Abstract
Cardiac thin filament deactivation is initiated by Ca2+ dissociation from troponin C (cTnC), followed by multiple structural changes of thin filament proteins. These structural transitions are the molecular basis underlying the thin filament regulation of cardiac relaxation, but the detailed mechanism remains elusive. In this study Förster resonance energy transfer (FRET) was used to investigate the dynamics and kinetics of the Ca2+-induced conformational changes of the cardiac thin filaments, specifically the closing of the cTnC N-domain, the cTnC-cTnI (troponin I) interaction, and the cTnI-actin interaction. The cTnC N-domain conformational change was examined by monitoring FRET between a donor (AEDANS) attached to one cysteine residue and an acceptor (DDPM) attached the other cysteine of the mutant cTnC(L13C/N51C). The cTnC-cTnI interaction was investigated by monitoring the distance changes from residue 89 of cTnC to residues 151 and 167 of cTnI, respectively. The cTnI-actin interaction was investigated by monitoring the distance changes from residues 151 and 167 of cTnI to residue 374 of actin. FRET Ca2+ titrations and stopped-flow kinetic measurements show that different thin filament structural transitions have different Ca2+ sensitivities and Ca2+ dissociation-induced kinetics. The observed structural transitions involving the regulatory region and the mobile domain of cTnI occurred at fast kinetic rates, whereas the kinetics of the structural transitions involving the cTnI inhibitory region was slow. Our results suggest that the thin filament deactivation upon Ca2+ dissociation is a two-step process. One step involves rapid binding of the mobile domain of cTnI to actin, which is kinetically coupled with the conformational change of the N-domain of cTnC and the dissociation of the regulatory region of cTnI from cTnC. The other step involves switching the inhibitory region of cTnI from interacting with cTnC to interacting with actin. The latter processes may play a key role in regulating cross-bridge kinetics.
Cardiac muscle utilizes troponin to sense the concentration changes of myoplasmic Ca2+ and translate the transient Ca2+ signal into a cascade of events within the thin filament that ultimately leads to force generation or relaxation. The cardiac thin filament is composed of the heterotrimeric troponin complex and tropomyosin bound to the double helical actin filament (1, 2). The cardiac troponin is formed by three subunits: troponin C (cTnC),2 troponin I (cTnI), and troponin T (cTnT). The subunit cTnC is the Ca2+-binding protein, cTnI binds actin and inhibits actomyosin ATPase in relaxed muscle, and cTnT anchors the troponin complex on the actin filament. A prominent feature of cardiac muscle regulation is the Ca2+-dependent dynamic interactions among the thin filament proteins and the multiple structural transitions at the interface between troponin and the actin filament. These structural transitions include opening/closing of the N-domain of cTnC (3, 4), changes in conformation of both the inhibitory region, and regulatory region of cTnI (5–7), switching of the inhibitory/regulatory regions of cTnI from interacting with actin to interacting with cTnC (8), and movement of tropomyosin on the actin surface (9), which permits cross-bridge cycling between actin and myosin. These Ca2+-induced structural transitions are the molecular basis of cardiac thin filament regulation. The strong cross-bridge formed between myosin heads and actin modulates the interactions among thin filament proteins and further affects thin filament regulation (10–12). This feedback has been identified as an important mechanism for the beat-to-beat regulation of cardiac output. However, the mechanism by which the thin filament regulation in cardiac muscle is fine tuned at a molecular level by cross-bridges remains to be determined.
It has been suggested recently that the rate of myoplasmic Ca2+ removal does not rate limit contraction and relaxation of the muscle (13). For example, the mechanistic studies on cardiac trabeculae (14) and myofibrils (15, 16) suggest that Ca2+ binding to cTnC induced switching on of the thin filament regulatory unit well before force generation. In corroboration of the conclusion, de Tombe and co-workers (17) recently reported that changes in myofilament Ca2+ sensitivity do not affect the kinetics of myofibrillar contraction and relaxation, i.e. the cross-bridge cycling rate is independent of the dynamics of thin filament activation. This notion is consistent with findings from a recent study where Ca2+-induced conformational changes of cTnC were measured simultaneously with force development of myofibril (18). It was found that kinetics of the Ca2+-induced conformational change of cTnC was much faster than cross-bridge kinetics. However, one study using photolysis of caged Ca2+ reported that the rate of Ca2+-induced muscle contraction (kCa) was slower than the rate of force redevelopment (ktr), suggesting the importance of the thin filament in regulating cross-bridge kinetics (19). These results raise questions as to how the thin filament regulation through Ca2+-cTnC interaction controls muscle contraction kinetics. If the kinetics of the cross-bridge formation and detachment determine the rate of cardiac muscle contraction and relaxation, what will be the regulatory role of thin filament in heart function? The fact is that a high percentage of cardiomyopathy mutations occur among the thin filament proteins, and some of these mutations can severely hinder the kinetics of heart contraction and relaxation (20). Without a link between Ca2+ regulation and dynamics of cross-bridge formation and detachment, it will be difficult to interpret the mechanism underlying how these mutations affect force development and relaxation in the diseased heart.
Signal transduction of Ca2+ activation/deactivation along the thin filament involves multiple structural transitions of the thin filament proteins (21). Each structural transition may have different dynamics that can differ from Ca2+ exchange with cTnC. Therefore, the dynamics of these structural transitions within the thin filament may provide insight into the dynamic linkage between the Ca2+ binding to cTnC and the activation state of the cardiac thin filament. Time-resolved Förster resonance energy transfer (FRET), which can quantitate the distribution of inter-probe distances (22), provides a clear metric for study of Ca2+-induced structural changes (on Å scale) in the thin filament. FRET involves two fluorophores (one is the FRET donor and the other is an acceptor) attached to two different sites of proteins. Because FRET provides information on the conformational changes of proteins only around a specific region of interest, it is a unique approach for monitoring specific structural changes associated with the functional activities of the thin filament. Especially when combined with fast time-resolved techniques, FRET can provide dynamic and kinetic information associated with a specific structural transition in a multiple structural transition system (23–26).
Accordingly, we focused our investigation on the relaxation kinetics of (a) cTnC N-domain closing, (b) cTnC-cTnI interaction, and (c) cTnI-actin interaction within the reconstituted thin filament upon Ca2+ removal from the regulatory binding site of cTnC. The kinetics of these structural transitions were measured using FRET stopped-flow to monitor structural changes associated with each transition in the reconstituted thin filament in the absence and presence of strongly bound myosin subfragment 1 (S1). Our results showed that all structural transitions occurred in two phases, one fast and the other slow. The fast phase transition accounted for more than two-thirds of the total FRET change, and the slow phase transition accounted for less than one-third of the total FRET change. Our study suggests that different structural transitions have different kinetics upon Ca2+ removal from cTnC. Structural transitions associated with the mobile domain and the regulatory region of cTnI occur at fast kinetic rates, whereas the structural transitions involving transversal movement of the inhibitory region of cTnI occur at slow rates.
