Abstract
Patients with locally advanced cancer or distant metastasis frequently receive prolonged treatment with chemotherapy and/or fractionated radiotherapy (RT). Despite the initial clinical response, treatment resistance frequently develops and cure in these patients is uncommon. Developments in RT technology allow for the use of high-dose (or ablative) RT to target local tumors, with limited damage to the surrounding normal tissue. We report that reduction of tumor burden after ablative RT depends largely on T-cell responses. Ablative RT dramatically increases T-cell priming in draining lymphoid tissues, leading to reduction/eradication of the primary tumor or distant metastasis in a CD8+ T cell–dependent fashion. We further demonstrate that ablative RT-initiated immune responses and tumor reduction are abrogated by conventional fractionated RT or adjuvant chemotherapy but greatly amplified by local immunotherapy. Our study challenges the rationale for current RT/chemotherapy strategies and highlights the importance of immune activation in preventing tumor relapse. Our findings emphasize the need for new strategies that not only reduce tumor burden but also enhance the role of antitumor immunity.
Introduction
The rationale for radiotherapy (RT) is based on inducing lethal DNA damage to tumor cells or tumor-associated stroma. Cancer patients often receive fractionated RT at low doses (1.5-3 Gy) that are administered daily over weeks, frequently in combination with chemotherapy. RT has traditionally been viewed as immunosuppressive,1 but studies published in recent years have suggested that the effects of RT on the immune system are complex. Lymphocyte radio-sensitivity is well established, but whether various doses or delivery methods can differentially target naive, effector, or regulatory T-cell populations and/or alternative regulatory molecules is still unclear.2–4 Although studies have investigated the potential immunomodulatory effects of localized RT on tumors, there have been conflicting reports as to whether these responses promote or interfere with tumor reduction.5–8
A recent study showed that radiation can trigger signals that stimulate toll-like receptor 4 on dendritic cells (DCs),9 suggesting another dimension of immune modulation by RT. Other studies reported a direct effect of radiation on tumors by either modifying the phenotype of tumor cells to render them more susceptible to vaccine-mediated T-cell killing,10 or altering the tumor microenvironment to promote greater infiltration of immune effector cells.11–13 A large tumor burden often creates severe suppression that prevents effective immune intervention. It has been proposed that local RT can induce a sufficient reduction in tumor burden to allow for therapeutic intervention by immunotherapy, such as vaccination or blockade of inhibitory molecules on immune cells.4,10,14 Whether RT can also generate significant cytotoxic T lymphocytes (CTLs) to kill metastasis has not been well documented.
Advances in RT technology allow for the use of ablative RT to be delivered very precisely to small tumors. For example, stereotactic body RT (SBRT) takes advantage of the technologic advancements in image guidance and radiation dose delivery to direct ablative doses to tumors with acceptable toxicity. Phase 1/2 trials have provided evidence that the potent doses delivered with SBRT may provide results that rival surgery for some localized primary tumors and have efficacy in the oligometastatic setting.15,16 The initial clinical reports indicate that the use of ablative RT is associated with better survival than conventional fractionated treatment, but the mechanisms remain unclear.15–18 Using an animal model, we unexpectedly observed that ablative RT (15-25 Gy × 1) alone generates strong enough CD8+ T cell–dependent immunity to lead to tumor reduction, reduced relapse of primary tumor, and even eradication of metastasis in some settings. The clinical implication of this study is that some current strategies using fractionated RT or chemotherapy may limit RT-mediated immunity and increase relapse over time. Indeed, we also demonstrate that ablative RT-mediated immunity is erased by current conventional chemotherapy and prolonged fractionated RT, resulting in early relapse because of inadvertent suppression of protective immune responses. On the other hand, ablative RT followed by proper immunotherapy can synergistically overcome the tumor barriers and generate more cytotoxic T cells that circulate systemically to eradicate micrometastasis. Therefore, we should revisit our current strategies and develop new approaches that can reduce tumor burden while boosting protective immunity.
