Abstract
11-cis-Retinol has previously been shown in physiological experiments to promote dark adaptation and recovery of photoresponsiveness of bleached salamander red cones but not of bleached salamander red rods. The purpose of this study was to evaluate the direct interaction of 11-cis-retinol with expressed human and salamander cone opsins, and to determine by microspectrophotometry pigment formation in isolated salamander photoreceptors. We show here in a cell-free system using incorporation of radioactive guanosine 5′-3-O-(thio)triphosphate into transducin as an index of activity, that 11-cis-retinol inactivates expressed salamander cone opsins, acting an inverse agonist. Similar results were obtained with expressed human red and green opsins. 11-cis-Retinol had no significant effect on the activity of human blue cone opsin. In contrast, 11-cis-retinol activates the expressed salamander and human red rod opsins, acting as an agonist. Using microspectrophotometry of salamander cone photoreceptors before and after bleaching and following subsequent treatment with 11-cis-retinol, we show that 11-cis-retinol promotes pigment formation. Pigment was not formed in salamander red rods or green rods (containing the same opsin as blue cones) treated under the same conditions. These results demonstrate that 11-cis-retinol is not a useful substrate for rod photoreceptors although it is for cone photoreceptors. These data support the premise that rods and cones have mechanisms for handling retinoids and regenerating visual pigment that are specific to photoreceptor type. These mechanisms are critical to providing regenerated pigments in a time scale required for the function of these two types of photoreceptors.
11-cis-Retinol is the precursor to 11-cis-retinal, the 11-cis-aldehyde form of vitamin A and the chromophore that combines covalently with rod and cone opsin proteins to form visual pigments. 11-cis-Retinal is consumed during visual signaling, and its continual synthesis is required. Photon absorption by the visual pigments causes the isomerization of its chromophore to the all-trans configuration. This initiates two processes critical for vision: activation of the photoreceptor cell and the eventual recovery of the original photosensitivity of the cells, requiring regeneration of the visual pigments. As cones are used for bright light vision, these two processes must work more rapidly in cones than in rods and thus cones have a higher requirement of 11-cis-retinoids as suggested by Rushton (1, 2).
Photoreceptor activation begins with photoisomerization of the chromophore within the visual pigment. This results in a subsequent conformational change of the protein part of the visual pigment that is able to activate its G protein transducin, which in turn activates a PDE that lowers the concentration of cGMP and closes cGMP-gated ion channels. These steps comprise the visual signal transduction cascade (see Ref. 3 for review).
The visual cycle involves regeneration of the visual pigment, which ultimately deactivates the protein and accomplishes the recovery of the photosensitivity of the photoreceptor cell. Classically, this process involves both the photoreceptor cell and the retinal pigment epithelium (RPE).4 After photoisomerization of the chromophore and formation of the active visual pigment, all-trans-retinal is released from the opsin and reduced to all-trans-retinol, which is then transported to the RPE where it is isomerized to 11-cis-retinol through a number of steps. In the RPE, 11-cis-retinol is oxidized to the aldehyde form, which is transported back to the photoreceptor cell and can be directly used by all of the opsins to regenerate an inactive pigment ready for photoactivation. The details of this model have been extensively reviewed (4, 5). Alternatively, recent work suggests that cones have an additional source of 11-cis-retinoids from Müller cells (6–8). Like the RPE cells, Müller cells have been shown to be able to convert all-trans-retinol to 11-cis-retinol (6). Unlike in the RPE cells, 11-cis-retinol is not oxidized to 11-cis-retinal in Müller cells.
Jones et al. (9) demonstrated that administration of 11-cis-retinol to bleached salamander red cones could restore photosensitivity. A logical conclusion was that red cones were able to oxidize 11-cis-retinol to the aldehyde and regenerate visual pigments although noncovalent binding of 11-cis-retinol to red cone opsins generating a light-sensitive complex could not be excluded. On the other hand, 11-cis-retinol does not restore photosensitivity to bleached salamander rod cells but appears to directly activate the cells (9, 10). The data suggested that the rods were not able to oxidize 11-cis-retinol, but that the retinol itself could activate the signal transduction cascade, and indeed we recently demonstrated that 11-cis-retinol acts as an agonist to expressed bovine rod opsin (11). Our aim here was to study the action of 11-cis-retinol on cone opsins and cone photoreceptor cells to determine the efficacy of an alternate visual cycle for cones.
The photoreceptor cells used in this study are from tiger salamander, and the expressed opsins used for biochemical experiments are those from salamander and human. Photoreceptor cells are generally identified by cell morphology and the type of opsin it contains that can be further complicated by the findings that some cone cells have multiple opsins (12, 13). Recently genetic analysis has determined that opsins fall into five classes (reviewed in Refs. 14 and 15). We have studied opsins falling into four of these classes and use common color-derived names for the opsins and photoreceptor cells. The classic rod cells used for scotopic vision contain rhodopsin, the visual pigment for the rod opsin (RH1 opsin) and appeared red and thus have been designated as red rods. Some species such as salamanders have an additional rod cell whose photosensitivity is blue-shifted from that of the red rod and thus designated as green rods. In the tiger salamander, the green rods contain the identical opsin (SWS2 opsin) found in blue cones (16). The human blue cones contain an opsin from a different class (SWS1 opsin), which is homologous to the salamander UV cone opsin. The human red and green and salamander red cone opsins all belong to the same class of opsins (M/LWS opsins). Absorption properties of visual pigments are further modulated in some animals including the tiger salamander by use of 11-cis-retinal with an additional double bond (3,4-dehydro or A2 11-cis-retinal) resulting in red-shifted absorbance from pigments containing 11-cis-retinal (A1 11-cis-retinal).
We show here that 11-cis-retinol is not an agonist to cone opsins and does not itself generate a light-sensitive opsin. We further show using microspectrophotometry that both red and blue salamander cone cells regenerate visual pigments from 11-cis-retinol, whereas pigments could not be regenerated with 11-cis-retinol in bleached salamander red and green rods even though the latter contains the same opsin as the salamander blue cone. Thus, rods and cones have mechanisms for handling retinoids and regenerating visual pigment that are specific to photoreceptor type, and these mechanisms are critical to providing regenerated pigments in a time scale required for the function of these two types of photoreceptors.
