Abstract
Alternative pre-mRNA splicing is often controlled by cell signals, for example, those activating the cAMP-dependent protein kinase (PKA) or the Ca2+/calmodulin-dependent protein kinase IV (CaMKIV). We have shown that CaMKIV regulates alternative splicing through short CA repeats and hnRNP L. Here we use a splicing reporter that shows PKA/CaMKIV promotion of exon inclusion to select from exons containing random 13-nt sequences for RNA elements responsive to the kinases in cultured cells. This selection not only identified both PKA- and CaMKIV-responsive elements that are similar to the CaMKIV-responsive RNA element 1 (CaRRE1) or CA repeats, but also A-rich elements not previously known to respond to these kinases. Consistently, hnRNP L is identified as a factor binding the CA-rich elements. Analyses of the motifs in the highly responsive elements indicate that they are indeed critical for the kinase effect and are enriched in alternative exons. Interestingly, a CAAAAAA motif is sufficient for the PKA/CaMKIV-regulated splicing of the exon 16 of the CaMK kinase β1 (CaMKK2) transcripts, implying a role of this motif in signaling cross-talk or feedback regulation between these kinases through alternative splicing. Therefore, these experiments identified a group of RNA elements responsive to PKA and CaMKIV from in vivo selection. This also provides an approach for selecting RNA elements similarly responsive to other cell signals controlling alternative splicing.
Alternative pre-mRNA splicing is a common means of gene expression regulation in metazoans (1, 2). The expression of splice variants is controlled not only in a tissue-, sex-, or developmental stage-specific way but also in response to stimulation of particular protein kinases by extracellular factors (3–5).
Many extracellular factors, including neurotransmitters and hormones, activate calcium and/or cAMP signals and downstream protein kinases. Besides membrane depolarization and the calcium/calmodulin-dependent protein kinase IV (CaMKIV)4 (5–11), the cAMP-dependent protein kinase A (PKA) has also been implicated in the regulation of alternative splicing. For example, dopamine induces alternative splicing in striatal neurons through the dopamine receptor that activates the PKA pathway (12–14). PKA phosphorylates and colocalizes with arginine/serine-rich (SR) splicing factors and regulates alternative splicing of an E1A minigene (15).
Alternative splicing in mammalian systems is controlled by multiple intronic and exonic regulatory elements called intronic or exonic splicing enhancers or silencers (1, 16, 17). The effect of a particular element can be either enhancer or silencer depending on its surrounding context in the pre-mRNA (5, 17–19). RNA elements that mediate cell signal-regulated splicing have also been isolated. These elements include the A-rich element in the CD44 exon 5, the CaMKIV-responsive RNA elements (CaRRE1 and CaRRE2) in the STREX (stress axis-regulated exon) of Slo and exons 5 and 21 of the NMDAR1 (N-methyl-d-aspartic acid receptor, type I) gene, the activation-responsive sequence in CD45, the G-tract in Bcl-x and the UAGG motif in NMDAR1 exon 21 (6–8, 10, 20–22). These elements have all been defined through intensive mutagenesis analyses of individual splicing reporter minigenes.
SELEX (systematic evolution of ligands by exponential enrichment) has been used for A/C-rich splicing enhancers and G-rich silencers (23–25), but not for cell signal-responsive RNA elements. It would be particularly difficult to enrich kinase-responsive elements from spliced mRNA in cells with SELEX when a kinase, like CaMKIV, shows exon-exclusion activity through the CaRREs in the current splicing reporter systems (6–8).
In this report, we selected PKA- and CaMKIV-responsive RNA elements using SELEX based on the context-dependent promotion of exon inclusion by the kinases and characterized the selected elements.
EXPERIMENTAL PROCEDURES
Plasmid Construction and Mutagenesis
The Flag-PKA (A), Flag-CaMKIV-dCT (CaMKIV or IV) or -dCTK75E (CaMKIVm or IVm, kinase-dead) and PKCγ are as described (6, 26, 27). Particularly for the CaMKIV and PKA plasmids, their activity or inactivity was confirmed by monitoring the phosphorylation levels of their target proteins CREB Ser133 and PTB Ser16, respectively (6, 27). In the Flag-PKA mutant (Am), the Lys73 and Lys169, phosphate-binding residues (28), were mutated to Glu73 and Glu169, respectively (numbered according to NCBI protein accession no. AAA39936, corresponding to Lys72 and Lys168 in the previous report (28)), using primers FlagPKA1 (5′-CGGGATCCGCCATGGACTACAAGGACGACGATGACAAAGCCGCCGCGGGCAA CGCCGCCGCCGCCAAG-3′), PKAK73Es (5′-GGGAACCACTACGCCATGGAGA TCTTAGACAAGCAGAAG-3′), PKAK73Ea (5′-CTCCATGGCGTAGTGGTTCCCACTC-3′), PKAK169Es (5′-CATCTACCGGGACCTGGAGCCCGAGAATCTTCTCATC-3′), PKAK169Ea (5′-CTCCAGGTCCCGGTAGATGAGGTC-3′), and PKA2 (5′-GCTCTAGACTAA AACTCAGTAAACTCCTTG-3′).
Splicing reporter minigene 33SB is a chimera of pDUP4-1 and pL53In (29, 30). The ApaI/BglII fragment of pDUP4-1 was cloned into the ApaI/BamHI sites of pL53In (a gift from Dr. Harold König), and SalI and BamHI restriction sites were introduced into the 33-nucleotide (nt) middle exon (23), resulting in an insert fragment: actgactctctctgcctattggtctattttcccacccttagGTCGACGGTGGTGCCATGGCAGGCCGGATCCAGgttggtatcaaggttacaagacaggtttaaggagaccaat (with exon in upper and intron in lower cases, SalI and BamHI sites in bold, and the replacement cassette for random sequences underlined). For cTNTβ splicing reporters, the SalI-BamHI fragment of the cTNTβ minigene (a kind gift of Dr. Thomas A. Cooper) (23), was substituted with that of 33SB-derived constructs containing cloned elements.
Cell Culture and Treatment
HEK293T cells were cultured in DMEM with 10% newborn calf serum in 12-well plates. Transfection was done with Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. To test PKA or PKCγ effect on splicing, 0.2 μg of a minigene splicing reporter construct was cotransfected with 0.6 μg of Flag-PKAm (Am), Flag-PKA (A), or pPKCγ-EGFP (Clontech Laboratories, Inc.) plasmid for 16–24 h. For PKCγ effect, cells were then washed, maintained in DMEM, and treated with TPA (120 ng/ml) or DMSO (TPA vehicle control) for 24 h. The translocation of PKCγ-EGFP from cytoplasm to the plasma membrane as a result of TPA activation of the PKC signal was monitored under a fluorescence microscope (27). PC12 cells were grown in DMEM supplemented with 10% horse serum and 5% fetal bovine serum. GH3 cells were cultured as described previously (9).
RNA Preparation and RT-PCR Analysis
Total RNA was extracted from cells following transfection/treatments with the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, Inc.) according to the supplier's protocol. About 2.5 μg of RNA was used in a 10-μl reverse transcription (RT) reaction containing M-MLV reverse transcriptase (Invitrogen) and oligo-dT18. 2 μl of the RT product was used in a 25-μl PCR reaction for 26 cycles at an annealing temperature of 60 °C. The RT-PCR products were resolved in 3.5% agarose gels containing ethidium bromide. For examination of endogenous exons, about 200 ng of RNA was used in a 10-μl RT reaction. 1 μl of the RT product was used in a 13-μl PCR reaction for 35 cycles at an annealing temperature of 55 °C, and the RT-PCR products were resolved in 3% agarose gels. Band intensities of the splice variants were quantified with the NIH Image J software 1.37v (developed at the United States National Institutes of Health). The percentages of exon inclusion were relative to the total band intensities of the spliced two products. The PCR primers for 33SB-derived splicing reporters: Insulin1, 5′-GATCCGCTTCCTGCCCCTG-3′; Insulin2a, 5′-ACCCAGCTCCAGTTGTGC-3′; for cTNTβ-derived splicing reporters: cTNT1, 5′-AGGAGTATGTGGAAGAAG-3′; cTNT2, 5′-GTCATCCTGAGTGTGGTTGGCAAAG-3′. Primers for endogenous exon STREX: RbSlo1, 5′-AGTGCCTTCGTGGGTCTGTCCTT-3′, QRA59, 5′-CACATTGGAGTCCATGTTGTC-3′; for CaMKK2 exon 16: 93437s, 5′-GATCCTAGTGAAGACCATG-3′, 93437a, 5′-CCACCTTTCACAAGAGCAC-3′; for LKAP exon 7: rh37944s, 5′-AGCTGATCAGATTTGAAG-3′, r37944a, 5′-GCTCCCATGGCCTTGCTG-3′.
