Abstract
Objective
Measurements of extravascular lung water (EVLW) correlate to the degree of pulmonary edema and have substantial prognostic information in critically ill patients. Prior studies using single indicator thermodilution have reported 21%–35% of patients with clinical acute respiratory distress syndrome (ARDS) have normal EVLW (<10mL/kg). Given that lung size is independent of actual body weight, we sought to determine whether indexing EVLW to predicted or adjusted body weight affects the frequency of increased EVLW in patients with ARDS.
Design
Prospective, observational cohort study.
Setting
Medical and surgical intensive care units at two academic hospitals.
Patients
30 patients within 72 hours of meeting AECC definition of ARDS and 14 severe sepsis patients without ARDS.
Interventions
None
Measurement and Main Results
EVLW was measured for 7 days by PiCCO™ transpulmonary thermodilution. 225 measurements of EVLW indexed to actual body weight (ActBW) were compared to EVLW indexed to predicted body weight (PBW) and adjusted body weight (AdjBW). Mean EVLW indexed to ActBW was 12.7 mg/kg for ARDS patients and 7.8 mg/kg for non-ARDS sepsis patients (p<0.0001). EVLW increased by 2.0±4.1mL/kg when indexed to PBW and 1.1±2.1mL/kg when indexed to AdjBW. Indexing EVLW to PBW or AdjBW increased the proportion of ARDS patients with elevated EVLW (each p<0.05) without increasing the frequency of elevated EVLW in non-ARDS patients. EVLW indexed to PBW had a stronger correlation to Lung Injury Score (r2=0.39 vs. r2=0.17) and PaO2/FiO2 ratio (r2=0.25 vs. r2=0.10) than did EVLW indexed to ActBW.
Conclusions
Indexing EVLW to PBW or AdjBW reduces the number of ARDS patients with normal EVLW and correlates better to LIS and oxygenation than using ActBW. Future studies are needed to confirm the presumed superiority of this method for diagnosing ARDS and to determine the clinical treatment implications.
Keywords: extravascular lung water, acute respiratory distress syndrome, sepsis, predicted body weight
Introduction
Acute respiratory distress syndrome (ARDS) is a life threatening form of injury to the lung caused by a variety of underlying diseases and affecting between 100,000 and 200,000 Americans each year. (1) Despite being relatively common and carrying a mortality of 30% to 60%, ARDS remains a heterogeneous clinical syndrome which is not easily defined or diagnosed. (1–3) A formal description of the syndrome was established in 1994 by the American European Consensus Conference (AECC). (4) The AECC defined ARDS as: [1] bilateral infiltrates on chest radiograph consistent with pulmonary edema, [2] ratio of partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) less than 200, and [3] absence of left atrial hypertension. This definition remains the most widely accepted description of the disease today. (5)
The hallmark of ARDS is protein rich pulmonary edema resulting from disruption of the alveolar capillary barrier and a decrease in net alveolar fluid clearance. (5–8) This pathologic accumulation of pulmonary edema can be quantified as extravascular lung water (EVLW). Although the physiologic derangements in ARDS necessarily lead to elevated EVLW, it is not part of the diagnostic criteria for ARDS. Previous studies using single indicator thermodilution have noted that 21% to 35% of clinical ARDS patients have normal EVLW (defined as < 10mL/kg). (9–12). The reason for this inconsistency has not been determined.
EVLW is calculated as milliliters per kilogram and historically is indexed to the actual body weight of the patient at the time of measurement. No scientific justification or clinical validation for indexing to actual body weight exists. Height and gender are known to be the most important factors in determining lung volumes (13,14) and excess body weight does not appreciably change functional lung volumes. As over half of ARDS patients are overweight (15), EVLW may be better represented by height-based calculations. If true, then indexing EVLW to actual body weight may not accurately depict the presence of pulmonary edema inherent to ARDS. We hypothesize that using patient height to calculate EVLW will more accurately characterize EVLW than current methodology, which may be important for both prognosis and institution of appropriate therapy.
Material and Methods
This study was reviewed and approved by the Institutional Review Board at Emory University School of Medicine and the Research Oversight Committee of Grady Memorial Hospital. Study participants were medical and surgical ICU patients at two large academic hospitals in Atlanta, Georgia (Grady Memorial Hospital and Emory Crawford Long Hospital). All ICU patients were screened for eligibility and participation was solicited within 72 hours for patients meeting the American-European Consensus definition of ARDS as described above. Exclusion criteria included contraindication to femoral artery catheterization, cause of ARDS >10 days prior to eligibility, Pneumocystis Carinii pneumonia, pneumonectomy, age <18 years, pregnancy, or inability to obtain consent from patient or surrogate. After informed consent, patient-specific data were obtained, such as past medical, social and surgical history, risk factors for ARDS, Acute Physiology and Chronic Health Evaluation (APACHE) II score, lung injury score (LIS) (16), and standard laboratory values. A cohort of severe sepsis patients (ACCP/SCCM consensus definition) (17), at risk for, but not meeting the AECC definition for ARDS, was simultaneously enrolled and used for comparison.