EXPERIMENTAL PROCEDURES
Retinoids
The retinoids used in this study, the A1 forms of 11-cis-retinal and 11-cis-retinol, were synthesized and purified as described (for review, see Ref. 17). Purity of retinoids was assessed by high performance liquid chromatography (18).
Animals and Preparation
Larval tiger salamanders (Ambystoma tigrinum) were purchased from the Charles D. Sullivan Company (Nashville, TN) and maintained, sacrificed, and processed to isolate intact photoreceptor cells as previously described (19). All experiments were performed according to protocols approved by the Animal Care and Use Committee of Boston University School of Medicine and in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.
Visual Pigment Bleaching and Regeneration
Cells were bleached on the microscope stage of the microspectrophotometer using a step of an unattenuated broad-band or “white” light. This light (duration 30–60 s) was provided from a tungsten-halogen light source (2.8 × 107 photons μm−2 s−1 at 500 nm) that was focused at the plane of the preparation. The extent of bleaching (>90% for all cell types) was confirmed by absorption spectroscopic measurements of single photoreceptor outer segments. This bleaching method taking place on the microscope stage had the advantage that it allowed spectral measurements from the same individual photoreceptor cell in the native state prior to bleaching as well as at different times after bleaching and following treatment with different retinoids.
In some experiments, all cells in the sample were fully bleached prior to making the spectral measurements. Cells in a 3-cm plastic Petri dish were exposed to overlapping light spots (about 1 cm diameter) from two tungsten light sources focused at the sample plane for 10 min. One light spot consisted of short-wavelength light obtained by passing the beam through a wide-band interference filter (>70% transmittance at 400–470 nm). The second light beam consisted of long-wavelength light obtained by passing the beam through a wide-band interference filter (Kodak Wratten filter 25, >70% transmittance in the range 620–700 nm). Light from these two sources were used to selectively bleach blue and red cones, respectively. The estimates for the minimum fraction of visual pigment bleached, Fmin, were calculated according to the relation: Fmin = 1 − exp(−I Pλt), where I is the light intensity (photons μm−2 s−1), Pλ is the photosensitivity of the pigment (μm2), and t = the duration of the light exposure (s). We used an estimate of Pλ = 6 × 10−9 μm2 (20) for the peak photosensitivity of all rod and cone pigments studied. By microspectrophotometric measurements, we confirmed that this method bleached the visual pigment within the outer segment to the same extent (>90%) as did the bleaching that took place on the stage of the microspectrophotometer.
For regeneration experiments, retinoids were supplied to bleached cells in Ringer solution containing 0.3% ethanol or in lipid vesicles as a retinoid carrier as described previously (21). Cells were exposed to retinoids (final concentration 20–40 μm) by adding a small amount (100–500 μl) of concentrated (40–350 μm) retinoid stock solution to Ringer solution containing photoreceptor cells in the recording chamber.
Microspectrophotometry
The absorption spectra from the outer segments of intact photoreceptors containing native pigment and after bleaching and addition of retinoid solutions were measured by a single-beam microspectrophotometer (22). The absorption spectra were recorded over the range of 380–740 nm at room temperature (21 °C) by procedures described earlier (23). The mean optical density (OD) was calculated automatically by the acquisition software as OD = log (I0/It), where I0 is the transmitted light in a cell-free space adjacent to the photoreceptor cell and It is the transmitted light through the photoreceptor outer segment. Each spectral measurement on photoreceptor cells as well as baseline measurements consisted of 10 individual spectral scans that were averaged. In the experiments where visual pigment bleaching was performed prior to placing the cells on the microscope state, the sample was divided into three parts: dark-adapted cells containing native pigment, cells that were bleached and kept in Ringer solution, and cells that were bleached and treated with retinoids following bleaching to promote pigment regeneration.
Estimation of Pigment Composition and Peak Optical Density (OD)
The absorption spectrum of each individual cell measured in the native state or following bleaching and exposure to retinoids was fitted by a sum of A1 and A2 visual pigment templates (24). The goodness of the fit was estimated based on least-square criteria in the following wavelength ranges: 390–705 nm (green rods and blue cones), 400–705 nm (red cones), and 450–705 nm (red rods). The following wavelengths of maximum absorbance for pure A1 and A2 pigments (λmax,A1 and λmax,A2) were used based on previous work (16, 25–27): λmax,A1 = 562 nm, λmax,A2 = 629 nm (red-sensitive cones); λmax,A1 = 432 nm, λmax,A2 = 440 nm (blue-sensitive cones, green rods); and λmax,A1 = 502 nm, λmax,A2 = 528 nm (red rods). The variation of peak ODs and A1:A2 ratios were characterized by calculating the S.E. based on template fitting to each individual spectrum.
Opsin Expression
Salamander and human rod and cone opsins were expressed in COS cells as described previously (28, 29). A discontinuous sucrose gradient was used to isolate the plasma membrane fraction (30, 31).
Transducin Activation Assay
The ability of a given opsin to activate transducin was determined using a radioactive filter binding assay using membrane preparations of opsin expressed in COS cells as described previously (30, 31). Bovine rod transducin was purified from bovine retinas as described previously (32). To assay the ability of the opsin to activate transducin, we followed the increase of radioactive GTPγS bound to transducin with time after addition of retinoid in the dark or after illumination. Typically, our reaction mixture contained 1–10 nm opsin, 2.5 μm transducin, 3.0 μm GTPγS-35 (∼5 nCi/μl), 20 μm retinoid, 10 mm Mes, pH 6.4, 100 mm NaCl, 1 mm dithiothreitol, and 5 mm MgCl2, pH 6.4, at a final volume of 50, 100, or 150 μl. The buffer, opsin membranes, and transducin were first mixed. Retinoids were added from a ×50 stock dissolved in ethanol 1 min prior to addition of GTPγS, at which time the clock was started. At specified times, a 10-μl aliquot was transferred onto a nitrocellulose filter membrane attached to a vacuum manifold, washed (3 times) with 4 ml of ice-cold buffer; and the amount of bound radioactivity was determined by scintillation counting. For light activation, samples were illuminated with either >530 nm (for rhodopsins and long wavelength-sensitive cone opsins) or >430 nm (for human and salamander blue cone opsins) light from a slide projector with a 300 watt bulb and long-pass filter for 12 s.
