Abstract
In selected mammalian tissues, long chain fatty acid transporters (FABPpm, FAT/CD36, FATP1, and FATP4) are co-expressed. There is controversy as to whether they all function as membrane-bound transporters and whether they channel fatty acids to oxidation and/or esterification. Among skeletal muscles, the protein expression of FABPpm, FAT/CD36, and FATP4, but not FATP1, correlated highly with the capacities for oxidative metabolism (r ≥ 0.94), fatty acid oxidation (r ≥ 0.88), and triacylglycerol esterification (r ≥ 0.87). We overexpressed independently FABPpm, FAT/CD36, FATP1, and FATP4, within a normal physiologic range, in rat skeletal muscle, to determine the effects on fatty acid transport and metabolism. Independent overexpression of each fatty acid transporter occurred without altering either the expression or plasmalemmal content of other fatty acid transporters. All transporters increased fatty acid transport, but FAT/CD36 and FATP4 were 2.3- and 1.7-fold more effective than FABPpm and FATP1, respectively. Fatty acid transporters failed to alter the rates of fatty acid esterification into triacylglycerols. In contrast, all transporters increased the rates of long chain fatty acid oxidation, but the effects of FABPpm and FAT/CD36 were 3-fold greater than for FATP1 and FATP4. Thus, fatty acid transporters exhibit different capacities for fatty acid transport and metabolism. In vivo, FAT/CD36 and FATP4 are the most effective fatty acid transporters, whereas FABPpm and FAT/CD36 are key for stimulating fatty acid oxidation.
Uptake of long chain fatty acids across the plasma membrane had long been considered to occur via passive diffusion. However, in recent years, there has been a fundamental shift in our understanding, and it is now widely recognized that long chain fatty acids cross the plasma membrane via a protein-mediated mechanism (for reviews, see Refs. 1–3). A number of fatty acid transporters have been identified, including fatty acid translocase/CD36 (FAT/CD36), plasma membrane-associated fatty acid binding proteins (FABPpm), and a family of fatty acid transport proteins (FATP1–6)5 (for reviews, see Refs. 1 and 4). Selected stimuli (muscle contraction, insulin, and AICAR) induce the translocation of selected fatty acid transporters (FABPpm, FAT/CD36, and FATP1) from an intracellular depot to the plasma membrane, in both heart and skeletal muscle, resulting in concurrently increased rates of fatty acid transport (for a review, see Ref. 1). Some fatty acid transporters have now also been implicated in the dysregulation of fatty acid metabolism in heart and skeletal muscle in models of insulin resistance and type 1 and 2 diabetes, including FAT/CD36 (5–9), FATP1 (10, 11), and possibly FATP4 (11, 12) but not FABPpm (5–7). Thus, in recent years, it has become widely accepted that (a) long chain fatty acids traverse the plasma membrane via a protein-mediated mechanism and (b) some of the fatty acid transporters are central to the dysregulation in skeletal muscle fatty acid metabolism in obesity and type 2 diabetes.
In vivo, many of the fatty acid transporters are frequently co-expressed in different tissues. FAT/CD36 and FABPpm are ubiquitously expressed (1), whereas FATP1–6 exhibit a somewhat tissue-specific distribution pattern (13, 14). The reason for the co-expression of different fatty acid transporters within the same tissue remains unclear. It has been speculated that selected fatty acid transporters may need to interact with each other (15, 16). Alternatively, it is also possible that (a) different fatty acid transporters have discrepant transport capacities, and (b) selected transporters may channel fatty acids differentially to fatty acid oxidation and esterification into triacylglycerols in mammalian tissue.
Recent evidence has shown that the transport capacities among FATPs can differ substantially, as revealed by overexpression (14, 17, 18) or knockdown studies (19), but there is little agreement as to which FATP is most effective. Extensive studies by DiRusso et al. (17) in yeast revealed that when FATP1–6 were overexpressed to similar levels (qualitative assessment), FATP4 exhibited 1.7- and 3-fold greater fatty acid transport effectiveness compared with FATP1 and FATP2, respectively, whereas no fatty acid transport capacities were attributable to FATP3, -5, and -6 (17). In contrast, in HEK293 cells, the FATP6 transport capacity was 3- and 6.5-fold greater than FATP1 and FATP4, respectively (14), whereas in 3T3-L1 adipocytes, a fatty acid transport role was evident only for FATP1 and not FATP4 (19). Others have also questioned the transport role of FATP4 (20). These discrepant findings with respect to the transport effectiveness of FATPs may reflect, in part, the use of diverse cell types with ill defined metabolic needs and/or machinery for fatty acid uptake and metabolism. Indeed, several recent reports indicate that fatty acid transport cannot be adequately examined in some cells, because these appear to lack accessory proteins that may be involved in fatty acid transport (21, 22). In addition, extrapolation of results from cultured cells to metabolically important tissue in vivo may also be problematic, since cells and mammalian tissues probably have different requirements for fatty acid utilization, and their regulation of fatty acid uptake may also differ. For example, the mechanisms regulating the acute contraction-induced up-regulation of fatty acid transport and oxidation, such as occurs in heart and skeletal muscle, is probably absent in selected cell cultures.
Assessment of fatty acid transporter effectiveness, in vivo, cannot be determined in knock-out animals, since compensatory responses in some fatty acid transporters (FATP1 and -4) occur when another fatty acid transporter (FAT/CD36) has been ablated (23, 24). Thus, the relative effectiveness of selected fatty acid transporters on fatty acid transport in vivo remains unknown. In addition, whether fatty acid transporters channel fatty acids to a particular metabolic fate, as has been suggested based on studies in cultured cells (18, 19, 25), may depend on the cell type being examined.
It is desirable to discern the effectiveness of selected fatty acid transporters in mammalian tissues that have a well known system for transporting and utilizing fatty acids and in which fatty acid transporters can be independently up-regulated without disturbing the expression of other fatty acid transporters. These criteria can be satisfied in rat skeletal muscle in which genes can be up-regulated under controlled conditions within a physiologically meaningful range (26–28). Therefore, in the present study, we have compared the independent transport effectiveness of fatty acid transporters (FABPpm, FAT/CD36, FATP1, and FATP4) in skeletal muscle, without disturbing the expression and plasmalemmal content of other fatty acid transporters. In addition, we also examined the contributions of these transporters to fatty acid oxidation and esterification into triacylglycerols. These are the first studies to reveal that in vivo (a) the fatty acid transport effectiveness of fatty acid transporters differs considerably, and (b) in skeletal muscle, these transporters serve to channel fatty acids to oxidation, not esterification into triacylglycerols.
