Abstract
The peripheral stalk of F1F0 ATP synthase is essential for the binding of F1 to FO and for proper transfer of energy between the two sectors of the enzyme. The peripheral stalk of Escherichia coli is composed of a dimer of identical b subunits. In contrast, photosynthetic organisms express two b-like genes that form a heterodimeric peripheral stalk. Previously we generated chimeric peripheral stalks in which a portion of the tether and dimerization domains of the E. coli b subunits were replaced with homologous sequences from the b and b′ subunits of Thermosynechococcus elongatus (Claggett, S. B., Grabar, T. B., Dunn, S. D., and Cain, B. D. (2007) J. Bacteriol. 189, 5463–5471). The spatial arrangement of the chimeric b and b′ subunits, abbreviated Tb and Tb′, has been investigated by Cu2+-mediated disulfide cross-link formation. Disulfide formation was studied both in soluble model polypeptides and between full-length subunits within intact functional F1F0 ATP synthase complexes. In both cases, disulfides were preferentially formed between TbA83C and Tb′A90C, indicating the existence of a staggered relationship between helices of the two chimeric subunits. Even under stringent conditions rapid formation of disulfides between these positions occurred. Importantly, formation of this cross-link had no detectable effect on ATP-driven proton pumping, indicating that the staggered conformation is compatible with normal enzymatic activity. Under less stringent reaction conditions, it was also possible to detect b subunits cross-linked through identical positions, suggesting that an in-register, nonstaggered parallel conformation may also exist.
F1F0 ATP synthases are found in the inner mitochondrial membrane, the thylakoid membrane of chloroplasts, and the cytoplasmic membrane of bacteria (1–4). These enzymes are responsible for harnessing an electrochemical gradient of protons across the membranes for the synthesis of ATP. In Escherichia coli F1F0 ATP synthase, the membrane-embedded F0 sector is composed of subunits ab2c10 and a soluble F1 portion composed of subunits α3β3γδϵ. The F0 sector houses a proton channel located principally in the a subunit, and the flow of protons through F0 generates torque used to rotate the c10 subunit ring relative to the ab2 subunits. The F1 γ and ϵ subunits are bound to the c10 ring and form the central or rotor stalk. Catalytic sites are located at the interfaces of each αβ pair in F1. The γ subunit extends into the center of the α3β3 hexamer, creating an asymmetry in the conformations of the αβ pairs (5). It is the rotation of the γ subunit and the resulting sequential conformational changes in each αβ pair that provides the driving force for the synthesis of ATP at the catalytic sites. The α3β3 hexamer is held stationary relative to the rotary stalk by the peripheral stalk consisting of the b2δ subunits.
The peripheral stalk is essential for binding F1 to F0 and for coupling proton translocation to catalytic activity (6–8). In the E. coli enzyme, the peripheral stalk is a dimer of identical b subunits. The stalk has been conceptually divided into functional domains called the membrane domain (bM1-I33), the tether domain (bE34-A61), the dimerization domain (bT62-K122), and the F1-binding domain (bQ123-L156) (9). Although there is ample evidence of direct protein-protein interactions between b subunits within the membrane, dimerization, and F1-binding domains, there is remarkably little evidence of tight packing between the b subunits in the tether domain. In fact, electron spin resonance studies suggested that the tether domains of the two b subunits may be separated by more than 20 Å in the F1F0 complex (10, 11). Much of what is known about the structure of the stalk has been inferred from analysis of the properties of polypeptides modeling segments of the b subunit. The structure of a peptide modeling the membrane domain, bM1-E34, has been determined by NMR (12), and a peptide based on the dimerization domain, bT62-K122, has been determined by x-ray diffraction (13). Both polypeptides assumed α-helical conformations, but neither structure directly revealed b subunit dimerization interactions. Recently, Priya et al. (14) reported a low resolution structure of a bM22-L156 dimer, but the extended conformation appears to be slightly too long to accurately reflect the dimensions of the peripheral stalk within the F1F0 complex.
Molecular modeling efforts supported by a variety of biochemical and biophysical experiments have yielded competing right-handed coiled coil (15, 16) and left-handed coiled coil (17, 18) models for the peripheral stalk. The parallel two-stranded left-handed coiled coli is a well known structure characterized by knobs-into-holes packing of the side chains of the two helices that are aligned in-register. An in-register conformation implies that any specific amino acid on the b subunit-subunit interface would occupy a position immediately adjacent to its counterpart in the other b subunit. In contrast, del Rizzo et al. (16) proposed a novel parallel right-handed coiled coil with the helices of the two b subunits offset by approximately one and a half turns of an α helix. This staggered model positions the two identical residues contributed by each of the b subunits in a homodimer into differing environments and at considerable distance from one another. Sequence analyses have been offered in support of both models (16, 18). In terms of experimental support, cross-linking studies of polypeptides have provided evidence that dimer packing could be in-register at many sites starting from residue Ala59 and continuing to the carboxyl termini in model bY24–L156 dimers and in bD53–K122 dimers (9, 16). Cross-linking at a few of the carboxyl-proximal positions has been confirmed within intact F1F0 ATP synthase complexes (19, 20). Recent electron spin resonance distance measurements on b24–156 have also been interpreted as support for the in-register arrangement (17, 18). Conversely, work with polypeptides modeling the b subunit has generated evidence favoring a staggered conformation in this section of the dimer (15, 16, 21). In mixtures of dimerization domain polypeptides with cysteines incorporated at different sites, disulfides preferentially formed between positions that were 4–7 residues apart. For example, bD53–L156 dimers were covalently locked into the offset conformation by the formation of disulfide bridges between cysteines introduced at positions bA79 and bR83 as well as bR83 and bA90 (15). These staggered dimers were more stable, melted with higher cooperativity, and bound soluble F1 with higher affinity than bD53–L156 dimers fixed in the in-register arrangement. Moreover, active and coupled F1F0 complexes were assembled with heterodimeric peripheral stalks using b subunits with tether domains varying in length by as many as 14 amino acids (22). These F1F0 complexes had peripheral stalks that were by definition out of register, at least within the tether domain.
In contrast to the homodimer of identical b subunits observed in the peripheral stalk of E. coli, photosynthetic organisms express two b-like subunits, b and b′, that are thought to form heterodimeric peripheral stalks in F1F0 ATP synthase. Previously, we generated heterodimeric peripheral stalks within the E. coli F1F0 by constructing chimeric b subunits (23). Segments of the tether and dimerization domains of the E. coli b subunit were replaced with the homologous regions of the Thermosynechococcus elongatus b and b′ subunits. The chimeric subunits formed heterodimeric peripheral stalks that were incorporated into intact, functional F1F0 ATP synthase complexes. The most active chimeric enzymes had T. elongatus primary sequences replacing residues bE39–I86 of the E. coli b subunit. For simplicity, these chimeric subunits will be referred to here as Tb and Tb′.
