Abstract
Smallpox vaccination with replication deficient vaccinia strains such as Modified Vaccinia-Ankara (MVA) may induce protective immunity with improved safety and tolerability profiles compared with currently available smallpox vaccines. Ninety subjects were randomized equally to 6 groups in a partially blinded, randomized control clinical trial. IMVAMUNE® (MVA-BN®, Bavarian Nordic A/S, Kvistgård, Denmark) vaccine or placebo was administered at Study Day 0 and 28 by subcutaneous or intramuscular injection and five groups were challenged with Dryvax® at study Day 112. Vaccination with two doses of IMVAMUNE® was safe and well-tolerated compared to Dryvax®. IMVAMUNE® produced comparable cellular and humoral immune responses to one dose of Dryvax® and the immunity induced appears robust 90 days post vaccination by evidence of attenuated primary cutaneous reaction responses following Dryvax®. IMVAMUNE® vaccination prior to Dryvax® reduced virus replication at the Dryvax® site, decreased the size of the primary cutaneous lesion, and decreased the time to healing but did not completely ameliorate the immune response.
Keywords: IMVAMUNE®, MVA, Dryvax®
2. Introduction
Stores of replicating vaccinia-based vaccines, Dryvax® and ACAM2000 are maintained in the United States to help counter the threat of re-emerging smallpox. The need to reduce the potential for serious systemic adverse events associated with replication-competent vaccinia, particularly in populations with compromised immune systems, has led to renewed interest in attenuated strains such as MVA. Modified vaccinia Ankara (MVA), an attenuated derivative of dermal chorioallantois vaccinia strain Ankara (CVA), was registered in Germany in 1976. MVA was tested in animals and humans towards the end of the WHO smallpox eradication campaign as an immune priming inoculation administered prior to a replicating vaccinia vaccine in an attempt to reduce the number and severity of adverse events from Lister strain based smallpox vaccines [1]. In 1974, 7,098 subjects, including 5,691 children under the age of three years, were vaccinated with MVA one to two weeks prior to administration of the replication competent Lister-Elstree vaccinia strain. Although most subjects developed a cutaneous lesion following scarification with the Lister-Elstree vaccine, no serious adverse reactions in the MVA primed, Lister-Elstree boosted subjects were noted and general symptoms following boost inoculation were reduced [2]. While as many as 120,000 persons received MVA [1], human efficacy clinical trials or epidemiological evidence for use of MVA in the prevention of variola infection were not accomplished prior to global eradication of smallpox.
MVA is protective against orthopoxviruses in animal models. Immunodeficient mice given a single dose of MVA survived intranasal challenge with vaccine virus strain Western Reserve (WR) [3] and animals administered intracranial injections of MVA experienced minimal encephalopathic responses [4,5]. MVA was efficacious in animal variola challenge models [6,2] and protected monkeys against a lethal monkeypox challenge [7,8].
In a recent study, IMVAMUNE®, a highly attenuated vaccinia strain derived from MVA-572 (obtained from Dr. Anton Mahr), does not replicate in human cells and was safe and immunogenic in humans [9]. The present study sought to evaluate the safety and immunogenicity of a range of doses and routes of administration of IMVAMUNE®. Potential surrogate efficacy of MVA-induced immune responses was evaluated by the ability of two doses of IMVAMUNE® to reduce the clinical effects of a Dryvax® challenge.
3. Methods
3.1 Vaccines and Diluents
IMVAMUNE® (Bavarian Nordic A/S, Kvistgård, Denmark), a modified vaccinia Ankara vaccine (lot no. 130303), is a non-replicating virus in human cells used as the MVA smallpox vaccine in this study. Lyophilized vaccine was reconstituted with sterile water for injection (WFI) (Impfstoffwerk Dessau-Thornau GmbH, Germany). The reconstituted vaccine contains approximately 2 × 108 TCID50 per ml. The placebo was sterile saline for injection (American Regent Laboratories, Inc, Shirley, NY). Licensed smallpox vaccine (Dryvax®, Wyeth Laboratories, Marietta, PA) (lot no. 4008284), a replicating vaccinia virus provided by the Centers for Disease Control and Prevention in Atlanta, GA, was used as both the comparator vaccine and the virus in the challenge portion of this study.
Reconstituted IMVAMUNE® was prepared by adding 0.6 ml of WFI directly into the vial of lyophilized vaccine; one half ml of reconstituted vaccine delivered 1 × 108 TCID50. Serial dilutions with saline were performed to make the lower doses of 5 × 107 TCID50 and 2 × 107 TCID50. IMVAMUNE® was used within 8 hours of reconstitution and stored at 2° to 8°C. Lyophilized Dryvax® was reconstituted by adding 250 μl of diluent (Chesapeake Biological Laboratories, Baltimore, MD) containing 50% glycerin and 0.25% phenol in Water for Injection, USP directly into the vial for a standard concentration of 108 pfu ml and stored at 2° to 8°C, for use up to 90 days after reconstitution. The titers of IMVAMUNE® and Dryvax® were verified by back titration.
3.2 Study Design and Subjects
The study was a phase I randomized, partially blinded placebo controlled trial conducted at the National Institute of Allergy and Infectious Diseases Vaccine and Treatment Evaluation Unit at Saint Louis University (SLU). The study was approved by the SLU Institutional Review Board; all subjects provided written informed consent. Ninety subjects were enrolled from 5/17/2004 to 6/21/2005. An unblinded vaccinator administered vaccine and had no further contact with the subjects. All other staff and subjects were blinded to IMVAMUNE® versus placebo for the groups receiving IMVAMUNE® by the subcutaneous route and Dryvax® versus placebo.
Healthy adults 18 – 32 years of age were eligible for enrollment if they had a negative history for receipt of vaccinia virus vaccination and did not have a vaccination scar; were hepatitis B, hepatitis C and HIV antibody negative; had normal urine glucose and protein, and serum creatinine; creatinine clearance ≥80 ml/minute; hemoglobin ≥11g/dl; white blood cell count ≥2,500 and ≤11,000/mm3; platelets ≥140,000/mm3; alanine transferase ≤1.25 times the institutional upper limit of normal; normal troponin I; and an electrocardiogram (ECG) without any clinically significant findings.
Exclusion criteria included military service prior to 1989 or after January 2003; known or suspected impairment of immunologic function; heart disease or stroke; ischemic heart disease in an immediate family member before the age of 50; ≥10% risk of developing a myocardial infarction or coronary death within the next 10 years based on the National Cholesterol Education Program’s risk assessment tool; and an abnormal ECG (e.g., complete left or right bundle branch block, AV-node block, QTc or PR prolongation, premature atrial contractions or other atrial arrhythmia, sustained ventricular arrhythmia or 2 sequential premature ventricular contractions or ST changes consistent with ischemia); history of illegal injection drug use; atopic dermatitis; receipt of an inactivated vaccine 14 days prior or receipt of a live attenuated vaccine 30 days prior to vaccination; had close contact with persons who were pregnant, <12 months of age, or who had atopic dermatitis or immunodeficiency. Women were excluded if they were pregnant or lactating.
Ninety subjects were enrolled. Fifteen subjects were randomized to one of six groups (A, B, C, D, E and F) to receive differing doses of IMVAMUNE® or saline placebo by two different routes followed by Dryvax® or saline placebo (Table 1). The subjects and investigators were blinded to the subject’s group assignment. Subjects in Groups A, B, C, and E received two subcutaneous doses of IMVAMUNE® (2 × 107, 5 × 107, 1 × 108 and 1 × 108 TICD50, respectively) or saline placebo (Group D) at days 0 and 28 post 1st vaccination, and Group F received two intramuscular doses (1 × 108 TICD50) of IMVAMUNE®. Groups A, B, C, D and F received Dryvax® and Group E received saline placebo by scarification at day 112 post 1st vaccination.
Table 1.