Once enrolled, a 5-F arterial catheter (Pulsiocath PV2015L20; Pulsion Medical Systems, Munich, Germany) was inserted into the descending aorta via the femoral artery using the Seldinger technique. A temperature sensor was connected to the distal port of a standard central venous catheter in either the internal jugular or subclavian location. The arterial and central venous catheters were connected to an integrated bedside monitor (PiCCO™, Pulsion Medical Systems) for continuous hemodynamic monitoring. All patient management decisions, including fluid and ventilator management, were made by the primary intensive care physicians caring for the patient.
Measurements of Extravascular Lung Water
The PiCCO™ catheter system operates via a single thermal indicator technique to determine EVLW, cardiac output (CO), and volumetric parameters. CO and EVLW measurements were obtained by triplicate central venous injections of 15–20cc of iced (<8°C) 0.9% saline and recorded as the mean of the three measurements. Iced saline was injected into the central venous catheter while the thermistor tip on the femoral artery catheter measured the downstream temperature change within the abdominal aorta. Cardiac output was then calculated by analysis of the temperature change of the thermodilution curve using the Stewart-Hamilton method. This has been shown to be comparable to pulmonary artery thermodilution. (18,19) Volumetric parameters were derived using cardiac output, mean transit time of the thermal indicator, and decay time of the thermodilution curve as previously described. (9,20,21) EVLW represents all fluid that diffuses and remains outside of the pulmonary vasculature during transit of the thermal indicator (i.e. summation of interstitial, intracellular, and alveolar fluid); its derivation has been previously described in detail. (20–22)All volumetric and hemodynamic parameters were indexed to body surface area, except for EVLW and CVP. Measurements were obtained immediately after catheter insertion and at least once every 24 hours for a period of 7 days. The catheter system was discontinued prior to 7 days in the event of patient transfer out of the ICU or death.
Outcome Variables
Patients were classified as having elevated EVLW if any measurement of EVLW was >10mL/kg during the study period. This value is based on previous studies of EVLW in animal models without evidence of lung injury (23–25) and has been established as a threshold for acute lung injury in previous clinical studies (9,26,27). All measurements of EVLW were initially recorded as absolute, unindexed values which were then divided by actual body weight, predicted body weight, or adjusted body weight to obtain specific indexed values. Actual body weight was obtained at time of enrollment into study by duplicate measurement using patient bedscale. The majority of patients were weighed on a Hill-ROM Century CC® hospital bed which has a maximal weight of 136kg and accuracy of ±1%. Patient height was recorded by duplicate measurements using a flexible tape measure and corroborated by self-reported patient height from admission paperwork when available.
Patient weight (kg) was calculated using the following formulas:
The equation for predicted body weight is based on the Devine Formula (30) and it should be noted that this formula is also frequently referred to as ideal body weight. We have selected the nomenclature predicted body weight to be consistent with recent ARDS clinical trials. (28,31)
Patients were followed until time of hospital discharge to determine duration of mechanical ventilation, hospital length of stay (LOS), and in-hospital mortality. Severity of ARDS was quantified by the Lung Injury Score (LIS) (16), PaO2/FiO2 ratio, PEEP, and static lung compliance. As ARDS is a dynamic process, a daily assessment of patients was performed to confirm whether they met the AECC criteria for ARDS on the same day that an EVLW measurement was recorded. This variable is referred to as “Daily ARDS” in the data analysis. All radiographs were interpreted by experienced critical care physicians. Fluid management and daily weights (if available) were recorded.
Statistical Analysis
Continuous data are presented as mean ± standard deviation and were compared using two-sample t-tests. The χ2 statistic was used to compare frequency proportions. Modeling by least squares linear regression for continuous outcome variables was used. Goodness of fit of linear regression model was measured by r-squared (r2). Comparison of sample correlations (r2) was performed by method derived by Cohen and Cohen (32). Multivariate linear regression was used for adjustment of differences in baseline characteristics, where noted in the text. Statistical analysis was performed using NCSS 2001 software (NCSS, Inc., Kaysville, UT, USA) and Microsoft® Excel 2003 (Microsoft Corporation, Redmond, Washington, USA). All statistical tests were two-sided; p values < 0.05 were considered significant and p values > 0.20 are reported as not significant (NS).
Results
Study Population
Forty-four patients were enrolled from July 2001 to December 2006. Thirty patients (68% of study population) with clinically diagnosed ARDS were prospectively enrolled within 72 hours of meeting AECC definition. The remainder of enrolled patients (n=14) had severe sepsis but did not meet criteria for ARDS. One patient was originally enrolled with severe sepsis only but developed ARDS on study day #2. This patient was included in the ARDS cohort, using data starting at day #2 and continuing for a total of eight days. Twenty-five (56%) patients completed all 7 days of the study. The study was terminated early in 19 patients (44%) due to patient death (n=8), transfer out of ICU (n=8), central line discontinuation (n=1), or study catheter malfunction (n=2). Patients not completing 7 days of the study had all data up to the time of discontinuation included in analysis. A total of 237 study days were available for analysis. Twelve measurements of EVLW were missing and were not imputed, therefore 225 independent determinations of EVLW were made during the study period (mean 5.1 measurements per patient).
