Abstract
Estrogen receptor (ER) α is an essential component in human physiology and is a key factor involved in the development of breast and endometrial cancers. ERα protein levels and transcriptional activity are tightly controlled by the ubiquitin proteasome system. Deubiquitinating enzymes, a class of proteases capable of removing ubiquitin from proteins, are increasingly being seen as key modulators of the ubiquitin proteasome system, regulating protein stability and other functions by countering the actions of ubiquitin ligases. Using mass spectrometry analysis of an ERα protein complex, we identified OTU domain-containing ubiquitin aldehyde-binding protein 1 (OTUB1) as a novel ERα-interacting protein capable of deubiquitinating ERα in cells and in vitro. We show that OTUB1 negatively regulates transcription mediated by ERα in transient reporter gene assays and transcription mediated by endogenous ERα in Ishikawa endometrial cancer cells. We also show that OTUB1 regulates the availability and functional activity of ERα in Ishikawa cells by affecting the transcription of the ERα gene and by stabilizing the ERα protein in the chromatin.
In mammals the effects of the steroid hormone 17β-estradiol (E2)2 are mediated by estrogen receptor (ER) α and estrogen receptor β. ERα is a member of the nuclear hormone receptor superfamily of transcription factors (1). In the cell ERα exists in either an inactive, unliganded form bound to the HSP90 chaperone or in an active liganded state in which ERα recruits coactivator protein complexes to gene promoters and initiates transcription of ERα target genes (2–15).
The ubiquitin proteasome system plays crucial and multifaceted roles in the regulation of both receptor pools (7, 16–24). The ubiquitin proteasome system is a multicomponent complex that regulates protein stability and degradation through the interplay of ubiquitin, ubiquitinating enzymes, deubiquitinating enzymes (DUBs), and 26 S proteasome (25–27). In the case of unliganded and inactive ERα, the E3 ligase CHIP maintains appropriate steady state receptor levels and is responsible for the clearance of misfolded ERα (18, 19). The effect that the ubiquitin proteasome system has on active, liganded ERα is complex because its activity is intimately tied to its stability. Our group and others have shown that inhibition of ERα degradation inhibits its function and that inhibition of ERα transcriptional activity stabilizes the receptor protein (21–23, 28–30). Métivier et al. (31) indicated that the initial steps of transcription are linked to monoubiquitination of the liganded ERα and that monoubiquitination may enhance receptor interaction with DNA or coactivators. As transcription progresses, ERα and chromatin surrounding the promoter are sequentially modified by successive coactivator complexes (8, 28, 29, 31) and degraded as a consequence of the recruitment of several ubiquitin E3 ligases: BRCA1/BARD1, MDM2, E6-AP, and EFP (16, 17, 32–35). In addition, Zhang et al. (24) showed that the LMP2 subunit of the 26 S proteasome is recruited to the gene by SRC coactivators and that its presence is necessary for ERα-mediated transcription and cycling on the promoter of the estrogen-responsive pS2 gene.
DUBs are cysteine proteases (with the exception of JAMM family DUBs, which are metalloproteases) that catalyze the removal of ubiquitin (Ub) from Ub-modified proteins and for the processing of tandemly linked nascently translated Ub precursors (36–41). Based on the structure of the active site and the mechanism of catalysis, DUBs are divided into five groups: UCHs, USPs, MJDs, OTUs, and JAMMs. Deconjugation of Ub-protein substrates is achieved either by removal of the entire Ub chain from the protein or by removal of individual or multiple ubiquitins from the chain in a process termed “editing.” Different DUBs exhibit preferences for mono and poly-Ub chains or for K48 or K63-based Ub-Ub linkages (40). DUBs also specifically target a distinct and wide range of ubiquitinated proteins, displaying a diverse array of DUB-specific biological functions (37).
Three DUBs have been reported to interact with steroid hormone receptors. Two of these, 2A-DUB and USP22, are part of histone acetyltransferase complexes (pCAF complex and STAGA, respectively), and both enhance AR transcription by removing histone H2A monoubiquitination (41, 42). USP10 has also been shown to coactivate AR-mediated transcription (43). However, in all these cases, the receptor itself has not been observed to be deubiquitinated.
In this study we have identified OTU domain-containing ubiquitin aldehyde-binding protein 1 (OTUB1) as an ERα-interacting DUB. OTUB1 is a deubiquitinating enzyme that has known in vitro deubiquitinating activity and a preference for K48-linked polyubiquitin chains (44–46). However, no protein substrates that are deubiquitinated by OTUB1 have been identified in living cells. Here, we show that OTUB1 interacts with ERα, deubiquitinates the receptor in cells and in vitro, and represses its transcriptional activity in transient reporter assays as part of its negative regulation of ERα activity. OTUB1 also regulates endogenous ERα both by regulating the transcription of ERα gene in Ishikawa cells and by stabilizing ERα protein in the chromatin.
EXPERIMENTAL PROCEDURES
Plasmids and siRNAs
The plasmid expression vectors pCR3.1-ERα, pCR3.1-FLAG-PR-B, pCR3.1-FLAG-ERα, pCR3.1-ERβ, pCR3.1-RAC3 (Src-3), and the reporter genes pERE-E1b-LUC, pGRE-E1b-LUC, and pG5-LUC were made and described before (28, 47). Constructs expressing HA-OTUB1 and V5-OTUB1 were generated by inserting 5′ HA or 5′ V5 tag into pCMV6 (Origene) using a sequential PCR method with overlapping primers. We used pCMV6-OTUB1 (Origene) as a template for these reactions. pCMV6-HA-OTUB1 C/S and pCMV6-V5-OTUB1 Ubmut were constructed using a sequential PCR method and overlapping primers to generate point mutations and deletion fragments. Point mutants and fragments were subsequently subcloned into the pCMV6 vector. The same technology was used for construction of GAL4-AF2 (pBIND-EF) and pCR3.1-ERα179C. All of the constructs were confirmed by sequencing. Details of our cloning scheme as well as the primer sequences used are available upon request. For knockdown experiments, siRNA ON TARGET SMART POOL against human OTUB1 (catalog number L-021061-00) and the appropriate siRNA ON TARGET SMART CONTROL POOL (catalog number D001810-10-05) were purchased form Dharmacon Research (Lafayette, CO).
siRNA Treatment, Transient Transfections, Immunoprecipitations, and Immunoblot Assays
All of the cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum or 10% charcoal-stripped serum for hormone treatment. For transient transfections, HeLa and HEK293T cells were transfected using Lipofectamine 2000 (Invitrogen). For the transfection of Ishikawa cells, we used FuGENE 6 (Roche Applied Science). For siRNA knockdown, the cells were transfected for 4 days with 50 nm of siRNA using TransientTKO (Mirus). Luciferase assays were performed with substrate purchased from Promega and used according to the manufacturer's instructions. Immunoprecipitations and Western blots were performed as described before using anti-FLAG or protein A beads (Invitrogen) and antibodies F10 and D12 (Santa Cruz) for ERα, anti-HA (Sigma), anti-FLAG (Affinity BioReagents), and rabbit and mouse control IgG (Santa Cruz). Antibody against human OTUB1 was custom produced and assayed by Genemed Synthesis, Inc. in rabbits using two synthetic peptides corresponding to the N-terminal variable domain of human OTUB1 (QQKIKDLHKKYSYIRKT and KQEPLGSDSEGVNC).