EXPERIMENTAL PROCEDURES
Sample Preparations and Characterizations
The double cysteine cTnC(L13C/N51C), and the single-cysteine cTnC(S89C), cTnI(S151C), and cTnI(S167C) mutants were generated purified from wild-type rat cTnC and cTnI clones using approaches previously described in the literature (8, 24, 27). The recombinant wild-type cTnT was purified as previously reported (3). The other proteins that comprise the thin filament, namely cTm (28), actin (29), and myosin subfragment 1 (S1) from chymotryptic digestion of myosin (30), were obtained from bovine cardiac tissue.
For the FRET measurements, the single cysteine residue of cTnI(S151C) and cTnI(S167C) mutants was modified with IAEDANS (5-iodoacetamidoethyl)aminonaphthalene-1-sulfonic acid) as FRET donor according to previously described procedures (3, 5). The single cysteine residue of cTnC(S89C) was modified either with DDPM (N-(4-dimethyamino-3,5-dinitrophenyl)maleimide) or DABM (4-dimethylaminophenylazophenyl-4′-maleimide) as FRET acceptor by following a previously described procedure (8). Cysteine 374 of actin was modified with DABM (31). The two cysteine residues of cTnC(L13C/N51C) were modified, as previously described, with AEDANS (FRET donor) attached to one cysteine residue and DDPM (FRET acceptor) attached to the other cysteine (24). The label ratio was determined using ϵ325 nm = 6,000 cm−1 m−1 for AEDANS, ϵ460 nm = 24,600 cm−1 m−1 for DABM, and ϵ442 nm = 2,930 cm−1 m−1 for DDPM, respectively. The identities of all cTnI mutants and the labeled proteins were verified using electrospray mass spectrometric analysis. Label ratios for all protein modification were >93%.
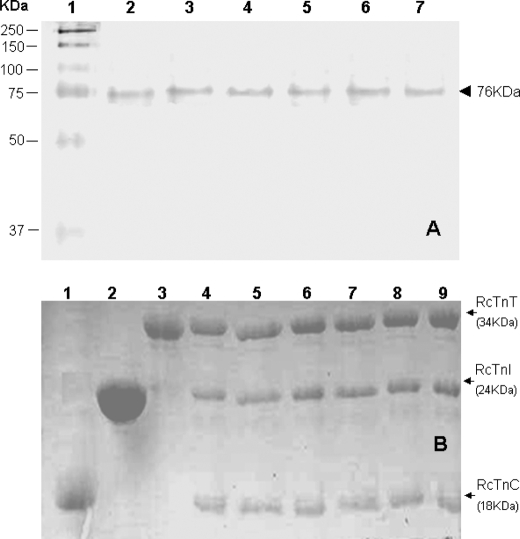
The troponin complexes and the thin filament containing different modified proteins were reconstituted using previously described procedures (8, 24). To examine whether labeled protein mutants have the same regulatory function as wild-type protein, Ca2+ regulation of the actin-activated S1-ATPase activity assay was carried out as described previously (26, 32). The results are summarized in Table 1. The ATPase activity of S1 in the presence of actin, but in the absence of troponin and tropomyosin, was taken as 100%. Ca2+ sensitivity was 0.827 for the control preparation containing wild-type troponin. Ca2+ sensitivities for all other preparations containing labeled protein mutants were similar to that of the control, suggesting that the effects of the mutations and modifications of these proteins on the Ca2+ regulatory activity were negligible. The stability and stoichiometry of the troponin complex reconstituted with the labeled mutant proteins were examined by SDS-PAGE and native gels (26). The gel analysis showed that both the wild-type and mutant troponin complexes existed as single complexes with correct stoichiometry, and there was no evidence of protein degradation (Fig. 1).
TABLE 1.
Effects of modified cTnC and cTnI mutants on reconstituted actomyosin ATPase activity
| Troponin complexes | ATPase activity |
Ca2+ sensitivity | |
|---|---|---|---|
| EGTA | Ca2+ | ||
| cTnC(wt)-cTnI(wt)-cTnT(wt) | 0.012 | 0.081 | 0.852 |
| cTnC(wt)-cTnI(S151C)AEDANS-cTnT(wt) | 0.013 | 0.079 | 0.835 |
| cTnC(wt)-cTnI(S167C)AEDANS-cTnT(wt) | 0.013 | 0.082 | 0.841 |
| cTnC(S89C)DDPM-cTnI(wt)-cTnT(wt) | 0.012 | 0.079 | 0.848 |
| cTnC(S89C)DABM-cTnI(wt)-cTnT(wt) | 0.014 | 0.082 | 0.829 |
| cTnC(L13C/N51C)AEDANS-DDPM-cTnI(wt)-cTnT(wt) | 0.015 | 0.080 | 0.813 |
Ca2+-de pend ent acto-S1 ATPase activity was measured at 30 °C in 60 mmKCl, 5.6 mmMgCl2, 2 mmATP, 30 mmimidazole (pH 7.0), 1 mmDTT, and either 500 μmCaCl2for the Ca2+state or 1 mmEGTA for the EGTA state. The protein concentrations used were 4.2 μmF-actin, 0.6 μmTm, 0.6 μmTn, and 0.5 μmS1. The amounts of inorganic phosphate released were determined colorimetrically and expressed in micromole of Pi(32). Ca2+sensitivity was defined as {1 − (ActivityEGTA/ActivityCa)} (32). cTnC(wt), cTnI(wt), and cTnT(wt), are wild-type cTnC, cTnI, and cTnT, respectively.
FIGURE 1.
Electrophoresis analysis of reconstituted cardiac troponin. Panel A, native PAGE gels (8% resolving and 4% stacking). Lanes 2–7, troponin reconstituted from wild-type cTnC, wild-type cTnI, wild-type cTnT, fluorophore-modified cTnC(L13C/N51C), cTnC(S89C), or AEDANS modified single cysteine cTnI mutants. Lanes 1, HMW protein standards (Amersham Biosciences); 2, wild-type troponin; 3, cTnC(L13C/N51C); 4, cTnC(S89C); 5, cTnI(S151C); 6, cTnI(S167C), and 7, cTnI(L129W/S151C). These gels showed that all six reconstituted samples ran as a single component with a mass of ∼76 kDa. Panel B, SDS-PAGE gels (18% resolving and 4% stacking). Lanes 1, wild-type cTnC; 2, wild-type cTnI; 3, wild-type cTnT; 4, wild-type troponin, and lanes 5–9, reconstituted troponin complex containing fluorophore-modified cTnC(131C/51C), cTnC(S89C), cTnI(S151C), cTnI(S167C), and cTnI(L129W/S151C), respectively. The six reconstituted troponin samples were each resolved into three bands. Densitometry analysis of the bands was done with Bio-Rad Quantity One Software, and the results showed that the three resolved bands corresponded to cTnC, cTnI, and cTnT with a molar ratio of 1:1:1. No significant degradation products were found on the gels.
