Abstract
One of the central problems in posttraumatic stress disorder (PTSD) is the inability to suppress fear even under safe conditions. The neural underpinnings of fear is a clinically relevant issue that is poorly understood. This study assessed fear potentiation and fear inhibition using fear-potentiated startle in a conditional discrimination procedure (AX+/BX-). We hypothesized that patients with PTSD would show normal fear potentiation and impaired fear inhibition. 28 healthy volunteers and 27 PTSD patients (14 with low current symptoms, 13 with high current symptoms) were presented with one set of colored lights (AX trials) paired with aversive air blasts to the throat, and a different series of lights (BX trials) presented without air blasts. We then presented A and B together (AB trials) to see whether B would inhibit fear potentiation to A. All groups showed robust fear potentiation in that they had significantly greater startle magnitude on AX trials compared to noise alone trials. However, the high symptom PTSD group did not show fear inhibition: these subjects had significantly greater fear potentiation on the AB trials than both the controls and the low symptom PTSD patients.
Keywords: Acoustic Startle Response, Classical Conditioning, Fear-Potentiated Startle, Electromyography, PTSD, Human
1. Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; APA, 1994), posttraumatic stress disorder (PTSD) is characterized by three major symptom clusters following an event that elicited fear, helplessness, or horror. The first category covers symptoms of re-experiencing the event, such as intrusive thoughts, nightmares, and flashbacks induced by reminders of the event. The second cluster includes avoidance of stimuli associated with the trauma, while the final category incorporates symptoms of increased arousal. As described above, one of the central problems in PTSD is the inability to suppress fear under safe conditions. Responses to fearful stimuli can be so powerful that the cortical inputs are not able to inhibit amygdala activity, as indicated by evidence of decreased prefrontal cortex activity (Bremner et al., 1999) and increased amygdala activity (Liberzon et al., 1999) during presentations of traumatic imagery. The amygdala is an integral part of the neural circuit that controls fear-potentiated startle (Davis et al., 1993). The present study used fear potentiation of the startle response to investigate impaired fear inhibition in PTSD.
The acoustic startle response (ASR) is characterized by an integrative, reflex contraction of the skeletal musculature in response to a strong auditory stimulus. ASR provides an excellent model to study emotional processing since the amygdala is directly connected with the startle circuit (Davis et al., 1993). It is controlled by a very small, three-synapse circuit that connects the auditory receptors to the nucleus reticularis pontis caudalis which then projects to the spinal cord and from there to the motor neurons. Both aversive and pleasant emotions can affect startle. Over the last decade numerous studies have been conducted used photographs with strong emotional content from the International Affective Picture System (IAPS, Bradley et al., 2001) and have found the same robust effect: when viewing unpleasant pictures such as threats and assaults startle is potentiated, whereas startle is attenuated during pleasant pictures.
In the present study, fear-potentiated startle is defined by the relative increase in the magnitude of the acoustic startle reflex when elicited in the presence of a conditioned stimulus (CS+) previously paired with an aversive unconditioned stimulus (US). Three studies have examined fear-potentiated startle in combat-related PTSD subjects. The first two studies used an instructed fear paradigm, in which the participants were told which stimuli were associated with the US. These studies found that PTSD subjects demonstrated larger startle magnitude during the entire startle session relative to control subjects. However, the PTSD subjects did not show a greater degree of fear potentiation to the specific threat stimuli compared to controls (Grillon et al., 1998; Morgan et al., 1995). The authors argued that heightened contextual anxiety in the PTSD subjects, rather than more explicit cue fear, accounted for their overall increase in startle. The third study (Grillon and Morgan, 1999) used a conditioning paradigm and found equivalent levels of fear potentiation to the CS+ in the PTSD and control groups. However, the PTSD subjects also potentiated to the CS-, whereas the controls did not. This result indicates that PTSD subjects may either have learning deficits that preclude them from learning about which specific cue predicts the US, or else cannot inhibit fear-potentiated startle even when they are aware of the safety condition. The former conclusion is consistent with the neuroimaging studies reporting decreased hippocampal volume (Bremner et al., 1995) and corresponding deficits in memory in PTSD subjects (Bremner et al., 1993). However, a study by Orr and colleagues that examined fear conditioning in PTSD subjects using skin conductance, heart-rate, and facial electromyogram found that PTSD subjects discriminated between the CS+ and the CS-better than controls (Orr et al., 2000). This finding argues for the alternative explanation, that PTSD subjects can learn safety but have difficulty inhibiting the fear response.
There are two major laboratory models that have been used for testing fear inhibition: extinction and conditioned inhibition. In fear extinction paradigms a stimulus that was paired with an aversive stimulus (the CS+) is repeatedly presented without the US, so that it no longer elicits a fear response (cf. Myers and Davis, 2006; Norrholm et al., 2006). In this case fear inhibition (safety) is learned through many trials of nonreinforced presentations of the CS+. In the conditioned inhibition paradigm, one cue is paired with the aversive stimulus when presented alone (A+), but not when presented in compound with a second cue (AX-). In this model X should become a safety signal because it signals the absence of the aversive stimulus. Moreover, X should transfer inhibition to a different CS+ when presented together (i.e. B+ in training, BX-in test). The problem with this test is that the discrimination can be solved configurally, where A is treated as one stimulus, and the compound, AX, as another unique stimulus. Because people tend to use this configural strategy, it has been difficult to show successful transfer on BX test trials following A+/AX-training (e.g. Grillon and Ameli, 2001).