Western Blot Analysis
To test the kinase activity of Flag-PKA and its mutant, overnight HEK293T cultures in 12-well plates were transfected with 0.6 μg of Myc-PTB plasmid, or cotransfected with 0.6 μg of pcDNA3.1(+), Flag-PKA, or Flag-PKA mutant plasmid. Cells were harvested about 1.5 days after transfection and sonication-lysed in Buffer E (20 mm HEPES KOH, pH 7.9, 100 mm KCl, 0.2 mm EDTA, 10% (v/v) glycerol, 1 mm dithiothreitol) (31), containing 1 mm Na3VO4, 10 mm Na4P2O7, and 10 mm NaF. A fraction of the cell lysates cotransfected with Myc-PTB plus Flag-PKA or PKA mutant plasmid was treated with calf intestinal alkaline phosphatase (CIAP) in 1× dephosphorylation buffer (Invitrogen) at 37 °C for about 30 min. For Western blot analysis, 5-μg cell lysates of each non-PKA cotransfected sample and 2.5 μg of each PKA-cotransfected sample (including those treated with CIAP) were resolved in Novex® 4–20% Tris-Glycine gels (Invitrogen) and transferred onto polyvinylidene difluoride membranes (Millipore), followed by blocking in TBS (500 mm NaCl, 20 mm Tris-Cl) containing 5% (w/v) dried skim milk. Total Myc-PTB and phospho-PTB were then probed by c-Myc monoclonal antibody (Clontech) and anti-phospho-Ser16-PTB antibody (27). After three washes in TBS, membranes were incubated with the appropriate secondary antibody for about 1 h, followed by three washes in TBS. Proteins were then visualized by applying Western blotting detection reagent (GE Healthcare) and exposing to x-ray films. For detection of PKA and PKA mutant protein, membranes were washed in TBS and reprobed by a monoclonal ANTI-FLAG® M2 antibody (Sigma-Aldrich Co).
Selection for CaMKIV- or PKA-responsive RNA Elements
To create a library of splicing reporters, a synthesized nominally random sequence of 13 nt in a primer was substituted for the 13-nt replacement cassette in 33SB in PCR reactions. The PCR products were digested with ApaI and BglII, and ligated with ApaI- and BamHI-digested pL53In. The resulting plasmid pool contained ∼1.16 × 107 reporter clones as estimated from transformation of Escherichia coli cells with an aliquot of the plasmids. The complexity of the sequence pool was verified by randomly sequencing 17 clones, with no identical sequences obtained. The pool was cotransfected with 10 μg of Flag-PKA or Flag-CaMKIV-dCT plasmid and 5 μg of an EGFP plasmid using Lipofectamine 2000 into HEK293T cells in a 10-cm dish (∼7 × 106 cells). The transfection efficiency was estimated 24 h later to be at least 90% by counting green cells under a fluorescence microscope. Total RNA was harvested using the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, Inc.) followed by digestion with RQ1 RNase-free DNase (Promega) to remove contaminating plasmid DNA, which was confirmed by the absence of PCR products using an RT sample without reverse transcriptase. RT-PCR was then performed to selectively amplify middle exon-included mRNA using primers targeted to splice junctions (primer SXenh-J1, 5′-GGAGGACCCACAAGGTCG-3′ and primer SXenh-J2, 5′-CCAGTTGTGCCACTGGAT-3′). The PCR products were cloned as SalI-BamHI fragments back into the 33SB vector and the selection cycle went through multiple rounds, repeating the same procedure in the subsequent rounds as in the first one except that about 1 × 106 plasmid clones (as estimated by transformation in E. coli) were cotransfected with 1.5 μg of Flag-PKA (or CaMKIV-dCT) and 1 μg of the EGFP plasmid into about 1 × 106 HEK293T cells.
UV cross-linking and immunoprecipitation
This assay was carried out as described previously using [α-32P]CTP-labeled RNA transcripts (9). The anti-hnRNP L antibody, 4D11 (32), was purchased from Santa Cruz Biotechnology. DNA templates for RNA probes were amplified from highly PKA or CaMKIV-responsive clones A3.15, C7, and C42 with a T7-tagged primer. RNA probes were in vitro transcribed with T7 RNA polymerase. The RNA probe A3.15 sequence (the 13-nt replacement cassette, underlined) is: gcuauuuucccacccuuagGUCGACGGUGCACAGCAAAAAUACCGGAUCCAGguugguaucaagguuacaagacagguuuaaggagaccaauagaucuucag. RNA probes C7 and C42 are different from A3.15 only at the 13-nt inserts. The control probe CG (CG repeat, underlined) is: GUCCAUGUGUCGCGCGGUUAAAUCUUCUGGAAAGUGUGUACACAAUGUUCCGGAAGGCUGACCUCCCUUCAAAUGUCACUCGCCAG; mCaRRE1 probe from the upstream 3′-splice site of DUP175ST is the same as described previously (9).
Primer Extension
A simplified procedure, modified from the previous method (7), was used. Briefly, total RNA (16 μl) was prepared from the splicing reporter-transfected HEK293T cells grown in 12-well plates, heated to 85 °C for 5 min followed by 2-min incubation on ice, and 5 μl was used for reverse transcription with Superscript III at 50 °C for 1.5 h. The resulting products were resolved in denaturing PAGE gel (5.3%), dried, exposed to phosphorimager plates, and scanned in a Bio-Rad phosphorimager scanner. The percent exon exclusion is calculated as relative to the total intensity of both exon-included and -excluded products. Comparison between 23 pairs of primer extension products of DUP175 or its derived splicing reporters showed no significant difference (p = 0.16) in the levels of their exon inclusion between samples transfected with each reporter alone and ones cotransfected with CaMKIVm in HEK293 cells; therefore, the kinase effect on splicing was examined by comparing the effect of kinase-dead mutants and active kinases.
Sequence Analyses and Data Base Search
The selected 13-nt sequences were analyzed with Mutiple Em Motif Elicitation (MEME) (33), for consensus motifs. The motif-containing human exons were identified from the ASAPII data base (34). The constitutive human exons were obtained from the HOLLYWOOD data base (35, 36).
RESULTS
A Selection Strategy Based on a Context-dependent Promotion of Exon Inclusion by CaMKIV and PKA
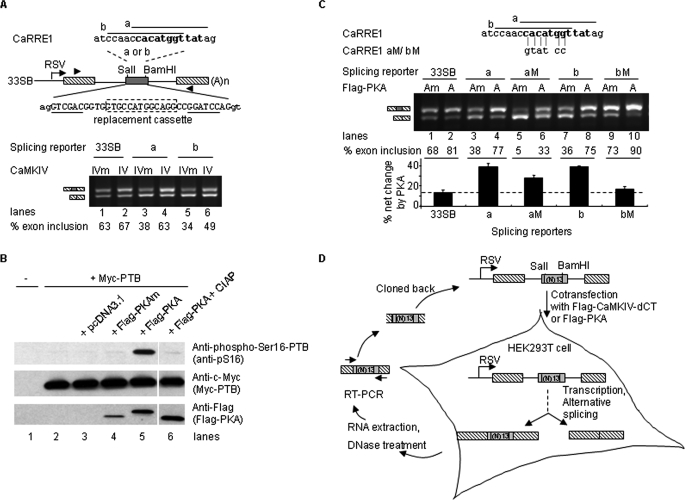
CaMKIV inhibited splicing through the CaRRE1 element in the DUP175-based reporters (6–9); however, in reporters derived from pL53In (29), CaMKIV enhanced splicing through the same element (Fig. 1A).