EXPERIMENTAL PROCEDURES
Materials
[14C]Mannitol and 1-14C- and 3H-labeled palmitate were purchased from GE Healthcare. Isoflurane was obtained from Baxter Corp. (Mississauga, Canada). Temgesic was obtained from Reckitt and Benckiser Healthcare Ltd. (Hull, UK). The monoclonal antibody MO25 (29) and antisera against FABPpm were used to detect FAT/CD36 and FABPpm, respectively, as we have done previously (7, 24, 30–32). Antibodies against FATP1 and FATP4 and rabbit anti-goat secondary antibodies were purchased (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The pcDNA3.1+ mammalian expression vector was obtained from Invitrogen. All other reagents were obtained from Sigma.
Animals
Female Sprague-Dawley rats were used in the present experiments (n = 270). All animals were bred on site and housed in a temperature-controlled room with a 12/12-h reversed light/dark cycle. Rats were fed water and standard rat chow ad libitum. All experimental procedures were approved by the Animal Care Committee at the University of Guelph.
Comparison of Fatty Acid Transporters with Muscle Oxidative Capacity, Carnitine Palmitoyltransferase I, and Triacylglycerol Formation
To ascertain whether FABPpm, FAT/CD36, FATP1, and FATP were expressed in relation to the muscles' oxidative capacity and their capacity for fatty acid metabolism (oxidation and esterification), we compared the protein expression of these four FATPs in six metabolically heterogeneous rat hind limb muscles (a) with the percentage of oxidative fibers, (b) with the rates of palmitate incorporation into intramuscular triacylglycerol, and (c) with carnitine palmitoyltransferase I protein expression, a marker of skeletal muscle fatty acid oxidation.
Tissue Harvesting and Tissue Protein Analyses
Rats were anesthetized with Somnotol administered intraperitoneally (6 mg/100 g of body weight). Metabolically heterogeneous skeletal muscles (extensor digitorum longus, red tibialis anterior, white tibialis anterior, plantaris, soleus, red gastrocnemius, and white gastrocnemius) were excised, freeze-clamped in liquid nitrogen, and stored at −80 °C for future analysis for selected proteins. We have recently reported the muscle fiber composition of these rat hind limb muscles (see Ref. 26).
Intramuscular Triacylglycerol Formation
In a subgroup of rats, we determined the rate of palmitate incorporation into intramuscular triacylglycerol in perfused hind limb muscles, as we have described previously (7, 24, 30). Briefly, after the animals were surgically prepared, the hind quarters of rats were preperfused with 0.1% BSA in Krebs-Henseleit buffer (pH 7.4) for 10 min at 37 °C while being gassed continuously with 95% O2 and 5% CO2. Thereafter, muscles were perfused for 60 min (flow rate 18 ml/min, pH 7.4, with Krebs-Henseleit buffer, 6 mm glucose, [1-14C]palmitate (0.1 μCi/ml; 0.5 mm), and 4% BSA). After 60 min, muscles (extensor digitorum longus, red tibialis anterior, white tibialis anterior, soleus, plantaris, red gastrocnemius, white gastrocnemius) were quickly harvested, rapidly frozen in liquid nitrogen, and stored at −80 °C. Palmitate accumulation in the intramuscular triacylglycerol depot was determined using thin layer chromatography, as we have described previously (7, 24). Briefly, after homogenizing muscle (50 mg in 2 ml of 1:1 chloroform/methanol, 2 × 15 s, 4 °C), the washed chloroform phase (500 μl) was dried under nitrogen and reconstituted (100 μl of 2:1 chloroform/methanol (v/v)). Samples were resolved (60/40/3, heptane/isopropylether/acetic acid) on silica gel plates. Chloroflurescein dye (0.02% (w/v) in ethanol) allowed triacylglycerol bands to be visualized under UV light and to be removed into scintillation vials for determining the radioactive counts. Standard calculations were used to determine palmitate incorporation into intramuscular triacylglycerol.
Fatty Acid Transporter Overexpression
The cDNAs for FAT/CD36, FABPpm, and FATP1 were generously donated by Dr. N. Abumrad (Washington University School of Medicine, St Louis, MO), Dr. A. Iriarte (University of Missouri, Columbia, MO), and Dr. J. Schaffer (Washington University School of Medicine, St. Louis, MO), respectively. These cDNAs were used as templates to generate expression plasmids. The open reading frame of FATP4 was amplified by PCR from skeletal muscle cDNA and cloned into pCR2.1 (Invitrogen) and was used to generate the expression vector. cDNAs were subcloned into the expression vector pcDNA3.1+ for overexpression. Large scale stocks of the expression plasmids were isolated using the Plasmid Giga Kit (Qiagen, Mississauga, Canada), following the manufacturer's instructions and as we have recently reported (26, 27). Plasmid DNA was resuspended in half-strength normal saline (0.75 mm NaCl), and the DNA concentration and integrity were verified by spectrophotometry and gel visualization of restriction digests.
For overexpression of fatty acid transporters, we used an electrotransfection procedure, as we have previously reported (26–28), to overexpress independently in four separate experiments, FABPpm, FAT/CD36, FATP1, or FATP4 in the tibialis anterior muscle. For these purposes, rats were anesthetized (2.5–3.0% isoflurane in oxygen), and their hind limbs were shaved and sterilized. Thereafter, we injected 250 μg of plasmid DNA, dissolved in 200 μl of half-strength normal saline solution (0.75 mm NaCl), through the skin into the muscle to independently overexpress FABPpm, FAT/CD36, FATP1, or FATP4 in four separate experiments. Studies with empty pcDNA 3.1+ plasmid were also performed. Electrotransfection of the intact muscle in vivo was performed immediately thereafter (i.e. using Tweezertrodes (8 pulses, 200 V/cm, 1 Hz, 20-ms duration (ECM 830 Square Wave Electroporator; BTX, Harvard Apparatus, Holliston, MA) (26–28, 33). After 2 weeks, muscles were harvested, and the expression of fatty acid transporter proteins (FABPpm, FAT/CD36, FATP1, and FATP4) was quantified in electrotransfected and contralateral control muscles by Western blotting of muscle homogenate and plasma membrane samples (see below), as we have described previously (34–37).
Giant Sarcolemmal Vesicle Preparation and Fatty Acid Transport Determinations
The effects of fatty acid transporter overexpression on fatty acid transport in the different experiments was determined in giant sarcolemmal vesicles prepared from control and transfected muscles. These vesicles have been thoroughly characterized (16, 34, 38) and are appropriate for determining rates of fatty acid transport, since fatty acids taken across the sarcolemma are not metabolized further (16, 34, 38).
