Abstract
Studies have attributed several functions to the Eaf family, including tumor suppression and eye development. Given the potential association between cancer and development, we set forth to explore Eaf1 and Eaf2/U19 activity in vertebrate embryogenesis, using zebrafish. In situ hybridization revealed similar eaf1 and eaf2/u19 expression patterns. Morpholino-mediated knockdown of either eaf1 or eaf2/u19 expression produced similar morphological changes that could be reversed by ectopic expression of target or reciprocal-target mRNA. However, combination of Eaf1 and Eaf2/U19 (Eafs)-morpholinos increased the severity of defects, suggesting that Eaf1 and Eaf2/U19 only share some functional redundancy. The Eafs knockdown phenotype resembled that of embryos with defects in convergence and extension movements. Indeed, knockdown caused expression pattern changes for convergence and extension movement markers, whereas cell tracing experiments using kaeda mRNA showed a correlation between Eafs knockdown and cell migration defects. Cardiac and pancreatic differentiation markers revealed that Eafs knockdown also disrupted midline convergence of heart and pancreatic organ precursors. Noncanonical Wnt signaling plays a key role in both convergence and extension movements and midline convergence of organ precursors. We found that Eaf1 and Eaf2/U19 maintained expression levels of wnt11 and wnt5. Moreover, wnt11 or wnt5 mRNA partially rescued the convergence and extension movement defects occurring in eafs morphants. Wnt11 and Wnt5 converge on rhoA, so not surprisingly, rhoA mRNA more effectively rescued defects than either wnt11 or wnt5 mRNA alone. However, the ectopic expression of wnt11 and wnt5 did not affect eaf1 and eaf2/u19 expression. These data indicate that eaf1 and eaf2/u19 act upstream of noncanonical Wnt signaling to mediate convergence and extension movements.
EAF1 (ELL-associated factor 1) was first discovered through its ability to associate with the protein ELL (eleven-nineteen lysine-rich leukemia), a fusion partner of MLL in the t(11;19)(q23;p13.1) chromosomal translocation associated with acute myeloid leukemia (1, 2). Subsequent studies found a second binding partner for ELL, EAF2, which was independently identified as an androgen up-regulated gene in the rat prostate and named human up-regulated 19 (U19) (3, 4). ELL binds to RNA polymerase II and acts as a transcriptional elongation factor whose targeted deletion leads to embryonic lethality in mice (5, 6). Both EAF1 and EAF2, which share significant sequence homology, stimulate ELL elongation activity (7). Studies by Luo et al. (8) argued that EAF proteins are important in MLL-ELL leukemogenesis, whereas our previous studies showed that EAF2/U19 inhibits xenograft prostate tumor growth and undergoes down-regulation in prostate cancer cell lines (9). These findings link the EAF2/U19 gene with two major human cancers: prostate cancer and acute myeloid leukemia. To investigate the function EAF2/U19 in vivo, we constructed a murine knock-out model. The EAF2/U19 knock-out mice develop B-cell lymphoma, lung adenocarcinoma, hepatocellular carcinoma, and prostate intraepithelial neoplasia with high frequency (10). In addition, we demonstrated that EAF2/U19 could bind to and stabilize the classic tumor suppressor-pVHL (11). These findings further support EAF2/U19 as a potential tumor suppressor; however, the molecular mechanisms underlying this activity remain poorly defined.
Evidence suggests that oncogenes and tumor suppressors may play a role in normal vertebrate embryogenesis. Thus, the evaluation of how these proteins function during embryogenesis would not only lead to a better understanding of development but may also shed light on how these proteins contribute to tumor initiation and progression (12, 13). Several studies have shown that eaf2/u19 appears to be important during embryogenesis, particularly in eye development (14, 15), but its function in vertebrate embryogenesis remains unclear.
In vertebrates, the body plan is established during gastrulation by a set of morphogenetic processes, including epiboly, internalization, and convergence and extension movements (16). Gastrulation starts with the epiboly of the enveloping layer and deep cells and the internalization of prospective mesendodermal cells, followed by convergence and extension movements. convergence and extension movements narrow embryonic tissues mediolaterally and lengthen them anteroposteriorly (17). By convergence movements, the precursor cells of many organs migrate toward midline from bilateral tissues and assemble to form the primitive organs (18, 19). During the convergence and extension movements, multiple signaling cascades control coordinated cell behaviors. silberblick/wnt11 (slb) and pipetail/wnt5 (ppt) zebrafish mutants show abnormal convergence and extension movements and display a short tail and body axis (20, 21). Thus, the noncanonical Wnt cascade has been proposed to be essential for convergence and extension movements. In addition, rhoA has been reported to act downstream of wnt5 and wnt11 to regulate convergence and extension movements (22).
We employed the zebrafish embryo as a model system to investigate the role of eaf1 and eaf2/u19 in vertebrate development. Based on initial findings, we focused our examination on the regulation of convergence and extension movements and midline convergence of the heart and pancreas primordia by Eaf1 and Eaf2/U19 during zebrafish embryogenesis. We also examined whether eaf1 and eaf2/u19 regulate convergence and extension movements by noncanonical Wnt signaling.
EXPERIMENTAL PROCEDURES
Maintenance of Fish Stocks and Embryo Collection
Breeding wild-type zebrafish (Danio rerio) (AB) were maintained and embryos rose under standard library conditions (23). Embryos were collected and staged as described (24).
Cloning of Zebrafish eaf1 and eaf2
Zebrafish eaf1 and eaf2/u19 genes (GenBankTM accession numbers AAI53593 and CAQ15588, respectively) were identified using human orthologue sequences to search the zebrafish Ensembl data base (available on the World Wide Web). To amplify zebrafish eaf1 and eaf2/u19, we used primers based on their cDNA open reading frame sequences. The primers for eaf1 were 5′-GGTACCGATGAACGGCAGCTCGAACC-3′ and 5′-CCCGGGCGTCGATGTCGCTGTCACTGC-3′. The primers for eaf2/u19 were 5′-GGTACCGTGGATTAGAATGAATGGAACAGC-3′ and 5′-CCCGGGCGTCATCATCGCTTTCACTTCCA-3′. Total RNA was isolated from zebrafish embryos using the RNeasy minikit (Qiagen), and cDNA was synthesized by the RevertAidTM first strand cDNA synthesis kit (Fermentas). The complete coding sequences were PCR-amplified, subcloned into the pTA2 vector (Toyobo), and then sequenced.
Whole Mount in Situ Hybridization
Probes for identifying zebrafish, eaf1, eaf2/u19, ntl, hgg, lefty2, gata5, foxo5, gsc, chd, foxa3, bon, and sox17 by in situ hybridization were PCR-amplified from cDNA pools using the appropriate sets of primers (sequences provided upon requested). Probes for otx1 and dlx3b were generous gifts from Dr. T. Whitefield (Medical Research Council Centre for Developmental and Biomedical Genetics, Sheffield, UK) (25). Dr Z. Yin (Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China) kindly provided probes for myoD, papc, bmp4, amhc, vmhc, cmlc, insulin, pdx-1, wnt5, and wnt11. The procedure for whole mount in situ hybridization was performed as described previously (26).
Morpholinos and mRNA Injection and Rescue Experiments
Two nonoverlapping, translation-blocking morpholinos (MOs)3 targeting the zebrafish eaf1 gene (Eaf1-MO1, GCGGCGGGTTCGAGCTGCCGTTCAT; Eaf1-MO2, GAATTCACCAACTTCACCCAAAATG; two nonoverlapping, translation-blocking morpholinos targeting the zebrafish eaf2 gene (Eaf2-MO1, ATATGCTGTTCCATTCATTCTAATC; Eaf2-MO2, TAGCGATTTTCAACTTTCTGCTTGG), and a standard control (STD) morpholino (CCTCTTACCTCAGTTACAATTTATA) were purchased from Gene Tools, LLC (Philomath, OR). All morpholinos were resuspended in 1× Danieau medium (58 mm NaCl, 0.7 mm KCl, 0.4 mm MgSO4, 0.6 mm Ca(NO3)2, and 5.0 mm HEPES, pH 7.6) and injected into embryos at the one-cell stage.
The full-length zebrafish eaf1 cDNA was cloned into pEGFP-N1 (Clontech) to generate wild-type Eaf1 tagged with GFP at the carboxyl terminus (WT) to validate the efficiency of Eaf1-MO1. The zebrafish GFP-tagged mutated Eaf1 (MT) was generated by PCR using a forward primer with five mismatched nucleotides, 5′-GGTACCGATGAGCCGCTGGTGGAACCCGCCGCTGG-3′ (mismatched nucleotides are underlined) to test for specificity of Eaf1-MO1. The full-length zebrafish eaf2/u19 cDNA was also cloned into pEGFP-N1 (Clontech) to generate GFP-tagged wild-type Eaf2/U19 (WT) to validate efficiency of Eaf2-MO1. The zebrafish GFP-tagged mutated Eaf2/U19 (MT) was generated by PCR using a forward primer with five mismatched nucleotides, 5′-GGTACCGTCGAATCGAATGATTCGAACAGCATATTCAAAC-3′ (mismatched nucleotides are underlined) to test the specificity of Eaf2-MO1.
For ectopic expression of zebrafish eaf1 (zEaf1), eaf2/u19 (zEaf2), wnt11, and wnt5 in embryos, full-length wild-type eaf1, eaf2/u19, wnt11, and wnt5 were subcloned into the Psp64 poly(A) vector (Promega). Capped mRNAs were synthesized using the Ampticap SP6 High Yield message maker kit (Epicenter). Capped mRNA (200–500 pg) was injected into one-cell stage embryos.