RESULTS
11-cis-Retinol Acts as an Agonist to Salamander Red Rod Opsin and Does Not Promote Regeneration of Visual Pigment in the Intact Rod
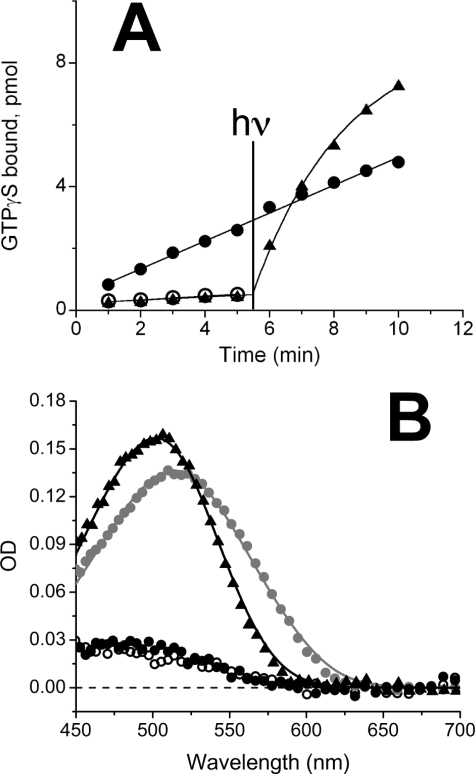
The data illustrated in Fig. 1A show that 11-cis-retinol behaves as an agonist to salamander red rod opsin. Transducin activation by opsin in the presence of 11-cis-retinol is higher than in its absence. Activation by the apoprotein (open circles) occurs at a low level indicating a low level of basal activity at pH 6.4. Addition of 11-cis-retinal (filled triangles) lowers transducin activation consistent with published results (33, 34), but subsequent exposure to light results in increased transducin activation due to photoactivation of the pigment. By contrast, exposure to 11-cis-retinol (filled circles) results in a roughly 5-fold elevation of transducin activation, which cannot be further incremented by light stimulation (λ > 530 nm) indicating that pigment had not been generated. These experiments were performed in a cell-free system that does not include an oxidoreductase capable of oxidizing 11-cis-retinol to 11-cis-retinal.
FIGURE 1.
Effect of 11-cis-retinol and -retinal on opsin activation and pigment regeneration in salamander red rods. A, transducin activation by expressed salamander red rod opsin (open circles), with 11-cis-retinol (filled circles) and with 11-cis-retinal (triangles). Each sample was assayed in triplicate and averaged; error bars reflect mean ± S.E. and often were smaller than the symbol size. At 5.5 min, samples with 11-cis-retinal and 11-cis-retinol were illuminated with light for 12 s as described under “Experimental Procedures” and illustrated in the figure with the vertical line. B, absorption spectra of isolated salamander red rods measured microspectrophotometrically in native state (gray circles, n = 28), measured ∼130 min following a complete (>90%) bleach (black filled circles, n = 3), measured 130–190 min after treatment with 11-cis-retinol following a complete bleach (open circles, n = 4), and measured 140–200 min after treatment with 11-cis-retinal following a complete bleach (triangles, n = 9). Retinoids were suspended in lipid vesicles or ethanolic solution. Continuous curves fitted to the data are sums of templates for A1 and/or A2 visual pigments (see “Experimental Procedures”).
To compare the effectiveness of 11-cis-retinal and -retinol in promoting pigment regeneration in isolated salamander rods, we recorded the absorption spectra of individual salamander red rods before and after bleaching, and following exposure to exogenous 11-cis-retinoids (Fig. 1B). After a >90% bleach of the native visual pigment, the main peak at around 514 nm (gray filled circles) is substantially reduced (closed filled circles). The native salamander red rod pigment spectrum is consistent with about 70:30% mixture of A2 and A1 chromophore-based pigments as shown by the template (continuous gray line) fitted to the data. Following bleaching, there were no significant differences between the spectra recorded in the absence and presence of 11-cis-retinol (compare filled and open circles) indicating that red rods do not regenerate visual pigment or do so only at a very low rate in the presence of 11-cis-retinol. Pigment regeneration was obtained following exposure to 11-cis-retinal (filled triangles). This spectrum is blue-shifted compared with the native visual pigment, consistent with pigment regeneration based on A1 chromophore alone (λmax at 502 nm) (fitted black template: λmax,A1 = 502 nm, 0% A2 pigment). The mean peak OD of the regenerated pigment (0.157) was higher than that of the native pigment (0.136) consistent with the higher molar extinction coefficient of A1-based pigment compared with the A2-based pigment.
11-cis-Retinol Inactivates Salamander Red Cone Opsin and Forms Pigment in the Intact Red Cone
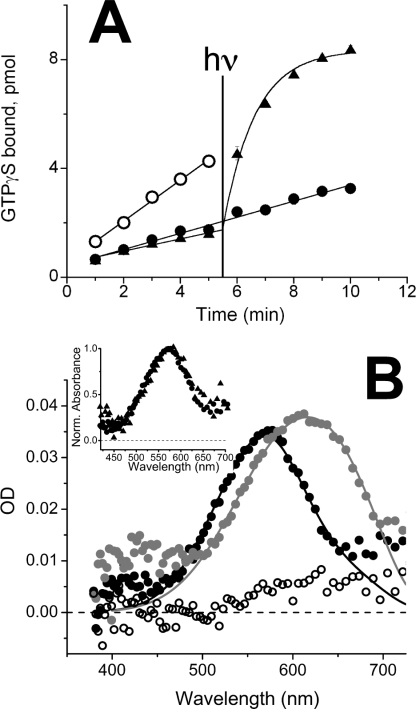
The data illustrated in Fig. 2A show that 11-cis-retinol behaves as an inverse agonist to salamander red cone opsin. Transducin activation by opsin in the presence of 11-cis-retinol (filled circles) is lower than in its absence (open circles). These latter data are indistinguishable from those obtained following the addition of 11-cis-retinal (filled triangles). However, when exposed to light (λ > 530 nm), 11-cis-retinal-treated opsin activated transducin, as expected. The sample treated with 11-cis-retinol did not, indicating that the pigment had not been generated. As in Fig. 1A, these experiments were performed in a cell-free system that does not include an oxidoreductase capable of oxidizing 11-cis-retinol to 11-cis-retinal.