We have recently developed a modified conditioned inhibition paradigm that allows for the evaluation of fear potentiation and inhibition of fear in humans (Jovanovic et al., 2005). The procedure, referred to as a conditional discrimination (abbreviated as AX+/BX-), was translated from a rodent model of fear inhibition (Myers and Davis, 2004) that was based on a paradigm used in earlier learning theory experiments (Wagner et al., 1968; Wagner and Rescorla, 1972). In this experiment, reinforcement of X is conditional upon the presence of either A or B. Stimulus A potentiates startle as the subject learns that A and X presented together predict the US. Stimulus B becomes the safety signal in that B presented with X predicts the absence of the US. The presentation of A and B together (AB) results in a reduced fear response to A because B transfers its inhibitory property to A. Thus the AB trials are referred to as conditioned inhibition test trials. Although this discrimination can also be solved configurally, we nonetheless found results in humans consistent with these predictions. Thus, we found greater startle magnitude in the presence of AX vs. BX (Jovanovic et al., 2005). More importantly, we also found that startle magnitude in the presence of AB was less than in the presence of AX, the critical transfer test used to determine whether stimulus B is indeed inhibitory. We used this novel procedure to assess whether patients with PTSD could transfer inhibition in a new situation. Current PTSD symptoms can vary largely both within and across individuals; thus the presence of a lifetime diagnosis of PTSD may not be the most relevant indication of the present clinical status. In order to capture the effects of this individual variability, we used a median split to divide the patients into high and low symptom groups according to the symptoms they were experiencing in the last month. We hypothesized that transfer of safety would be related to the current severity of the illness in that the patients with the greatest symptom severity would have the most difficulty inhibiting fear.
2. Methods
2. 1. Participants
Sixty-four male subjects participated in the study after signing a consent form approved by the Emory University Institutional Review Board and the Atlanta VA Research and Development Committee as an indication of their informed consent. The sample included 31 healthy control subjects and 33 PTSD patients. The PTSD subjects were Vietnam veterans seeking treatment for PTSD at the Atlanta VA Medical Center. Controls subjects had no current or lifetime Axis I disorders, including substance abuse and dependence, as ascertained by the Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID) (First et al., 1998) interview. Data from 20 control subjects have been presented in our previous studies (Jovanovic et al., 2005; 2006).
In the patients, lifetime PTSD was confirmed, and comorbid Axis I diagnoses assessed by the SCID, while current PTSD symptoms were assessed using the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1990). Additionally, PTSD subjects rated the severity of their combat exposure with the Combat Exposure Scale (Lund et al., 1984). The PTSD patients were not excluded if they had past or current depression, or past substance abuse or dependence. They were excluded from the study if they ever had a diagnosis of schizophrenia or bipolar depression, or if they had current substance abuse or dependence in the last 3 months. The PTSD patients who were medicated continued to use the medication as prescribed.
All subjects were screened for auditory or visual impairment. Using an audiometer, (Grason-Stadler, Model GS1710) the subjects were required to detect tones at 30 dB[A]SPL at frequencies ranging from 250 to 4000 Hz. Four subjects (3 PTSD patients and 1 control) were screened out due to hearing loss. The subjects were not color-blind and had at least 20/40 vision as tested by eyechart, in both eyes (using correction, if necessary) at day of testing. In addition, all subjects had negative urine toxicology screens. The Wechsler Abbreviated Scale of Intelligence (WASI, Harcourt Assessment, Inc., San Antonio, TX) and the Finger Tapping Test (Raitan and Wolfson, 1985) were used to screen for low IQ and impaired motor performance, respectively. Two PTSD subjects had incomplete interview data and were excluded from the analysis. Furthermore, two control subjects and one PTSD subject were excluded due to IQ scores below 80, resulting in a final sample of 28 controls and 27 patients.
2.2. Startle Procedure
The acoustic startle response (eyeblink component) was measured via electromyography (EMG) of the right orbicularis oculi muscle. Two 5 mm Ag/AgCl electrodes filled with electrolyte gel were positioned approximately 1 cm under the pupil and 1 cm below the lateral canthus and a ground electrode was placed behind the right ear over the mastoid. All resistances were less than 6 kilo-ohms. EMG activity was amplified and digitized using a computerized EMG startle response monitoring system (SR-LAB, San Diego Instruments). The EMG signal was filtered with 30 and 1000 Hz. All acoustic stimuli were delivered binaurally through headphones (Maico, TDH-39-P) while subjects were seated in a sound attenuated booth.
There were three criteria that had to be met for a valid blink response. First, the onset latency (latency from stimulus to commencement of blink reflex) was defined by a shift of 7.33 mV from the baseline value, occurring 21 to 120 ms after the startle stimulus. The baseline value was calculated by taking the average of the minimum and maximum values recorded during the first 20ms after the pulse stimulus. Peak latency (latency from stimulus to maximum blink amplitude) was defined as the point of maximal amplitude occurring no more than 150ms after the pulse alone stimulus. Second, the minimum response criterion for a peak was set at 12.21 mV. On trials in which no scorable blink occurred, amplitude was recorded as zero. Finally, responses in which onset and peak latencies differed by more than 95 ms were considered to be artifactual (not generated by the stimulus) and were discarded. If any of these three criteria were violated, the trial was discarded. Trials were also discarded if excessive EMG activity was observed during the first 20ms of recording, that is, there was a high baseline prior to the startle probe. Less than 5% of the trials were discarded using these parameters. Missing data were replaced with the mean of the corresponding condition.