For the rescue experiments, full-length wild-type cDNAs of zebrafish eaf1 (zEaf1), zebrafish eaf2/u19 (zEaf2), human EAF1 (hEaf1), human EAF2/U19 (hEaf2), zebrafish wnt5, zebrafish wnt11, and zebrafish wild-type rhoA (provided by Zhan Yin) were subcloned into the Psp64 poly(A) vector (Promega). To avoid quenching by the Eaf1-MO1 in these rescue experiments, we used a primer to introduce five mismatched nucleotides in the zebrafish eaf1 mRNA, 5′-AAGCTTATGAATGGATCTTCGAACCCGCCGCTG-3′ (mismatched nucleotides are underlined), but Eaf2-MO1 itself has seven nucleotides mismatching to zebrafish eaf2/u19 open reading frame, so it cannot quench the zebrafish eaf2/u19 mRNA. Capped mRNAs synthesized using the Ampticap SP6 High Yield message maker kit (Epicenter) mixed with corresponding morpholinos were co-injected into one-cell stage embryos. Different amounts of synthetic mRNA, varying from 10 to 200 pg, were titrated by co-injection with morpholinos to reach an optimal level that could rescue the defects of the eaf1 and eaf2/u19 morphants effectively. All of the microinjection was performed using a Harvard apparatus PLI-100.
Semiquantitative RT-PCR
Using the RNeasy minikit (Qiagen), we isolated total RNAs from embryos at the 50% epiboly stage that had been injected with eaf1 mRNA, eaf2 mRNA, GFP mRNA, Eafs-MO1(Eaf1-MO1 + Eaf2-MO1), or a standard morpholino (STD-MO). Aliquots of RNA were subjected to 1% agarose gel electrophoresis and stained by ethidium bromide to verify RNA quantity and quality. For RT-PCR detection, about 1 μg of each RNA was reverse transcribed by the reverse transcriptase Moloney murine leukemia virus (Invitrogen) at 37 °C with oligo(dT) primers. We used 18 S RNA to adjust the concentrations of these first strand cDNAs so they could be used as templates for semiquantitative PCR. PCR reactions using gene-specific primers were carried out in a Chromo 4TM detector for the PTC DNA EngineTM system (Bio-Rad) in the presence of SYBR green. All PCRs were run in triplicate and repeated at least three times. Differences were calculated according to the ΔΔCt relative quantization method using 18 S RNA as calibrators. The primers for the zebrafish wnt5 (wnt5b) gene were 5′-CTTCGCCCGGGAGTTTGTGGA-3′ and 5′-CGGCGGCGCTGTCGTATTTC-3′. The primers for zebrafish wnt11 gene were 5′-CCGTCTTCACCAATAGACCTTG-3′ and 5′-CCCAGTCTCTTCCCCTCAGT-3′. The primers for zebrafish 18 S were 5′-GAGAAACGGCTACCACATCC-3′ and 5′-CACCAGACTTGCCCTCCAA-3′.
Cell Tracing Experiments
For the cell tracing experiments, full-length wild-type kaeda was subcloned into the pCS2+ vector (27). Capped mRNA was synthesized using the Ampticap SP6 High Yield message maker kit (Epicenter). 200 pg of capped kaeda mRNA was co-injected with STD-MO or Eafs-MO1 (Eaf1-MO1 + Eaf2-MO1, 4 ng + 4 ng) into one-cell stage embryos. Injected embryos were maintained in a dark room until the shield stage. To convert green fluorescence to red, a beam of UV light, generated by a UV filter set (350–400 nm) on a Zeiss fluorescence microscope was directed for 1 s at the dorsal or lateral blastoderm margin of embryos (27). The location of red fluorescent cells was then photographed at the indicated time points using the Zeiss fluorescence microscope.
RESULTS
Expression of eaf1 and eaf2/u19 during Zebrafish Development
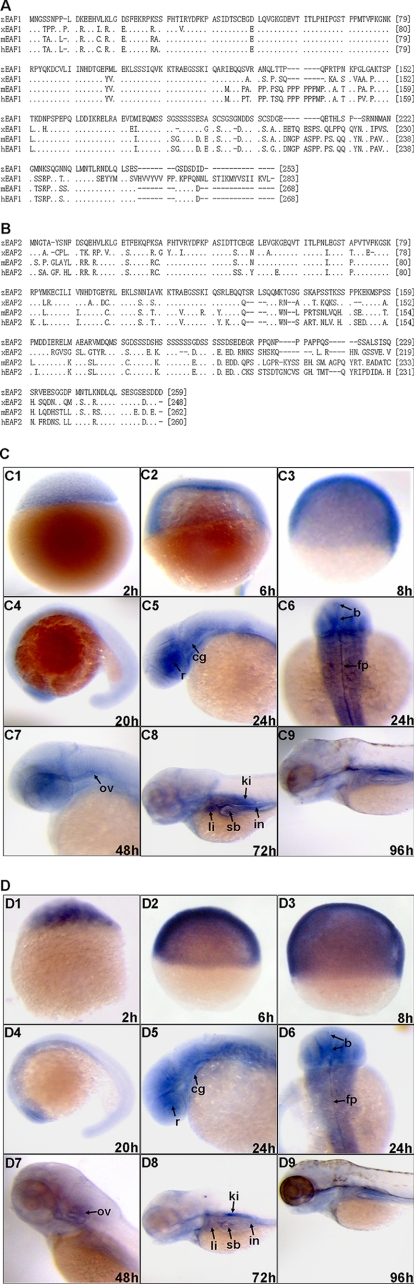
We chose the zebrafish model to examine the function of eaf1 and eaf2/u19 during vertebrate development. An orthologous gene search of the zebrafish Ensembl data base with human EAF1 and EAF2/U19 sequences yielded a single copy of each gene. We then cloned the zebrafish eaf1 and eaf2/u19 genes by RT-PCR. Zebrafish eaf1 encodes a predicted protein 253 amino acids in length that has a high degree of identity with Eaf1 from Xenopus (67.83%), mice (74.44%), and humans (74.81%), with the amino terminus (amino acids 21–126) exhibiting much greater conservation than the carboxyl terminus (Fig. 1A). Similarly, the predicted protein encoded by zebrafish eaf2/u19 is 259 amino acids in length and has a high degree of identity with Eaf2/U19 from Xenopus (62.31%), mice (62.08%), and humans (64.04%). Again we found that the greatest degree of conservation occurred in the amino terminus (amino acids 29–126) (Fig. 1B).
FIGURE 1.
Alignment and expression of zebrafish (D. rerio) Eaf1 and Eaf2/U19. A, zebrafish eaf1 (zEAF1; GenBankTM accession number AAI53593) codes for a 253-amino acid protein with orthologues in X. laevis (xEAF1; GenBankTM accession number AAI25802), Mus musculas (mEAF1; GenBankTM accession number AAH41329), and Homo sapiens (hEAF1; GenBankTM accession number AAH79658). B, zebrafish eaf2/u19 (zEAF2; GenBankTM accession number CAQ15588) codes for a 259-amino acid protein with orthologues in X. laevis (xEAF2; GenBankTM accession number CAE22450), M. musculas (mEAF2; GenBankTM accession number AAH04721), and H. sapiens (hEAF2; GenBankTM accession number AAO63811). C, whole mount in situ hybridization analysis of zebrafish eaf1 expression. D, whole mount in situ hybridization analysis of zebrafish eaf2/u19 expression. C1 and D1, blastula stage, lateral view; C2 and D2, shield stage embryos, lateral view; C3 and D3, 75% epiboly, lateral view; C4 and D4, 20 hpf, lateral view with anterior to the left; C5 and D5, 24 hpf, lateral view with anterior to the left; C6 and D6, 24 hpf, dorsal view with anterior on top; C7 and D7, 48 hpf, lateral view with anterior to the left; C8 and D8, 72 hpf, lateral view with anterior to the left; C9 and D9, 96 hpf, lateral view with anterior on top. b, brain; cg, cranial ganglion; fp, floor plate; ki, kidney; in, intestine; li, liver; sb, swim bladder; r, retina; ov, otic vesicle.
Examination of zebrafish embryos by in situ hybridization revealed a similar pattern of expression for embryonic eaf1 and eaf2/u19 as well as for maternal transcripts in all cells at early developmental stages (Fig. 1, C (C1, C2, and C3) and D (D1, D2, and D3)). By 20 h postfertilization (hpf), the anterior head region expressed the highest level of eaf1 and eaf2/u19, whereas other regions of the body expressed lower, homogenous levels (Fig. 1, C (C4) and D (D4)). By 24 hpf, eaf1 and eaf2/u19 transcripts were predominantly distributed in the central and ventral nervous system, including the brain, cranial ganglion, retina, otic vesicle, and floor plate (Fig. 1, C (C5 and C6) and D (D5 and D6)). By 48 hpf, the strongest signals were still distributed in the central nervous system and sensory organs (Fig. 1, C (C7) and D (D7)). The expression of eaf1 and eaf2/u19 in the nervous system became weaker by 72 hpf and remained low at 96 hpf. At the same time, expression increased in strength at sites with an active interaction between the epithelium and mesenchyme, such as the liver, kidney, swim bladder, and intestine (Fig. 1, C (C8 and C9) and D (D8 and D9)).