FIGURE 2.
Effect of 11-cis-retinol and -retinal on salamander opsin activation and pigment regeneration in red cones. A, transducin activation by expressed salamander red cone opsin (open circles) with 11-cis-retinol (filled circles) and 11-cis-retinal (triangles). Each sample was assayed in triplicate and averaged; error bars reflect mean ± S.E. and often were smaller than the symbol size. At 5.5 min, samples with 11-cis-retinal and 11-cis-retinol were illuminated with light for 12 s as described under “Experimental Procedures” and illustrated in the figure with the vertical line. B, absorption spectra measured on salamander red cones in the native dark-adapted state (gray circles, n = 41), about 44 min after >90% bleach (black open circles, n = 23), and after treatment of bleached cones for 50–100 min with 11-cis-retinol (filled black circles, n = 44). Inset, normalized absorption spectra of salamander red cones before and after bleaching and regeneration with 11-cis-retinol (circles, n = 44) or 11-cis-retinal (triangles, n = 4).
To compare the effectiveness of 11-cis-retinal and -retinol in promoting pigment regeneration in isolated salamander red cones, we recorded the absorption spectra of individual salamander red cones before (filled gray circles) and after bleaching (open circles), and following exposure to exogenous 11-cis retinoids (filled circles and filled triangles) (Fig. 2B and inset). Both 11-cis-retinol and 11-cis-retinal are effective substrates for pigment regeneration. As shown in the main figure, the average unbleached pigment spectrum peaks at about 620 nm. As was the case for rod spectra illustrated in Fig. 1B, this spectrum reflects a mixture of A1- and A2-based visual pigments dominated by the A2 pigment. A new absorption band peaking at about 560 nm is obtained after 11-cis-retinol was added, consistent with the regeneration of native pigment with mostly the A1 form of 11-cis-retinal. These data were fitted with pigment template curves calculated with the same λmax,A1 and λmax,A2 values as in the native state but with a significantly higher A1 pigment content following treatment with 11-cis-retinol compared with the native state. Template fits to each individual spectrum give the following estimates for OD and A2% (mean ± S.E.): OD = 0.038 ± 0.002 (native) and 0.034 ± 0.001 (after 11-cis-retinol treatment); A2% = 87 ± 2 (native) and 34 ± 3 (after 11-cis-retinol treatment). The normalized absorption spectra obtained from cells bleached and regenerated with 11-cis-retinal and 11-cis-retinol were indistinguishable (compare filled triangles and filled circles, Fig. 2B, inset) suggesting that the regenerated pigments in both cases were identical. Based on the fits, some A2 pigment was always present and may be due to a small amount of A2 retinoid remaining within the photoreceptor membranes that was available for pigment regeneration, or it may be due to photoregeneration of a small fraction of pigment upon by the intense bleaching light. Our data did not allow us to distinguish between these possibilities. In some cells, bleaching and regeneration were repeated several times, and the resulting spectra showed reduced levels of A2 pigment with each cycle (data not shown).
11-cis-Retinol Does Not Activate the Salamander Blue Cone/Green Rod Opsin but Only Forms Pigment in Blue Cones
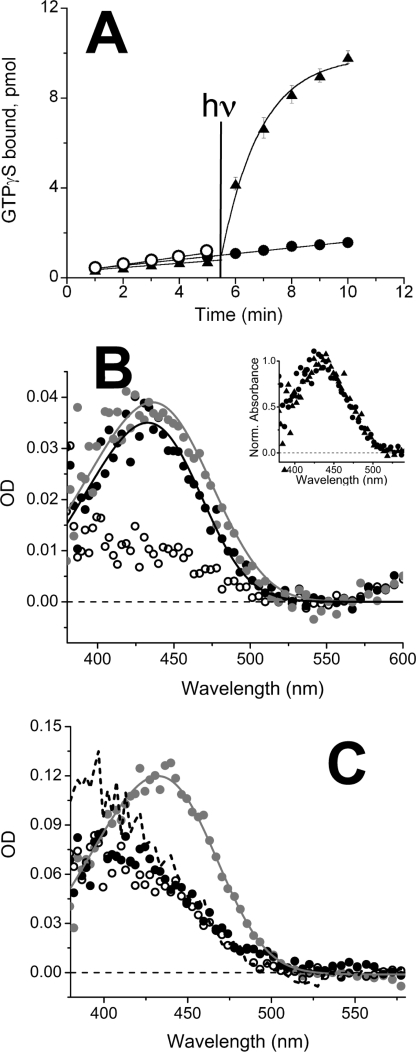
The data illustrated in Fig. 3 show that 11-cis-retinol does not behave as an agonist to the salamander green rod/blue cone opsin. Salamander blue cone and green rod visual pigments contain the same opsin (16). Constitutive activation of transducin by the blue cone/green rod opsin is low (open circles), more consistent with the red rod opsin data (see Fig. 1A) and consistent with previous work (35). The retinoid effect on transducin activation of this opsin is qualitatively consistent with the result obtained with red cone opsin: no increase (if anything a decrease) following treatment with both 11-cis-retinol (filled circles) and 11-cis-retinal (filled triangles). However, the effect is much less dramatic than in red cones as the blue cone opsin itself is less active (35). Because the in vitro cell-free system has no oxidoreductase (see Figs. 1A and 2A), 11-cis-retinal, but not 11-cis-retinol treatment, results in the formation of visual pigment, making possible the light-dependent activation of transducin.
FIGURE 3.