RNA Isolation and Real Time Quantitative PCR
Ishikawa cells total RNA was extracted using Aurum Total RNA Mini Kit (Bio-Rad). The PR, GREB1, OTUB1, ERα mRNA, and the cyclophilin mRNA (as an internal control) were quantitated as in Yi et al. (47) by TaqMan-based reverse transcriptase PCR using the AIB1 Prism 7700 sequence detection system (Applied Biosystems). TaqMan primer-probe sets for PR, GREB1, and OTUB1 were purchased from Applied Biosystems. The primers for the ERα mRNA are as follows: forward, 5′-GACAGGGAGCTGGTTCACATG; reverse, 5′-GGAGGGTCAAATCCACAAAGC; and probe, 5′-FAM-TGGCACCCTCTTCGCCCA-TAMRA. The primers for the cyclophilin mRNA were described previously (47).
Ubiquitin-AFC Analysis of Immunoprecipitated ERα Complex
For the purpose of analyzing deubiquitinating activity associated with ERα, ERα was immunoprecipitated from HEK293T cells transfected with pCR3.1-hERα or empty vector for 2 days or from MCF-7 cells. The cells were lysed in a lysis buffer (25 mm Tris, pH 8.0, 150 mm NaCl, 0.5% Triton X-100, 1 mm EDTA, 5% glycerol, and 1 mm DTT) without protease inhibitors at 4 °C. The lysates were sonicated at low power (10% duty cycle, output 2, using a Branson sonifier 250) and spun down, and the supernatants precleared with protein A beads for 30 min. ERα antibody (2.5 μg of each F10 and D12) or normal mouse IgG (5 μg) was then added to supernatants and incubated for 2 h. Protein A beads (Invitrogen) were added for 1 h. Protein A beads were spun down and washed twice with 0.2% TBS with Tween 20 detergent and twice with TBS. The beads were then resuspended in 100 μl of ubiquitin-AFC reaction buffer (48). 50 mm HEPES, pH 8.0, 0.5 mm EDTA, 0.5 mm DTT, and 1 mg bovine serum albumin. Finally, ubiquitin-AFC (Boston Biochem) was added at a concentration of 0.5 μm. A control reaction was pretreated for 5 min with 2 μm of ubiquitin-aldehyde (Boston Biochem) at 37 °C before the addition of ubiquitin-AFC. The reactions were incubated in the dark at room temperature with shaking for 30 min. Measurement of AFC release was performed by excitation at 405 nm and measured at an emission wavelength of 505 nm using a fluorescent plate reader. Statistical analysis was performed using Student's t test.
In Vitro Deubiquitination Assay
Recombinant ERα (300 ng/reaction) from Invitrogen was in vitro ubiquitinated using Boston Biochem ubiquitination kit (catalog number K-960) and FLAG-ubiquitin (catalog number U-120). The ubiquitination reaction was then diluted to 400 μl with lysis buffer without protease inhibitors and ERα antibodies (2.5 μg of each F10 and D12; Santa Cruz) were added for 2 h. Protein A beads were then added for 1 h and washed afterward twice with 0.2% TBS with Tween 20 detergent and twice with TBS. The beads were then resuspended in deubiquitination reaction buffer: 20 mm Tris-Cl, pH 8.0, 0.5 mm DTT, and 10% energy solution (Boston Biochem from K-960). Finally, 2 μg of recombinant purified GST-OTUB1 (Abgent) was added. The reaction was incubated at room temperature for 4 h or overnight. Following incubation, 5× SDS loading buffer was added to the reaction and analyzed by Western blotting using anti-FLAG and Anti-ERα antibodies and chemiluminscence.
Alkaline Phosphatase Assay
Alkaline phosphatase assays were performed using an alkaline phosphatase kit from AnaSpec (71230). Ishikawa cells were transfected with siOTUB1 or a control siRNA pool as described above. E2 (100 nm) or ethanol was added to the cells 2 days after transfection for 48 h. The cells were then harvested and processed according to the manufacturer's instructions.
Absorbance was measured at 405 nm. Absorbance values were then normalized with a protein concentration that was determined by Bradford analysis (Bio-Rad). Statistical analysis was performed using the Student's t test.
Nuclear and Chromatin Fractionation
Nuclear and chromatin fractionations were performed according to the protocol published by Wysocka et al. (49). In short, cells were washed in phosphate-buffered saline and resuspended in solution A (without Triton X-100). Triton X-100 0.1% (final) was then added to cells for 5 min on ice. The cells were spun down at 1300 × g for 4 min. Precipitate (P1) was washed with solution A without Triton X-100 and lysed using solution B for 10 min on ice. Lysate was then spun down at 1700 × g for 4 min, precipitate-washed (chromatin fraction) with solution B once, and then centrifuged at 10,000 × g for 1 min. The remaining chromatin was then resuspended in SDS loading buffer, sonicated, and analyzed using SDS-PAGE. Solution A contained 10 mm HEPES, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 0.34 m sucrose, 10% glycerol, 1 mm DTT, 10 mm NaF, 1 mm Na2VO3, and protease inhibitor mixture (Roche Applied Science). Solution B contained 3 mm EDTA, 0.2 mm EGTA, 1 mm DTT, 10 mm NaF, 1 mm Na2VO3, and protease inhibitors (Roche Applied Science).
Mass Spectrometry Analysis of the ERα Protein Complex
HEK293T cells were grown in Dulbecco's modified Eagle's medium without phenol red supplemented with 10% charcoal-stripped fetal calf serum. The cells were transfected with ERα for 48 h and treated with E2 (1 nm) where indicated for 2 h. The cells were lysed in radioimmune precipitation assay buffer, and ERα was immunoprecipitated using ERα antibodies (F10 and D12; Santa Cruz) for 2 h and protein A beads (Invitrogen). The lysates were then run on SDS-PAGE. The whole gel was cut in five parts, trypsinized, and prepared for electrospray ionization-liquid chromatography/liquid chromatography (ESI-LTQ) mass spectrometry. The peptides were identified using SeQuest software and the human RefSeq library.