Morpholino-mediated Knockdown of eaf1 and eaf2/u19 Results in Abnormal Axis Formation and Disrupted Convergence and Extension Movements during Gastrulation
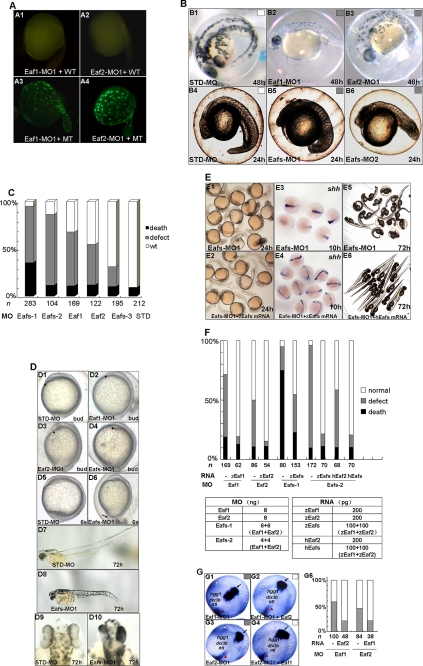
To investigate the roles of eaf1 and eaf2/u19 during zebrafish embryogenesis, we knocked down their expression using translation-blocking morpholinos. As a first step, we evaluated the efficiency of the morpholino targeting eaf1 (Eaf1-MO1) and eaf2/u19 (Eaf2-MO1) by co-injecting each into zebrafish embryos along with a vector expressing the target protein tagged with GFP at the carboxyl terminus. We also evaluated the specificity by co-injecting the morpholinos with vectors expressing a mutated form of GFP-tagged Eaf1 or Eaf2/U19; each eaf target sequence had five mismatched nucleotides in the 5′ region. Eaf1-MO1 (8 ng) and Eaf2-MO1 (8 ng) successfully blocked expression of GFP-tagged Eaf1 and Eaf2/U19, respectively, but not expression of the mutated targets (Fig. 2A).
FIGURE 2.
Knockdown of Eaf1 and Eaf2/U19 caused defects in gastrulation. A, validation of the morpholinos. A1, embryos were injected with Eaf1-MO1 (8 ng) and a wild-type eaf1-GFP fusion protein expression vector (WT) and then examined by fluorescence microscopy. A2, embryos were injected with Eaf2-MO1 (8 ng) and a wild-type eaf2/u19-GFP fusion protein expression vector. A3, embryos were injected with Eaf1-MO1 and a mutated eaf1-GFP fusion protein expression vector (MT). A4, embryos were injected with Eaf2-MO1 and a mutated eaf2/u19-GFP fusion protein expression vector. B, morphology of representative morphants. B1 and B4, morphants of the STD-MO (8 ng) at 48 hpf (B1) and 24 hpf (B4). B2, an eaf1 morphant (Eaf1-MO1; 8 ng) at 48 hpf. B3, an eaf2/u19 morphant (Eaf2-MO1; 8 ng) at 48 hpf. B5, an eafs morphant (Eaf1-MO1 plus Eaf2-MO1; 4 ng each) at 24 hpf. B6, an eafs morphant 2 (Eaf1-MO2 plus Eaf2-MO2; 4 ng each) at 24 hpf. C, embryos were injected with morpholinos: Eafs-1 (Eaf1-MO1 plus Eaf2-MO1; 6 ng each), Eafs-2 (Eaf1-MO1 plus Eaf2-MO1; 4 ng each), Eaf1 (Eaf1-MO1, 8 ng), Eaf2 (Eaf2-MO1, 8 ng), Eafs-3 (Eaf1-MO1 and Eaf2-MO1, 2 ng each), and STD-MO (8 ng). Black box, dead embryos at 24 hpf; gray box, embryos with defects at 3 days postfertilization (dpf) with defects characterized by mild cyclopia, no forebrain structures anterior to the eyes, and reduced body length; white box, embryos with no discernable defects. D, morpholinos, including Eaf1-MO1 (8 ng), Eaf2-MO1 (8 ng), Eafs-MO1 (Eaf1-MO1 plus Eaf2-MO1; 4 ng each), and STD-MO (8 ng) were injected at the one-cell stage, and the morphology was assessed at different embryonic stages. D1, D2, D3, and D4, bud stage embryos, lateral view, dorsal to the right; the arrowhead marks the anterior-most structure. D5 and D6, six somite stage embryos, lateral view, dorsal to the right. The arrow indicates the misprotruded tail. D7 and D8, 72 hpf, lateral view, anterior to the left. D9 and D10, 72 hpf, dorsal view, anterior to the top. E, both zebrafish and human eaf1 and eaf2/u19 mRNA completely rescued the Eaf1-MO1-, Eaf2-MO1-, and Eafs-MO1 (Eaf1-MO1 plus Eaf2-MO1)-induced defects. E1 and E2, 24 hpf, morphology of embryos injected with Eafs-MO1 alone and injected with Eafs-MO1 combined with both zebrafish eaf1 and eaf2/u19 mRNA (zEafs mRNA). E3 and E4, 10 hpf, whole mount in situ hybridization assays for shh expression in embryos treated as the same as that of E1 and E2. E5 and E6, 72 hpf, morphology of embryos injected with Eafs-MO1 alone and injected with Eafs-MO1 combined with human Eaf1 and Eaf2/U19 mRNA (hEafs mRNA). F, the embryos were scored morphologically from four independent experiments. Black bar, percentage of death; gray bar, percentage of defects; white bar, percentage of embryos with no discernable defects. The dosage and composition of the injected mRNA and morpholino are indicated. To eliminate error among different experiments, we used the same batch of embryos produced by a select number of zebrafish. G, eaf1 mRNA rescued Eaf2-MO-mediated convergence and extension movement defects during gastrulation, and eaf2/u19 mRNA rescued Eaf1-MO-mediated convergence and extension movement defects during gastrulation. G1, Eaf1-MO1 (8 ng)-injected embryos stained with the markers ntl, hgg, and dlx3b. G2, Eaf1-MO1 (8 ng) plus eaf2/u19 mRNA (50–100 pg)-injected embryos stained with the markers ntl, hgg, and dlx3b. G3, Eaf2-MO1 (8 ng)-injected embryos stained with the markers ntl, hgg, and dlx3b. G4, Eaf2-MO1 (8 ng) plus eaf1 mRNA (50–100 pg)-injected embryos stained with the markers ntl, hgg, and dlx3b. Black arrow, prechordal plate (stained by hgg probe); white arrowhead, edges of the neural plate (stained by dlx3b); red arrowhead, axial chorda (stained by ntl). All embryos are shown at 9 hpf in dorsal view, anterior to the top. G5, the ratio of defects in convergence and extension movements rescued by eaf1 and eaf2/u19 mRNA was scored by the three markers, hgg1, dlx3b, and ntl.
We next injected Eaf1-MO1 (8 ng) or Eaf2-MO1 (8 ng) into zebrafish embryos at the one-cell stage and evaluated morphology at 48 hpf. Knockdown of Eaf1 and Eaf2 resulted in a similar phenotype; both morphants had a shorter tail and a reduced head as compared with the control embryo injected with STD-MO (Fig. 2B (B1–B3)). A combination of 4 ng each of Eaf1-MO1 and Eaf2-MO1 (Eafs-MO1) produced a more severe defect in the eafs morphants (Fig. 2B (B4 and B5)). To validate the effects seen with Eaf1-MO1 and Eaf2-MO1, we repeated the assay using two additional nonoverlapping, translation-blocking morpholinos targeting eaf1 and eaf2/u19 mRNA: Eaf1-MO2 and Eaf2-MO2. Injections with the individual morpholinos (data not shown) or a combination of both resulted in a phenotype similar to what was seen with Eaf1-MO1 and Eaf2-MO1 (Fig. 2B (B6)). Next, embryos were injected with varying amounts of Eaf1-MO1 and/or Eaf2-MO1 to determine if the changes in morphology occurred in a dose-dependent manner. We evaluated the embryos at 24 hpf for viability and at 3 days postfertilization for defects; Fig. 2C clearly shows a dose-dependent effect.
We further characterized the morphological changes seen with Eaf1 and Eaf2/U19 knockdown. Embryos at the one-cell stage were injected with a total of 8 ng of morpholinos (Eaf1-MO1, Eaf2-MO1, or a combination of both) and then examined at different stages: at the bud stage (Fig. 2D (D1–D4)), at the six-somite stage (Fig. 2D (D5 and D6)), and at 72 hpf (Fig. 2D (D7–D10). Knockdown of expression of the eaf1 and eaf2/u19 genes resulted in a shorter anterior-posterior axis of body (Fig. 2D (D1–D6)), which resembled the convergence and extension defects seen with knockdown of wnt11 gene expression (28). Moreover, embryos injected with Eafs-MO1 had a shorter tail and a fusion of the eyes, resembling that of slb/wnt11 mutants (20).
Rescue experiments represent the gold standard for demonstrating a direct relationship between morpholino-mediated knockdown of a specific protein and the changes in phenotype attributed to the knockdown. Thus, we injected embryos with Eafs-MO1 alone or with Eafs-MO1 mixed with capped zebrafish full-length eaf1 mRNA and/or eaf2/u19 mRNA. To avoid quenching, the eaf1 mRNA contained five mismatched synonym nucleotides, whereas the eaf2/u19 mRNA could not be quenched by Eaf2-MO1 due to seven nucleotides mismatched. Individually, both mRNAs rescued the phenotype induced by their “matched” MO (data not shown). Also, a mixture of eaf1 and eaf2/u19 mRNA (zeafs) rescued the phenotype of eafs morphants (Fig. 2, E (E1 and E2) and F). We also evaluated shh expression as a marker for convergence and extension movements by in situ hybridization and showed that zebrafish eaf1 and eaf2/u19 mRNA also rescued convergence and extension movement defects (Fig. 2E (E3 and E4)). Interestingly, human EAF1 and EAF2/U19 mRNA could also rescue the defects of eafs morphants, suggesting that its function is conserved across species (Fig. 2, E (E5 and E6) and F).
The 57.25% amino acid identity between zebrafish Eaf1 and Eaf2/U19, their equivalent mRNA expression patterns during embryogenesis, and the similar phenotype produced by their knock-out supports the possibility of redundant functions for Eaf1 and Eaf2/U19 in development. To test this possibility, we did reciprocal rescue experiments in which embryos were co-injected with Eaf1-MO1 and varying amounts of eaf2/u19 mRNA or with Eaf2-MO1 and varying amounts of eaf1 mRNA. Embryos were then stained with markers for convergence and extension movement: ntl to show the axial chorda, hgg to show the prechordal plate, and dlx3b to show the edges of the neural plate. We found that 50–100 pg of eaf2/u19 mRNA rescued Eaf1-MO1-mediated convergence and extension movement defects (Fig. 2G (G1, G2, and G5)). Similarly, 50–100 pg of eaf1 mRNA rescued Eaf2-MO-mediated defects (Fig. 2G (G3–G5)). This suggested that Eaf1 and Eaf2/U19 performed some of the same functions in embryogenesis. However, the morpholinos did not induce higher expression of the reciprocal eaf mRNAs (data not shown), suggesting that the functions of the two were not completely redundant. This was not surprising, given that either Eaf1-MO1 or Eaf2-MO1 alone could induce a phenotype, which increased in severity upon combining the two morpholinos (Fig. 2, B and C).