FIGURE 1.
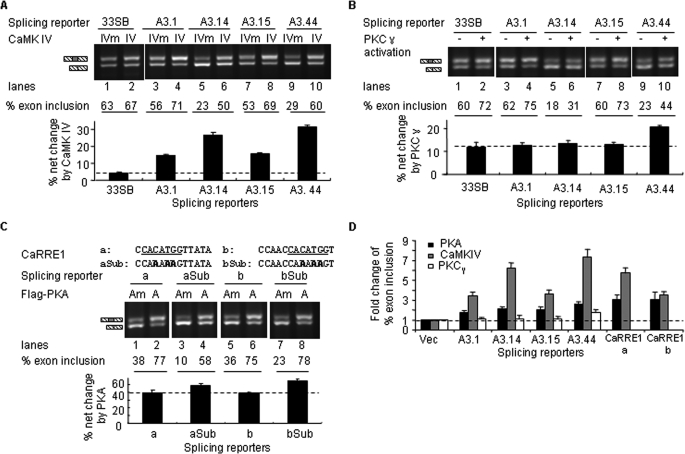
Selection of CaMKIV- or PKA-responsive RNA elements based on a context-dependent promotion of exon inclusion by the kinases. A, CaMKIV promotion of exon inclusion through CaRRE1 in 33SB. Upper, diagram of CaRRE1 (consensus motif from the previous reports in bold) in 33SB derived from the vector pL53In based on the insulin gene (exons as black-slashed boxes). Each of the two 13-nt sequences, CaRRE1a (a) and CaRRE1b (b), replaced the 13-nt cassette (boxed in dotted line) between the SalI and BamHI sites (underlined) in the middle exon. RSV, Rous Sarcoma Virus promoter. Exon sequences are in upper case and intron in lower case. Lower, agarose gel of RT-PCR products from HEK293T cells cotransfected with the splicing reporters and Flag-CaMKIV-dCTK75E (IVm) or Flag-CaMKIV-dCT (IV) as indicated above the gel. Spliced products are indicated to the left and percentages of exon inclusion below each gel. Lines, introns. Arrowheads, locations of PCR primers. (A)n, poly(A). B, phosphorylation activity of the wild type Flag-PKA (PKA) or inactivity of the kinase-dead Flag-PKA mutant (PKAm), respectively. Shown are Western blots indicating the phosphorylation level of the PKA target Ser16 of the Myc-tagged PTB protein (top panel) compared with the total protein (middle panel) expressed in HEK293T cells with vector pcDAN3.1(+), PKAm, or PKA (bottom panel), as indicated above the gels. The sample for the last lane was pretreated with calf intestine alkaline phosphatase (CIAP) before loading. PTB phosphorylation is increased about 7-fold by PKA, about 4-fold of that induced by forskolin on the phosphorylation of the endogeneous PTB Ser16 as shown in Xie et al. (27). C, PKA effect on the 33SB-CaRRE1 reporters. Shown is an agarose gel of RT-PCR products of CaRRE1 or its mutant splicing reporters cotransfected with Flag-PKAm (Am) or Flag-PKA (A) into HEK293T cells as indicated above the gel. The mutated CaRRE1 nucleotides are indicated below the CaRRE1 sequences. Below the gel are the percentages of exon inclusion for each lane and a bar graph of the percent net changes by PKA for each reporter. D, strategy for in vivo SELEX enrichment of CaMKIV- or PKA-responsive RNA elements controlling alternative splicing.
In this test, CaRRE1 responses to coexpressed constitutively active Flag-CaMKIV-dCT (CaMKIV or IV) or its kinase-dead mutant (IVm) were examined in the pL53In-based splicing reporter 33SB-CaRRE1a or b (Fig. 1A), containing exons/introns and promoters different from the previous DUP175 (6, 29, 37). In the 33SB vector transcripts, inclusion of the middle exon (63%) was essentially unchanged by CaMKIV (lanes 1 and 2). Replacing part of the middle exon with CaRRE1a or CaRRE1b sequences reduced the level of exon inclusion to 38 and 34% (lanes 3 and 5), respectively, indicating that the CaRRE1 sequences are splicing silencers. However, unlike in DUP175, the exon inclusion was increased by overexpressed CaMKIV (63 and 49% exon inclusion, lanes 4 and 6, respectively).
A similar promoting effect on exon inclusion by coexpressed Flag-PKA was also observed in the 33SB-CaRRE1 splicing reporters (Fig. 1, B and C). For this assay, we made a double mutant of PKA (PKAm, K73E, K169E) from the wild type Flag-PKA, which phosphorylates the Ser16 of the polypyrimidine tract-binding protein (PTB, Fig. 1B) (27, 38). In the PKAm, the residues Lys73 and Lys169, essential for phosphate-binding (28), were mutated to Glu73 and Glu169, respectively. The PKAm showed no kinase activity on the PKA target Ser16 of PTB (Fig. 1B) and exhibited no effect on the exon inclusion (Fig. 1, A and C, compare lanes 1, 3, 5 in A with 1, 3, 7 in C, respectively, p = 0.25, paired Student's t test), in comparison with the CaMKIVm that is known to be kinase-dead and have no effect on splicing (6, 8, 26). In contrast, the wild type Flag-PKA increased the exon inclusion level to 77 and 75%, respectively (Fig. 1C, lanes 4 and 8), similarly to the effect by CaMKIV (Fig. 1A). Importantly, this increase was reduced to that of the 33SB vector level by mutations in CaRRE1 (Fig. 1C, lanes 5, 6, 9, and 10). Because these clones contain the same promoter, flanking exons/introns and poly(A) signals, their differential responses to CaMKIV or PKA are properties of the insert elements themselves rather than those of the transcriptional unit.
The promoting effect of CaMKIV and PKA suggested that these protein kinases would likely enrich exons containing kinase-responsive RNA elements in a pool of random sequences during repeated selections. Moreover, because the vector already showed about 65% exon inclusion with CaMKIVm or PKAm (Fig. 1, A and C), constitutive enhancers in the random pool were expected to cause 100% exon inclusion soon in early rounds of selection and could not be further increased by the kinases during subsequent rounds of selection. Thereby this system would likely allow enrichment of silencer elements preferably targeted by the kinases through repeated selections.
According to this approach, a pool of 13-nt random sequences was cloned into the middle exon of the 33SB to obtain a library of splicing reporters (containing about 1.16 × 107 clones). The reporters were cotransfected into HEK293T cells with the constitutively active CaMKIV or PKA plasmids. The spliced products were amplified by RT-PCR, cloned back into the vector, and cycled through five selection rounds (Fig. 1D). Because no PKA-responsive splicing element has been reported yet and also for reasons as found out later (more net changes of exon inclusion by PKA and mostly shared elements between these two kinases as shown below), we analyzed the PKA effect in more detail in the following experiments.