The ability to generate F1F0 ATP synthases with Tb/Tb′ heterodimeric peripheral stalks provided a means to investigate the positions of the two subunits in the peripheral stalk. In the present work, we show that the Tb and Tb′ subunits assumed preferred positions relative to one another within the F1F0 complex. The staggered conformation appears to be a favored and functional conformation for the peripheral stalk. However, within a population of F1F0 complexes, some complexes with peripheral stalks in the in-register conformation are likely to exist.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
The host strain for all membrane preparations was KM2 (Δb), an E. coli strain that carries a chromosomal deletion of the uncF gene (b subunit) (24). Strain MM294 was used for the expression of peptides modeling the b subunit (25). F1F0 ATP synthase viability was assessed in vivo by growing cells on minimal A medium supplemented with succinate (0.2%, w/v). The cells for membrane preparations were grown in Luria broth supplemented with glucose (0.2%, w/v) and isopropyl-1-thio-β-d-galactopyranoside (40 μg/ml). Ampicillin (100 μg/ml) and chloramphenicol (25 μg/ml) were added as needed. All of the cells were grown at 37 °C.
Expression Plasmid Construction
A b subunit gene was synthesized by GenScript that contained a C21S mutation and a V5 epitope tag (GKPIPNPLLGLDST) on the carboxyl terminus. The C21S mutation is present in all b, Tb, and Tb′ subunit gene constructs but will not be included in the subunit designations throughout the paper for clarity. Plasmid pSBC127 (bV5) was generated by ligating the synthetic uncF(b) subunit gene from GenScript into the EcoRI/KpnI restriction sites of pUC19. Cysteine substitutions were engineered into b subunits by site-directed mutagenesis of plasmid pSBC127 (bV5) using the oligonucleotides listed in supplemental Table S1. The plasmids were constructed for the expression of chimeric Tb or Tb′ subunits with a cysteine substituted for residue Ala83 or Ala90 (see Table 1). Tb subunit substitutions were made in plasmid pSBC97 (Tbhis) that has T. elongatus b subunit sequence substituted for residues Glu39–Ile86 and a histidine tag at the amino terminus. Tb′ constructs were based on plasmid pSBC98 (Tb′V5) that has the T. elongatus b′ subunit sequence substituted for the same region and a V5 epitope tag on the carboxyl terminus. All of the mutations were confirmed by direct nucleotide sequencing.
TABLE 1.
Growth of bacterial strains
| Strain/plasmid(s) | Gene producta–e | Antibiotic resistancef | Growth on succinateg | Source or reference |
|---|---|---|---|---|
| KM2/pTAM37/pTAM46 | bhis/bV5 | Ap+Cm | +++ | Ref. 22 |
| KM2/pBR322 | Δb | Ap | − | New England Biolabs |
| KM2/pSBC127 | bV5 | Ap | ++ | This study |
| KM2/pSBC128 | bI76C, V5 | Ap | ++ | This study |
| KM2/pSBC129 | bR83C, V5 | Ap | ++ | This study |
| KM2/pSBC130 | bA90C, V5 | Ap | ++ | This study |
| KM2/pSBC131 | bE97C, V5 | Ap | + | This study |
| KM2/pSBC97 | Tb | Cm | + | [23] |
| KM2/pSBC123 | TbA83C | Cm | + | This study |
| KM2/pSBC124 | TbA90C | Cm | + | This study |
| KM2/pSBC98 | Tb′ | Ap | ++ | [23] |
| KM2/pSBC125 | Tb′A83C | Ap | ++ | This study |
| KM2/pSBC126 | Tb′A90C | Ap | ++ | This study |
| KM2/pSBC97/pSBC98 | Tb/Tb′ | Ap + Cm | ++ | [23] |
| KM2/pSBC123/pSBC125 | TbA83C/Tb′A83C | Ap + Cm | ++ | This study |
| KM2/pSBC123/pSBC126 | TbA83C/Tb′A90C | Ap + Cm | ++ | This study |
| KM2/pSBC124/pSBC125 | TbA90C/Tb′A83C | Ap + Cm | + | This study |
| KM2/pSBC124/pSBC126 | TbA90C/Tb′A90C | Ap + Cm | ++ | This study |
| MM294/pSD359 | Db+GGGC | Cm | N/A | This study |
| MM294/pSD358 | Db′+GGGC | Ap | N/A | This study |
| MM294/pSD361 | PbA83C | Ap | N/A | This study |
| MM294/pSD363 | PbA90C | Ap | N/A | This study |
| MM294/pSD362 | Pb′A83C | Ap | N/A | This study |
| MM294/pSD364 | Pb′A90C | Ap | N/A | This study |
a Tb, wild-type E. coli b subunit with sequence from the b subunit of T. elongatus substituted for amino acids Glu39–Ile86, a C21S mutation, and a six-histidine epitope tag at the amino terminus.
b Tb′, wild-type E. coli b subunit with sequence from the b′ subunit of T. elongatus substituted for amino acids Glu39–Ile86, a C21S mutation, and a V5 epitope tag (GKPIPNPLLGLDST) at the carboxyl terminus.
c The native cysteine at bC21has been mutated to serine in all pSBC plasmids.
d The Db and Db′ genes produce peptides modeling the dimerization domains of the Tb and Tb′ subunits. These peptides have the sequence MSY at the amino terminus, contain Tb/Tb′ residues Asp53–Lys122in the middle, and terminate with the sequence GGGC at the carboxyl terminus.
e The Pb and Pb′ genes produce peptides modeling the D53-L156 regions of the Tb and Tb′ subunits. These peptides also start with the sequence MSY and have cysteine residues substituted at either Ala83 or Ala90 as specified.
f Ap, ampicillin; Cm, chloramphenicol.
g +++, wild-type growth; ++, colonies smaller than wild-type; +, small colony formation; −, no growth.
Plasmids were also constructed to facilitate expression of soluble polypeptides modeled on the Tb and Tb′ subunits (see Table 1). Regions of interest from the parent plasmids were amplified by PCR using the primers listed in supplemental Table S1 and cloned into the expression plasmid pJB3 (26) using the unique NdeI and HindIII sites. The forward primers for all of the Tb chimeras, SD101, and the Tb′ chimeras, SD102, included an NdeI site and encoded a protein starting with residues MSY followed by Tb or Tb′ sequence beginning at residue Asp53. Four plasmids were constructed to express peptides modeling the Tb or Tb′ subunit residues Asp53–Leu156 by PCR amplification of plasmids pSBC123 (TbE39–I86,A83C), pSBC124 (TbE39–I86,A90C), pSBC125 (Tb′E39–I86,A83C) and pSBC126 (Tb′E39–I86,A90C). Primers SD101/SD102 were used as the forward primer and SD104 as the reverse primer to generate plasmids pSD361 (PbA83C), pSD362 (Pb′A83C), pSD363 (PbA90C), and pSD364 (Pb′A90C), abbreviated as Pb and Pb′. Two additional plasmids expressing peptides modeling the Tb and Tb′ dimerization domain residues Asp53–Lys122 and containing a GGGC extension on the carboxyl terminus were constructed using SD103 as the reverse PCR primer and pSBC97 (Tb) or pSBC98 (Tb′) as the template DNA. The resulting plasmids pSD357 (Db+GGGC) and pSD358 (Db′+GGGC) are abbreviated as Db and Db′. Plasmid pSD357 (Db+GGGC) was moved into the expression plasmid pACT3 (27) using the EcoRI and HindIII sites to produce pSD359 (Db+GGGC) and allow coexpression with pSD358 (Db′+GGGC).
Preparation and Assay of Membranes
Membrane vesicles were prepared as described previously (28). Membrane protein concentrations were determined using the bicinchoninic acid method (29). ATP-driven proton pumping assays were conducted by adding 500 μg of membrane protein to 3.5 ml of reaction buffer (50 mm MOPS,2 pH 7.3], 10 mm MgCl2) and monitoring the fluorescence quenching of 9-amino-6-chloro-2-methoxyacridine (30). Fluorescence quenching was detected using a Photon Technologies International QuantaMaster 4 spectrofluorometer.