Further Characterization of the Convergence and Extension Movement Defect Caused by eaf1 and eaf2/u19 Knockdown
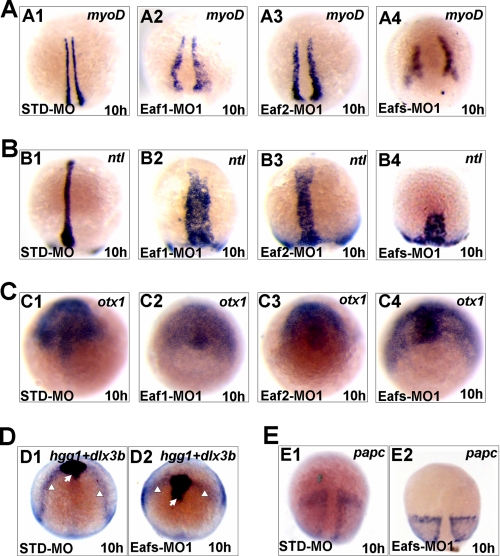
To more thoroughly investigate convergence and extension movement defects, we used whole mount in situ hybridization to analyze the expression patterns of several marker genes. We found that knockdown of Eaf1, Eaf2/U19, or both caused a widening in the distance between bilateral adaxial cells, as visualized by mesodermal myoD expression, and the axial chorda mesoderm could not extend to reach the anterior region, as visualized by expression of the mesodermal marker ntl (Fig. 3, A and B). In addition, the neural plate, expressing the neuroectodermal marker otx1, became broader (Fig. 3C). As before, simultaneous knockdown of Eaf1 and Eaf2/U19 protein levels produced a phenotype similar to the one that resulted from knockdown of a single Eaf, but the double knockdown caused more severe defects in convergence and extension movements (Figs. 2, B (B4) and C (C4), and 3A (A4)). Furthermore, double knockdown of Eaf1 and Eaf2/U19 disrupted convergence of the edges of the neural plate, as visualized by dlx3b expression (Fig. 3D) and resulted in a more posterior positioned prechordal plate (staining by hgg) (Fig. 3D). Embryos injected with Eafs-MO1 also showed shorter presomitic mesoderm domains than controls (staining by papc) (Fig. 3E). The more severe phenotype observed in the double knockdown embryos indicates that Eaf1 and Eaf2/U19 have at least some nonoverlapping functions.
FIGURE 3.
Whole mount in situ hybridization assays for different markers in 10-hpf-old embryos injected with STD-MO, Eaf1-MO1, Eaf2-MO1, or Eafs-MO1 (Eaf1-MO1 plus Eaf2-MO1). A, myoD (staining the adaxial cell). B, ntl (staining the forerunner cell group, axial chorda mesoderm). C, otx1 (staining the anterior axial hypoblast and neural plate). D, dlx3b (white arrowheads) and hgg probe (staining the prechordal plate, indicated by white arrows). E, papc (staining the paraxial mesoderm). Shown are dorsal views with the anterior to the top.
Role for Eaf1 and Eaf2/U19 in Cell Migration
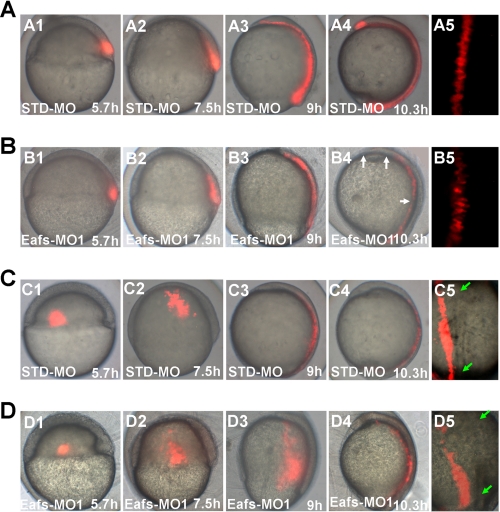
To assess the effect of eaf1 and eaf2/u19 loss on cell migration, we performed cell tracing experiments using Eafs-MO1 and capped kaeda mRNA. The kaeda gene encodes a protein with green fluorescence that changes to a red upon stimulation with a beam of UV light (350–400 nm) (27). At 5.7 hpf, we directed the UV light at the dorsal shield of injected embryos to analyze the extension of axial mesoderm cells at the indicated time points (Fig. 4, A and B). In STD-MO-injected embryos, the labeled cells extended along the AP axis and intercalated in nonlabeled cells to form an elongated and interrupted cell array (Fig. 4A (A4 and A5); n = 3). In addition, the AP length measured by labeled cells dramatically increased by the end of the gastrula period (Fig. 4A (A3)), and continued to increase during the segmentation period (Fig. 4A (A4)). In Eafs-MO1-injected embryos, the initial size of the labeled cells was the same as that in the STD-MO1-injected embryos, but its AP axis length was obviously shorter at the end of gastrulation (Fig. 4, A (A3) and B (B3)). Furthermore, the subsequent elongation of the labeled cells in the array during early segmentation was almost completely inhibited in the eafs morphants (Fig. 4B (B4); n = 5). The dorsal labeled cells in eafs morphants also displayed defects in polarization and intercalation (Fig. 4B (B5)). To measure dorsal-ward movements of lateral cells (convergence), a cluster of cells, located in the lateral margin, 90° from the dorsal shield, was labeled by UV light, and the dorsal translocation of the cells was analyzed at the indicated time points (Fig. 4, C and D). In STD-MO-injected embryos, the labeled cells reached the dorsal site, along with the mediolateral axis at the end of gastrula period (Fig. 4C (C3)) and then underwent mediolateral intercalation with the axial tissue (Fig. 4C (C4 and C5); n = 5). In contrast, the labeled cells in the eafs morphants remained at the lateral site with a broader distribution at the end of the gastrula period (Fig. 4D (D3)). At the early segmentation stage, although the labeled cells reached the dorsal site (Fig. 4D (D4)), they could not undergo mediolateral intercalation due to the long distance from the dorsal axis; thus, they could not get into the axial tissue (Fig. 4D (D5); n = 5). The extension of the lateral tissue was not inhibited, but it was disorganized (Fig. 4D (D2–D4)). The impaired dorsal-ward movements of lateral cells not only resulted in a significant reduction of the AP length (Fig. 2B) but also resulted in a shorter dorsal mesendoderm during gastrulation (Fig. 3A). Taken together, the analysis of cell migration and morphology provided evidence that both convergence and extension movements in lateral and dorsal regions of the gastrula require Eaf1 and Eaf2/U19.
FIGURE 4.
Eaf1 and Eaf2/U19 were required for normal convergence and extension movements during gastrulation. A and B, knockdown of Eafs (Eaf1 and Eaf2/U19) disrupted extension of mesendoderm. C and D, knockdown of Eafs disrupted convergence. kaede mRNA (200 pg) was co-injected with 8 ng of the indicated MOs into wild-type embryos. Cell labeling was performed by UV activation of kaede at 5.7 hpf. Pictures were taken directly after labeling. A and C, STD-MO; B and D, eafs-MO1; A1, B1, C1, and D1, shield (6 hpf); A2, B2, C2, and D2, 60% epiboly (7.5 hpf); A3, B3, C3, and D3, 90% epiboly (9 hpf); A4, B4, C4, and D4, bud (10.3–10.5 hpf); A5, B5, C5, and D5, bud, dorsal view with the anterior to the top. Each experiment was repeated five times. B4, the gaps of labeling extension mesendoderm cells are indicated by white arrows. A5 and B5, magnification of chordal region in A4 and B4, respectively. C5 and D5, dorsal view of the labeling convergence cells of C4 and D4, respectively, chorda indicated by green arrows.
Eaf1 and Eaf2/U19 Knockdown Does Not Affect Cell Fate Specification at Early Gastrula
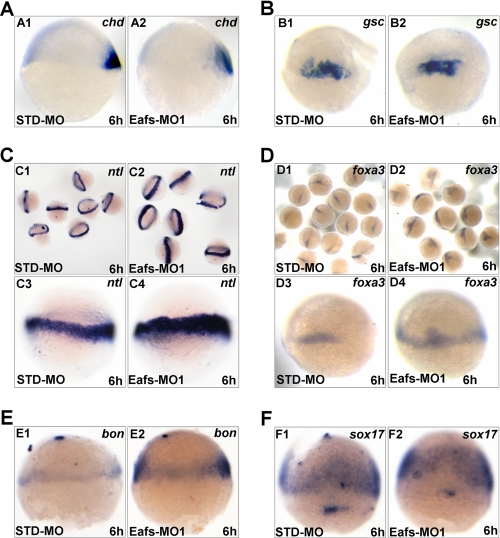
To determine whether knockdown of Eaf1 and Eaf2/U19 could also affect cell fate specification at the beginning of gastrulation, we examined the expression of chd and gsc, two cell fate specification markers at early gastrula. In eafs morphants, the expression level of chd (Fig. 5A (A2)) and gsc (Fig. 5B (B2)) was not significantly changed compared with STD-MO-injected controls (Fig. 5A (A1) and 5B (B1)), resembling the results observed in shp2 (29) and csk (30) morphants. Both shp2 and csk have been showed to regulate convergence and extension movement without affecting cell fate specification during gastrulation.
FIGURE 5.