Effect of 11-cis-retinol and -retinal on opsin activation and pigment regeneration in salamander blue cones and green rods. A, transducin activation by expressed salamander blue cone/green rod opsin (open circles) with 11-cis-retinol (filled circles) and 11-cis-retinal (triangles). Each sample was assayed in triplicate and averaged; error bars reflect mean ± S.E. and often were smaller than the symbol size. At 5.5 min, samples with 11-cis-retinal and 11-cis-retinol were illuminated with light for 12 s as described under “Experimental Procedures” and illustrated in the figure with the vertical line. B, averaged spectra of single salamander blue cones: dark adapted (gray circles, n = 8), 1–9 min after >90% bleach (open circles, n = 13), and 60 min following exposure to 11-cis-retinol applied 85 min following the bleach (black filled circles, n = 7). Inset, normalized absorption spectra of salamander blue cones before and after bleaching and regeneration with 11-cis-retinol (circles, n = 7) or 11-cis-retinal (triangles, n = 5). C, spectra of a single dark-adapted salamander green rod before bleaching (gray circles), after bleaching measured at 2 (black dashes) and 32 min (open circles), and after treatment with 11-cis-retinol in vesicles for 90 min (black filled circles).
Because in salamanders blue cones and green rods contain the same opsin (16), we compared the effectiveness of 11-cis-retinal and 11-cis-retinol in promoting pigment regeneration in these photoreceptor cells. We recorded the absorption spectra of individual salamander blue cones and green rods before and after bleaching, and following exposure to exogenous 11-cis-retinoids (Fig. 3, B and C). The filled gray circles in Fig. 3B illustrate the native blue cone visual pigment spectrum. Following bleaching (open circles), most of the native blue cone pigment disappeared, with only a small fraction of the native pigment or bleaching products remaining. As in red-sensitive cones, exposure to 11-cis-retinol (filled circles) resulted in regeneration of mainly A1-based visual pigment. The normalized absorption spectra obtained from cells bleached and regenerated with 11-cis-retinal and 11-cis-retinol were indistinguishable (Fig. 3B, inset) suggesting that the regenerated pigments in both cases were identical.
With salamander green rods, similar experiments were performed (Fig. 3C) except we waited longer to obtain the final bleached spectrum. Representative data from a single cell are shown. The native pigment spectrum is illustrated by the gray filled circles. Among all native cells studied, we obtained the following results (mean ± S.E.): A2% = 35 ± 19 and OD = 0.095 ± 0.017 shown by the pigment template (continuous gray line). It should be noted that the λmax values of pure A1 and A2 pigment spectra of salamander green rod pigment are only about 8 nm apart (λmax,A1 = 432 nm and λmax,A2 = 440 nm (26)), which makes it difficult to obtain accurate estimates of A1 and A2 pigment contributions based on template fits. The initial spectrum taken 2 min after bleaching (dashed line) has a spectrum that appears to change with time, but this change is not due to the absorbance of the original dark pigment. This is likely due to a combination of reduction of all-trans-retinal to retinol, which blue shifts the spectrum and slows clearance of retinal/retinol from the rod cell to the bulk solution (36). Retinoids diffuse from cone cells about 7 times faster than in rod-shaped cells (36), and thus by 2 min the bleached spectrum appears more complete in cones (Fig. 3B). Because of this, the bleached spectrum shown in Fig. 3C was taken at 32 min (open circles). We observed no significant difference between the absorbance measurements performed on bleached cells before and after the addition of 11-cis-retinol (cf. open and filled black circles) indicating that 11-cis-retinol did not regenerate any significant amount of the green rod pigment within 90 min of treatment. We also carried out a second bleach in 4 of the 5 green rods studied after bleaching and exposure to 11-cis-retinol. The difference spectrum after the second bleach in the cell shown in panel C (90 min after the addition of 11-cis retinol time, not shown) had an OD of about 0.03 at 432 nm. This OD may represent a small fraction of visual pigment present in the cell. However, we observed no significant difference between the spectrum recorded 32 min after bleaching in the absence of 11-cis-retinol and the spectrum recorded 130 min after bleaching and 90 min following the addition of 11-cis-retinol indicating that the small amount of pigment may not be due to the addition of 11-cis-retinol. Based on these data as well as other data on all of the green rods we studied, we deduced the maximum regeneration rate of pigment under these conditions to be 0.00015 OD units min−1. Full pigment regeneration at this rate would require about 17 h (0.157/0.00015 min−1) assuming a similar peak OD value as observed in the rods that were bleached and regenerated with 11-cis-retinal. These results demonstrate that the expression of the oxidoreductase responsible for the oxidation of 11-cis-retinol to 11-cis-retinal depends on photoreceptor type and that it is not present in salamander rods in sufficient amounts to result in pigment regeneration on a physiologically relevant time scale.