RESULTS
Deubiquitinating Enzymes Are Part of ERα Complex
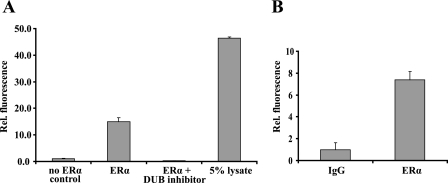
To determine whether DUBs are associated with ERα protein, complexes were immunoprecipitated from 293T cells transiently transfected with an ERα expression vector and incubated with a recombinant DUB substrate, ubiquitin-AFC. Fluorometry-detected release of the AFC moiety revealed that the ERα complex contains DUB activity (Fig. 1A). Next, we repeated this experiment using an endogenous ERα protein complex immunoprecipitated from MCF-7 cells, indicating that DUBs are associated with the endogenous receptor as well (Fig. 1B).
FIGURE 1.
ERα is associated with DUBs. A, HEK293T cells were transfected with empty vector (no ERα control) or ERα. ERα was immunoprecipitated form the cells and exposed to recombinant DUB substrate ubiquitin-AFC. Ubiquitin aldehyde (0.5 μm) was used to demonstrate the specificity of deubiquitinating activity (ERα and DUB inhibitor). 5% of cellular lysate was used as a positive control for deubiquitinating activity. Release of the fluorescent AFC moiety was measured at an excitation level of 405 nm and an emission level of 505 nm. B, endogenous ERα complexes were immunoprecipitated with either nonspecific IgG (IgG) or with ERα antibody (ERα) from MCF7 cells and exposed to ubiquitin-AFC as described previously.
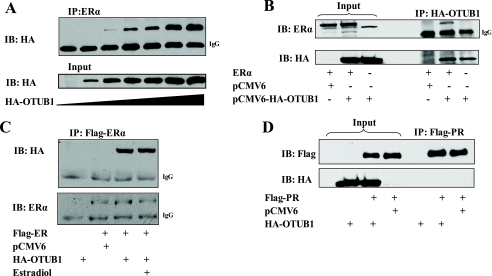
To identify the ERα-interacting DUB(s), ERα immunoprecipitated from the 293T cells was analyzed by mass spectrometry. Mass spectrometric analysis revealed that ERα interacts in its unliganded state with OTUB1 (supplemental Fig. S1 and supplemental Table S1). Subsequent immunoprecipitations and reciprocal immunoprecipitation experiments of OTUB1 and ERα confirmed the mass spectrometry analysis, finding that OTUB1 binds ERα (Fig. 2, A and B). Next, we investigated whether ERα interaction with OTUB1 is dependent on the presence of E2. Because the receptor interaction with OTUB1 did not change in the presence of E2 (Fig. 2C), we concluded that the ERα-OTUB1 interaction is hormone-independent. Additionally, we tested whether OTUB1 would bind progesterone receptor B (PR-B). Under the same experimental conditions no observable binding of OTUB1 to PR-B was detected (Fig. 2D).
FIGURE 2.
OTUB1 interacts with ERα but not with PR-B. HEK293T cells were transfected with FLAG-ERα and HA-OTUB1 or empty vector controls. A, ERα was immunoprecipitated and analyzed by SDS-PAGE using antibodies against the HA epitope tag. B, reciprocal coimmunoprecipitation. HA-OTUB1 was immunoprecipitated and analyzed using indicated antibodies. C, ERα interacts with OTUB1 irrespective of E2. FLAG-ERα and HA-OTUB1 were expressed in the presence of 1 nm estradiol (E2) or vehicle and analyzed with indicated antibodies. D, OTUB1 does not bind to PR-B. FLAG-PR-B and HA-OTUB1 were transfected in HEK293T cells as previously described for ERα. PR-B was immunoprecipitated and analyzed for OTUB1 binding using the indicated antibodies. IB, immunoblot.
OTUB1 Deubiquitinates ERα in HEK 293T Cells and in Vitro
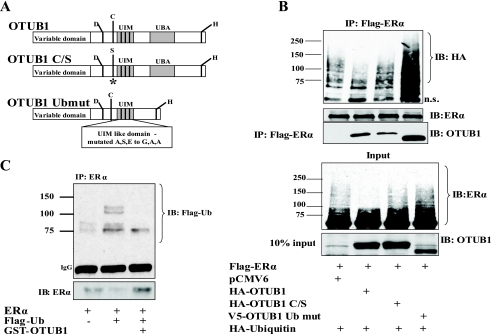
Because OTUB1 is a DUB with confirmed in vitro deubiquitinating activity toward polyubiquitin chains (44–46, 50), we investigated whether OTUB1 can deubiquitinate ERα. To study this process we used wild type OTUB1 and two OTUB1 mutant molecules: a catalytically inactive OTUB1 C/S and an OTUB1 Ubmut that cannot bind ubiquitin (Fig. 3A). A catalytically inactive OTUB1 (OTUB1-C/S) expression vector was constructed by substituting active site cysteine 91 to serine (44) (Fig. 3A). Balakirev et al. (44) identified two conserved OTUB1 domains capable of binding ubiquitin: an UIM domain located immediately next to the active site and an UBA-like domain located toward the C terminus of the molecule. In the UIM domain we substituted conserved alanine, serine, and glutamate residues to glycine, alanine, and alanine, respectively. In the same molecule we deleted the other UBA-like domain to create an OTUB1 protein that cannot interact with ubiquitin (OTUB1 Ubmut) (Fig. 3A).
FIGURE 3.
OTUB1 deubiquitinates ERα in cells and in vitro. A, schematic representation of wild type OTUB1, catalytically inactive OTUB1 C/S, and OTUB1 that cannot bind ubiquitin (OTUB1 Ubmut). B, HEK293T cells were transfected with the indicated vectors or empty vector controls. FLAG-ERα was immunoprecipitated and precipitates were analyzed using antibodies against ERα, HA tag, and OTUB1. The lower panels represent Western analysis of inputs. C, OTUB1 deubiquitinates ERα in vitro. Purified recombinant ERα protein was in vitro ubiquitinated with FLAG-ubiquitin and then exposed to purified recombinant GST-OTUB1 as described under “Experimental Procedures.” Western blot analysis was done using antibodies against ERα and FLAG-ubiquitin.
We examined ERα ubiquitination in HEK293T cells in the presence of overexpressed wild type OTUB1 or OTUB1 mutants. As shown in Fig. 3B (first two lanes) overexpression of OTUB1 substantially reduced slower migrating ubiquitinated ERα species. The expression of catalytically inactive OTUB1 (OTUB1-C/S) restored ERα ubiquitin levels (Fig. 3B, third lane), indicating that deubiquitination of ERα by OTUB1 is dependent on OTUB1 catalytic activity. The expression of the OTUB1 Ubmut that is incapable of ubiquitin binding further increased the amount of ubiquitinated ERα (Fig. 3B, fourth lane), suggesting that the OTUB1 Ubmut may act as a dominant negative with respect to receptor deubiquitination.