Eaf1 and Eaf2/U19 knockdown did not affect cell fate specification at the beginning of gastrulation. A, chd expression in embryos injected with STD-MO (A1) or Eafs-MO1 (A2). B, gsc expression in embryos injected with STD-MO (B1) or Eafs-MO1 (B2). C, ntl expression in embryos injected with STD-MO (C1 and C3) or Eafs-MO1 (C2 and C4). D, foxa3 expression in embryos injected with STD-MO (D1 and D3) or Eafs-MO1 (D2 and D4). E, bon expression in embryos injected with STD-MO (E1) or Eafs-MO1 (E2). F, sox17 expression in embryos injected with STD-MO (F1) or Eafs-MO1 (F2). Injected embryos were fixed at 6 hpf, and in situ hybridization was performed with the indicated probes, respectively. A1, A2, C1–C4, E1, and E2, lateral view, animal pole top; B1, B2, D1–D4, F1, and F2, dorsal-lateral view, animal pole top.
Subsequently, we examined the expression of the panmesodermal marker ntl. As showed in Fig. 5C, the expression domain of ntl was apparently expanded (Fig. 5C (C2 and C4)) compared with the controls (Fig. 5C (C1 and C3)). These results indicated that the specification of mesoderm occurred in eafs morphants, implying that the cell fate specification at the early gastrula was indeed not affected by eafs knockdown.
Furthermore, we examined the expression of three endoderm specification markers, including foxa3, bon, and sox17. As shown in Fig. 5D, the expression of foxa3 in eafs morphants (Fig. 5C (C2 and C4)) as well as that of bon (Fig. 3E (E2)) exhibited obviously expanded expression compared with the controls (Fig. 5, D (D1 and D3) and E (E1)). The expression of another endoderm-specific gene, sox17, was also enriched at the ventro-lateral region in eafs morphants (Fig. 5F (F2)) compared with the controls (Fig. 5F (F1)). These results indicated that the specification of endoderm also occurred in eafs morphants, further suggesting that cell fate specification at early gastrula was not affected by eafs knockdown. Taken together, the knockdown of eafs did not affect cell fate specification at the beginning of gastrulation.
eaf1 and eaf2/u19 Are Required for the Midline Migration of Heart Precursors and Pancreas Precursors
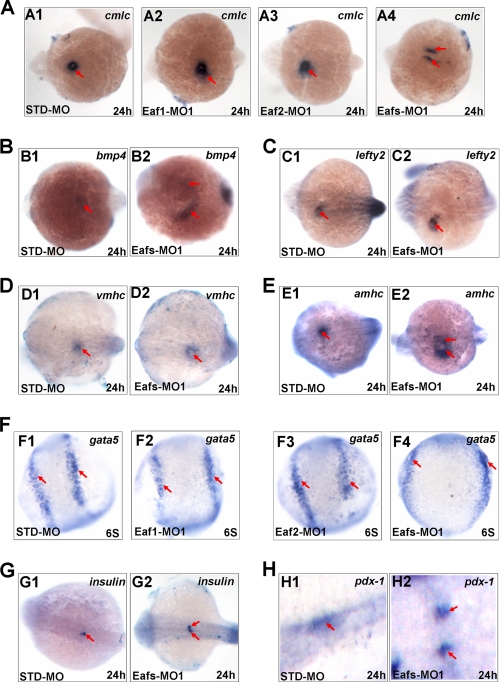
During embryogenesis, the mesendoderm and midline structure strongly expressed eaf1 and eaf2/u19 transcripts (Fig. 1, C (C3–C6) and D (D3–D6)). We also observed that decrease in expression of these genes led to severe defects in convergence and extension movement (Figs. 2–4). Together, these findings suggest a role for Eaf1 and Eaf2/U19 in both cardiac and pancreas development. To assess this possibility, we used whole mount in situ hybridization to analyze expression of several cardiac markers, including cmlc, bmp4, lefty2, vmhc, and amhc. Both the eaf1 and eaf2/u19 morphants had a broader and more dispersed expression of cmlc than the control morphants (Fig. 6A (A1–A3)). However, loss of Eaf1 and Eaf2/U19 in eafs morphants resulted in the formation of two separate sites of cmlc expression (Fig. 6A (A4)). Similarly, both bmp4 expression (Fig. 6B (B2)) and amhc expression (Fig. 6E (E2)) also separated into two parts in eafs morphants. In addition, the expression of lefty2 (Fig. 6C (C2)) and vmhc (Fig. 6D (D2)) was much broader in eafs morphants as compared with control morphants. This evidence indicates that Eaf1 and Eaf2/U19 play very important roles in regulating the migration of cardiac cells.
FIGURE 6.
Eaf1 and eaf2/u19 regulated myocardial cell migration (A–F) and midline convergence of pancreas precursors (G and H) without affecting their cell fate. Shown is cmlc (A1–A4) bmp4 (B1 and B2), lefty2 (C1 and C2), vmhc (D1 and D2), amhc (E1 and E2), gata5 (F1–F4), insulin (G1 and G2), and pdx-1 (H1 and H2) expression in 24 hpf embryos injected with STD-MO or Eafs-MO1, in dorsal view, with anterior to the left. The red arrows in A–F indicate myocardial cells, and the red arrows in G and H indicate pancreas precursors.
To further explore whether the myocardial migration defects were a consequence of altered specification of myocardial precursors, we analyzed the expression levels of gata5, an early marker of myocardial specification. As shown in Fig. 6F, none of the embryos injected with Eaf1-MO1 (Fig. 6F (F2)), Eaf2-MO1 (Fig. 6F (F3)), or Eafs-MO1 (Fig. 6F (F4)) displayed significant changes in gata5 expression levels, although heart primordia cells failed to converge at the midline under these experimental conditions. These results indicated that specification of the myocardial cell fate proceeded normally after ablation of Eaf1and Eaf2/U19 in embryos.
The migration of myocardial precursors toward the midline had been proposed to depend on endoderm specification (18) or on midline convergence of the anterior endoderm (31); thus, we next investigated the effect of Eaf1 and Eaf2/U19 down-regulation on the expression of endoderm markers. Given that gata5, one of the earliest markers of endoderm fate determination (32) as well as a myocardial precursor marker, did not change in the eafs morphants (Fig. 6F), we evaluated markers of later stages in endoderm specification and pancreatic differentiation, insulin and pdx-1. Although the expression levels of insulin and pdx-1 were similar between eafs morphants and control morphants, the pattern of expression varied (Fig. 6, G and H). Eaf1 and Eaf2/U19 knockdown resulted in the failure of fusion of the bilateral pancreas primordia, giving rise to a bifid pancreas (Fig. 6, G (G2) and H (H2)). Therefore, eaf1 and eaf2/u19 regulated midline convergence of organ primordia, including the heart and pancreas.
eaf1 and eaf2/u19 Regulate Expression of Noncanonical Wnt Ligands
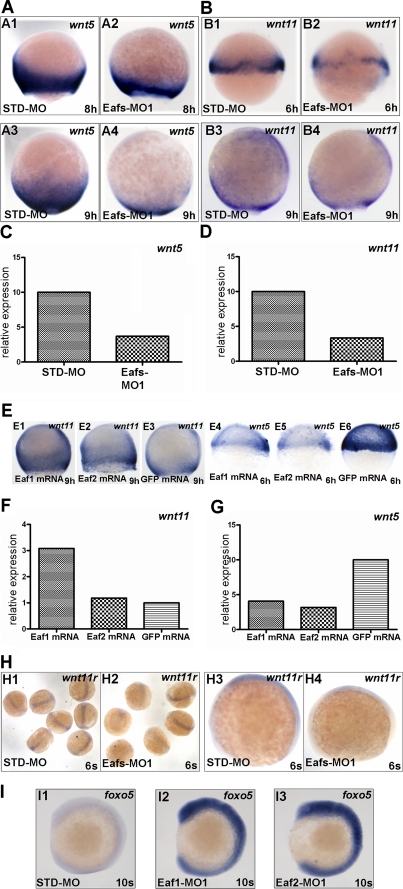
Studies have shown that the noncanonical Wnt ligands, wnt11 and wnt5, were functionally essential for the regulation of convergence and extension movement (20, 21, 33), and we have shown that eafs morphants phenocopied wnt11 mutants and morphants, prompting us to investigate if eaf1 and eaf2/u19 affected convergence and extension movements through noncanonical Wnt signaling. Through in situ hybridization, we found that wnt5 (wnt5b) and wnt11 transcripts were markedly reduced in Eafs-MO1-injected embryos as compared with STD-MO-injected embryos (Fig. 7, A and B). In addition, semiquantitative RT-PCR showed a remarkable decline in expression of both wnt5 (Fig. 7C) and wnt11 (Fig. 7D). We next investigated the expression level of wnt5 and wnt11 in embryos injected with eaf1 and eaf2/u19 mRNA. As compared with overexpression of ectopic GFP, overexpression of either eaf1 or eaf2/u19 mRNA led to a dramatic increase in wnt11 mRNA in embryos, as determined by in situ hybridization and semiquantitative RT-PCR (Fig. 7, E (E1 and E2) and F). Surprisingly, as with Eafs knockdown, overexpression eaf1 or eaf2/u19 down-regulated wnt5 levels (Fig. 7, E (E4 and E5) and G). These data suggest that eaf1 and eaf2/u19 act upstream of wnt11 to regulate its expression, but the regulation of wnt5 occurs through a different mechanism.
FIGURE 7.
