Abstract
Olfactory receptors (ORs) are expressed not only in the sensory neurons of the olfactory epithelium, where they detect volatile substances, but also in various other tissues where their potential functions are largely unknown. Here, we report the physiological characterization of human OR51E2, also named prostate-specific G-protein-coupled receptor (PSGR) due to its reported up-regulation in prostate cancer. We identified androstenone derivatives as ligands for the recombinant receptor. PSGR can also be activated with the odorant β-ionone. Activation of the endogenous receptor in prostate cancer cells by the identified ligands evoked an intracellular Ca2+ increase. Exposure to β-ionone resulted in the activation of members of the MAPK family and inhibition of cell proliferation. Our data give support to the hypothesis that because PSGR signaling could reduce growth of prostate cancer cells, specific receptor ligands might therefore be potential candidates for prostate cancer treatment.
Excessive signaling by G-protein-coupled receptors (GPCRs)3 such as endothelin A receptor (1), bradykinin 1 receptor (2), follicle-stimulating hormone receptor (3), and thrombin receptor (4, 5) is known to occur in prostate cancers due to strong overexpression of the respective receptors. Activation of some of these GPCRs results in androgen-independent androgen receptor activation, thus promoting the transition of prostate cancer cells from an androgen-dependent to an androgen-independent state (6, 7).
The prostate-specific G-protein-coupled receptor (PSGR) is a class A GPCR that was initially identified as a prostate-specific tumor biomarker (8–10). It is specifically expressed in prostate epithelial cells, and its expression increases significantly in human prostate intraepithelial neoplasia and prostate tumors, suggesting that PSGR may play an important role in early prostate cancer development and progression (9, 11). Although expression of the human PSGR was found to be prostate-specific (10, 12), mRNA can also be detected in the olfactory zone and the medulla oblongata of the human brain (12). Human PSGR shares 93% amino acid homology to the respective mouse and rat homologues, which are also expressed in the brain (12). Interestingly, PSGR has numerous sequence motifs in common with the large superfamily of olfactory receptors (ORs), which build the largest class of human GPCRs and allow the recognition of a wide range of structurally diverse molecules in the nasal epithelium (13–15). Recently, also the steroid hormones androstenone and androstadienone were identified as OR ligands (16). In addition to their role in the sensory neurons of the nose, ORs have been found in different tissues throughout the body (17, 18). Their function(s) in these extranasal locations are questionable except for in a few cases where functional studies have been performed in spermatozoa (19, 20) and in enterochromaffin cells of the gastrointestinal tract (21).
Here, we report the identification of steroid ligands of heterologously expressed PSGR and investigate the functional relevance of PSGR expression in prostate tissue. Steroid hormones elicited rapid Ca2+ responses in the LNCaP prostate cancer cell line and in primary human prostate epithelial cells. Moreover, activated PSGR causes phosphorylation of p38 and stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) mitogen-activated protein kinases (MAPKs), resulting in reduced proliferation rates in prostate cancer cells.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
Reagents for cell culture use were purchased from Invitrogen, unless stated otherwise. HEK293 cells were maintained under standard conditions in minimum Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin and streptomycin, and 2 mm l-glutamine. LNCaP cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 units/ml penicillin and streptomycin. PC-3 cells were maintained in Ham's F12/RPMI 1640 (1:1) supplemented with 10% fetal bovine serum and 100 units/ml penicillin and streptomycin. HEK293 cell transfections with the PSGR-containing plasmid were performed using a standard calcium phosphate precipitation technique; for siRNA experiments, LNCaP cells were transiently transfected with either targeted or scrambled siRNAs using Exgen 500 (Fermentas). 2 days after transfection, the growth medium was removed and replaced with standard Ringer solution.
Prostate cancer epithelial cells were isolated from freshly collected prostate tissue, which was obtained from radical prostatectomy specimens (adenocarcinoma of the prostate pT2c, pN0, Gleason score 4 + 4) after written consent. The tissue pieces were minced to 1-mm3 pieces and digested for 30 min at 37 °C in Ringer solution containing 0.1% trypsin-EDTA. The tissue was dissociated by trituration and washed, and single cell suspensions were prepared by centrifugation of the remaining tissue pieces. Cells were seeded in the serum-free keratinocyte medium, supplemented with 50 ng/ml human recombinant epidermal growth factor 1–53 and 50 mg/ml bovine pituitary extract and kept at 37 °C in a humidified incubator with 5% CO2. When they reached 70–80% confluence, the cells were trypsinized and subcultured either in Petri dishes for Ca2+-imaging experiments or in 96-well plates for cell proliferation. Cell morphology was checked with Zeiss Axioskop 2 microscope and viewed with ×20 magnification.
Antibodies
The following primary antibodies were used: (a) rabbit polyclonal antibodies against p44/42 MAPK and against phosphorylated p44/42 MAPK (New England Biolabs); (b) rabbit polyclonal antibodies against p38 MAPK and phosphorylated p38 MAPK (New England Biolabs); (c) rabbit polyclonal antibodies against SAPK/JNK and phosphorylated SAPK/JNK (New England Biolabs); and (d) rabbit polyclonal against PSGR (Abcam). Secondary goat anti-rabbit antibodies conjugated to horseradish peroxidase (Bio-Rad) or Alexa Fluor 546 or Alexa Fluor 488 (Invitrogen) were used.