The ATP hydrolysis activities in membrane samples were determined using the 7-methyl-6-thioguanosine (MESG) method (31). This assay determines the rate of ATP hydrolysis by using the enzyme purine nucleotide phosphorylase (PNP) to catalyze the reaction of inorganic phosphate (Pi) with MESG to form 7-methyl-6-thioguanine. The appearance of product was detected as an absorbance increase at 360 nm using a Varian Cary 50 spectrophotometer. A PNP stock solution was made by dissolving the solid enzyme in water to a concentration of 0.1 units/ml and stored at 4 °C. An MESG stock solution was made in water to a final concentration of 1 mm and stored in 5-ml aliquots at −20 °C. The MESG solution was thawed prior to use, and both MESG and PNP were kept on ice until needed. Each reaction consisted of 10–25 mg of membrane protein, 50 ml of 20× TM buffer (1 m Tris-HCl, pH 7.5, 200 mm MgSO4), 300 ml of MESG, 50 ml of PNP, and water to a final volume of 1 ml. The components were combined in a disposable glass tube, agitated briefly to mix, and poured into a cuvette prewarmed to 37 °C. Each reaction was allowed to sit for 1 min to stabilize the temperature before starting by the addition of 30 ml of 0.15 m ATP made in TM buffer. Absorbance data points were collected continuously for 5 min. The slope of the linear portion of the reaction was determined in units of absorbance/min using the software associated with the instrument. Stimulation of ATP hydrolysis by N,N-dimethyldodecylamine N-oxide (LDAO) was determined by adding LDAO to each reaction to a final concentration of 0.5% as described previously (32).
Heterodimeric peripheral stalks were detected using a purification procedure essentially as described previously (22). Membrane samples were solubilized and purified over a high capacity nickel chelate affinity matrix (Sigma) to selectively retain F1F0 ATP synthase complexes containing at least one histidine tag. Membrane- and nickel resin-purified proteins were separated on a 15% SDS-PAGE gel for immunoblot analysis (33). Primary antibodies used were an antibody against the b subunit (a gift from G. Deckers-Hebestreit) and an antibody against the V5 epitope tag (Invitrogen). Secondary antibodies used were horseradish peroxidase-linked anti-rabbit and anti-mouse antibodies, respectively. The presence of bound secondary antibody was detected by chemiluminescence with the Amersham Biosciences ECL Plus Western blotting detection system.
Expression and Purification of Soluble b Subunits
The chimeric dimerization domains Db+GGGC and Db′+GGGC were coexpressed by isopropyl-1-thio-β-d-galactopyranoside induction in cells carrying both pSD359 (Db+GGGC) and pSD359 (Db′+GGGC). The Tb and Tb′ chimeras were separated by ion exchange chromatography, exploiting their substantial difference in charge. The cell extracts were first fractionated by procedures similar to those that have been previously described (16) and then clarified by ultracentrifugation. Proteins that precipitated with ammonium sulfate at concentrations between 40 and 60% of saturation were collected. Anion exchange chromatography on DEAE-Sepharose at pH 8.0 separated the domains into two peaks. Further fractionation of the first peak by cation exchange chromatography on SP-Sepharose at pH 5.0 yielded essentially pure Tb dimerization domain. Application of the cation exchange step to the second peak resulted in partially purified b′ chimera; subsequent size exclusion chromatography on a column of Sephadex G-75 yielded the pure polypeptide. Chimeric polypeptides consisting of residues Asp53–Leu156 and containing internal cysteine substitutions at either position Ala83 or Ala90 were expressed by isopropyl-1-thio-β-d-galactopyranoside induction of cells carrying plasmids pSD361, pSD362, pSD363, or pSD364. Following cell breakage and clarification of the extracts by ultracentrifugation, the supernatant solutions were brought to 45% saturation with ammonium sulfate. The precipitates were collected by centrifugation, dissolved, and dialyzed against 50 mm Tris-HCl, pH 7.5, containing 1 mm EDTA and 1 mm dithiothreitol. These partially purified samples were used for disulfide formation studies without further purification. The identity and purity of each product was confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry; the masses of the Tb and Tb′ subunits differ by 167 Da. All of the buffers included dithiothreitol to a final concentration of 1 mm to maintain the cysteines in a reduced state. Protein concentrations were determined by the Advanced Assay (Cytoskeleton, Inc.) as described previously (16). Purified proteins were analyzed on 15% SDS-PAGE gels using a Tris-Tricine buffer system (9).
Disulfide Cross-linking
Disulfide cross-links between b subunits in the peripheral stalk were formed by incubating membrane samples with 50 mm Cu2+ for time periods specified for each experiment. The cross-linking reactions were carried out in open 1.5-ml microcentrifuge tubes with mixing at 300 rpm in an Eppendorf Thermomixer R heated to 37 °C. Each reaction was stopped by adding fresh N-ethylmaleimide (NEM) to a final concentration of 5 mm, through mixing and cooling on ice. Two time points were taken for each sample, a zero time point and a 2- or 10-min time point. The zero time point sample was obtained by diluting the concentrated membranes to 5 mg/ml in TM buffer and adding NEM to react with free cysteine residues and prevent cross-linking. The reaction was mixed and allowed to sit at room temperature for 15 s before adding Cu2+ and placing on ice. Cross-linking for 2 or 10 min was done by diluting the membrane sample to 5 mg/ml in TM buffer and warming to 37 °C in the thermomixer. Prewarmed Cu2+ was added, and the samples were allowed to incubate with mixing for 2 or 10 min before further cross-linking was stopped by the addition of NEM. A total of 1 μg of each cross-linked sample was analyzed by Western blot using an antibody against the V5 epitope tag. Each band in the Western blot was quantified using the program UN-SCAN-IT (Silk Scientific).
Soluble b Subunit Cross-linking
The propensity of the soluble Tb and Tb′ dimerization domains with carboxyl-terminal GGGC (DbGGGC and Db′GGGC) additions to form homodimers and heterodimers was determined by dialysis of either individual polypeptides, or an equimolar mixture of the Tb and Tb′ constructs, overnight at 4 °C against buffer containing 50 mm sodium phosphate, 10 μm CuCl2, and cysteine at concentrations of 0, 1, or 10 mm. The total protein concentration was 0.5 mg/ml in each case. The samples were treated with SDS sample buffer containing NEM to prevent further cross-link formation. The tendency of chimeric polypeptides consisting of residues Asp53–Leu156 and containing internal cysteine substitutions at either residue Ala83 or Ala90 to form disulfide cross-links was assessed by dilution of partially purified protein samples, or mixtures, into a sulfhydril/disulfide buffer prepared by the addition of 10 mm β-mercaptoethanol and 2.5 mm dithiobisnitrobenzoate to 50 mm Tris-HCl buffer, pH 7.4. Following incubation at 25 °C for 30 min, the samples were treated with SDS sample buffer containing 15 mm NEM to prevent further cross-link formation.