The Selected PKA- or CaMKIV-responsive RNA Elements Are Mostly Splicing Silencers
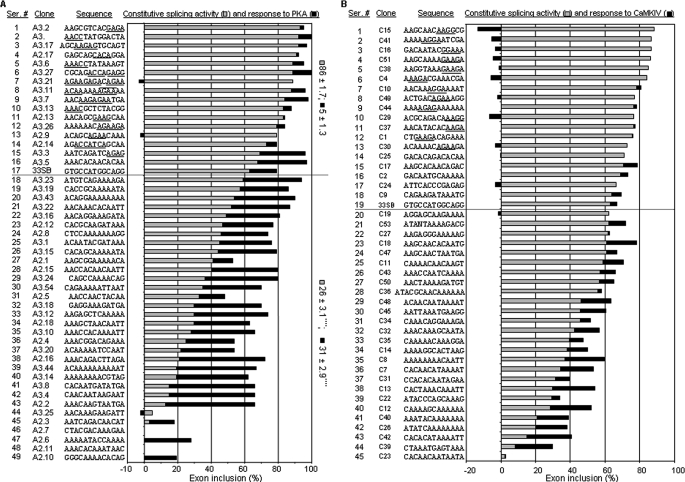
Preliminary analyses with RT-PCR of the spliced mRNA pools from each selection round (of five rounds in total) indicated that the net changes of exon inclusion by PKA increased and peaked at round 3 (about 3-fold, compared with the starting pool). We therefore randomly picked up 48 clones from the 2nd and 3rd rounds of selection plus the 33SB vector, and analyzed their constitutive splicing and response to PKA coexpression (Fig. 2A).
FIGURE 2.
Selected PKA/CaMKIV-responsive elements. A, elements from the selection by PKA, with their serial numbers, clone numbers, sequences, and bar graphs of constitutive levels of exon inclusion (gray bars) and PKA responses (black bars, net changes of percent exon inclusion by PKA) of each clone indicated. The graph is aligned from top to bottom with decreasing constitutive levels of exon inclusion. The average levels of constitutive splicing (±S.E.) or response to PKA of the clones with at least 10% less (silencers) or higher (enhancers) constitutive level than the vector 33SB are indicated to the right of the graph. ****, p < 0.0001 compared with the enhancer group, in two tailed Student's t test. B, elements from the selection by CaMKIV, indicated similarly in A. In both groups, the vector 33SB is also included, as nos. 17 and 19, respectively, above the dotted lines. Motifs similar to the known purine- or A/C-rich enhancers are underlined in both groups.
The constitutive level of exon inclusion of these clones ranges from 0 to 93% (Fig. 2A, from bottom to top). A small group of clones (nos. 1–16) showed higher constitutive level of exon inclusion than the 33SB vector. Most of these clones contain motifs that are similar to the purine- or A/C-rich enhancers in the previous selection (23), as expected. For these clones, the PKA effect to increase exon inclusion is hard to assess since they already have almost 100% exon inclusion even without Flag-PKA coexpression. However, a larger group of clones (nos. 18–49) showed lower constitutive level of exon inclusion than the 33SB vector. Of the 32 clones in this group, 29 (nos. 21–49) showed at least 10% less constitutive exon inclusion than the vector, likely containing splicing silencers. PKA coexpression substantially increased the inclusion of exons in 22 clones (with net changes at least 10% more than the vector) except 7 clones (nos. 27, 31, 44–46, and 48–49). Therefore, PKA appears to relieve the splicing repression effect to promote the inclusion of exons containing PKA-responsive silencer elements.
Analyses of 44 clones of the CaMKIV group showed similar promotion of exon inclusion through silencer elements though the net change of exon inclusion by CaMKIV is generally less than that by PKA (Fig. 2B, nos. 21–44). Besides, CaMKIV also appeared to generally repress the exon inclusion of the clones containing enhancer elements (nos. 1–13), an effect not seen for the silencers or by PKA.
Overall, the occurrence of clones responsive to PKA or CaMKIV increased from 39% or 38% in the starting pool to 57% (for both kinases) in the 3rd round, supporting that the selection indeed enriched RNA elements responsive to PKA or CaMKIV.
Consensus Motifs of PKA- or CaMKIV-responsive RNA Elements
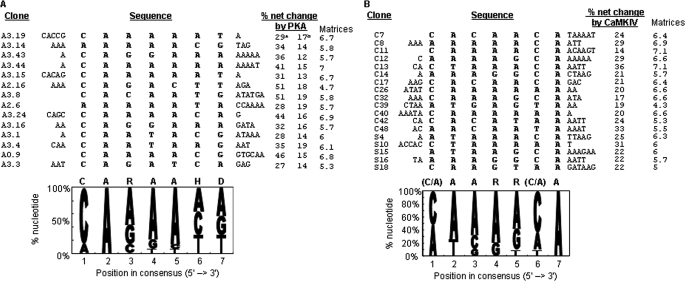
To identify consensus motifs of the elements that are PKA-responsive, we first transferred the highly PKA-responsive elements (net changes at least 10% more than the 33SB by PKA, same as in the following texts) from selection rounds 2 and 3 to a heterologous minigene splicing reporter cTNTβ. This experiment identified 14 out of 20 elements that conferred PKA response to this heterologous gene as well (Fig. 3A, b). These transferable elements appeared mostly CA- and A-rich. From these elements, a consensus heptamer motif CARAAHD (R: A or G; H: A, C or T; D: A, G or T, Fig. 3A) was identified using Mutiple Em Motif Elicitation (MEME) (33). In contrast, MEME analysis of the 13nt insert sequences of 17 clones from the starting random pool did not identify any consensus motif that could be found in most clones.
FIGURE 3.
Selected highly PKA/CaMKIV-responsive RNA elements and their consensus motif. A, consensus motif shared by highly PKA-responsive elements. Upper, sequences of fourteen elements, with the nucleotides for the consensus motif aligned in the middle and sequence names to the left and net changes of exon inclusion by PKA in 33SB (a). PKA responses (net changes of percent exon inclusion by PKA) of the elements in another reporter cTNTβ minigene is also listed (b) to the right. Lower, frequency of each nt at each position of the heptamer motif. The height of each nucleotide letter is proportional to its nt frequency, in decreasing order from top to bottom. The consensus sequence is above the graph (R, A or G; H, A, C, or T; D, A, G, or T). The numbers in the clone/element names indicate, from left to right, selection round, number in 33SB, number in cTNTβ. B, consensus motif shared by highly CaMKIV-responsive elements. Upper, sequences of the elements from selection round 3 with CaMKIV (C) are listed with the nucleotides for the consensus motif aligned in the middle, followed by the net changes of exon inclusion by CaMKIV. Also included are 5 highly responsive sequences from the starting pool (S). Lower, consensus motif of the highly CaMKIV-responsive elements. The matrices (out of max. 10) of the motifs are indicated to the right of each panel. The CaRRE1 has a matrix score of 5.1 with the “aaccaca” motif.
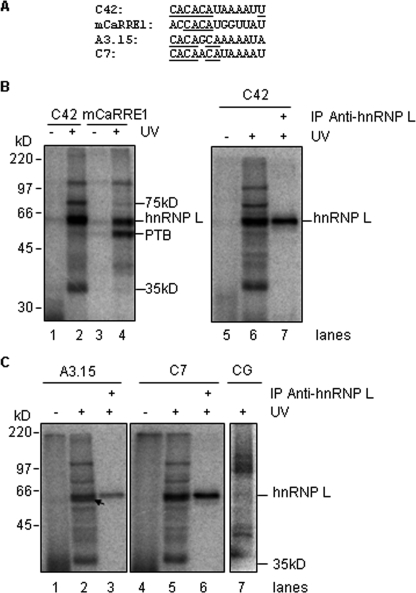
Similar examination of the 13-nt inserts of a group of highly CaMKIV-responsive clones also identified a consensus motif (C/A)AARR(C/A)A (Fig. 3B, R: purine). This motif is similar to the CA repeats of CaRRE1 (for example, the “CACAT” in clone C42). Moreover, RNA transcripts containing this motif were strongly cross-linked to a protein similar in size to the hnRNP L (Fig. 4, A and B), which binds the CaRRE1 (9). This protein was immunoprecipitated by 4D11 (Fig. 4B, right), a monoclonal anti-hnRNP L antibody (32). In contrast, barely any PTB signal was detected by the C42 probe compared with mCaRRE1 (compare lanes 2 and 4).
FIGURE 4.