Dose, Route of Administration and Timing of IMVAMUNE® and Dryvax® Challenge
| IMVAMUNE®a | Dryvax®b | |||||
|---|---|---|---|---|---|---|
| Group | # Volunteers | Dose | Route | Day 0 | Day 28±2 | Day 112±7 |
| A | 15 | 2×107 | SCc | IMVAMUNE® | IMVAMUNE® | Dryvax® |
| B | 15 | 5×107 | SC | IMVAMUNE® | IMVAMUNE® | Dryvax® |
| C | 15 | 1×108 | SC | IMVAMUNE® | IMVAMUNE® | Dryvax® |
| D | 15 | N/A | SC | Placebod | Placebod | Dryvax® |
| E | 15 | 1×108 | SC | IMVAMUNE® | IMVAMUNE® | Placebod |
| F | 15 | 1×108 | IMe | IMVAMUNE® | IMVAMUNE® | Dryvax® |
IMVAMUNE® = Modified vaccinia Ankara (IMVAMUNE®), Bavarian Nordic GmbH, Kvistgard, Denmark)
Dryvax® = Replicating vaccinia (Dryvax®, Wyeth, Philadelphia, PA) given intradermally by scarification
SC = subcutaneous
Placebo = sterile normal saline
IM = intramuscular
Dryvax® or saline placebo was administered by fifteen punctures with a bifurcated needle into the skin over the deltoid muscle. The Dryvax® vaccination sites were covered with gauze and two semi-permeable dressings, a 6 × 7 cm Tegaderm® (Health Care, St. Paul, MN) and a 10 × 12 cm Tegaderm® to avoid autoinoculation or inoculation of close contacts with vaccinia. Revaccination with Dryvax® is considered successful if a vesicular or pustular lesion or an area of definite palpable induration or congestion surrounding a central lesion, which may be a scar or ulcer, is present on examination 6–8 days after revaccination.
Subjects were followed closely for adverse events including cardiac related events at each follow-up visit. In addition to safety labs, a total of nine screening and follow-up ECGs were planned for each subject. Cardiac status was assessed using standardized questions to evaluate the presence of new onset chest pain, dyspnea, and signs of congestive heart failure. If a subject answered yes to one of the standardized questions, a cardiac evaluation was initiated per study alogorithm.
The primary endpoints of the study were to evaluate local and systemic adverse events following vaccination, to quantify cellular and humoral immune responses, and to compare routes of administration. The secondary endpoint was to evaluate the attenuation of cutaneous reactions following challenge with Dryvax® as a signal of surrogate efficacy of IMVAMUNE®.
3.3 Assays for the Detection of Neutralizing Antibody and for Total IgG by ELISA
Plaque Reduction Neutralizing Antibody Assay
Serum samples were collected prior to vaccination (Day 0) and Days 14, 28, 42, 56, 112 and 140. The plaque reduction neutralizing antibody assay using Dryvax® as the neutralizing antigen was described previously [10]. The endpoint was a 60 percent reduction in the number of plaque forming units calculated by linear regression interpolation. The MVA plaque reduction assay used MVA VR-1508 (ATCC) as the neutralizing antigen. Seroconversion was defined as a titer of ≥20 for the plaque reduction neutralizing antibody (PRNT) for both the MVA VR-1508 and Dryvax® antigen assays.
IMVAMUNE® Specific Total IgG ELISA
IMVAMUNE® specific IgG antibodies were measured using an automated direct ELISA on a Biomek FX (Beckman Coulter, Krefeld, Germany). Ninety-six well plates (Transparent Maxisorb Fluoronunc, Nunc, Wiesbaden, Germany) were coated overnight at 4°C with 100μl of IMVAMUNE® or CEF antigen in coating buffer (200mM Na2CO3, pH 9.6). Plates were blocked with 200μl of PBS-FCS 5% (PAA Laboratories, Linz, Austria) plus 0.05% Tween 20 (Sigma, Taufkirchen, Germany) for 30 minutes at room temperature. After washing the plates, heat-inactivated (56°C for 30 min), test sera were titrated in duplicate using 2-fold serial dilutions starting at 1:50. Plates were incubated for 1 hour at room temperature, washed and incubated for 1 hour with the detection antibody goat anti-human HRP IgG (Sigma, Taufkirchen, Germany), before development with TMB substrate (Sigma, Taufkirchen, Germany) for 15 minutes. After stopping the reaction with 1M H2SO4 the optical density (OD) was measured at 450nm. Plates were produced on multiple days with appropriate controls for inter-assay variability: Each plate within a run and between runs was required to have a mean OD value within +/− 3 Standard Deviations (SD) of the mean OD value established for the specific positive control in use at the time. The OD value was measured at 450nm. The antibody titers were calculated using linear regression over the linear region of the dilution curve and defined as the serum dilution that resulted in an OD of 0.35. Seroconversion was defined as a titer of ≥50 for the ELISA.
Dryvax® Total IgG ELISA
Dryvax® was used as the antigen in the ELISA assay as previously described [11]. The endpoint ELISA titers were computed based on an Optical Density (OD) cut-off set at 0.30. Seroconversion was defined as a titer of ≥100 for the binding ELISA antibody.
3.4 Cell Mediated Immunity
Quantitation of Vaccinia-specific IFN-γ producing T cells
A modified enzyme-linked immunospot (ELISPOT) assay was used to detect live virus-specific release of interferon-γ (IFN-γ) in cryopreserved peripheral-blood mononuclear cells (PBMCs). The IFN-γ ELISPOT assays were performed, with modifications, as previously described [12, 13]. Briefly, cryopreserved PBMCs were thawed, washed, plated (250,000 cells per well), and stimulated in triplicate using two levels of vaccinia virus at multiplicity of infection (MOI) of 1 and 2, for comparative purposes. Vaccinia virus was prepared as a single lot in CV-1 cells. Donor cells were incubated with virus for 18 hours. Plates were read by automated ELISPOT reader (CTL, Inc., Cleveland, Ohio). A limit of detection for the assay of 5 spots per million PBMCs was calculated as mean ±2 SD of the sum of the Day 0 virus response in previously naïve individuals. The mean Day 0 response for virus was low (<5 spots per million PBMCs). Values obtained for an MOI=1 are reported, unless quality controls allowed for accepting MOI=2. The limit of quantitation for the assay in this study was <16 IFN-gamma(+) cells per million PBMCs. Values above this level were considered positive virus-specific T-cell interferon-gamma vaccine responses. A cut-off of >15 spots was set as the positive conversion for the ELISPOT assay for Dryvax® assay antigen.
3.5 Cutaneous Lesion Viral Titer
The cutaneous lesions occurring after Dryvax® scarification were swabbed for virus quantification by plaque assay using BSC 40 cells.
4. Statistical Analysis
The objective of this Phase I study was to obtain preliminary safety and immunogenicity data on IMVAMUNE® responses using different dose levels, and routes of administration. The primary outcomes of the study are adverse events and side effects to IMVAMUNE® and Dryvax®. Secondary outcomes include immunogenicity testing of antibody and cellular responses to the vaccines.
Fifteen subjects were enrolled into each of the six treatment groups. Although the study was not designed with adequate statistical power to make a final determination of the optimal dose and route of the administration, it was adequate to collect preliminary safety and immunogenicity data on IMVAMUNE® responses using different dose levels and routes of administrations. Due to the small sample size it is likely that some differences could not be detected due to the small sample size.
All data are analyzed in two ways. First, comparisons were made among the groups to detect effects from vaccine, dose, or route. Treatment groups were combined when appropriate to increase the power of the test. For example, the IMVAMUNE®/IMVAMUNE®/Dryvax® groups are combined in the comparisons with the placebo/placebo/Dryvax® group after the first two vaccinations. The IM IMVAMUNE®/IMVAMUNE®/Dryvax® group is compared with the combined SC IMVAMUNE®/IMVAMUNE®/Dryvax® groups using the same dose. Significance level was adjusted using Bonferroni method when necessary. Fisher’s exact tests were used for binary outcomes such as take rates, rates of local and systemic symptoms and seroconversion rates. Wilcoxon Rank Sum test was used for continuous outcomes such as lesion size after Dryvax®, geometric mean titers (GMT) of plaque reduction neutralizing antibody assay (PRNT), ELISA and ELISPOT results, lesion titers and healing time (defined as the time from the day of Dryvax® challenge to the visit date when lesion was scabbed or healed).