The average age of study participants was 51 years, with a majority of patients (84%) being African-American. Sepsis was the primary cause of ARDS in 22 (73%) patients. Other risk factors for development of ARDS included pneumonia (n=5), trauma (n=1), pancreatitis (n=1), and aspiration (n=1). Fourteen patients (47%) had extrapulmonary causes for ARDS. The sources of non-ARDS sepsis included pneumonia (n=5), primary bloodstream infection (n=4), urinary system (n=2), and intra-abdominal infection (n=3). The mean APACHE II score was 25.8 and overall mortality was 45.5%. Chronic alcohol abuse was present in 19 out of 44 patients (43%) and HIV infection was present in 13 (30%) patients. At the time of enrollment, ARDS patients were taller (mean = 174.5cm) and weighed more (mean actual body weight 83.5 kg) than non-ARDS severe sepsis patients (166cm, 70.5kg respectively). This baseline imbalance produced a disparity in calculated body weight between the ARDS and non-ARDS patients. Baseline characteristics including demographic and physiologic parameters are included in Table 1.
Table 1.
Baseline characteristics and physiology of enrolled patients. Data presented are mean ± standard deviation or proportions (%)
| All | ARDS | Non-ARDS Sepsis | p-value | |
|---|---|---|---|---|
| Total Number Patients | 44 | 30 | 14 | |
| Gender (% male) | 52% | 57% | 50% | NS |
| Age (years, mean) | 50.9 ± 14.7 | 47.8 ± 14.2 | 57.6 ± 13.8 | 0.04 |
| Race (% African American) | 84% | 83% | 86% | NS |
| Chronic Heart Disease | 13% | 7% | 33% | 0.07 |
| Pulmonary Disease | 20% | 21% | 17% | NS |
| Renal Disease | 7% | 0% | 27% | 0.02 |
| Hepatic Disease | 10% | 13% | 0% | NS |
| HIV infection | 30% | 30% | 29% | NS |
| Chronic alcoholism | 43% | 37% | 57% | NS |
| Height (cm) | 171.9 ± 11.1 | 174.5 ± 10.6 | 166.2 ± 10.2 | 0.02 |
| Enrollment BSA (Dubois Formula) | 1.91 ± 0.23 | 1.98 ± 0.21 | 1.78 ± 0.19 | 0.005 |
| Actual Body Weight (ActBW), kg | 79.4 ± 20.0 | 83.5 ± 20.8 | 70.6 ± 15.5 | 0.05 |
| Predicted Body Weight (PBW), kg | 65.7 ± 11.9 | 68.2 ± 11.5 | 60.3 ± 11.2 | 0.04 |
| Adjusted Body Weight (AdjBW), kg | 69.7 ± 10.9 | 72.8 ± 10.2 | 63.1 ± 9.6 | 0.005 |
| APACHE II (Day 1) | 25.8 ± 7.0 | 24.9 ± 6.4 | 27.7 ± 8.3 | NS |
| LIS (Day 1) | 2.10 ± 1.14 | 2.66 ± 0.83 | 0.85 ± 0.63 | <0.001 |
| PaO2/FiO2 ratio (Day 1) | 178.0 ± 132.3 | 117.9 ± 63.4 | 352.1 ± 126.8 | <0.0001 |
Hemodynamic measurements were similar between ARDS and non-ARDS patients with no significant differences in mean study values of cardiac index (CI), systemic vascular resistance index (SVRI), global end-diastolic volume index (GEDVI), and intrathoracic blood volume index (ITBVI). Fluid balance (net input minus output) was consistently positive and not significantly different in both ARDS and non-ARDS patients with a daily mean fluid balance of 2.1 ± 2.8 L. ICU length of stay (LOS) was longer in the ARDS group (20 days vs. 11 days, p=0.02) as was the duration of mechanical ventilation (17 days vs. 9 days, p=0.05). Hospital LOS (mean 30 days) and overall mortality (45.5%) were not significantly different between ARDS and non-ARDS patients.
