Abstract
Pancreatic phospholipase A2 (phospholipase A2 group 1B, G1B) belongs to the superfamily of secreted phospholipase A2 (PLA2) enzymes. G1B has been proposed to be a potential target for diseases such as hypertension, obesity, and diabetes. Human pancreatic prophospholipase A2 (pro-hG1B) is activated by cleavage of the first seven-residue propeptide (phospholipase A2 propeptide, PROP). However, questions still remain on the mode of action for pro-hG1B. In this work, we expressed pro-hG1B in Pichia pastoris and determined the crystal structure at 1.55-Å resolution. The x-ray structure demonstrates that pro-hG1B forms a trimer. In addition, PROP occupies the catalytic cavity and can be self-cleaved at 37 °C. A new membrane-bound surface and activation mechanism are proposed based on the trimeric model of pro-hG1B. We also propose a new autoproteolytic mechanism for pro-hG1B by the reaction triad Asp49-Arg0-Ser(-2) that is similar to the serine protease catalytic triad.
Phospholipase A2 (PLA2,5 EC 3.1.1.4) hydrolyzes glycerophospholipids at the sn-2 acyl bond to produce free fatty acids. The PLA2 family currently comprises five categories: the secreted PLA2s, the cytosolic PLA2s, the Ca2+-independent PLA2s, the platelet-activating factor acetyl hydrolases, and the lysosomal PLA2s. To date, based on the catalytic mechanism as well as functional and structural information, 15 different groups of PLA2 have been reported and named (1).
G1B is a member of the secreted PLA2 enzymes. This lipolytic enzyme releases glycerophospholipids and arachidonic acid that serve as the precursors of signal molecules that mediate a multitude of biological functions, such as inflammation. The G1B gene has been reported to be linked to hypertension in three sample populations (2). The concentration of G1B protein in serum is a potential marker for pancreatic acinar cell carcinoma (3). Knock-out mice experiments showed that G1B knockdown can prevent diet-induced obesity and obesity-related insulin resistance (4). After being fed with glucose-rich meals, knock-out mice showed lower postprandial glycemia than wild-type mice (5). A recent report also pointed to the linkage between hG1B and ophthalmic diseases (6). Therefore, hG1B is considered to be a potential target for treatment of obesity, diabetes (4,5), or ophthalmic diseases (6).
Pro-hG1B is a digestive zymogen secreted from pancreatic acinar cells in its inactive form (7). It is activated by trypsin in the duodenum. The activation of pro-hG1B by cleavage of the PROP, a heptapeptide of the sequence DSGISPR, is linked to diseases like pancreatitis (8) and acute lung injury (9). Circulating hG1B, mostly in the form of pro-hG1B, indicates pancreatic injury in acute pancreatitis (10). PROP can be used in assays to characterize the severity of acute pancreatitis (11). The C-terminal pentapeptide of PROP (GISPR) is essential for the inhibition of enzyme activity (12). Besides trypsin, pro-hG1B can also be activated by thrombin (13) and 29-kDa trypsin-like endogenous type 1-proPLA2 activator (11). PROP may also play an important role in the folding process as suggested by the refolding experiments of pro-hG1B and hG1B (14).
Helix 1 at the N-terminal of hG1B is known to play an important role in enzyme function. An engineered hG1B lacking the N-terminal helix 1 bound to membranes with weaker affinity and exhibited ∼100-fold lower enzymatic activity compared with that of the full-length hG1B. It is inferred that this helix 1 facilitates the membrane binding, thus enhances the enzymatic activity based on polarized infrared spectroscopic experiments (15). Experiments using semi-synthetic hG1B demonstrated that helix 1 residues act as a regulatory domain and mediate interfacial activation (16).
It has been generally believed that PLA2 possesses an interfacial binding surface (i-face), which orientates PLA2 for binding to membrane so that PLA2 subsequently hydrolyzes the sn-2 acyl bond of glycerophospholipids (17). A model for this i-face was postulated based on the crystal structure of dimeric porcine G1B (18). Questions still remain on the details of this i-face and the mechanism of its binding to membrane.
To date, numerous structures of G1B proteins from various species, including bovine (17, 19–21) and porcine (22,23), have been published. However, there is no report of structure for hG1B or pro-hG1B. The crystal structure of pro-hG1B presented here will illustrate the structural difference of G1B between the human and other species. It will provide structural insight into the activation mechanism of pro-hG1B and potentially facilitate the structure-based design of inhibitor for the treatment of a number of diseases including hypertension, obesity, and diabetes.