Interestingly, we observed that mutation of a single conserved residue in the catalytic site of OTUB1 significantly decreased the interaction of OTUB1 with ERα (Fig. 3B, compare second lane and third lane), indicating that the active site cysteine plays an essential role as a substrate-interacting surface. More surprisingly, we found that the ubiquitin-binding mutant OTUB1 Ubmut bound ERα substantially more strongly than the wild type OTUB1 (Fig. 3B, compare second and fourth lane). This finding is significant because it shows that ubiquitin binding is not necessary for a deubiquitinating enzyme to recognize and bind its substrate. Analysis of the crystal structure of human OTUB1 (45) suggests that OTUB1 is catalytically inactive in an unliganded state (in the absence of K48-linked chains). We speculate that because the OTUB1 Ubmut cannot recognize ubiquitin chains, it remains auto-inhibited and unable to release ERα.
To show that OTUB1 is capable of deubiquitinating ERα in vitro, we ubiquitinated recombinant ERα under cell-free conditions using an in vitro ubiquitin kit consisting of ubiquitin-activating, conjugating, and E3 ligase enzymes (Boston Biochem). This ubiquitinated ERα was then incubated with recombinant purified GST-OTUB1. As shown in Fig. 3C recombinant GST-OTUB1 is capable of deubiquitinating ERα in vitro.
OTUB1 Attenuates ERα-mediated Transcription in HeLa and HEK 293T Cell Lines
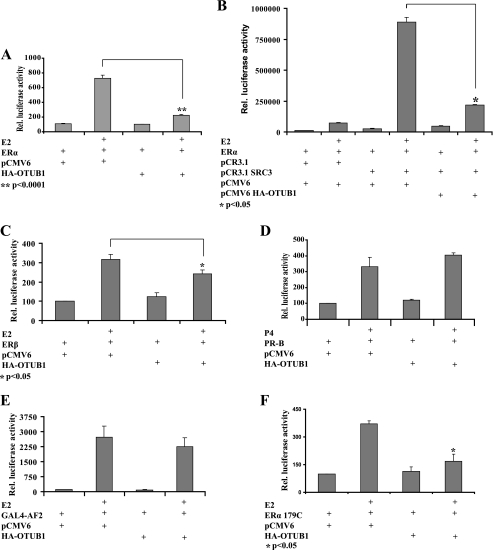
To examine the potential role of OTUB1 in ERα-mediated transcription, we performed ERE-LUC reporter assays in HeLa cells. Expression of OTUB1 significantly attenuated ERα transcriptional activity even in the presence of coactivator (Fig. 4, A and B). In addition, OTUB1 affected the transcriptional activity of ERβ (Fig. 4C). However, OTUB1 did not have an effect on PR-B mediated transcription in the same assay (Fig. 4D), suggesting that OTUB1 transcriptional repression is specific for ERα. Furthermore, OTUB1 failed to suppress the activity of the activation function 2 (AF2) domain of ERα fused to GAL4 DNA-binding domain (Fig. 4E). To examine the potential role of activation function 1 (AF1) in OTUB1 repression of ERα, we evaluated the effect of OTUB1 expression on the ERα179C construct lacking the AF1 region of the ERα molecule. Fig. 4F indicates that the absence of AF1 does not contribute substantially to the ability of OTUB1 to repress ERα and that this effect is most likely conveyed by the DNA-binding domain and hinge regions of ERα. However, the AF1 may still play a role in OTUB1 repression, given that ERβ is repressed less than ERα, and it has low AF1 activity (13).
FIGURE 4.
OTUB1 represses ERα mediated transcription. A, HeLa cells were transfected with an ERE-luciferase reporter construct, ERα, OTUB1, or vector control. ERα-dependent transcription was measured in the presence of 1 nm estradiol (E2) or ethanol vehicle. B, OTUB1 represses ERα activity in the presence of coactivator SRC-3. HeLa cells were transfected with an ERE-luciferase reporter construct and indicated vectors and analyzed for luciferase activity. Representative result of three independent experiments is shown. C, OTUB1 represses ERβ activity. HeLa cells were transected with ERE-luciferase, ERβ, and the indicated vectors, and luciferase activity was analyzed. D, OTUB1 does not affect PR-B transcriptional activity. HeLa cells were transfected with a GRE-Luciferase reporter construct, PR-B, OTUB1, or vector control. PR-B dependent transcriptional activity was measured in the presence of 100 nm progesterone (P4) or ethanol vehicle. E, OTUB1 does not repress AF-2 domain of ERα. HeLa cells were transfected with pG5-luciferase reporter, pGAL4-AF2, and OTUB1 or empty vector. F, OTUB1 represses ERα lacking the AF1 domain. HeLa cells were transfected with ERE-luciferase, pCR3.1 ERα179C, and OTUB1 or empty vector.
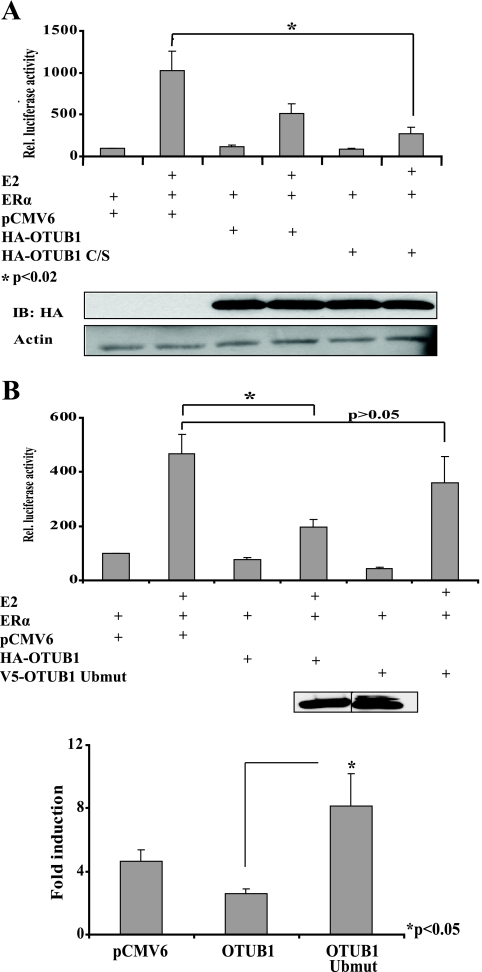
OTUB1 is a deubiquitinating enzyme, and its effect on protein targets is presumably mediated by its ability to bind and cleave ubiquitin chains. Therefore, we assessed the impact of OTUB1 C/S on the activity of ERα in HeLa cells. Surprisingly, the expression of the OTUB1 catalytic mutant (OTUB1 C/S) also suppressed ERα mediated transcription (5A). Because we were unable to express the OTUB1 ubiquitin-binding mutant (OTUB1 Ubmut) in HeLa cells, possibly because of its increased protein degradation, we evaluated the effect of OTUB1 and the OTUB1 Ubmut on transcription in 293T cells. As indicated in Fig. 5B, the expression of the OTUB1 Ubmut restored ERα-mediated transcription and even increased ERα estradiol-dependent coactivation. These results indicate that OTUB1 is capable of inhibiting ERα-mediated transcription and that its inhibitory effect is dependent on ubiquitin binding.