Giant Sarcolemmal Vesicles
Giant sarcolemmal vesicles were prepared, as we have previously described (34, 35). Briefly, muscle tissues were cut into thin layers (1–3 mm), incubated, and shaken (1 h, 34 °C; 140 mm KCl, 10 mm MOPS (pH 7.4), aprotinin (30 μg/ml), and collagenase type VII (150 units/ml)). Thereafter, the supernatant fraction was collected. Percoll, KCl, and aprotinin were added to final concentrations of 3.5% (v/v), 28 mm, and 10 μg/ml, respectively, and the suspension was placed at the bottom of a density gradient consisting of a 4% Nycodenz (w/v) middle layer and a KCl-MOPS upper layer. Samples were centrifuged at low speed (60 × g, 45 min), after which the vesicles were harvested from the interface of the upper and middle layers, diluted in KCl-MOPS, and recentrifuged (12,000 × g, 5 min). To obtain sufficient vesicles for the fatty acid transport studies and the determination of fatty acid transporters on the plasma membrane of the giant vesicles, it was necessary to pool muscles from eight animals for each independent experiment. This pooling of muscle was done for each of the six independent experiments for each of the four fatty acid transporters and for the experiments with empty plasmids.
Fatty Acid Transport
The rates of palmitate transport into giant sarcolemmal vesicles were determined in freshly obtained giant vesicles, as we have described previously (5, 34, 35). For this purpose, KCl-MOPS (40 μl, containing 0.1% BSA, unlabeled palmitate, (14 μm), radiolabeled 0.3 μCi of [3H]palmitate, and 0.06 μCi of [14C]mannitol), was added to 40 μl of vesicle suspension for 15 s. Palmitate uptake was terminated by the addition of 1.4 ml of ice-cold KCl-MOPS containing 2.5 mm HgCl2 and 0.1% BSA. The sample was quickly centrifuged, and radioactivity was determined in the remaining pellet. Nonspecific uptake was measured by adding the stop solution before the addition of the radiolabeled palmitate solution. Fatty acid transporters were measured on the plasma membrane of the giant vesicles, using Western blotting.
Fatty Acid Oxidation and Esterification in Transfected Muscle
To determine the effect of fatty acid transporter overexpression on the rates of fatty acid oxidation and esterification into triacylglycerols, we used an incubated soleus muscle preparation, which has been well characterized in our laboratory for metabolic studies (39, 40). To keep muscle sizes relatively small, as is required for studies with isolated skeletal muscle to prevent hypoxia (41), female rats weighing ∼80 g were used. The independent overexpression of the fatty acid transporters and studies with empty plasmid were performed using electrotransfection, as described above. However, the anatomic location of the soleus muscle required minor additional surgery to expose this muscle for injection with plasmid DNA and to perform the electrotransfection. After the electrotransfection, the overlying superficial muscle was sutured, and the skin incision was closed. After surgery, animals were provided with analgesic (Temgesic).
Because growth rates are very rapid in young animals, small soleus muscles were removed from anesthetized rats (Somnotol; 6 mg/100 g, body weight) 7 days after transfection. Muscles were subdivided longitudinally into thin strips (<20 mg) for incubation (41). In these muscles, we examined the protein expression of fatty acid transporters and rates of esterification into triacylglycerols and palmitate oxidation. These fatty acid metabolism studies were performed as we have described previously (39, 40). Briefly, muscles were placed in warmed (30 °C), pregassed (95% O2, 5% CO2) Medium 199 containing 4% BSA and 0.5 mm palmitate, pH 7.4. After a 30-min equilibration period, muscles were transferred to freshly pregassed medium 199 (4% BSA, 0.5 mm palmitate, pH 7.4, 30 °C) supplemented with 0.5 μCi/ml [1-14C]palmitate for 40 min. Palmitate oxidation was determined as we have previously described (39, 40) by summing the 14CO2 trapped in benzothium hydroxide, the 14CO2 released from acidified incubating medium, and the water-soluble 14C-intermediates extracted from muscles. Fatty acid incorporation into phospholipids and triacylglycerols was determined by extracting lipids from muscle and resolving them on silica gel plates, as described above.
Western Blotting
To detect FATP expression, muscles were homogenized, and proteins were separated using SDS-PAGE and detected using Western blotting, as we have previously described (34, 35). FATPs were also determined on the plasma membranes of giant sarcolemmal vesicles. Signals were detected using enhanced chemiluminescence (PerkinElmer Life Sciences) and were subsequently quantified by densitometry as per the manufacturer's instructions (Gene Tool, SynGene, and ChemiGenius2; PerkinElmer Life Sciences). Equal quantities of muscle homogenate (20 μg) or plasma membrane proteins (5 μg) were loaded. Membranes were stained with Ponceau S to confirm equal loading.
Statistics
Correlational analyses were performed using least squares linear regression. For these purposes, the means of the data were used, since the data were necessarily obtained from different animals. Repeated measures analyses of variance were used to compare the effects of transfection on FATP expression, plasmalemmal fatty acid transporters, and transport as well as on rates of fatty acid esterification and oxidation. Post hoc analyses, when warranted, were performed using Fisher's least significant difference test. All data are reported as mean ± S.E.
RESULTS
Relationship of Fatty Acid Transporters with Muscle Oxidative Capacity and Fatty Acid Metabolism
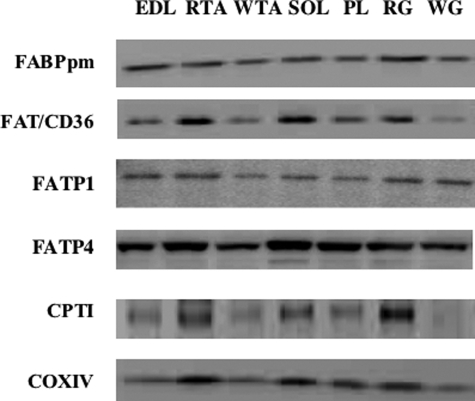
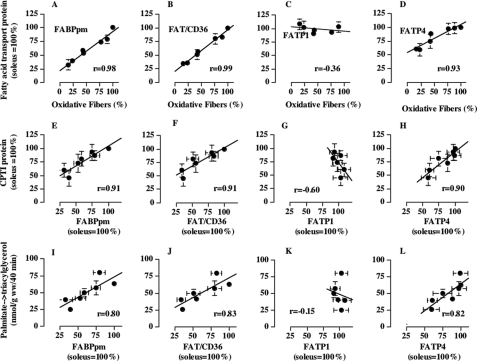
Initially, we compared the fatty acid transporter protein expression in metabolically heterogeneous muscles (Fig. 1) with the oxidative muscle fiber composition of these muscles, a characteristic that is known to be very highly correlated with the ability to metabolize fatty acids (34, 42). The protein expression of FABPpm, FAT/CD36, and FATP4 but not FATP1 was highly correlated with the oxidative phenotype of metabolically heterogeneous muscle (r ≥ 0.93; Fig. 2, A–D). Similarly, fatty acid transporters, except for FATP1, also correlated highly with carnitine palmitoyltransferase I protein expression (r ≥ 0.89; Fig. 2, E–H), an index of mitochondrial fatty acid oxidation, and with the different capacities for fatty acid esterfication (r ≥ 0.80; Fig. 2, I–L). High correlations were also observed between fatty acid transporters (FABPpm, FAT/CD36, and FATP4) and PGC-1α (r = 0.85–0.97) (data not shown), the nuclear encoded transcriptional co-activator that has been implicated in the regulation of mitochondrial biogenesis and lipid metabolism (43).