The second type of analyses tested for linear trend among SC IMVAMUNE®/IMVAMUNE®/Dryvax® groups receiving increasing dosages of IMVAMUNE® (2×107, 5×107, and 1×108 TCID50) in the first and the second vaccinations. In such analyses, the Cochran-Armitage trend test was used for binary outcomes at single time points. Since immunogenicity data were obtained at multiple time points, tests for trends were performed in a generalized estimating equation model (PROC GENMOD in SAS) with repeated measurements for the time points following each dose to account for within-subject correlations. All tests were two-sided and overall significance level is 5%.
5. Results
5.1 Characteristics of the Subjects
Among the 90 subjects enrolled in the study and dosed as in Table 1, fifty-eight (64.4%) subjects were male (Table 2). Eighty (88.9%), 6 (6.7%), and 4 (4.4%) subjects were white, black and other, respectively. The mean (± SD) age was 24.8 ± 3.8 years. Ninety (100%), 87 (96.7%) and 72 (80.0%) received the first and second IMVAMUNE® vaccinations and the Dryvax® challenge, respectively. Eleven subjects terminated early: one subject was found to have a cardiac anomaly and 10 subjects were non-compliant or lost to follow-up, one of whom refused Dryvax® vaccination.
Table 2.
Demographic Characteristics
| Category | Sub-category | 2×10^7 SC IMVAMUNE® IMVAMUNE® Dryvax®a (N=15) | 5×10^7 SC IMVAMUNE® IMVAMUNE® Dryvax®a (N=15) | 1×10^8 SC IMVAMUNE® IMVAMUNE® Dryvax®a (N=15) | SC Placebo Placebo Dryvax®a (N=15) | 1×10^8 SC IMVAMUNE® IMVAMUNE® Placeboa (N=15) | 1×10^8 IM IMVAMUNE® IMVAMUNE® Dryvax®a (N=15) | Overall (N=90) |
|---|---|---|---|---|---|---|---|---|
| Group | A | B | C | D | E | F | G | |
| Genderb | Male | 13 (86.7%) | 11 (73.3%) | 8 (53.3%) | 11 (73.3%) | 5 (33.3%) | 10 (66.7%) | 58 (64.4%) |
| Female | 2 (13.3%) | 4 (26.7%) | 7 (46.7%) | 4 (26.7%) | 10 (66.7%) | 5 (33.3%) | 32 (35.6%) | |
| Age | Mean (S.D.) | 24.1 (3.3) | 22.9 (3.5) | 24.5 (4.1) | 27.1 (3.9) | 25.1 (3.5) | 24.8 (3.8) | 24.8 (3.8) |
| Range | [20, 31] | [19, 31] | [18, 33] | [22, 32] | [20, 32] | [20, 30] | [18, 33] | |
| Race | American Indian/Alaskan Native | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Asian | 0 (0.0%) | 0 (0.0%) | 1 (6.7%) | 1 (6.7%) | 0 (0.0%) | 0 (0.0%) | 2 (2.2%) | |
| Native Hawaiian/Pacific Islander | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.7%) | 1 (1.1%) | |
| Black | 1 (6.7%) | 1 (6.7%) | 0 (0.0%) | 2 (13.3%) | 1 (6.7%) | 1 (6.7%) | 6 (6.7%) | |
| White | 14 (93.3%) | 13 (86.7%) | 14 (93.3%) | 12 (80.0%) | 14 (93.3%) | 13 (86.7%) | 80 (88.9%) | |
| Multiracial | 0 (0.0%) | 1 (6.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.1%) |
Vaccine or placebo is given intradermally by scarification.
Difference among the groups is marginally significant (p=0.04) using chi-square test.
6. Safety Results
6.1 Reactogenicity
Local signs and symptoms after IMVAMUNE®
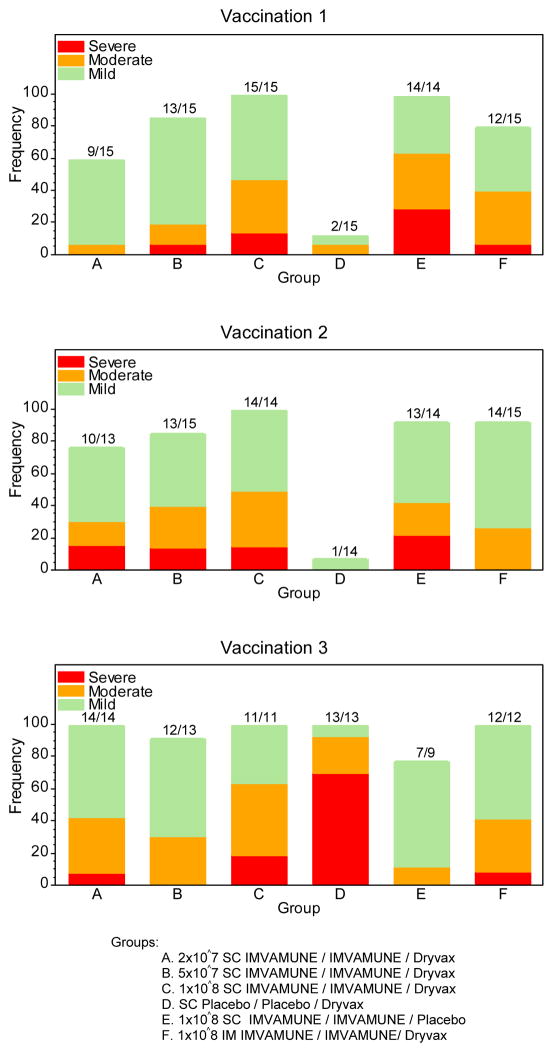
Significant differences (p<0.0001) were observed for having at least mild pain between placebo/placebo/Dryvax® and IMVAMUNE®/IMVAMUNE®/Dryvax® groups combined after each of the IMVAMUNE® doses (Figure 1, Panel A). The rates of observed moderate or severe symptoms were significantly different (p=0.003) between the placebo/placebo/Dryvax® group and the highest dose IMVAMUNE® groups combined. Significant differences (p=0.003) were found between the IM and the high dose SC groups for having at least mild induration post Dose 1. Significant differences (p=0.001) were observed in rates of having any local symptoms (pain, underarm pain, underarm swelling, itchiness, erythema, or induration) between the highest SC dose groups and the lowest SC dose. Trends for dose response (Table 3; Figure 1, Panel A) were observed for groups receiving subcutaneous IMVAMUNE® for erythema (p=0.01), induration (p=0.053) and pain (0.0007), respectively. The differences of symptom rates and trends among the IMVAMUNE®/IMVAMUNE®/Dryvax® groups were not statistically significant after Dose 2 (p>0.05).
Figure 1.
Figure 1, Panel A: Percentage of local adverse events experienced by subjects according to the number of vaccinations received and group assignment.
