [11] and brain tumors [12-16] were found to aberrantly express ALK. The most common mechanism of ALK overexpression is through formation of a fusion protein with a partner due to chromosomal translocations. However, activation through point mutation and gene amplification has also been demonstrated.
ALK was initially believed to be expressed only in anaplastic large cell lymphoma. In 1997, Delsol et al reported a small series of diffuse large B-cell lymphoma with expression of ALK (ALK+ LBCL) [17]. To date, approximately 40 cases of ALK+ LBCL have been described in the English literature and those cases share similar morphologic, immunophenotypic and molecular genetic characteristics. In fact, ALK+ LBCL is now considered to be a distinct entity of mature B-cell neoplasms in the new WHO classification of hematolymphoid neoplasm [18]. Most patients with ALK+ LBCL presented with stage III/IV disease and were clinically worse than the more common ALK+ anaplastic large cell lymphoma, particularly in the pediatric population. Therefore, recognition of this rare entity will further our understanding of its pathobiology and development of more efficient treatment including targeted therapy.
Clinical Features
Since the initial description of ALK+ LBCL by Delsol et al in 1997 [17], about 40 cases have been described. Their clinical features are summarized in Table 1. The youngest patient affected was 9 years old and the oldest one was 71 years old, with a mean age of 44.5 years. Approximately 27% of the cases occurred in the pediatric population (younger than 18 years). There is a male predominance with a male to female ratio of about 3.6:1 (32 vs 9). 23 patients presented with higher stage disease (III and IV) while 15 with lower stage disease (I and II or IIE). Interestingly, in patients younger than 18 years old, more patients presented with lower stage than higher stage diseases, a fact that may be attributed to early diagnosis in the pediatric population.
Table 1.
Clinical features of the reported ALK+ LBCL cases
| Authors | Case | Age/sex | Sites of disease | Stage | Outcome |
|---|---|---|---|---|---|
| Delsol et al [17] | 1 | 53/M | Systemic lymph nodes and spleen | IVA | DOD 26 months after CHX and BMT |
| 2 | 14/M | N/A | I | Alive without disease 156 months after CHX | |
| 3 | 37/M | Mediastinal lymph node | II | DOD after CHX | |
| 4 | 44/M | N/A | III-IV | DOD after CHX | |
| 5 | 67/M | N/A | III-IV | Lost to followup 11 months after CHX | |
| 6 | 51/M | N/A | III-IV | Alive without disease 14 months after CHX | |
| 7 | 60/M | N/A | III-IV | DOD after CHX | |
| Gascoyne et al [19] | 1 | 46/M | Supraclavicular and abdominal lymph nodes | III | Alive without disease 27 months after CHX and XRT |
| 2 | 45/F | Inguinal lymph node | N/A | N/A | |
| 3 | 49/M | Systemic lymph nodes and epidural mass | IV | Alive with disease 9 months after CHX and XTR | |
| 4 | 48/M | Axillary lymph node | IA | Alive without disease 27 months after CHX | |
| 5 | 58/M | Supraclavicular lymph node | IV | DOD 6 months after CHX | |
| De Paepe et al [20] | 1 | 10/M | Cervical mass | II | Alive without disease 6 months after CHX |
| 2 | 13/F | Cervical lymph node | III | DOD 3 months after CHX and BMT | |
| 3 | 26/M | Cervical lymph node | II | Alive without disease 44 months after CHX and BMT | |
| Chikatsu et al [21] | 1 | 36/F | Intramuscular and bilateral ovarian masses | IV | DOD 11 months after CHX |
| Onciu et al [22] | 1 | 16/M | Systemic lymph node and multiple lytic skeletal lesions | IV | DOD 24 months after CHX and XTR |
| 2 | 10/M | Head and neck lymph nodes | II | Alive without disease 156 months after CHX and XTR | |
| Adam et al [23] | 1 | 35/M | Cervical and supraclaviclular lymph nodes | IIA | DOD 14 months after CHX and BMT |
| McManus et al [24] | 1 | 21/M | Pyloric mass | IIE | Alive without disease 2 years after CHX |
| Colomo et al [25] | 1 | 34/M | Systemic lymph nodes | N/A | DOD 8 months after therapy |
| Ishii et al [26] | 1 | 33/M | Right neck lymph node | N/A | DOD 31 months after CHX, XTR and BMT |
| Rudzki et al [27] | 1 | 48/M | Neck mass | IIIB | DOD 3 months after CHX |
| 2 | 49/M | Abdominal lymph nodes | IV | Alive on CHX | |