The CA-rich RNA elements are bound by hnRNP L, a mediator of CaMKIV-regulated splicing through CA repeats. A, 13-nt elements in the RNA probes made from the kinase-responsive clones as indicated. Control probe CG is from the gene PRKDC with its CACACA motif mutated to CGCGCG; mCaRRE1 in DUP175 is a positive control for hnRNP L binding. B, hnRNP L binds to the RNA probe C42 containing the CACAT motif as in mCaRRE1. HeLa nuclear extracts were subjected to UV-cross-linking with [α-32P]CTP-labeled RNA probes (left), and 6 times of the nuclear extracts cross-linked to C42 were immunoprecipitated with the anti-hnRNP L antibody 4D11 (right). C, hnRNP L binds to the RNA probes containing similar CA repeats but not the CG probe. Similar to those in B, HeLa nuclear extracts were cross-linked to RNA probes A3.15, C7, and CG, and immunoprecipitated with the antibody 4D11. The arrow pointing to the ∼62-kDa band below hnRNP L is specific for the A3.15 probe.
Interestingly, similar UV cross-linking immunoprecipitation with the CA-rich A3.15 and C7 RNA probes, but not a control transcript containing CG repeats, also identified hnRNP L as a prominent binding factor (Fig. 4C). In addition, a ∼62-kDa protein band just below hnRNP L is clearly visible in the A3.15 sample as well (Fig. 4C, lane 2, arrow). Moreover, hnRNP L binding to C7 and C42 appeared stronger than to the A3.15 probe (compare 4B, lane 7 and 4C, lane 6 with 4C, lane 3).
Of the other two bands (75 and 35 kDa) that are specific for the enriched element probes but not the mCaRRE1 or the CG probe, the 35 kDa band showed no difference in relative intensity among the element probes (Fig. 4C), suggesting that it is not an element-specific band; however, the 75 kDa band showed a much higher relative intensity in the C42 sample.
Thus, the CA-rich motifs enriched in the PKA- and CaMKIV-responsive elements likely bind proteins differentially; one of the common protein factor is the hnRNP L. Taken the random enrichment of these elements together with the essential role of hnRNP L in CaMKIV-regulated splicing through CA repeats (9), hnRNP L is likely a preferred target of PKA and CaMKIV in regulating splicing. Together, the CA-rich content and the identification of hnRNP L as a binding factor suggest that the selection indeed identified consensus motifs that are known to be CaMKIV-responsive.
PKA and CaMKIV Specifically Share Their Responsive Elements
As shown above, both CaMKIV and PKA promote inclusion of exons containing CaRRE1 (Fig. 1, A and C), and their consensus motifs appear similar in the CA- and A-rich content. Moreover, hnRNP L was identified as one binding factor for both kinase-selected elements (Fig. 4). These together suggest that the two kinases may share similar elements.
To examine the kinase specificity of these elements, four highly PKA-responsive clones were tested with CaMKIV and PKCγ (Fig. 5, A and B). These clones showed clear responses to CaMKIV (Fig. 5A), with CaMKIV-induced net changes ranging from 15 to 31%, well above that of the 33SB vector alone (4%). In contrast, three clones had no response to PKCγ above the 33SB level (Fig. 5B, lanes 1–8), and one clone (A3.44) showed only 9% increase of exon inclusion over the vector (lanes 9 and 10). Thus, the PKA-responsive elements are preferably responsive to both PKA and CaMKIV but not PKCγ.
FIGURE 5.
Kinase specificity of the PKA- or CaMKIV-responsive elements. A and B, CaMKIV and PKCγ effects on the splicing of the individual PKA-responsive clones. Shown are agarose gels of RT-PCR products of the splicing reporters contransfected into HEK293T cells with CaMKIV-dCTK75E (IVm), CaMKIV-dCT (IV) as indicated above the gels. In B, PKCγ-EGFP was cotransfected with all the reporters but was either not activated (−) or activated with TPA (+) as indicated. Below the gels are the percentages of exon inclusion for each lane and a bar graph of the percent net changes by CaMKIV or PKCγ for each reporter. C, CaRRE1 motif is replaceable by a consensus motif of the PKA-responsive elements. Shown on top are the 13-nt insert sequences of CaRRE1 (a and b) and its core motif substituted by CAAAAAG (aSub or bSub). Below are agarose gels of the splicing reporter assay for PKA response indicated as in A and B. D, comparison between the effects of the kinases on splicing through the RNA elements. Graphed are the fold changes of the percentages of exon inclusion by PKA, CaMKIV, or PKCγ, each normalized to that of vector 33SB (taken as 1.0). Data are from the splicing reporter assays in Figs. 1 and 5, A–C.
The sharing of motifs between PKA and CaMKIV is also supported by that substituting the CaRRE1 core sequence with CAAAAAG, from the consensus motif of PKA-responsive elements (Fig. 3A), retained the PKA effect (Fig. 5C). Therefore, the CaRRE1 core motif is replaceable by a PKA consensus motif.
Comparisons of these kinase effects through the elements relative to that through the vector sequence indicate that the PKA and CaMKIV effects on splicing are significantly stronger than PKCγ (Fig. 5D). Therefore PKA and CaMKIV specifically share most of these tested RNA elements in controlling alternative splicing.
The Consensus Motifs of the Selected RNA Elements Are Critical for Kinase-dependent Splicing and Are Significantly Enriched in Alternative Exons
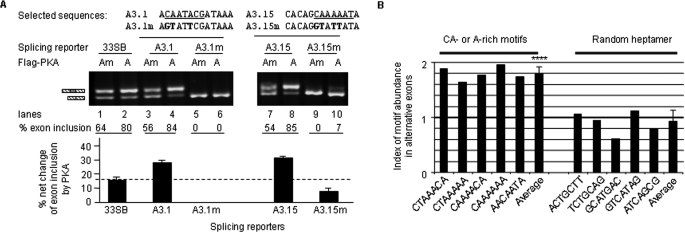
To verify that the consensus motifs are critical for kinase effects on splicing, two strongly PKA-responsive clones containing CA dinucleotides or A-rich motifs were mutated and tested in splicing reporter assays (Fig. 6A). The wild type clones showed significantly increased exon inclusion by PKA as expected (compare lanes 3 and 4, 7 and 8). Importantly, their responses to PKA were abolished or reduced by mutations in the consensus motifs (compare lanes 5 and 6, 9 and 10). Therefore, these selected motifs are essential for the PKA-dependent splicing changes.
FIGURE 6.
The kinase-responsive heptamer motifs are critical for the kinase effect on splicing and are significantly enriched in alternative exons. A, splicing reporter assay with highly PKA-responsive clones and their mutants. Above the gel are the 13-nt sequences of each clone and mutated nucleotides (bold). The dotted horizontal lines in the graphs mark the baseline percentages of net changes of the vector 33SB by PKA. Below each lane are the percentages of exon inclusion and a bar graph of net changes (aligned between every two lanes) by PKA for each reporter. B, bar graph of motif abundance demonstrating the enrichment of the kinase-responsive CA-containing or A-rich motifs in alternative exons in comparison to the random sequences in the control group. Each motif (as indicated) was searched against 6125 alternative or 3775 constitutive exons of 7 to 500 nt in length, and the abundance index was taken as the result of the element occurrences per kilobases of alternative exons divided by the corresponding occurrences of constitutive exons. Therefore, an index number of 1 equals the abundance of the elements in constitutive exons. The averages of the index numbers of the selected and non-selected motif groups are shown to the right of each group. ****, p < 0.0001, two tailed Student's t test between the two groups.
As would be expected from the above results, these motifs should be enriched in alternative exons compared with constitutive exons. We thus searched five CA-containing or A-rich heptamers from the consensus region of the PKA- or CaMKIV-responsive elements against subsets of alternative (6125 exons total) as well as constitutive (3775 exons total) exons from the published splicing databases (34–36). In total, 276 heptamers were found in the alternative exons while as only 108 found in the constitutive exons. On average, there are 45 heptamers in every 1000 alternative exons versus 29 in the same number of constitutive exons; or 38 versus 21 heptamers per 100 kilobases of alternative versus constitutive exon sequences. Relatively, all the five heptamers are present between 1.5 and 2-fold (1.8 ± 0.12, average ± S.D.) in the alternative exons over the constitutive exons (Fig. 6B). In contrast, five random heptamers all show about 1-fold (0.91 ± 0.2, average ± S.D.) in the alternative exons. Therefore, these CA-containing or A-rich motifs are significantly (p < 0.0001) enriched in alternative exons.