Fluorescence Measurements
Steady-state measurements were carried out at 10 ± 0.1 °C on an ISS PCI photon-counting spectrofluorometer equipped with a microtitrator (3). FRET was used in titration experiments to monitor Ca2+-induced changes in each distance. The procedures previously described were used to convert titration data to FRET efficiency (25). In a typical titration experiment, the fluorescence intensity of the donor (AEDANS) excited at 343 nm was monitored at 480 nm in a Ca2+-EGTA-nitrilotriacetic acid buffer containing 50 mm Mops, pH 7.0, 1 mm DTT, 2 mm EGTA, 5 mm nitrilotriacetic acid, 5 mm MgCl2, and 0.15 m KCl. The free [Ca2+] was calculated using the pCa Calculator program provided by Dweck et al. (33).
Stopped-flow Measurements
The kinetic measurements were carried out at 10.0 °C in a KinTek F2004 spectrometer with a 1.5-ms dead time. In the Ca2+ dissociation experiments monitored by FRET, a protein sample saturated with Ca2+ in a buffer of 50 mm Mops, pH 7.0, containing 1 mm DTT, 5 mm MgCl2, 0.15 m KCl, and 0.16 mm Ca2+ (pCa 3.8) was mixed with an equal volume of the same buffer in which Ca2+ was replaced with 2 mm BAPTA. After mixing, [protein] = 2 μm and [BAPTA] = 1000 μm. The kinetic tracings of donor AEDANS fluorescence intensity (FD(t)) were first determined from a donor-only sample, followed by determination of the kinetic tracing (FDA(t)) for the corresponding donor-acceptor sample. Eight to 10 kinetic tracings were collected for each set of donor only and donor-acceptor samples, and the averages of each set of samples were used to calculate the time-dependent FRET efficiency, E(t).
The resultant FRET efficiency kinetic decays were fitted to a sum of exponentials by a nonlinear least squares method (34).
RESULTS
Ca2+-dependent Conformational Transitions in Cardiac Thin Filament
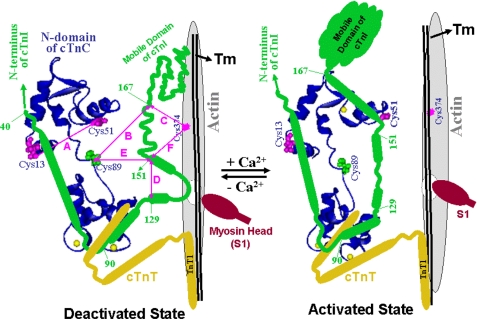
Several Ca2+-induced protein conformational changes and protein-protein interactions within the cardiac thin filament were investigated by reconstituting the constituent proteins and subjecting them to FRET measurements. These Ca2+-dependent changes include conformation of the cTnC N-domain and the inhibitory region of cTnI, changes in interaction between cTnC and cTnI, and interaction between cTnI and actin. To facilitate discussion of our results, a schematic (Fig. 2) showing proximity relationships between cTnC (blue ribbon), cTnI (green), and actin filament (gray) in cardiac thin filament in the deactivated and activated states will be referred. Also depicted in Fig. 2 are fragments of cTnT (yellow) and the residues that were modified with FRET donors or acceptors for our FRET studies. The conformational change in the cTnC N-domain (A) was monitored by measuring the FRET between AEDANS (donor) attached at one cysteine and DDPM (acceptor) attached to the other cysteine of the cTnC(L13C/N51C) mutant. The cTnC-cTnI interactions (B and E) were monitored by two FRET measurements, one from AEDANS attached to the cysteine of cTnI(S151C), to DDPM attached to the cysteine of cTnC(S89C), and the other from AEDANS attached to the cysteine 167 of cTnI to the same DABM-labeled cTnC(S89C). The cTnI-actin interactions (C and F) were monitored by measuring FRET from AEDANS attached to residues Cys-151 or Cys-167 of cTnI to DABM attached to Cys-374 of actin. Residue 151 of cTnI is located at the junction of the inhibitory region and the regulatory region of cTnI and residue 167 is located at the junction of the regulatory region and the second actin binding or the mobile domain of cTnI. The measurements of FRET distances from cTnI to cTnC or from cTnI to actin allowed us to examine the dynamic and kinetic roles of different regions of cTnI upon removal of Ca2+ from cTnC. The Ca2+-induced structural transition of the inhibitory region of cTnI (D) within the reconstituted thin filament was previously studied using FRET-sensitized emission of the acceptor (AEDANS) attached to the Cys-151 of mutant cTnI(L129W/S151C) (23). For comparison we also re-examined this transition at different Ca2+ states.
FIGURE 2.
A model for the proximity relationship of troponin, tropomyosin (Tm), and actin to illustrate Ca2+-induced changes in the tertiary structure of cTnC (blue) and the secondary structure of cTnI (green). For simplicity, cTnT is shown as a fragment. In the deactivated state, the N-domain of cTnC is unoccupied by Ca2+. In the activated state, the N-domain of cTnC is saturated by Ca2+ at its single binding site. The disposition of myosin head (S1) is arbitrary. Residues in cTnC, cTnI, and actin that are involved in FRET measurements are shown. The six distances (A–F) monitored in this study are indicated to show changes in secondary structures of cTnC and cTnI.
The steady-state FRET measurements of the reconstituted thin filaments containing cTnC(L13C/N51C)AEDANS-DDPM (panel A), cTnI(S167C)AEDANS plus cTnC(S89C)DDPM (panel B), and cTnI(S167C)AEDANS plus actin(Cys-374)DABM (panel C) are depicted in Fig. 3. In the sample containing cTnC(L13C/N51C)AEDANS-DDPM, the fluorescence intensity of AEDANS (donor) in the absence of Ca2+ at 480 nm was quenched by more than 50% when compared with that of AEDANS in the donor only sample (data not shown). This suggested that residues 13 and 51 were in close proximity to each other. But, upon Ca2+ binding to the regulatory site of cTnC, the quenched donor fluorescence was significantly recovered (compare dot to circle of Fig. 3A), suggesting a decrease in FRET, thereby indicating an increase in distance between the two residues. When strongly bound S1 was present, similar spectral change (solid square to hollow square of Fig. 3A) was observed upon Ca2+ binding to the regulatory site of cTnC. The results of these steady-state measurements were consistent with the quantitative analysis of the distance changes between these two residues obtained from a sample reconstituted with TnC from chicken slow skeletal muscle (24).
FIGURE 3.