Methods
Mice, cell line, and reagents
C57BL/6, Nude, B6/Rag, OTI, and Balb/c mice were purchased from Jackson Laboratory at 6 to 7 weeks of age. 2C T-cell receptor (TCR)–transgenic mice were provided by Jianzhu Chen (Massachusetts Institute of Technology, Cambridge, MA) and maintained in the specific pathogen–free facility at the University of Chicago. For all experiments, mice were between the ages of 6 to 16 weeks of age, bred under specific pathogen-free conditions, and used in accordance to the animal experimental guidelines set by and with the approval of the Institute of Animal Care and Use Committee. 4T1 is a 6-thioguanine–resistant cell line derived from spontaneous mammary carcinoma.19 B16 lines were obtained from ATCC and maintained by Y.-X.F. and R.R.W. B16-SIY melanoma cells and anti-2C TCR (1B2) antibody were obtained from Tom Gajewski (University of Chicago). B16-CCR7 melanoma cell was generously provided by Sam T. Hwang (National Cancer Institute, Rockville, MD).20 The human lung tumor cell line A549 was purchased from ATCC. All other antibodies for fluorescence-activated cell sorter (FACS) were purchased from BD Biosciences. The generation of ad-LIGHT was described previously.21
Harvesting of mouse lymphoid DCs
For DC harvest for FACS, draining lymph nodes (DLNs) and spleen were digested with 1.5 mg/mL collagenase and 100 μg/mL DNase for 20 minutes at 37°C and then gently pipetted in the presence of 0.01 M ethylenediaminetetraacetic acid for 1 minute. Single-cell suspensions were stained and analyzed by flow cytometry on a FACSCanto (BD Biosciences).
Adoptive transfer of T cells
LN cells and splenocytes were isolated from 2C TCR Tg mice. A total of 2 × 106 2C cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and then adoptively transferred intravenously into B16-SIY tumor-bearing C57BL/6 mice as described previously.21,22 Cells were isolated from the inguinal LNs (DLNs), spleen, or tumors at the time indicated. CFSE dilution was evaluated as described previously.21,22
TCR tetramer and FACS staining
For tetramer staining, tumor, DLN, and spleen were excised from mouse, chopped, and collagenase digested (1.5 mg/mL) for 20 minutes in shaking incubator at 37°C. Single-cell suspensions of cells were incubated with 2.4G2 to block antibody binding to the Fc receptors, CD11c+-allophycocyanin, 1 μg SIY-Kb–specific m67 TCR tetramer-phycoerythrin, and monoclonal antibody CD11b+-peridinin chlorophyll protein-Cy5.5. Samples were analyzed on a FACSCanto (BD Biosciences), and data were analyzed with FlowJo software (TreeStar). The m67 antibody was a generous gift from David Kranz (University of Illinois) and Hans Schreiber (University of Chicago).
Local tumor irradiation and systemic chemotherapy
Mice were irradiated using an x-ray generator (PCM 1000; Pantak) at the doses indicated by each experiment. Each mouse was protected with a lead cover with only tumor exposed, allowing local irradiation. For systemic chemotherapy, tumor-bearing mice were injected intraperitoneally with 20 mg/kg paclitaxel (Ameristat Pharmaceuticals) for 4T1-bearing mice and 200 mg/kg dacarbazine (Bedford Laboratories) for B16-bearing mice.
Tumor injection, treatments, and evaluation of metastases by colonogenic assay
Cultured cancer cells were trypsinized, washed with media, and injected subcutaneously on the back. Tumor size was determined at 3- to 4-day intervals. Tumor volumes were measured along 3 orthogonal axes (a, b, and c) and calculated as tumor volume = abc/2. The tumor nodules were inoculated with indicated amount of ad-LIGHT or ad-control virus intratumorally. For surgical excision of primary 4T1 and B16-CCR7 tumors, mice were anesthetized and tumors were resected with sterilized instruments. A colonogenic assay was used to evaluate metastases in 4T1 and B16-CCR7 tumors as previously described.19 Briefly, lungs for 4T1 tumor or DLN for B16-CCR7 were collected, chopped, and dissociated in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum containing 1.5 mg/mL collagenase type D (Sigma-Aldrich) for 20 minutes in 37°C shaking incubator. Single-cell suspensions were plated at various dilutions in media supplemented with 10% fetal calf serum and selection drug. 4T1 is resistant to 6-thioguanine (60 mM), and B16-CCR7 is resistant to G418 (0.7 mg/mL). Individual colonies representing micrometastases were counted after 5 to 10 days.19
Statistical analysis
Statistics were done using an unpaired Student 2-tailed t test. Error bars represent SD. For survival curves, statistics were done using the log-rank (Mantel-Cox) test.