Western Blotting
LNCaP cells were treated with 500 mm β-ionone for the indicated times, harvested, pelleted, and homogenized in lysis buffer (50 mm Tris HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100) with protease inhibitors (Roche Applied Science Complete® protease inhibitor mixture). Sample aliquots of the cells were mixed with Laemmli buffer (30% glycerol, 3% SDS, 125 mm Tris/Cl, pH 6.8), resolved by 10% SDS-PAGE, and transferred to nitrocellulose membrane (Protran; Schleicher & Schuell). The nitrocellulose membranes were stained with Ponceau S (Sigma), blocked with TBST (150 mm NaCl, 50 mm Tris-Cl, Tween 20, pH 7.4) containing 5% nonfat dried milk (Bio-Rad), and incubated with primary antibodies diluted in 3% dry milk in TBST. After washing and incubation with horseradish peroxidase-coupled secondary antibodies, detection was performed with ECL plus or ECL Advance (Amersham Biosciences) on Hyperfilm ECL (Amersham Biosciences).
Single Cell Ca2+ Imaging
Cells were incubated (45 min/37 °C) in Ringer solution containing 3 mm Fura-2-AM (Molecular Probes). After removal of extracellular Fura-2, cells were washed with extracellular solution. For the inhibitor experiments, cells were treated with 10 μm flutamide (Sigma). Ratiofluorometric Ca2+ imaging was performed as described using a Zeiss inverted microscope equipped for ratiometric imaging. Images were acquired from randomly selected fields of view, and integrated fluorescence ratios (F340/F380 ratio) were measured. Exposure to odorants was accomplished using a specialized microcapillary application system. Odorants used were a gift of Dr. T. Gerke (Henkel, Düsseldorf, Germany) and Dr. J. Panten (Symrise, Holzminden, Germany). Steroid hormones were purchased from Steraloids (Newport, RI). Odorants and steroids assayed for potential activation of PSGR were tested in at least five transfection experiments in HEK293 cells and tested for activation of LNCaP cells afterward. All compounds regarded as ligands led to clear Ca2+ responses in several different imaging experiments (n > 7), whereas they did not elicit any Ca2+ signals in untransfected dishes. 200 μm ATP was applied as the positive control at the end of each experiment in HEK293 cells.
DNA and siRNA Constructs and Plasmids
Human PSGR (NM-030774) was amplified from human genomic DNA by PCR using specific primers that amplify the complete open reading frame and contain EcoRI restriction sites for further subcloning into pcDNA3 (Invitrogen); the generated plasmid was verified by sequencing. PSGR targeted and scrambled hairpin siRNA designs were done with siRNA Target Designer, Version 1.51 (Promega); oligonucleotides were synthesized by Invitrogen and ligated into the pGeneClipTM hMGFP vector (Promega) according to the manufacturer's instructions. The best working siRNA sequence of PSGR was GCTGCCTCCTGTCATCAAT; the oligonucleotide sequences to generate 5′-target-loop-reverse-complement-3′ hairpins were 5′- TCTCGCTGCCTCTGTCATCAATAAGTTCTCTATTGATGACAGGAGGCAGCCT-3′ and 5′-CTGCAGGCTGCCTCCTGTCATCAATAGAGAACTTATTGATGACAGGAGGCAGC-3′. The following scrambled versions of the siRNA sequence was used as control: 5′-TCTCGTACACTGACCCCCTTTGTAAGTTCTCTACAAAGGGGGTCAGTGTACCT-3′ and 5′-CTGCAGGTACACTGACCCCCTTTGTAGAGAACTTACAAAGGGGGTCAGTGTAC-3′.
Cell Proliferation
LNCaP cells and primary human prostate epithelial cells were plated in 96-well plates at a density of 5 × 103 cells/well. After 24 h at 37 °C with 5% CO2, cells were treated with different concentrations of β-ionone (50–250 μm), dihydrotestosterone (DHT, 10 nm), or with a mixture of β-ionone (250 and 100 μm) and DHT (10 nm). Alternatively, cells were simultaneously stimulated with β-ionone (250 μm) and varying concentrations of inhibitors for p38 ((RS)-(4-[5-(4-fluorophenyl)-2-methylsulfanyl-3H-imidazol-4-yl]pyridin-2-yl)-(1phenylethyl) amine]), Calbiochem) and JNK (Anthra[1,9-cd]pyrazol-6(2H)-one1,9-pyrazoloanthrone, Calbiochem). Cell proliferation was investigated after 3 and 6 days using the CyQUANT cell proliferation assay kit (Invitrogen).
RT-PCR
RNA of human olfactory epithelium, LNCaP and human primary prostate cells was isolated with TRIzol reagent (Invitrogen), digested with DNaseI (Fermentas), and purified again with TRIzol before isolation of poly(A)+ mRNA with oligo(dT)-coated paramagnetic particles (Dynal). cDNA was synthesized by using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and oligo(dT)12–18 primer. PCR was performed with 2 ng of template cDNA and specific intron-spanning primer pairs for PSGR (forward, 5′-CCTCAGCCTTCTGAGTCAGC-3′; reverse, 5′-GAGACTGTGACAAGCCCTGG-3′), prostate-specific antigen (PSA), androgen receptor, cytokeratin 8, and cytokeratin 18, respectively. The amplifications were done for 35 cycles (1 min, 94 °C; 1 min, 58 °C; 45 s, 72 °C).