Donor (AEDANS) fluorescence spectra of FRET in the cardiac thin filament reconstituted with cTnC(L13C/N51C)AEDANS-DDPM (A), cTnI(S167C)AEDANS plus cTnC(S89C)DABM (B), and cTnI(S167C)AEDANS with actin labeled with DABM at cysteine residue 374 (C). Dots, thin filament devoid of Ca2+; circles, thin filament in the presence of Ca2+; solid square, thin filament devoid of Ca2+ in the presence of strongly bound S1; and hollow square, thin filament plus Ca2+ in the presence of strongly bound S1. Excitation wavelength was 343 nm, and the emission was monitored at 485 nm. Samples were in 50 mm Mops, pH 7.0, 1 mm DTT, 1 mm EGTA, 5 mm MgCl2, 0.15 m KCl. When Ca2+ was present, it was at 2 mm CaCl2.
The Ca2+-induced changes in the interaction between cTnC and cTnI in the reconstituted thin filament were monitored by FRET measurements of two distances between the two proteins. The first distance was from residue 151 of cTnI labeled with AEDANS as the donor to residue 89 of cTnC labeled with DDPM as acceptor. The other was from residue 167 of cTnI labeled with AEDANS to the same residue 89 of cTnC labeled with DABM. Because the distance between residue 167 of cTnI and residue 89 of cTnC was longer than that between residue 151 of cTnI to the same residue of cTnC, different donor-acceptor pairs were used to monitor these two distances. The Förster distance (R0), which determines sensitivity of FRET between a specific donor and acceptor pair to distance changes, was about 28 Å for the AEDANS-DDPM pair and 39 Å for the AEDANS-DABM pair. The steady-state donor fluorescence intensity changes of cTnI(S167C)AEDANS in the presence of cTnC(S89C)DABM at different biochemical states is shown in Fig. 3B. In the absence of the acceptor, change in the donor fluorescence was negligible in response to Ca2+ binding (data not shown), but the presence of an acceptor decreased the donor fluorescence by 16% in the absence of Ca2+ (data not shown). Upon Ca2+ binding to the regulatory site of cTnC, the intensity at 480 nm further decreased by 30% (dot to circle of Fig. 3B) suggesting an increase in FRET and a decrease in the intersite distance. When strongly bound S1 was present, the observed FRET increased in both the apo state (solid square curve) and the Ca2+-saturated state (hollow square curve) compared with that observed in thin filament samples. This result suggests that strongly bound S1 conferred a structural effect on the conformation of the interface between cTnC and cTnI. Similar Ca2+ and strongly bound S1-induced changes were observed to the FRET between cTnI(S151C)AEDANS and cTnC(S89C)DDPM (data not shown), which is consistent with our previous quantitative analysis of the same system (8).
The Ca2+-induced interaction between cTnI and actin in the reconstituted thin filament was examined by measuring the FRET from cTnI(S151C)AEDANS and cTnI(S167C)AEDANS to DABM attached to Cys-374 of actin. Fig. 3C shows the fluorescence changes due to the FRET between cTnI(S167C)AEDANS and actin(Cys-374)DABM at different biochemical states. In the absence of Ca2+, the donor fluorescence intensity of cTnI(S167C)AEDANS in the reconstituted thin filament was quenched by about 45% when the acceptor (DABM) was attached to actin at cysteine 374 (data not shown). Upon Ca2+ binding to the regulatory site of cTnC, the quenched donor fluorescence recovered by about 30% (dot to circle in Fig. 3C), indicating the departure of cTnI from actin. In the presence of strongly bound S1, a similar extent of change in FRET was observed. However, FRET efficiency at both the apo state (solid square curve) and the Ca2+-saturated state (hollow square curve) was less than that observed in the thin filament sample. This was because feedback effects of the strongly bound S1 pushed cTnI further away from actin in both Mg2+ and Ca2+ states. The FRET from cTnI(S151C)AEDANS to actin(Cys-374)DABM showed similar changes upon Ca2+ binding to cTnC and in the presence of strongly bound S1 (data not shown).
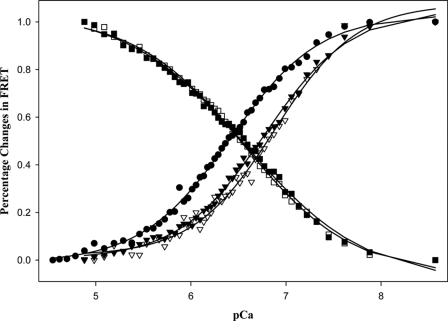
The normalized changes in the FRET between the five donor-acceptor pairs versus [Ca2+] in the absence of strongly bound S1 are depicted in Fig. 4. The Ca2+ sensitivity in each of these conformational transitions in the thin filament was measured using FRET-sensed Ca2+ titration. As Ca2+ levels increased, the FRET between the donor and acceptor attached to residues 13 and 51 of cTnC decreased. In a similar manner, FRET between the donor attached to cTnI and acceptor attached to actin in the reconstituted thin filament decreased in response to increasing free Ca2+ concentration. In contrast, FRET between the donor attached to cTnI and the acceptor attached to cTnC increased because Ca2+ binding induced cTnI to move away from actin and move closer to cTnC within the thin filament. These titration curves were fitted to the Hill equation, and the recovered pCa50 and the Hill coefficients are given in Table 2. From their sensitivity to Ca2+, these structural transitions were divided into two groups. One group, with pCa50 of 6.21–6.38 and the Hill coefficients of 1.11–1.23, included a conformational change of the N-domain of cTnC, structural transitions measured by FRET between cTnC(S151C)AEDANS and cTnC(S89C)DDPM, and FRET between cTnI(S167C)AEDANS and cTnC(S89C)DABM. The other group, with pCa50 of 6.58–6.68 and Hill coefficients of 1.29–1.34, involved structural transitions monitored by FRET between cTnI(S151C)AEDANS and actin(Cys-374)DABM and FRET between cTnI(S167C)AEDANS and actin(Cys-374)DABM. These results suggest that the structural transitions associated with different thin filament proteins may have different sensitivity to Ca2+ activation, and the presence of strongly bound S1 significantly increased sensitivity of each structural transition to Ca2+ binding due to feedback modulation of strongly bound S1 on the thin filament regulation.
FIGURE 4.
FRET-based Ca2+ titration of the changes of FRET in the reconstituted thin filaments containing different donor- and acceptor-labeled proteins. ●, from thin filament containing cTnC(L13C/N51C)AEDANS-DDPM; ■, from thin filament containing cTnI(S151C)AEDANS plus cTnC(S89C)DDPM; ▾, from thin filament containing cTnI(S151C)AEDANS with actin labeled with DABM at cysteine 374; □, from thin filament containing cTnI(S167C)AEDANS plus cTnC(S89C)DABM; and ▿, from thin filament containing cTnI(S151C)AEDANS with actin labeled with DABM at cysteine 374. The curves were fitted with the Hill equation to obtain values of pCa50 and the Hill coefficient. These parameters are listed in Table 2.
TABLE 2.