FIGURE 1.
Representative Western blots of selected proteins in metabolically heterogeneous rat muscles. EDL, extensor digitorum longus; RTA, red tibialis anterior; WTA, white tibialis anterior; SOL, soleus; PL, plantaris; RG, red gastrocnemius; WG, white gastrocnemius. Equal loading was confirmed by Ponceau staining (not shown). COXIV, cytochrome oxidase IV; CPTI, carnitine palmitoyltransferase I.
FIGURE 2.
Relationship between fatty acid transporter protein expression (FABPpm, FAT/CD36, FATP1, and FATP4) in metabolically heterogeneous rat muscles and their oxidative muscle fiber composition (A–D), the protein expression of CPTI (E–H), and the rate of palmitate incorporation into intramuscular triacylglycerol depots (I–L) (mean ± S. E.). Fatty acid transporter protein expression and CPTI protein expression were determined in muscles of four or five animals, and the muscle fiber composition data were determined in red gastrocnemius, white gastrocnemius, extensor digitorum longus, soleus, plantaris, red tibialis anterior, and white tibialis anterior muscles, as we have recently reported (26). For Western blotting, equal protein concentrations were loaded for each muscle. For the fatty acid transporter protein expression the soleus muscle data in each animal were set to 100, and the data for the remaining muscles were expressed relative to the soleus muscle.
Effects of Overexpressing Fatty Acid Transporters on Rates of Fatty Acid Transport
We overexpressed fatty acid transporters in the muscle of one hind limb while the contralateral muscle served as control. In separate experiments, we established that transfection with empty plasmid did not affect any of the parameters under consideration (data not shown). This concurs with studies by us (26, 28, 44) and others (45, 46), which have shown that electrotransfection does not alter substrate transport and/or metabolism.
Overexpression of Fatty Acid Transporters
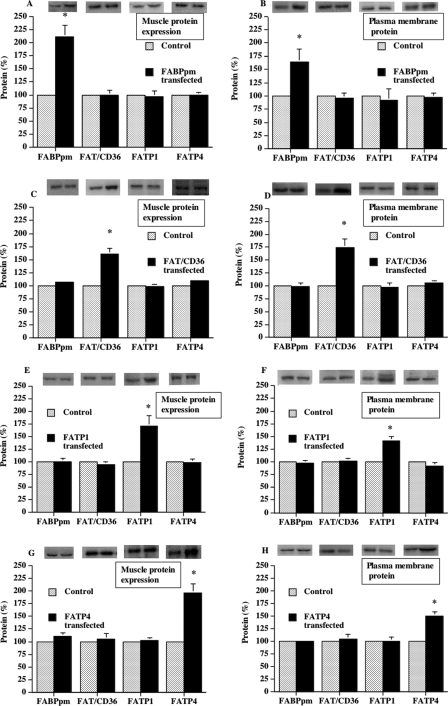
In four separate sets of experiments, FABPpm, FAT/CD36, FATP1, or FATP4 was independently overexpressed. The increase in fatty acid transporter protein expression relative to the contralateral control muscles was as follows: FABPpm (+112%), FAT/CD36 (+60%), FATP1 (+71%), or FATP4 (+97%) (Fig. 3A, C, E, and G). This independent overexpression also resulted in concomitant, independent increases at the plasma membrane for FABPm (+64%), FAT/CD36 (+75%), FATP1 (+41%), or FATP4 (+50%) (Fig. 3, B, D, F, and H). It is important to note that whenever one fatty acid transporter was independently overexpressed, there were no compensatory changes in any of the other three fatty acid transporters, either at the whole muscle level or at the plasma membrane (Fig. 3, A–H). Therefore, a change in fatty acid transport in a given experiment was attributable to the concomitant change in a given fatty acid transporter in that experiment.
FIGURE 3.
Fatty acid transporter overexpression in rat muscle and presence at the plasma membrane (mean ± S.E.). The fatty acid transporters FABPpm (A), FAT/CD36 (C), FATP1 (E), FATP4 (G) were independently overexpressed in separate experiments using an electrotransfection procedure as described under “Experimental Procedures.” To obtain sufficient plasma membrane for Western blotting of plasma membranes (present figure) FABPpm (B), FAT/CD36 (D), FATP1 (F), FATP4 (H) and determinations of fatty acid transport into giant vesicles (Fig. 4), it was necessary to combine muscles from eight animals for each independent determination. In each of the four fatty acid transporter transfection experiments, six independent determinations were made with such pooled muscles. Isolation of giant sarcolemmal vesicles from muscle was performed as described under “Experimental Procedures.” Muscle FATP expression and plasma membrane content was determined in six independent determinations, using the pooled muscle samples. For the protein expression data and the plasmalemmal fatty acid transporter content, each fatty acid transporter in the control muscles was normalized to 100, and the data in the transfected muscles were expressed relative to the control muscle. *, p < 0.05, transfected muscle versus control (paired analysis).
Fatty Acid Transport
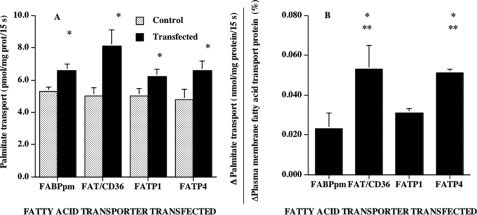
The independent overexpression of each transporter increased the rate of fatty acid transport into giant sarcolemmal vesicles. Specifically, transport rates were increased by 24, 62, 25, and 38% in muscles that independently overexpressed FABPpm, FAT/CD36, FATP1, or FATP4, respectively (Fig. 4A). These differences were due, in part, to differences in the overexpression of the individual fatty acid transporters and the resultant differences in their plasmalemmal content. However, since we measured the rates of fatty acid transport (Fig. 4A) and the plasma membrane content of the fatty acid transporters in the same giant vesicles (Fig. 3, B, D, F, and H), we were able to determine the change in palmitate transport relative to the change of a given fatty acid transporter at the sarcolemma. This revealed that the transport effectiveness of FAT/CD36 and FATP4 were similar and that these were 2.3-fold greater than that of FABPpm and 1.7-fold greater than that of FATP1 (Fig. 4B).
FIGURE 4.