EXPERIMENTAL PROCEDURES
Expression and Purification of Pro-hG1B
The pro-hG1B gene (GenBankTMID NM_000928) was cloned into the Pichia pastorias expression vector pPIC9k (Invitrogen). The recombinant plasmid pPIC9K-pro-hGIB was linearized with SacI. CompetentP. pastorisGS115 cells were prepared according to the multicopy Pichia expression manual (Invitrogen) and transformed with 1–2 μg of linearized plasmids by electroporation with a Bio-Rad Gene Pulser at 1500 V using a 0.1-cm cuvette (Bio-Rad). Transformants of GS115/pro-hG1B were selected on four YPD plates containing 0.25, 0.50, 0.75, and 1.0 mg/ml of G418, respectively. After incubation for 2 days at 30 °C, colonies resistant to G418 were further selected by PCR and directly transferred to BMGY medium, then switched to BMMY medium for pro-hG1B expression. The colony with the highest expression was selected based on time course experiments by SDS-PAGE. To scale up, the selected strain of pro-hG1B was expressed in a 2.0-liter shake flask. Seed cultures were generated by inoculating 10 ml of YPD with 100 μl of cells from a glycerol stock. After 24 h, 1 ml of this culture was used to inoculate 400 ml of BMGY and allowed to grow for 2 days. Cells were collected by centrifugation at 2500 ×g for 5 min at 4 °C. Then cells were resuspended in 400 ml of BMMY to begin induction for 3 days with shaking at 30 °C. 1% (v/v) fresh methanol was replenished following each day of induction. After 72 h of induction, the cell suspension was centrifuged at 12,000 ×g for 45 min at 4 °C. The cell pellets were discarded, and the crude media were frozen immediately at −80 °C. All cultures were incubated at 30 °C and shaken at 200 rpm.
The culture supernatant contained the pro-hG1B was thawed from −80 °C and centrifuged at 15,000 ×g to pellet any precipitant. The supernatant was then buffer-exchanged to 50 mmTris-HCl, pH 9.5 at 4 °C. Purification was achieved by Q-Sepharose XL (GE Healthcare) and then by Superdex 75 (GE Healthcare).
Crystallization, X-Ray Data Collection, and Structure Determination
Initial crystallization screening was done by vapor diffusion method using sparse-matrix crystallization screening kits from Hampton Research. The protein sample, concentrated to 45 mg·ml−1in 20 mmTris-HCl, 150 mmNaCl, pH 8.5, was mixed with equal volumes of precipitant in 2-μl sitting drops on a 96-well Microplate (Grenier) at 25 °C. Crystals were grown in a mix of 1-μl protein solution, 1 μl of 0.1mTris, 0.2mlithium sulfate, 33% PEG 4000, pH 8.4 in 14 days.
The data for the best crystal were collected at 110 K using the Oxford Diffraction Gemini R Ultra system. The space group of the crystal was determined to beP63, with unit cell parametersa = b = 56.55 Å,c = 60.63 Å. All data were processed by CrysAlisPro (24), then scaled by SCALA (25) using CCP4 (26). 5% of data were randomly selected for theR-free calculation. The initial structure solution was obtained by molecular replacement using the porcine pancreatic PLA2 (PDB code: 1HN4) as the search model with program Molrep (27,28). The model was rebuilt to completion by ARP/wARP (29) and then refined by refmac5 (30) with cycles of manual fitting using Coot (31). The structure was finalized with a round of TLS refinement based on the analysis carried out via TLS motion determination server (32,33). Crystallographic results are shown in Table 1. The final model was refined to anR-work of 14.53%, and anR-free value of 18.29%. Analysis with the program Molprobity (34) shows that the final model has 97.7% of the residues in the favored region and no outlier in the Ramachandran plot (supplemental TableS2 and Fig.S1). Figures were prepared with PyMOL (35) unless otherwise stated. Coordinates for the pro-hG1B has been deposited to Protein Data Bank with accession number 3ELO. The simulated annealing omit map calculation for pro-hG1B was carried out by CNS (36).
TABLE 1.
Data collection and refinement statistics
| Data collection | |
| Space group | P63 |
| Unit cell parameters | |
| a,b,c (Å) | 56.55, 56.55, 60.63 |
| α,β,γ (°) | 90, 90, 120 |
| Resolution (Å) | 18.68-1.55 |
| Total observations | 84309 |
| Unique reflections | 16042 (2323)a |
| Completeness (%) | 99.9 (100) |
| Mean I/σ(I) | 22.4 (2.4) |
| R-merge (%) | 7.2 (51.3) |
| Multiplicity | 5.2 (3.8) |
| Refinement statistics | |
| Resolution (Å) | 18.68-1.55 (1.59-1.55) |
| Completeness for range (%) | 99.99 |
| Data cutoff (σ(F)) | None |
| No. of reflections forR-work calculation | 15232 |
| R-free value test set size (%) | 5.0 |
| No. of reflections forR-free calculation | 810 |
| R-work (%) | 14.53 (24.3) |
| R-free (%) | 18.29 (24.3) |
| No. atoms | |
| Protein | 1054 |
| Ligand/ion | 5 |
| Water | 164 |
| B-factor (Å2) | |
| Mean B value | 14.008 |
| Protein | 13.268 |
| Ion | 12.830 |
| Water | 18.802 |
| R.M.S. deviations | |
| Bond lengths (Å) | 0.010 |
| Bond angles (°) | 1.365 |
a Values in parentheses indicate data in the highest resolution shell.