The startle session began with a one-minute acclimation period consisting of 70-dB A broadband noise, which continued as the background noise throughout the session. The startle probe was either a 104 or 108-dB [A] SPL, 40-ms burst of broadband noise with a near instantaneous rise time. According to methods established by Grillon and Ameli (1998) and used in our prior studies (Jovanovic et al., 2005; 2006; Norrholm et al., 2006) the aversive stimulus (US) was a 250-ms airblast with an intensity of 140 psi directed to the larynx. A, B, C and X were green, purple, orange or blue lights ranging in light transmission from 4.0% to 4.2% (counterbalanced color assignment across subjects). The lights were mounted on the wall of the booth approximately 5 feet from the subject’s seat.
The test session began with a habituation phase consisting of six startle probes (3 at 104 dB and 3 at 108 dB) to reduce initial startle reactivity. In order to minimize individual variability in baseline startle, subjects were assigned either to the 104-dB session or the 108-dB session, based on startle level in the habituation phase. Of the 55 subjects included in the analysis, 38 received the 108 dB sessions, while 17 received the 104 dB sessions. There was no difference in the distribution of the sessions across groups (χ2(2)=2.42, P>0.1).
The conditioning phase included six startle probes presented alone, six trials in which stimuli A and X were paired with the US (AX+), and six trials in which stimuli B and X were not paired with the US (BX-). The AX+ stimuli were presented serially within a trial, and the order of A and X alternated randomly across trials. Figure 1 shows a diagram of the AX+ and BX-trials. The testing phase immediately followed the conditioning phase and consisted of two blocks: each block included six startle probes presented alone and six presentations of AB or AC, respectively. The order of the two blocks (i.e., one with AB trials and one with AC trials) was counterbalanced across subjects. Of the 55 subjects included in the analyses, 30 received AB first, while 25 received AC trials first. A Chi-square analysis of the counterbalancing across the groups indicated equal distribution (χ2(2)=4.64, P=0.1). In all phases of the experiment, inter-trial intervals were of randomized duration ranging from 9 to 22 seconds. There were no breaks between phases and the entire session was under 15 minutes in length. After the session a subset of the subjects (N=33: 13 controls, and 10 in each PTSD group) were asked to rate the aversiveness of the airblast and the startle probe on a scale from one to five.
Figure 1.
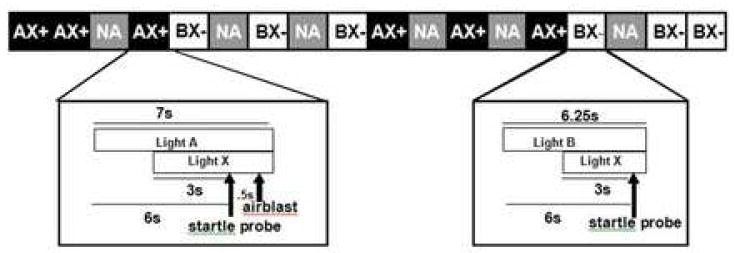
Diagram of the AX+/BX-startle session.
2.3. Expectancy ratings
A three-button response keypad (SuperLab, Cedrus Corp.) was used in the startle sessions in coordination with the EMG startle response monitoring system (SR-LAB, San Diego Instruments) to collect trial by trial ratings of US-expectancy similar to previously published methods (Jovanovic et al., 2005; Jovanovic et al., 2006; Norrholm et al., 2006). Each trial contained two light components (e.g. A and X). Subjects were instructed to respond to each light separately on each trial by pressing one of three buttons: the “+” key when they expected a light to be followed by the airblast (threat), the “—“ key when they did not expect the light to be followed by the airblast (safe), and the “0” key when they were uncertain of what to expect (don’t know). For the purposes of data analysis, subject responses of “+” were scored as +1, responses of “0” were scored as 0, and responses of “—“ were scored as -1 as in our previous work (Norrholm et a., 2006).
Statistical analyses
In order to assess fear potentiation of startle to the reinforced stimulus (AX+), we used a repeated measures analysis of variance (ANOVA) with Trial Type (two levels: noise alone, NA, and AX) as a within-subjects independent variable, Group (three levels: control, low symptom PTSD, and high symptom PTSD) as a between-groups independent variable, and startle magnitude as the dependent variable. The two PTSD groups were derived by performing a median split of their current CAPS scores.). Repeated measures ANOVAs were also used to compare the effects of different stimuli with Trial Type (four levels: AX, BX, AB, and AC) as the within-subjects variable, and Group as a between-groups independent variable. The dependent variable was the percent potentiation from baseline startle calculated using the following formula:
where the mean of the startle magnitude on noise alone (NA) trials (probes delivered in the absence of the light) is subtracted from the startle magnitude during light presentation during the conditioning and testing phase, and this difference is divided by NA, and multiplied by 100. Thus each person’s response was calculated as a ratio of their baseline startle (i.e., startle to NA). Due to the variable nature of the startle response we did not want to use a single data point for each individual and thus averaged three trials of each trial type. For AX+ and BX-trial types we wanted to capture the results of training; therefore we calculated the mean of the last three trials of each type. For AB and AC trial types we wanted to test immediate transfer of safety (without the effects of learning) and thus used only the first three trials of each type.