Indexing EVLW measurements to body weight
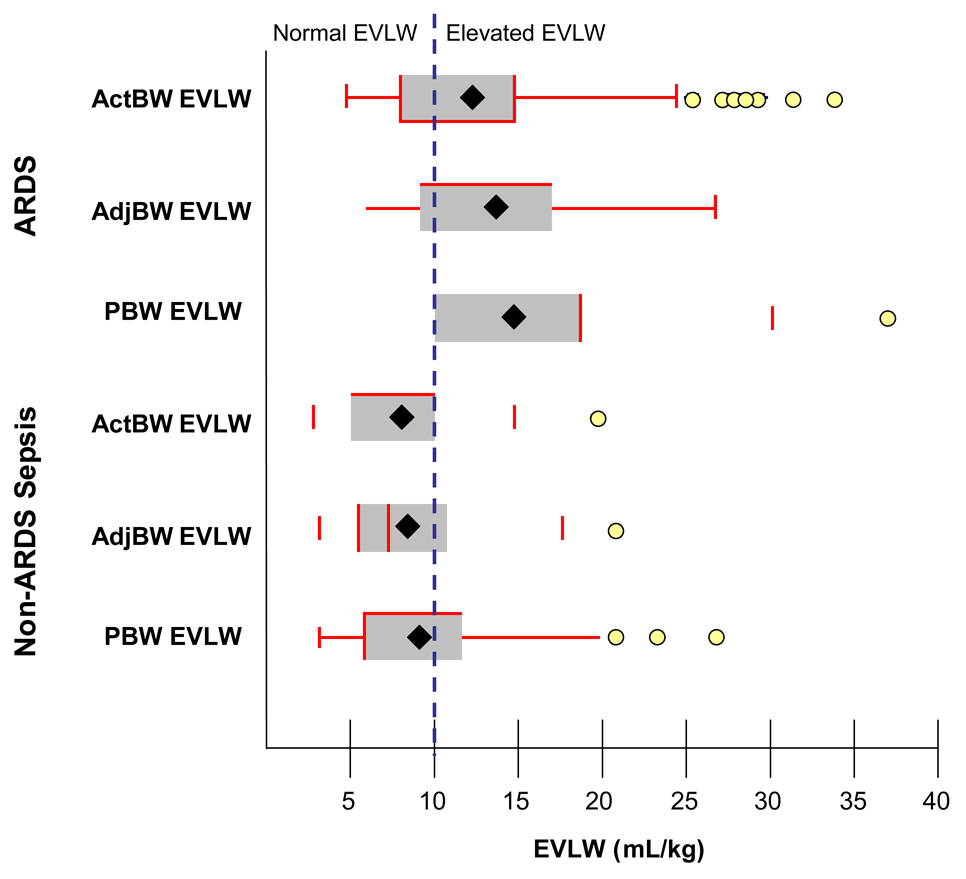
There were 164 individual measurements of EVLW in ARDS patients (123 “Daily ARDS” measurements) and 61 measurements in non-ARDS sepsis patients. Mean EVLW indexed to actual body weight was 12.7 for ARDS patients and 7.8 for non-ARDS sepsis patients (p<0.0001). Mean EVLW increased for both ARDS and non-ARDS sepsis patients when EVLW was indexed to PBW or AdjBW as demonstrated in Figure 1. When using actual body weight to determine EVLW, 7 of 30 (23%) ARDS patients had normal EVLW (<10 mL/kg) throughout the study and 6 of 14 (43%) non-ARDS sepsis patients had normal EVLW throughout the study. In ARDS patients, 45 of 123 (37%) “Daily ARDS” measurements were normal and in non-ARDS sepsis patients, 46 of 61 (75%) of measurements were normal when EVLW was indexed to ActBW. In all patients, EVLW increased by an average of 1.1 ± 2.1 mL/kg when indexed to AdjBW and 2.0 ± 4.1 mL/kg when indexed to PBW. The absolute change in EVLW was similar between patients with or without ARDS. By indexing EVLW to PBW or AdjBW, all but one patient with ARDS (97% of all ARDS patients) had at least one elevated measurement of EVLW, whereas more non-ARDS sepsis patients had normal EVLW measurements (50% of non-ARDS sepsis patients). The proportion of ARDS patients with elevated EVLW was significantly greater when indexed to PBW or AdjBW compared to ActBW (each p<0.05). See Table 3 for details.
Figure 1.
Extravascular lung water (EVLW) in patients with ARDS and non-ARDS sepsis. EVLW is conventionally indexed to actual body weight (ActBW). Mean (represented as ♦ in figure) and median EVLW increased most when indexing EVLW to predicted body weight (PBW) followed by adjusted body weight (AdjBW). Normal EVLW is ≤ 10mL/kg.
Table 3. Effect of indexing extravascular lung water (EVLW) to various body weights.
EVLW calculated using predicted body weight (PBW) and adjusted body weight (AdjBW) compared to actual body weight (ActBW) results in significantly more ARDS patients with elevated EVLW (>10mL/kg) and fewer non-ARDS sepsis patients with elevated EVLW. Daily measurements represent individual EVLW measurements stratified by whether ARDS was confirmed present by AECC criteria on that day (“Daily ARDS”) or whether ARDS was absent on that day. For Daily ARDS measurements, indexing EVLW to PBW or AdjBW results in a significantly greater proportion of measurements with elevated EVLW; daily measurements in patients without ARDS led to non-significant changes in EVLW measurements.