Groups: A. 2×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
B. 5×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
C. 1×10^8 SC IMVAMUNE/IMVAMUNE/Dryvax
D. SC Placebo/Placebo/Dryvax
E. 1×10^8 SC IMVAMUNE/IMVAMUNE/Placebo
F. 1×10^8 IM IMVAMUNE/IMVAMUNE/Dryvax
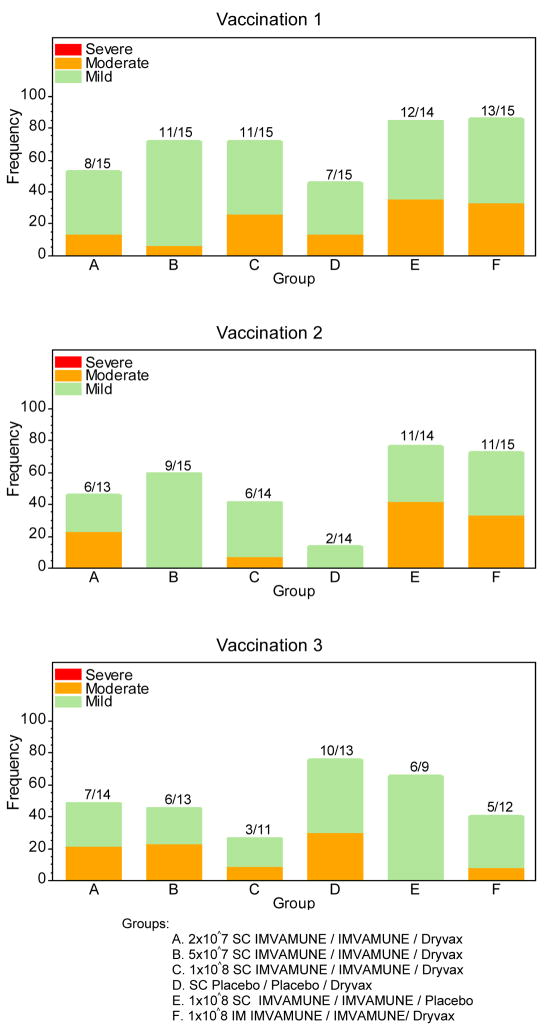
Figure 1, Panel B: Percentage of systemic adverse events experienced by subjects according to the number of vaccinations received and group assignment.
Groups: A. 2×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
B. 5×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
C. 1×10^8 SC IMVAMUNE/IMVAMUNE/Dryvax
D. SC Placebo/Placebo/Dryvax
E. 1×10^8 SC IMVAMUNE/IMVAMUNE/Placebo
F. 1×10^8 IM IMVAMUNE/IMVAMUNE/Dryvax
Table 3.
Numbers of Subjects with Erythema or Induration ≥15 mm following IMVAMUNE® or Placebo According to Dose and Route of Vaccine
| Dose 1 (MVA/Placebo) | Dose 2 (MVA or Placebo) | ||||
|---|---|---|---|---|---|
| Group | Vaccine Dose, (Route) | Erythema n/N (%) | Induration n/N (%) | Erythema n/N (%) | Induration n/N (%) |
| A | 2 × 107 (SC) | 0/15 (0.0) | 1/15 (6.7) | 2/13 (15.4) | 1/13 (7.7) |
| B | 5 × 107 (SC) | 2/15 (13.3) | 1/15 (6.7) | 4/15 (26.7) | 2/15 (13.3) |
| C | 1 × 108 (SC) | 3/15 (20.0) | 3/15 (20.0) | 3/14 (21.4) | 2/14 (14.2) |
| D | Placebo (SC) | 0/15 (0.0) | 0/15 (0.0) | 0/14 (0.0) | 0/14 (0.0) |
| E | 1 × 108 (SC) | 5/14 (35.7) | 5/14 (35.7) | 5/14 (35.7) | 4/14 (28.6) |
| F | 1 × 108 (IM) | 0/15 (0.0) | 0/15 (0.0) | 1/15 (6.7) | 0/15 (0.0) |
n/N = numbers (n) erythema or induration ≥15mm over total number (N) vaccinated by dose and group
Local signs and symptoms after Dryvax®
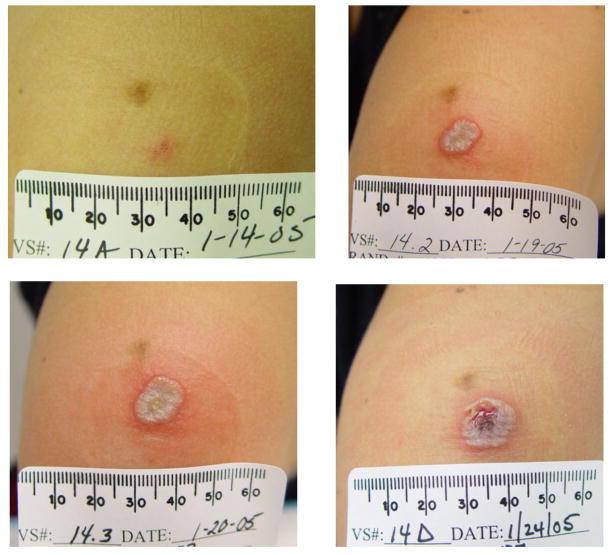
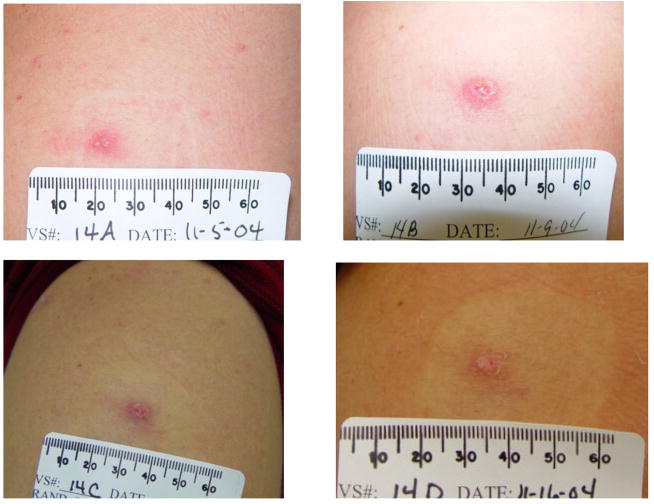
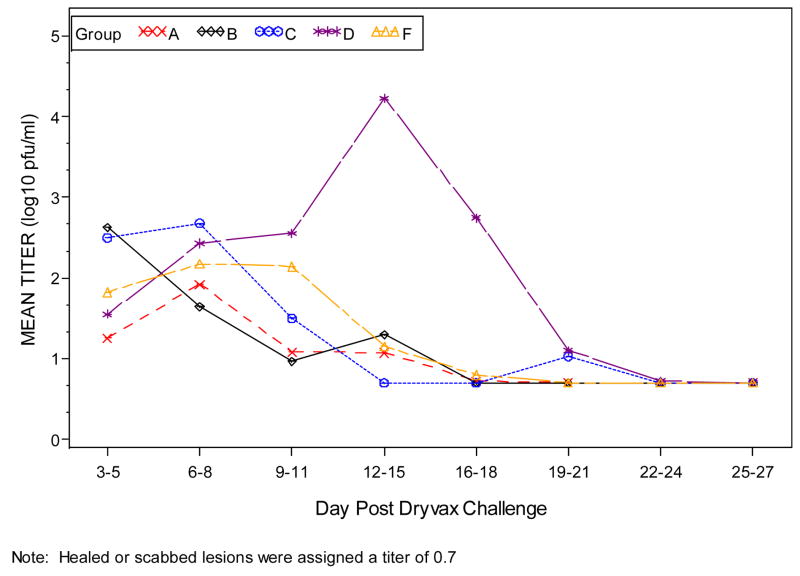
When the combined groups receiving IMVAMUNE®/IMVAMUNE®/Dryvax® are compared to the placebo/placebo/Dryvax® group, there was no significant difference in the rate of primary lesions (p=0.10) after Dryvax® challenge (Table 4) or whether IMVAMUNE® was given SC or IM (p=0.42); however, the vaccination lesion size was significantly smaller after receiving IMVAMUNE® (Table 4, Figure 2). IMVAMUNE® priming significantly reduced (p = 0.0009) Dryvax® lesion viral titers compared to placebo (Figure 3) at the site of scarification. There was no significant trend in dose response for lesion size (p=0.55) or titer (p=0.05) between the three IMVAMUNE® dose groups. There were significantly larger sizes in the diameter of erythema (p<0.0001), induration (p<0.0004), and lesion size (0.0001) when the placebo/placebo/Dryvax® group was compared to the combined IMVAMUNE®/IMVAMUNE®/Dryvax® groups (Table 4). The IMVAMUNE® prime reduced (p< 0.0001) the time to healing of Dryvax® lesions (Table 4; Figure 2) compared to administration of Dryvax® alone.
Table 4.