RESULTS
Recombinant PSGR Responds to Steroid Hormones
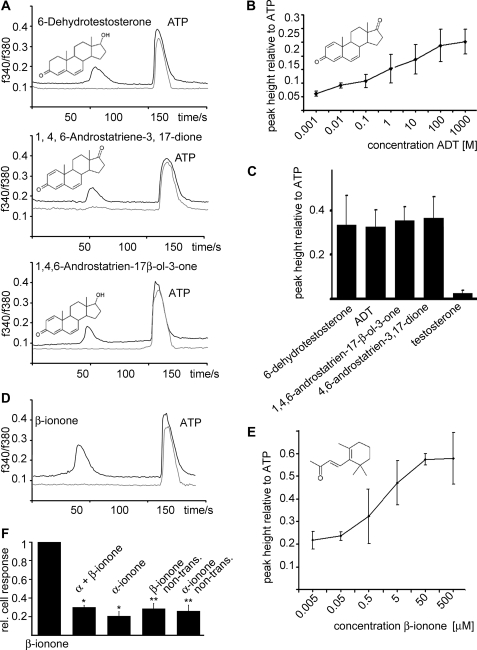
To investigate the function of PSGR in prostate tissue, we first had to identify a ligand for this “orphan” G-protein-coupled receptor. We therefore transiently expressed PSGR in HEK293 cells and determined its ligand specificity by measuring the cell responses to a mixture of chemical stimuli using ratiofluorimetric Ca2+ imaging, similar to the approach we used previously to find stimuli for orphan GPCRs (20, 22, 23). In this case, the complex mixture used for ligand screening included a mixture of 100 steroid hormones from different structural classes (see supplemental materials). By subdividing this mixture, we identified certain steroids as active ligands (Fig. 1A). 1,4,6-Androstatriene-3,17-dione (ADT) was tested at different concentrations for activation of PSGR and found to function as a ligand already in the low nanomolar range (Fig. 1B). The presence of an aldehyde group at position 3, together with at least two double bonds at positions 4 and 6, which are present in 6-dehydrotestosterone, 1,4,6-androstatriene-3,17-dione, and 1,4,6-androstatriene-17β-ol-3-one, were key determinants for effective PSGR ligands (Fig. 1C). Some modifications of the group at position 17 of the steroid were tolerated, whereas the absence of the double bonds at position 6, e.g. in testosterone, abolished the ability of the ligand to activate PSGR (Fig. 1C). Additional single substances, which were tested and found to be inactive, were androstenedione, 4,16-androstadiene-3-one, 5α-androstan-3β,16β- diol, etiocholanolone, 4-androstene-3,6,17-trione, Fernholtz acid, and 4-estren-3α-17β-diol. All steroids were tested in a concentration of 10 mm in untransfected HEK293 cells and did not elicit similar Ca2+ responses (Fig. 1, A, gray curves, and F).
FIGURE 1.
Heterologously expressed PSGR responds to steroid ligands and β-ionone. A, testing a steroid library composed of 100 structurally diverse steroids for the activation of recombinant PSGR resulted in the identification of the active substances 6-dehydrotestosterone, ADT, and 1,4,6-androstatriene-17β-ol-3-one (all 10 μm) in HEK293 cells. In randomly selected fields of view, the substances induced transient Ca2+ signals in PSGR-transfected HEK293 cells (black curves) but not in non-transfected HEK293 cells (gray curves); ATP (200 μm) served as a control. The integrated fluorescence ratio (F340/F380) for Fura-2-loaded cells is shown as a function of time. Substances were applied for 5 s. B, ADT was tested at different concentrations for activation of recombinant PSGR; the peak height of the Ca2+ signal relative to the ATP (200 μm)-induced control signal is displayed as a function of the applied concentration. C, different steroids containing double bonds at positions 6 and 4 and a keto group at position 3 were tested for activation of recombinant PSGR; the peak heights relative to ATP are given (concentrations are 10 μm each). D, β-ionone was identified as an odorant ligand for PSGR expressed in HEK293 cells; ATP (200 μm) served as a control. The integrated fluorescence ratio (F340/F380) for Fura-2-loaded cells is shown as a function of time. Substances were applied for 5 s. E, β-ionone was tested at different concentrations for activation of PSGR; the peak height of the Ca2+ signal relative to the ATP (200 μm)-induced Ca2+ signal is displayed as a function of the applied concentration. F, the response of recombinantly expressed PSGR to β-ionone (50 μm) could be inhibited by the co-application of α-ionone (100 μm), which was identified as specific inhibitor. The application of α-ionone (100 μm) alone did not cause a significant calcium rise in PSGR-transfected HEK293 cells. The application of either β-ionone or α-ionone to non-transfected (non-trans.) HEK293 cells did not cause an intracellular calcium increase. Shown are the numbers of responding cells relative to the number of PSGR-transfected HEK293 cells responding to β-ionone (50 μm). Error bars represent S.E., *, p < 0.05; **, p < 0.01.
Because PSGR has clear sequence characteristics of an olfactory receptor (OR51E2) and because ORs can typically be activated by multiple ligands with different receptor affinities, we tested the transiently expressed PSGR in HEK293 cells with a mixture of odorant stimuli using ratiofluorimetric Ca2+ imaging. This complex mixture includes 100 compounds, mainly aromatic and short chain aliphatic hydrocarbons, which was previously used to find stimuli for ORs (20, 22, 23). By subdividing the mixture, we found β-ionone to be an active ligand in this mixture (Fig. 1D). β-Ionone elicited receptor-induced Ca2+ responses at concentrations as low as ∼100 nm (Fig. 1E). Other components of the same mixture were tested as single substances and did not result in Ca2+ signals in PSGR-expressing HEK293 cells. In untransfected HEK293 cells, β-ionone did not induce Ca2+ signals. We additionally discovered that the response of recombinantly expressed PSGR to β-ionone can be inhibited by the structurally related substance α-ionone, which alone did not activate recombinantly expressed PSGR (Fig. 1E and supplemental Fig. S1).