Results
Ablative RT induces strong T-cell responses leading to tumor rejection
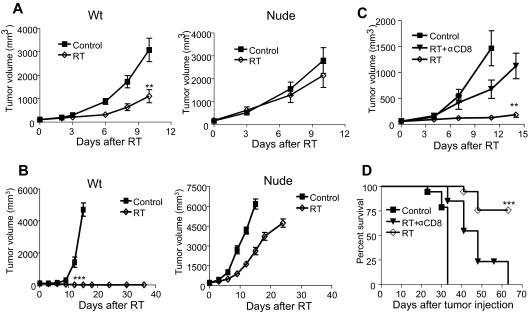
B16 melanoma is well established to be a highly aggressive, rapidly growing, poorly immunogenic, radio-resistant tumor and also known to resist various treatments, including immunotherapies.14,23 Intriguingly, we observed that, after ablative RT (20 Gy × 1), B16 tumors show significant regression in wild-type (WT) mice (Figure 1A) and an increase of infiltrating T cells to the tumor microenvironment and the lymphoid tissues 1 to 2 weeks after treatment (data not shown). This raised the possibility that the sensitivity to RT observed in vivo was T cell–mediated. Therefore, nude mice (T cell–deficient) were used to determine the role of T cells in RT-mediated B16 tumor reduction. Impressively, the tumor remained radio-resistant to ablative RT in the absence of T cells (Figure 1A). Similar results were observed in B and T cell–deficient B6/Rag−/− mice (data not shown). These findings reveal that the therapeutic effect of ablative RT requires T cells.
Figure 1.
Immunodeficiency abrogates the antitumor effect of RT. (A) WT C57BL/6 or nude mice (n = 10) were injected with 2 × 106 B16 melanoma cells and treated 7 days later with 20 Gy. The radiation group in WT but not in nude mice showed significantly smaller tumor size (**P = .002 at day 10 after RT). (B) WT or nude mice (n = 8-12) were injected with 2 × 105 B16-SIY and treated 10 days later with 25 Gy. The radiation group showed significantly smaller tumor size (***P < .001 on day 12 after RT). A similar trend of the inhibition was also detected with single 20 Gy. A total of 60% WT mice were cured, whereas 100% nude mice die with 20 Gy. (C) Tumor growth curve and (D) survival for WT mice injected with 105 B16 and treated on day 14 with 15 Gy given on days 0, 1, and 2 after RT. A total of 200 μg/mouse anti-CD8 antibody was administered on days 0, 4, and 8 after RT (n = 5-9 per group). After RT plus depletion of CD8, the size of tumor increased significantly from RT alone (**P = .007 at day 14). Survival increased after RT (***P < .001), but with CD8 depletion survival was significantly reduced: *P < .05; **P < .01; ***P < .001. Similar experiments were repeated 3 times (A-D).
We wondered whether RT-mediated regression could be influenced by the immunogenicity of the tumor. To test this, we introduced a Kb-binding peptide SIYRYYGL (SIY) into B16 cells (B16-SIY), and tumors remained very aggressive in both WT and nude mice (Figure 1B). We demonstrate that a single dose of RT alone is sufficient to completely reject B16-SIY tumors in 9 of 10 mice, whereas in nude mice, the tumors grew progressively, rapidly killing the host in 9 of 9 mice (Figure 1B). To test whether CD8+ cells, the major killer T cells, are essential for RT-mediated tumor reduction, we treated WT mice bearing established B16 tumors with ablative RT in conjunction with antibody-mediated CD8+ T-cell depletion. The therapeutic effect of RT was largely diminished, and survival decreased by more than 75% in the absence of CD8+ T cells (Figure 1C-D). With CD8+ cell depletion, the tumors become radio-resistant. The depletion of NK cells by anti-NK1.1 does not increase resistance of the tumor (data not shown). These results suggest that ablative RT can elicit a strong CTL response accountable for the reduction or even eradication of established tumor.
RT matures DCs for priming of Ag-specific cells
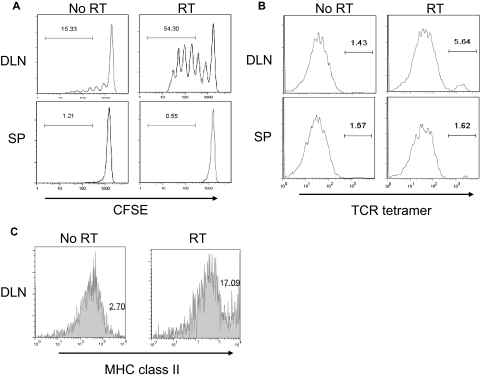
It is unclear how localized ablative RT generates such a strong T-cell response that mediates tumor regression because it has been shown that, by 14 days after implantation, immunity against B16 is lost and immunosuppression becomes dominant.23 Potential immune-stimulatory effects of localized RT on tumors have been reported to be involved in various phases of the immune response.5–8 To address whether a high dose of RT reenergizes the priming phase of naive T cells, naive CD8+ 2C transgenic T cells, which recognize the SIY antigen, were CFSE labeled and then adoptively transferred into B16-SIY tumor-bearing mice that received a high dose of RT on local tumor. The degree of CFSE dilution was determined on CFSE+1B2+CD8+ cells in the DLN and spleen of RT or no-RT mice 4 to 5 days after adoptive transfer. Nominal proliferation was detected in the DLN of the nonirradiated tumor-bearing group, but impressively, transferred Ag-specific naive T cells demonstrated robust priming in the DLNs of the localized RT group (Figure 2A). To test whether the increase of priming occurs not only in non–self-antigens (such as SIY) but also shared self-antigens, T cells from pmel mice specific for endogenous gp100, an antigen expressed on normal melanocytes and the majority of malignant melanomas, were used and also found to exhibit robust proliferation in the DLN (data not shown). Therefore, large single-dose RT on the tumor can alter the tumor microenvironment from that of immune suppressive to immune activating, ultimately resulting in vigorous priming and the expansion of effector T cells with both low and high affinity to antigens.