Effects of fatty acid transporter overexpression on the rates of palmitate transport into giant sarcolemmal vesicles (A) and the change in palmitate transport per unit change in fatty acid transporters (B) (mean ± S.E.). The fatty acid transporters FABPpm, FAT/CD36, FATP1, and FATP4 were independently overexpressed in separate experiments using an electrotransfection procedure as described under “Experimental Procedures.” To obtain sufficient giant sarcolemmal vesicles for Western blotting of plasma membranes (Fig. 3) and for determinations of fatty acid transport into giant vesicles (present figure), it was necessary to combine tibialis anterior muscles from eight animals for each independent determination. In each of the four fatty acid transporter transfection experiments, six independent determinations were made with such pooled muscles. Isolation of giant sarcolemmal vesicles from muscle and rates of fatty acid transport were performed as described under “Experimental Procedures.” Since the overexpression of the fatty acid transporters differed somewhat among the four experiments, we determined (B) the change (%) in palmitate transport (Δ = transfected − control) in relation to the change in the plasma membrane content of the overexpressed fatty acid transporter (Δ = transfected − control), since both of these measures were made on the same preparation. A, *, p < 0.05, transfected versus control (paired analysis). B, *, p < 0.05, FAT/CD36 versus FABPpm and FATP4 versus FABPpm; **, p < 0.05, FAT/CD36 versus FATP1 and FATP4 versus FATP1.
Effects of Overexpressing Fatty Acid Transporters on Fatty Acid Metabolism
Based on studies in cultured cells (18, 19, 25, 47–49), it has been suggested that fatty acid transporters may channel fatty acids to specific metabolic fates. However, FATP1 to -6 overexpression in yeast (17) and FATP1 overexpression in the heart (50) has shown that there is not necessarily a direct concordance between fatty acid transport and metabolism. Therefore, we examined the effects of fatty acid transporter overexpression on the rates of fatty acid metabolism in muscles transfected independently with each of the four fatty acid transporters. In these studies, the overexpression of each of the fatty acid transporters (data not shown) was comparable with those shown in Fig. 3.
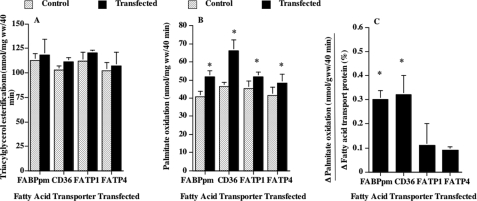
The independent overexpression of each of the fatty acid transporters failed to alter the rate of palmitate incorporation into intramuscular triacylglycerols (Fig. 5A) and phospholipids (data not shown). In contrast, the rates of palmitate oxidation were increased by 28, 42, 14, and 17%, in muscles transfected with FABPpm, FAT/CD36, FATP1, and FATP4, respectively (Fig. 5B). These relative differences among the transporters were due in part to differences in fatty acid transporter overexpression. Therefore, we compared the net increase in palmitate oxidation (Δ = transfected − control) relative to the change in fatty acid transporter expression (Δ = transfected − control). This revealed that FABPpm and FAT/CD36 contributed similarly to the increase in fatty acid oxidation, and their effects on fatty acid oxidation were 3-fold greater than those elicited by FATP1 or by FATP4 (Fig. 5C).
FIGURE 5.
Effects of fatty acid transporter overexpression on the rates of palmitate incorporation into intramuscular triacylglycerols (A), the rates of palmitate oxidation (B), and the change in oxidation in relation to the change in fatty acid transporter protein expression (C) (mean ± S.E.). Palmitate oxidation and incorporation into intramuscular triacylglycerol were determined in isolated muscles as described under “Experimental Procedures.” In each of the four fatty acid transporter transfection experiments, six independent determinations were made. Since the overexpression of the fatty acid transporters differed somewhat among the four experiments, we determined the change in palmitate oxidation (Δ = transfected − control) in relation to the change (%) in the overexpressed fatty acid transporter (Δ = transfected − control) (C). B, *, p < 0.05, transfected versus control (paired analysis). C, *, p < 0.05, FABPpm and FAT/CD36 versus FATP1 and FATP4.
DISCUSSION
Our studies show that in skeletal muscle, (a) the fatty acid transporters FABPpm, FAT/CD36, and FATP4 but not FATP1 are expressed in relation to the abilities of hind limb muscles to metabolize long chain fatty acids; (b) the fatty acid transport effectiveness of FAT/CD36 and FATP4 were similar, and were greater than for FABPpm (2.3-fold) and FATP1 (1.7-fold); and (c) the overexpression of any of the fatty acid transporters did not channel fatty acids into intramuscular triacylglycerol depots. In contrast, (d) fatty acid transporter overexpression did result in an increased rate of fatty acid oxidation, an effect that was similar for FAT/CD36 and for FABPpm and 3-fold greater than for FATP1 and -4. These studies indicate that selected fatty acid transporters have unique roles in fatty acid transport and metabolism in vivo.
Relationship between Fatty Acid Transporters and Indices of Fatty Acid Metabolism
A comparative approach was used initially to gain insight into the relationship between fatty acid transporter protein expression and the metabolic capacities for fatty acid oxidation in metabolically heterogeneous skeletal muscles. This indicated that, except for FATP1, there is a broad, positive association between oxidative capacities of muscle tissues and fatty acid transporter protein expression. In addition, the transporter expression was also highly correlated with fatty acid esterification into triacylglycerols and with PGC-1α and carnitine palmitoyltransferase I, markers of mitochondrial density and fatty acid oxidation capacities. This comparative approach indicates that the protein expression of selected fatty acid transporters supports the differential capacities for fatty acid metabolism among metabolically heterogeneous skeletal muscles. We recognize that correlations between FATPs and indices of fatty acid metabolism do not necessarily imply a causative relationship. Therefore, we also examined the effects of fatty acid transporter overexpression on fatty acid transport and metabolism.
Comparative Effects of Fatty Acid Transporters on Fatty Acid Transport
In order to study the independent transport effectiveness of FABPpm, FAT/CD36, FATP1, and FATP4, we have used an electrotransfection procedure to overexpress independently these four fatty acid transporters in rat skeletal muscle. Conveniently, the contralateral muscle in the same animal served as control. This approach has been used previously in our laboratory (26–28) to overexpress selected proteins. A key feature in the present study is that we overexpressed each fatty acid transporter independently without altering the expression of the other fatty acid transporters, thereby avoiding the compensatory responses that have been observed in animals in which a fatty acid transporter has been ablated (23, 24). We also show that the independent fatty acid transporter overexpression targeted these proteins to the plasma membrane, without any compensatory subcellular redistribution of the other fatty acid transporters. Hence, we were able to relate the change in fatty acid transport directly to change in a given fatty acid transporter at the plasma membrane.
We examined rates of fatty acid transport into giant sarcolemmal vesicles, a preparation that has been well characterized as being suitable for this purpose, since fatty acids transported across the plasma membrane are not metabolized further (16, 34). The present study documents that FABPpm, FAT/CD36, FATP1, and FATP4 all exhibit fatty acid transport capacities in skeletal muscle. This concurs with studies in FABPpm-overexpressing muscle, in which fatty acid transport was increased (28), and with studies in FAT/CD36 null (23) and FATP1 null mice (51), in which fatty acid transport was reduced. Unlike some studies in cultured cells, in which FATP4 appears not to be located at the plasma membrane and/or has no fatty acid transport role per se (19, 20), we and others find that FATP4 is present at the plasma membrane in rat (present study) (11) and human skeletal muscle (12). Moreover, in skeletal muscle, this protein is now shown to be involved in transporting fatty acids across the plasma membrane (present study). This transport role for FATP4 concurs with studies in which this transporter was expressed in yeast (17) and HEK293 cells (14). Taken altogether, our work shows clearly that FABPpm, FAT/CD36, FATP1, and FATP4 each exhibit transport capacity for long chain fatty acids in skeletal muscle.