Eaf1 and eaf2/u19 were required for maintaining wnt5 and wnt11 during gastrulation. Shown are wnt5 (A) and wnt11 (B) expression in embryos injected with STD-MO or Eafs-MO1. In embryos with eafs-knockdown, the expression levels of both wnt5 (A2 and A4) and wnt11 (B2 and B4) were obviously lower than that of embryos injected with STD-MO (A1, A3, B1, and B3). Shown is wnt5 (C) and wnt11 (D) expression in embryos injected with STD-MO or Eafs-MO1, as determined by RT-PCR. E, overexpression of eaf1 and eaf2/u19 modulates wnt5 and wnt11 expression. Shown is a lateral view of wnt11 (E1–E3) and wnt5 (E4–E6) expression in embryos injected with eaf1 mRNA (E1 and E4), eaf2/u19 mRNA (E2 and E5), or GFP mRNA (control) (E3 and E6), respectively. Shown are wnt11 (F) and wnt5 (wnt5b) (G) expression in embryos injected with eaf1 mRNA, eaf2/u19 mRNA, and GFP mRNA, as determined by RT-PCR. H, wnt11r expression in embryos injected with STD-MO (H1 and H3) or Eafs-MO1 (H2 and H4). I, foxo5 expression in embryos injected with STD-MO (I1), Eaf1-MO1 (I2), or Eaf2-MO1 (I3).
We have shown that eaf1 and eaf2/u19 contribute to midline convergence of organ precursors, a process regulated by the noncanonical Wnt ligands wnt4a, wnt11, and wnt11r (31). To establish if eaf1 and eaf2/u19 regulate expression of wnt4a and wnt11r, we measured expression in Eaf1 and Eaf2/U19 knockdown embryos. Eafs-MO1 injection produced a decrease in wnt11r levels (Fig. 7H) but an unexpected increase in wnt4a(wnt4) levels,4 again underscoring the complexity of the signaling pathways.
It has been reported that eaf1 and eaf2/u19 could serve as regulators of transcriptional elongation by in vitro assays (7). This fact raised a question of whether the down-regulation of noncanonical Wnt ligands wnt11, wnt5, and wnt11r in eafs morphants was due to the disruption of the function of eafs in regulating transcriptional elongation. If this is the case, then eafs regulating wnts appears to be nonspecific. Although we already observed that the expression of a noncanonical Wnt ligand wnt4a was increased in eafs morphants, contrary to that of wnt11, wnt5, and wnt11r,4 we still wanted to further clarify this issue. Through microarray analysis, we identified not only down-regulated genes but also up-regulated genes in eafs morphants.5 From up-regulation genes, we chose foxo5, one of the highest up-regulated genes, for further verification by in situ hybridization. As shown in Fig. 7I, either Eaf1 or Eaf2/U19 knockdown could up-regulate foxo5 expression (Fig. 7I (I2 and I3)), similar to that of wnt4a,4 which was also consistent with the data obtained from microarray analysis (data not shown). Thus, eaf1 and eaf2/u19 could also inhibit gene expression. Taken together, these observations ruled out the possibility that eafs regulating noncanonical Wnt ligands, wnt11, wnt5, and wnt11r, was nonspecific as a result of disrupting the activity of eafs for regulating transcriptional elongation in eafs morphants. Taking into consideration that the knockdown of eafs did not affect cell fate specification at early gastrula, we think that eaf1 and eaf2/u19 probably regulate noncanonical Wnt ligands specifically.
Expression of eaf1 and eaf2/u19 Is Not Affected by Overexpression of wnt11 and wnt5
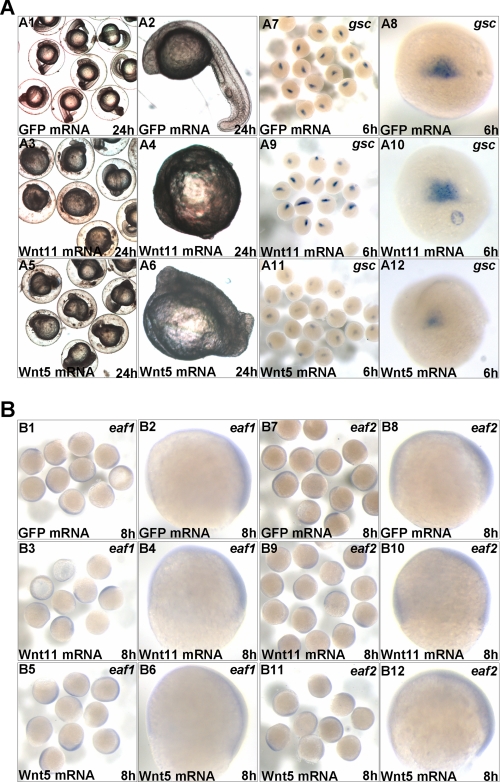
To completely understand the relationship between noncanonical Wnt ligands (wnt11 and wnt5) and the Eaf gene family, we set out to determine whether overexpression of wnt11 or wnt5 would have an impact on eaf1 or eaf2/u19 expression. First, we performed functional evaluation for synthesized zebrafish wnt11 and wnt5 mRNA. As showed in Fig. 8A, the embryos injected with either wnt11 (Fig. 8A (A3 and A4)) or wnt5 mRNA (Fig. 8A (A5 and A6)) exhibited malformed phenotypes as reported previously (34). In addition, compared with the control embryos injected with GFP mRNA (Fig. 8A (A7 and A8)), the expression of organizer marker gsc reduced dramatically in embryos injected with wnt5 mRNA (Fig. 8A (A11 and A12)) but not in embryos injected with wnt11 mRNA (Fig. 8A (A9 and A10)), which was also consistent with the results reported previously (34, 35). These observations indicated that the synthesized wnt11 and wnt5 mRNA had intact function. Subsequently, we checked the expression of eaf1 or eaf2/u19 in embryos injected with either wnt11 mRNA or wnt5 mRNA. As showed in Fig. 8B, compared with the control embryos injected with GFP mRNA (Fig. 8B (B1, B2, B7, and B8)), both expression pattern and expression level of eaf1 (Fig. 8B (B3–B6)) and eaf2/u19 (Fig. 8B (B9–B12)) were not changed in wnt11 and wnt5 overexpression embryos. These results further suggested that eaf1 and eaf2/u19 function upstream of wnt11 and wnt5.
FIGURE 8.
The expression of eaf1 and eaf2/u19 was not affected by overexpression of wnt5 and wnt11. A, the functional evaluation of synthesized wnt11 and wnt5 mRNA. Embryos overexpressing wnt11 were evaluated by morphology (A3 and A4) and whole mount in situ hybridization (gsc staining) (A9 and A10). Embryos overexpressing wnt5 were evaluated by morphology (A5 and A6) and whole mount in situ hybridization (gsc staining) (A11 and A12). B, examination of eaf1 and eaf2/u19 expression in wnt11- and wnt5-overexpressed embryos by whole mount in situ hybridization. The expression pattern and level of eaf1 and eaf2/u19 (B3, B4, B9, and B10) were not changed in wnt11-overexpressing embryos compared with the control embryos injected with GFP mRNA (B1, B2, B7, and B8). The expression pattern and level of eaf1 and eaf2/u19 (B5, B6, B11, and B12) were not changed in wnt5-overexpressing embryos compared with the control embryos injected with GFP mRNA (B1, B2, B7, and B8).
eaf1 and eaf2/u19 Mediate Convergence and Extension Movements and Convergence of Organ Primordial through Wnt Signaling
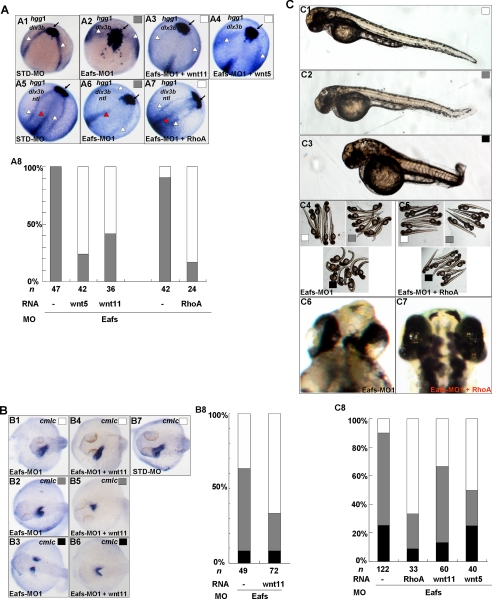
To gain a better understanding of how eaf1 and eaf2/u19 may regulate convergence and extension movements during gastrulation, we performed rescue experiments using expression of wnt11 and wnt5 as well as wild-type rhoA, a down-stream gene of both wnt5 and wnt11. We co-injected zebrafish embryos with Eafs-MO1 and wnt5 mRNA, wnt11 mRNA, or rhoA mRNA. We then scored embryos for expression of the convergence and extension movement markers hgg1, dlx3b, and ntl using in situ hybridization (Fig. 9A) as well as for general morphological characteristics (Fig. 9C). In eafs morphants, we found that wnt11 mRNA not only suppressed the reduction in the anterior-posterior extension of the prechordal plate (Fig. 9A (A3), indicated by an arrow) but also suppressed cyclopia and body shortening (Fig. 9C). Furthermore, wnt5 mRNA (Fig. 9, A (A4) and C) also rescued these defects effectively. Interestingly, rhoA rescued the defects even more effectively than wnt11 and wnt5. In comparison with embryos injected with Eafs-MO1 alone (Fig. 9A (A7)), rhoA-rescued embryos exhibited a U-shaped prechordal plate with a normal anterior extension (Fig. 9A (A7), indicated by arrows). At the same time, both the edges of the neural crest (Fig. 9A (A7), indicated by white triangles) and notochord (Fig. 9A (A7), indicated by red triangles) narrowed in the embryos co-injected with rhoA mRNA (Fig. 9A (A6)). The cyclopia and shortened body defects seen in the eafs morphants were also significantly suppressed by co-injection of rhoA mRNA (Fig. 9C (C5, C7, and C8)). These results suggested that the ability of eaf1 and eaf2/u19 to govern convergence and extension movements during zebrafish gastrulation required the convergence of wnt5 and wnt11 on rhoA.