Dynamic Light Scattering (DLS) Experiments
DLS experiments were carried out on a DynaPro Titan instrument (Wyatt Technology Corp.). Purified pro-hG1B was first concentrated to 30 mg/ml, and then diluted to 15 mg/ml, 7.5 mg/ml, and 3.7 mg/ml sequentially. All samples were prepared in duplicate. The solution buffer was 20 mmTris, pH 8.0, 150 mmNaCl, with or without 200 mmlithium sulfate added. The assays were performed at 4 °C.
The hG1B sample was obtained by thrombin cleavage of the pro-hG1B and then purified on a Superdex 75 column. The same protein concentrations were prepared, and DLS assays were performed according to the procedure used for pro-hG1B.
The hydrodynamic radius was measured, and the molecular weight was calculated by DYNAMICS V6 Version 6.7.7.9. The data were analyzed by GraphPad Prism 5.
Mass Spectrometry Analysis
After purification by Superdex 75 column, pro-hG1B was buffer-exchanged to 10 mmTris-HCl, pH 8.0, by Amicon Ultra 5k (Millipore). The sample was divided into four aliquots. Each aliquot contained 100 μg of pro-hG1B in 10 μl of buffer. One aliquot was stored at 4 °C. The other three aliquots were incubated at 37 °C. Among these three samples, two were treated with protease, 2 μg of trypsin (Sigma), and thrombin (GE healthcare), respectively. After an overnight reaction, the four samples were analyzed on an Agilent 1200 LC/MS system (single quadrupole mass spectrometer, ESI ion source).
SDS-PAGE for PROP Cleavage and Thermostability Analysis
100 μg of purified pro-hG1B was divided into two aliquots in 100 μl of 10 mmTris-HCl, pH 8.0, 150 mmNaCl with or without 2 μg of thrombin. 20 μl of the samples were taken in a time course of 0, 2, 4, and 12 h at 37 °C incubation. Samples from different time points were divided equally, and each was mixed with 2.5 μl of 5× loading buffer (60 mmTris-HCl, pH 6.8, 25% glycerol, 2% SDS, 715 mm2-mercaptoethanol, 0.1% bromphenol blue) with or without boiling. All the samples were stored on ice immediately after treatment and subject to SDS-PAGE analysis.
To test thermostability of pro-hG1B, purified pro-hG1B was dissolved in 10 mmTris-HCl, pH 8.0, 150 mmNaCl and incubated at 37 °C overnight, and then the samples were divided into eight aliquots. After 2.5 μl of 5× loading buffer was added to each of the 10-μl samples, the samples were then incubated at 25, 50, 64, 74, 80, 84, and 90 °C for 15 min, respectively, and then analyzed on SDS-PAGE. All the SDS-PAGE analyses were carried out on a Mini PROTEAN 3 Cell system (Bio-Rad) with 15% separating gel and 5% stacking gel.
RESULTS
Structure of Pro-hG1B
The structure of pro-hG1B shown inFig. 1 includes one monomer of pro-hG1B and one sulfate ion. Very similar to other reported structures of the PLA2 family, including bovine pancreatic PLA2 (21) and porcine pancreatic PLA2 (23), the structure of pro-hG1B consists of a typical set of four major helices (Helix 1 (α1), from residue 3 to 12; Helix 2 (α2), from residue 40 to 57; Helix 3 (α3), from residue 59 to 61; Helix 4 (α4), from residue 90 to 108) and two β-strands (β-strand 1 (β1), from residue 76 to 78; β-strand 2 (β2), from residue 81 to 83). Two short helices were found in the C terminus (Helix 5 (α5), from residue 113 to 115, and one 310 helix, Helix 6 (α6), from residue 120 to 123).
FIGURE 1.
Structure of pro-hG1B. The overall structure of human pancreatic prophospholipase A2 is shown. Helices are in red, and β-strands are in yellow. Loops are colored in green. The sulfate ion is presented as a CPK model in magenta.
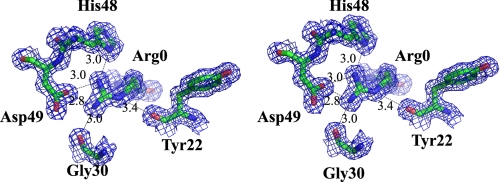
One unique feature of this pro-hG1B structure is the position of PROP. The PROP (from Asp6 to Arg0) occupies the active site that was used for inhibitor binding in porcine pancreatic PLA2 (18). In porcine pancreatic PLA2 structure, there are six amino acid (aa) residues (Phe22, Gly30, His48, Asp49, Tyr52, and Phe106) interacting with the inhibitor MJ33, and these residues form the active site cavity. However, in the pro-hG1B structure, these aa residues (Tyr22, Gly30, His48, Asp49, Tyr52, and Phe106) are all buried due to the folding back of PROP. An active site water molecule that was conserved in other G1B structure is not observed in pro-hG1B structure as the position is displaced by the Nδof Arg0. Residue Arg0 also interacts with residue Tyr22, Gly30, His48, and Asp49 through a hydrogen-bonding network as shown inFig. 2.
FIGURE 2.
Arg0 occupies the active site. Stereo view of the catalytic site with 2Fo-Fc electron density map contoured at 1σ. Arg0 interacts with Tyr22, Gly30, His48, and Asp49 through hydrogen bonds.