FIGURE 5.
OTUB1 C/S represses ERα-dependent transcription, whereas OTUB1 Ubmut rescues ERα mediated transcription. A, OTUB1 C/S represses ERα-dependent transcription. HeLa cells were transfected with empty vector, OTUB1, or OTUB1 C/S in the presence of ethanol or E2. The inset contains Western blot showing equal expression of OTUB1 and OTUB1 C/S in this experiment. B, OTUB1 Ubmut rescues ERα-mediated transcription. HEK293T cells were transfected with an ERE-luciferase reporter construct, ERα, OTUB1, and OTUB1 Ubmut or vector controls. ERα transcriptional activity was analyzed in the presence of E2 or ethanol vehicle. The inset contains a Western blot showing equal expression of OTUB1 and OTUB1 Ubmut in this experiment. The lower panel indicates E2-dependent ERα fold induction of transcription. IB, immunoblot.
Loss of Endogenous OTUB1 Increases Production of PR mRNA and Activity of Placental Alkaline Phosphatase
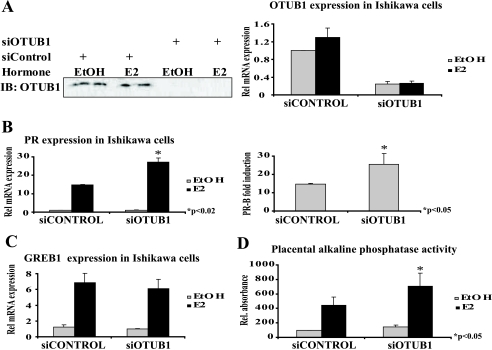
To evaluate the effect of the loss of OTUB1 on transcription mediated by endogenous ERα, we knocked down OTUB1 in the endometrial Ishikawa cancer cell line using siRNA (Fig. 6A). Partial loss of OTUB1 protein in Ishikawa cells caused a significant increase in E2-dependent ERα-mediated transcription of PR mRNA (Fig. 6B). This effect is specific to the PR gene because no observable effect was seen on transcription of the E2-responsive GREB1 gene (Fig. 6C). In addition to increasing PR mRNA, the loss of endogenous OTUB1 also increased placental alkaline phosphatase activity (Fig. 6D).
FIGURE 6.
Knockdown of OTUB1 in Ishikawa endometrial cancer cells increases transcription of PR gene and placental alkaline phosphatase activity. Ishikawa cells were transfected with ON TARGET SMART POOL siOTUB1 or adequate siRNA control pool (Dharmacon). A, successful knockdown of OTUB1 protein and OTUB1 mRNA. B, knockdown of OTUB1 increases E2-dependent transcription of PR mRNA. The right panel indicates increase in estrogen-dependent PR mRNA fold induction. C, knockdown of OTUB1 does not increase E2-dependent production of GREB1 mRNA. D, knockdown of OTUB1 increases E2-dependent placental alkaline phosphatase activity.
OTUB1 Mediates ERα Levels by Regulating the Transcription of ERα Gene
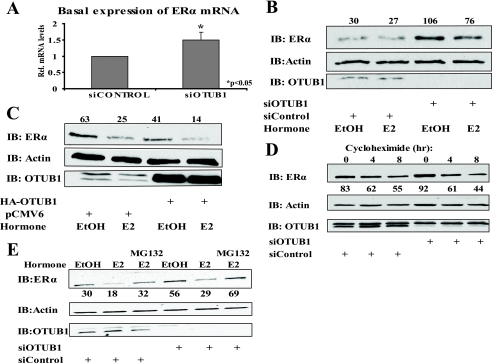
While examining the effects that OTUB1 has on the expression of endogenous ERα target genes in Ishikawa cells, we noticed that the loss of OTUB1 caused increased production of ERα mRNA (Fig. 7A). This observation prompted us to investigate the potential role of OTUB1 in the regulation of the ERα protein level. Consistent with our previous finding, we observed that knockdown of OTUB1 increased, and overexpression of OTUB1 decreased the level of endogenous ERα in the cells (Fig. 7,B and C). To confirm that the observed change in the ERα protein level is transcriptional rather than post-translational, we performed knockdown of OTUB1 in the presence of the translation inhibitor cycloheximide. Knockdown of OTUB1 did not change the ERα protein level in the presence of cycloheximide, indicating that OTUB1 affects ERα at the transcriptional level (Fig. 7D). Similarly, knockdown of OTUB1 in the presence of MG132 resulted in an overall increase in ERα protein, further suggesting that this increase is likely caused by an increase in ERαmRNA (Fig. 7E).
FIGURE 7.
OTUB1 changes ERα protein levels by affecting expression of ERα mRNA. A, loss of OTUB1 in Ishikawa cells increases levels of ERα mRNA. Cells were treated with siOTUB1 or siRNA control for 4 days and then collected for analysis by Q-PCR. B, cells were transfected with siOTUB1 or siRNA control for 4 days and treated with E2 or ethanol (EtOH) for 2 h. The cell lysates were analyzed by Western blotting using the indicated antibodies. The protein bands were quantified by Image J and normalized to actin. C, Ishikawa cells were transfected with HA-OTUB1 or empty vector and treated for 2 h with E2 or ethanol vehicle. The lysates were analyzed as in B. D, Ishikawa cells were transfected with siOTUB1 or siRNA control for 4 days. Cycloheximide (15 μm) was then added for indicated time points. The lysates were analyzed as in B. E, Ishikawa cells were treated with siOTUB1 or siRNA as described previously. The cells were then treated with 4 μm MG132 for 4 h, and EtOH or E2 for 2 h as indicated. The cell lysates were analyzed as described in B.
Expression of OTUB1 in Ishikawa Cells Increases ERα Retention in the Insoluble Nuclear Fraction
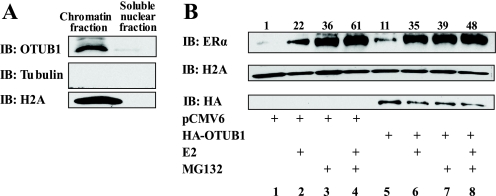
To gain an insight into the mechanism by which OTUB1-mediated deubiquitination of ERα is affecting ERα biology and to differentiate between the effect of OTUB1 on ERα function and its protein stability, we investigated the cellular localization of OTUB1. OTUB1 contains a bona fide nuclear localization signal and resides in the nucleus of porcine kidney cells (44, 46). However, the precise localization of OTUB1 within the nucleus has not been examined. We used a nuclear fractionation protocol to find out whether OTUB1 is present in the chromatin (Fig. 8A). OTUB1 was determined to reside in the insoluble nuclear fraction. Interestingly, overexpression of OTUB1 caused an increase in unliganded ERα presence in the insoluble chromatin fraction (Fig. 8B, lanes 1 and 5). This increase is particularly intriguing given that overexpression of OTUB1 decreased the overall level of ERα (Fig. 7C). The increased presence of ERα in the chromatin can be due to ERα retention in the nuclear matrix and the reduction in ERα turnover (16, 29). We evaluated the ERα presence in the chromatin fraction in the presence of the proteasome inhibitor MG132. As shown in the Fig. 8B (lanes 3 and 7), the addition of MG132 did not cause further stabilization of ERα, indicating that OTUB1 protects chromatin-bound ERα from proteasome-mediated degradation and that overexpression of OTUB1 abrogates the ability of MG132 to illicit any further increase in ERα association with chromatin. These data, together with the finding that OTUB1 acts through the ERα DNA-binding domain, are consistent with results by Reid et al. (29) showing that MG132 stabilization of ERα represses its activity and increases its residence in the nuclear matrix.