Figure 2.
RT promotes priming of Ag-specific cells. (A) A total of 5 × 105 B16-SIY tumor cells were subcutaneously injected into the lower back of C57BL/6 (n = 8-9 per group). Fourteen days after tumor challenge, mice received localized RT (20 Gy) on the tumors and were transferred intravenously with CFSE-labeled naive 2C cells. Four to 5 days after adoptive transfer, mice were killed for analysis of DLN and spleen. The degree of CFSE dilution via FACS was determined by gating on the 1B2+CD8+ lymphocyte population. The RT group has more proliferative T cells than the no RT group (***P < .001). (B) A total of 5 × 105 B16-SIY tumor cells were subcutaneously injected into the lower back of C57BL/6 (n = 5 or 6 per group). Fourteen days after tumor challenge, mice received local RT (20 Gy) on the tumors and were killed 5 days later for tetramer+ cell analysis. DLN and spleen were harvested, collagenase digested, and then stained for FACS. Cells were gated on CD11c+ cells. Similar experiments were repeated twice. The RT group has more positive cells than the no-RT group (***P < .001). (C) A total of 2 × 105 B16 tumor cells were subcutaneously injected into the lower back of C57BL/6 mice (n = 4-6 per group). Fourteen days after tumor challenge, mice received localized RT (20 Gy) on the tumors and were analyzed 48 hours later. DLN was isolated, collagenase-digested (1.5 mg/mL), and then stained for FACS. Cells were gated on CD11c+ cells. Mean ± SD for the no-RT group was 6.8 ± 4, and for the RT group 14.6 ± 2. Similar experiments were repeated at least twice.
To determine whether increased priming in the RT group is attributed to reduced T-cell suppression, improved cytokine milieu, increased maturation of DC, or increased cross-presentation of endogenously acquired tumor-derived SIY peptide, we harvested tumor, DLN, and spleen after local RT and stained for antigen-presenting cells expressing SIY peptide using a unique TCR tetramer.24 This tetramer binds to SIY peptide presented by major histocompatibility complex (MHC) class I molecules, thereby allowing us to determine whether SIY+ DCs are increased in tumor or DLN after RT. The results indicate that, 5 days after RT, there was an increase in SIY peptide presenting CD11c+ cells in the DLN (Figure 2B). This increase could be the result of residential DCs in the DLN picking up free-floating antigens or RT-activated DCs inside the tumor that picked up antigens and then migrated to the DLN. To test this, we harvested DCs from tumor tissues and found increased intratumoral CD11c+TCR tetramer+ cells and that they increased their ability to stimulate T cells after local RT (data not shown). Increased T-cell priming in DLN suggests that high-dose RT might activate DCs inside the tumor, which then promotes maturation and migration to the DLN to present antigens to awaiting T cells. To determine whether local RT on the tumor can promote maturation of myeloid DC (mDCs) in the DLN, we analyzed the level of MHC class II on mDCs after RT. Within 48 hours after local RT, there was an increased percentage of MHC class II on mDCs, but not plasmacytoid DCs compared with no RT (Figure 2C; and data not shown). Together, ablative RT on the tumor can activate and mature DCs to subsequently facilitate better T-cell priming.