RESULTS
Cysteine Substitutions in the E. coli b Subunit
A plasmid carrying a synthetic cysteine-less b subunit gene equipped with a carboxyl-terminal V5 epitope tag (bV5) was generated to facilitate construction and expression of recombinant subunits. To look for evidence of an in-register conformation in the dimerization domain of the peripheral stalk of the E. coli F1F0 ATP synthase, four mutations were constructed within the synthetic gene for expression of cysteine substitution subunits bI76C,V5, bR83C,V5, bA90C,V5, and bE97C,V5 (Table 1). These positions were selected based on earlier work with model polypeptides (16). Each of the new plasmids was transformed into strain E. coli strain KM2 (Δb). Complementation of the strain was examined by growth on minimal A medium supplemented with succinate. The succinate growth experiments indicated that all cysteine insertion b subunits were functionally incorporated into F1F0 ATP synthase complexes (Table 1). However, it should be noted that the colonies of KM2/pSBC127 (bV5) expressing the synthetic uncF(b) gene were typically smaller than a strain with the epitope tags added to native uncF(b) genes. Subsequent inclusion of the cysteine substitution mutations had no further discernable effect on growth.
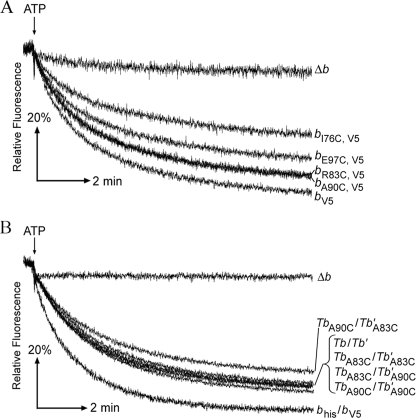
The membranes from each strain were prepared and studied for ATP hydrolysis activity in both the absence of presence of 0.5% LDAO as a measure of the relative amounts of intact F1F0 complexes (Table 2). Membranes from KM2/pSBC128 (bI76C,V5) had only two-thirds of the activity seen in the positive control membranes, but F1F0-ATPase activities in the other cysteine mutation membrane samples were not statistically different from values obtained for control KM2/pTAM37/pTAM46 (bhis/bV5) membranes. The extent of coupling between ATP hydrolysis at F1 and proton pumping at F0 was quantified using a fluorescence-based ATP-driven proton pumping assay (Fig. 1A). Although very significant levels of coupled activity were evident in all of the samples, the b subunit cysteine substitution membranes had somewhat reduced proton pumping activity. As might be expected, strain KM2/pSBC128 (bI76C,V5) membranes showed the most significant reduction, with only ∼50% of activity found in KM2/pSBC127 (bV5) membranes. Nevertheless, all four cysteine substitution b subunits supported enzyme complex assembly and provided readily detectable levels of F1F0 ATP synthase activity.
TABLE 2.
ATP hydrolysis activity of engineered F1F0
| Strain/Plasmid(s) | Gene producta | ATP hydrolysisb |
Fold induction | |
|---|---|---|---|---|
| −LDAO | +LDAOc | |||
| KM2/pTAM37/pTAM46 | bhis/bV5 | 0.30 ± 0.02 | 1.2 ± 0.06 | 4.0 |
| KM2/pBR322 | Δb | 0.07 ± 0.01 | 0.2 ± 0.01 | 2.3 |
| KM2/pSBC127 | bV5 | 0.29 ± 0.01 | 1.3 ± 0.08 | 4.4 |
| KM2/pSBC128 | bI76C, V5 | 0.19 ± 0.01 | 0.9 ± 0.03 | 4.6 |
| KM2/pSBC129 | bR83C, V5 | 0.28 ± 0.07 | 1.2 ± 0.07 | 4.3 |
| KM2/pSBC130 | bA90C, V5 | 0.29 ± 0.04 | 1.2 ± 0.11 | 4.0 |
| KM2/pSBC131 | bE97C, V5 | 0.33 ± 0.07 | 1.1 ± 0.07 | 3.4 |
| KM2/pSBC97 | Tb | 0.30 ± 0.02 | 1.0 ± 0.15 | 3.4 |
| KM2/pSBC123 | TbA83C | 0.19 ± 0.03 | 0.7 ± 0.05 | 3.7 |
| KM2/pSBC124 | TbA90C | 0.20 ± 0.04 | 0.7 ± 0.06 | 3.5 |
| KM2/pSBC98 | Tb′ | 0.17 ± 0.02 | 0.5 ± 0.06 | 2.9 |
| KM2/pSBC125 | Tb′A83C | 0.10 ± 0.01 | 0.2 ± 0.01 | 2.2 |
| KM2/pSBC126 | Tb′A90C | 0.10 ± 0.01 | 0.2 ± 0.01 | 2.3 |
| KM2/pSBC97/pSBC98 | Tb/Tb′ | 0.24 ± 0.02 | 0.9 ± 0.04 | 3.7 |
| KM2/pSBC123/pSBC125 | TbA83C/Tb′A83C | 0.19 ± 0.01 | 0.6 ± 0.01 | 3.2 |
| KM2/pSBC123/pSBC126 | TbA83C/Tb′A90C | 0.23 ± 0.02 | 0.8 ± 0.02 | 3.4 |
| KM2/pSBC124/pSBC125 | TbA90C/Tb′A83C | 0.19 ± 0.02 | 0.7 ± 0.05 | 3.8 |
| KM2/pSBC124/pSBC126 | TbA90C/Tb′A90C | 0.21 ± 0.02 | 0.8 ± 0.07 | 3.6 |
a See Table 1 for gene product definitions.
b Reported in μmol of Pi/mg of membrane protein/min.
c Used to release F1from the inhibitory effect of F0.
FIGURE 1.
Effects of b subunit engineering on enzyme coupling as measured by ATP-driven energization of inverted membrane vesicles (34). Membranes prepared from strains KM2/pTAM37/pTAM46 (bhis/bV5) and KM2/pBR322 (Δb) were used as positive and negative controls, respectively. The point where ATP was added is indicated above each graph, and the expressed b subunits are listed on the right. The traces were obtained in triplicate using a Photon Technologies International spectrofluorimeter, and the results shown are individual representative results. A, traces shown are from membranes prepared from strains KM2/pSBC127 (bV5), KM2/pSBC128 (bI76C,V5), KM2/pSBC129 (bR83C,V5), KM2/pSBC130 (bA90C,V5), and KM2/pSBC131 (bE97C,V5). B, traces shown are from membranes prepared from strains KM2/pSBC97 (Tb), KM2/pSBC123 (TbA83C), KM2/pSBC124 (TbA90C), KM2/pSBC98 (Tb′), KM2/pSBC125 (Tb′A83C), KM2/pSBC126 (Tb′A90C), KM2/pSBC97/pSBC98 (Tb/Tb′), KM2/pSBC123/pSBC125 (TbA83C/Tb′A83C), KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C), KM2/pSBC124/pSBC125 (TbA90C/Tb′A83C), and KM2/pSBC124/pSBC126 (TbA90C/Tb′A90C).