Pro-hG1B Forms a Trimer
There is one pro-hG1B molecule in the asymmetric unit. Through the symmetry operation, however, it is clearly shown that pro-hG1B forms a trimer (Fig. 3). On one side of the trimer (which will be referred to as the front side), there are three chains of PROP that protrude out of the plane (Fig. 3A). Whereas on the back side of the trimer, which includes the proposed membrane-binding N-terminal helix 1 (helix α1) (15,16), there is a largely positive charge surface with a center opening that is currently occupied by a sulfate ion (Fig. 3B). The interactions involved in the trimer formation consist of hydrogen bonds and hydrophobic contacts (Fig. 3E). Key residues forming the hydrogen bonds include Ser-5, Asp17, Lys113, Ala114, Lys116 of one chain and Ser-5, Trp3, Lys7, Asn67, Thr70, Lys89, and Glu92 of the other chain. Residues involved in the hydrophobic contacts include Asp-6, Ala1, Asp17, Phe19, Leu20, Asn24, Lys113, Asn117, and Lys121 of one chain and Asp-6, Trp3, Lys62, Tyr69, Thr70, His71, and Glu92 of the other chain. These residues clearly suggest that the N terminus, C terminus, and the 62–75 loop (also called “69-loop”) are contributing to the trimer formation.
FIGURE 3.
The trimeric form of pro-hG1B. A, electrostatic surface potential representation of pro-hG1B with the PROP occupying the active site cavity. The PROP is shown as a scheme and is colored in green. The surface potential is calculated using ABPS (47) and color-coded, where red and blue represent net negative and positive charges, and white represents overall neutral positions, respectively. The total charge is represented from −8 kbT/ec to 8 kbT/ec as the color change, in which kb is the Boltzmann constant, T is the temperature, and ec is the electron charge. B, view of molecule with 180 degree rotation around the y axis of A. The sulfate ion is shown as a magenta CPK model. C, calculated molecular weight of pro-hG1B obtained from DLS experiments in various protein concentrations, 30, 15, 7.5, 3.7, and 0 mg/ml, respectively, and with/without lithium sulfate. All data shown are the average of triplicates. Error bars denote the S.E. D, calculated molecular weight of hG1B obtained from DLS experiments in various protein concentrations, 30, 7.5, 3.7, and 0 mg/ml, respectively, with lithium sulfate. All data shown are the average of triplicates. Error bars denote the S.E. Panels C and D were prepared by GraphPad Prism 5. E, subunit interface of pro-hG1B trimer. Two monomer chains, chain B and chain C, are colored in blue and red, respectively. The key residues involved in the subunit interaction are labeled. The figure was prepared by LIGPLOT (48).
The DLS measurement further confirmed the trimer formation in solution. Pro-hG1B at concentrations above 7.5 mg/ml assumed a tri meric form (average molecular mass of 42 kDa); whereas it assumed a monomeric form at the lower protein concentration (3.7 mg/ml). Interestingly, trimerization exists only when sulfate ion is present (Fig. 3C).
Pro-hG1B Can Be Autoproteolytic by Cleavage of PROP
PROP of pro-hG1B can be cleaved by trypsin and thrombin leading to pro-hG1B activation (13,37). In our experiment, after treatment with trypsin or thrombin, PROP with a molecular mass of 731 Da was detected by mass spectrometry. Surprisingly, when pro-hG1B was incubated at 37 °C for 18 h, a peak at 731 Da was also detected by mass spectrometry (Fig. 4A). This autocleavage, however, was not observed when the sample was incubated at 4 °C.
FIGURE 4.
Autocleavage of PROP. A, mass spectrometry data of pro-hG1B (100 μg) digested with 2 μg of trypsin or thrombin, pro-hG1B at 37 °C for 18 h in 100 μl of 10 mm Tris, pH 8. 5. Pro-hG1B stored at 4 °C was used as a blank control. B, purified pro-hG1B and hG1B (activated by thrombin) were dissolved in 10 μl of 10 mm Tris, pH 8.0, 150 mm NaCl, then added to an equal volume of SDS-PAGE loading buffer that contained 2% (w/v) SDS and heated for 15 min when marked with +. C, hG1B (activated by autocleavage) was dissolved in 10 μl of 10 mm Tris, pH 8.0, 150 mm NaCl, then added to an equal volume of SDS-PAGE loading buffer that contained 2% (w/v) SDS and heated for 15 min at the indicated temperature.
To confirm the autocleavage of pro-hG1B, pro-hG1B was analyzed by SDS-PAGE after different treatments. As shown inFig. 4B, in the SDS-PAGE gel, the sample migrated to two bands (hereby referred to “upper” and “lower” band) without pre-boiling, and only one band (the lower band) with pre-boiling. In addition, the amount of upper band increased along with the time of 37 °C treatment and thrombin treatment, while the amount of lower band decreased. These results indicated that the upper band was hG1B, and the lower band was pro-hG1B. Furthermore, the lower band and the upper band samples were analyzed by N-terminal amino acid sequencing. The upper band was confirmed to be hG1B, and the lower band without pre-boiling was pro-hG1B. The single band at the lower position with pre-boiling treatment was identified to be the mixture of pro-hG1B and hG1B.