Radiation-initiated immunity and antitumor effects can be suppressed by either chemotherapy or fractionated radiation
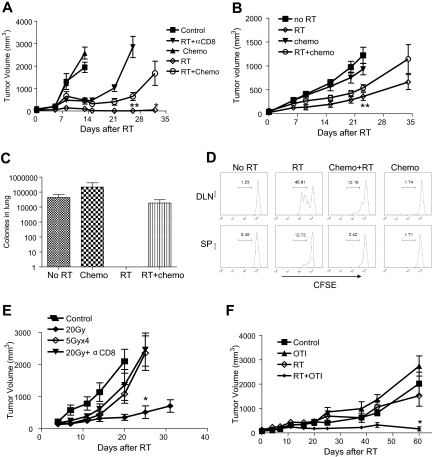
Because chemotherapy is often combined with RT in clinical practice, our results raised concern about the potential immunosuppressive effects of prolonged chemotherapy on RT-mediated immunity. Indeed, chemotherapy given as adjuvant after localized RT significantly hindered tumor regression and promoted melanoma outgrowth (Figure 3A). One of the goals of adjuvant chemotherapy after surgery and radiation is to reduce subclinical metastasis. In the 4T1 breast tumor model, the influence of RT-mediated tumor regression was less pronounced in regards to the primary tumor (Figure 3B), yet impressively, lung metastases with ablative RT were completely eliminated (Figure 3C). The prolonged effect of ablative RT on metastasis suggests a probable immune effect at distal sites more than local ablative effect. Indeed, distant metastasis is dramatically increased with CD8 depletion (data not shown). Unexpectedly, the addition of chemotherapy (paclitaxel) actually increased the number of tumor colonies and exclusively erased the suppressive effect of ablative RT on metastasis (Figure 3C). To test whether the negating effect of chemotherapy (dacarbazine) was directly on CD8+ T-cell priming, naive CFSE-labeled 2C cells were adoptively transferred into B16-SIY tumor-bearing mice that received RT on day 0, chemotherapy on day 2 after transfer, and were then killed on day 4 to determine the degree of T-cell priming in the DLN and the spleen. Our results illustrate that the addition of chemotherapy to the RT group does indeed abolish priming of CD8+ T cells and chemotherapy given alone does not elicit CTL proliferation (Figure 3D). Therefore, it is possible that many types of chemotherapy regimens may forestall CTL generation, especially when followed by local RT, leading to the increased incidence of recurrent tumor.
Figure 3.
Chemotherapy diminishes the effect of radiation-mediated eradication of metastases and T-cell priming. (A) A total of 2 × 105 B16-CCR7 cells were subcutaneously injected; and on days 14, 15, and 16, mice received 15 Gy. On days 7 and 14 after RT, 200 mg/kg dacarbazine (also for human melanoma) was administered intraperitoneally. The radiation group showed a significantly smaller tumor size (***P < .001 at day 13 after RT). Additional dacarbazine after RT led to significant regrowth (**P < .007 at day 26 after RT, *P = .015 day 32 after RT; n = 3-5). (B) Tumor growth curve: 105 4T1 tumor cells were injected; and on days 15, 16, and 17, mice received 15 Gy. On days 7 and 14 after RT, 20 mg/kg paclitaxel was administered intraperitoneally. The radiation group showed significantly smaller tumor size (**P = .008 at day 23; n = 4-9 per group). (C) Metastasis assay: 105 4T1 tumor cells were subcutaneously injected; and on days 12, 13, and 14, Balb/c mice received local RT of 15 Gy. The tumors were removed on day 21. On days 7 and 12 after RT, 20 mg/kg paclitaxel was administered intraperitoneally No colonies were detected after radiation, whereas addition of chemotherapy completely eliminated the effect of radiation (n = 4 or 5 per group). (D) A total of 5 × 105 B16-SIY melanoma cells were injected subcutaneously. On day 17, mice were transferred with 2 × 106 CFSE-labeled 2C cells and locally RT with 20 Gy. A total of 200 mg/kg dacarbazine intraperitoneally was given 2 days after adoptive transfer. DLN and spleen were harvested on day 21 for analysis. (E) A total of 5 × 105 B16-SIY melanoma cells were injected subcutaneously. Mice received local tumor RT of 20 Gy once or 5 Gy × 4. Single-treatment 200 μg/mouse of anti-CD8 antibody was administered on days 0, 4, 8, and 12 after RT. Repeated treatment of radiation showed significant regrowth of tumor mass (*P = .03 at day 25; n = 4-6). (F) A total of 8 × 106 human lung tumor A549 cells were subcutaneously injected into B6/Rag−/− mice; and 4 weeks later, the mice were adoptively transferred with 2 × 106 LN cells from OT-I transgenic mice. Three days later, mice received 20 Gy of local RT. RT (P = .48) or T cells (P = .3) alone showed no significant differences from the no treatment group, whereas the radiation + T-cell group showed significantly smaller tumor size (*P = .018 at day 60). Similar experiments were repeated at least twice (A-F).