The present study is the first to determine the relative fatty acid transport effectiveness of a number of fatty acid transporters in vivo. We increased fatty acid transporters within physiologic limits (5, 6, 36). Relative comparisons among the fatty acid transporters revealed that the fatty acid transport effectiveness of FAT/CD36 and FATP4 were similar and were 1.7-fold greater than that FATP1. These results correspond to those reported by DiRusso et al. (17), who had previously shown a 1.7-fold greater fatty acid transport effectiveness for FATP4 compared with FATP1 in yeast. This excellent agreement between our work in mammalian skeletal muscle and in yeast (17) gives considerable confidence to the approach taken in the present study and the results obtained. We recognize that neither our present studies in skeletal muscle nor studies by others using nonmammalian cell preparations (14, 17–20) reveal whether fatty acid transporters collaborate to transport fatty acids, as has been suggested (15, 16). This remains to be determined. Nevertheless, it is clear that fatty acids can be transported with a different effectiveness by selected transporters that are co-expressed in mammalian skeletal muscle.
Fatty Acid Transporters and Metabolism
The overexpression of the FABPpm, FAT/CD36, FATP1, and FATP4 transporters did not alter the rates of fatty esterification into triacylglycerols, whereas fatty acid oxidation was increased. We (28, 52) and others (50) have previously shown that overexpression of FABPpm in skeletal muscle (28, 52) and FATP1 in the heart (50) increased the rate of fatty acid oxidation. It is perhaps not surprising that in muscle, fatty acid transporters channel fatty acids to oxidation, since this sustains ATP synthesis, without which muscle cannot sustain contractile activity for long.
In agreement with the studies of FATP1 to -6 by DiRusso et al. (17) in yeast, we also find that the fatty acid transporter-mediated changes in fatty acid transport and metabolism (esterification and oxidation) in skeletal muscle were not always completely congruent with each other, since the fatty transporter-induced changes in fatty acid transport (FAT/CD36 = FATP4 > FABPpm = FATP1) were not directly matched with the fatty transporter-induced changes in skeletal muscle fatty acid oxidation (FAT/CD36 = FABPpm > FATP1 = FATP4). The role for FABPpm in the stimulation of fatty acid oxidation observed in the present study was tentatively proposed in several previous studies (7, 53), although how this occurs is presently unclear. Others have also reported a dissonance between fatty acid uptake and utilization in hearts that overexpress FATP1 (50), since in these studies fatty acid incorporation into triacylglycerols was not altered, and the increased rate of fatty acid oxidation was less than the FATP1-mediated increase in fatty acid uptake (50). Although the reasons for the discrepancies between fatty acid transport and metabolism (present study) (17, 50) are not known, these observations do suggest that in the absence of other complementary metabolic responses, muscle cells cannot adequately metabolize the additional fatty acids that are taken up when a plasmalemmal fatty acid transporter is increased. DiRusso et al. (17) have reasonably suggested that a multiprotein complex is required to channel fatty acids within the cell. For this reason, there is probably not always a direct correlation between fatty acid transport and metabolism, particularly when only fatty acid transporters are overexpressed.
Do Fatty Acid Transporters Collaborate?
The present study and others (14, 17–20) are limited in that transport of fatty acids is being linked with an individual fatty acid transporter only. This may not be the case, since some of these proteins may interact with each other. For example, blocking either FABPpm or FAT/CD36 in giant vesicles is sufficient to inhibit fatty acid transport, and these effects are not additive (16). This suggests that these proteins may act in a concerted fashion to transport fatty acids into muscle cells. Some studies have begun to suggest that FABPpm may be required to stimulate fatty acid transport and oxidation, possibly in collaboration with FAT/CD36 (7, 15, 53).6 It is unknown whether FABPpm also interacts with other fatty acid transporters or whether all fatty acid transporters interact with each other to stimulate fatty acid oxidation. This remains to be determined.
Implications of Increasing Plasmalemmal Fatty Acid Transporters and Transport for Insulin Resistance
We and others have linked the increase in plasmalemmal FAT/CD36 with insulin resistance in rodent (6, 11) and human skeletal muscle (5, 12), since a fatty acid transporter-mediated increase in the rates of fatty acid transport can exceed the capacity for fatty acid oxidation (5, 6, 11), thereby allowing intramuscular fatty acids to accumulate where they can interfere with insulin signaling and GLUT4 translocation (40, 54). This mismatch between fatty acid uptake and oxidation may well be at the root of insulin resistance in skeletal muscle. Hence, reducing fatty acid transport and/or some plasmalemmal fatty acid transporters would seem to be a potential therapeutic strategy for lowering insulin resistance, as we have recently shown when FAT/CD36 was lowered in metformin- and exercise-treated Zucker diabetic fatty rats (55). FATP4 might also be a therapeutic target, since it effectively transports fatty acids into muscle but fails to contribute to fatty acid oxidation. In contrast, FATP1 appears to have minimal effects on fatty acid transport and metabolism. Finally, it is possible that increasing FABPpm could provide an effective therapeutic strategy, since, despite its limited transport effectiveness, it contributes markedly to stimulating fatty acid oxidation, which is associated with improvement in insulin sensitivity (for a review, see Ref. 56).
Conclusions
In vivo FABPpm, FAT/CD36, and FATP1 and -4 each increased the rate of fatty acid transport in rat skeletal muscle, with FAT/CD36 and FATP4 being the most effective. Each of the transporters also contributed to increasing fatty acid oxidation, but not esterification, with the greatest effects being attributed to FAT/CD36 and FABPpm. Thus, in vivo, FABPpm, FAT/CD36, FATP1, and FATP4 (a) exhibit specific abilities for transporting long chain fatty acids and (b) contribute differentially to the regulation of fatty acid metabolism.
This work was supported in part by grants from the Canadian Institutes of Health Research (to A. B.), the Natural Sciences and Engineering Research Council of Canada (to A. B.), the Heart and Stroke Foundation of Ontario (to A. B.), the Netherlands Organization for Health Research and Development (NWO-ZonMw Grant 40-00812-98-03075) (to J. L. and J. G.), the European Commission (Integrated Project LSHM-CT-2004-005272, Exgenesis) (to J. L. and J. G.), and the Canada Research Chair program (to A. B.).
A. Bonen, unpublished data.
- FATP
- fatty acid transport protein
- BSA
- bovine serum albumin
- MOPS
- 4-morpholinepropanesulfonic acid.