Evidence for an In-register Parallel Peripheral Stalk
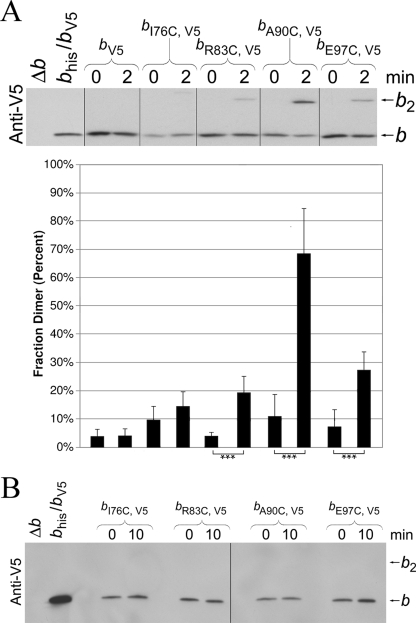
The approach to looking for an in-register conformation for b subunits within the peripheral stalk was straightforward. In these experiments, E. coli b subunits with the single cysteine substitutions were expressed forming a homodimeric peripheral stalk. Disulfide cross-link formation was induced by treating membranes prepared from strains expressing subunits bV5, bI76C,V5, bR83C,V5, bA90C,V5, or bE97C,V5 for 2 min with high concentrations (500 μm) of the oxidizing agent CuCl2. Levels of monomeric and cross-linked dimeric subunits were determined by Western blot analysis using an antibody against the V5 epitope tag (Fig. 2A). Analysis of multiple experiments revealed that ∼70% of the bA90C,V5 subunits could be cross-linked. Only 20–25% of the bR83C,V5 and bE97C,V5 subunits were cross-linked, and very little if any cross-link formation was detectable in the bI76C,V5 sample. The results provided additional evidence of the existence of an in-register conformation of the peripheral stalk in intact F1F0 ATP synthase. To test whether the disulfide cross-links were capable of forming under more selective conditions, the oxidizing agent concentration was lowered to 50 μm, and the reaction time was increased to 10 min. No apparent dimer formation was observed with cysteine substitutions at bI76C, bR83C, bA90C, or bE97C (Fig. 2B). Therefore, even the most efficient cross-link observed in the homodimeric bA90C,V5/bA90C,V5 peripheral stalk was not detectable under more stringent reaction conditions.
FIGURE 2.
Disulfide cross-link formation in F1F0 ATP synthase complexes containing engineered E. coli peripheral stalks. A, membranes were prepared in the presence of 5 mm Tris (2-carboxyethyl) phosphine from strains KM2/pSBC127 (bV5), KM2/pSBC128 (bI76C,V5), KM2/pSBC129 (bR83C,V5), KM2/pSBC130 (bA90C,V5), and KM2/pSBC131 (bE97C,V5). Cross-link formation was induced by diluting samples to 5 mg/ml and adding Cu2+ to a final concentration of 500 μm. The reactions were carried out at 37 °C with shaking for 2 min, and the cross-linking reaction was quenched by adding NEM. The fraction dimer was calculated for each sample by dividing the dimer band by the sum of the monomer and dimer bands. The vertical lines indicate the removal of intervening lanes from the gel in silico. The samples with a low probability of being identical are indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 6). B, samples were prepared and cross-linked essentially as described for A with the exception that the Cu2+ concentration was reduced to 50 μm, and the reaction time was increased to 10 min.
Cross-linking in Tb and Tb′ Polypeptides
Cross-linking in the E. coli b subunits produced evidence supporting an in-register model for the peripheral stalk, but the staggered model cannot be readily tested in a homodimeric stalk because the two b subunits are indistinguishable from each other. Hence, we took advantage the heterodimeric chimeric Tb/Tb′ subunit enzyme (20) to devise a specific test of the staggered conformation hypothesis.
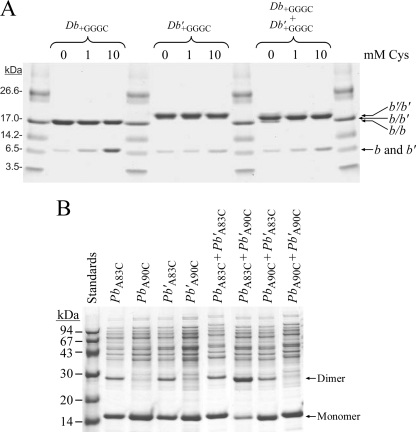
To correlate the properties of Tb and Tb′ with the extensive work on polypeptides modeling the native E. coli b subunit, the dimerization domains containing residues bD53-K122 of the Tb and Tb′ subunits were expressed and purified to homogeneity. Circular dichroism spectra of both forms and of mixtures exhibited strong minima at 208 and 222 nm (data not shown), implying that the chimeric peptides could form both homodimers and heterodimers. To determine whether formation of heterodimer was favored, the dimerization domains were expressed with carboxyl-terminal GGGC additions. A previous study (16) using the E. coli dimerization domain showed that disulfide formation between the added GGGC extensions produced stably linked dimers without perturbing the size and shape of the native structure. Disulfide formation tests were conducted on the individual polypeptides and mixtures using Cu2+-mediated oxidation in the presence of various levels of free cysteine to provide specificity (Fig. 3A). In the absence of any cysteine, both individual domains formed disulfides effectively. The reduced forms of the domains migrated identically, but the disulfide-linked (Db+GGGC)2 dimer was distinguished from the (Db′+GGGC)2 dimer by a difference in migration speed. The prominent product obtained with an equimolar mixture of the two domains showed an intermediate mobility and was assigned as the Db+GGGC/Db′+GGGC heterodimer. The specificity of dimer formation in this experiment can be increased by the addition of free cysteine to the buffer, because peptidyl cysteines that are not located proximal to a partner peptidyl cysteine are more likely to make a mixed disulfide with the free cysteine. The addition of 10 mm cysteine increased the specificity of heterodimer formation in the mixture. These results imply that each of the individual chimeric peptides was capable of forming homodimers under the conditions of the experiment but that the formation of heterodimers was favored in mixtures because of the T. elongatus portion of the sequence.
FIGURE 3.
Disulfide cross-linking of peptide fragments modeling the chimeric Tb and Tb′ subunits. A, the propensity of peptides modeling the dimerization domain of chimeric Tb and Tb′ subunits and containing carboxyl-terminal GGGC extensions to form homodimeric and heterodimeric arrangements was investigate using disulfide cross-link formation. The peptide fragments Db+GGGC and Db′+GGGC were purified from strain MM294/pSD359/pSD358 (Db+GGGC/Db′+GGGC) as described under “Experimental Procedures.” The location of the chimeric b and b′ subunits, both monomeric and dimeric, are labeled on the right side of the gel. Assignment of the dominant species was confirmed by mass spectrometry. B, disulfide cross-link formation between cysteines substituted at residues Ala83 or Ala90 in peptides modeling the dimerization and F1-binding domains of the chimeric subunits was analyzed by SDS-PAGE. Peptides from strains MM294/pSD361 (PbA83C), MM294/pSD362 (Pb′A83C), MM294/pSD363 (PbA90C), and MM294/pSD364 (Pb′A90C) were purified as described under “Experimental Procedures.” The proteins were diluted individually or in combination into a sulfhydril/disulfide buffer and incubated at 25 °C for 30 min. The cross-linking reaction was terminated by treated with SDS sample buffer containing 15 mm NEM, and each sample was analyzed by SDS-PAGE. The positions of the monomeric and dimeric bands are indicated on the right side of the gel.