FIGURE 8.
OTUB1 stabilizes ERα in the chromatin. A, OTUB1 is located in the insoluble chromatin fraction in Ishikawa cells. The nuclear fraction was isolated from untreated Ishikawa cells and subfractioned to insoluble chromatin fraction and a soluble nuclear fraction as described under “Experimental Procedures.” The fractions were analyzed by Western blot with indicated antibodies. B, expression of OTUB1 in Ishikawa cells causes retention of ERα in the chromatin. Ishikawa cells were transfected with pCMV6 or HA-OTUB1 and treated as indicated with MG132 (4 μm) for 4 h and with estradiol (E2) or ethanol (EtOH) for 2 h. The chromatin fractions were isolated as in A and analyzed using indicated antibodies. The protein bands were quantified using Image J and normalized to histone. IB, immunoblot.
DISCUSSION
Using the recombinant DUB substrate ubiquitin-AFC, we show for the first time that ERα can be isolated in a complex with deubiquitinating enzymes (Fig. 1). Furthermore, utilizing mass spectrometry analysis, we identified OTUB1 as a novel ERα-interacting protein capable of deubiquitinating ERα in cells and in vitro (supplemental Fig. S1 and Fig. 3). We also show that OTUB1 binding to ERα is dependent on an intact OTUB1 active site, because OTUB1 C/S has diminished binding affinity. On the other hand, binding of OTUB1 Ubmut to ERα is enhanced, indicating that OTUB1 likely recognizes ERα independent of the ubiquitination status of the receptor (Fig. 3). We also show that OTUB1 negatively affects ERα-mediated transcription in transient reporter assays and from an endogenous ERα target gene (Figs. 4–6). Finally, we show that OTUB1 regulates ERα levels in the Ishikawa endometrial cancer cell line containing endogenous ERα, both at the level of the expression of ERα gene and by increasing the residence of the ERα protein in the chromatin (Figs. 7 and 8).
ERα Interacts with Deubiquitinating Enzymes
Because ERα is an ubiquitinated protein (2, 21–24), we hypothesized that ERα may also be regulated by deubiquitinating enzymes. Although nuclear receptors have been shown to recruit DUBs to regulate histone H2A and H2B ubiquitination as in the case of androgen receptor and 2A-DUB, USP22, and USP10 (41–43), DUBs have not been shown to target nuclear receptors themselves.
Our initial step in this study was to coimmunoprecipitate ERα complexes from ERα expressed 293T cells and incubate them with the recombinant DUB substrate ubiquitin-AFC. This novel approach that utilizes ubiquitin-AFC probe enabled us to detect a robust deubiquitinating activity associated with ERα complex, indicating that one or more DUBs are being recruited to ERα both in its unliganded and liganded state (data not shown). We further extended this by performing mass spectrometry analysis of ERα complexes, identifying OTUB1 as an ERα-interacting DUB. Furthermore, we showed that both intact OTUB1 active site and ubiquitin recognition are important modulators of OTUB1-ERα interaction. The observation that OTUB1 C/S has decreased affinity for ERα is in accordance with findings by Edelmann et al. (45) that showed that the OTUB1 active site undergoes a conformational change upon binding to protein substrate. Point mutations in the active site may therefore interfere with this conformational change and decrease the binding of ubiquitin. Based on our findings with OTUB1 Ubmut, we conclude that presence of an ubiquitin chain is not necessary for OTUB1 to recognize and bind ERα, but that it may be necessary for OTUB1 to release ERα after deubiquitination. In light of this interpretation and the fact that OTUB1 binds ERα in both the unliganded and liganded states, it is tempting to speculate that OTUB1 resides on ERα prior to ubiquitination and that it leaves ERα after deubiquitination together with the cleaved polyubiquitin chain.
OTUB1 Deubiquitinates ERα
OTUB1 is a DUB with an in vitro affinity for K48-linked (and to the lesser extent to K63-linked) polyubiquitin chains (44–46, 50). Although OTUB1 deubiquitinating activity has been confirmed in vitro, no protein substrates have been identified to undergo deubiquitination by OTUB1. Soares et al. (50) implicated OTUB1 involvement in T cell anergy where OTUB1 binds and destabilizes the E3 ligase GRAIL through a mechanism that does not directly involve the DUB activity of OTUB1. In addition, in the proteomic study by Edelmann et al. (45), two more OTUB1 interactors have been identified: FUS/TLS and Rack1, both of which are involved in RNA splicing. However, the significance of these interactions and whether FUS/TLS and Rack1 are deubiquitinated by OTUB1 remains unknown at this time. In this study, we identify ERα as a protein substrate that is deubiquitinated by OTUB1 in vitro and in vivo. We show that the wild type OTUB1, but neither its catalytic (OTUB1 C/S) nor ubiquitin binding (OTUB1 Ubmut) mutant, is capable of efficiently removing polyubiquitin aggregates from ERα (Fig. 3).
OTUB1 Negatively Regulates ERα-mediated Transcription
Our data indicate that OTUB1 has a negative effect on ERα-mediated transcription in transient reporter assays. We also show that the OTUB1 effect on transcription is specific for ERα because OTUB1 does not repress PR-B- or glucocorticoid receptor-mediated (Fig. 4 and data not shown) transcription. We also show that OTUB1 repression is not mediated by the AF2 domain of ERα because GAL4-AF2 (encompassing amino acids 302–595) fails to be repressed. In the subsequent experiment using ERα179C that lacks the AF1 domain, we determined that the majority of OTUB1 repression is mediated by the ERα DNA-binding domain and possibly by the ERα hinge region (Fig. 4).