PSGR Activation in Prostate Cells
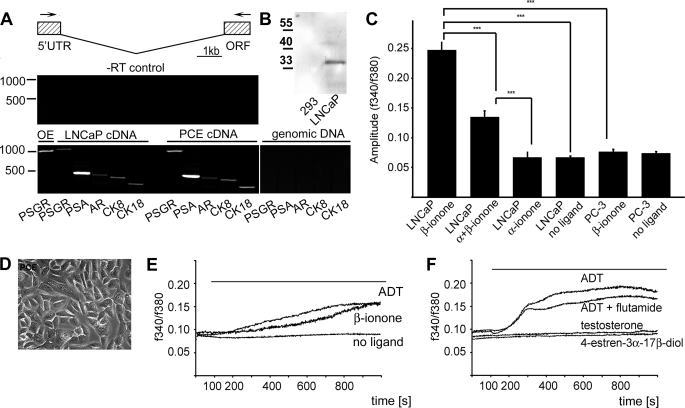
Next we investigated whether PSGR is expressed in cultured prostate epithelial cells. The prostate consists of glandular epithelium embedded in a fibromuscular stroma. The epithelium is composed of two different layers, a basal layer that contains a stem cell-like population and is responsible for the development of the epithelial cells, and the secretory luminal layer, which is responsible for the production of PSA and other parts of the seminal fluid (24). The luminal layer consists of androgen-dependent cells, which express low molecular mass cytokeratins (CKs), mostly CK8 and CK18, the androgen receptor (24), as well as PSGR (10). We cultured human prostate epithelial cells from freshly collected prostate tissue, obtained from a radical prostatectomy specimen. Additionally, we tested the LNCaP cell line, which is derived from a prostate cancer metastasis and was also shown to express PSGR (10). RT-PCR analysis revealed that our primary culture, similar to the LNCaP cell line, showed expression of the prostate epithelial cell markers CK8, CK18, PSA, and androgen receptor (AR) (Fig. 2A). Moreover, both cell types express PSGR (Fig. 2A), whereas another prostate cancer cell line, PC-3, did not (supplemental Fig. S2). PSGR protein can be detected in LNCaP prostate cells, but not in HEK293 cells as negative control, by Western blotting (Fig. 2B).
FIGURE 2.
PSGR can be activated in primary prostate epithelial cells. To investigate PSGR signaling in primary prostate cancer epithelial cells, epithelial cell culture was performed from prostatic tissue that was obtained from resections arising from prostate cancer (prostate cancer epithelial, PCE). To investigate whether the cultured cells resemble differentiated secretory epithelial cells, we checked for expression of marker RNAs by RT-PCR. A, the cultured primary cells, as well as the prostate cancer cell line LNCaP, express androgen receptor (AR), PSA, PSGR, and the cytokeratins 8 and 18, which are hallmarks of prostatic secretory cells. Shown are a schematic representation of the intron spanning primers for PSGR detection and PCR control experiments with the isolated RNAs (no reverse transcription, −RT). No amplification could be obtained from genomic DNA as control. PSGR transcripts could also be detected in human olfactory epithelium (OE). The size of the PSGR amplificant was 1050 (cDNA) or >15,000 (genomic DNA). UTR, untranslated region; ORF, open reading frame. B, Western blot showing expression of PSGR in LNCaP cells; untransfected HEK293 cells are shown as negative control. C, activation of LNCaP and PC-3 prostate cancer cells lines to the identified PSGR ligand β-ionone. Shown are the amplitudes of the intracellular calcium signals caused by ligand application in the respective cell lines. β-Ionone (500 μm) caused an increase in the calcium concentration of (PSGR-expressing) LNCaP cells, which could be reduced by the co-application of the specific inhibitor α-ionone in a ratio of 2:1. The application of α-ionone (1.5 mm) alone did not cause a similar increase in the calcium concentration. The application of β-ionone (500 μm) to PC-3 cells, which do not express PSGR, did not result in an intracellular calcium increase in these cells. The experiment was performed three times, and n > 100 cells were analyzed for each ligand application (error bars represent S.E., ***, p < 0.001). D, phase-contrast picture of the epithelial cell culture from prostate cancer tissue. E, primary prostate epithelial cells were investigated for activation by the identified PSGR ligands using Ca2+ imaging. β-Ionone- and ADT-induced Ca2+ signals are displayed as a function of time. The ligands are applied from 100 s on for the entire duration of the experiment (1000 s). F, responses of LNCaP cells to the PSGR ligand ADT and to ADT + flutamide, a well known antagonist for the classical androgen receptor, were undistinguishable from each other, showing that the Ca2+ signals do not originate from androgen receptor activation. Moreover, testosterone and 4-estren-3α-17β-diol, which did not activate recombinant PSGR, did not induce similar Ca2+ signals in LNCaP cells. The experiment was performed three times, and n > 100 cells were analyzed for each ligand application.
LNCaP cells responded to the application of β-ionone with an increase in the intracellular calcium concentration, which could be inhibited by the simultaneous application of the specific inhibitor α-ionone; the application of α-ionone alone did not cause an increase in the calcium concentration (Fig. 2C and supplemental Fig. 2). The application of β-ionone did moreover not cause an increase in the intracellular calcium concentration in PC-3 cells, which were shown not to express PSGR (Fig. 2C and supplemental Fig. 2).
The primary cell culture displayed the typical morphology of prostate epithelial cells (Fig. 2D). Applying the PSGR agonists ADT and β-ionone to prostate cancer cells elicited a slow, gradual increase in the intracellular Ca2+ concentration (Fig. 2E), similar to the Ca2+ responses in the LNCaP cell line. Together, these results show that PSGR is expressed in primary prostate epithelial cells and can be activated by the identified ligands.
To ensure that the steroid-induced Ca2+ rise is not influenced or mediated by androgen receptor activation, we performed Ca2+ imaging in the presence of the classical androgen receptor inhibitor flutamide, which did not change the amplitude or the kinetics of the ADT-induced signal (Fig. 2F). Moreover, steroids that are well known to effectively activate the androgen receptor, e.g. 4-estren-3α,17β-diol and DHT, but do not activate the heterologously expressed PSGR, elicited no significant Ca2+ increases in LNCaP cells (Fig. 2F). ADT induced increases in intracellular Ca2+ already at concentrations in the nanomolar range (supplemental Fig. S2E). All steroids that have been identified as PSGR ligands in HEK293 cells were able to induce an increase in the intracellular Ca2+ concentration in LNCaP cells, whereas androgens without double bonds at position 6 or 4 (e.g. in testosterone) did not cause similar Ca2+ signals (Fig. 2F).