FIGURE 9.
wnt5, wnt11, and rhoA partially rescued the defects of eafs morphants. A, wnt5, wnt11, and rhoA partially rescued the convergence and extension movement defects of eafs morphants. Embryos were injected with STD-MO (A1 and A5) or with Eafs-MO1 (A2 and A6). A3, embryos were co-injected with Eafs-MO1 and wnt11 mRNA (100 pg). A4, embryos were co-injected with Eafs-MO1 and wnt5 mRNA (100 pg). A7, embryos were co-injected with Eafs-MO1 and rhoA mRNA (10 pg). A1–A4, 9 hpf, dorsal view, anterior to the top right; embryos were stained for hgg1 and dlx3b. A5–A7, 12 hpf, dorsal view, anterior to the top right; the expression of hgg1, dlx3b, and ntl was stained. Black arrow, prechordal plate (stained by hgg probe); white arrowhead, edges of the neural plate (stained by dlx3b); red arrowhead, the axial chorda (stained by ntl). A8, the percentage of eafs morphants exhibiting convergence and extension movement defects was scored by the three markers: hgg1, dlx3b, and ntl. Gray box, defects; white box, wild-type or wild-type likely staining. B, wnt11 partially rescued the defects of myocardial cell migration induced by Eafs-MO1 injection. B1–B3, 30 hpf embryos, injected with Eafs-MO1, stained by a cmlc probe, dorsal view, anterior to the left. B4–B6, 30 hpf embryos, co-injected with Eafs-MO1 and wnt11 mRNA, stained by a cmlc probe, dorsal view, anterior to the left. B7, 30 hpf embryos, injected with STD-MO, stained by cmlc probe, dorsal view, anterior to the left. B8, the percentage of defects of myocardial cell migration in embryos injected with Eafs-MO1 alone or co-injected with Eafs-MO1 and wnt11 mRNA based on the whole mount in situ hybridization assays. White box, wild-type or wild-type likely phenotypes; gray box, moderate defects; black box, severe defects. C, rhoA, wnt5, and wnt11 rescued the general defects induced by Eafs-MO1 injection. C1, C2, and C3, 3 days postfertilization, the embryos were morphologically scored for defects into three categories: 1) wild-type, no discernable defect (white box); 2) moderate defect (gray box), characterized by slightly reduced head and anterior-posterior axis and slightly reduced distance between two eyes; and 3) severe defect (black box), characterized by dramatically reduced head and anterior-posterior axis and dramatically reduced distance between two eyes. C4 and C6, embryos injected with Eafs-MO1. C5 and C7, embryos co-injected with Eafs-MO1 and rhoA mRNA. C8, the embryos were scored morphologically; the percentages of WT (white bar), moderate (gray bar), and severe (black bar) are indicated. All of the injections, including MO alone or MO combined with mRNA, were performed using the same batch of embryos produced by a select number of zebrafish to eliminate the error caused by embryo variation.
We also tested whether eaf1 and eaf2/u19 rely on noncanonical Wnt signaling to regulate convergence of organ primordia by co-injecting embryos with Eafs-MO1 and wnt11 mRNA and then evaluating expression of the cardiac marker cmlc. In the 8.1% of eafs morphants exhibiting severe defects in myocardial cell migration, cmlc expression occurred in two separate locations (Fig. 9B (B3 and B8)). eafs morphants with moderate defects, 55.1% in all, had a more dispersed cmlc expression (Fig. 9B (B2 and B8)). Co-injection with wnt11 mRNA widened the distance between the eyes in morphants with no discernable defects or moderate defects (Fig. 9B (B4 and B5)). In the case of eafs morphants without discernable defects, co-injection with wnt11 mRNA resulted in cmlc staining identical to that in the control morphants (Fig. 9B (B4 and B7)). Introduction of ectopic Wnt11 mRNA decreased the frequency of eafs morphants with moderate defects (22.2%; Fig. 9B (B8)), and in those morphants, the expression of cmlc was more concentrated (Fig. 9B (B5)). Last, in embryos co-injected with Eafs-MO1 and wnt11 mRNA that exhibited severe defects, cmlc expression was only mildly bifida (Fig. 9B (B6)). These results suggested that wnt11 mRNA could rescue the midline convergence defects of organ primordia induced by Eafs-MO1.
DISCUSSION
Previous studies have established that EAF2/U19 contributes to tumor suppression (9, 10). Other studies suggest that EAF1 and EAF2/U19, like other tumor suppressors, may play a role in vertebrate development (14, 15). However, their exact function in embryogenesis remains unclear. To better understand the cellular function of EAF1 and EAF2/U19 and subsequently how these proteins suppress tumors will require a more extensive investigation of their roles in embryogenesis. Here, we are the first to present data showing that eaf1 and eaf2/u19 mediate effective convergence and extension movements through the maintenance of wnt11 and wnt5 expression during zebrafish gastrulation.
Eaf family members demonstrated a high degree of conservation across species and with each other, so not surprisingly, the expression of eaf1 and eaf2/u19 observed in the zebrafish mirrored the pattern seen in the mouse (14). However, intercrosses of EAF2/U19 heterozygous knock-out mice yielded Eaf2/U19-null offspring at Mendelian ratios at birth, suggesting that early mouse development did not require EAF2/U19 (10). Eaf2/U19 knockdown in zebrafish embryos alone caused defects in convergence and extension movements in our study. This phenotypic difference between mouse and zebrafish might result from either the redundant function of EAF1 and EAF2/U19 in mouse embryogenesis or from slightly different functions of EAF2/U19 in mammalians and lower vertebrate fish. Indeed, functional differences between mammalian and fish genes are not uncommon (36). In addition, Eaf2/U19 knockdown in Xenopus laevis caused defects in eye development, which was a pure eye phenotype unrelated to the defect of convergence and extension movement (15). However, eaf2/u19 knockdown in zebrafish caused shorter eye distance or eye fusion (partial cyclopia) resulting from the defect in convergence and extension movements (Fig. 8C). Together, eaf1 and eaf2/u19 might play different roles in eye development during embryogenesis. Further demonstrating the different mechanism of eaf1 and eaf2/u19 playing their roles in eye formation among species probably will give us a more complete picture about the function of eaf1 and eaf2/u19 in embryogenesis.
eaf1 and eaf2/u19 appeared to play partially redundant roles in the regulation of convergence and extension movements. Knockdown of both eaf1 and eaf2/u19 resulted in abnormal cell behavior in both the head and trunk mesendoderm (Fig. 2). The phenotypes observed in eafs morphants included disorganized head structure, reduced body axis, and shorter tail and were similar to those seen in embryos with a disruption of convergence and extension movements (20, 21, 33, 37–39). On the basis of phenotype, the marker gene expression patterns, and cell tracing experiments, we concluded that eaf1 and eaf2/u19 served as novel regulators of convergence and extension movements (Figs. 2–4). In reciprocal rescue experiments, eaf1 mRNA could rescue Eaf2-MO1 knockdowns, and eaf2/u19 mRNA could rescue Eaf1-MO knockdowns, suggesting redundant roles for Eaf1 and Eaf2/U19 in the regulation of convergence and extension movements. However, knockdown of either eaf1 or eaf2/u19 produced similar defects that increased in severity with the combined knockdown of both Eaf1 and Eaf2/U19 (Figs. 2–4), indicating that these proteins do not have completely redundant functions.
In this study, we found that eaf1 and eaf2/u19 might contribute to the regulation of convergence and extension movements through noncanonical Wnt signaling. Eaf1 and Eaf2/U19 knockdown dramatically down-regulated wnt11 expression, whereas introduction of ectopic eaf1 and eaf2/u19 mRNA increased wnt11 expression (Fig. 7). Moreover, wnt11 mRNA rescued convergence and extension movement defects caused by Eaf1 and Eaf2/U19 knockdown with high frequency (Fig. 9A). However, overexpression of wnt11 by mRNA injection did not affect the expression of eaf1 and eaf2/u19 (Fig. 8). These findings imply that eaf1 and eaf2/u19 act upstream of wnt11 to control its expression and to govern convergence and extension movements. Furthermore, Eaf1 and Eaf2/U19 knockdown down-regulated wnt5 expression (Fig. 7, A and C), and wnt5 mRNA, in turn, also rescued defects in convergence and extension movements caused by the loss of eaf1 and eaf2/u19 (Fig. 9A (A4)). However, overexpression of eaf1 and eaf2/u19 also down-regulated wnt5 expression, with expression levels comparable with those seen in the eafs morphants (Fig. 7, E and G). This suggests that the maintenance of wnt5 might require a specific level or ratio of eaf1 and eaf2/u19. Similar to that of wnt11, wnt5 overexpression did not affect the expression of eaf1 and eaf2/u19, suggesting that eaf1 and eaf2/u19 also act upstream of wnt5. As a downstream activator of noncanonical Wnt ligands, RhoA rescued convergence and extension movement defect more effectively than Wnt5 and Wnt11 (Fig. 8), indicating a convergence of wnt5 and wnt11 on rhoA during the regulation of convergence and extension movements. Of note, the expression patterns of eaf1 and eaf2/u19 were similar to that of rhoA during zebrafish and Xenopus embryogenesis (22, 40).
Notably, eaf1 and eaf2/u19 have activity in stimulating ELL transcriptional elongation (7). Thus, if knockdown of eaf1 and eaf2/u19 only caused inhibition of gene expression, eafs regulating the expression of Wnts (wnt11, wnt5, and wnt11r) presented in this study might be nonspecific as a result of disrupting the general function of eafs as regulators of transcriptional elongation. However, in addition to wnt4a,4 foxo5 (Fig. 9A) as well as other genes5 were up-regulated by eafs knockdown, unrelated to their function as regulators of transcriptional elongation. Therefore, eafs knockdown inhibiting the expression of Wnts appears to be specific. Taking into consideration that the knockdown of eafs did not affect cell fate specification at the beginning of gastrulation, it suggests that eafs might directly regulate noncanonical Wnt ligands (especially for wnt11). However, this conclusion needs to be further verified by more direct ways, such as promoter chromatin immunoprecipitation assays, etc.