| Gesk et al [28] | 1 | 13/M | Cervical lymph node | II | Alive with partial remission on CHX |
| 2 | 12/F | Mediastinal and cervical lymph nodes | II | Alive without disease 4 years after CHX | |
| 3 | 16/M | Systemic lymph nodes | IV | DOD 1 year after CHX and BMT | |
| Isimbaldi et al [29] | 1 | 9/F | Left cervical mass | I | DOD 9 months after CHX |
| Bubala et al [30] | 1 | 9/M | Systemic lymph nodes with bony lesions | III | DOD 5 months after CHX |
| Reichard et al [31] | 1 | 41/F | Cervical lymph node | I | Alive without disease 58 months after CHX and local XTR |
| 2 | 49/F | Cervical lymph node | I | Alive without disease 36 months after CHX and local XTR | |
| 3 | 71/M | Nasopharyngeal mass | IV | DOD 22 months after CHX and local XTR | |
| 4 | 53/M | Cervical lymph node | I | Alive on CHX | |
| Stachurski et al [32] | 1 | 33/M | Right neck lymph node | IV | Alive with recurrent disease 10 months after CHX |
| Lee et al [33] | 1 | 26/F | Axillary lymph node | IV | Lost to followup 6 months after CHX |
| 2 | 35/M | Axillary lymph node | IV | DOD 18 months after CHX and XTR | |
| 3 | 24/M | Neck lymph node | IV | DOD 17 months after CHX, BMT and local XTR | |
| Momose et al [34] | 1 | 53/M | Left supraclavicular lymph node | IV | Alive with recurrent disease 4 months after CHX |
| 2 | 41/M | Abdominal lymph nodes | IIE | Alive with CHX | |
| Personal experience | 1 | 57/M | Inguinal lymph node | IV | DOD 2 years after CHX |
| 2 | 14/M | Cervical lymph node | I | Alive without disease 14 months after CHX |
CHX, chemotherapy with various BMT, bone marrow or peripheral cytotoxic and/or cytostatic agents; XTR, radiation therapy; DOD, died of diease; blood stem cell transplantation; N/A, not available.
The most common anatomic site of involvement is cervical lymph node. However, any lymph node can be involved and systemic lymphadenopathy and extranodal presentation is not uncommon. Despite aggressive treatment, approximately half of the patients died of disease 4-26 months after therapy, a prognosis similar to other diffuse large B-cell lymphomas, but worse than the more common ALK-positive anaplastic large cells lymphoma [35, 36]. The outcome is not much different in patients younger than 18 with relatively early stage disease at diagnosis (Table 1).
Histopathology
The lymph node architecture in almost all cases is partially or completely effaced by a diffuse proliferation of large neoplastic lymphoid cells (Figure 1). Focal sinusoidal infiltration, coagulative necrosis and starry-sky pattern may be present [17, 31]. Cases with a prominent intravascular component has also been described [31].
Figure 1.
Partial architectural effacement of the inguinal lymph node from a 57 year old male by ALK+ LBCL with an immunoblastic morphology (H&E staining. A, 4×; B, 40×). Another ALK+LBCL from a 14 year old male with diffuse architectural effacement and a piasmabiastic morphology (H&E staining. C. 4×; D, 40×).
Cytologically, the lymphoma cells in all reported cases exhibit either an immunoblastic or plasmablastic morphology with a round, centrally or eccentrally located nucleus, a prominent central nucleolus and a moderate amount of eosinophilic or amphophilic cytoplasm (Figure 1). Occasional binucleated or multinucleated cells mimicking Reed-Stern berg cells may also be seen [31]. In extranodal sites, the lymphoma cells in ALK+ LBCL cells may form cohesive sheets resembling nonhematolymphoid neoplasms [21, 24].
Immunophenotype
Extensive immunophenotypic profiling of ALK+ LBCL by flow cytometry has been unsuccessful due to the cohesiveness and immunoblastic/plasmablastic morphology of the lymphoma cells. Among the reported cases, only one had a flow cytometric immunophenotyping analysis performed successfully [31]. The lymphoma cells in this case demonstrated increased side angle light scatter properties, and expressed CD45, CD4, HLA-DR and moderate density CD38. As expected, they were negative for mature B cells markers including CD19, CD20, CD23, FMC7 and surface immunoglobulin light chains. They also failed to express T cell (CD2, CD3, CD5, CD7 and CD8), myelomonocytic (CD14 and CD33) lineage markers as well as CD10.