| ARDS Patients | EVLW indexed to ActBW |
EVLW indexed to AdjBW |
EVLW indexed to PBW |
|
|---|---|---|---|---|
| Patients with elevated EVLW | 23/30 (77%) | 29/30 (97%)* | 29/30 (97%)* | |
| Mean Δ EVLW§(mL/kg) ± std dev | Reference | 1.1 ± 2.3 | 2.0 ± 4.4 | |
| Mean % change§ EVLW | Reference | 14% | 26% | |
| Daily ARDS# measurements with elevated EVLW (>10mL/kg) | 78/123 (63%) | 97/123 (79%)** | 106/123 (86%)** | |
| Non-ARDS Sepsis Patients | EVLW indexed to ActBW |
EVLW indexed to AdjBW |
EVLW indexed to PBW |
|
| Patients with elevated EVLW | 8/14 (57%) | 7/14 (50%) | 7/14 (50%) | |
| Mean Δ EVLW§(mL/kg) ± std dev | Reference | 1.0 ± 1.5 | 1.8 ± 3.2 | |
| Mean % change§ EVLW | Reference | 13% | 23% | |
| Daily measurements of elevated EVLW (>10mL/kg) | 15/61 (25%) | 16/61 (26%) | 20/61 (33%) | |
change compared to EVLW indexed to ActBW
Daily ARDS means that ARDS was confirmed each day with simultaneous measurement of EVLW
p <0.01 for comparison to EVLW indexed to ActBW
p <0.01 for comparison to EVLW indexed to ActBW
Multivariate linear regression was performed to adjust for baseline differences in age, actual body weight and height between ARDS and non-ARDS sepsis patients. After adjusting for age, actual body weight, and height, 97% of ARDS patients had elevated EVLW when indexed AdjBW and 93% when indexed to PBW. This was not significantly different than the unadjusted measurements (97%, p=NS) reported above. The percentage of non-ARDS sepsis patients with normal EVLW when indexed to PBW or AdjBW was not significantly affected by adjusting for baseline differences in age, actual body weight, and height.
Correlation of extravascular lung water to lung injury and oxygenation
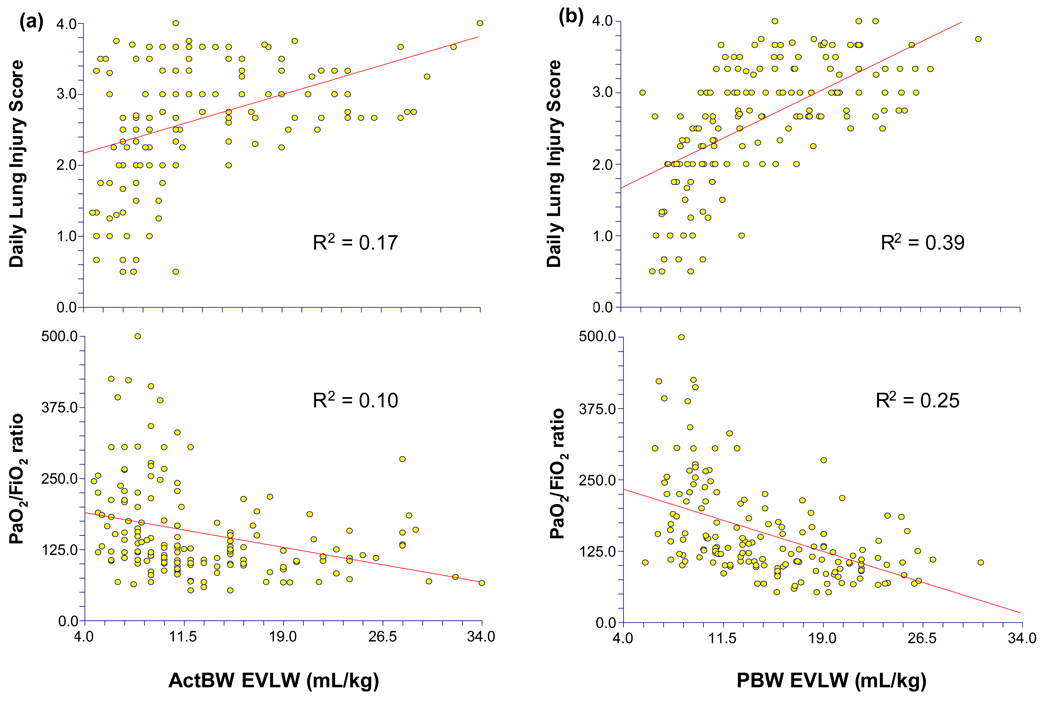
In ARDS patients, there was a significant correlation between EVLW measurements indexed to all body weights and the severity of lung injury (LIS) (ActBW r2=0.17, p<0.0001; AdjBW r2=0.30, p<0.0001; PBW r2=0.39, p<0.0001. This correlation between LIS and EVLW was statistically greater when EVLW was indexed to AdjBW or PBW than ActBW (p<0.0001). There was an inverse relationship between EVLW and oxygenation. The correlation between PaO2/FiO2 ratio and EVLW indexed to all body weights was modest (ActBW r2=0.10, p<0.0001; AdjBW r2=0.19, p<0.0001; PBW r2=0.25, p<0.0001). This correlation was statistically greater when EVLW was indexed to AdjBW or PBW (p<0.0001). Representative scatter plots are included in Figure 2.
Figure 2.
Scatter plots demonstrating the relationship between oxygenation (PaO2/FiO2) or lung injury score (LIS) and extravascular lung water (EVLW) indexed to (a) actual body weight (ActBW) and (b) predicted body weight (PBW) in patient with ARDS (R2 by linear regression, all p<0.0001). There was a statistically greater correlation between EVLW indexed to PBW than EVLW indexed to ActBW for both oxygenation and LIS (p<0.0001).