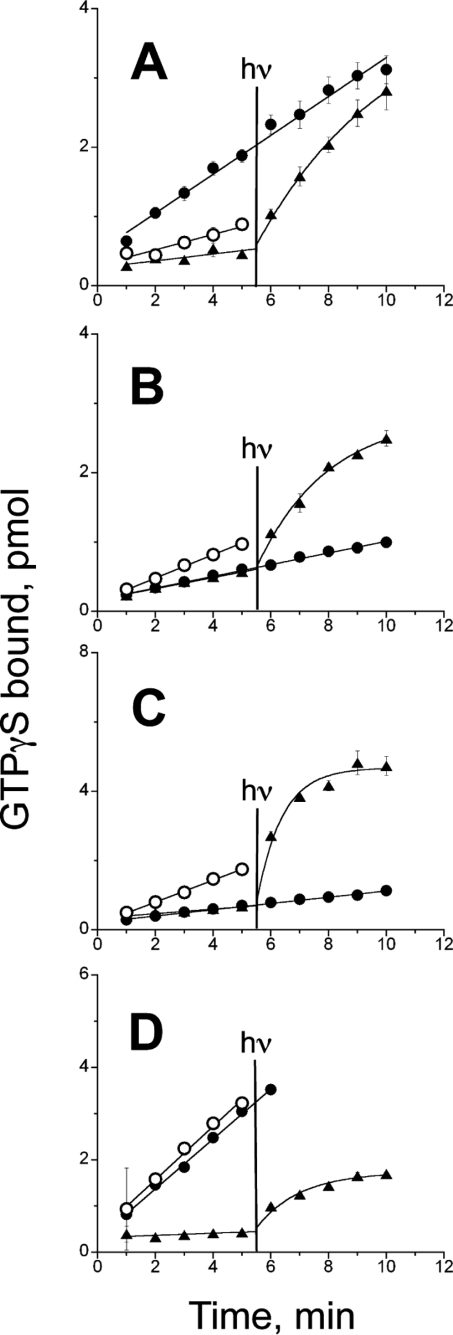
11-cis-Retinol Activates Human Rod Opsin and Deactivates Human Cone Opsins
Figs. 1–3 show the effects of 11-cis-retinol on salamander rod and cone opsins as well as on intact photoreceptor cells. The experiments illustrated in Fig. 4 were designed to test if the same phenotype holds for expressed human visual opsins. Human rod opsin (Fig. 4A) is constitutively active (open circles). Transducin activation by the opsin decreased in the presence of 11-cis-retinal (filled triangles), which also makes pigment that can activate transducin in a light-dependent manner. However, transducin activation by opsin increased in the presence of 11-cis-retinol (filled circles). All of the expressed human cone opsins are constitutively active as addition of 11-cis-retinal results in lower activation of transducin by the opsins (Fig. 4, B–D). The red and green cone opsins are also clearly deactivated by 11-cis-retinol. However, only opsins exposed to 11-cis-retinal form a pigment and can be activated with light. The effect of 11-cis-retinol on the human blue-expressed opsin is somewhat different from that for the red and green opsins in that 11-cis-retinol has little to no effect on the activity of the blue cone opsin, but it does not further activate the human blue cone opsin (Fig. 4D).
FIGURE 4.
Effect of 11-cis-retinoids on expressed human rod and cone opsin activities. Transducin activation by expressed human (A) rod, (B) red cone, (C) green cone, and (D) blue cone opsins (open circles) with 11-cis-retinol (filled circles) and with retinal (triangles). At 5.5 min, the samples with 11-cis-retinoids were illuminated for 12 s, and only opsins with 11-cis-retinal showed a light-dependent increase in transducin activation. Each sample was assayed in triplicate and averaged; error bars reflect mean ± S.E. and often were smaller than the symbol size. At 5.5 min, samples with 11-cis-retinal and 11-cis-retinol were illuminated with light for 12 s as described under “Experimental Procedures” and illustrated in the figure with the vertical line.
DISCUSSION
Recent studies with cone-dominant retinas have indicated that cones likely have a second visual cycle involving the Müller cells as an additional source of 11-cis-retinoids (6). The earliest evidence of the utility of an additional pathway for cone pigment regeneration came from direct measurements of human visual pigment regeneration by retinal densitometry, which showed that bleached cones and rods can compete with one another for the supply of 11-cis-retinoid (1), which under severe conditions of bleaching, appears to be limited (2). According to Rushton (1), a retinoid “famine” occurs during bleaching in which photoreceptors with the highest rate of retinoid uptake will steal substrate from others who bind it less strongly. One way out of this competition dilemma would be for the cones to have privileged access to retinoids and be able to utilize a separate retinoid pathway not available to rods (7, 8), and an extra source of 11-cis-retinoids from the Müller cells could fulfill this role. Müller cells have not been shown to generate 11-cis-retinal but have been shown to be able to convert all-trans-retinol to 11-cis-retinol (6). 11-cis-Retinol is able to restore photosensitivity to bleached salamander red cones suggesting that at least red cones have the ability to oxidize 11-cis-retinol (9).
This study documents two important findings. First, 11-cis-retinol-mediated activation or deactivation of salamander and human rod and cone opsins can occur without forming a light-sensitive complex as judged by changes in their abilities to activate transducin. Its behavior as an agonist or inverse agonist differs between rod and cone opsins. The efficacy as an inverse agonist also varies among the different cone opsin types. Second, microspectrophotometric measurements demonstrate the formation of visual pigments in bleached salamander red and blue cone photoreceptors following treatment with 11-cis-retinol but not in bleached rod photoreceptors, even rods containing cone opsins. The efficient oxidation of 11-cis-retinol and hence pigment formation occurs only in cone cells.
11-cis-Retinol acts as an agonist to salamander red rod and human rod opsins and does not regenerate rhodopsin pigment in bleached salamander red rods. 11-cis-Retinol is known to bind noncovalently with rod opsin (37) and has been shown to activate both phosphodiesterase and guanylyl cyclase in intact bleached salamander red rods (10). On the other hand, 11-cis-retinol does not activate any cone opsin that we tested and clearly deactivates long wavelength-sensitive opsins. In a cell-free environment, cone pigments do not form with 11-cis-retinol, and there was no light-dependent activation. In bleached salamander cone photoreceptors, full recovery of photosensitivity occurred with 11-cis-retinol indicating that the red cone cell likely oxidized the retinol to retinal to regenerate pigment (9). Here we show directly pigment formation in bleached red cones with 11-cis-retinol by microspectrophotometry. We further show pigment regeneration in bleached salamander blue cones indicating that 11-cis-retinol oxidation is not unique to red cones. However, bleached salamander green rods do not regenerate pigment on a physiologically relevant time scale following treatment with 11-cis-retinol. It is notable that the green rod failed to form pigment because it contains the same pigment as the blue cone (16). Therefore, the cones but not the rods contain an as yet unspecified retinal oxidoreductase capable of oxidizing 11-cis-retinol to 11-cis-retinal thereby allowing pigment regeneration to occur.