Thermostability of pro-hG1B was tested on an SDS-PAGE gel, and results were analyzed by N-terminal amino acid sequencing. Pro-hG1B was autocleaved to form hG1B through incubation at 37 °C overnight.Lane 1 of Fig. 4C showed that the lower band corresponded to uncleaved pro-hG1B, and the upper band corresponded to cleaved hG1B. This sample was then incubated in various temperatures, 50, 64, 70, 74, 80, 84, and 90 °C for 15 min, respectively. Two bands are shown in the SDS-PAGE gel for the lower temperature treatment, from 50 to 74 °C (Fig. 4C). N-terminal amino acid sequencing showed that the upper band is hG1B, and the lower band is the mixture of hG1B and pro-hG1B. When the incubation temperature is above 74 °C, the upper band disappeared completely.
DISCUSSION
Pro-hG1B Is a Functional Trimer
Trimeric form of PLA2 has been reported only for cobra PLA2 (38) and Naja naja PLA2 (39). The current study reveals the first tri meric association for pancreatic PLA2. When compared with the cobra PLA2 and Naja naja naja PLA2, the tri meric structure of pro-hG1B shows a significantly different subunit association and a much higher percentage of buried surfaces. Analyzed by the PISA Server (40), 22% of the solvent accessible area (4760 Å2) is buried in the trimer of pro-hG1B. The buried surface area of pro-hG1B is significantly higher than those for the cobra PLA2 trimer and the Naja naja naja PLA2 trimer (15 and 16%, respectively).
To date, most reported pancreatic PLA2 structures adopt a dimeric association. In its apo form, the dimer of porcine pancreatic PLA2 (PDB code 1HN4), which contains the PROP peptide, has only 9% buried surface area. When the inhibitor MJ33 is bound to the dimer, the buried surface area increases to 23%, and the protein becomes a much tighter dimer. In comparison, when PROP is removed, the buried surface area in the hypothetical tri meric hG1B can still reach 18.3% when calculated. We deduce that this PROP attributes to the trimer formation similar to the contribution of MJ33 to the dimerization of porcine pancreatic PLA2. Sequence alignment shows differences between the PROP sequences in the human and other species (Fig. 5A). Two residues in PROP of pro-hG1B (Asp-6 and Ser-5) are involved in the hydrogen-bonding network and hydrophobic contract in the trimer interface (Fig. 3E). These two key residues are unique for the human enzyme (Fig. 5A). Based on this, it is postulated that the tri meric formation of pro-G1B only exists in the human form of enzyme. To date, there is no report of trimer formation for any other pancreatic PLA2, including porcine pro-G1B and bovine pro-G1B.
FIGURE 5.
69-loop adopts a unique conformation. A, sequence alignment between human pro-G1B and pro-G1B from porcine and bovine, color-coded as following: blue for identical alignment, red shading for semi-conserved substitution, and other colors for no similarity. The alignment was performed using BIOEDIT (49). B, superposition of all published pancreatic PLA2 in the 69-loop region. Pro-hG1B is colored in red, pro-pG1B (PDB code: 1HN4) is colored in blue, others (porcine and bovine G1B) are colored in green. Tyr69 is presented as a stick model.
Additionally, DLS experiments show that trimerization of pro-hG1B depends on the protein concentration. And hG1B required a higher protein concentration for trimerization (30 mg/ml compared with 7.5 mg/ml for pro-hG1B) (Fig. 3,C and D). This finding corroborates with the hypothesis that PROP enhances the trimer formation. Sulfate ion, an anionic ion that may mimic the membrane-charged surface, is crucial for trimer formation in our DLS experiments. It is in agreement with previous reports and a review by Bahnson (41) that the sulfate is critical for bridging the adjacent subunits along the i-face.
The protein concentration of 30 mg/ml is highly non-physiological for hG1B; therefore, trimer formation of hG1B alone is unlikely to be stable. However, PROP occupies the active site in pro-hG1B structure and presumably mimics an inhibitor or substrate. As a result, pro-hG1B presents as a model for hG1B binding with a substrate molecule. When hG1B becomes activated in the reaction system, the substrate binding will stabilize the trimerization. The local concentration of hG1B on the membrane-binding surface will only need to be as low as 7.5 mg/ml (or even lower).