PSGR Mediates Non-genomic Steroid Actions in a Prostate Cancer Cell Line
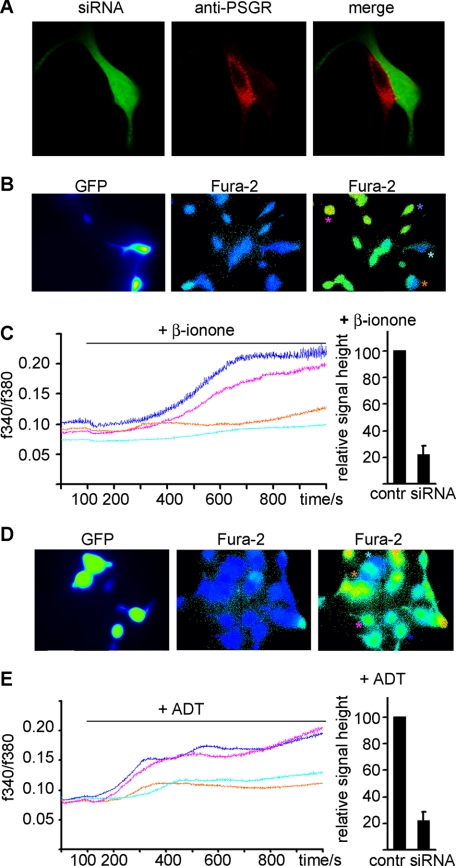
We next tried to prove that stimulation of prostate cells with β-ionone and ADT affects intracellular Ca2+ homeostasis via activation of PSGR (Fig. 3). Applying the PSGR agonist β-ionone to LNCaP cells elicited a slow, gradual increase in the intracellular Ca2+ concentration within 5–8 min. To confirm that the β-ionone-induced Ca2+ increase was due to PSGR activation, we reduced the expression level using a plasmid for in vivo expression of short interfering RNAs. This vector contains GFP as an internal fluorescent marker, which enables the determination of the transfection efficiency and facilitates recording of Ca2+ responses specifically in the siRNA-expressing cells. Immunohistochemical staining revealed that siRNA-expressing cells did express lower levels of PSGR (Fig. 3A). β-Ionone-induced Ca2+ signals in siRNA-expressing LNCaP cells were then compared with the Ca2+ increase in neighboring cells not expressing GFP (Fig. 3, B and C). Quantification of the Ca2+ signals revealed that siRNA expression strongly (∼80%) reduced the β-ionone-mediated Ca2+ increase (Fig. 3C). The responses from the control cells varied in strength but had on average significantly higher amplitudes than in siRNA-expressing (GFP-labeled) cells. Expression of GFP alone did not alter the β-ionone-induced Ca2+ increase, nor did the expression of scrambled control siRNA (data not shown). Similarly, ADT also induced a PSGR-dependent Ca2+ increase in LNCaP cells, already in the nanomolar range (Fig. 3, D and E).
FIGURE 3.
PSGR-dependent induction of Ca2+ signals in LNCaP cells by ADT and β-ionone. LNCaP cells were transfected with a plasmid encoding siRNA directed against PSGR. Because the plasmid also codes for GFP, siRNA-expressing cells can be detected via GFP fluorescence. A, immunohistochemical staining against PSGR showed that siRNA-expressing cells have a reduced PSGR expression level. The cells were then investigated for activation by the identified PSGR ligands using Ca2+ imaging. B, fluorescence pictures of Fura-2-loaded LNCaP cells transfected with the PSGR-siRNA/GFP-encoding plasmid. GFP-expressing cells (turquoise and orange stars) show no change in the Fura-2-ratio after the application of the PSGR ligand β-ionone, whereas non-transfected cells (blue and pink stars) do. C, β-ionone-induced Ca2+ signals in transfected (turquoise and orange stars) and non-transfected (blue and pink stars) LNCaP cells, displayed as a function of time. The ligand is applied from 100 s on for the entire duration of the experiment (1000 s). The relative signal strength of the Ca2+ signals in siRNA-transfected and non-transfected cells was quantified, showing an 80% decrease of the signal after expression of PSGR-siRNA (data are the mean from four independent transfections, n = 22 siRNA-expressing cells, n = 186 control (contr) cells). Error bars represent S.E. D, fluorescence pictures of Fura-2-loaded LNCaP cells transfected with the PSGR-siRNA/GFP-encoding plasmid. GFP-expressing cells (turquoise and orange stars) show no change in the Fura-2 ratio after the application of the PSGR ligand ADT, whereas non-transfected cells (blue and pink stars) do. E, ADT-induced Ca2+ signals in transfected (turquoise and orange stars) and non-transfected (blue and pink stars) LNCaP cells, displayed as a function of time. The ligand is applied from 100 s on for the entire duration of the experiment (1000 s). Quantification of ADT-induced Ca2+ signals in PSGR-siRNA-transfected LNCaP cells was done as described for β-ionone (data are the mean from four independent experiments, n = 26 siRNA-expressing cells, n = 201 control cells). Error bars represent S.E.