Significant Trial Type by Group interactions and main effects of Group were followed-up by one-way ANOVAs and LSD post-hoc tests. Main effects of Trial Type were followed-up with specific contrasts comparing AX to BX, AB to AX, AC to AX, and AB to AC. Effect sizes of the individual effects are reported using partial Eta square (η2). In order to correct for violations of the sphericity assumption in the repeated-measures ANOVAs, we used Huynh-Feldt epsilon adjustments. Chi-square analyses were used to analyze categorical data. All analyses were conducted using SPSS 12.0 for Windows (SPSS, Inc.).
3. Results
3.1. Demographics
Table 1 summarizes the demographic and clinical data. Control subjects were slightly younger (mean age=48.29, SD=11.50) than the low symptom PTSD patients (mean age=53.21, SD=6.29) and the high symptom PTSD patients (mean age=53.85, SD=7.30); however, this difference was not significant (F(2,54)=2.09, P>0.1). Race was distributed equally across the three groups (χ2(4)=6.55, P>0.1). The control subjects had more years of education (mean=15.43, SD=1.87) than the low symptom PTSD group (mean=13.36, SD=1.08; F(2,52)=8.70, post-hoc P<0.01) and the high symptom PTSD group (mean=13.96, SD=1.42; F(2,52)=8.70, post-hoc P<0.01).
Table 1.
Demographic and symptom characteristics of subject groups.
| CONTROL | LOW SYM PTSD | HIGH SYM PTSD | F | df | P | |
|---|---|---|---|---|---|---|
| AGE | 48.29 (11.50)a | 53.21 (6.29) | 53.85 (7.30) | 2.09 | 2, 54, | ns |
| EDUCATION | 15.43 (1.87) | 13.36 (1.08) | 13.96 (1.42) | 8.70 | 2, 54 | <0.01 |
| RACE | 8 AA, 20 C | 8 AA, 1 AS, 5 C | 5 AA, 9 C | χ2=6.55 | 4 | ns |
| CAPSb TOTAL | N/A | 49.21 (23.34) | 106.15 (15.51) | 54.77 | 1, 26 | <0.001 |
| CAPS FREQUENCY | N/A | 26.00 (12.66) | 54.85 (9.65) | 43.82 | 1, 26 | <0.001 |
| CAPS INTENSITY | N/A | 23.21 (11.62) | 51.31 (8.00) | 52.71 | 1, 26 | <0.001 |
| CAPS B: RE-EXPERIENCING | N/A | 12.36 (8.38) | 23.54 (4.37) | 18.44 | 1, 26 | <0.001 |
| CAPS C: AVOIDANCE | N/A | 15.07 (9.97) | 31.00 (6.51) | 23.77 | 1, 26 | <0.001 |
| CAPS D: HYPERAROUSAL | N/A | 13.86 (7.49) | 28.92 (3.88) | 42.00 | 1, 26 | <0.001 |
| CESc | N/A | 22.67 (9.29) | 22.91 (6.27) | 0.02 | 1, 26 | ns |
Numbers are means and (SD)
Abbreviations:
Values are mean (SD).
CAPS=Clinician Administered PTSD Scale,
CES=Combat Exposure Scale. AA=African American, C=Caucasian.
3.2. Clinical Assessment
The median CAPS score in the PTSD patients was 82 and the low and high symptom PTSD groups were defined by this criterion. Thus the two symptom groups had significantly different CAPS total scores, as well as CAPS frequency and intensity scores, and CAPS cluster scores. However, the two groups did not differ on severity of combat exposure as assessed by the Combat Exposure Scale (CES).
3.3. Medication
Twenty-five of the 27 PTSD patients were taking psychiatric medication at the time of the study, with the greatest proportion of patients taking antidepressants in the selective serotonin reuptake inhibitor (SSRI) class (11 of 27). A Chi-square analysis indicated that the distribution of prescriptions did not differ between the two PTSD groups (the most common pharmacotherapies were: SSRIs, χ2(1)=0.58, P>0.1; atypical antipsychotics, χ2(1)=1.71, P>0.1; benzodiazepines, χ2(1)=2.19, P>0.1; and anxiolytics, χ2(1)=0.70, P>0.1). Benzodiazepines have been found to reduce baseline startle without affecting fear-potentiated startle (Baas et al., 2002). In our sample, the five patients who were taking benzodiazepines did not have significantly lower baseline startle compared to the other patients (F(1,26)<1, P=0.35).
3. 4. Aversiveness Ratings
The subjects rated the airblast as more aversive (mean=3.09, SE=0.26) than the startle probe (mean=2.57, SE=0.21), (F(1, 30)=6.59, P<0.05). While the airblast was equally aversive to all participants, the PTSD patients rated the startle probe as more aversive (low symptom mean=2.90, SE=1.29; high symptom mean=3.05, SE=1.21) than the control subjects (mean=1.77, SE=1.17), F(2,32)=3.91, P<0.05. Post hoc analyses showed that both patient groups differed from the control group; however, the low symptom patients found the startle equally aversive as the high symptom patients.