Knockdown of Eaf1 and Eaf2/U19 protein levels also resulted in the failure of fusion of the heart and pancreas primordia (Fig. 6), but neither endoderm fate determination genes nor myocardial precursor marker genes displayed significant changes in their expression levels (Fig. 6, F–H). These observations imply that eaf1 and eaf2/u19 act as novel regulators of midline convergence of both endoderm- and mesoderm-derived organ primordia without affecting the specification of progenitors.
Although a primary role of non-canonical Wnt signaling was to govern convergence and extension movements, studies had also shown that the three Wnt noncanonical ligands, wnt4a, wnt11, and wnt11r, redundantly regulated midline convergence of organ primordia, in zebrafish embryos (31, 41). In this study, we showed that the midline migration of heart precursors and pancreas precursors required expression of eaf1 and eaf2/u19 (Fig. 6). We also showed that eaf1 and eaf2/u19 mediated midline convergence through noncanonical Wnt signaling, specifically by regulating expression of wnt11 and wnt11r (). Unexpectedly, however, knockdown of eaf1 and eaf2/u19 up-regulated wnt4a expression.4 This observation suggested that, unlike wnt11 and wntllr, wnt4a was not a downstream factor of eaf1 and eaf2/u19 in the signaling pathway that regulated midline convergence of organ precursors.
In conclusion, our data are the first to demonstrate that eaf1 and eaf2/u19 have essential roles in regulating embryonic cell behavior and migration. Although the complete pathway has yet to be defined, our study shows that eaf1 and eaf2/u19 function in this pathway by specifically modulating expression of wnt11 and wnt5, which in turn converge on rhoA for the positive regulation of convergence and extension movements.
Acknowledgments
We thank Drs. Zhan Yin, Yonghua Sun, and T. Whitfield for the generous gifts of various reagents. We also thank Moira Hitchens for editing.
X. Wan and W. Xiao, unpublished data.
J.-X. Liu and W. Xiao, unpublished data.
- MO
- morpholino
- WT
- wild type
- GFP
- green fluorescent protein
- RT
- reverse transcription
- hpf
- hours postfertilization
- STD-MO
- standard morpholino.
REFERENCES
- 1.Thirman M. J., Levitan D. A., Kobayashi H., Simon M. C., Rowley J. D. ( 1994) Proc. Natl. Acad. Sci. U. S. A. 91, 12110– 12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simone F., Polak P. E., Kaberlein J. J., Luo R. T., Levitan D. A., Thirman M. J. ( 2001) Blood 98, 201– 209 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z., Tufts R., Haleem R., Cai X. ( 1997) Proc. Natl. Acad. Sci. U. S. A. 94, 12999– 13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simone F., Luo R. T., Polak P. E., Kaberlein J. J., Thirman M. J. ( 2003) Blood 101, 2355– 2362 [DOI] [PubMed] [Google Scholar]
- 5.Shilatifard A., Lane W. S., Jackson K. W., Conaway R. C., Conaway J. W. ( 1996) Science 271, 1873– 1876 [DOI] [PubMed] [Google Scholar]
- 6.Mitani K., Yamagata T., Iida C., Oda H., Maki K., Ichikawa M., Asai T., Honda H., Kurokawa M., Hirai H. ( 2000) Biochem. Biophys. Res. Commun. 279, 563– 567 [DOI] [PubMed] [Google Scholar]
- 7.Kong S. E., Banks C. A., Shilatifard A., Conaway J. W., Conaway R. C. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 10094– 10098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo R. T., Lavau C., Du C., Simone F., Polak P. E., Kawamata S., Thirman M. J. ( 2001) Mol. Cell. Biol. 21, 5678– 5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao W., Zhang Q., Jiang F., Pins M., Kozlowski J. M., Wang Z. ( 2003) Cancer Res. 63, 4698– 4704 [PubMed] [Google Scholar]
- 10.Xiao W., Zhang Q., Habermacher G., Yang X., Zhang A. Y., Cai X., Hahn J., Liu J., Pins M., Doglio L., Dhir R., Gingrich J., Wang Z. ( 2008) Oncogene 27, 1536– 1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao W., Ai J., Habermacher G., Volpert O., Yang X., Zhang A. Y., Hahn J., Cai X., Wang Z. ( 2009) Cancer Res. 69, 2599– 2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H., Kimelman D. ( 2002) Dev. Cell 2, 607– 616 [DOI] [PubMed] [Google Scholar]
- 13.Langheinrich U., Hennen E., Stott G., Vacun G. ( 2002) Curr. Biol. 12, 2023– 2028 [DOI] [PubMed] [Google Scholar]
- 14.Li M., Wu X., Zhuang F., Jiang S., Jiang M., Liu Y. H. ( 2003) Dev. Dyn. 228, 273– 280 [DOI] [PubMed] [Google Scholar]
- 15.Maurus D., Héligon C., Bürger-Schwärzler A., Brändli A. W., Kühl M. ( 2005) EMBO J. 24, 1181– 1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers D. C., Sepich D. S., Solnica-Krezel L. ( 2002) Trends Genet. 18, 447– 455 [DOI] [PubMed] [Google Scholar]
- 17.Keller R., Shih J., Domingo C. ( 1992) Dev. Suppl. 81– 91 [PubMed] [Google Scholar]
- 18.Stainier D. Y. ( 2001) Nat. Rev. 2, 39– 48 [DOI] [PubMed] [Google Scholar]
- 19.Ober E. A., Olofsson B., Mäkinen T., Jin S. W., Shoji W., Koh G. Y., Alitalo K., Stainier D. Y. ( 2004) EMBO Rep. 5, 78– 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heisenberg C. P., Tada M., Rauch G. J., Saúde L., Concha M. L., Geisler R., Stemple D. L., Smith J. C., Wilson S. W. ( 2000) Nature 405, 76– 81 [DOI] [PubMed] [Google Scholar]
- 21.Kilian B., Mansukoski H., Barbosa F. C., Ulrich F., Tada M., Heisenberg C. P. ( 2003) Mech. Dev. 120, 467– 476 [DOI] [PubMed] [Google Scholar]
- 22.Zhu S., Liu L., Korzh V., Gong Z., Low B. C. ( 2006) Cell. Signal. 18, 359– 372 [DOI] [PubMed] [Google Scholar]
- 23.Westerfield M., Doerry E., Douglas S. ( 1999) Trends Genet. 15, 248– 249 [DOI] [PubMed] [Google Scholar]
- 24.Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. ( 1995) Dev. Dyn. 203, 253– 310 [DOI] [PubMed] [Google Scholar]
- 25.Hammond K. L., Loynes H. E., Folarin A. A., Smith J., Whitfield T. T. ( 2003) Development 130, 1403– 1417 [DOI] [PubMed] [Google Scholar]
- 26.Yang Z., Liu N., Lin S. ( 2001) Dev. Biol. 231, 138– 148 [DOI] [PubMed] [Google Scholar]
- 27.Ando R., Hama H., Yamamoto-Hino M., Mizuno H., Miyawaki A. ( 2002) Proc. Natl. Acad. Sci. U. S. A. 99, 12651– 12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jopling C., den Hertog J. ( 2005) EMBO Rep. 6, 426– 431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jopling C., van Geemen D., den Hertog J. ( 2007) PLoS Genet. 3, e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jopling C., Hertog J. ( 2007) Mech. Dev. 124, 129– 136 [DOI] [PubMed] [Google Scholar]
- 31.Matsui T., Raya A., Kawakami Y., Callol-Massot C., Capdevila J., Rodríguez-Esteban C., Izpisúa Belmonte J. C. ( 2005) Genes Dev. 19, 164– 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiter J. F., Kikuchi Y., Stainier D. Y. ( 2001) Development 128, 125– 135 [DOI] [PubMed] [Google Scholar]
- 33.Wallingford J. B., Fraser S. E., Harland R. M. ( 2002) Dev. Cell 2, 695– 706 [DOI] [PubMed] [Google Scholar]
- 34.Westfall T. A., Brimeyer R., Twedt J., Gladon J., Olberding A., Furutani-Seiki M., Slusarski D. C. ( 2003) J. Cell Biol. 162, 889– 898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao Q., Yokota C., Puck H., Kofron M., Birsoy B., Yan D., Asashima M., Wylie C. C., Lin X., Heasman J. ( 2005) Cell 120, 857– 871 [DOI] [PubMed] [Google Scholar]
- 36.Correa R. G., Matsui T., Tergaonkar V., Rodriguez-Esteban C., Izpisua-Belmonte J. C., Verma I. M. ( 2005) Curr. Biol. 15, 1291– 1295 [DOI] [PubMed] [Google Scholar]
- 37.Yamashita S., Miyagi C., Carmany-Rampey A., Shimizu T., Fujii R., Schier A. F., Hirano T. ( 2002) Dev. Cell 2, 363– 375 [DOI] [PubMed] [Google Scholar]
- 38.Topczewski J., Sepich D. S., Myers D. C., Walker C., Amores A., Lele Z., Hammerschmidt M., Postlethwait J., Solnica-Krezel L. ( 2001) Dev. Cell 1, 251– 264 [DOI] [PubMed] [Google Scholar]
- 39.Jessen J. R., Topczewski J., Bingham S., Sepich D. S., Marlow F., Chandrasekhar A., Solnica-Krezel L. ( 2002) Nat. Cell Biol. 4, 610– 615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wünnenberg-Stapleton K., Blitz I. L., Hashimoto C., Cho K. W. ( 1999) Development 126, 5339– 5351 [DOI] [PubMed] [Google Scholar]
- 41.Miyasaka K. Y., Kida Y. S., Sato T., Minami M., Ogura T. ( 2007) Proc. Natl. Acad. Sci. U. S. A 104, 11274– 11279 [DOI] [PMC free article] [PubMed] [Google Scholar]