Effect of β-Ionone Proliferation of Prostate Cells
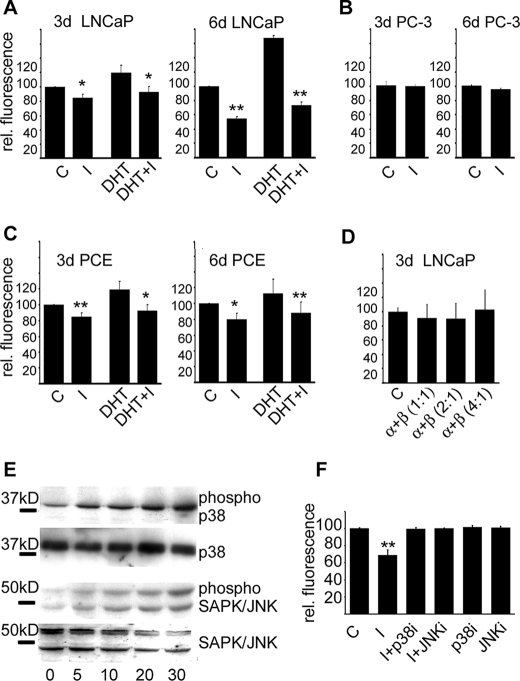
To examine the effect of PSGR signaling in prostate cells, we treated the LNCaP and PC-3 cell lines and the primary prostate cells with 250 μm β-ionone for 72 and 144 h, which resulted in a significant reduction of cell proliferation in all tested PSGR-expressing prostate cells, with a maximal inhibition at 144 h (∼50%) for the LNCaP cell line (Fig. 4, A and C). Moreover, the proliferative effect of DHT could be suppressed in LNCaP or primary cells by β-ionone treatment (Fig. 4, A and C). Similar effects were observed when stimulating the cells with ADT or 6-dehydrostestosterone (supplemental Fig. S3). The substances did not influence proliferation rates of HEK293 cells or prostate cancer cells not expressing PSGR (Fig. 4B). Moreover, the antiproliferative effects of β-ionone on LNCaP cells could be reversed by the co-application of the specific inhibitor α-ionone (Fig. 4D).
FIGURE 4.
Activation of PSGR in LNCaP and primary prostate epithelial cells reduces cell proliferation via MAPK signaling. A, proliferation of LNCaP cells after the application of DHT, a well known inducer of prostate cell proliferation, β-ionone (I), and a mixture of DHT and β-ionone for 3 and 6 days, relative to control conditions. β-Ionone slowed cell proliferation and could even reduce the DHT-stimulated increased proliferation rates. C, control cells. B, proliferation of PC-3 cells, which do not express PSGR, was not affected by the application of the same concentration of β-ionone. C, the same experiments as for the LNCaP cells were performed with primary prostate cancer epithelial (PCE) cells. D, the effect of 250 μm β-ionone on cell proliferation of LNCaP cells could be reversed by the co-application of α-ionone in ratios of 1:1 to 4:1. E, Western blot analysis showing that PSGR stimulation results in a time-dependent phosphorylation of SAPK/JNK and p38 MAPKs in LNCaP cells. Detection of the total amounts of SAP/JNK and p38 is shown as controls. F, inhibition of p38 and SAPK/JNK MAPKs abolished the effect of β-ionone on LNCaP cell proliferation. The inhibitors were applied together with β-ionone (I+p38; I+JNK) or without β-ionone (p38, JNK) for 6 days, and cell proliferation rates were measured. Control cells and β-ionone-exposed cells were treated with equal concentrations of DMSO as used for the inhibitors (error bars represent S.E., *, p < 0.05; **, p < 0.01). rel. fluorescence, relative fluorescence.
To elucidate the intracellular effectors of β-ionone signaling in LNCaP cells, we studied the phosphorylation of members of the MAP kinase family, which are known to be involved in the regulation of cell cycle and apoptosis in prostate cells. LNCaP cells showed no modulation of the extracellular stress-regulated kinase (ERK1/2) pathway after ligand exposure (data not shown) but showed a marked increase in phosphorylation of p38 and SAPK/JNK within 5–10 min, which remained elevated up to 30 min of continued ligand exposure (Fig. 4E).
Treatment of LNCaP cells with inhibitors of p38 and SAPK/JNK kinases simultaneously to stimulation with β-ionone abolished the effect of β-ionone on cell proliferation (Fig. 4F). The p38 MAPK inhibitor reversed the β-ionone-induced proliferation effect at concentrations near the half-maximal inhibitory concentration (used concentration, 0.5 μm; IC50, 0.38 μm); whereas only relatively high concentrations of the SAPK/JNK inhibitor (used concentration, 1 μm; IC50, 40 nm for JNK-1/2 and 90 nm for JNK3) were effective, lower concentrations had only partial effects (data not shown). The inhibitors did not influence cell proliferation of non-stimulated LNCaP cells at the concentrations used (Fig. 4F).
DISCUSSION
We deorphanized the PSGR and identified steroid hormones and the odorant β-ionone as ligands. Moreover, we identified β-ionone as specific inhibitor for the receptor. PSGR is therefore a novel membrane receptor for rapid, non-genomic effects of steroids on intracellular signaling cascades. Rapid, non-genomic, steroid actions have already been identified both in cells bearing intracellular steroid receptors and in those without. With the exception of progesterone and estradiol, for which GPCRs had been identified as receptors, considerable controversy exists about the identity of the receptor proteins that mediate these responses (25). In the present study, we show the existence of another G-protein-coupled membrane steroid receptor, which belongs to the superfamily of odorant receptors. According to the Mannheim Criteria (26), non-genomic responses to steroids must be observed within minutes, at low steroid concentrations, should not be affected by inhibitors of transcription or translation, and should be present in the presence of antagonists for the classic receptors. All of these criteria hold true for the activation of PSGR in LNCaP cells by the androstenone derivatives that we identified as PSGR ligands.
One of the steroid ligands we identified as a PSGR ligand is 6-dehydrotestosterone, which can be made endogenously by conversion of testosterone. Testosterone is produced by Leydig cells of the testes and is the major androgen in most mammalian species. Androgen metabolites can make up a significant fraction of circulating steroids. Testosterone metabolism and conversion to DHT is known to amplify the testosterone action on androgen receptors. Testosterone was shown to be converted to 6-dehydrotestosterone by a cytochrome P-450 belonging to the CYP3A family (27, 28). The organ that expresses the highest levels of cytochrome P-450 is the liver, which plays the dominant role in steroid hormone metabolism. 6-Dehydrotestoste-rone binds the steroid binding domain of rat androgen-binding protein, indicating that it might have physiological relevance (29). The other steroid structures that we identified, solely based on similarity to β-ionone and 6-dehydrotestoste-rone, are not known to occur in the human body as major metabolites. ADT is known to function as an aromatase (CYP19) inhibitor preventing the aromatization of androgens to estrogens and is used in pharmaceutical treatments of cancer, including cancer of the prostate (30).