Ca2+dissociation-induced FRET-sensed kinetic and equilibrium parameters
| FRET monitored transition | Thin filament |
Thin filament + S1-ADP |
||||
|---|---|---|---|---|---|---|
| Transition ratesa | p Ca50 | Hill coefficient | Transition ratesa | p Ca50 | Hill coefficient | |
| s−1 | s−1 | |||||
| (A) cTnC(L13C/N51C)AEDANS-DDPM | 147.1 ± 13 (0.70), 24.3 ± 3 (0.30) | 6.21 ± 0.06 | 1.23 ± 0.11 | 98.7 ± 5.2 (0.62), 15.3 ± 2 (0.38) | 6.58 ± 0.07 | 1.07 ± 0.06 |
| (B) cTnC(S89C)DABM-cTnI(S167C)AEDANS | 132 ± 7 (0.71), 24.9 ± 3 (0.29) | 6.36 ± 0.04 | 1.11 ± 0.0.8 | 84.7 ± 7 (0.67), 16.2 ± 2 (0.33) | 6.60 ± 0.05 | 1.04 ± 0.05 |
| (C) cTnI(S167C)AEDANS-actin(Cys-374C)DABM | 141.0 ± 12 (0.85), 25.4 ± 2 (0.15) | 6.68 ± 0.08 | 1.34 ± 0.12 | 109.1 ± 9 (0.68), 15.2 ± 2 (0.32) | 6.84 ± 0.07 | 1.06 ± 0.07 |
| (D) cTnI(L129W/S151C)AEDANS | 134.2 ± 10 (0.77), 22.2 ± 3 (0.23) | 6.32 ± 0.04 | 1.24 ± 0.0.9 | 92.7 ± 6 (0.64), 15.7 ± 2 (0.36) | 6.56 ± 0.04 | 1.09 ± 0.08 |
| (E) cTnC(S89C)DDPM-cTnI(S151C)AEDANS | 56.3 ± 3 (0.65), 20.9 ± 3 (0.35) | 6.38 ± 0.07 | 1.12 ± 0.08 | 37.5 ± 2 (0.60), 7.7 ± 2 (0.40) | 6.63 ± 0.08 | 1.02 ± 0.09 |
| (F) cTnI(S151C)AEDANS-actin(Cys-374)DABM | 69.0 ± 4 (0.84), 18.5 ± 2 (0.16) | 6.58 ± 0.05 | 1.29 ± 0.10 | 27.3 ± 2 (0.52), 9.3 ± 2 (0.48) | 6.81 ± 0.04 | 1.12 ± 0.07 |
a In the column of Transition rates, two sets of numbers were given. The numbers of the top row were the kinetic rates of the fast component of each distance transition, whereas the numbers of the bottom row were the kinetic rates of the slow component of each distance transition. The numbers in parentheses were the amplitudes of the corresponding transition components relative to the total distance transitions.
Kinetics of Ca2+ Dissociation-induced Conformational Transitions in Cardiac Thin Filament
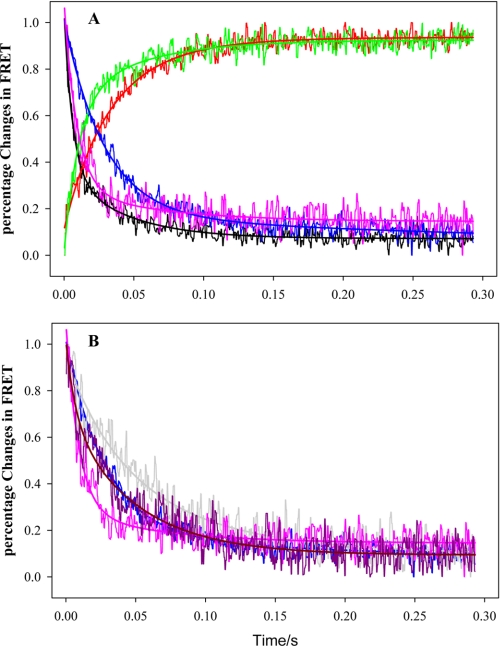
The results from equilibrium steady-state FRET measurements on the Ca2+-induced conformational change in the cTnC N-domain, cTnI-cTnC interaction, and cTnI-actin interaction provided a basis to use FRET to determine the kinetics of these structural transitions upon Ca2+ dissociating from the regulatory site of cTnC. These experiments were performed by mixing a buffer containing BAPTA, a strong Ca2+ chelating agent, with a reconstituted thin filament sample containing donor- and acceptor-labeled proteins at pCa 3.8. Under this condition, the Ca2+-specific site and the two Ca2+/Mg2+ sites were saturated with Ca2+. The stopped-flow experiments were monitored with the fluorescence intensity of the donor AEDANS in different sample preparations. Shown in Fig. 5 are FRET kinetic tracings of these structural transitions obtained from the reconstituted thin filament containing different donor and donor-acceptor-labeled proteins. All these tracings may be described with two transition phases, one fast and the other slow. Generally the fast transient accounted for more than two-thirds of the total FRET changes in each monitored structural transition, whereas the slow phase accounted for the rest of the total FRET change. The recovered parameters from these FRET tracing fittings are summarized in Table 2.
FIGURE 5.
FRET-based stopped-flow tracings of normalized FRET changes triggered by Ca2+ dissociation from cTnC in reconstituted thin filaments containing different donor- and acceptor-labeled proteins. A, the data were collected from the reconstituted thin filament samples without the presence of strongly bound S1. Black, from sample containing cTnC(L13C/N51C)AEDANS-DDPM; red, from sample containing cTnI(S151C)AEDANS plus cTnC(S89C)DDPM; green, from sample containing cTnI(S167C)AEDANS plus cTnC(S89C)DABM; blue, from sample containing cTnI(S151C)AEDANS with actin labeled with DABM at cysteine residue 374; and pink, from sample containing cTnI(S1671C)AEDANS with actin labeled with DABM at cysteine residue 374. B, comparing selected FRET kinetic tracings collected from the reconstituted thin filament samples in the absence of S1 (blue and red tracings) to the tracings obtained in the same samples in the presence of strongly bound S1 (gray and dark pink tracings). Blue and red, from reconstituted thin filament samples containing cTnI(S151C)AEDANS with actin labeled with DABM at cysteine residue 374; and gray and dark pink, from reconstituted thin filament samples containing cTnI(S167C)AEDANS with actin labeled with DABM at cysteine residue 374. All experimental data were fitted with a two-exponential function (solid smooth lines) and recovered rate constants and amplitudes are given in Table 2.