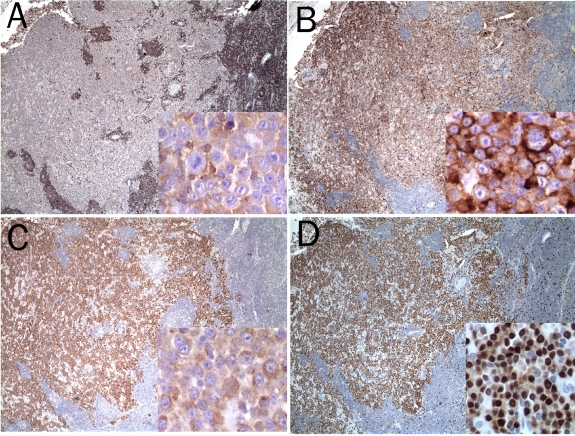
Immunohistochemical staining with a panel of antibodies has been the mainstay to characterize the neoplastic cells of ALK+ LBCL (Figures 2 and 3). As summarized in Table 2, the lymphoma cells are uniformly positive for CD138/VS38c, MUM1 and epithelial membrane antigen (EMA). Most of them demonstrate intracellular immunoglobulin light chain restriction and show a preferential usage of IgA over IgG (18 vs 3). They have lost the B cell lineage-specific markers CD19, CD20, CD22 and CD79a with only focal and weak expression of these markers in few cases, suggesting terminal plasma cell differentiation. Interestingly, CD4 expression was observed in three quarter and CD57 in about one third of the cases examined. The expression of other T cell, natural killer cell and myelomonocytic cell lineage markers, including CD56 and CD68, was absent. There was no evidence of EBV infection.
Figure 2.
Immunohistochemical stains showing the lymphoma cells to be weakly positive for CD45 (A), strongly positive for CD138 (B), ALK (C) and MUM1 (D) (4× and 40× for insets).
Figure 3.

Immunohistochemical stains for kappa (A) and lambda (B) immunoglobulin light chains showing the lymphoma cells to be kappa light chain-restricted (20×).
Table 2.
Immunophenotypic profile of the reported ALK+ LBCL cases
| Antibody | Number of positive cases/total (%) | Staining pattern |
|---|---|---|
| CD20 | 2/41 (5) | Focal and weak |
| CD22 | 0/3 | |
| CD79a | 6/40 (15) | Focal and weak |
| CD19 | 1/3 (33) | Membrane |
| CD138/VS38C | 41/41 (100) | Strong and cytoplasmic |
| CD38 | 5/7 (71) | Membrane and cytoplasmic |
| PAX5 | 2/8 (25) | Focal nuclear |
| MUM1 | 8/8 (100) | Strong nuclear |
| EMA | 39/39 (100) | Strong membrane/cytoplasmic |
| KAPPA/LAMBDA | 29/38 (76) | Strong cytoplasmic |
| IGA/G | 21/30 (70) | Strong cytoplasmic |
| CD45 | 15/21 (71) | Focal and weak |
| CD30 | 4/41 (10) | Focal |
| ALK | 41/41 (100) | Cytoplasmic granular (majority) |
| CD3 | 0/41 (0) | |
| CD4 | 17/23 (74) | Focal and weak |
| CD5 | 0/38 (0) | |
| CD57 | 8/21 (38) | Focal |
| CD43 | 4/15 (27) | Focal |
| CD56 | 0/8 (0) | |
| TIA1 | 0/6 (0) | |
| Perforin | 1/2 (50) | Strong cytoplasmic |
| KI-67 | 4/4 | 50-90% |
| AE1/AE3 | 1/5 (20) | Focal |
| BCL2 | 0/5 (0) | |
| EBV | 0/13 (0) |
Figure 4.
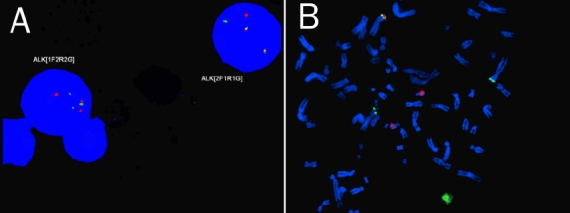
Fluorescent in situ hybridization of interphase (A) and metaphase (B) nuclei using ALK breakapart probe (LSI Alk, Vysis/Abbott, Downers Grove, USA) demonstrating the reciprocal transiocation of t(2;17) involving/ALKon chromosome 2 and presumably CLTC on chromosome 17.