Our study also raised concern about fractionated RT, another potentially immunosuppressive conventional treatment. Fractionated RT delivers low daily doses of radiation to the tumor over weeks, in contrast to ablative hypofractionated radiation, which uses 1 to 5 larger doses usually administered in 1 to 7 days. To test the relative effectiveness of these treatment schemes in immune-competent models, we designed 2 protocols delivering the same total RT dose: 5 Gy × 4 over 2 weeks or a single 20-Gy dose. Surprisingly, even though the 5 Gy × 4–treated mice initially responded to RT, over time tumors relapsed in a manner analogous to the CD8-depleted 20-Gy RT condition (Figure 3E). However, in the absence of lymphocytes, B6/Rag−/− showed comparable progressive growth of B16-SIY tumor irrespective of being treated with ablative RT dose of 20 Gy or low dose of 5 Gy × 4. Conversely, WT mice showed considerable delay in 100% of cases (26 of 26 mice), and even complete tumor regression in 35% (9 of 26 mice) when given 20 Gy, but had nominal therapeutic impact with 5 Gy × 4 (0 of 15). It is possible that fractionated low-dose RT may continuously kill off infiltrating effector T cells over time, leading to early relapse or recurrence. It is also possible that the dose of 5 Gy × 4 may not be equivalent to one dose of 20 Gy for direct tumor cell killing. It is important to note that the repair of sublethal damage or proliferation between doses may have accounted for a portion of the inferior tumor control in the fractionated treatment groups. However, we found tumor outgrowth to be comparable with 5 Gy × 4 even when RT was extended to 7 times (data not shown). It is difficult to determine the relative magnitude of these direct effects vs indirect effect via activation of antitumor immunity; but based on the data in Figure 3, it is highly probable that antitumor immunity significantly contributes to the superior response induced by one dose of 20 Gy. Nonetheless, these findings suggest that the current standard practice of fractionated RT may hinder RT-initiated antitumor immunity, resulting in an early relapse of tumor growth or recurrence at both local and distant sites.
RT on human tumor also causes immune-mediated rejection: a new immune xenograft model for preclinical study
Human tumors might respond to various anticancer treatments differently from murine tumors. The most commonly used model for preclinical testing of anticancer agents before clinical trials involves xenografts of human tumors into the immunodeficient nude mouse as required by the US Food and Drug Administration. Indeed, many of our assumptions about the behavior of human tumors in vivo are derived from these types of xenograft models. Our current study now questions whether such models can provide comprehensive evaluation of treatments in the absence of T cells. To overcome the current problem, we developed a novel xenograft model whereby the immune response to RT can be assessed. It is estimated that there are at least 80 mutated antigens per growing tumor, some of which can be presented to T cells in each patient.25,26 Each antigenic epitope has 20 to 200 specific T cells per host.27 Therefore, there are 300 to 3000 tumor-reactive T cells in each immunocompetent host. Transfer of such small numbers of T cells into B6/Rag−/− mice can result in rapid homeostatic proliferation that might artificially activate T cells and reject tumor. To overcome this limitation, our new immune xenograft model integrates a sufficient number of nonresponsive T cells to limit homeostatic proliferation of responsive T cells in B6/Rag−/− mice. CD8+ T cells from MHC class I–restricted OVA specific T-cell receptor transgenic mice (OT-I) contain 97% to 98% OVA-specific T cells that cannot respond to antigens from human tumor but can still effectively inhibit homeostatic proliferation. The remaining 2% to 3% non-OT-I T cells have the potential to recognize antigens from human tumor. We injected human lung tumor A549 cells subcutaneously into B6/Rag−/− mice. After tumors were established for 4 weeks, mice were transferred with 2 × 106 total LN cells (300-3000 tumor-reactive vs 1 million -nonreactive cells from OT-I transgenic mice), which approximates the number of tumor-reactive T cells in human patients. Three days later, mice received localized 20 Gy to the tumor. Interestingly, tumor growth was inhibited only when RT was administered in the presence of CD8+ T cells, and failed when RT was given alone or with adoptive transfer of T cells alone (Figure 3F). These data suggest that the immune-stimulating effects of RT are also applicable to human tumors and accordingly convey the need to revisit conclusions based on models that used immunodeficient mice.