Summary of Primary Cutaneous Lesions Post Dryvax® Challenge of Subjects Vaccinated with IMVAMUNE® or Placebo
| Groupa | Vaccine, Dose, Route | Number with Primary Lesions by Number Challenged with Dryvax® (%, [C.I.]) | Median (Range) [Mean (Range)] Maximum Lesion Size (mm) | Median (Range) [Mean (Range)] Maximum Erythema Size (mm) | Median (Range) [Mean (Range)] Maximum Induration Size (mm) | Median (Range) [Mean (Range)] Heal Timeb Post Dryvax® Time (Days) |
|---|---|---|---|---|---|---|
| A | 2 × 107 SC IMVAMUNE®/IMVAMUNE®/Dryvax® | 13/14 (93[66,100]) | 8.0 (3–15) [8.2 (3–15)] |
12.0 (5–45) [13.9 (5–45)] |
9 (0–40) [11.5 (0–40)] |
14.0 (10–21) [13.8 (10–21)] |
| B | 5 × 107 SC IMVAMUNE®/IMVAMUNE®/Dryvax® | 7/13 (54[25,81]) | 7.0 (5–15) [8.1 (5–15)] |
11.0 (5–20) [11.4 (5–20)] |
9.0 (2–12) [8.0 (2–12)] |
15.0 (6–19) 14.1 (6–19)] |
| C | 1 × 108 SC IMVAMUNE®/IMVAMUNE®/Dryvax® | 10/11 (91[59, 100]) | 10.0 (4–15) [8.7 (4–15)] |
11.0 (4–55) [15.7 (4–55)] |
11.0 (0–40) [13.0 (0–40)] |
13.0 (8–21) [13.0 (8–21)] |
| D | SC placebo/placebo/Dryvax® | 13/13 (100[75,100]) | 13.9c (10–20) [14.0c(10–20)] |
40.0c (12–70) [36.8c(12–70)] |
30.0c (10–70) [30.0c(10–70)] |
24.0c (14–31) [22.6c (14–31)] |
| F | 1 × 108 IM IMVAMUNE®/IMVAMUNE®/Dryvax® | 8/12 (67[35,90]) | 7.5 (3–12) [7.5 (3–12)] |
11.0 (5–20) [11.9 (5–20)] |
11.5 (5–35) [14.1 (5–35)] |
14.0 (9–24) [14.8 (9–24)] |
No subjects in the 1 × 108 SC IMVAMUNE®/IMVAMUNE®/Placebo group had signs of a primary cutaneous reaction since Dryvax® was not administered.
Heal time = The period of time to scabbing of lesion after Dryvax® Challenge.
Significantly larger lesion, erythema, induration and time to healing compared with other groups.
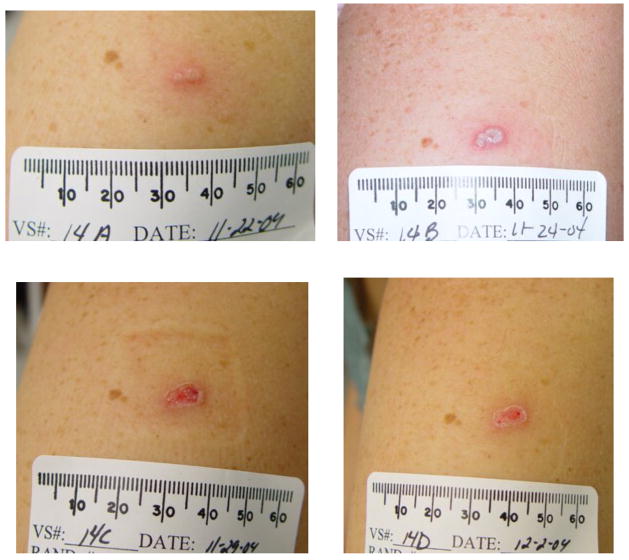
Figure 2.
Figure 2A: The subject received placebo/placebo/Dryvax®. Dryvax® was administered on January 11, 2005
Figure 2B: The subject received IMVAMUNE®/IMVAMUNE®/Dryvax®. IMVAMUNE® was administered subcutaneously. Dryvax® was administered on November 2, 2004.
Figure 2C: The subject received IMVAMUNE®/IMVAMUNE®/Dryvax®. IMVAMUNE® was administered intramuscularly. Dryvax® was administered on November 18, 2004.
Figure 3.
Plots of Dryvax® vaccination lesion titers (Log 10 pfu/ml) by time (days) post Dryvax® challenge. Only subjects with a Dryvax® skin lesion are included.
Systemic symptoms
Systemic adverse events are shown in Figure 1, Panel B. There were no significant differences observed among groups after any dose for any systemic symptom or grade except for muscle ache. A significant difference (p=0.005) was noted between placebo/placebo/Dryvax® and IM IMVAMUNE®/IMVAMUNE®/Dryvax® groups after Dose 2 for mild muscle ache but not for moderate or severe muscle aches.
6.2 Serious Adverse Events and Possible Cardiac Related Events
A total of 27 noncardiac related serious adverse events were reported in 20 subjects and included any medically attended event. All events were considered unrelated except a case of thyroid cancer that was considered unrelated by the investigator and related by the medical monitor.
Subjects were evaluated for cardiac symptoms and ECG changes possibly related to vaccine induced cardiac events after each vaccination. Twenty-four subjects experienced 32 events including chest pain or tightness (11 events); shortness of breath (3 events); T wave, non-specific ST-T wave and Q wave changes (16 events); pericarditis (1 event) and pericardial effusion (1 event). Twelve (10 vaccine and 2 placebo recipients), 10 (9 vaccine and 1 placebo recipients) and 10 (10 vaccine recipients) events occurred after the first and second IMVAMUNE® vaccinations and Dryvax® challenge, respectively. Only one subject was deemed to have cardiac symptoms possibly related to vaccination. Four days after receiving Dryvax vaccination a subject with a previously unknown congenital malformation of the heart experienced shortness of breath and limited chest pain associated with a small pericardial effusion and normal ECG, CK-MB and troponin I. A transesophageal echocardiogram revealed a bicuspid aortic valve, congenital left ventricular malformation and a small pericardial effusion. Follow-up ECG (0.3mm ST-segment depression in lead III) and cardiac enzymes were normal. This constellation of symptoms was reported as pericarditis possibly related to Dryvax®. Another subject developed symptoms including sternal chest pain, dyspnea and lightheadedness 10 days after receiving Dryvax® while playing softball which caused the subject to sit out an inning. Although the symptoms were deemed related to Dryvax®, the cardiologist did not attribute the symptoms to a cardiac etiology.