In contrast to ALK-positive anaplastic large cell lymphoma, CD30 expression is essentially absent. Though a few cases were reportedly positive for CD30, the expression was either focal or weak. Expression of CD45 is absent in about a quarter of the cases reported. In light of its cytomorphology, uniform expression of EMA and, in rare cases, focal expression of cytokeratins, ALK+ LBCL may occasionally be confused with metastatic carcinoma.
As the name implies, the neoplastic cells in all cases of ALK+ LBCL strongly express ALK (Figure 2C). The majority of them have a granular cytoplasmic staining pattern with rare exceptions, which demonstrate cytoplasmic and nuclear staining [19, 22, 33].
Molecular Genetics
Initial studies by Delsol et al failed to demonstrate the presence of NPM-ALK by RT-PCR in 3 of 7 ALK+ LBCL cases studied [17]. Subsequent reports have shown that the majority of ALK+ LBCL cases contain CLTC-ALK fusion gene secondary to t(2;17) transiocation as demonstrated by either conventional karyotyping, FISH or RT-PCR (Table 3). It is possible that the cases originally reported by Delsol et al may instead harbor the t(2;17) and RT-PCR designed specifically for NPM-ALK fusion gene may have failed to detect CLTC-ALK. A small subset of ALK-positive ana plastic large cell lymphoma and inflammatory myofibroblastic tumor also harbor the t(2;17) transiocation and aberrantly overexpress ALK [38-41], suggesting a broader role of CLTC-ALK fusion protein in tumorigenesis.
Table 3.
Cytogenetic and molecular features of the reported ALK+ LBCL cases
| Authors | Case studied/total | Cytogenetics | Molecular genetics |
|---|---|---|---|
| Delsol et al [17] | 3/7 | N | ND |
| Gascoyne et al [19] | 5/5 | t(2;17;7)(p23;q23;q?22) | CLTC-ALK by FISH |
| De Paepe et al [20] | 3/3 | t(2;17)(p23;q23) | CLTC-ALK by FISH |
| Chikatsu et al [21] | 1/1 | t(2;17)(p23;q23) | CLTC-ALK by RT-PCR |
| Onciu et al [22] | 2/2 | t(2;5)(p23;q35) | NPM-ALK by RT-PCR |
| Adam et al [23] | 1/1 | t(2;5)(p23;q35) | NPM-ALK by RT-PCR |
| McManus et al [24] | 1/1 | N/A | CLTC-ALK by RT-PCR |
| Colomo et al [37] | 0/1 | N/A | N/A |
| Ishii et al [26] | 0/1 | N/A | N/A |
| Rudzki et al [27] | 1/2 | N/A | NPM-ALK by RT-PCR |
| Gesk et al [28] | 3/3 | N/A | CLTC-ALK by FISH |
| Isimbaldi et al [29] | 1/1 | N/A | CLTC-ALK by RT-PCR |
| Bubala et al [30] | 1/1 | N/A | CLTC-ALK by FISH |
| Reichard et al [31] | 1/4 | N/A | CLTC-ALK by FISH |
| Stachurski et al [32] | 1/1 | t(2;17)(p23;q23) | CLTC-ALK by FISH |
| Lee et al [33] | 3/3 | N/A | CLTC-ALK by FISH |
| Momose et al [34] | 2/2 | N/A | CLTC-ALK by RT-PCR |
| Personal experience | 1/2 | t(2;17)(p23;q23) | CLTC-ALK by FISH |
FISH, fluorescence in situ hybridization; RT-PCR, reverse transcription polymerase chain reaction; ALK, anaplastic lymphoma kinase; CLTC, clathrin; N/A, not available; N, negative for translocation involving ALK by karyotyping; ND, No NPM-ALK by RT-PCR
The ALK gene is located on chromosome 2p23. It is normally expressed in the developing central nervous system, but not in hematopoietic cells [3-5]. Aberrant expression of ALK has been demonstrated in ALK-positive anaplastic large cell lymphoma via chromosomal translocations, causing fusion of ALK with a variety of partners [42]. The most common transiocation is t(2;5)(p23;q25), resulting in a 80 kd fusion NPM-ALK [1]. It occurs in about 75% of anaplastic large cell lymphoma. Other translocations include t(1;2)(q25;q23)[TPM3-ALK], t(2;3)(p23;q21) [TGF-ALK], inv2(p23;q35)[ATIC-ALK], t(2;X)(p23;q11-12) [MSN-ALK], t(2;19)(p23;q13) [TPM4-ALK], t(2;17)(p23;q25)[AL017-ALK] and t(2;22)(p23;q11.2)[MYH9-ALK].