The results from Fig. 5 and Table 2 suggest that the five Ca2+ dissociation-induced structural transitions of the thin filament examined by this study may be divided into two groups based on their structural kinetics. One group comprises the structural transitions involving the cTnC N-domain closing, the structural transitions monitored by FRET between cTnI(S167C)AEDANS-cTnC(S89C)DABM, and FRET between cTnI(S167C)AEDANS-DABM attached to cysteine 374 of actin. In the absence of strongly bound S1, the Ca2+ dissociation induced cTnC N-domain closing in the thin filament in two phases. The fast phase had an observed rate constant of 147 s−1 accounting for 70% of the total FRET change and the slow phase had a rate constant of 24 s−1 accounting for the rest of the total FRET changes. Similar kinetic rates were also observed for the other two structural transitions in this group (Table 2). The second group involves structural transitions monitored by FRET between cTnI(S151C)AEDANS-cTnC(S89C)DDPM and FRET between cTnI(S151C)AEDANS-DABM attached to cysteine 374 of actin. These two structural changes were two-phase transitions with fast kinetic rates of 56–69 s−1 and slow rates of 21–24 s−1. The fast components of the structural transitions from this group were much slower than those observed in the first group, suggesting that different structural regions of thin filament may have different kinetic roles in Ca2+ regulation of thin filament. For comparison, Ca2+ dissociation-induced kinetics of structural transition between residues Trp-129 and Cys-151 of cTnI(L129W/S151C) labeled with AEDANS within the reconstituted thin filament was also measured by monitoring FRET-sensitized acceptor (AEDANS) emission and using BAPTA as Ca2+ chelator (Table 2). The results were consistent with our previous report in which EGTA was used as Ca2+ chelator to rapidly remove Ca2+ from thin filament (23). The kinetic rates recovered from this measurement were close to the rates of the structural transitions in the first group.
Because kinetics of these thin filament structural transitions were acquired by monitoring FRET distance changes between two residues of interest, modification of one residue with the FRET donor might have a different effect on the observed structural kinetics than the effect that the FRET acceptor might cause. To exclude this possibility, we switched the labeling positions of the donor and acceptor to monitor distance changes from cTnC(S89C) to cTnI(S151C) and cTnI(S167C), respectively. The FRET stopped-flow measurements (data not shown) suggested no significant differences in the kinetics obtained from these measurements compared with the results in Table 2.
The presence of strongly bound S1 significantly reduced the kinetic rates for each structural transition. Fig. 5B shows that the kinetic effects of strongly bound S1 on Ca2+ dissociation induced structural transition monitored by FRET between cTnI(S151C)AEDANS-actin(Cys-374)DABM and FRET between cTnI(S167C)AEDANS-actin(Cys-374)DABM. In the presence of S1, kinetics of structural transition between cTnI(S167C)-actin(Cys-374) reduced from 141 s−1 and 25 s−1 to 109 s−1 and 15 s−1, respectively, whereas the kinetics of the structural transition between cTnI(S151C)-actin(Cys-374) changed from 69 s−1 and 21 s−1 to 27 s−1 and 9 s−1, respectively. The effects of the strongly bound S1 on other structural transitions are summarized in Table 2.
DISCUSSION
The current dogma suggests that cardiac muscle contracts upon Ca2+ binding to cTnC, which regulates an “on” process in the thin filament, which leads myosin heads in the thick filament to interact with actin in the thin filament to generate force. The reverse process, cardiac relaxation or cardiac force decay, is regulated by an “off” process in the thin filament triggered by rapid dissociation of Ca2+ from cTnC. However, this regulatory role of Ca2+-induced thin filament deactivation in cardiac relaxation is not consistent with recent studies from the single cardiac myofibril preparation (13, 18). These experiments showed that the kinetics of Ca2+ dissociation from cTnC and Ca2+ dissociation-induced conformational change of cTnC were too fast to regulate cross-bridge detachment in cardiac muscle. This raises the question of how the thin filament regulation through Ca2+ dissociation from cTnC controls the cross-bridge detachment during muscle relaxation. Because muscle relaxation is regulated by thin filament through Ca2+ dissociation from cTnC and subsequent structural transitions of thin filament proteins, it is likely that structural transitions involving different proteins may have different kinetics and play different roles in thin filament regulation. In this study we used FRET Ca2+ titration and FRET stopped-flow measurements to examine the dynamics and the kinetics of different structural transition of thin filament proteins induced by Ca2+ dissociation. In total, four structural transitions in the thin filament were examined by monitoring changes in six FRET distances (Fig. 2): (i) the Ca2+ dissociation-induced closing of the N-domain of cTnC was monitored by measuring the FRET distance change between residues 13 and 51 of the cTnC(L13C/N51C) mutant, (ii) structural change of the inhibitory region of cTnI was monitored by measuring FRET distance between residues 129 and 151 of the cTnI(L129W/S151C) mutant, (iii) the cTnC-cTnI interaction was monitored by measuring FRET distances from residues 151 and 167 of cTnI to residue 89 of cTnC, and (iv) the cTnI-actin interaction was monitored by measuring FRET distances from residues 151 and 167 of cTnI to cysteine residue 374 of actin.
Equilibrium FRET Ca2+ titration measurements indicated that different structural regions of thin filament have different sensitivity to Ca2+ binding to the regulatory site of cTnC. Based on measured Ca2+ sensitivity (Table 2), we divided these structural transitions into two groups. One group includes the FRET-monitored structural transitions from (i) to (iii), which occurred at the interface between cTnC and cTnI, and the other group involved the FRET-monitored structural transition (iv), which occurred at the interface between cTnI and actin of the thin filament. The structural transitions at the interface between cTnI and actin were more sensitive to Ca2+ than the structural transitions at the interface between cTnC and cTnI. Because the structural transitions (iv) at the interface between cTnI and actin were investigated by FRET distance measurements, which involved probes attached to actin, our results may indicate contributions of actin to Ca2+ sensitivity of thin filament regulation. This contribution may result from a conformational change of actin filament in response to Ca2+-activated thin filament regulation. However, there is no experimental evidence from this study to support this mechanism. All structural transitions in the two groups were sensitive to the modulation of strong cross-bridge formation. The presence of strongly bound S1 significantly increased the Ca2+ sensitivity of each structural transition. These results were consistent with previous findings (8, 23, 24) and suggested that these Ca2+-induced structural changes of the thin filament conveyed the feedback mechanism of strong cross-bridge on the regulation of the thin filament.