The specificity of disulfide formation between cysteines substituted for residues Ala83 and Ala90 was tested using soluble forms of the chimeric subunits that consisted of residues bD53–L156 (Fig. 3B). Partially purified peptides were incubated, either individually or as mixtures, for 30 min in a thiol-disulfide buffer and analyzed by nonreducing SDS-PAGE. Both the individual homodimeric (PbA83C)2 and (Pb′A83C)2 polypeptides and the mixture of them both showed modest tendencies to form disulfides, whereas no dimer formation was observed for either of the individual constructs with cysteine at position 90. Of all the possible cross-linking arrangements tested, dimer formation was found to be the strongest in the PbA83C/Pb′A90C mixture, implying a proximity for position 83 of Pb and position 90 of Pb′. Dimer formation for the PbA90C/Pb′A83C peptide mixture was substantially weaker. Importantly, the T. elongatus sequences appeared to be dictating positioning of the chimeric peptides relative to one another.
Evidence for a Staggered Parallel Peripheral Stalk
Plasmids were constructed to express the chimeric Tbhis,A83C, Tbhis,A90C, Tb′A83C,V5, and Tb′A90C,V5 subunits. The chimeric subunits were expressed in deletion strain KM2 (Δb) individually and in combination and then assayed for in vivo F1F0 activity by growth on succinate as the sole carbon source. All of the cysteine-containing subunits complemented the deletion strain and supported colony formation comparable with the unmodified chimeric subunits (Table 1). The membranes were prepared from the paired chimeric subunit samples and analyzed for ATP hydrolysis activity in both the absence and the presence of 0.5% LDAO (Table 2). All four combinations of A83C and A90C chimeric subunits yielded membrane vesicles with activities approaching levels found in control KM2/pSBC97/pSBC98 (Tbhis/Tb′V5) membranes. Analysis of enzyme coupling using the proton pumping assay indicated that the cysteine insertion subunits produced no significant effect on coupling (Fig. 1B).
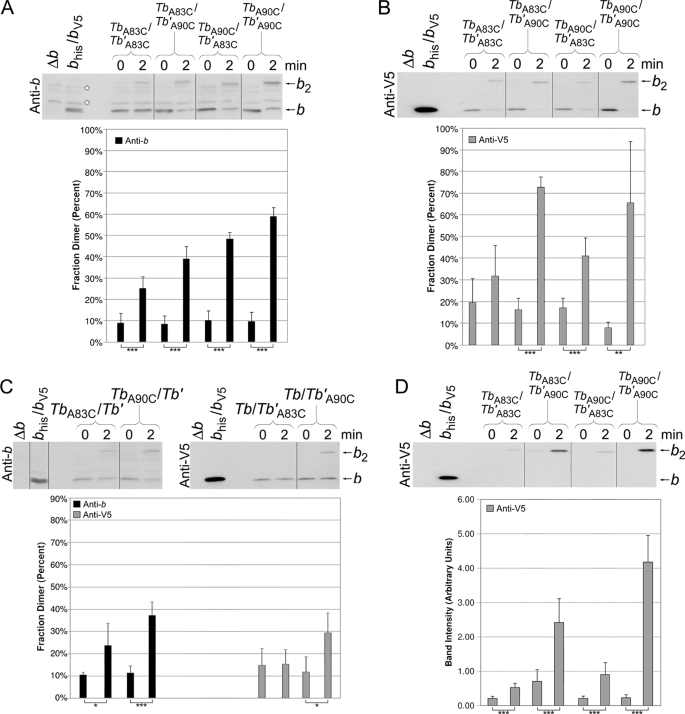
The levels of disulfide formation in F1F0 ATP synthase peripheral stalks were examined in membranes prepared from the strains expressing both chimeric Tb and Tb′ subunits. The initial experiments were conducted by cross-linking under high Cu2+ concentration conditions (Fig. 4, A and B). Cross-linking was evident in membranes containing all four chimeric subunit combinations: Tbhis,A83C/Tb′A83C,V5, Tbhis,A83C/Tb′A90C,V5, Tbhis,A90C/Tb′A83C,V5, and Tbhis,A90C/Tb′A90C,V5. Interpretation of the experiment was complicated because each sample contained a mixture of F1F0 complexes with heterodimeric Tb/Tb′ stalks and homodimeric Tb/Tb and Tb′/Tb′ peripheral stalks. Membranes prepared from strains expressing a pair of chimeric subunits where only one type of subunit contained a cysteine substitution produced a significant degree of cross-link formation (Fig. 4C), indicating that homodimeric peripheral stalks likely contributed to the total cross-linked product observed in the membrane fractions where both subunits contained cysteines. Therefore, to detect only cross-link formation specifically attributable to heterodimeric peripheral stalks, each sample was purified over a nickel resin after cross-linking and analyzed by Western blot using an antibody against the V5 epitope tag (Fig. 4D). Strong dimer formation was observed for both the Tbhis,A90C/Tb′A90C,V5 and Tbhis,A83C/Tb′A90C,V5 peripheral stalks. The data set cannot be easily reconciled with respect to either an exclusively in-register conformation or an exclusively staggered conformation but arguably offers support for both competing models. It should be noted that the cross-linked Tbhis,A90C/Tb′A83C,V5 peripheral stalk was barely detectable in comparison with the Tbhis,A83C/Tb′A90C,V5. The result was in accord with the apparent orientation of the stagger observed in the polypeptide work (Fig. 3B). Together the evidence indicates that the chimeric subunits assume preferred and distinguishable positions within the heterodimeric stalks. The evidence further supports a staggered conformation within the dimerization domain that has the Tb subunits offset in the carboxyl direction with respect to the Tb′ subunit.
FIGURE 4.
Disulfide cross-link formation in F1F0 ATP synthase peripheral stalks composed of chimeric Tb and Tb′ subunits. The membranes were prepared from strains coexpressing cysteine-containing Tb and Tb′ subunits. Samples from KM2/pSBC123/pSBC125 (TbA83C/Tb′A83C), KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C), KM2/pSBC124/pSBC125 (TbA90C/Tb′A83C), and KM2/pSBC124/pSBC126 (TbA90C/Tb′A90C) were cross-linked with 500 μm Cu2+ exactly as described for Fig. 2 and analyzed by Western blot using antibodies against the b subunit (A) and V5 epitope tag (B) (10 and 1 μg of protein/lane, respectively). The vertical lines indicate the removal of intervening lanes in silico. Samples with a low probability of being identical are indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 5). C, membranes were prepared from strains coexpressing a cysteine-containing chimeric subunit with the complementary cysteine-free chimeric subunit. Samples from KM2/pSBC123/pSBC98 (TbA83C/Tb′), KM2/pSBC124/pSBC98 (TbA90C/Tb′), KM2/pSBC97/pSBC125 (Tb/Tb′A83C), and KM2/pSBC97/pSBC126 (Tb/Tb′A90C) were cross-linked as before and analyzed by Western blot. Antibodies against either the b subunit or the V5 epitope tag were used for the detection of (Tb)2 and (Tb′)2 dimers, respectively. The experiment was done a total of four times, and the average fraction dimer is graphed below each lane. D, disulfide cross-link formation in heterodimeric Tb/Tb′ peripheral stalks was detected using a Nickel-resin purification assay as previously described (22). Samples KM2/pSBC123/pSBC125 (TbA83C/Tb′A83C), KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C), KM2/pSBC124/pSBC125 (TbA90C/Tb′A83C), and KM2/pSBC124/pSBC126 (TbA90C/Tb′A90C) were prepared and cross-linked as before. Each sample was then solubilized and purified over a high capacity nickel chelate affinity matrix to retain only F1F0 complexes containing at least one histidine tag. A total of 10% of the protein retained by the high capacity nickel chelate affinity matrix resin was analyzed by Western blot using an antibody against the V5 epitope tag. The average fraction dimer is graphed below each lane (n = 8).