Abnormal expression of ALK has also been observed in solid tumors [6-16]. Soda et al first demonstrated inv(2)(p21;p23) in a subset of non-small cell carcinoma of lung, resulting in formation EML4-ALK fusion protein [11, 43]. More recently, several groups have identified ALK gene mutation and amplification in familiar as well as sporadic neuroblastomas [12, 13, 15, 16]. NPM-ALK transgenic mice developed both B and T cell lymphomas [44-48]. These findings suggest an important role of ALK activation in the pathogenesis of malignant lymphoma and solid tumors.
The physiological target(s) or substrate(s) of ALK remains elusive. Much of the studies have been focusing on the fusion protein NPM-ALK with constitutive tyrosine kinase activity [42, 49]. NPM-ALK has been shown to interact with numerous intracellular targets involved in signal transduction pathways important for cell proliferation, cell cycle progression and apoptosis [42, 44, 50]. These include the JAK/STAT pathway, m-TOR pathway as well as the SHIP2 tyrosine phosphatase negative regulatory loop. More recently, NPM-ALK has been shown to upregulate the expression of an immunosuppressive molecule on the cell surface, CD274 [51], suggesting a role in tumor evasion of the human immune surveillance. Little is known about the targets of CLTC-ALK fusion protein. Momose et al [34] demonstrated hyperactivation of STAT3 in ALK+ LBCL compared to ALK- LBCL, suggesting that the CLTC-ALK fusion protein may also act through the JAK/STAT pathway to induce malignant transformation.
Differential Diagnosis
The characteristic morphologic and immunophenotypic profiles should allow for distinction of ALK+ LBCL from other entities including anaplastic large cell lymphoma, plasmablastic myeloma, metastatic carcinoma and other morphologic variants of diffuse large B-cell lymphoma (immunoblastic, plasmablastic and anaplastic). Anaplastic large cell lymphoma is usually strongly positive for CD30 with a T-cell phenotype, negative for plasma cell markers CD138, MUM1 and intracellular monoclonal immunoglobulin light or heavy chain proteins, and frequently demonstrates molecular evidence of clonal T-cell receptor gene rearrangement. Plasmablastic myeloma has not been reported to express ALK, and would be associated with other myeloma features such as lytic bone lesions and serum or urine paraproteins. Plasmablastic lymphoma has an immunophenotype similar to ALK+ LBCL, but they tend to occur in the oral cavity of patient with HIV infection. They are usually EBV-positive and always ALK-negative. Anaplastaic variant of diffuse large B-cell lymphoma can be easily distinguished from ALK+ LBCL because B-cell lineage specific markers such as CD20 and CD79 are strongly positive, and ALK is always negative. Occasionally, metastatinc carcinoma may enter the differential diagnosis because focal cytokeratin staining has been seen in rare ALK+ LBCL cases. However, evidence of plasma cell differentiation with light chain or heavy chain restriction distinguishes ALK+ LBCL from metastatic carcinoma.
In conclusion, ALK+ LBLC is a rare subtype of diffuse large B-cell lymphoma with a characteristic histomorphology, immunophenotypic profile, recurrent cytogenetic abnormality and dismal prognosis. It should be distinguished from other subtypes of diffuse large B-cell lymphoma, ALK-positive anaplastic large cell lymphoma, plasmablastic myeloma, and nonhematolymphoid neoplasms using a panel of antibodies and molecular techniques if needed. Recent in vitro and animal studies have shown promise of immunotherapy using ALK as a vaccine or targeted therapy with small molecule inhibitors of ALK [52-55], providing potential new treatment modalities for ALK+ LBCL.