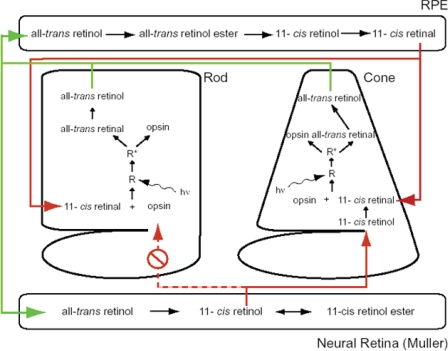
An interpretation of how these different and contrasting findings may relate to the behavior of intact rod and cone photoreceptor cells within the retina is illustrated in Fig. 5. This model takes into account our two principal observations: that 11-cis-retinol is an effective substrate for visual pigment regeneration in cone but not rod photoreceptors and that 11-cis-retinol behaves as an agonist to red rod opsin and is thereby expected to have a deleterious effect on sensitivity recovery in rods by perpetuating the activation of transducin beyond the time of light exposure to produce cell desensitization. 11-cis-Retinol is expected to have the opposite effect in bleached cones where it will not activate the signal cascade and be oxidized within the cell to 11-cis-retinal to promote pigment formation. Consideration of these findings together imposes constraints on the translocation mechanisms whereby cis-retinoids are moved from their sources of production, i.e. in the RPE and Müller cells to rod and cone photoreceptor outer segments. 11-cis-Retinol derives from Müller cells, whose processes generally form baskets around the inner segments and ellipsoid regions of photoreceptors. On the other hand, 11-cis-retinal is provided from the RPE, whose processes are intimate with the outer segments of photoreceptors.
FIGURE 5.
Schematic representation of separate visual cycles involving the retinal pigment epithelium and the neural retina (Müller cells). See text for details.
Selective translocation of 11-cis-retinol from Müller cells into cones over rods is consistent with results of Jin et al. (38) on salamander rods and cones. These experiments showed that 11-cis-retinal can enter rods only through their outer segments (as would occur through their contact with processes from the RPE), whereas cones can take up retinoid either through their outer segments (as would occur through the RPE) or through their inner segments (as would occur from Müller cell processes). Although such a polar transport mechanism facilitating translocation of 11-cis-retinol from Müller cells selectively to cones but not to rods through the inner segments is attractive and consistent with our present findings, direct experimental support is presently lacking.
It is important to note that 11-cis-retinal has been observed as well to interact with opsin and to impede the recovery of sensitivity in intact salamander rods (10). However, this effect is transient; as the visual pigment is formed and the concentration of 11-cis-retinal decreases, the desensitizing effect of 11-cis-retinal disappears. Such is not the case with 11-cis-retinol. Its persistent presence within rod outer segments would compromise dark adaptation and not support pigment regeneration.
Recent studies have indicated an additional important role for 11-cis-retinal and hence the necessity for having a constant supply. It is critical for the maintenance of cone cell viability (39). A number of membrane proteins, including opsin, mislocalize if 11-cis-retinal is not present in cones (40). This phenomenon is not present in the rods as opsins and other membrane proteins traffic normally in the absence of 11-cis-retinal. This is a further demonstration that cones require a constant supply of 11-cis-retinol/11-cis-retinal to maintain photoreceptor viability.
Our results support the proposals (7, 8) that two retinoid cycles are rational and indeed required for the support of a constant retinoid supply for both types of photoreceptors. The findings that 11-cis-retinol acts as an agonist to the rhodopsin apoprotein, generating a nonphotic response and cannot be oxidized to 11-cis-retinal in the intact rods show that this form of the retinoid is not useful and is even detrimental to the dark adaptation of rod photoreceptors. The finding that 11-cis-retinol is not an agonist but rather an inverse agonist to all of the human cone opsins and to two of the salamander cone opsins provides evidence that this retinoid is likely to have two related physiological roles in cones. First, 11-cis-retinol, following its oxidation to 11-cis-retinal by a yet unidentified retinal oxidoreductase provides substrate for pigment regeneration. Expression of at least one retinol dehydrogenase abundant in bovine cones (33) and 11-cis-retinol oxidase activity in carp cones (41, 42) have been reported. Second, the noncovalent association of 11-cis-retinol promotes the deactivation of transducin, thereby assisting in recovery of sensitivity following bright bleaching light. Finally, our data suggest that the cone pathway that provides 11-cis-retinol to cones must be isolated from the rods, whereas the rod pathway involving the RPE provides chromophore for both receptors.
Acknowledgments
We thank Alecia Gross of the University of Alabama, Birmingham, AL, for the human rhodopsin gene and Patrice Goletz for technical help.
This work was supported, in whole or in part, by National Institutes of Health Grants EY01157 (to M. C. C.), EY04939 (to R. K. C.), and EY14793 (vision core at the Medical University of South Carolina). This work was also supported by Academy of Finland Grant 123231 (to P. A.-L.), a grant from the Foundation Fighting Blindness, Inc., Owings Mills, MD (to R. K. C.), and an unrestricted grant to the Department of Ophthalmology at the Medical University of South Carolina from Research to Prevent Blindness, New York.
- RPE
- retinal pigment epithelium
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- Mes
- 2-(N-morpholino)ethanesulfonic acid.