Based on sequence comparison, PSGR clearly belongs to the superfamily of odorant receptors. In general, ORs allow the recognition of a wide range of odorants in the olfactory sensory neurons of the nose (31). Recently, another human OR was identified as a steroid receptor, detecting the steroid hormones androstenone and androstadienone in a recombinant expression system (16). Although activation of the endogenous receptor by steroid hormones was not investigated, it is very likely that this receptor also initiates rapid steroid actions because genetic variation in the receptor gene alters the perception of the respective steroids by the sense of smell, and therefore, may initiate the classical OR G-protein-mediated signaling cascade in the sensory neurons (16).
Despite the fact that ORs were found to be ectopically expressed in different non-olfactory tissues (17, 18, 32, 33), uncertainties exist about the possible functions of these ectopic receptors due to the generally low amounts of the transcripts. Functional expression could only be shown for human OR17-4 (OR1D2) (20) and mouse MOR267-13 (19), which are involved in sperm chemotaxis. Here, we show that PSGR, clearly belonging to the OR superfamily (OR51E2), can be activated by recombinantly identified specific receptor ligands in prostate cells. Moreover, PSGR transcripts could also be detected in human olfactory epithelium, indicative of the dual role of this receptor also in the olfactory system. Our data give new support for the hypothesis that at least some ectopically expressed ORs may have additional functions and demonstrate a role for OR51E2/PSGR in cancerogenesis.
Pertinent to its sequence similarity to odorant receptors, PSGR can be activated by the odorant ligand β-ionone, having a characteristic odor of violet. β-Ionone is an isoprenoid widely found in plants and plant products as a degradation product of carotenoids (34). Generally, plant isoprenoids have been suggested to inhibit cancer growth and development through inhibition of hydroxymethylglutaryl-CoA reductase activity (35, 36), but the isoprenoids β-ionone and geraniol have been shown to inhibit mammary carcinogenesis by a so far unknown mechanism that is not causally related to hydroxymethylglutaryl-CoA reductase activity (37). The antiproliferative and cell cycle regulatory effects of β-ionone and geraniol are therefore caused by another, so far unknown mechanism. With the detection of a β-ionone responsive OR in prostate epithelial cells, we identify here a new way by which β-ionone, and possibly other isoprenoids, can influence growth of tumor cells.
In the present study, we show that PSGR is a G-protein-coupled membrane steroid receptor in prostate cells. PSGR belongs to the superfamily of ORs and thereby constitutes a new example for the functionality of at least some ectopic ORs. In addition, we found that major intracellular signaling cascades involved in cell survival are activated by PSGR, which might possibly be used in the future as a therapeutic target in prostate cancer control strategies.
Supplementary Material
Acknowledgments
We thank H. Bartel and J. Gerkrath (Ruhr-University Bochum) for excellent technical assistance, B. Ache (University of Florida) for helpful comments on the manuscript, and J. Panten (Symrise, Holzminden, Germany) and T. Gerke (Henkel, Düsseldorf, Germany) for providing odorants.
This study was supported by Grant SFB642 from the Deutsche Forsch ungs ge mein schaft (to E. M. N. and H. H.) and a grant from the Max-Planck-Research School for Chemical Biology.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 .
- GPCR
- G-protein-coupled receptor
- PSGR
- prostate-specific G-protein-coupled receptor
- PSA
- prostate-specific antigen
- OR
- olfactory receptor
- CK
- cytokeratin
- MAP
- mitogen-activated protein
- MAPK
- MAP kinase
- SAPK
- stress-activated protein kinase
- JNK
- c-Jun NH2-terminal kinase
- siRNA
- small interfering RNA
- DHT
- dihydrotestosterone
- RT
- reverse transcription
- ADT
- 1,4,6-androstatriene-3,17-dione
- GFP
- green fluorescent protein.