Motif-containing Endogenous Exons Are Regulated by cAMP and/or Depolarization in Cell Culture
Of these motifs, the A-rich ones in endogenous exons have not been shown to respond to cAMP and depolarization. In examination of seven A-rich consensus motif-containing exons (from ASAPII data base), we found three exons/genes with detectable expression and two of the three were alternatively spliced (with both exon-included and -excluded products detectable) in the PC12 and GH3 cells used. Interestingly, both of the alternatively spliced exons were regulated by cAMP and/or depolarization (Fig. 7).
FIGURE 7.
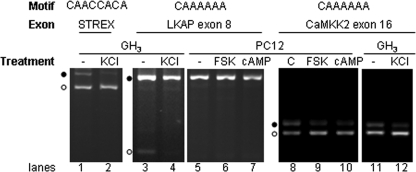
Regulation of the motif-containing endogeneous exons by cAMP or depolarization in cell cultures. Inclusion levels of endogenous exons STREX, LKAP exon 8, and CaMKK2 exon 16 were analyzed by RT-PCR. Motifs and exon names are above the gels. −, nontreated. C, ethanol, as the vehicle control for forskolin; FSK, forskolin (10 μm), an activator of adenylyl cyclase; cAMP, 8-cpt-cAMP (100 μg/ml), a membrane-permeable cAMP analogue. Exon-included products are indicated by black dots, and exon-excluded products by open circles. STREX-included and -excluded products are 597 and 423 nt, respectively; LKAP, 554 nt and 125 nt; and CaMKK2, 225 nt and 182 nt. The gels are representative of three experiments.
One of them, the exon 8 of the Limkain b1 (Lkap, GenBankTM accession no.: AB012133) gene encoding an autoantigen with RNA binding domains (39), contains 2 copies of CAAAAAA. This exon is differentially included in PC12 and GH3 cells (Fig. 7, lanes 3 and 5), suggesting that its inclusion is regulated. Depolarization of GH3 cells with KCl led to its complete inclusion (Fig. 7, lanes 3 and 4). In PC12 cells, this exon is already 100%-included before treatment and the forskolin or cAMP effect on its inclusion, if similarly promoting as the depolarization effect, cannot be assessed (lanes 5–7).
Another A-rich motif-containing exon identified is the CaMK kinase β1 (CaMKK2) exon 16 (40). This exon was regulated by both forskolin and cAMP, as well as by depolarization (Fig. 7, lanes 8–12), and was examined in more detail with hot-PCR and primer extension.
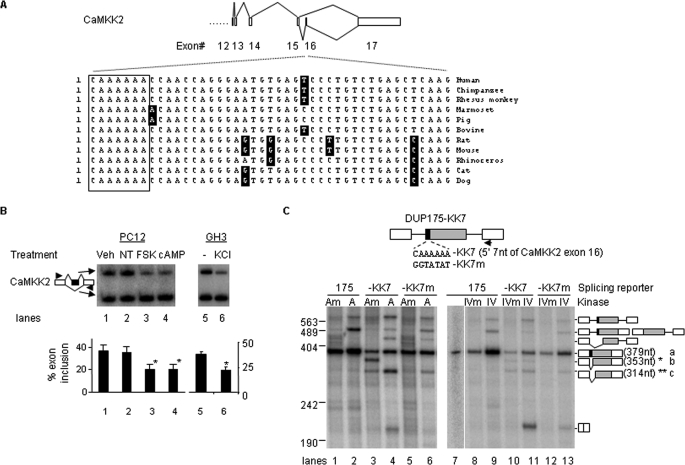
This exon, containing a conserved CAAAAAA motif at its 5′-end in mammalian genes (Fig. 8A), showed an inclusion level of about 37 and 36% in vehicle- and non-treated PC12 cells, respectively in hot-PCR (Fig. 8B, lanes 1 and 2). Upon treatment with PKA stimulus forskolin or cpt-cAMP, the exon inclusion level was reduced to about 21% (Fig. 8B, lanes 3 and 4). Therefore, the CaMKK2 exon 16 is likely controlled by the PKA pathway in PC12 cells. A similar effect was observed by treatment of GH3 cells with a depolarizing concentration of KCl (25 mm, lane 6), which controls splicing through CaMKIV (6).
FIGURE 8.
An A-rich motif mediates splicing regulation of CaMKK2 by PKA and CaMKIV. A, diagram of splicing pattern and sequences of CaMKK2 exon 16 with the conserved motif in mammals boxed. B, changes in the inclusion level of the endogenous CaMKK2 exon 16 in PC12 cells upon treatment with PKA stimuli, or in GH3 cells upon depolarization. Shown is the phosphorimages of denaturing PAGE gels of RT-PCR products of the CaMKK2 gene expressed in PC12 cells not-treated (−) or treated with various chemicals for 7 h as indicated above the gel, or in GH3 cells untreated (−) or treated with KCl (25 mm). Exon 16-included or -excluded product is diagrammed to the left, and a bar graph of the percent net changes is below the gel. Treatments are indicated as in the legend to Fig. 7. C, primer extension assay of the splicing reporters containing the CAAAAAA element from the CaMKK2 exon 16 at the 5′-end of the middle exon in the presence of PKAm (Am), PKA (A), CaMKIVm (IVm), CaMKIV (IV). Shown are phosphorimages of denaturing PAGE gels of primer extension products from mRNA of HEK293T cells transfected with the reporters and kinases as indicated above the gel. Products resulted from the pre-mRNA containing one or both unspliced introns with the deduced structures are diagrammed, based on sequencing similar bands of DUP175CaRRE1 products in other experiments; Asterisks, cryptically spliced products truncating 26 nt (*, band b) and 65 nt (**, band c) of the 5′-end of the 175-nt middle exon. Arrow, location of the primer for primer extension. The gels are representative of three experiments.
To verify the role of the CAAAAAA motif in response to the kinases, we replaced the 5′-end 7 nt of the DUP175 middle exon with the motif and tested its responses to PKA or CaMKIV in HEK293T cells (Fig. 8C). The PKA cotransfection did not induce exon skipping of the DUP175 transcripts (lanes 1 and 2). Transferring the CAAAAAA motif to DUP175 promoted two cryptically spliced products in the presence of PKA mutant (lane 3), but with barely visible complete exon-skipping products (about 7%, without counting the cryptic products, same as below). Coexpressed wild type PKA led to substantial amount of exon skipping (lane 4, about 31% exon-skipping product). Importantly, mutation of the CAAAAAA completely abolished the PKA effect (lane 6). Thus, the CAAAAAA motif is sufficient for the PKA effect on splicing. Coexpressed wild type CaMKIV induced exon skipping as well, with an even stronger effect (lane 11, about 60% exon skipping product), and also in a CAAAAAA-dependent way (lanes 12 and 13).
Taken together, these data indicate that the CAAAAAA motif mediates the control of alternative splicing of the CaMKK2 gene by PKA and CaMKIV. Therefore, the motif provides a converging point for PKA and CaMKIV to cross-talk or feedback regulate CaMKK2 through alternative splicing.
DISCUSSION
Several Considerations about the in Vivo Selection System and Identified RNA Elements
Previous reports selected constitutive enhancer or silencer elements in alternative splicing (23, 25). There has been no reported selection system to enrich and refine pre-mRNA elements responsive to activated protein kinases. We thus attempted to select kinase-responsive elements by modifying the method used for the constitutive enhancers (23).
The rationale of selection is based on the kinase promotion of exons containing kinase-responsive silencers in a splicing reporter already having about 65% exon inclusion (Fig. 1). As expected, most of the selected clones contain constitutive splicing silencers. The exon inclusion levels of a smaller group of enhancer-containing clones mostly either reached about 100% and not to be increased further by the protein kinase PKA or even reduced by CaMKIV (Fig. 2). Based on this observation, similar selection could be applied to enrich RNA elements responsive to other signaling protein kinases.