REFERENCES
- 1.Rushton W. A. H. ( 1968) J. Physiol. 198, 219– 236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rushton W. A. H., Henry G. H. ( 1968) Vision Res. 8, 617– 631 [DOI] [PubMed] [Google Scholar]
- 3.Burns M. E., Arshavsky V. Y. ( 2005) Neuron 48, 387– 401 [DOI] [PubMed] [Google Scholar]
- 4.McBee J. K., Palczewski K., Baehr W., Pepperberg D. R. ( 2001) Prog. Retinal Eye Res. 20, 469– 529 [DOI] [PubMed] [Google Scholar]
- 5.Lamb T. D., Pugh E. N., Jr. ( 2004) Prog. Retin. Eye Res. 23, 307– 380 [DOI] [PubMed] [Google Scholar]
- 6.Das S. R., Bhardwaj N., Kjeldbye H., Gouras P. ( 1992) Biochem. J. 285, 907– 913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mata N. L., Radu R. A., Clemmons R. C., Travis G. H. ( 2002) Neuron 36, 69– 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniz A., Villazana-Espinoza E. T., Hatch A. L., Trevino S. G., Allen D. M., Tsin A. T. C. ( 2007) Exp. Eye Res. 85, 175– 184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones G. J., Crouch R. K., Wiggert B., Cornwall M. C., Chader G. J. ( 1989) Proc. Natl. Acad. Sci. U. S. A. 86, 9606– 9610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kefalov V. J., Crouch R. K., Cornwall M. C. ( 2001) Neuron 29, 749– 755 [DOI] [PubMed] [Google Scholar]
- 11.Kono M., Goletz P. W., Crouch R. K. ( 2008) Biochemistry 47, 7567– 7571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Applebury M. L., Antoch M. P., Baxter L. C., Chun L. L. Y., Falk J. D., Farhangfar F., Kage K., Krzystolik M. G., Lyass L. A., Robbins J. T. ( 2000) Neuron 27, 513– 523 [DOI] [PubMed] [Google Scholar]
- 13.Makino C. L., Dodd R. L. ( 1996) J. Gen. Physiol. 108, 27– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebrey T., Koutalos Y. ( 2001) Prog. Retin. Eye Res. 20, 49– 94 [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama S. ( 2000) Prog. Retin. Eye Res. 19, 385– 419 [DOI] [PubMed] [Google Scholar]
- 16.Ma J., Znoiko S., Othersen K. L., Ryan J. C., Das J., Isayama T., Kono M., Oprian D. D., Corson D. W., Cornwall M. C., Cameron D. A., Harosi F. I., Makino C. L., Crouch R. K. ( 2001) Neuron 32, 451– 461 [DOI] [PubMed] [Google Scholar]
- 17.Crouch R. K., Kefalov V., Gärtner W., Cornwall M. C. ( 2002) Methods Enzymol. 343, 29– 48 [DOI] [PubMed] [Google Scholar]
- 18.Moiseyev G., Crouch R. K., Goletz P., Oatis J., Jr., Redmond T. M., Ma J. X. ( 2003) Biochemistry 42, 2229– 2238 [DOI] [PubMed] [Google Scholar]
- 19.Estevez M. E., Ala-Laurila P., Crouch R. K., Cornwall M. C. ( 2006) J. Gen. Physiol. 128, 671– 685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones G. J., Fein A., MacNichol E. F., Jr., Cornwall M. C. ( 1993) J. Gen. Physiol. 102, 483– 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornwall M. C., Jones G. J., Kefalov V. J., Fain G. L., Matthews H. R. ( 2000) Methods Enzymol. 316, 224– 252 [DOI] [PubMed] [Google Scholar]
- 22.MacNichol E. F., Jr. ( 1978) in Frontiers of Visual Science ( Cool S. J., Smith E. L. eds) pp. 194– 208, Springer, Berlin [Google Scholar]
- 23.Cornwall M. C., MacNichol E. F., Jr., Fein A. ( 1984) Vision Res. 24, 1651– 1659 [DOI] [PubMed] [Google Scholar]
- 24.Govardovskii V. I., Fyhrquist N., Reuter T., Kuzmin D. G., Donner K. ( 2000) Vis. Neurosci. 17, 509– 528 [DOI] [PubMed] [Google Scholar]
- 25.Hárosi F. I. ( 1975) J. Gen. Physiol. 66, 357– 382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makino C. L., Groesbeek M., Lugtenburg J., Baylor D. A. ( 1999) Biophys. J. 77, 1024– 1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ala-Laurila P., Donner K., Crouch R. K., Cornwall M. C. ( 2007) J. Physiol. 585, 57– 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oprian D. D. ( 1993) Methods Neurosci. 15, 301– 306 [Google Scholar]
- 29.Das J., Crouch R. K., Ma J. X., Oprian D. D., Kono M. ( 2004) Biochemistry 43, 5532– 5538 [DOI] [PubMed] [Google Scholar]
- 30.Robinson P. R. ( 2000) Methods Enzymol. 315, 207– 218 [DOI] [PubMed] [Google Scholar]
- 31.Kono M. ( 2006) FEBS Lett. 580, 229– 232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H., Kono M., McKee T. D., Oprian D. D. ( 1995) Biochemistry 34, 14963– 14969 [DOI] [PubMed] [Google Scholar]
- 33.Cohen G. B., Yang T., Robinson P. R., Oprian D. D. ( 1993) Biochemistry 32, 6111– 6115 [DOI] [PubMed] [Google Scholar]
- 34.Melia T. J., Jr., Cowan C. W., Angleson J. K., Wensel T. G. ( 1997) Biophys. J. 73, 3182– 3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isayama T., Chen Y., Kono M., Degrip W. J., Ma J. X., Crouch R. K., Makino C. L. ( 2006) Vis. Neurosci. 23, 899– 908 [DOI] [PubMed] [Google Scholar]
- 36.Ala-Laurila P., Kolesnikov A. V., Crouch R. K., Tsina E., Shukolyukov S. A., Govardovskii V. I., Koutalos Y., Wiggert B., Estevez M. E., Cornwall M. C. ( 2006) J. Gen. Physiol. 128, 153– 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daemen F. J. M. ( 1978) Nature 276, 847– 848 [DOI] [PubMed] [Google Scholar]
- 38.Jin J., Jones G. J., Cornwall M. C. ( 1994) Vis. Neurosci. 11, 389– 399 [DOI] [PubMed] [Google Scholar]
- 39.Fan J., Rohrer B., Frederick J. M., Baehr W., Crouch R. K. ( 2008) Invest. Ophthalmol. Vis. Sci. 49, 2384– 2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Fan J., Li S., Karan S., Rohrer B., Palczewski K., Frederick J. M., Crouch R. K., Baehr W. ( 2008) J. Neurosci. 28, 4008– 4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haeseleer F., Huang J., Lebioda L., Saari J. C., Palczewski K. ( 1998) J. Biol. Chem. 273, 21790– 21799 [DOI] [PubMed] [Google Scholar]
- 42.Miyazono S., Shimauchi-Matsukawa Y., Tachibanaki S., Kawamura S. ( 2008) Proc. Natl. Acad. Sci. U. S. A. 105, 16051– 16056 [DOI] [PMC free article] [PubMed] [Google Scholar]