Local immunotherapy can amplify radiation-initiated immunity to eradicate disseminated metastasis
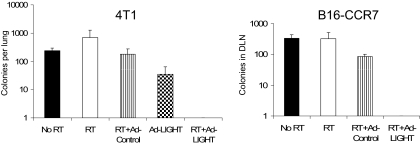
Our study opens the avenue for new strategies, such as RT and immunotherapy, to synergize antitumor effects. Homologous to lymphotoxin, exhibiting inducible expression, and competing with herpes virus glycoprotein D for herpes viral entry mediator on T cells (LIGHT), a tumor necrosis factor superfamily member that is a ligand of stromal cell–expressed lymphotoxin β receptor and T cell–expressed herpes viral entry mediator, has been shown to greatly enhance host responses to progressively growing tumor.21,22 Therefore, we tested whether targeting tumor with ad-LIGHT after a suboptimal dose of RT could amplify radiation-initiated immunity to control metastasis. Mice bearing established 4T1 or B16-CCR7 tumors, which are both spontaneously metastatic tumor lines, received 2 consecutive doses of 12 Gy followed by intratumoral injections of relatively low-dose ad-LIGHT, concomitant with the second dose of radiation. No metastatic colonies in lung or DLN were detected on day 35 only in the RT + ad-LIGHT group (Figure 4). Impressively, most mice (86%) treated with RT and ad-LIGHT showed prolonged survival (> 100 days), whereas all mice treated with either radiation or ad-LIGHT died in less than 60 days (data not shown). Together, these data demonstrate that RT in combination with immunotherapy can better control metastasis than either single treatment.
Figure 4.
The synergy of RT plus ad-LIGHT immunotherapy eradicates distant metastases. 4T1 tumor: Balb/c mice were subcutaneously injected with 105 cells on the lower back. Mice received local RT (12 Gy) on days 14 and 15 and intratumoral injection with ad-control (2 × 1010 virus particles [vp]) or ad-LIGHT (2 × 1010 vp) on days 15 and 16 (n = 24-41 pooled from 5 experiments). B16-CCR7 tumor: C57BL/6 mice were subcutaneously injected with 105 cells on the lower back. Mice received local RT (12 Gy) on days 14 and 15 and intratumoral injection with ad-control (2 × 1010 vp) or ad-LIGHT (2 × 1010 vp) on days 15, 16, and 17 (n = 6-9 pooled from 2 experiments). On day 25 after tumor injection, tumors were surgically removed. Mice were killed on day 35 for tumor colonogenic assay (n = 4 or 5 per group). No colonies were detected in combination group in both types of tumor. Similar experiments were repeated 3 times.
Discussion
Recently, enabled by technologic advances in targeting of RT, there has been an increased interest in using 1 to 3 high doses of radiation in contrast to low-dose fractionation. Initially applied to arteriovenous malformations, benign brain tumors, and brain metastasis (referred to as radiosurgery), there are now clinical trials applying this concept to extracranial targets. Recently, it has been suggested that high-dose single-fraction RT achieves better local control than would be predicted, implicating alternative mechanisms beyond direct killing of tumor cells.28 It has been suggested that damage to the tumor-associated endothelium contributes to the superior local antitumor effect of high-dose radiation.29 We have recently observed that SBRT effectively targets ablative doses of RT to isolated oligometastasis.17,18 Most patients with oligometastasis die in 6 months because of the lack of treatment and rapid progression of tumor. Our clinical data showed that better survival of patients with oligometastasis is closely associated with higher dose of RT: 1 of 6 (15%) of patients progressed with 12 Gy 3 times, 4 of 19 (20%) with 10 Gy 3 times, whereas 19 of 31 (60%) progressed in 6 months when treated with 8 Gy 3 times of RT. In this study, we have now revealed the essential role of the immune response in tumor reduction in modified SBRT, shifting the goal of targeting tumors beyond local control toward generating systemic immunity for the eradication of distant metastases. Further clinical trials are urgently needed to study the role of T cells, chemotherapy, and immunotherapy in SBRT-mediated control of oligometastasis.
Considering that many cancer patients are under immune suppression or will be treated by immunosuppressive drugs before RT, the current ongoing trials using high-dose radiation might underestimate its potency. Many cancer patients with potential metastasis routinely receive prolonged cycles of chemotherapy and conventional/prolonged RT. This combination therapy, whether delivered concurrently or sequentially, is aimed at direct cytotoxic reduction of tumor cells. Indeed, studies have shown that radiation used in conjunction with chemotherapy can synergistically reduce tumor burden in in vitro cultures and in vivo using immune-deficient xenograft models. Our results have revealed that the use of certain immunosuppressive adjuvant chemotherapy actually erases radiation-initiated T-cell priming, challenging the rationale of some current combinations. Studies of RT and chemotherapy using standard lymphocyte-deficient xenograft models or immune-suppressive patients fail to consider an integral effect of the immune response, which might cater to misleading interpretations and conclusions as well as overestimate the therapeutic effect of RT/chemotherapy in immunocompetent hosts. Therefore, our newly generated immune xenograft animal model will allow evaluation of the effect of RT in various types of human tumor in the presence of immune system.