Pro-hG1B Has an Autoproteolytic Mechanism Similar to Serine Protease
In the pro-hG1B structure, PROP fills the active site cavity in a manner similar to inhibitor binding to other G1B structures. It is known that PROP can be cleaved by trypsin or thrombin for the enzyme to become fully active. As shown earlier, when the sample was heated to 37 °C, PROP can be cleaved. This suggests a potential autoproteolytic mechanism in the activation of pro-hG1B. In the typical serine protease, there is an Asp-His-Ser catalytic triad involved in catalysis. In the structure of pro-hG1B, there is a strikingly similar set of residues that may be involved in the cleavage of the Arg0-Ala1 peptide bond. As shown inFig. 6, comparison between Asp102-His57-Ser95 of serine protease (PDB code 1K1I) and Asp49-Arg0-Ser(-2) of pro-hG1B in the current structure shows that the R.M.S.D. over all atoms between these two sets is only 1 Å. On the basis of the spatial arrangement of these three side chains, we propose a mechanism for the self-activation of pro-hG1B. In pro-hG1B, the salt bridge between Asp49 and Arg0 may orient the NH2 of Arg0 such that the guanidinium group of Arg0 can obtain a proton from the hydroxyl group of Ser-2. Thus, the deprotonated OG of Ser-2 may attack the carbonyl carbon of Arg0, leading to the formation of oxyanion hole. The unstable peptide oxygen may promote the breakage of the peptide bond and the dissociation of the amine product. As discussed above, temperature is likely a key factor for the self-activation mechanism.
FIGURE 6.
Asp49-Arg0-Ser(-2) reaction triad. Pair fitting of the proposed pro-hG1B triad with the trypsin catalytic triad is shown. Asp49, Ser-2, and Arg0 of pro-hG1B are colored in orange. Asp102, His57, and Ser95 of trypsin (PDB code: 1K1I) are colored in cyan.
Conformational Change upon Activation of Pro-hG1B
Two SDS-PAGE gel results (Fig. 4,B and C) showed that hG1B can migrate to two different positions under various temperature treatments. However, pro-hG1B showed only one band. The molecular mass difference between the cleaved and un-cleaved protein is 731 Da, which is significantly smaller than the apparent molecular size difference observed on SDS-PAGE. Interestingly, pro-hG1B migrates faster than hG1B under the same conditions on SDS-PAGE. These results suggest that pro-hG1B and hG1B adopt two different conformations that correspond to the two bands on the gel. We predict that the upper band corresponds to the conformation the active hG1B adopts while the lower band corresponds to the conformation pro-hG1B adopts. When hG1B was heated, its conformation would become similar to pro-hG1B so it migrated to the same lower band as pro-hG1B. It has been indicated that upon activation and membrane binding, helix 1 of hG1B will undergo a conformational change (16). Our experimental results confirmed the conformational change between pro-hG1B and hG1B. The conformational change is expected to be large enough to affect the migration rate on SDS-PAGE for a protein of this small size.
Aside from helix 1 that may affect the conformation of hG1B, 69-loop, which is in proximity to helix 1, may also contribute to the conformation of hG1B. Solution NMR study suggested that 69-loop is flexible and play an important role in the substrate recognition (42). It has also been previously observed by Tomooet al.(43) that the 69-loop is disordered in pro-pG1B. In the current crystal structure, excellent electron density is observed for residues of the 69-loop as shown in the 2Fo-Fc map and simulated-annealing OMIT map (supplemental Fig.S2). The 69-loop of pro-hG1B showed a significantly different conformation from that of other PLA2 structures. There is a noticeable positional movement in the 69-loop, whereas the sequence of this loop shows a high degree of similarity across different species (Fig. 5,A and B). In our pro-hG1B structure, the 69-loop packs against PROP. Residues in the 69-loop (Lys62, Asn67, Tyr69, Thr70, His71) are involved in key hydrogen bonding and hydrophobic interactions in the trimer interface, indicating that the 69-loop contributes to trimer formation. Apart from mediating the PROP interaction and trimer formation, this loop clearly affects the conformation of hG1B. Tyr69, the key residue in the 69-loop reported to be important for enzyme binding to the membrane (42,44), adopted a significantly different side chain conformation from those in other PLA2 structures as shown inFig. 5B.
New Model for G1B Membrane Binding and Activation
The anion-assisted dimerization interface of the porcine PLA2 dimer was proposed to be an analog to the interfacial-bound surface of G1B (45,46). Our current study demonstrates that pro-hG1B is able to trimerize, and the anionic sulfate ion is required for the trimer formation. Our model also suggests that hG1B is capable of trimerization when an additional substrate molecule is present. The trimerization of pro-hG1B and hG1B may provide a new model for membrane attachment. We hereby propose a new interface for the membrane association that is crucial for the fatty acid hydrolysis. The monomeric form of pro-hG1B or hG1B exists before its binding to membrane. When membrane is available in the reaction system, pro-hG1B or hG1B will bind to the membrane in the tri meric form using the back side of the trimer, the positively charged surface. The temporal order of trimer formation and membrane attachment will require further investigation. When the tri meric form of enzyme is anchored to the membrane surface, for pro-hG1B, PROP will need to be cleaved either by trypsin or itself. The resultant hG1B will undergo conformational changes that further open up the center hole of the trimer to form a channel for the entry of substrate. The previous study indicated that hG1B activity is significantly increased compared with that of pro-hG1B when membrane is available in the reaction system (13). Our current model provides a reasonable explanation for this difference. Based on this model, PROP physically occupies the active site and also hinders the conformational change that is required for the opening of the substrate entry channel. When PROP is cleaved and removed as a leaving group, the efficiency of substrate entry and product release will be greatly increased. Most PLA2 family proteins have very conserved catalytic sites and the design of the inhibitor solely based on this conserved site will need to address the specificity issue. Therefore, blocking the substrate entry will confer improved specificity for potential inhibitor design. This tri meric form, the membrane-bound model, provides new insights into the activation mechanism of hG1B and a rational basis for the design of hG1B inhibitors.