Although the OTUB1 C/S catalytic mutant does not reverse the inhibitory effect on transcription, we speculate that it is still able to inhibit ERα-mediated transcription because of its continued ability to interact with the receptor and interfere with the dynamic exchange of ubiquitin required for efficient transcription to ensue. To this end, Balakirev et al. (44) and others (45, 46, 50) have shown that expression of OTUB1 slightly reduces the cellular ubiquitin pool and that expression of OTUB1 C/S does not completely reverse this phenotype (data not shown). On the other hand, expression of an OTUB1 Ubmut that cannot bind ubiquitin completely restores ERα transcription, a result that is in concordance with its effect on ERα ubiquitination. While performing these experiments we noticed that transient expression of both OTUB1 and OTUB1 C/S causes a spindle like morphology in HeLa but not in HEK293T cells and Ishikawa cells. This occurrence in HeLa cells was separate from our observation that OTUB1 represses ERα because we were able to detect this repression in all of the cell lines tested. Finally, transient reported data are consistent with our finding that the loss of OTUB1 in Ishikawa cells containing endogenous ERα up-regulates ERα-mediated transcription of PR gene promoter and increases the activity of estrogen-dependent placental alkaline phosphatase.
OTUB1 Regulates ERα Stability Both at the Transcriptional and Post-translational Level
Using overexpression and knockdown of OTUB1 in Ishikawa cells, we show that OTUB1 regulates transcription of ERα gene (Fig. 7). We find that expression of OTUB1 decreases, and knockdown increases overall levels of ERα in Ishikawa cells, further implicating OTUB1 in regulating the biological response to estrogens. Although we hypothesize that OTUB1 is directly affecting the transcription of the ERα gene in Ishikawa cells, we cannot exclude the possibility that OTUB1 may have an effect on ERα mRNA stability. Regardless, this coordinated event on both the transcriptional and post-translational level specifically down-regulates estrogen action because it does not impact PR-B activity. Furthermore, utilizing a chromatin fractionation protocol, we show that OTUB1 resides in the chromatin and that its overexpression increases ERα stability and residency in the chromatin. In light of our result that OTUB1 may interfere with ERα DNA binding, we conclude that OTUB1 stabilizes ERα and causes its immobilization to the nuclear matrix. This is consistent with the effect of MG132 on ERα stabilization, activity, and localization to the nuclear matrix (29).
Overall, our data indicate that OTUB1 affects ERα both at the level of expression of the ERα gene and at the level of protein stability in the chromatin. Our group and others (2, 7, 21–24, 28, 29, 31) have shown that ERα protein turnover is necessary for the ERα function and that stabilization of ERα protein abrogates its transcriptional activity. Here we find that OTUB1 stabilizes ERα protein in the insoluble nuclear fraction, thereby reducing ERα transcriptional activity. This negative effect that OTUB1 has on ERα functional output is further reinforced by OTUB1-mediated reduction in transcription of the ERα gene itself and consequently, the reduction in availability of the overall ERα pool in the cell. Because ERα is known to regulate its own promoter (51), it is possible that OTUB1 reduces ERα mRNA levels by inhibiting ERα transcription of its own promoter, thereby creating an inhibitory feedback loop. In conclusion, OTUB1 has evolved as a specific negative regulator of ERα-mediated transcription by decreasing the overall amount of ERα present in the cell and by preventing ERα turnover in the chromatin.
ERα transcriptional activity is regulated by numerous post-translational modifications including phosphorylation, SUMOylation, glycosylation, methylation, acetylation, and ubiquitination. For most of these modes of post-translational modification of ERα, processes that reverse these modifications also exist. Here, we see that receptor deubiquitination also exists and plays a critical role in ERα function as a transcription factor.
Supplementary Material
This work was supported by National Institutes of Health Grants HD08818 and HD07857 and Welch Foundation Grant FY09-Q1521.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S3.
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen-response element
- DUB
- deubiquitinating enzymes
- E3
- ubiquitin-protein isopeptide ligase
- Ub
- ubiquitin
- siRNA
- small interfering RNA
- HA
- hemagglutinin
- DTT
- dithiothreitol
- TBS
- Tris-buffered saline
- PR
- progesterone receptor
- LUC
- luciferase
- AF
- activation function
- TAMRA
- 6-carboxytetramethylrhodamine
- AFC
- 7-amido-4-methylcourmain.
REFERENCES
- 1.Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. ( 1995) Cell 83, 835– 839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass C. K., Rosenfeld M. G. ( 2000) Genes Dev. 14, 121– 141 [PubMed] [Google Scholar]
- 3.McKenna N. J., Lanz R. B., O'Malley B. W. ( 1999) Endocr. Rev. 20, 321– 344 [DOI] [PubMed] [Google Scholar]
- 4.Nilsson S., Mäkelä S., Treuter E., Tujague M., Thomsen J., Andersson G., Enmark E., Pettersson K., Warner M., Gustafsson J. A. ( 2001) Physiol. Rev. 81, 1535– 1565 [DOI] [PubMed] [Google Scholar]
- 5.Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. ( 1987) Cell 51, 941– 951 [DOI] [PubMed] [Google Scholar]
- 6.Pratt W. B., Toft D. O. ( 1997) Endocr. Rev. 18, 306– 360 [DOI] [PubMed] [Google Scholar]
- 7.Alarid E. T. ( 2006) Mol. Endocrinol. 20, 1972– 1981 [DOI] [PubMed] [Google Scholar]
- 8.Lonard D. M., O'Malley B. W. ( 2008) Nature 452, 946– 947 [DOI] [PubMed] [Google Scholar]
- 9.McDonnell D. P., Norris J. D. ( 2002) Science 296, 1642– 1644 [DOI] [PubMed] [Google Scholar]
- 10.McKenna N. J., Xu J., Nawaz Z., Tsai S. Y., Tsai M. J., O'Malley B. W. ( 1999) J. Steroid Biochem. Mol. Biol. 69, 3– 12 [DOI] [PubMed] [Google Scholar]