Other correlates with extravascular lung water
EVLW (indexed to any body weight) in ARDS patients was not significantly correlated with admission albumin (r2=0.03, p=0.48), days of mechanical ventilation (r2=0.007, p=0.28), daily fluid balance (r2=0.002, p=0.56), cumulative fluid balance (r2=0.005, p=0.69), or GEDVI (r2=0.003, p=0.51).
Discussion
Extravascular lung water (EVLW) is a useful method to quantify the accumulation of parenchymal lung edema. Its measurement is more sensitive than chest radiograph (33,34) and more accurate than pulmonary artery occlusion pressure at estimating non-hydrostatic edema in patients with ARDS (35). By current convention, measurements of EVLW are indexed to actual body weight (ActBW), despite a lack of scientific basis or clinical validation. In this study, we have found that indexing EVLW to predicted body weight (PBW) or adjusted body weight (AdjBW) increases the proportion of ARDS patients with elevated EVLW while reducing the proportion of non-ARDS sepsis patients with elevated EVLW, as would be expected clinically. Therefore, the conventional method of indexing to ActBW may mis-classify both ARDS and non-ARDS patients, and recalculating EVLW using PBW may improve the diagnosis of ARDS in critically ill patients.
We additionally examined the relationship between oxygenation [PaO2 /FiO2 ratio] or severity of lung damage (LIS) and the various methods of indexing EVLW in patients with ARDS. We found a much stronger relationship between EVLW indexed to PBW and either LIS or oxygenation compared to EVLW indexed to other weights. Both LIS and oxygenation are routinely measured in ARDS patients to determine severity and progression of disease. The improved correlation to LIS and oxygenation when EVLW is indexed to PBW further demonstrates the superiority of this method over indexing EVLW to actual body weight.
Only a minority of ARDS patients are expected to have normal EVLW due to alveolar-capillary barrier dysfunction and impaired fluid clearance inherent in the disease process. (5) We found that 23% of patients with ARDS had normal EVLW when indexed to ActBW, which is consistent with previous studies (9–12), reporting 21%–35% of ARDS patients with normal EVLW. These findings reflect the imperfection in the current calculation of EVLW using actual body weight, and the current data suggest that the method for measuring EVLW can be improved by simply adopting a more appropriate method for indexing EVLW. As height and gender are the main components in determining lung volumes (13,14), excess body weight should not influence extravascular capacity of the lungs. Therefore, calculations of PBW and AdjBW, which use height and gender as the main determinants of body weight, may favorably influence indexing EVLW for categorization of ARDS and non-ARDS sepsis patients and better correlate with oxygenation and lung injury.
The findings of our study have both prognostic and therapeutic implications. EVLW has been found to be an independent predictor of survival in the ICU with mortality greater than 60% when EVLW exceeds 10mg/kg. (9,22,27) The current method for calculating EVLW may underestimate the degree of lung water in ARDS patients. Changing the method for indexing EVLW will improve our prognostic capabilities by providing more accurate estimations of lung edema in ARDS patients. EVLW measurements have been used therapeutically to assist in fluid management with subsequent decreases in duration of mechanical ventilation and ICU length of stay. (36,37) There are case reports of relevant changes in clinical management based on re-indexing of EVLW. (38) Imprecise measurements of EVLW using actual body weight may equally have adverse therapeutic ramifications.
There are important limitations to this study. There is no consensus for an abnormal level of EVLW. We have chosen the common 10 mL/kg as a cut-off for normal EVLW (9,23–27), although others have also used 7 mL/kg. (10,11,36,37) We used the higher threshold to be more strict in classifying cases of elevated EVLW, as in previous studies (9,23–27). If the lower threshold was used, more cases of elevated EVLW would potentially be identified in the non-ARDS sepsis group. This, however, would cause a misclassification of severe sepsis patients to elevated lung water that may not be clinically justified. Similarly, although the AECC definition for ARDS is an accepted international standard, it captures a heterogeneous group of patients. To mitigate these effects, we compared three different measurements of body weight to equally consider the accuracy of each, and we included patients with both ARDS and non-ARDS (controls) to examine the clinical relevance of any observed changes. In addition, this relatively small study found important and clinically relevant differences in EVLW depending on body weight index, thus diminishing concerns about sample size.
Another limitation is the baseline anthropometric imbalance between the ARDS and non-ARDS sepsis patients in this study. ARDS patients were, on average, 8 cm taller and weighed 13kg more than non-ARDS sepsis patients. The difference is attributed the small sample size as there is no physiologic explanation for this in ARDS. As calculations for PBW and AdjBW are all dependent on height, the difference between groups persisted across these values as well. Despite these baseline differences, the study data and its interpretation should not be affected. As this was not a therapeutic study, the classification of patients into ARDS or non-ARDS sepsis categories did not affect how patients were treated or how data was collected. The groupings merely served as a basis for comparison to show that adjusting EVLW was not haphazard; that is, in patients with ARDS, EVLW was elevated in all patients after adjusting body weights whereas in non-ARDS patients, the number of patients with normal EVLW increased. Furthermore, after adjusting for baseline differences in height and actual body weight using multivariate regression modeling, the study results remained unchanged. Finally, race is also a predictor of lung size as evidenced by smaller lung volumes in Africian-Americans for a given height and gender. (39) The smaller predicted lung volumes could lead to a global underestimation of EVLW in our predominantly African-American population, however, this affect is minor compared to the influence of gender and height and thus should not significantly influence the study results.