REFERENCES
- 1.Bonen A., Chabowski A., Luiken J. J., Glatz J. F. ( 2007) Physiology 22, 15– 29 [DOI] [PubMed] [Google Scholar]
- 2.Kampf J. P., Kleinfeld A. M. ( 2007) Physiology 22, 7– 14 [DOI] [PubMed] [Google Scholar]
- 3.Kampf J. P., Kleinfeld A. M., Bonen A., Chabowski A., Luiken J. J. F. P., Glatz J. F. C. ( 2007) Physiology 22, 29 [Google Scholar]
- 4.Doege H., Stahl A. ( 2006) Physiology 21, 259– 268 [DOI] [PubMed] [Google Scholar]
- 5.Bonen A., Parolin M. L., Steinberg G. R., Calles-Escandon J., Tandon N. N., Glatz J. F., Luiken J. J., Heigenhauser G. J., Dyck D. J. ( 2004) FASEB J. 18, 1144– 1146 [DOI] [PubMed] [Google Scholar]
- 6.Luiken J. J., Arumugam Y., Dyck D. J., Bell R. C., Pelsers M. M., Turcotte L. P., Tandon N. N., Glatz J. F., Bonen A. ( 2001) J. Biol. Chem. 276, 40567– 40573 [DOI] [PubMed] [Google Scholar]
- 7.Han X. X., Chabowski A., Tandon N. N., Calles-Escandon J., Glatz J. F., Luiken J. J., Bonen A. ( 2007) Am. J. Physiol. Endocrinol. Metab. 293, E566– 575 [DOI] [PubMed] [Google Scholar]
- 8.Coort S. L., Hasselbaink D. M., Koonen D. P., Willems J., Coumans W. A., Chabowski A., van der Vusse G. J., Bonen A., Glatz J. F., Luiken J. J. ( 2004) Diabetes 53, 1655– 1663 [DOI] [PubMed] [Google Scholar]
- 9.Luiken J. J., Arumugam Y., Bell R. C., Calles-Escandon J., Tandon N. N., Glatz J. F., Bonen A. ( 2002) Am. J. Physiol. Endocrinol. Metab. 283, E612– 621 [DOI] [PubMed] [Google Scholar]
- 10.Kim J. K., Gimeno R. E., Higashimori T., Kim H. J., Choi H., Punreddy S., Mozell R. L., Tan G., Stricker-Krongrad A., Hirsch D. J., Fillmore J. J., Liu Z. X., Dong J., Cline G., Stahl A., Lodish H. F., Shulman G. I. ( 2004) J. Clin. Invest. 113, 756– 763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holloway G. P., Benton C. R., Mullen K. L., Yoshida Y., Snook L. A., Han X. X., Glatz J. F., Luiken J. J., Lally J., Dyck D. J., Bonen A. ( 2009) Am. J. Physiol. Endocrinol. Metab. 296, E738– 747 [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay G. K., Yu J. G., Ofrecio J., Olefsky J. M. ( 2006) Diabetes 55, 2277– 2285 [DOI] [PubMed] [Google Scholar]
- 13.Hirsch D., Stahl A., Lodish H. F. ( 1998) Proc. Natl. Acad. Sci. U. S. A. 95, 8625– 8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimeno R. E., Ortegon A. M., Patel S., Punreddy S., Ge P., Sun Y., Lodish H. F., Stahl A. ( 2003) J. Biol. Chem. 278, 16039– 16044 [DOI] [PubMed] [Google Scholar]
- 15.Chabowski A., Górski J., Luiken J. J., Glatz J. F., Bonen A. ( 2007) Prostaglandins Leukot. Essent. Fatty Acids 77, 345– 353 [DOI] [PubMed] [Google Scholar]
- 16.Luiken J. J., Turcotte L. P., Bonen A. ( 1999) J. Lipid Res. 40, 1007– 1016 [PubMed] [Google Scholar]
- 17.DiRusso C. C., Li H., Darwis D., Watkins P. A., Berger J., Black P. N. ( 2005) J. Biol. Chem. 280, 16829– 16837 [DOI] [PubMed] [Google Scholar]
- 18.Hall A. M., Wiczer B. M., Herrmann T., Stremmel W., Bernlohr D. A. ( 2005) J. Biol. Chem. 280, 11948– 11954 [DOI] [PubMed] [Google Scholar]
- 19.Lobo S., Wiczer B. M., Smith A. J., Hall A. M., Bernlohr D. A. ( 2007) J. Lipid Res. 48, 609– 620 [DOI] [PubMed] [Google Scholar]
- 20.Milger K., Herrmann T., Becker C., Gotthardt D., Zickwolf J., Ehehalt R., Watkins P. A., Stremmel W., Füllekrug J. ( 2006) J. Cell Sci. 119, 4678– 4688 [DOI] [PubMed] [Google Scholar]
- 21.van Oort M. M., van Doorn J. M., Bonen A., Glatz J. F., van der Horst D. J., Rodenburg K. W., Luiken J. J. ( 2008) Biochim. Biophys. Acta 1781, 61– 71 [DOI] [PubMed] [Google Scholar]
- 22.Eyre N. S., Cleland L. G., Mayrhofer G. ( 2008) Biochem. Biophys. Res. Commun. 370, 404– 409 [DOI] [PubMed] [Google Scholar]
- 23.Habets D. D., Coumans W. A., Voshol P. J., den Boer M. A., Febbraio M., Bonen A., Glatz J. F., Luiken J. J. ( 2007) Biochem. Biophys. Res. Commun. 355, 204– 210 [DOI] [PubMed] [Google Scholar]
- 24.Bonen A., Han X. X., Habets D. D., Febbraio M., Glatz J. F., Luiken J. J. ( 2007) Am. J. Physiol. Endocrinol. Metab. 292, E1740– 1749 [DOI] [PubMed] [Google Scholar]
- 25.Richards M. R., Harp J. D., Ory D. S., Schaffer J. E. ( 2006) J. Lipid Res. 47, 665– 672 [DOI] [PubMed] [Google Scholar]
- 26.Benton C. R., Nickerson J. G., Lally J., Han X. X., Holloway G. P., Glatz J. F., Luiken J. J., Graham T. E., Heikkila J. J., Bonen A. ( 2008) J. Biol. Chem. 283, 4228– 4240 [DOI] [PubMed] [Google Scholar]
- 27.Benton C. R., Yoshida Y., Lally J., Han X. X., Hatta H., Bonen A. ( 2008) Physiol. Genomics 35, 45– 54 [DOI] [PubMed] [Google Scholar]
- 28.Clarke D. C., Miskovic D., Han X. X., Calles-Escandon J., Glatz J. F., Luiken J. J., Heikkila J. J., Bonen A. ( 2004) Physiol. Genomics 17, 31– 37 [DOI] [PubMed] [Google Scholar]
- 29.Matsuno K., Diaz-Ricart M., Montgomery R. R., Aster R. H., Jamieson G. A., Tandon N. N. ( 1996) Br. J. Haematol. 92, 960– 967 [DOI] [PubMed] [Google Scholar]