7. Immunogenicity Results
7.1 Plaque Reduction Neutralizing Geometric Mean Titer Antibody Results Using MVA VR-1508 or Dryvax® as the Neutralizing Viruses
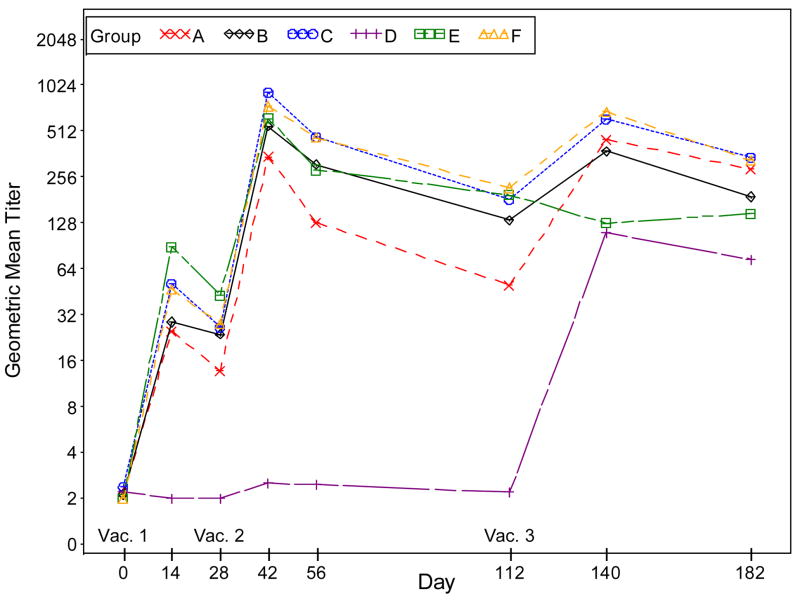
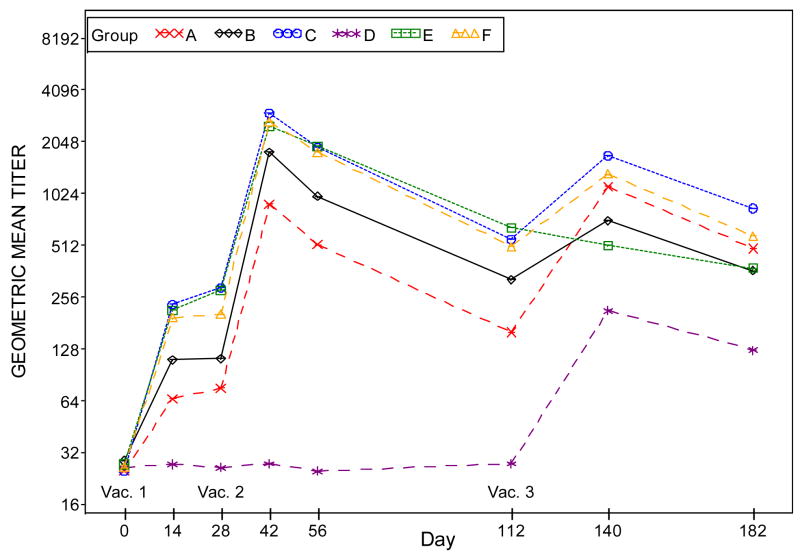
The GMTs in the placebo/placebo/Dryvax® group are significantly lower than those in the combined IMVAMUNE®/IMVAMUNE®/Dryvax® group after Doses 1 and 2 (p<0.0001). Compared to the GMT in the combined IMVAMUNE®/IMVAMUNE®/Dryvax® groups, the GMT in the placebo/placebo/Dryvax® group is significantly higher (p<0.0001) after Dose 3, when Dryvax® (Figure 4, Panel B) is used as the antigen assay whereas the GMT in the placebo/placebo/Dryvax® group is significantly lower (p<0.0001) when MVA VR-1508 (Figure 4, Panel A) is used as the assay antigen.
Figure 4.
Figure 4, Panel A: Plots of plaque reduction neutralizing antibody geometric mean titers by time (days) post vaccination when VR-1508 is used as the assay antigen. Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140 and 182. Subjects who did not receive the third vaccination were excluded in the results for Days 140 and 182.
Groups: A. 2×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
B. 5×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
C. 1×10^8 SC IMVAMUNE/IMVAMUNE/Dryvax
D. SC Placebo/Placebo/Dryvax
E. 1×10^8 SC IMVAMUNE/IMVAMUNE/Placebo
F. 1×10^8 IM IMVAMUNE/IMVAMUNE/Dryvax
Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140, 182.
Subjects who did not receive the third vaccination were excluded in the results for Days 140, 182.
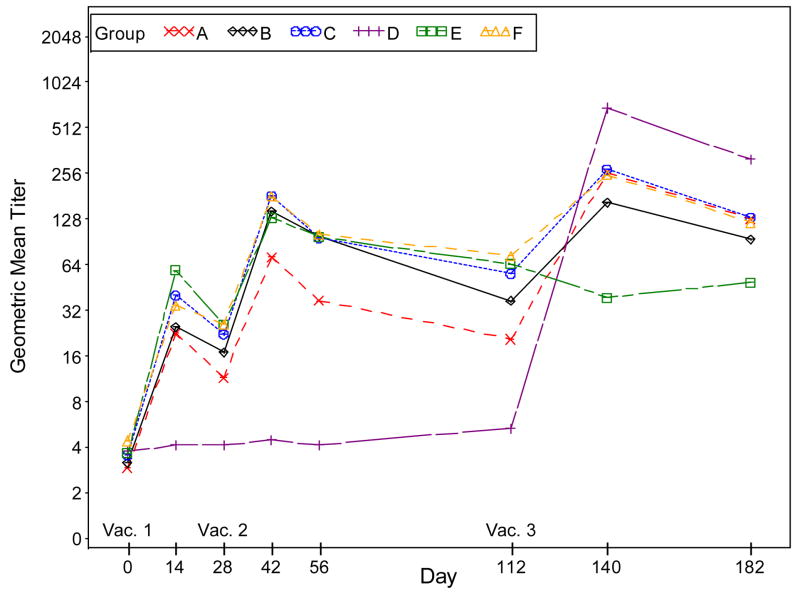
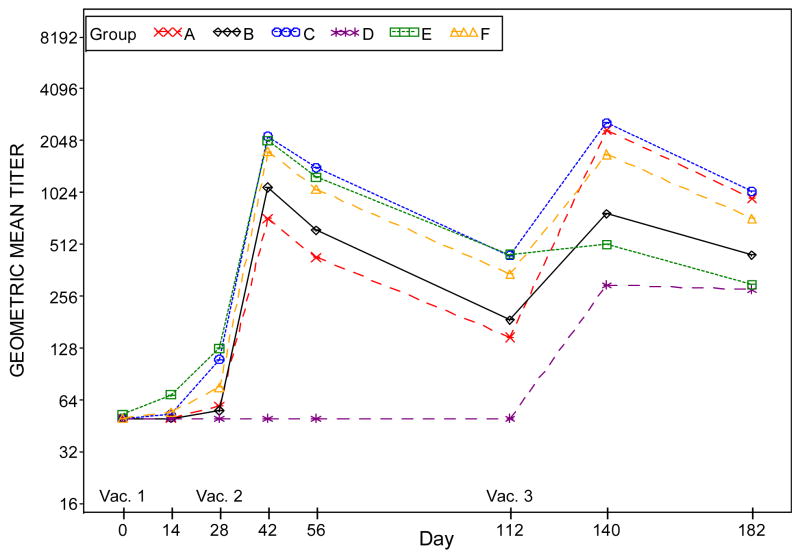
Figure 4, Panel B: Plots of plaque reduction neutralizing antibody geometric mean titers by time (days) post vaccination when Dryvax® is used as the assay antigen. Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140 and 182. Subjects who did not receive the third vaccination were excluded in the results for Days 140 and 182.
Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140, 182.
Subjects who did not receive the third vaccination were excluded in the results for Days 140, 182.
Groups: A. 2×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
B. 5×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
C. 1×10^8 SC IMVAMUNE/IMVAMUNE/Dryvax
D. SC Placebo/Placebo/Dryvax
E. 1×10^8 SC IMVAMUNE/IMVAMUNE/Placebo
F. 1×10^8 IM IMVAMUNE/IMVAMUNE/Dryvax
Figure 4, Panel C: Plots of ELISA antibody geometric mean titers by time (days) post-vaccination when IMVAMUNE® is used as the assay antigen. Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140 and 182. Subjects who did not receive the third vaccination were excluded in the results for Days 140 and 182.
Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140, 182.
Subjects who did not receive the third vaccination were excluded in the results for Days 140, 182.
Groups: A. 2×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
B. 5×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
C. 1×10^8 SC IMVAMUNE/IMVAMUNE/Dryvax
D. SC Placebo/Placebo/Dryvax
E. 1×10^8 SC IMVAMUNE/IMVAMUNE/Placebo F. 1×10^8 IM IMVAMUNE/IMVAMUNE/Dryvax
Figure 4, Panel D: Plots of ELISA antibody geometric mean titer by time (days) when Dryvax® is used as the assay antigen. Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140 and 182. Subjects who did not receive the third vaccination were excluded in the results for Days 140 and 182.
Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140, 182.
Subjects who did not receive the third vaccination were excluded in the results for Days 140, 182.