In conclusion, RT disrupts physical and immunologic barriers, introduces danger signaling, increases DC cross-presentation of tumor antigen, and possibly reverses T-cell unresponsiveness in tumor-bearing hosts, leading to the rejection of local and distal tumors. Our study reveals critical insight into the immune-mediated therapeutic effect of RT, potential mobilization of immune response against established tumor, and challenges current intensive RT/chemotherapy protocols. It raises the possibility that, although immunotherapy is a viable alternative, current conventional cancer treatment strategies may cause attenuating effects on the immune system. Data analyzed in the context of immune-suppressed patients for various clinical trials may undermine the potency of immune responses against cancer and lead to misguided interpretation of the results. Therefore, our study unveils a paradigm shift in combined modality treatment of cancer and opens new strategies to mobilize the host immune system and potentially cure patients with metastasis.
Acknowledgments
The authors thank Drs David M. Kranz for the TCR tetramer and Bin Zhang for technical assistance, and Drs Hans Schreiber, Tom Gajewski, and Ping Yu for critical reading. We would like to acknowledge Dr Xinzhong Wang and Campbell Kaynor for preparation of ad-LIGHT.
This work was supported in part by the National Institutes of Health (grants AI062026, CA115540, and CA97296, Y.-X.F; CA111423, R.R.W.) and the Ludwig Foundation (R.R.W., Y.-X.F.). S.L.A. is part of the Medical Scientist Training Program at the University of Chicago and is supported by a Medical Scientist National Research Service Award (5 T32 GM07281).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.L. and S.L.A. designed and performed most experiments and wrote the paper; Yugang Wang and Yang Wang performed the human xenograft model; Y.M. and M.B. performed experiments on nude mice; R.S., B.B., R.C., and T.T. performed some experiments; and R.R.W. and Y.-X.F. organized, designed, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ralph R. Weichselbaum, Department of Radiation and Cellular Oncology, Ludwig Center for Metastasis Research, University of Chicago, 5841 S Maryland Ave, Chicago, IL 60637; e-mail: rrw@radonc.bsd.uchicago.edu; and Yang-Xin Fu, Department of Pathology, University of Chicago, 5841 S Maryland Ave, Chicago, IL 60637; e-mail: yfu@uchicago.edu.
References
- 1.Wasserman J, Blomgren H, Rotstein S, Petrini B, Hammarstrom S. Immunosuppression in irradiated breast cancer patients: in vitro effect of cyclooxygenase inhibitors. Bull N Y Acad Med. 1989;65:36–44. [PMC free article] [PubMed] [Google Scholar]
- 2.Lowenthal JW, Harris AW. Activation of mouse lymphocytes inhibits induction of rapid cell death by x-irradiation. J Immunol. 1985;135:1119–1125. [PubMed] [Google Scholar]
- 3.North RJ. Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation: preferential elimination of suppressor T cells allows sustained production of effector T cells. J Exp Med. 1986;164:1652–1666. doi: 10.1084/jem.164.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 5.Ohuchida K, Mizumoto K, Murakami M, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–3222. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- 6.Merrick A, Errington F, Milward K, et al. Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naive T-cell priming. Br J Cancer. 2005;92:1450–1458. doi: 10.1038/sj.bjc.6602518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 8.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 11.Hatfield P, Merrick A, Harrington K, et al. Radiation-induced cell death and dendritic cells: potential for cancer immunotherapy? Clin Oncol (R Coll Radiol) 2005;17:1–11. doi: 10.1016/j.clon.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Liao YP, Wang CC, Butterfield LH, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–2469. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 13.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 14.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 16.Papiez L, Timmerman R. Hypofractionation in radiation therapy and its impact. Med Phys. 2008;35:112–118. doi: 10.1118/1.2816228. [DOI] [PubMed] [Google Scholar]
- 17.Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Salama JK, Chmura SJ, Mehta N, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res. 2008;14:5255–5259. doi: 10.1158/1078-0432.CCR-08-0358. [DOI] [PubMed] [Google Scholar]
- 19.Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169:5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 20.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 21.Yu P, Lee Y, Wang Y, et al. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol. 2007;179:1960–1968. doi: 10.4049/jimmunol.179.3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu P, Lee Y, Liu W, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 23.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 26.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 27.Moon JJ, Chu HH, Pepper M, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys. 2008;71:324–325. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]