REFERENCES
- 1.Godara G., Cannon G. W., Cannon G. M., Jr., Bies R. R., Nelson J. B., Pflug B. R. ( 2005) Prostate 65, 27– 34 [DOI] [PubMed] [Google Scholar]
- 2.Taub J. S., Guo R., Leeb-Lundberg L. M., Madden J. F., Daaka Y. ( 2003) Cancer Res. 63, 2037– 2041 [PubMed] [Google Scholar]
- 3.Ben-Josef E., Yang S. Y., Ji T. H., Bidart J. M., Garde S. V., Chopra D. P., Porter A. T., Tang D. G. ( 1999) J. Urol. 161, 970– 976 [PubMed] [Google Scholar]
- 4.Chay C. H., Cooper C. R., Gendernalik J. D., Dhanasekaran S. M., Chinnaiyan A. M., Rubin M. A., Schmaier A. H., Pienta K. J. ( 2002) Urology 60, 760– 765 [DOI] [PubMed] [Google Scholar]
- 5.Cooper C. R., Chay C. H., Gendernalik J. D., Lee H. L., Bhatia J., Taichman R. S., McCauley L. K., Keller E. T., Pienta K. J. ( 2003) Cancer 97, 739– 747 [DOI] [PubMed] [Google Scholar]
- 6.Dai J., Shen R., Sumitomo M., Stahl R., Navarro D., Gershengorn M. C., Nanus D. M. ( 2002) Clin. Cancer Res. 8, 2399– 2405 [PubMed] [Google Scholar]
- 7.Lee L. F., Guan J., Qiu Y., Kung H. J. ( 2001) Mol. Cell. Biol. 21, 8385– 8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Weng J., Cai Y., Penland R., Liu M., Ittmann M. ( 2006) Prostate 66, 847– 857 [DOI] [PubMed] [Google Scholar]
- 9.Weng J., Wang J., Cai Y., Stafford L. J., Mitchell D., Ittmann M., Liu M. ( 2005) Int. J. Cancer 113, 811– 818 [DOI] [PubMed] [Google Scholar]
- 10.Xu L. L., Stackhouse B. G., Florence K., Zhang W., Shanmugam N., Sesterhenn I. A., Zou Z., Srikantan V., Augustus M., Roschke V., Carter K., McLeod D. G., Moul J. W., Soppett D., Srivastava S. ( 2000) Cancer Res. 60, 6568– 6572 [PubMed] [Google Scholar]
- 11.Xu L. L., Sun C., Petrovics G., Makarem M., Furusato B., Zhang W., Sesterhenn I. A., McLeod D. G., Sun L., Moul J. W., Srivastava S. ( 2006) Prostate Cancer Prostatic. Dis. 9, 56– 61 [DOI] [PubMed] [Google Scholar]
- 12.Yuan T. T., Toy P., McClary J. A., Lin R. J., Miyamoto N. G., Kretschmer P. J. ( 2001) Gene 278, 41– 51 [DOI] [PubMed] [Google Scholar]
- 13.Buck L., Axel R. ( 1991) Cell 65, 175– 187 [DOI] [PubMed] [Google Scholar]
- 14.Firestein S. ( 2004) Sci. STKE 2004, pe15. [DOI] [PubMed] [Google Scholar]
- 15.Malnic B., Godfrey P. A., Buck L. B. ( 2004) Proc. Natl. Acad. Sci. U. S. A. 101, 2584– 2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller A., Zhuang H., Chi Q., Vosshall L. B., Matsunami H. ( 2007) Nature 449, 468– 472 [DOI] [PubMed] [Google Scholar]
- 17.Feldmesser E., Olender T., Khen M., Yanai I., Ophir R., Lancet D. ( 2006) BMC Genomics 7, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., De la Cruz O., Pinto J. M., Nicolae D., Firestein S., Gilad Y. ( 2007) Genome Biol. 8, R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda N., Yomogida K., Okabe M., Touhara K. ( 2004) J. Cell Sci. 117, 5835– 5845 [DOI] [PubMed] [Google Scholar]
- 20.Spehr M., Gisselmann G., Poplawski A., Riffell J. A., Wetzel C. H., Zimmer R. K., Hatt H. ( 2003) Science 299, 2054– 2058 [DOI] [PubMed] [Google Scholar]
- 21.Braun T., Voland P., Kunz L., Prinz C., Gratzl M. ( 2007) Gastroenterology 132, 1890– 1901 [DOI] [PubMed] [Google Scholar]
- 22.Neuhaus E. M., Mashukova A., Zhang W., Barbour J., Hatt H. ( 2006) Chem. Senses 31, 445– 452 [DOI] [PubMed] [Google Scholar]
- 23.Wetzel C. H., Oles M., Wellerdieck C., Kuczkowiak M., Gisselmann G., Hatt H. ( 1999) J. Neurosci. 19, 7426– 7433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long R. M., Morrissey C., Fitzpatrick J. M., Watson R. W. ( 2005) Clin. Sci. 108, 1– 11 [DOI] [PubMed] [Google Scholar]
- 25.Losel R. M., Falkenstein E., Feuring M., Schultz A., Tillmann H. C., Rossol-Haseroth K., Wehling M. ( 2003) Physiol. Rev. 83, 965– 1016 [DOI] [PubMed] [Google Scholar]
- 26.Falkenstein E., Norman A. W., Wehling M. ( 2000) J. Clin. Endocrinol. Metab. 85, 2072– 2075 [DOI] [PubMed] [Google Scholar]
- 27.Halvorson M., Greenway D., Eberhart D., Fitzgerald K., Parkinson A. ( 1990) Arch. Biochem. Biophys. 277, 166– 180 [DOI] [PubMed] [Google Scholar]
- 28.Nagata K., Liberato D. J., Gillette J. R., Sasame H. A. ( 1986) Drug Metab. Dispos. 14, 559– 565 [PubMed] [Google Scholar]
- 29.Danzo B. J., Parrott J. A., Skinner M. K. ( 1991) Endocrinology 129, 690– 696 [DOI] [PubMed] [Google Scholar]
- 30.Brodie A. M., Garrett W. M., Hendrickson J. R., Tsai-Morris C. H., Williams J. G. ( 1983) J. Steroid Biochem. 19, 53– 58 [PubMed] [Google Scholar]
- 31.Buck L. B. ( 1992) Curr. Opin. Neurobiol. 2, 282– 288 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Rogers M., Tian H., Zhang X., Zou D. J., Liu J., Ma M., Shepherd G. M., Firestein S. J. ( 2004) Proc. Natl. Acad. Sci. U. S. A. 101, 14168– 14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderhaeghen P., Schurmans S., Vassart G., Parmentier M. ( 1997) Genomics 39, 239– 246 [DOI] [PubMed] [Google Scholar]
- 34.Sacchettini J. C., Poulter C. D. ( 1997) Science 277, 1788– 1789 [DOI] [PubMed] [Google Scholar]
- 35.Elson C. E., Peffley D. M., Hentosh P., Mo H. ( 1999) Proc. Soc. Exp. Biol. Med. 221, 294– 311 [DOI] [PubMed] [Google Scholar]
- 36.Mo H., Elson C. E. ( 1999) J. Nutr. 129, 804– 813 [DOI] [PubMed] [Google Scholar]
- 37.Duncan R. E., Lau D., El-Sohemy A., Archer M. C. ( 2004) Biochem. Pharmacol. 68, 1739– 1747 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.