Groups: A. 2×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
B. 5×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
C. 1×10^8 SC IMVAMUNE/IMVAMUNE/Dryvax
D. SC Placebo/Placebo/Dryvax
E. 1×10^8 SC IMVAMUNE/IMVAMUNE/Placebo
F. 1×10^8 IM IMVAMUNE/IMVAMUNE/Dryvax
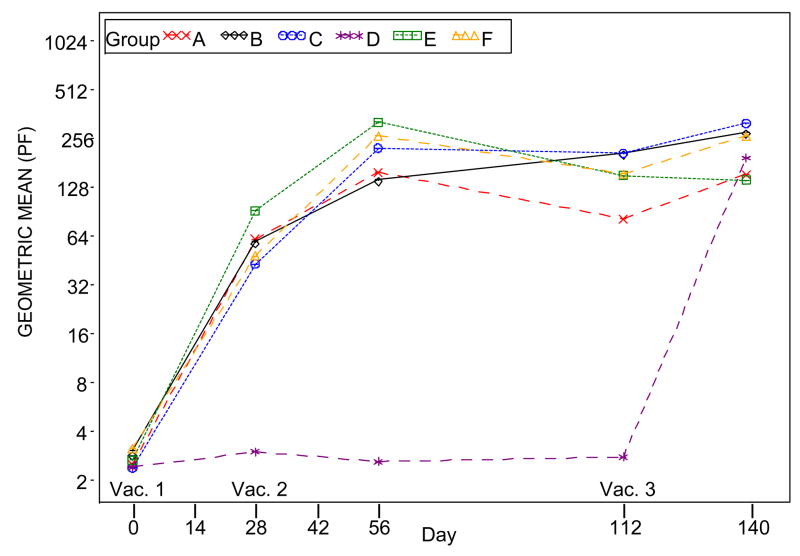
Figure 4, Panel E: Plot of ELISPOT results by time (days) post-vaccination when Dryvax® is used as the assay antigen. Subjects who did not receive the second vaccination were excluded in the results for Days 56, 112, 140 and 365. Subjects who did not receive the third vaccination were excluded in the results for Days 140 and 365.
Subjects who did not receive the second vaccination were excluded in the results for Days 42, 56, 112, 140.
Subjects who did not receive the third vaccination were excluded in the results for Day 140.
Groups: A. 2×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
B. 5×10^7 SC IMVAMUNE/IMVAMUNE/Dryvax
C. 1×10^8 SC IMVAMUNE/IMVAMUNE/Dryvax
D. SC Placebo/Placebo/Dryvax
E. 1×10^8 SC IMVAMUNE/IMVAMUNE/Placebo
F. 1×10^8 IM IMVAMUNE/IMVAMUNE/Dryvax
Significant trends in geometric mean titers were observed after each dose for groups in order of dosage level for Doses 1 and 2 (all p<0.01). The dose responses were not significant for Dose3 (p=0.93 and 0.05, respectively) when MVA VR-1508 or Dryvax® was used as the assay antigen.
7.2 ELISA GMT Antibody Results Using IMVAMUNE® or Dryvax® as the Assay Antigen
At Day 28 post Dose 1, no significant difference was observed in GMTs among all the groups when Dryvax® (Figure 4, panel D) was used as the assay antigen. When IMVAMUNE® (Figure 4, Panel C) was used as the assay antigen, a difference in favor of IMVAMUNE® is seen (p<.0001) between the placebo/placebo/Dryvax® group and IMVAMUNE®/IMVAMUNE®/Dryvax® groups combined.
Regardless of antigen, GMTs in the placebo/placebo/Dryvax® group were significantly lower (p<0.0001) than the combined IMVAMUNE®/IMVAMUNE®/Dryvax® group at two weeks post Dose 2 (Days 39–45) and four weeks post Dose 3 (Days 133–147) (all p-values <0.0001). Significant trends of increasing GMTs were observed for increasing priming doses of IMVAMUNE® after Doses 1 and 2 (p<0.002 each) but not after Dose 3 (p<0.35 and 0.25) when IMVAMUNE® and Dryvax® were used as the antigen, respectively.
7.3 ELISPOT Using Dryvax® as the Assay Antigen
Significant differences in IFN-γ forming ELISPOT unit GMTs were observed between the placebo/placebo/Dryvax® group and the IMVAMUNE®/IMVAMUNE®/Dryvax® groups after Doses 1 and 2 (p<0.0001) but not after Dose 3 (p=0.47) when vaccinia virus derived from Dryvax® was used for the assay antigen. Trend analyses for increasing IFN-γ production with increasing doses of IMVAMUNE® were statistically insignificant (Figure 4, Panel E) after any of the doses (p=0.95, 0.11 and 0.21 for Dose 1, 2, and 3, respectively).
7.4 Seroconversion Rates (data not shown)
PRNT seroconversion
When Dryvax® was used as the neutralizing antigen, 87% to 100% of the subjects in the IMVAMUNE®/IMVAMUNE®/Dryvax® groups converted at two weeks post the second IMVAMUNE® dose and 100% four weeks after Dryvax® challenge. One person in the placebo/placebo/Dryvax® group had a positive PRNT antibody titer of 49 (cutoff 20) two weeks post second placebo dose when the neutralizing antigen was MVA but not when Dryvax was used as the neutralizing virus. All of the remaining subjects in this group seroconverted four weeks after Dryvax® challenge. The seroconversion rate in the IMVAMUNE®/IMVAMUNE®/placebo group remained 100% 10 weeks after placebo challenge. When MVA VR-1508 was used as the neutralizing antigen, 100% of subjects receiving IMVAMUNE® seroconverted by two weeks post second IMVAMUNE® dose and 85% of the subjects seroconverted after Dryvax®
ELISA seroconversion
By two weeks post second vaccination, 100% of subjects receiving IMVAMUNE® seroconverted when either Dryvax® or IMVAMUNE® was used as the assay antigen. Ten weeks post Dryvax® challenge, 84.6% (group D) and 100% (Groups A, B, C, E and F) of subjects remained seroconverted when Dryvax® or IMVAMUNE®, was used as the assay antigen, respectively.
8. Discussion
The results of the current study suggest that IMVAMUNE® is safe, well-tolerated and immunogenic at doses of 2 × 107 to 1 × 108 TCID50 and attenuates the cutaneous Dryvax® reaction when given by either the SC or IM routes. In general, there were no significant differences between 1 × 108 TCID50 per ml IMVAMUNE® when given by the IM or SC routes except for a significant difference in the number with mild induration in SC group. Slightly fewer cases of erythema and induration were observed after the first or second IMVAMUNE® injections given IM compared to SC. In a recent trial [9] to evaluate two doses of IMVAMUNE® given subcutaneously or intramuscularly on Days 0 and 28, IMVAMUNE® was found to be generally well-tolerated albeit, a higher incidence of systemic symptoms was reported in the IM versus the SC group.
IMVAMUNE® given prior to Dryvax® reduces virus replication at the site of vaccination, local reactogenicity and the time to healing. Although MVA may reduce the size of a primary cutaneous lesion after vaccination with Dryvax®, it does not completely ameliorate the response. Partial attenuation of primary takes in some vaccinia experienced individuals receiving MVA [2] or replicating virus [11,14] vaccination has been documented in previous studies and may depend on the level of pre-existing immunity. While heterologous vaccination protects animals from a variety of generalized orthopoxvirus infections, Dryvax® does not completely protect against the formation of skin lesions after a second vaccination [15] nor did it protect against monkeypox during the Wisconsin outbreak [16]. Lack of complete protection against skin lesions after vaccination may be dependent on the high dose of inoculum in the skin (e.g. vaccinia vaccination) versus a small dose via the respiratory tract (e.g. variola infection) [15]. Nonetheless the partial amelioration of the Dryvax® lesions provides limited evidence that two doses of MVA may be protective against vaccinia [17]. Further studies to determine the efficacy of MVA against other orthopox viruses such as variola and monkeypox are warranted.