The selected elements appear CA- and A-rich. The consensus motif heptamers are critical for the PKA effect and are significantly enriched in alternative exons (Figs. 5 and 6), supporting their role in regulated splicing. Interestingly, CA repeats have been recently found to mediate CaMKIV-regulated splicing, with the hnRNP L as an essential component (9). Consistently, hnRNP L is identified as a factor binding to the enriched CA-rich elements as well (Fig. 4). Therefore, CA repeats or the CA-rich elements and hnRNP L are likely preferred targets of CaMKIV in controlling splicing. The consensus sequence and the matrices should be helpful for recognizing these regulatory elements in the “sea” of the genomic sequences.
The CA-rich content also bears some similarity to the A/C-rich elements but the latter are splicing enhancers, as tested in a similar 33-nt exon derived from the β-globin gene as well (23). Moreover, the A/C-rich enhancer-binding protein YB-1 (∼50 kDa) is not observed as a prominent band in UV-cross-linking (Fig. 4). The A-rich contents of these elements have not been observed in PKA- or CaMKIV-regulated splicing. A known A-rich element is the AAAAUU in the exon v5 of the CD44 gene (20), but it is an enhancer. Therefore, the similarities and differences between the selected and these known elements need further investigation.
The number of clones contained in the starting pool is about one-sixth of a full coverage (6.7 × 107 clones) of 13-nt random sequences. The CaMKIV selection did not enrich elements similar to the G-rich CaRRE2 (8). Moreover, this system does not allow the selection and assessment of constitutive splicing enhancers in kinase-promoted exons. Therefore, besides the selected elements, the existence of other sequences that are also responsive to these kinases is not ruled out. Despite these considerations, this selection does provide the first example to enrich RNA elements that are responsive to protein kinases.
Context Dependence of Cell Signal-regulated Alternative Splicing
Context dependence of pre-mRNA elements on splicing has been observed in many cases (5, 17–19), but opposite effects of a kinase in splicing through the same element in different minigenes have not been observed. We have shown here that, through the CaRRE1, CaMKIV inhibits exon inclusion in one minigene, DUP175ST (6), but promotes exon inclusion in another minigene, 33SB-CaRRE1 (Fig. 1). The promoting effect of CaMKIV, as well as PKA, is also seen through the SELEX-enriched elements (Figs. 2, 5, and 6). Consistent with the context-dependent effect through these elements, PKA and CaMKIV also inhibit the inclusion of the motif-containing exon in DUP175 (Fig. 8).
The molecular mechanisms underlying the context-dependence of a protein kinase through an element is unknown. In the case of SRp38, the splicing enhancing or repressing effects can be determined by the phosphorylation/dephosphorylation status of the protein (41). In our splicing reporter assays, the protein kinases used for the reporters DUP175ST and 33SB-CaRRE1 are the same (PKA or CaMKIV). Therefore, any changes of the phosphorylation status of a splicing factor, for example hnRNP L, are likely to be the same for both reporter systems. However, these reporters differ in their promoters, lengths/sequences of the middle exons, flanking exons/introns, and the locations of the CaRRE1 element relative to the splice sites (Fig. 1). Interestingly, the mCaRRE1 in DUP175 and the selected elements in 33SB have several different bound factors: PTB specific for mCaRRE1 while as the 75-kDa protein specific for the enriched element probes (Fig. 4). Moreover, a ∼62-kDa protein smaller than hnRNP L is specific for the A3.15 element (Fig. 4C, lane 2). Taking these differences together with the known combinatorial control of mammalian splicing by multiple elements and influence by promoters (16, 17), one possibility may involve differential recruitment of trans-acting factors in addition to hnRNP L to the target and/or its surrounding elements/promoters by the kinases, resulting in either inhibition or promotion of splicing depending on the balance between the positive and negative regulators.
Effect of the Enriched Elements in Cells and Shared Target Elements/Exons in PKA- and CaMKIV-regulated Alternative Splicing
Similar to the previously identified depolarization-responsive elements that respond to depolarization only with multiple copies of the elements or together with other elements (6, 8, 10), a splicing reporter containing only one copy of an hnRNP L high affinity sequence, as used previously(9), was not sufficient to respond to depolarization (data not shown). It is thus likely that the CA-rich elements also require multiple copies (such as 2 copies of CAAAAAA in the LKAP exon 8, Fig. 7) or with the help of other elements in alternative exons to confer response to endogeneous signals.
The ASAPII exons containing these enriched motifs have average MaxEntScan scores of 7.5 (±2.98) for the 3′-splice sites and 7.9 (±2.8) for the 5′-splice sites (n = 263) as measured according to the maximum entropy model (42). These scores are not significantly lower than that of the constitutive exons (7.9 ± 3.6, 8.3 ± 3.1, for the 3′- and 5′-splice sites, respectively)(43). The relatively strong splice sites may make the exons mostly included as the LKAP exon but with the presence of the CA- or A-rich elements can still be regulated by the activation of protein kinases.
The cAMP and Ca2+ signaling pathways are known to converge at the Ser133 of the CREB protein in transcriptional control (44). Their effects on alternative splicing through common RNA elements have not been observed. Interestingly, the 33SB-derived reporters used here demonstrate preferred responses of the CA- and A-rich elements to both kinases, but not PKCγ. The control of alternative splicing by PKA and CaMKIV through the same RNA elements adds another possible point of convergence between these two important signaling pathways.
Among the common target elements shown here however, their responses to PKA and CaMKIV overexpression are not always the same (Fig. 5D). Specific elements with variations in nucleotide sequences are preferred by each of the two kinases (Fig. 5D). Related to this variation, in the exon of a DUP175-derived splicing reporter, a CA repeat element strongly responded to CaMKIV but barely to PKA (9), unlike in the 33SB-derived reporters (Figs. 1, 5, and 6). In this case, the reporter context appears to play an important role on the PKA effect. The shared, but differential, splicing regulation through these RNA elements by PKA and CaMKIV likely helps to refine gene expression profiles tailored for precise functions of genes and cells.
PKA is known to control the CaMKK1 (CaMKKα) through direct protein phosphorylation (45–50). The control of CaMKK2 splicing by PKA and CaMKIV through a common RNA element adds a novel way of signal cross-talk/feedback between the PKA and CaM kinase pathways. Compared with direct phosphorylation, one likely advantage of this splicing regulation is that it can be temporally longer and involved in cell functions requiring gene expression to take effect. Therefore, this regulation provides an example where the selected RNA element could play a role in regulating the important signaling networks in cells.
In summary, we used in vivo selection for PKA/CaMKIV-responsive RNA elements controlling alternative splicing and identified consensus motifs that are similar to the previously characterized CaRRE1 and CA repeats, as well as novel A-rich elements. This selection also provides an approach that could be applied to other signal pathways.
Acknowledgments
We thank Thomas Cooper for the cTNT plasmid, Doug Black for DUP splicing reporters and kinase plasmids, Harold König for the pL53In plasmid, and Christopher Lee for ASAPII database exons. We thank Mary-Lynn Duckworth and Doug Black for helpful comments.
This work was supported by the Canadian Institutes of Health Research (CIHR, MOP 68919) (to J. X.).
- CaMKIV
- calcium/calmodulin-dependent protein kinase IV
- PKA
- cAMP-dependent protein kinase
- nt
- nucleotide
- DMEM
- Dulbecco's modified Eagle's medium
- RT
- reverse transcription
- TPA
- 12-O-tetradecanoylphorbol-13-acetate
- STREX
- stress axis-regulated exon
- SELEX
- systematic evolution of ligands by exponential enrichment
- PKC
- protein kinase C
- PTB
- polypyrimidine tract-binding protein
- CaRRE1
- CaMKIV-responsive RNA element 1
- CaMKK2
- CaMK kinase β1.