- 11.Lonard D. M., O'Malley B. W. ( 2008) Sci. Signal. 1, pe16. [DOI] [PubMed] [Google Scholar]
- 12.Lonard D. M., O'Malley B W. ( 2007) Mol. Cell 27, 691– 700 [DOI] [PubMed] [Google Scholar]
- 13.Dahlman-Wright K., Cavailles V., Fuqua S. A., Jordan V. C., Katzenellenbogen J. A., Korach K. S., Maggi A., Muramatsu M., Parker M. G., Gustafsson J. A. ( 2006) Pharmacol. Rev. 58, 773– 781 [DOI] [PubMed] [Google Scholar]
- 14.Herynk M. H., Fuqua S. A. ( 2004) Endocr. Rev. 25, 869– 898 [DOI] [PubMed] [Google Scholar]
- 15.Pinzone J. J., Stevenson H., Strobl J. S., Berg P. E. ( 2004) Mol. Cell. Biol. 24, 4605– 4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calligé M., Richard-Foy H. ( 2006) Nucl. Recept. Signal. 4, e004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima A., Maruyama S., Bohgaki M., Miyajima N., Tsukiyama T., Sakuragi N., Hatakeyama S. ( 2007) Biochem. Biophys. Res. Commun. 357, 245– 251 [DOI] [PubMed] [Google Scholar]
- 18.Tateishi Y., Kawabe Y., Chiba T., Murata S., Ichikawa K., Murayama A., Tanaka K., Baba T., Kato S., Yanagisawa J. ( 2004) EMBO J. 23, 4813– 4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan M., Park A., Nephew K. P. ( 2005) Mol. Endocrinol. 19, 2901– 2914 [DOI] [PubMed] [Google Scholar]
- 20.Berry N. B., Fan M., Nephew K. P. ( 2008) Mol. Endocrinol. 22, 1535– 1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nawaz Z., Lonard D. M., Dennis A. P., Smith C. L., O'Malley B. W. ( 1999) Proc. Natl. Acad. Sci. U. S. A. 96, 1858– 1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laïos I., Journé F., Nonclercq D., Vidal D. S., Toillon R. A., Laurent G., Leclercq G. ( 2005) J. Steroid Biochem. Mol. Biol. 94, 347– 359 [DOI] [PubMed] [Google Scholar]
- 23.Wijayaratne A. L., McDonnell D. P. ( 2001) J. Biol. Chem. 276, 35684– 35692 [DOI] [PubMed] [Google Scholar]
- 24.Zhang H., Sun L., Liang J., Yu W., Zhang Y., Wang Y., Chen Y., Li R., Sun X., Shang Y. ( 2006) EMBO J. 25, 4223– 4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhananjayan S. C., Ismail A., Nawaz Z. ( 2005) Essays Biochem. 41, 69– 80 [DOI] [PubMed] [Google Scholar]
- 26.Nandi D., Tahiliani P., Kumar A., Chandu D. ( 2006) J. Biosci. 31, 137– 155 [DOI] [PubMed] [Google Scholar]
- 27.Hershko A., Ciechanover A. ( 1998) Annu. Rev. Biochem. 67, 425– 479 [DOI] [PubMed] [Google Scholar]
- 28.Lonard D. M., Nawaz Z., Smith C. L., O'Malley B. W. ( 2000) Mol. Cell 5, 939– 948 [DOI] [PubMed] [Google Scholar]
- 29.Reid G., Hübner M. R., Métivier R., Brand H., Denger S., Manu D., Beaudouin J., Ellenberg J., Gannon F. ( 2003) Mol. Cell 11, 695– 707 [DOI] [PubMed] [Google Scholar]
- 30.Shao W., Keeton E. K., McDonnell D. P., Brown M. ( 2004) Proc. Natl. Acad. Sci. U. S. A. 101, 11599– 11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Métivier R., Penot G., Hübner M. R., Reid G., Brand H., Kos M., Gannon F. ( 2003) Cell 115, 751– 763 [DOI] [PubMed] [Google Scholar]
- 32.Zheng L., Annab L. A., Afshari C. A., Lee W. H., Boyer T. G. ( 2001) Proc. Natl. Acad. Sci. U. S. A. 98, 9587– 9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saji S., Okumura N., Eguchi H., Nakashima S., Suzuki A., Toi M., Nozawa Y., Saji S., Hayashi S. ( 2001) Biochem. Biophys. Res. Commun. 281, 259– 265 [DOI] [PubMed] [Google Scholar]
- 34.Eakin C. M., Maccoss M. J., Finney G. L., Klevit R. E. ( 2007) Proc. Natl. Acad. Sci. U. S. A. 104, 5794– 5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L., Li Z., Howley P. M., Sacks D. B. ( 2006) J. Biol. Chem. 281, 1978– 1985 [DOI] [PubMed] [Google Scholar]
- 36.Amerik A. Y., Hochstrasser M. ( 2004) Biochim. Biophys. Acta 1695, 189– 207 [DOI] [PubMed] [Google Scholar]
- 37.Ventii K. H., Wilkinson K. D. ( 2008) Biochem. J. 414, 161– 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Love K. R., Catic A., Schlieker C., Ploegh H. L. ( 2007) Nat. Chem. Biol. 3, 697– 705 [DOI] [PubMed] [Google Scholar]
- 39.Nijman S. M., Luna-Vargas M. P., Velds A., Brummelkamp T. R., Dirac A. M., Sixma T. K., Bernards R. ( 2005) Cell 123, 773– 786 [DOI] [PubMed] [Google Scholar]
- 40.Kim J. H., Park K. C., Chung S. S., Bang O., Chung C. H. ( 2003) J. Biochem. 134, 9– 18 [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y., Lang G., Ito S., Bonnet J., Metzger E., Sawatsubashi S., Suzuki E., Le Guezennec X., Stunnenberg H. G., Krasnov A., Georgieva S. G., Schüle R., Takeyama K., Kato S., Tora L., Devys D. ( 2008) Mol. Cell 29, 92– 101 [DOI] [PubMed] [Google Scholar]
- 42.Zhou W., Zhu P., Wang J., Pascual G., Ohgi K. A., Lozach J., Glass C. K., Rosenfeld M. G. ( 2008) Mol. Cell 29, 69– 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faus H., Meyer H. A., Huber M., Bahr I., Haendler B. ( 2005) Mol. Cell. Endocrinol. 245, 138– 146 [DOI] [PubMed] [Google Scholar]
- 44.Balakirev M. Y., Tcherniuk S. O., Jaquinod M., Chroboczek J. ( 2003) EMBO Rep. 4, 517– 522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelmann M. J., Iphöfer A., Akutsu M., Altun M., di Gleria K., Kramer H. B., Fiebiger E., Dhe-Paganon S., Kessler B. M. ( 2008) Biochem. J. 418, 379– 390 [DOI] [PubMed] [Google Scholar]
- 46.Shan T. L., Tang Z. L., Guo D. Z., Yang S. L., Mu Y. L., Ma Y. H., Guan W. J., Li K. ( 2008) Mol. Biol. Rep., in press [DOI] [PubMed] [Google Scholar]
- 47.Yi P., Feng Q., Amazit L., Lonard D. M., Tsai S. Y., Tsai M. J., O'Malley B. W. ( 2008) Mol. Cell 29, 465– 476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson C., Crimmins S., Wilson J. A., Korbel G. A., Ploegh H. L., Wilson S. M. ( 2005) J. Neurochem. 95, 724– 731 [DOI] [PubMed] [Google Scholar]
- 49.Wysocka J., Reilly P. T., Herr W. ( 2001) Mol. Cell. Biol. 21, 3820– 3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soares L., Seroogy C., Skrenta H., Anandasabapathy N., Lovelace P., Chung C. D., Engleman E., Fathman C. G. ( 2004) Nat. Immunol. 5, 45– 54 [DOI] [PubMed] [Google Scholar]
- 51.Eeckhoute J., Keeton E. K., Lupien M., Krum S. A., Carroll J. S., Brown M. ( 2007) Cancer Res. 67, 6477– 6483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.