Supplementary Material
Acknowledgments
We thank Dr. Xinhua Ji and Dr. Changyou Chen for reading the manuscript.
This work was supported by the National Basic Research Program of China (973 Program) (No. 2007CB914301, 2006CB910202, and 2005CB523008).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S2 and Figs. S1 and S2.
The atomic coordinates and structure factors (code 3ELO) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- PLA2
- phospholipase A2
- G1B
- pancreatic phospholipase A2 Group 1B
- pro-G1B
- pancreatic prophospholipase A2
- h
- human
- p
- porcine
- b
- bovine
- PROP
- phospholipase A2 propeptide
- aa
- amino acid
- 69-loop
- 62–75 loop
- R.M.S.D.
- root mean-square deviation
- DLS
- dynamic light scattering.
REFERENCES
- 1.Schaloske R. H., Dennis E. A. ( 2006) Biochim. Biophys. Acta 1761, 1246– 1259 [DOI] [PubMed] [Google Scholar]
- 2.Frossard P. M., Lestringant G. G. ( 1995) Clin. Genet. 48, 284– 287 [DOI] [PubMed] [Google Scholar]
- 3.Kuopio T., Ekfors T. O., Nikkanen V., Nevalainen T. J. ( 1995) Apmis. 103, 69– 78 [DOI] [PubMed] [Google Scholar]
- 4.Huggins K. W., Boileau A. C., Hui D. Y. ( 2002) Am. J. Physiol. Endocrinol. Metab. 283, E994– 1001 [DOI] [PubMed] [Google Scholar]
- 5.Labonte E. D., Kirby R. J., Schildmeyer N. M., Cannon A. M., Huggins K. W., Hui D. Y. ( 2006) Diabetes 55, 935– 941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolko M., Prause J. U., Bazan N. G., Heegaard S. ( 2007) Acta Ophthalmol. Scand. 85, 317– 323 [DOI] [PubMed] [Google Scholar]
- 7.Eskola J. U., Nevalainen T. J., Aho H. J. ( 1983) Clin. Chem. 29, 1772– 1776 [PubMed] [Google Scholar]
- 8.Mayer J. M., Rau B., Siech M., Beger H. G. ( 2000) Digestion 62, 164– 170 [DOI] [PubMed] [Google Scholar]
- 9.Rae D., Sumar N., Beechey-Newman N., Gudgeon M., Hermon-Taylor J. ( 1995) Clin. Biochem. 28, 71– 78 [DOI] [PubMed] [Google Scholar]
- 10.Schoenberg M. H., Mayer J. M., Beger H. G. ( 1997) Chirurg 68, 1112– 1118 [DOI] [PubMed] [Google Scholar]
- 11.Rae D., Beechey-Newman N., Burditt L., Sumar N., Hermon-Taylor J. ( 1996) Scand. J. Gastroenterol. Suppl. 219, 24– 27 [DOI] [PubMed] [Google Scholar]
- 12.Gudgeon A. M., Patel G., Hermon-Taylor J., Hurley P., Bowyer R. C., Jehanli A. M. ( 1991) Ann. Clin. Biochem. 28, 497– 503 [DOI] [PubMed] [Google Scholar]
- 13.Grataroli R., Dijkman R., Dutilh C. E., van der Ouderaa F., De Haas G. H., Figarella C. ( 1982) Eur. J. Biochem. 122, 111– 117 [DOI] [PubMed] [Google Scholar]
- 14.Cheng H. Q., Xu G. J. ( 2004) Acta Biochim. Biophys. Sin. (Shanghai) 36, 583– 588 [DOI] [PubMed] [Google Scholar]
- 15.Qin S., Pande A. H., Nemec K. N., Tatulian S. A. ( 2004) J. Mol. Biol. 344, 71– 89 [DOI] [PubMed] [Google Scholar]
- 16.Qin S., Pande A. H., Nemec K. N., He X., Tatulian S. A. ( 2005) J. Biol. Chem. 280, 36773– 36783 [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra B. W., Drenth J., Kalk K. H. ( 1981) Nature 289, 604– 606 [DOI] [PubMed] [Google Scholar]
- 18.Epstein T. M., Yu B. Z., Pan Y. H., Tutton S. P., Maliwal B. P., Jain M. K., Bahnson B. J. ( 2001) Biochemistry 40, 11411– 11422 [DOI] [PubMed] [Google Scholar]
- 19.Dijkstra B. W., Drenth J., Kalk K. H., Vandermaelen P. J. ( 1978) J. Mol. Biol. 124, 53– 60 [DOI] [PubMed] [Google Scholar]
- 20.Dijkstra B. W., Kalk K. H., Hol W. G., Drenth J. ( 1981) J. Mol. Biol. 147, 97– 123 [DOI] [PubMed] [Google Scholar]
- 21.Steiner R. A., Rozeboom H. J., de Vries A., Kalk K. H., Murshudov G. N., Wilson K. S., Dijkstra B. W. ( 2001) Acta Crystallogr. D 57, 516– 526 [DOI] [PubMed] [Google Scholar]