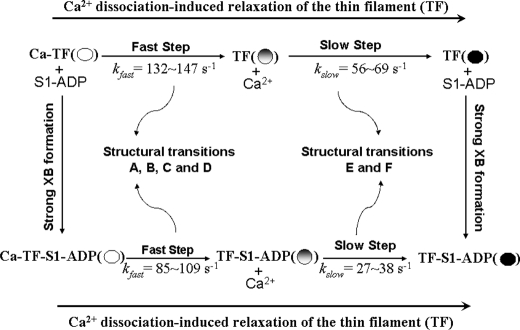
Our FRET kinetic analysis showed that the structural kinetics of thin filament induced by Ca2+ dissociation varied with different structural transitions, and they may also be divided into two groups. Each group had similar fast components of the structural kinetic transitions (Table 2). The first group of structural transitions comprises the N-domain closing of cTnC (Table 2, A), the conformation change of the inhibitory region of cTnI (Table 2, D), and the structural transitions monitored by FRET distance between cTnI(S167C)AEDANS and cTnC(S89C)DABM (Table 2, B) and the distance between cTnI(S167C)AEDANS and actin(Cys-374)DABM (Table 2, C). The second group comprises the structural transitions monitored by FRET distance between cTnI(S151C)AEDANS and cTnC(S89C)DABM (Table 2, E) and the distance between cTnI(S151C)AEDANS and actin(Cys-374)DABM (Table 2, F). The structural transitions in the first group had fast kinetics (132–147 s−1), whereas the structural transitions in the second group had slow kinetics (56–69 s−1). The fact that each group has similar kinetics indicates that these structural transitions in the same group are kinetically coupled to each other in response to Ca2+ dissociation. Based on these results, we propose a mechanistic scheme of Ca2+-induced thin filament deactivation (Scheme 1). In this scheme, the open circle represents the Ca2+-activated thin filament, the partially filled circle is partially activated thin filament, and the filled circle represents the deactivated thin filaments. The proposed scheme suggests that the mechanism of thin filament regulating muscle relaxation may involve a two-step structural transition of thin filament at the interface between troponin and actin. The first step is a fast process and involves rapid Ca2+-induced interactions between the C-domain (the mobile domain and the regulatory region) of cTnI and actin, which are structurally and kinetically coupled with the closing of the N-domain of cTnC. Immediately following this fast step, a much slower second step involves the dissociation of the inhibitory region of cTnI from cTnC and binding to actin.
SCHEME 1.
Based on the “drag and release” model (8, 35, 36), the inhibitory region of cTnI located at the cTnC-cTnI interface provides the main Ca2+-induced switching mechanism between relaxation and activation. The interaction between the regulatory region of cTnI and the N-domain of cTnC plays a key role in eliciting the Ca2+-induced opening/closing of the N-domain of cTnC (3, 4), and this leads to conformational change of the inhibitory region of cTnI (5, 6). The interaction between the regulatory region of cTnI and the N-domain of cTnC also regulates the Ca2+-induced interaction between the second actin binding domain or mobile domain (residues 165–210) of cTnI and actin (37). An NMR structural study suggested that the mobile domain of skeletal troponin I was unstructured and flexible when the thin filament was activated by Ca2+ binding to troponin C (38). This result promoted a hypothesis that the flexible structure allowed the mobile domain to transiently contact with actin without binding; therefore, the mobile domain can rapidly bind to actin through mechanisms of flycasting upon Ca2+ dissociated from the N-domain of cTnC (39). It is likely that the observed fast transition step of the thin filament described in our proposed scheme is related to the Ca2+ dissociation-induced dynamic interaction between the mobile domain of cTnI and actin, which facilitates the dissociation of the regulatory region of cTnI from the N-domain of cTnC, and the close of the cTnC N-domain. These structural transitions are likely kinetically coupled to each other with rapid rates. More studies are underway to verify the rapid interaction between the mobile domain of cTnI and actin. It is also possible that this fast step leads to movement of tropomyosin from the “open” position to the “closed” position on actin surface based on the “three states” model of thin filament activation (40). Immediately following the dissociation of the regulatory region of cTnI from the N-domain of cTnC, the inhibitory region of cTnI experiences a movement from interacting with cTnC to the interacting with actin, which may shift the tropomyosin from the closed state to the “blocked” state to block cross-bridge binding sites on actin. The observed slow step of the structural transitions in our proposed scheme may reflect the transversal movement of the inhibitory region of cTnI. It is possible that the slow step may also involve the movement of the regulatory region because residue 151 is located at the junction of the inhibitory and regulatory regions. Further experiments are needed to address this possibility.
Our FRET kinetic measurements suggest that in the absence of strongly bound S1, all Ca2+ dissociation-induced structural changes at the interface between troponin and actin can be best described by a two-phase transition. One, as a fast transition, accounted for about two-thirds of the total FRET change, and the other, as slow transition, accounted for the rest of the total FRET change. The presence of strong cross-bridge significantly decreased both the fast and slow kinetics of each structural transition. The amplitude of the slow phase of each structural transition increased in response to the presence of the strong cross-bridge (Table 2). These kinetic changes are likely caused by feedback modulation of strong cross-bridge and are related to cross-bridge kinetics. However, how the cross-bridge kinetics are regulated by two-phase structural transition of the thin filament remains unknown. The observed two-phase conformational changes of thin filament proteins in our FRET kinetic measurements may reflect a true nature of each Ca2+-induced structural transition of thin filament, because a similar two-phase transition associated with the Ca2+-induced conformational change of cTnC was also observed in a single myofibril study (18). It could also be the consequence of lack of the protein lattice structure found in the cardiac sarcomere in our reconstituted sample preparations. The protein lattice environment provides realistic geometric and mechanical constraints on protein-protein interactions. These constraints may have a tremendous effect on the structural dynamics of interest. Lack of such constraints may be the reason for the observed multiple phase and fast structural transitions upon Ca2+ dissociation. To answer this issue requires further investigation with muscle fiber or myofibril preparation.
In summary, the current dilemma in understanding thin filament regulation is that the kinetics of Ca2+ dissociation from cTnC and subsequent conformational change of cTnC are too fast to regulate cross-bridge detachment in cardiac muscle. This study provides evidence that different structural transitions of thin filament proteins may have different kinetics and play different roles in regulating cross-bridge kinetics. Our FRET equilibrium and FRET kinetic results suggest that the mechanism of thin filament regulating muscle relaxation involves a two-step structural transition of thin filament at the interface between troponin and actin. The fast step involves Ca2+-induced interaction between the mobile domain of cTnI and actin, the dissociation of the regulatory region of cTnI from the N-domain of cTnC, and the closing of the N-domain of cTnC. The second slow step involves the dissociation of the inhibitory region of cTnI from cTnC and binding to actin. These novel findings provide new insights into the mechanism of thin filament regulating cross-bridge kinetics and force development in cardiac muscle.
Acknowledgment
We greatly appreciate Dr. Kenneth Campbell for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant HL80186 (to W.-J. D.).
- cTnC
- cardiac troponin C
- cTnI
- cardiac troponin I
- cTnT
- cardiac troponin T
- FRET
- Förster resonance energy transfer
- DTT
- dithiothreitol
- Mops
- 3-(N-mopholino)propanesulfonic acid
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- IAEDANS
- 5-(iodoacetamidoethyl)aminonaphthelene-1-sulfonic acid
- DDPM
- N- (4-dimethylamino-3,5-dinitrophenyl)maleimide
- DABM
- 4-dimethylaminophenylazophenyl-4′-maleimide
- S1
- subfragment 1.