References
- 1.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 2.Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, Mori N, Fujimoto J, Miyauchi J, Mikata A, Nanba K, Takami T, Yamabe H, Takano Y, Izumo T, Nagatani T, Mohri N, Nasu K, Satoh H, Katano H, Yamamoto T, Mori S. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood. 1995;86:1954–1960. [PubMed] [Google Scholar]
- 3.Mourali J, Benard A, Lourenco FC, Monnet C, Greenland C, Moog-Lutz C, Racaud-Sultan C, Gonzalez-Dunia D, Vigny M, Mehlen P, Delsol G, Allouche M. Anaplastic lymphoma kinase is a dependence receptor whose proapoptotic functions are activated by caspase cleavage. Mol Cell Biol. 2006;26:6209–6222. doi: 10.1128/MCB.01515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernersson E, Khoo NK, Henriksson ML, Roos G, Palmer RH, Hallberg B. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr Patterns. 2006;6:448–461. doi: 10.1016/j.modgep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Souttou B, Carvalho NB, Raulais D, Vigny M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:9526–9531. doi: 10.1074/jbc.M007333200. [DOI] [PubMed] [Google Scholar]
- 6.Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, Hill DA. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–1371. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Tsuzuki T, Magi-Galluzzi C, Epstein JL. ALK-1 expression in inflammatory myofibroblastic tumor of the urinary bladder. Am J Surg Pathol. 2004;28:1609–1614. doi: 10.1097/00000478-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Tan LH, Tan PH, Tan SY, Ventura R, Yip GW, Zhou YC, Do E, Koay ES, Kwan C, Poh BK, Pen S. Inflammatory myofibroblastic tumour of the bladder may express anaplastic lymphoma kinase by translocation-dependent and translocation-independent mechanisms: a report of two cases. Histopathology. 2007;50:278–282. doi: 10.1111/j.1365-2559.2007.02575.x. [DOI] [PubMed] [Google Scholar]
- 9.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 10.Li XQ, Hisaoka M, Shi DR, Zhu XZ, Hashimoto H. Expression of anaplastic lymphoma kinase in soft tissue tumors: an immunohistochemical and molecular study of 249 cases. Hum Pathol. 2004;35:711–721. doi: 10.1016/j.humpath.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 12.George RE, Sanda T, Hanna M, Frohling S, Luther W, 2nd, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, Xue L, Zozulya S, Gregor VE, Webb TR, Gray NS, Gilliland DG, Diller L, Greulich H, Morris SW, Meyerson M, Look AT. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamant L, Pulford K, Bischof D, Morris SW, Mason DY, Delsol G, Mariame B. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am J Pathol. 2000;156:1711–1721. doi: 10.1016/S0002-9440(10)65042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, Combaret V, Raynal V, Puisieux A, Schleiermacher G, Pierron G, Valteau-Couanet D, Frebourg T, Michon J, Lyonnet S, Amiel J, Delattre O. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, Nakagawara A, Hayashi Y, Mano H, Ogawa S. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 17.Delsol G, Lamant L, Mariame B, Pulford K, Dastugue N, Brousset P, Rigal-Huguet F, al Saati T, Cerretti DP, Morris SW, Mason DY. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2; 5 translocation. Blood. 1997;89:1483–1490. [PubMed] [Google Scholar]
- 18.Delsol G, Campo E, Gascoyne RD. ALK-positive large B-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stain H, Thiele J, Vardiman JW, editors. WHO Classification of Tumors of Haematopoietic and Lymphoid tissues. 4th ed. Lyon: IARC; 2008. pp. 254–255. [Google Scholar]
- 19.Gascoyne RD, Lamant L, Martin-Subero JI, Lestou VS, Harris NL, Muller-Hermelink HK, Seymour JF, Campbell LJ, Horsman DE, Auvigne I, Espinos E, Siebert R, Delsol G. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003;102:2568–2573. doi: 10.1182/blood-2003-03-0786. [DOI] [PubMed] [Google Scholar]