3.5. Fear-Potentiated Startle
Baseline startle magnitude, i.e. startle to the sound probe alone, without the presence of lights, did not differ between the three groups (F(2,54)=0.09, P>0.1).However, startle magnitude was robustly potentiated in the presence of the reinforced stimulus, NA vs. AX (Trial Type F(1,53)=59.93, P<0.001, η2=0.49). Figure 2 shows fear-potentiated startle in the three groups.
Figure 2.
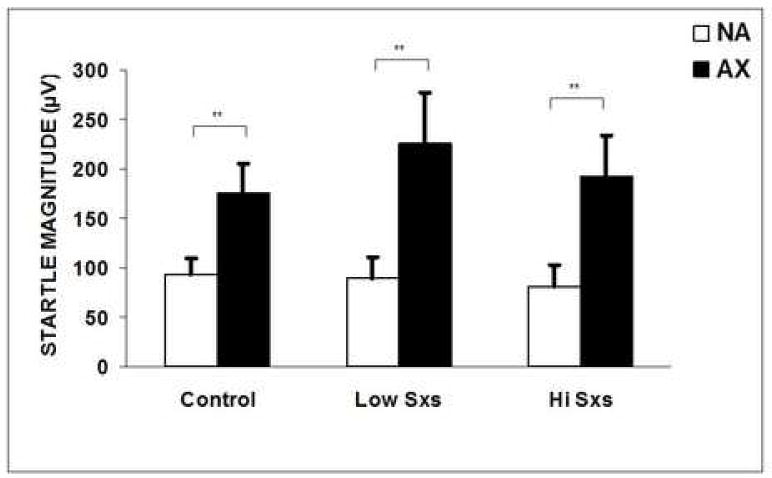
Startle amplitude (mean + SEM) to noise alone (NA) and in the presence of the reinforced stimulus (AX+) in controls, low symptom, and high symptom PTSD subjects. Brackets indicate significant contrasts. *P<0.05. **P<0.01.
There was no interaction effect of Trial Type and Group; i.e., startle was potentiated in the control group (F(1,27)=19.89, P<0.001, η2=0.42), the low symptom PTSD group (F(1,13)=15.50, P<0.01, η2=0.54), and in the high symptom PTSD group (F(1,12)=14.89, P<0.01, η2=0.55).
Figure 3 shows the percent startle potentiation from noise alone in the presence of the four trial types (AX, BX, AB, and AC) in the controls and the two PTSD groups.
Figure 3.
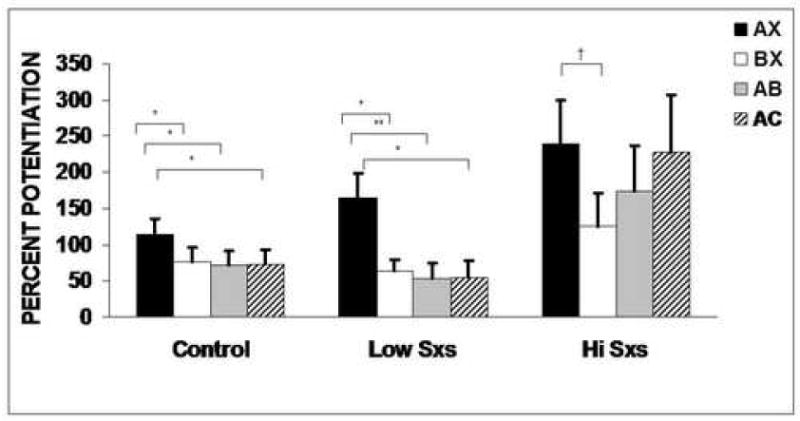
Percent potentiation (mean + SEM) from noise alone to AX+, BX-, AB, and AC trial types in control, low symptom, and high symptom PTSD subjects. Brackets indicate significant contrasts. *P<0.05. **P<0.01. †P<0.1
Analyses of percent potentiation to the different trial types revealed a significant main effect of Trial Type (F(3, 156)=8.08, P<0.001, η2=0.13), and Group (F(2, 52)=4.26, P<0.05, η2=0.14), but there was no Group by Trial Type interaction. Given our apriori hypotheses that high symptom patients will show impaired fear inhibition, we tested planned comparisons for Trial Type separately in each group. The overall effect of trial type was significant in the controls (F(3, 81)=2.96, P<0.05, η2=0.10). However, among the patients, only the low symptom group had a significant effect of trial type (F(3, 39)=4.75, P<0.01, η2=0.27), while the high symptom group did not (F(3, 36)=1.91, P>0.1, η2=0.14). Follow-up contrasts comparing the different trial types within each group showed that startle was potentiated more in the presence of AX than BX in the controls (F(1,27)=4.54, P<0.05, η2=0.14) and the low symptom PTSD group (F(1,13)=6.27, P<0.05, η2=0.32), with a trend for discrimination in the high symptom PTSD group (F(1,12)=3.28, P=0.09, η2=0.21). Potentiation in the presence of AB was significantly inhibited relative to AX in the controls (F(1,27)=5.67, P<0.05, η2=0.17) and the low symptom PTSD group (F(1,13)=9.35, P<0.01, η2=0.42), but not in the high symptom PTSD group (F(1,12)=1.68, P>0.1, η2=0.12). Unexpectedly, in the control and low symptom group startle potentiation was also decreased in the presence of AC relative to AX (F(1,27)=5.39, P<0.05, η2=0.17 and F(1,13)=7.00, P<0.05, η2=0.35, respectively). On the other hand, in the high symptom group, startle to AC trials was not inhibited relative to AX trials (F(1,12)<1, P>0.1, η2=0.00). In all three groups, startle was equally potentiated in the presence of AB and AC trials.