Conclusion
Indexing EVLW to body weight that is based on height (predicted or adjusted body weight) rather than actual body weight improves the classification of ARDS patients with elevated EVLW and has a neutral or beneficial effect on the classification of severe sepsis patient without ARDS. Adjusting EVLW to reflect height and gender rather than body weight is justified in clinical practice as it is well known that height and gender are the main predictors of lung volume. EVLW measurements indexed to PBW, or AdjBW correlate better to patient oxygenation and lung injury score than do measurements indexed to actual body weight. Precise measurements of EVLW are important as they are used for prognostic purposes and could be the basis for therapeutic interventions. Further investigation is required to confirm these findings and to determine whether additional hemodynamic and volumetric parameters need to be similarly recalculated.
Table 2.
Fluid balance, hemodynamics, and outcome variables of enrolled patients. Data presented are mean +/− standard deviation (SD) or proportions (%)
| All (n=44) |
ARDS (n=30) |
Non-ARDS Sepsis (n=14) |
p-value | |
|---|---|---|---|---|
| Fluid Balance | ||||
| Daily net fluid balance (L/d) | 2.1 ± 2.8 | 2.0 ± 2.8 | 2.5 ± 2.7 | NS |
| Mean Hemodynamic Values | ||||
| CI | 3.9 ± 1.2 | 3.9 ± 1.2 | 4.1 ± 1.1 | NS |
| SVRI | 1628 ± 563 | 1637 ± 574 | 1574 ± 495 | NS |
| GEDVI | 712 ± 203 | 706 ± 224 | 728 ± 130 | NS |
| ITBVI | 885 ± 245 | 876 ± 269 | 910 ± 162 | NS |
| SVI | 57 ± 25 | 53 ± 25 | 67 ± 22 | 0.0004 |
| Outcome Measurements | ||||
| ICU length of stay (days, mean) | 17 ± 12 | 20 ± 12 | 11 ± 8 | 0.02 |
| Hospital length of stay (days, mean) | 30 ± 23 | 32 ± 12 | 25 ± 20 | NS |
| Duration of mechanical ventilation (days, mean) | 15 ± 12 | 17 ± 13 | 9 ± 6 | 0.05 |
| Hospital Mortality | 45.5% | 50.0% | 35.7% | NS |
CI: cardiac index; SVRI: systemic vascular resistance index; GEDVI: global end diastolic volume index; ITBVI: intrathoracic blood volume index; SVI: stroke volume index
Acknowledgments
Supported by grants: National Institutes of Health, HL 067739 (Martin) and AA 011660 (Moss)
Footnotes
The authors have no financial relationship to disclose relative to this work.
Reference List
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995;152:1818–1824. doi: 10.1164/ajrccm.152.6.8520742. [DOI] [PubMed] [Google Scholar]
- 3.Zilberberg M, Epstein S. Acute Lung Injury in the Medical ICU . Comorbid Conditions, Age, Etiology, and Hospital Outcome. American Journal of Respiratory and Critical Care Medicine. 1998;157:1159–1164. doi: 10.1164/ajrccm.157.4.9704088. [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 5.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 6.Anderson WR, Thielen K. Correlative study of adult respiratory distress syndrome by light, scanning, and transmission electron microscopy. Ultrastruct Pathol. 1992;16:615–628. doi: 10.3109/01913129209023751. [DOI] [PubMed] [Google Scholar]
- 7.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 8.Tate RM, Repine JE. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983;128:552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- 9.Martin GS, Eaton S, Mealer M, Moss M. Extravascular lung water in patients with severe sepsis: a prospective cohort study. Crit Care. 2005;9:R74–R82. doi: 10.1186/cc3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzkov VV, Kirov MY, Sovershaev MA, et al. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit Care Med. 2006;34:1647–1653. doi: 10.1097/01.CCM.0000218817.24208.2E. [DOI] [PubMed] [Google Scholar]
- 11.Groeneveld AB, Verheij J. Extravascular lung water to blood volume ratios as measures of permeability in sepsis-induced ALI/ARDS. Intensive Care Med. 2006;32:1315–1321. doi: 10.1007/s00134-006-0212-8. [DOI] [PubMed] [Google Scholar]
- 12.Patroniti N, Bellani G, Maggioni E, Manfio A, Marcora B, Pesenti A. Measurement of pulmonary edema in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:2547–2554. doi: 10.1097/01.ccm.0000186747.43540.25. [DOI] [PubMed] [Google Scholar]