- 20.De Paepe P, Baens M, van Krieken H, Verhasselt B, Stul M, Simons A, Poppe B, Laureys G, Brons P, Vandenberghe P, Speleman F, Praet M, De Wolf-Peeters C, Marynen P, Wlodarska I. ALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102:2638–2641. doi: 10.1182/blood-2003-04-1050. [DOI] [PubMed] [Google Scholar]
- 21.Chikatsu N, Kojima H, Suzukawa K, Shinagawa A, Nagasawa T, Ozawa H, Yamashita Y, Mori N. ALK+, CD30-, CD20- large B-cell lymphoma containing anaplastic lymphoma kinase (ALK) fused to clathrin heavy chain gene (CLTC) Mod Pathol. 2003;16:828–832. doi: 10.1097/01.MP.0000081729.40230.1F. [DOI] [PubMed] [Google Scholar]
- 22.Onciu M, Behm FG, Downing JR, Shurtleff SA, Raimondi SC, Ma Z, Morris SW, Kennedy W, Jones SC, Sandlund JT. ALK-positive plasmablastic B-cell lymphoma with expression of the NPM-ALK fusion transcript: report of 2 cases. Blood. 2003;102:2642–2644. doi: 10.1182/blood-2003-04-1095. [DOI] [PubMed] [Google Scholar]
- 23.Adam P, Katzenberger T, Seeberger H, Gattenlohner S, Wolf J, Steinlein C, Schmid M, Muller-Hermelink HK, Ott G. A case of a diffuse large B-cell lymphoma of plasmablastic type associated with the t(2;5)(p23;q35) chromosome translocation. Am J Surg Pathol. 2003;27:1473–1476. doi: 10.1097/00000478-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 24.McManus DT, Catherwood MA, Carey PD, Cuthbert RJ, Alexander HD. ALK-positive diffuse large B-cell lymphoma of the stomach associated with a clathrin-ALK rearrangement. Hum Pathol. 2004;35:1285–1288. doi: 10.1016/j.humpath.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Colomo L, Loong F, River S, Pittaluga S, Martinez A, Lopes-Guillermo A, Ojanguren J, Romagosa V, Jaffe ES, Campo E. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogenous group of disease entities. Am J Surg Pathol. 2004;28:736–747. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]
- 26.Ishii K, Yamamoto Y, Nomura S. [CD30-negative diffuse large B-cell lymphoma expressing ALK] Rinsho Ketsueki. 2005;46:501–506. [PubMed] [Google Scholar]
- 27.Rudzki Z, Rucinska M, Jurczak W, Skotnicki AB, Maramorosz-Kurianowicz M, Mruk A, Pirog K, Utych G, Bodzioch P, Srebro-Stariczyk M, Wlodarska I, Stachura J. ALK-positive diffuse large B-cell lymphoma: two more cases and a brief literature review. Pol J Pathol. 2005;56:37–45. [PubMed] [Google Scholar]
- 28.Gesk S, Gascoyne RD, Schnitzer B, Bakshi N, Janssen D, Klapper W, Martin-Subero JI, Parwaresch R, Siebert R. ALK-positive diffuse large B-cell lymphoma with ALK-Clathrin fusion belongs to the spectrum of pediatric lymphomas. Leukemia. 2005;19:1839–1840. doi: 10.1038/sj.leu.2403921. [DOI] [PubMed] [Google Scholar]
- 29.Isimbaldi G, Bandiera L, d'Amore ES, Conter V, Milani M, Mussolin L, Rosolen A. ALK-positive plasmablastic B-cell lymphoma with the clathrin-ALK gene rearrangement. Pediatr Blood Cancer. 2006;46:390–391. doi: 10.1002/pbc.20540. [DOI] [PubMed] [Google Scholar]
- 30.Bubala H, Maldyk J, Wlodarska I, Sonta-Jakimczyk D, Szczepanski T. ALK-positive diffuse large B-cell lymphoma. Pediatr Blood Cancer. 2006;46:649–653. doi: 10.1002/pbc.20396. [DOI] [PubMed] [Google Scholar]
- 31.Reichard KK, McKenna RW, Kroft SH. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. Mod Pathol. 2007;20:310–319. doi: 10.1038/modpathol.3800742. [DOI] [PubMed] [Google Scholar]
- 32.Stachurski D, Miron PM, Al-Homsi S, Hutchinson L, Harris NL, Woda B, Wang SA. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma with a complex karyotype and cryptic 3' ALK gene insertion to chromosome 4 q22-24. Hum Pathol. 2007;38:940–945. doi: 10.1016/j.humpath.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Lee HW, Kim K, Kim W, Ko YH. ALK-positive diffuse large B-cell lymphoma: report of three cases. Hematol Oncol. 2008;26:108–113. doi: 10.1002/hon.841. [DOI] [PubMed] [Google Scholar]
- 34.Momose S, Tamaru J, Kishi H, Mikata I, Mori M, Toyozumi Y, Itoyama S. Hyperactivated STAT3 in ALK-positive diffuse large B-cell lymphoma with clathrin-ALK fusion. Hum Pathol. 2009;40:75–82. doi: 10.1016/j.humpath.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Nasr MR, Laver JH, Chang M, Hutchison RE. Expression of anaplastic lymphoma kinase, tyrosine-phosphorylated STAT3, and associated factors in pediatric anaplastic large cell lymphoma: A report from the children's oncology group. Am J Clin Pathol. 2007;127:770–778. doi: 10.1309/FNY8Y4H6PK1V2MGE. [DOI] [PubMed] [Google Scholar]