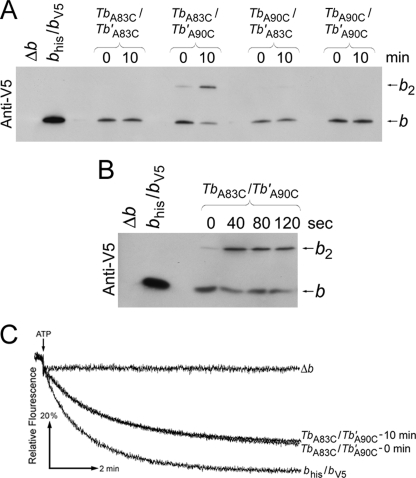
The Cu2+ concentration was reduced to 50 μm to more selectively form only those cross-links that occur with the highest efficiency (Fig. 5A). Higher concentrations of Cu2+ inhibited F1F0 ATPase, but 50 μm Cu2+ allowed for direct measurement of enzymatic activity. Under these conditions the only sample that was successfully cross-linked contained Tbhis,A83C/Tb′A90C,V5 peripheral stalks. The dimer band observed was the cross-linked product of Tbhis,A83C/Tb′A90C,V5 heterodimer F1F0 complex and not cross-linking in a homodimeric stalk, a fact that can be deduced from the lack of dimer formation in samples expressing either Tbhis,A83C and Tbhis,A83C subunits or Tb′A90C,V5 and Tb′A90C,V5 subunits. A reaction time course study showed that the TbA83C/Tb′A90C cross-linking reached its maximum within 40 s (Fig. 5B). Image analysis of the Western blots indicated that ∼70% of the Tb′A90C subunit was paired with a TbA83C subunit and cross-linked upon treatment with oxidizing agent. Indeed, the zero time point samples show a degree of background cross-link formation occurring even in the presence of the Tris (2-carboxyethyl) phosphine reducing agent. Clearly, the rapid, high efficiency Tbhis,A83C/Tb′A90C,V5 cross-linking was reporting on an offset structure extant in the peripheral stalk, and not less than two-thirds of the peripheral stalks were in a staggered rather than in-register conformation.
FIGURE 5.
Disulfide cross-link formation at low Cu2+ concentration in F1F0 ATP synthase peripheral stalks composed of chimeric Tb and Tb′ subunits. A, the membranes were prepared from strains KM2/pSBC123/pSBC125 (TbA83C/Tb′A83C), KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C), KM2/pSBC124/pSBC125 (TbA90C/Tb′A83C), and KM2/pSBC124/pSBC126 (TbA90C/Tb′A90C). Each sample was cross-linked with 50 mm Cu2+ for 10 min and analyzed by Western blot using an antibody against the V5 epitope tag exactly as described for Fig. 2B. B, membrane sample were prepared from strain KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C) and cross-linked with 50 μm Cu2+ for increasing lengths of time. Each sample was allowed to react for the length of time specified above the Western blot before the reaction was quenched by the addition of NEM to a final concentration of 5 mm. C, membranes prepared from strain KM2/pSBC123/pSBC126 (TbA83C/Tb′A90C) were assayed for ATP-driven proton pumping activity both before and after disulfide cross-link formation using 50 μm Cu2+ for 10 min. The traces shown are individual representative results (n = 3).
If it were necessary to revert to an in-register conformation for enzyme function, then trapping 70% of the enzyme in the stagger by disulfide bridge formation would be expected to have a readily detectable impact on activity. The effect of cross-link formation on coupled enzyme activity was analyzed using the proton pumping assay (Fig. 5C). Importantly, the formation of this cross-link had no effect on enzyme coupling, demonstrating that a peripheral stalk covalently locked in the offset arrangement represented a fully functional form of the enzyme.
DISCUSSION
The goal of this study was to provide direct tests of two competing structural models of the peripheral stalk of F1F0 ATP synthase. One model proposed that the b subunits are in a staggered parallel conformation (15, 16, 21), and the other suggested an in-register parallel arrangement (9, 19, 20). We have provided evidence compatible with both conformations, but the staggered positions appeared to be highly preferred. The in-register model was supported by the observation of disulfide bridge formation in homodimeric E. coli peripheral stalks where both b subunits had identical cysteine substitutions. Disulfide cross-link formation was observed at multiple sites within the dimerization domain including bR83C, bA90C, and bE97C. However, it was not possible to conduct a comparable experiment to test the offset model using the wild-type E. coli peripheral stalk because both b subunits are identical and hence indistinguishable within the holoenzyme. To overcome this limitation, we employed Tb and Tb′ chimeric subunits constructed by replacing residues Glu39–Ile86 of the E. coli b subunit with a homologous sequence from the organism T. elongatus as previously described (23). The cross-links favoring the in-register arrangement were reproducible in F1F0 complexes containing homodimeric TbA83C, TbA90C, Tb′A83C and TbA90C chimeric subunits. However, studies utilizing both polypeptides modeling the chimeric Tb/Tb′ subunits and the chimeric subunits incorporated into intact functional F1F0 ATP synthases demonstrated that the chimeric subunits assume preferred positions relative to one another as dictated by the T. elongatus sequence. Very efficient cross-linking was observed in the Tbhis,A83C/Tb′A90C,V5 heterodimeric peripheral stalk, strongly favoring a staggered conformation with the Tb subunit shifted in the carboxyl direction relative to Tb′. To use the nomenclature introduced previously (16), these results suggest that in the heterodimeric bb′ stator stalk of cyanobacteria, the b′ subunit preferentially assumes the bN position, and the b subunit occupies the bC position. The rapid, high yield dimer formation within holoenzyme argues that the predominant arrangement of the heterodimeric peripheral stalks is the staggered conformation. Moreover, covalently fixing the offset in place did not have any affect on the coupled activity of F1F0 ATPase. Therefore, the staggered parallel conformation is a viable functional structure for the peripheral stalk. The evidence from our current data set is not sufficient to be interpreted as definitive in terms of establishing either a left- or right-handed coiled coil in the peripheral stalk.
Disulfide cross-link formation induced by larger amounts of oxidizing agent produced evidence of the existence of an in-register conformation in both chimeric and wild-type peripheral stalks. The most efficient cross-link was formed between cysteines substituted at residues Ala90 in both cases, TbA90C/Tb′A90C and (bA90C,V5). Approximately 70% of the TbA83C/Tb′A90C was very rapidly cross-linked upon exposure to Cu2+ in peripheral stalks in the staggered conformation (Fig. 5), but using a much higher concentration of oxidizing agent as much as 70% of the TbA90C/Tb′A90C and (bA90C,V5) peripheral stalks were also cross-linked in the in-register arrangement. Regardless of whether one assumes a left- or right-handed coiled coil, residues 83 and 90 would be located not less than 10 Å apart in the in-register conformation. Given that the average disulfide bond is only 2.2 Å, residue Tb′A90C cannot be within cross-linking distance of both TbA83C and TbA90C. A model where the peripheral stalk exists in equilibrium between the staggered and in-register conformations might explain the observation that both arrangements can be detected. However, the fact that the staggered arrangement can be trapped rapidly upon treatment with far less oxidizing agent implies that this more efficient cross-link represents the predominant conformation.