REFERENCES
- 1.Black D. L. ( 2003) Annu. Rev. Biochem. 72, 291– 336 [DOI] [PubMed] [Google Scholar]
- 2.Maniatis T., Tasic B. ( 2002) Nature 418, 236– 243 [DOI] [PubMed] [Google Scholar]
- 3.Shin C., Manley J. L. ( 2004) Nat. Rev. Mol. Cell Biol. 5, 727– 738 [DOI] [PubMed] [Google Scholar]
- 4.Stamm S. ( 2008) J. Biol. Chem. 283, 1223– 1227 [DOI] [PubMed] [Google Scholar]
- 5.Xie J. ( 2008) Biochim. Biophys. Acta 1779, 438– 452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J., Black D. L. ( 2001) Nature 410, 936– 939 [DOI] [PubMed] [Google Scholar]
- 7.Xie J., Jan C., Stoilov P., Park J., Black D. L. ( 2005) RNA 11, 1825– 1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J. A., Xing Y., Nguyen D., Xie J., Lee C. J., Black D. L. ( 2007) PLoS Biol 5, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J., Hai Y., Liu G., Fang T., Kung S. K., Xie J. ( 2009) J. Biol. Chem. 284, 1505– 1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An P., Grabowski P. J. ( 2007) PLoS Biol 5, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKee A. E., Neretti N., Carvalho L. E., Meyer C. A., Fox E. A., Brodsky A. S., Silver P. A. ( 2007) Genome Biol. 8, R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Küppers E., Sabolek M., Anders U., Pilgrim C., Beyer C. ( 2000) Brain Res. Mol. Brain Res. 81, 19– 28 [DOI] [PubMed] [Google Scholar]
- 13.Berke J. D., Sgambato V., Zhu P. P., Lavoie B., Vincent M., Krause M., Hyman S. E. ( 2001) Neuron 32, 277– 287 [DOI] [PubMed] [Google Scholar]
- 14.Sgambato V., Minassian R., Nairn A. C., Hyman S. E. ( 2003) J. Neurochem. 86, 153– 164 [DOI] [PubMed] [Google Scholar]
- 15.Kvissel A. K., Ørstavik S., Eikvar S., Brede G., Jahnsen T., Collas P., Akusjärvi G., Sk̊lhegg B. S. ( 2007) Exp. Cell Res. 313, 2795– 2809 [DOI] [PubMed] [Google Scholar]
- 16.Smith C. W., Valcárcel J. ( 2000) Trends Biochem. Sci. 25, 381– 388 [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Burge C. B. ( 2008) RNA 14, 802– 813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goren A., Ram O., Amit M., Keren H., Lev-Maor G., Vig I., Pupko T., Ast G. ( 2006) Mol. Cell 22, 769– 781 [DOI] [PubMed] [Google Scholar]
- 19.Underwood J. G., Boutz P. L., Dougherty J. D., Stoilov P., Black D. L. ( 2005) Mol. Cell. Biol. 25, 10005– 10016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matter N., Herrlich P., König H. ( 2002) Nature 420, 691– 695 [DOI] [PubMed] [Google Scholar]
- 21.Rothrock C., Cannon B., Hahm B., Lynch K. W. ( 2003) Mol. Cell 12, 1317– 1324 [DOI] [PubMed] [Google Scholar]
- 22.Hai Y., Cao W., Liu G., Hong S. P., Elela S. A., Klinck R., Chu J., Xie J. ( 2008) Nucleic Acids Res. 36, 3320– 3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulter L. R., Landree M. A., Cooper T. A. ( 1997) Mol. Cell. Biol. 17, 2143– 2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stickeler E., Fraser S. D., Honig A., Chen A. L., Berget S. M., Cooper T. A. ( 2001) EMBO J. 20, 3821– 3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Rolish M. E., Yeo G., Tung V., Mawson M., Burge C. B. ( 2004) Cell 119, 831– 845 [DOI] [PubMed] [Google Scholar]
- 26.Chatila T., Anderson K. A., Ho N., Means A. R. ( 1996) J. Biol. Chem. 271, 21542– 21548 [DOI] [PubMed] [Google Scholar]
- 27.Xie J., Lee J. A., Kress T. L., Mowry K. L., Black D. L. ( 2003) Proc. Natl. Acad. Sci. U. S. A. 100, 8776– 8781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng J., Knighton D. R., ten Eyck L. F., Karlsson R., Xuong N., Taylor S. S., Sowadski J. M. ( 1993) Biochemistry 32, 2154– 2161 [DOI] [PubMed] [Google Scholar]
- 29.König H., Ponta H., Herrlich P. ( 1998) EMBO J. 17, 2904– 2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modafferi E. F., Black D. L. ( 1997) Mol. Cell. Biol. 17, 6537– 6545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kataoka N., Dreyfuss G. ( 2008) Methods Mol. Biol. 488, 357– 365 [DOI] [PubMed] [Google Scholar]
- 32.Piñol-Roma S., Swanson M. S., Gall J. G., Dreyfuss G. ( 1989) J. Cell Biol. 109, 2575– 2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey T. L., Williams N., Misleh C., Li W. W. ( 2006) Nucleic Acids Res. 34, W369– 373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim N., Alekseyenko A. V., Roy M., Lee C. ( 2007) Nucleic Acids Res. 35, D93– 98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holste D., Huo G., Tung V., Burge C. B. ( 2006) Nucleic Acids Res. 34, D56– 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo G. W., Van Nostrand E., Holste D., Poggio T., Burge C. B. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 2850– 2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominski Z., Kole R. ( 1991) Mol. Cell. Biol. 11, 6075– 6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma S., Liu G., Sun Y., Xie J. ( 2007) Biochim. Biophys. Acta 1773, 912– 923 [DOI] [PubMed] [Google Scholar]
- 39.Dunster K., Lai F. P., Sentry J. W. ( 2005) Clin. Exp. Immunol 140, 556– 563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu L. S., Chen G. D., Lee L. S., Chi C. W., Cheng J. F., Chen J. Y. ( 2001) J. Biol. Chem. 276, 31113– 31123 [DOI] [PubMed] [Google Scholar]
- 41.Feng Y., Chen M., Manley J. L. ( 2008) Nat. Struct. Mol. Biol. 15, 1040– 1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo G., Burge C. B. ( 2004) J. Comput. Biol. 11, 377– 394 [DOI] [PubMed] [Google Scholar]
- 43.Bortfeldt R., Herrmann A., Pospisil H., Schuster S. ( 2007) in Mathematical Modeling of Biological Systems ( Deutsch A. B., Byrne H., de Vries G., Herzel H. ed) Vol. I, pp. 337– 349, Birkhäuser, New York [Google Scholar]
- 44.West A. E., Chen W. G., Dalva M. B., Dolmetsch R. E., Kornhauser J. M., Shaywitz A. J., Takasu M. A., Tao X., Greenberg M. E. ( 2001) Proc. Natl. Acad. Sci. U. S. A. 98, 11024– 11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soderling T. R. ( 1999) Trends Biochem. Sci 24, 232– 236 [DOI] [PubMed] [Google Scholar]
- 46.Davare M. A., Saneyoshi T., Guire E. S., Nygaard S. C., Soderling T. R. ( 2004) J. Biol. Chem. 279, 52191– 52199 [DOI] [PubMed] [Google Scholar]
- 47.Matsushita M., Nairn A. C. ( 1999) J. Biol. Chem. 274, 10086– 10093 [DOI] [PubMed] [Google Scholar]
- 48.Kitani T., Okuno S., Fujisawa H. ( 2001) J. Biochem. 130, 515– 525 [DOI] [PubMed] [Google Scholar]
- 49.Okuno S., Kitani T., Fujisawa H. ( 2001) J. Biochem. 130, 503– 513 [DOI] [PubMed] [Google Scholar]
- 50.Wayman G. A., Tokumitsu H., Soderling T. R. ( 1997) J. Biol. Chem. 272, 16073– 16076 [DOI] [PubMed] [Google Scholar]