There was a significant between-groups effect of Group on percent potentiation (F(2,52)=4.26, P<0.05, η2=0.14). Follow-up one-way ANOVAs with LSD post-hoc tests indicated that the high symptom PTSD group potentiated more to AX+ compared to the control group (P<0.05), and had less fear inhibition to AB than either the control group (P<0.05) or the low symptom group (P<0.05). Finally, the high symptom group also potentiated more to the AC trials compared to the control group (P<0.05) and the low symptom PTSD group (P<0.05) (see Figure 3). The low symptom PTSD group did not differ from the control group on any trial types (see Figure 3).
Since the clinical presentation of PTSD is characterized by 3 different symptom clusters, we wanted to see whether fear inhibition in patients with high symptoms was evident for each of the individual clusters. Thus we compared percent potentiation in the presence of AX to potentiation to AB in patients who were categorized as high using the median split on the Re-experiencing, Avoidance, and Hyperarousal subscores of the CAPS. Patients with high Hyperarousal symptoms (F(1,14)=10.73, P<0.01, η2=0.43) demonstrated fear inhibition, while patients with high Re-experiencing (F(1,14)=1.93, P>0.1, η2=0.12) and Avoidance symptoms (F(1,13)=2.79, P>0.1, η2=0.18) showed a lack of fear inhibition.
3.6. Expectancy ratings
Figure 4 shows the subjects’ US-expectancy ratings for each trial type. An analysis of subject’s keypad responses to the final conditioning block (i.e., AX+ and BX-) showed that all groups discriminated between AX and BX, i.e., they expected to receive an airblast on AX trials, and did not expect an airblast on BX trials (Controls: F(1,27)=17.56, P<0.01); low symptom PTSD: F(1,12)=5.73, P<0.05); and high symptom patients: F(1,12)=20.53, P<0.01). Furthermore, on the first block of AB trials the expectancy of the airblast was reduced relative to AX in all groups (Controls: F(1,27)=17.34, P<0.01); low symptom PTSD: F(1,12)=7.92, P<0.05); and high symptom patients: F(1,12)=28.96, P<0.01). Finally, on the first block of AC trials, the subjects had a reduction in airblast expectancy relative to AX; however, this difference was significant only in the controls (F(1,27)=22.39, P<0.01) and the high symptom group (F(1,12)=23.11, P<0.01). We used chi-square analyses in order to examine the subjects’ expectancy on the first presentation of B and C. We found that in all groups, a majority of subjects rated B as “safe” (Controls: 78%; low symptom PTSD: 75%; high symptom PTSD: 89% of the responders). On the other hand, C was rated as “don’t know” in a majority of the controls (55% of responders) and high symptom PTSD patients (63% of responders); while the low symptom PTSD patients were more likely to rate C as “threat” (56% of responders), although this difference was only at trend level χ2(4)=8.32, P=0.08.
Figure 4.
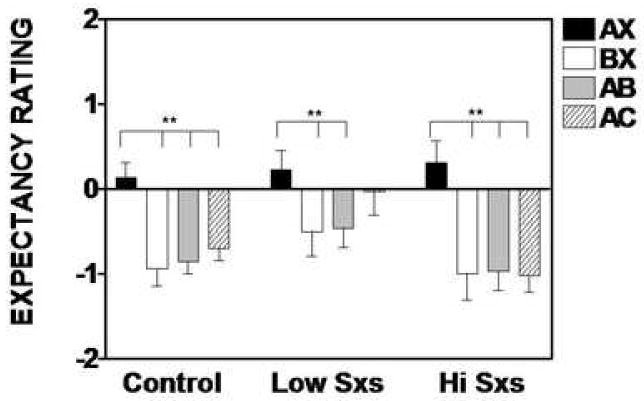
Expectancy ratings (mean + SEM) for AX+, BX-, AB, and AC trial types in low symptom and high symptom PTSD subjects. Positive score indicate airblast expectancy, negative scores indicate that there is no expectancy of airblast, and zero indicates uncertainty. Brackets indicate significant contrasts. **P<0.01.
4. Discussion
Our objective in this study was to translate an experimental paradigm that would independently measure fear potentiation and fear inhibition to a clinical population. Because fear dysregulation is one of the key symptoms of posttraumatic stress disorder (PTSD), we examined this paradigm in a population of Vietnam veterans with chronic PTSD. We used a modified conditional discrimination procedure (Wagner et al., 1968) that was adapted from the rodent model (Myers and Davis, 2004) and developed for use in a human startle paradigm (Jovanovic et al., 2005). In our previous study using this paradigm, we found that healthy controls showed fear-potentiated startle in the presence of AX+, discriminated between AX+ and BX-, and transferred safety on the AB test trials (Jovanovic et al., 2005). We used the same paradigm in the present study to test fear potentiation and fear inhibition in PTSD patients.