- 30.Luiken J. J., Dyck D. J., Han X. X., Tandon N. N., Arumugam Y., Glatz J. F., Bonen A. ( 2002) Am. J. Physiol. Endocrinol. Metab. 282, E491– 495 [DOI] [PubMed] [Google Scholar]
- 31.Chabowski A., Coort S. L., Calles-Escandon J., Tandon N. N., Glatz J. F., Luiken J. J., Bonen A. ( 2004) Am. J. Physiol. Endocrinol. Metab. 287, E781– 789 [DOI] [PubMed] [Google Scholar]
- 32.Chabowski A., Momken I., Coort S. L., Calles-Escandon J., Tandon N. N., Glatz J. F., Luiken J. J., Bonen A. ( 2006) Mol. Cell Biochem. 288, 201– 212 [DOI] [PubMed] [Google Scholar]
- 33.Holloway G. P., Lally J., Nickerson J. G., Alkhateeb H., Snook L. A., Heigenhauser G. J., Calles-Escandon J., Glatz J. F., Luiken J. J., Spriet L. L., Bonen A. ( 2007) J. Physiol. 582, 393– 405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonen A., Luiken J. J., Lui S., Dyck D. J., Kiens B., Kristiansen S., Turcotte L., van der Vusse G. J., Glatz J. F. ( 1998) Am. J. Physiol. Endocrinol. Metab. 275, E471– 478 [DOI] [PubMed] [Google Scholar]
- 35.Bonen A., Luiken J. J., Arumugam Y., Glatz J. F., Tandon N. N. ( 2000) J. Biol. Chem. 275, 14501– 14508 [DOI] [PubMed] [Google Scholar]
- 36.Koonen D. P., Benton C. R., Arumugam Y., Tandon N. N., Calles-Escandon J., Glatz J. F., Luiken J. J., Bonen A. ( 2004) Am. J. Physiol. Endocrinol. Metab. 286, E1042– 1049 [DOI] [PubMed] [Google Scholar]
- 37.Benton C. R., Koonen D. P., Calles-Escandon J., Tandon N. N., Glatz J. F., Luiken J. J., Heikkila J. J., Bonen A. ( 2006) J. Physiol. 573, 199– 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koonen D. P., Coumans W. A., Arumugam Y., Bonen A., Glatz J. F., Luiken J. J. ( 2002) Mol. Cell Biochem. 239, 121– 130 [PubMed] [Google Scholar]
- 39.Alkhateeb H., Chabowski A., Bonen A. ( 2006) Appl. Physiol. Nutr. Metab. 31, 467– 476 [DOI] [PubMed] [Google Scholar]
- 40.Alkhateeb H., Chabowski A., Glatz J. F., Luiken J. F., Bonen A. ( 2007) Am. J. Physiol. Endocrinol. Metab. 293, E783– 793 [DOI] [PubMed] [Google Scholar]
- 41.Bonen A., Clark M. G., Henriksen E. J. ( 1994) Am. J. Physiol. Endocrinol. Metab. 266, E1– 16 [DOI] [PubMed] [Google Scholar]
- 42.Dyck D. J., Peters S. J., Glatz J., Gorski J., Keizer H., Kiens B., Liu S., Richter E. A., Spriet L. L., van der Vusse G. J., Bonen A. ( 1997) Am. J. Physiol. Endocrinol. Metab. 272, E340– 351 [DOI] [PubMed] [Google Scholar]
- 43.Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O., Ahmad F., Matsui T., Chin S., Wu P. H., Rybkin I. I., Shelton J. M., Manieri M., Cinti S., Schoen F. J., Bassel-Duby R., Rosenzweig A., Ingwall J. S., Spiegelman B. M. ( 2005) Cell Metab. 1, 259– 271 [DOI] [PubMed] [Google Scholar]
- 44.Benton C. R., Holloway G. P., Campbell S. E., Yoshida Y., Tandon N. N., Glatz J. F., Luiken J. J., Spriet L. L., Bonen A. ( 2008) J. Physiol. 586, 1755– 1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kramer H. F., Witczak C. A., Taylor E. B., Fujii N., Hirshman M. F., Goodyear L. J. ( 2006) J. Biol. Chem. 281, 31478– 31485 [DOI] [PubMed] [Google Scholar]
- 46.Kramer H. F., Taylor E. B., Witczak C. A., Fujii N., Hirshman M. F., Goodyear L. J. ( 2007) Diabetes 56, 2854– 2862 [DOI] [PubMed] [Google Scholar]
- 47.Bastie C. C., Hajri T., Drover V. A., Grimaldi P. A., Abumrad N. A. ( 2004) Diabetes 53, 2209– 2216 [DOI] [PubMed] [Google Scholar]
- 48.Hatch G. M., Smith A. J., Xu F. Y., Hall A. M., Bernlohr D. A. ( 2002) J. Lipid Res. 43, 1380– 1389 [DOI] [PubMed] [Google Scholar]
- 49.Hall A. M., Smith A. J., Bernlohr D. A. ( 2003) J. Biol. Chem. 278, 43008– 43013 [DOI] [PubMed] [Google Scholar]
- 50.Chiu H. C., Kovacs A., Blanton R. M., Han X., Courtois M., Weinheimer C. J., Yamada K. A., Brunet S., Xu H., Nerbonne J. M., Welch M. J., Fettig N. M., Sharp T. L., Sambandam N., Olson K. M., Ory D. S., Schaffer J. E. ( 2005) Circ. Res. 96, 225– 233 [DOI] [PubMed] [Google Scholar]
- 51.Wu Q., Ortegon A. M., Tsang B., Doege H., Feingold K. R., Stahl A. ( 2006) Mol. Cell Biol. 26, 3455– 3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibrahimi A., Bonen A., Blinn W. D., Hajri T., Li X., Zhong K., Cameron R., Abumrad N. A. ( 1999) J. Biol. Chem. 274, 26761– 26766 [DOI] [PubMed] [Google Scholar]
- 53.Chabowski A., Coort S. L., Calles-Escandon J., Tandon N. N., Glatz J. F., Luiken J. J., Bonen A. ( 2005) FEBS Lett. 579, 2428– 2432 [DOI] [PubMed] [Google Scholar]
- 54.Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., Summers S. A. ( 2007) Cell Metab. 5, 167– 179 [DOI] [PubMed] [Google Scholar]
- 55.Smith A. C., Mullen K. L., Junkin K. A., Nickerson J., Chabowski A., Bonen A., Dyck D. J. ( 2007) Am. J. Physiol. Endocrinol. Metab. 293, E172– 181 [DOI] [PubMed] [Google Scholar]
- 56.Houmard J. A. ( 2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1111– 1116 [DOI] [PMC free article] [PubMed] [Google Scholar]