- 13.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 14.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien JM, Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34:738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 17.The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Bone RC, Balk RA, Cerra FB. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Goedje O, Hoeke K, Lichtwarck-Aschoff M, Faltchauser A, Lamm P, Reichart B. Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: comparison with pulmonary arterial thermodilution. Crit Care Med. 1999;27:2407–2412. doi: 10.1097/00003246-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Sakka SG, Reinhart K, Meier-Hellmann A. Comparison of pulmonary artery and arterial thermodilution cardiac output in critically ill patients. Intensive Care Medicine. 1999;V25:843–846. doi: 10.1007/s001340050962. [DOI] [PubMed] [Google Scholar]
- 20.Neumann P. Extravascular lung water and intrathoracic blood volume: double versus single indicator dilution technique. Intensive Care Medicine. 1999;V25:216–219. doi: 10.1007/s001340050819. [DOI] [PubMed] [Google Scholar]
- 21.Sakka SG, Ruhl CC, Pfeiffer UJ, et al. Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Medicine. 2000;V26:180–187. doi: 10.1007/s001340050043. [DOI] [PubMed] [Google Scholar]
- 22.Sturm JA. Development and Significance of Lung Water Measurement in Clinical and Experimental Practice. In: Lewis FR, Pfeiffer UJ, editors. Practical Applications of Fiberoptics in Critical Care Monitoring. New York: Springer-Verlag; 1990. pp. 129–139. [Google Scholar]
- 23.Elings VB, Lewis FR. A single indicator technique to estimate extravascular lung water. J Surg Res. 1982;33:375–385. doi: 10.1016/0022-4804(82)90052-x. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Mondejar E, Castano-Perez J, Rivera-Fernandez R, et al. Quantification of lung water by transpulmonary thermodilution in normal and edematous lung. J Crit Care. 2003;18:253–258. doi: 10.1016/j.jcrc.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Katzenelson R, Perel A, Berkenstadt H, et al. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med. 2004;32:1550–1554. doi: 10.1097/01.ccm.0000130995.18334.8b. [DOI] [PubMed] [Google Scholar]
- 26.Holm C, Tegeler J, Mayr M, Pfeiffer U, Henckel vD, Muhlbauer W. Effect of crystalloid resuscitation and inhalation injury on extravascular lung water: clinical implications. Chest. 2002;121:1956–1962. doi: 10.1378/chest.121.6.1956. [DOI] [PubMed] [Google Scholar]
- 27.Sakka SG, Klein M, Reinhart K, Meier-Hellmann A. Prognostic value of extravascular lung water in critically ill patients. Chest. 2002;122:2080–2086. doi: 10.1378/chest.122.6.2080. [DOI] [PubMed] [Google Scholar]
- 28.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz SN, Pazin GJ, Lyon JA, Ho M, Pasculle AW. A controlled investigation of the pharmacokinetics of gentamicin and tobramycin in obese subjects. J Infect Dis. 1978;138:499–505. doi: 10.1093/infdis/138.4.499. [DOI] [PubMed] [Google Scholar]
- 30.Devine BJ. Gentamicin therapy. Drug Intelligence & Clinical Pharmacy. 1974;8:650–655. [Google Scholar]
- 31.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Second ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- 33.Halperin BD, Feeley TW, Mihm FG, Chiles C, Guthaner DF, Blank NE. Evaluation of the portable chest roentgenogram for quantitating extravascular lung water in critically ill adults. Chest. 1985;88:649–652. doi: 10.1378/chest.88.5.649. [DOI] [PubMed] [Google Scholar]
- 34.Baudendistel L, Shields JB, Kaminski DL. Comparison of double indicator thermodilution measurements of extravascular lung water (EVLW) with radiographic estimation of lung water in trauma patients. J Trauma. 1982;22:983–988. doi: 10.1097/00005373-198212000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sibbald WJ, Short AK, Warshawski FJ, Cunningham DG, Cheung H. Thermal dye measurements of extravascular lung water in critically ill patients. Intravascular Starling forces and extravascular lung water in the adult respiratory distress syndrome. Chest. 1985;87:585–592. doi: 10.1378/chest.87.5.585. [DOI] [PubMed] [Google Scholar]
- 36.Eisenberg PR, Hansbrough JR, Anderson D, Schuster DP. A prospective study of lung water measurements during patient management in an intensive care unit. Am Rev Respir Dis. 1987;136:662–668. doi: 10.1164/ajrccm/136.3.662. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 1992;145:990–998. doi: 10.1164/ajrccm/145.5.990. [DOI] [PubMed] [Google Scholar]
- 38.Beutler S, Schmidt U, Michard F. Hemodynamic monitoring in obese patients: a big issue. Crit Care Med. 2004;32:1981. doi: 10.1097/01.ccm.0000139622.85728.c1. [DOI] [PubMed] [Google Scholar]
- 39.Harik-Khan RI, Fleg JL, Muller DC, Wise RA. The effect of anthropometric and socioeconomic factors on the racial difference in lung function. Am J Respir Crit Care Med. 2001;164:1647–1654. doi: 10.1164/ajrccm.164.9.2106075. [DOI] [PubMed] [Google Scholar]