In this study a dose response effect was observed for both PRNT and ELISA titers after each vaccination. For the IFN-γ producing ELISPOT assay, a dose response was apparent for the first and second vaccination but not the third vaccination. The CMI response after two doses of IMVAMUNE® compared favorably with one dose of Dryvax® alone, indicating that IMVAMUNE® inoculation did not attenuate T-cell IFN-γ responses to Dryvax®, and that diminished skin response to challenge with Dryvax® suggests that the T-cell responses generated during priming were protective in nature. In addition, following two priming doses of IMVAMUNE®, Dryvax® did not boost T-cell responses after 60 days between vaccinations, however, earlier intervals when a boost in T-cell responses may have been detectable were not evaluated.
When comparing assays, the antigen chosen to perform the antibody assay, i.e. MVA or Dryvax®, affects the magnitude of the GMTs after IMVAMUNE® or Dryvax® vaccination. When MVA is used as the assay antigen, significantly higher GMTs occur post first and second dose of IMVAMUNE® compared to responses when Dryvax® is used as the assay antigen, and a diminished response is observed in subjects receiving Dryvax® alone. When Dryvax® is used as the assay antigen, there is a significantly higher antibody response in naive individuals receiving a single dose of Dryvax® compared to individuals receiving an IMVAMUNE® prime.
One subject in the placebo/placebo/Dryvax® group appeared to be antibody positive after the second dose of placebo, but this phenomenon only occurred when MVA was used as the neutralizing antigen. Although the antibody titer to MVA was low (49) a PRNT titer of ≥20 was considered to be seropositive, regardless of the antigen used for the PRNT assay. Previously, the appropriate threshold to use to consider a serum sample positive for vaccinia antibody was evaluated. It was found that the sensitivity and specificity were very similar regardless of whether <20 or < 50 was used as the cut-off value to be considered seronegative. The more conservative value of 20 is used as the threshold to determine serostatus, although occasionally a higher value is observed in individuals with no known exposure or vaccination with vaccinia.
These data may suggest that immunologic determinants for humoral immunity may be different for MVA versus replication efficient vaccinia such as Dryvax®. Recent studies suggest that MVA does not produce all the major vaccinia proteins targeted by VIG [18]. These investigators determined one protein to be the vaccinia gene fragment corresponding to the cowpox A-type inclusion body. They showed that this cowpox gene is highly conserved across poxvirus strains including variola major virus, India strain (92% homology). These data combined with the recent work on variola survivors [19] suggest that there are differences in the humoral immune responses in humans following exposure to MVA, Dryvax® or variola that may have important implications to the degree and duration of protective immunity. Robust cellular responses in the immediate years following vaccination may compensate for diminished humoral response, but the waning of circulating memory T-cells following MVA or other modified vaccinia-based approaches might result in less durable or less prolonged immunity seen historically in vaccinated individuals.
IMVAMUNE® priming acts to limit local reactogenicity to Dryvax® challenge. The power of the study was too small to evaluate whether IMVAMUNE® could decrease both systemic and local reactogenicity as well as serious adverse events. Larger studies would be needed to determine the timing of vaccination and the number of doses of IMVAMUNE® that are sufficient to protect against significant adverse events due to vaccination with replicating virus. However, studies to evaluate whether priming with MVA reduces the rate of uncommon or life threatening adverse effects of Dryvax® are not currently feasible. Clinical trials using MVA conducted during the 1970’s in Germany reported no severe adverse events in children and did not reveal an increased risk for immunocompromised persons. Further trials are under development to evaluate the optimal route, dose and schedule for IMVAMUNE® priming followed by replicating vaccinia boost and to evaluate the protective immunity of IMVAMUNE® as a stand-alone smallpox vaccine without replicating vaccinia boost.
Acknowledgments
The study is funded by NIH contracts #N01-AI-25464 and #U19-AI-05719, and Bavarian Nordic.
Special thanks to Irene Graham, M.D., Elizabeth Babusis M.D., Janice Tennant, nurse coordinator and the staff of the VTEU for clinical support; Mahendra Mandava, Brandt Gormley, Tammy Blevins, Yinyi Yu, and Laura Orphin for laboratory support; Carolyn Novotny for manuscript preparation; and Mark Challberg, Lydia Falk, Wendy Fanaroff-Ravick, Stephen Heyse, Michael Kennedy, Gerald Kovacs, Catherine Laughlin, Carmen Maher, Carol Ostrye, Gerald Poley, and Janet Shimko from the DMID. We are especially thankful to the volunteers without whom the study would not be possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayr A, Stickl H, Müller HK, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defense mechanism. Zentralbl Bakteriol. 1978;167(5–6):375–90. [PubMed] [Google Scholar]
- 2.Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schäfer H, Holzner A. MVA-vaccination against smallpox: clinical trials of an attenuated live vaccinia virus strain (MVA) Dtsch Med Wschr. 1974;99(47):2386–92. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Nat Acad Sci. 2004;101:4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayr A, Hochstein M, Mintzel V, Stickl H. Passage history, properties, and aplicability of the attenuated vaccinia virus strain MVA. Infection. 1975;3:6–14. [Google Scholar]
- 5.Mayr A, Danner K. Vaccination against pox diseases under immunosuppressive conditions. Dev Biological Standardization. 1978;41:225–34. [PubMed] [Google Scholar]
- 6.Hochstein-Mintzel V, Hanichen T, Huber HC, Stickl H. An attenuated strain of vaccinia virus (MVA). Successful intramuscular immunization against vaccinia and variola. Zentralbl Bakteriol. 1975;230:283–97. [PubMed] [Google Scholar]
- 7.Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 8.Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, Pistoor FH, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005 Jun;79(12):7845–51. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, et al. Safety and immunogenicity of IMVAMUNE®, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24(12):2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Newman FK, Frey SE, Blevins TP, Mandava M, Bonifacio JRA, Yan L, et al. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax®) vaccine. J Clin Microbiol. 2003;41(7):3154–7. doi: 10.1128/JCM.41.7.3154-3157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey SE, Newman FK, Yan L, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. J Amer Med Assoc. 2003;289(24):3295–9. doi: 10.1001/jama.289.24.3295. [DOI] [PubMed] [Google Scholar]
- 12.Ennis FA, Cruz J, Demkowitz WE, Rothman AL, McClain DJ. Primary induction of human CD8+ cytotoxic T lymphocytes and interferon- producing T cells after smallpox vaccination. J Infect Dis. 2002;185(11):1657–9. doi: 10.1086/340517. [DOI] [PubMed] [Google Scholar]
- 13.Frey SE, Newman FK, Curz J, Shelton WB, Tennant JM, Polach T, et al. Dose-Related Effects of Smallpox Vaccine. New Engl J Med. 2002;346(17):1275–1280. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh K, Cherry JD, Benenson AS, Connor JD, Alling DW, Rolfe UT, et al. Standard percutaneous revaccination of children who received primary percutaneous vaccination. J Infect Dis. 1977;135(1):155–166. doi: 10.1093/infdis/135.1.155. [DOI] [PubMed] [Google Scholar]
- 15.Fenner F, Henderson DA, Aritra I, Ježek Z, Ladnyi ID. Smallpox and its Eradication. Geneva: World Health Organization; 1988. [Google Scholar]
- 16.Hammarlund E, Lewis MW, Carter SV, Amanna I, Hansen SG, Strelow LI, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nature Med. 2005;11(9):1005–11. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 17.Parrino J, McCurdy LH, Larkin BD, Gordon IJ, Rucker SE, Enama ME, et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax® challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine. 2007;25:1513–1525. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones-Trower A, Garcia A, Meseda CA, He Y, Weiss C, Kumar A, et al. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005;343(1):128–140. doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Sivapalasingam S, Kennedy JS, Borkowsky W, Valentine F, Zhan M-X, Pazoles P, et al. Immunological Memory after Exposure to Variola Virus, Monkeypox Virus, and Vaccinia Virus. J Infect Dis. 2007;195(8):1151–1159. doi: 10.1086/512161. [DOI] [PMC free article] [PubMed] [Google Scholar]