We used a response keypad to induce the subjects to use elemental strategies during conditioning (Williams et al., 1995), so that A would be perceived as the threat stimulus and B would be perceived as the safety signal. The keypad also allowed us to see whether the subjects were aware of the contingencies in the experiment. Thus we could examine whether PTSD subjects have difficulty learning safety signals, or difficulty inhibiting a physiological response to a known safety cue.
The studies that have analyzed fear-potentiated startle in combat-related PTSD found that PTSD subjects demonstrated larger startle amplitude over the entire session compared to control subjects but did not show increased fear potentiation (Grillon et al., 1998; Morgan, Grillon et al., 1995). The present study found that subjects with a history of PTSD, but with low current symptoms responded similarly to healthy controls, showing both fear potentiation to the danger signal (AX+), discrimination between danger (AX+) and safety (BX-) trials, and transfer of safety on conditioned inhibition trials (AB). On the other hand, the PTSD patients with high symptoms showed strong fear potentiation to danger, but no significant difference in discrimination between danger and safety (AX+ vs. BX-), and did not transfer safety on the AB test trials. This impairment appeared to be related mostly to high symptoms in the Re-experiencing (Intrusive) and Avoidance symptom cluster rather than the Hyperarousal symptom cluster. While this might be initially surprising, given that exaggerated startle response is one of the Hyperarousal symptoms, the Hyperarousal cluster is actually more related to difficulties in sleeping and concentration, while the Re-experiencing cluster is related to physiological arousal in response to trauma (i.e. fear-related) stimuli, and the inability to control this arousal.
Another interesting finding is that startle to the AC trials, in which the danger cue is paired with a novel (presumably neutral) stimulus was not suppressed in the highly symptomatic PTSD group, whereas it was in the other two groups. In the animal literature, a novel stimulus reduces the fear potentiation generated by the danger cue, a phenomenon known as external inhibition (Pavlov, 1927). In terms of external inhibition to the AC trials, the healthy subjects showed significantly less startle to AC compared to AX. Thus, in this experiment, we cannot distinguish conditioned from external inhibition. However, one of the issues in the design we have used is that C is completely novel when first presented in compound with A. In contrast, because B has been presented several times in compound with X in BX-training, B is not completely novel when first presented in compound with A. This might, therefore, promote more external inhibition on AC compared to AB test trials. Future studies will expose the subjects to the stimuli in the experiment prior to conditioning in order to minimize the effect of novelty. While the low symptom PTSD subjects, like the controls, demonstrate external inhibition, the high symptom PTSD subjects show no inhibition to AC relative to AX+. Since the AC trials represent danger coupled with a novel stimulus, this finding may correspond to the PTSD symptom of hyper-vigilance, in that the novel stimuli are perceived as threatening. Again, this possibility needs to be tempered with the general lack of significant effects on any of the test trials in the high symptom PSTD subjects.
Nonetheless, the lack of significant inhibition in the high symptom PTSD subjects with any stimulus combination may be a measure of their inability to process safety signals more generally, especially because as a group they seemed to be cognitively aware of the stimulus contingencies. Thus, we found that the expectancy ratings during the conditioning trials corresponded to the startle data, i.e. in all groups the subjects expected an airblast on the AX trials and did not expect airblasts on the BX trials. Therefore the discrimination in startle was consistent with the discrimination on the response pad. However, on the inhibition test trials there was dissociation between the expectancy ratings and startle data. On the first AB test trial, all subjects indicated that they did not expect to get an airblast: while this corresponded to the inhibition in startle observed in the controls and low symptom patients, the startle in the high symptom patients was still increased regardless of their expectancy.
On the first AC trial, the majority of low symptom patients indicated that they expected the airblast but inhibited startle anyway; the majority of controls and high symptom patients indicated that they were uncertain. As seen by the responses averaged for the entire AC block, by the third AC trial all subjects were aware that they would not receive the airblast; however only the high symptom patients did not inhibit startle. This finding indicates that the high symptom patients appeared to be unable to suppress the fear-potentiated startle response even when they were cognitively aware of safety. The categorical analyses of the first presentation of AB and AC indicate that B was perceived as a safety signal while C was perceived as novel, suggesting that, in the controls and low symptom patients, AB was governed by conditioned inhibition, while the reduced response to AC was due to external inhibition. The fact that startle was potentiated for both AB and AC trials in the high symptom group indicates a failure of inhibition, regardless of the inhibition mechanism, either conditioned or external inhibition.
In our previous studies of fear-potentiated startle and US-expectancy ratings, we have found that startle and expectancy can be dissociated, especially with regard to safety cues (Jovanovic, et al., 2006; Norrholm et al., 2006). Awareness of safety appears to be necessary in order to inhibit fear-potentiated startle (Jovanovic et al., 2006); however, it may not always be sufficient. Previous work from our lab has shown that subjects can have zero US-expectancy after extinction, but still demonstrate startle potentiation, as a result of partial reinforcement during acquisition which may increase uncertainty (Norrholm et al., 2006). These findings taken together suggest that the high symptom PTSD patients may have more uncertainty or more latent fear on the AB and AC trials, even though they are cognitively aware that they are safe. It is possible that the high symptom PTSD subjects have an inability to effectively use prefrontal cortex to inhibit amygdala output and hence have persistently increased startle to AB and AC. A limitation of the present study is the lack of additional psychophysiological measures of the fear response, such as skin conductance. It is possible that the inability to inhibit the fear response is limited to the startle response; future studies should expand this research paradigm to include other measures.