An obvious concern is whether the conformations detected using the Tb/Tb′ chimeric subunit enzymes are representative of conformations present in authentic F1F0 ATP synthases. There are several lines of evidence that address this contention. First, the Tb and Tb′ subunits were efficiently assembled into the E. coli F1F0 ATP synthase in vivo (23). Second, the chimeric enzymes displayed fully coupled ATP-driven proton pumping activity, which requires that the chimeric subunits not only be incorporated into F1F0 but also be positioned such that they can participate in all of the protein-protein interactions within both F1 and F0 needed for a functional enzyme complex. Finally, the cross-linking results for the E. coli (bA83C,V5)2 and (bA90C,V5)2 peripheral stalks are nearly identical to those of the chimeric TbA83C/Tb′A83C and TbA90C/Tb′A90C peripheral stalks, suggesting that the conformation of the chimeric peripheral stalk may be the same as the wild-type peripheral stalk. Therefore, it seems that the most reasonable interpretation is that the properties of the chimeric Tb and Tb′ subunits accurately reflect the normal functions of a wild-type b subunit. A conclusive proof for the staggered conformation awaits similar experiments conducted in a photosynthetic organism with native b and b′ subunits.
Supplementary Material
Acknowledgment
We are pleased to acknowledge the excellent technical assistance of Yumin Bi.
This work was supported by United States Public Health Service Grant GM70978 (to B. D. C.), a University of Florida Alumni Fellowship (to S. B. C.), and Canadian Institutes of Health Grant MOP-10237 (to S. D. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
- MOPS
- morpholinepropanesulfonic acid
- LDAO
- N,N-dimethyldodecylamine N-oxide
- MESG
- 7-methyl-6-thioguanosine
- NEM
- N-ethylmalemide
- PNP
- purine nucleotide phosphorylase
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1.von Ballmoos C., Cook G. M., Dimroth P. ( 2008) Annu. Rev. Biophys. 37, 43– 64 [DOI] [PubMed] [Google Scholar]
- 2.Devenish R. J., Prescott M., Rodgers A. J. ( 2008) Int. Rev. Cell. Mol. Biol. 267, 1– 58 [DOI] [PubMed] [Google Scholar]
- 3.Nakamoto R. K., Scanlon J. A., Al-Shawi M. K. ( 2008) Arch. Biochem. Biophys. 476, 43– 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimroth P., von Ballmoos C., Meier T. ( 2006) EMBO Rep. 7, 276– 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahams J. P., Leslie A. G., Lutter R., Walker J. E. ( 1994) Nature 370, 621– 628 [DOI] [PubMed] [Google Scholar]
- 6.Weber J. ( 2006) Biochim. Biophys. Acta 1757, 1162– 1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber J. ( 2007) Trends. Biochem. Sci. 32, 53– 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker J. E., Dickson V. K. ( 2006) Biochim Biophys. Acta 1757, 286– 296 [DOI] [PubMed] [Google Scholar]
- 9.Revington M., McLachlin D. T., Shaw G. S., Dunn S. D. ( 1999) J. Biol. Chem. 274, 31094– 31101 [DOI] [PubMed] [Google Scholar]
- 10.Zaida T., Hornung T., Volkov O., Hoffman A., Pandey S., Wise J., Vogel P. ( 2008) J. Bioenerg. Biomembr. 40, 551– 559 [DOI] [PubMed] [Google Scholar]
- 11.Steigmiller S., Börsch M., Gräber P., Huber M. ( 2005) Biochim. Biophys. Acta 1708, 143– 153 [DOI] [PubMed] [Google Scholar]
- 12.Dmitriev O., Jones P. C., Jiang W., Fillingame R. H. ( 1999) J. Biol. Chem. 274, 15598– 15604 [DOI] [PubMed] [Google Scholar]
- 13.Del Rizzo P. A., Bi Y., Dunn S. D., Shilton B. H. ( 2002) Biochemistry 41, 6875– 6884 [DOI] [PubMed] [Google Scholar]
- 14.Priya R., Tadwal V. S., Roessle M. W., Gayen S., Hunke C., Peng W. C., Torres J., Grüber G. ( 2008) J. Bioenerg. Biomembr. 40, 245– 255 [DOI] [PubMed] [Google Scholar]
- 15.Wood K. S., Dunn S. D. ( 2007) J. Biol. Chem. 282, 31920– 31927 [DOI] [PubMed] [Google Scholar]
- 16.Del Rizzo P. A., Bi Y., Dunn S. D. ( 2006) J. Mol. Biol. 364, 735– 746 [DOI] [PubMed] [Google Scholar]
- 17.Hornung T., Volkov O. A., Zaida T. M., Delannoy S., Wise J. G., Vogel P. D. ( 2008) Biophys. J. 94, 5053– 5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wise J. G., Vogel P. D. ( 2008) Biophys. J. 94, 5040– 5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers A. J., Wilkens S., Aggeler R., Morris M. B., Howitt S. M., Capaldi R. A. ( 1997) J. Biol. Chem. 272, 31058– 31064 [DOI] [PubMed] [Google Scholar]
- 20.Rodgers A. J., Capaldi R. A. ( 1998) J. Biol. Chem. 273, 29406– 29410 [DOI] [PubMed] [Google Scholar]
- 21.McLachlin D. T., Dunn S. D. ( 1997) J. Biol. Chem. 272, 21233– 21239 [DOI] [PubMed] [Google Scholar]
- 22.Grabar T. B., Cain B. D. ( 2003) J. Biol. Chem. 278, 34751– 34756 [DOI] [PubMed] [Google Scholar]
- 23.Claggett S. B., Grabar T. B., Dunn S. D., Cain B. D. ( 2007) J. Bacteriol. 189, 5463– 5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick K. A., Cain B. D. ( 1991) J. Bacteriol. 173, 7240– 7248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meselson M., Yuan R. ( 1968) Nature 217, 1110– 1114 [DOI] [PubMed] [Google Scholar]
- 26.Dunn S. D., Chandler J. ( 1998) J. Biol. Chem. 273, 8646– 8651 [DOI] [PubMed] [Google Scholar]
- 27.Dykxhoorn D. M., St Pierre R., Linn T. ( 1996) Gene. 177, 133– 136 [DOI] [PubMed] [Google Scholar]
- 28.Caviston T. L., Ketchum C. J., Sorgen P. L., Nakamoto R. K., Cain B. D. ( 1998) FEBS Lett. 429, 201– 206 [DOI] [PubMed] [Google Scholar]
- 29.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. ( 1985) Anal. Biochem. 150, 76– 85 [DOI] [PubMed] [Google Scholar]
- 30.Sorgen P. L., Bubb M. R., McCormick K. A., Edison A. S., Cain B. D. ( 1998) Biochemistry 37, 923– 932 [DOI] [PubMed] [Google Scholar]
- 31.Webb M. R. ( 1992) Proc. Natl. Acad. Sci. U. S. A. 89, 4884– 4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner J. L., Cain B. D. ( 1999) Arch. Biochem. Biophys. 361, 302– 308 [DOI] [PubMed] [Google Scholar]
- 33.Grabar T. B., Cain B. D. ( 2004) J. Biol. Chem. 279, 31205– 31211 [DOI] [PubMed] [Google Scholar]
- 34.Sorgen P. L., Caviston T. L., Perry R. C., Cain B. D. ( 1998) J. Biol. Chem. 273, 27873– 27878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.