- 22.Dijkstra B. W., Renetseder R., Kalk K. H., Hol W. G., Drenth J. ( 1983) J. Mol. Biol. 168, 163– 179 [DOI] [PubMed] [Google Scholar]
- 23.Thunnissen M. M., Franken P. A., de Haas G. H., Drenth J., Kalk K. H., Verheij H. M., Dijkstra B. W. ( 1993) J. Mol. Biol. 232, 839– 855 [DOI] [PubMed] [Google Scholar]
- 24.Oxford Diffraction ( 2008) CrysAlis, Version 1.171.32.24, Oxford Diffraction Ltd., Yarnton, UK [Google Scholar]
- 25.Kabsch W. ( 1988) J. Appl. Crystallogr. 21, 916– 924 [Google Scholar]
- 26.CCP4 ( 1994) Acta Crystallogr. D 50, 760– 763 [DOI] [PubMed] [Google Scholar]
- 27.Vagin A., Teplyakov A. ( 1997) J. Appl. Crystallogr. 30, 1022– 1025 [Google Scholar]
- 28.Lebedev A. A., Vagin A. A., Murshudov G. N. ( 2008) Acta Crystallogr. D 64, 33– 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langer G., Cohen S. X., Lamzin V. S., Perrakis A. ( 2008) Nat. Protoc. 3, 1171– 1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murshudov G. N., Vagin A. A., Dodson E. J. ( 1997) Acta Crystallogr. D 53, 240– 255 [DOI] [PubMed] [Google Scholar]
- 31.Emsley P., Cowtan K. ( 2004) Acta Crystallogr. D 60, 2126– 2132 [DOI] [PubMed] [Google Scholar]
- 32.Painter J., Merritt E. A. ( 2006) Acta Crystallogr. D 62, 439– 450 [DOI] [PubMed] [Google Scholar]
- 33.Painter J., Merritt E. A. ( 2006) J. Appl. Crystallogr. 39, 109– 111 [Google Scholar]
- 34.Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., III, Snoeyink J., Richardson J. S., Richardson D. C. ( 2007) Nucleic Acids Res. 35, W375– 383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLano W. L. ( 2002) PyMol, Version 1.0r1, DeLano Scientific LLC, Palo Alto, CA [Google Scholar]
- 36.Brunger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. ( 1998) Acta Crystallogr. D 54, 905– 921 [DOI] [PubMed] [Google Scholar]
- 37.de Haas G. H., Postema N. M., Nieuwenhuizen W., van Deenen L. L. ( 1968) Biochim. Biophys. Acta 159, 118– 129 [DOI] [PubMed] [Google Scholar]
- 38.Fremont D. H., Anderson D. H., Wilson I. A., Dennis E. A., Xuong N. H. ( 1993) Proc. Natl. Acad. Sci. U. S. A. 90, 342– 346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segelke B. W., Nguyen D., Chee R., Xuong N. H., Dennis E. A. ( 1998) J. Mol. Biol. 279, 223– 232 [DOI] [PubMed] [Google Scholar]
- 40.Krissinel E., Henrick K. ( 2007) J. Mol. Biol. 372, 774– 797 [DOI] [PubMed] [Google Scholar]
- 41.Bahnson B. J. ( 2005) Arch Biochem. Biophys 433, 96– 106 [DOI] [PubMed] [Google Scholar]
- 42.Peters A. R., Dekker N., van den Berg L., Boelens R., Kaptein R., Slotboom A. J., de Haas G. H. ( 1992) Biochemistry 31, 10024– 10030 [DOI] [PubMed] [Google Scholar]
- 43.Tomoo K., Yamane A., Ishida T., Fujii S., Ikeda K., Iwama S., Katsumura S., Sumiya S., Miyagawa H., Kitamura K. ( 1997) Biochim. Biophys. Acta 1340, 178– 186 [DOI] [PubMed] [Google Scholar]
- 44.Egmond M. R., Hore P. J., Kaptein R. ( 1983) Biochim. Biophys. Acta 744, 23– 27 [DOI] [PubMed] [Google Scholar]
- 45.Pan Y. H., Epstein T. M., Jain M. K., Bahnson B. J. ( 2001) Biochemistry 40, 609– 617 [DOI] [PubMed] [Google Scholar]
- 46.Winget J. M., Pan Y. H., Bahnson B. J. ( 2006) Biochim. Biophys. Acta 1761, 1260– 1269 [DOI] [PubMed] [Google Scholar]
- 47.Baker N. A., Sept D., Joseph S., Holst M. J., McCammon J. A. ( 2001) Proc. Natl. Acad. Sci. U. S. A. 98, 10037– 10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace A. C., Laskowski R. A., Thornton J. M. ( 1995) Protein Eng. 8, 127– 134 [DOI] [PubMed] [Google Scholar]
- 49.Hall T. A. ( 1999) Nucleic Acids Symp. Ser. 41, 95– 98 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.