In most studies that have examined fear conditioning in combat-related PTSD, the patients with the disorder were compared to combat veterans who did not develop the disorder, in order to establish that the effect was due to the disorder, rather than to trauma exposure (Grillon et al., 1998; Grillon and Morgan, 1999; Morgan et al., 1995; Orr et al., 2000). In the present study the critical comparison group was another patient sample with lower current symptoms, indicating that the physiological effects may be related to the current state of the disorder, rather than ever having had the disorder. One of the advantages of this approach is that the two groups have equivalent levels of combat exposure, which is frequently significantly lower in non-PTSD combat controls. In addition, the two groups are matched in terms of treatment history and medication use, as well as demographics. Another advantage is that the focus on current symptoms provides an opportunity to see whether treatment can improve the physiological response as well as the PTSD symptoms.
The present study is the first to examine fear inhibition in PTSD patients and to relate this inhibition to symptom severity. Given the exploratory nature of this study, the follow-up comparisons were driven by the hypothesis rather than higher-order interactions. Therefore, the results of this study must be replicated and validated in another sample of PTSD patients.
In conclusion, we found that the PTSD patients with greater current symptoms had difficulty inhibiting fear-potentiated startle to a safety cue. Future studies should examine fear potentiation and fear inhibition in relation to treatment outcome. It is possible that the ability to inhibit fear, and the potentiation to novel stimuli, may improve with treatment.
Acknowledgments
This research was supported by the Mental Health Service, Atlanta DVA Medical Center; the STC Program, the Center for Behavioral Neuroscience, of the National Science Foundation under Agreement No. IBN-9876754 (Venture grant, PI, E. Duncan); the American Psychiatric Association/Glaxo SmithKline (PI, E. Duncan); National Institute of Mental Health Grants 1R24MH067314-01A1 (PI, B. Rothbaum), R37 MH47840 (PI, M. Davis), Kirschstein National Research Service Award Individual Fellowship 1F32 MH070129-01A2 (PI, T. Jovanovic) and the Woodruff Foundation, Emory University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- Baas J, Grillon C, Bocker KB, Brack AA, Morgan CA, 3rd, Kenemans JL, Verbaten NM. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2002;161(3):233–247. doi: 10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weather FW, Nagy LM, Kaloupek DG, Klauminker G, Charney DS, Keane TM. A clinical rating scale for assessing current and lifetime PTSD. The CAPS-1. Behavioral Therapy. 1990;13:187–188. [Google Scholar]
- Bradley MM, Codispoti M, Cutberth BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. American Journal of Psychiatry. 1995;152(7):973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS. Deficits in short-term memory in posttraumatic stress disorder. American Journal of Psychiatry. 1993;150(7):1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. New York: Wiley-Liss; 1992. [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: A neural and pharmacological analysis. Behavioral Brain Research. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Effects of threat and safety signals on startle during anticipation of aversive shocks, sounds, or airblasts. J Psychophysiology. 1998;12:329–337. [Google Scholar]
- Grillon C, Ameli R. Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology. 2001;38(5):807–815. [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Effects of explicit and contextual cue conditioning following paired vs. unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Falls WA, Ameli R, Davis M. Safety signals and human anxiety: a fear-potentiated startle study. Anxiety. 1994;1:13–21. doi: 10.1002/anxi.3070010105. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108(1):134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry. 1998;44(10):1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan E. Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry. 2005;57:1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos AM, Jovanovic S, Myers KM, Davis M, Duncan E. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behavioral Neuroscience. 2006;120:995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavolvian conditioning: empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- Lund M, Foy D, Sipprelle C, Strachan A. The combat exposure scale: a systematic assessment of trauma in the Vietnam war. Journal of Clinical Psychology. 1984;40:1323–1328. doi: 10.1002/1097-4679(198411)40:6<1323::aid-jclp2270400607>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry. 1995;38(6):378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. AX+, BX-discrimination learning in the fear-potentiated startle paradigm: Possible relevance to inhibitory fear learning in extinction. Learning and Memory. 2004;11:464–75. doi: 10.1101/lm.74704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. doi: 10.1038/sj.mp.4001939. in press. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning and Memory. 2006;13(6):681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109(2):290–298. [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. London: Oxford University Press; 1927. [Google Scholar]
- Wagner AR, Logan FA, Haberlandt K, Price T. Stimulus selection in animal discrimination learning. Journal of Experimental Psychology. 1968;76:177–186. doi: 10.1037/h0025414. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Rescorla RA. Inhibition in Pavlovian conditioning: application of a theory. In: Boakes RA, Halliday MS, editors. Inhibition and Learning. London: Academic Press; 1972. pp. 301–336. [Google Scholar]
- Williams DA, Sagness KE, McPhee JE. Configural and elemental strategies in predictive learning. J Experimental Psychology: Learning, Memory, Cognition. 1995;20:694–709. [Google Scholar]