- 36.Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 37.Colomo L, Loong F, River S, Pittaluga S, Martinez A, Lopes-Guillermo A, Ojanguren J, Romagosa V, Jaffe ES, Campo E. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogenous group of disease entities. Am J Surg Pathol. 2004;28:736–747. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]
- 38.Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B, De Wolf-Peeters C, Pauwels P, Hagemeijer A, Marynen P. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2002;34:354–362. doi: 10.1002/gcc.10033. [DOI] [PubMed] [Google Scholar]
- 39.Bellezza G, Cavaliere A, Del Sordo R, Sidoni A. Inflammatory myofibroblastic tumor of the larynx with anaplastic lymphoma kinase (ALK) protein overexpression. A case report. Tumori. 2006;92:449–451. [PubMed] [Google Scholar]
- 40.Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 41.Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, Lui MY, Colleoni GW, Antonescu CR, Ladanyi M, Morris SW. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol. 2001;159:411–415. doi: 10.1016/S0002-9440(10)61711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 43.Inamura K, Takeuchi K, Togashi Y, Nomura K, Ninomiya H, Okui M, Satoh Y, Okumura S, Nakagawa K, Soda M, Choi YL, Niki T, Mano H, Ishikawa Y. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 44.Chiarle R, Gong JZ, Guasparri I, Pesci A, Cai J, Liu J, Simmons WJ, Dhall G, Howes J, Piva R, Inghirami G. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood. 2003;101:1919–1927. doi: 10.1182/blood-2002-05-1343. [DOI] [PubMed] [Google Scholar]
- 45.Jager R, Hahne J, Jacob A, Egert A, Schenkel J, Wernert N, Schorle H, Wellmann A. Mice transgenic for NPM-ALK develop non-Hodgkin lymphomas. Anticancer Res. 2005;25:3191–3196. [PubMed] [Google Scholar]
- 46.Turner SD, Alexander DR. What have we learnt from mouse models of NPM-ALK-induced lymphomagenesis? Leukemia. 2005;19:1128–1134. doi: 10.1038/sj.leu.2403797. [DOI] [PubMed] [Google Scholar]
- 47.Turner SD, Merz H, Yeung D, Alexander DR. CD2 promoter regulated nucleophosmin-anaplastic lymphoma kinase in transgenic mice causes B lymphoid malignancy. Anticancer Res. 2006;26:3275–3279. [PubMed] [Google Scholar]
- 48.Turner SD, Tooze R, Maclennan K, Alexander DR. Vav-promoter regulated oncogenic fusion protein NPM-ALK in transgenic mice causes B-cell lymphomas with hyperactive Jun kinase. Oncogene. 2003;22:7750–7761. doi: 10.1038/sj.onc.1207048. [DOI] [PubMed] [Google Scholar]
- 49.Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110:2259–2267. doi: 10.1182/blood-2007-04-060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu F, Wang P, Young LC, Lai R, Li L. Proteome-wide identification of novel binding partners to the oncogenic fusion gene protein, NPM-ALK, using tandem affinity purification and mass spectrometry. Am J Pathol. 2009;174:361–370. doi: 10.2353/ajpath.2009.080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li R, Morris SW. Development of anapiastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev. 2008;28:372–412. doi: 10.1002/med.20109. [DOI] [PubMed] [Google Scholar]
- 53.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anapiastic lymphoma kinase and c-Met, in experimental models of anapiastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 54.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, Koudriakova TB, Alton G, Cui JJ, Kung PP, Nambu MD, Los G, Bender SL, Mroczkowski B, Christensen JG. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 55.Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, Xia G, Steensma R, Chopiuk G, Jiang J, Wan Y, Ding P, Liu Y, Sun F, Schultz PG, Gray NS, Warmuth M. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci USA. 2007;104:270–275. doi: 10.1073/pnas.0609412103. [DOI] [PMC free article] [PubMed] [Google Scholar]