REFERENCES
- 1.Ebashi S., Endo M., Otsuki I. ( 1969) Q. Rev. Biophys. 2, 351– 384 [DOI] [PubMed] [Google Scholar]
- 2.Farah C. S., Reinach F. C. ( 1995) FASEB J. 9, 755– 767 [DOI] [PubMed] [Google Scholar]
- 3.Dong W. J., Xing J., Villain M., Hellinger M., Robinson J. M., Chandra M., Solaro R. J., Umeda P. K., Cheung H. C. ( 1999) J. Biol. Chem. 274, 31382– 31390 [DOI] [PubMed] [Google Scholar]
- 4.Li M. X., Spyracopoulos L., Sykes B. D. ( 1999) Biochemistry 38, 8289– 8298 [DOI] [PubMed] [Google Scholar]
- 5.Dong W. J., Xing J., Robinson J. M., Cheung H. C. ( 2001) J. Mol. Biol. 314, 51– 61 [DOI] [PubMed] [Google Scholar]
- 6.Dong W. J., Robinson J. M., Stagg S., Xing J., Cheung H. C. ( 2003) J. Biol. Chem. 278, 8686– 8692 [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T., Kobayashi M., Gryczynski Z., Lakowicz J. R., Collins J. H. ( 2000) Biochemistry 39, 86– 91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson J. M., Dong W. J., Xing J., Cheung H. C. ( 2004) J. Mol. Biol. 340, 295– 305 [DOI] [PubMed] [Google Scholar]
- 9.Lehman W., Craig R., Vibert P. ( 1994) Nature 368, 65– 67 [DOI] [PubMed] [Google Scholar]
- 10.Güth K., Potter J. D. ( 1987) J. Biol. Chem. 262, 13627– 13635 [PubMed] [Google Scholar]
- 11.Hannon J. D., Martyn D. A., Gordon A. M. ( 1992) Circ. Res. 71, 984– 991 [DOI] [PubMed] [Google Scholar]
- 12.Gordon A. M., Ridgway E. B. ( 1987) J. Gen. Physiol. 90, 321– 340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stehle R., Iorga B., Pfitzer G. ( 2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1125– 1128 [DOI] [PubMed] [Google Scholar]
- 14.Palmer S., Kentish J. C. ( 1998) Circ. Res. 83, 179– 186 [DOI] [PubMed] [Google Scholar]
- 15.Stehle R., Krüger M., Pfitzer G. ( 2002) Biophys. J. 83, 2152– 2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stehle R., Krüger M., Scherer P., Brixius K., Schwinger R. H., Pfitzer G. ( 2002) Basic Res. Cardiol. 97, Suppl. 1, I127– 135 [DOI] [PubMed] [Google Scholar]
- 17.de Tombe P. P., Belus A., Piroddi N., Scellini B., Walker J. S., Martin A. F., Tesi C., Poggesi C. ( 2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1129– 1136 [DOI] [PubMed] [Google Scholar]
- 18.Solzin J., Iorga B., Sierakowski E., Gomez Alcazar D. P., Ruess D. F., Kubacki T., Zittrich S., Blaudeck N., Pfitzer G., Stehle R. ( 2007) Biophys. J. 93, 3917– 3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regnier M., Martin H., Barsotti R. J., Rivera A. J., Martyn D. A., Clemmens E. ( 2004) Biophys. J. 87, 1815– 1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes A. V., Potter J. D. ( 2004) Ann. N.Y. Acad. Sci. 1015, 214– 224 [DOI] [PubMed] [Google Scholar]
- 21.Gordon A. M., Homsher E., Regnier M. ( 2000) Physiol. Rev. 80, 853– 924 [DOI] [PubMed] [Google Scholar]
- 22.Cheung H. C., Wang C. K., Gryczynski I., Wiczk W., Laczko G., Johnson M. L., Lakowicz J. R. ( 1991) Biochemistry 30, 5238– 5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong W. J., An J., Xing J., Cheung H. C. ( 2006) Arch. Biochem. Biophys. 456, 135– 142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong W. J., Jayasundar J. J., An J., Xing J., Cheung H. C. ( 2007) Biochemistry 46, 9752– 9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong W. J., Robinson J. M., Xing J., Cheung H. C. ( 2003) J. Biol. Chem. 278, 42394– 42402 [DOI] [PubMed] [Google Scholar]
- 26.Dong W. J., Xing J., Ouyang Y., An J., Cheung H. C. ( 2008) J. Biol. Chem. 283, 3424– 3432 [DOI] [PubMed] [Google Scholar]
- 27.Xing J., Chinnaraj M., Zhang Z., Cheung H. C., Dong W. J. ( 2008) Biochemistry 47, 13383– 13393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smillie L. B. ( 1982) Methods Enzymol. 85, 234– 241 [DOI] [PubMed] [Google Scholar]
- 29.Pardee J. D., Spudich J. A. ( 1982) Methods Enzymol. 85, 164– 181 [DOI] [PubMed] [Google Scholar]
- 30.Xing J., Cheung H. C. ( 1994) Arch. Biochem. Biophys. 313, 229– 234 [DOI] [PubMed] [Google Scholar]
- 31.Tao T., Gong B. J., Leavis P. C. ( 1990) Science 247, 1339– 1341 [DOI] [PubMed] [Google Scholar]
- 32.Wang C. K., Cheung H. C. ( 1986) J. Mol. Biol. 191, 509– 521 [DOI] [PubMed] [Google Scholar]
- 33.Dweck D., Reyes-Alfonso A., Jr., Potter J. D. ( 2005) Anal. Biochem. 347, 303– 315 [DOI] [PubMed] [Google Scholar]
- 34.Bevington P. R., Robinson D. K. ( 1992) Data Reduction and Error Analysis for the Physical Sciences, 2 Ed., McGraw Hill, Boston [Google Scholar]
- 35.Kobayashi T., Solaro R. J. ( 2005) Annu. Rev. Physiol. 67, 39– 67 [DOI] [PubMed] [Google Scholar]
- 36.Li M. X., Wang X., Sykes B. D. ( 2004) J. Muscle Res. Cell Motil. 25, 559– 579 [DOI] [PubMed] [Google Scholar]
- 37.Tripet B., Van Eyk J. E., Hodges R. S. ( 1997) J. Mol. Biol. 271, 728– 750 [DOI] [PubMed] [Google Scholar]
- 38.Murakami K., Yumoto F., Ohki S. Y., Yasunaga T., Tanokura M., Wakabayashi T. ( 2005) J. Mol. Biol. 352, 178– 201 [DOI] [PubMed] [Google Scholar]
- 39.Hoffman R. M., Blumenschein T. M., Sykes B. D. ( 2006) J. Mol. Biol. 361, 625– 633 [DOI] [PubMed] [Google Scholar]
- 40.McKillop D. F., Geeves M. A. ( 1993) Biophys. J. 65, 693– 701 [DOI] [PMC free article] [PubMed] [Google Scholar]