Abstract
The physiological actions of brain Angiotensin II AT2 receptors and their relationship to Angiotensin II AT1 receptors remain controversial. To further clarify their role, we determined to what extent systemic administration of an AT2 receptor antagonist affected AT2 receptor binding within the brain and the expression of AT1 receptors. For this purpose, we subcutaneously administered the AT2 receptor antagonist PD123319 (1 mg/kg/day) to adult male rats for two weeks via osmotic minipumps. We also studied the content of pituitary adrenocorticotropic hormone and vasopressin, representative of hypothalamic–pituitary–adrenal axis activation, and the tyrosine hydroxylase gene expression in the locus coeruleus as a measure of central norepinephrine function. We found significant decreases in AT2 receptor binding in brain areas inside the blood brain barrier, the inferior olive and the locus coeruleus. AT2 receptor blockade increased AT1 receptor binding and mRNA expression not only in the subfornical organ and the median eminence, situated outside the blood brain barrier, but also in the hypothalamic paraventricular nucleus, located inside the blood brain barrier. These changes paralleled decreased expression of tyrosine hydroxylase mRNA in the locus coeruleus and decreased pituitary adrenocorticotropic and vasopressin content. Our results demonstrate that sustained peripheral administration of an AT2 antagonist decreases binding to brain AT2 receptors, indicating that this drug is a useful tool for the study of their central role. AT2 receptor activity inhibition up-regulates AT1 receptor expression in specific brain areas. Blockade of brain AT2 receptors is compatible with enhanced hypothalamic–pituitary–adrenal axis and decreased central sympathetic system activity.
Keywords: Brain Angiotensin II receptor, Central sympathetic system, Pituitary hormone, Locus coeruleus, Paraventricular nucleus, Inferior olive
1. Introduction
Brain Angiotensin II (Ang II) is a multitasking peptide controlling central sympathetic system activity, pituitary hormone formation and release, stress and cerebrovascular flow (Saavedra, 2005). There are two Ang II receptor types, the AT1 and AT2 receptors (de Gasparo et al., 2000). AT1 receptors predominate in the adult rodent brain, and their expression is high in areas involved in autonomic, hormone, cerebrovascular, and behavior regulation (Tsutsumi and Saavedra, 1991a; Rowe et al., 1992; Lenkei et al., 1998). In particular, AT1 receptors are the only Ang II receptor type expressed in brain areas regulating pituitary hormone formation and release (Tsutsumi and Saavedra, 1991b). For this reason, and because the effects of centrally-administered Ang II were prevented by peripherally administered AT1 receptor blockers (Toney and Porter, 1993; Seltzer et al., 2004), the effects of Ang II in the brain are considered as a consequence of AT1 receptor stimulation (McKinley et al., 1996; de Gasparo et al., 2000).
On the other hand, the function of brain AT2 receptors remains elusive. AT2 receptors are very highly expressed in the immature rodent brain and dramatically decrease in number during the first two weeks of postnatal life, an indication of a still undetermined role in brain development (Tsutsumi and Saavedra, 1991a; Millan et al., 1991). In the adult rat brain, AT2 receptors remain significantly expressed in sensory and motor areas (Tsutsumi and Saavedra, 1991a; Lenkei et al., 1996). The high number of AT2 receptors in the locus coeruleus of the rat suggests a role in the regulation of central norepinephrine activity (Tsutsumi and Saavedra, 1991a; Lenkei et al., 1996). It has also been reported that the AT2 receptor modulates neuronal differentiation and nerve degeneration, protecting against neuronal injury during ischemia, and regulates water intake and vasopressin (AVP) release (Hogarty et al., 1992; Li et al., 2003, 2005). These results, however, are still controversial (de Gasparo and Siragy 1999; Jung et al., 2007).
The overall effect of Ang II was originally proposed to be the result of a balance or crosstalk between AT1 and AT2 receptor function (Gelband et al., 1997). The original “cross talk” hypothesis was based on results obtained with cell culture studies. The hypothesis suggested an intracellular interaction between AT1 and AT2 receptors regulating opposing signal transduction mechanisms, and that the balance between AT1 and AT2 activity was important to ensure intracellular homeostasis upon stimulation by Ang II (Gelband et al., 1997; Inagami et al. 1999). AT1 and AT2 receptors were also considered to stimulate distinct, but converging intracellular pathways with some responses simultaneously mediated by both receptor types (Bleuel et al. 1995).
In the brain, however, most if not all AT1 and AT2 receptors are expressed in different brain nuclei and in different neurons (Tsutsumi and Saavedra 1991a; Jöhren et al. 1995; Lenkei et al., 1996, 1998). The selective neuronal localization of brain AT1 and AT2 receptors makes the universality of the intracellular “cross-talk” hypothesis highly unlikely. Nevertheless, it appears that the expression of AT1 and AT2 receptors in the brain is reciprocally regulated, because AT1 receptor blockade enhances AT2 receptor expression in selected areas (Seltzer et al., 2004; Zhou et al., 2006; Bregonzio et al., 2008). In addition, AT2 receptor −/− mice express higher numbers of AT1 receptors in the hypothalamic paraventricular nucleus (PVN) and subfornical organ (SFO), leading to hypothalamic–pituitary–adrenal (HPA) axis hyperactivity (Armando et al., 2002).
Functional studies on brain AT2 receptors have been hampered by the limited number of selective ligands (de Gasparo et al., 2000) and by questionable characterization and specificity of AT2 receptor antibodies (Reagan et al., 1994; Yiu et al., 1997). Experimental studies on the effects of AT2 receptor agonists and antagonists directly injected into the brain yielded controversial results (Toney and Porter, 1993; Li et al., 2003; Saad et al., 2005; Kerr et al., 2005). There are few reports of central effects of peripherally administered AT2 agonists or antagonists (Li et al., 2005), and there is no direct evidence that these compounds actually enter the brain, decreasing binding to central AT2 receptors. For this purpose, we decided to determine to what extent the non-peptidic AT2 receptor antagonist PD123319, administered peripherally, can cross the blood brain barrier to inhibit central AT2 receptors. Because of the proposed mutual influence in brain binding expression between AT1 and AT2 receptors, we studied the effects of PD123319 on AT1 receptor expression in selected areas expressing AT1 receptors. The locus coeruleus (LC) is a very important site for expression and transcription of the catecholamine rate-limiting enzyme tyrosine hydroxylase (TH), and TH transcription in this area regulates the production of forebrain norepinephrine (Sabban and Serova, 2007). In the rat, the LC expresses large numbers of AT2 receptors (Tsutsumi and Saavedra, 1991a,b), indicating that this receptor type may be involved in central norepinephrine function. For this reason we studied TH mRNA expression in the locus coeruleus after PD123319 administration. AT2−/− mice showed increased PVN AT1 receptor expression and signs of enhanced HPA axis activation. Consequently we studied levels of pituitary AVP and adrenocorticotropic hormone (ACTH), hormones partially regulated by AT1 receptor activity (Armando et al., 2001).
2. Results
2.1. Peripheral administration of an AT2 receptor antagonist decreases AT2 binding in the brain
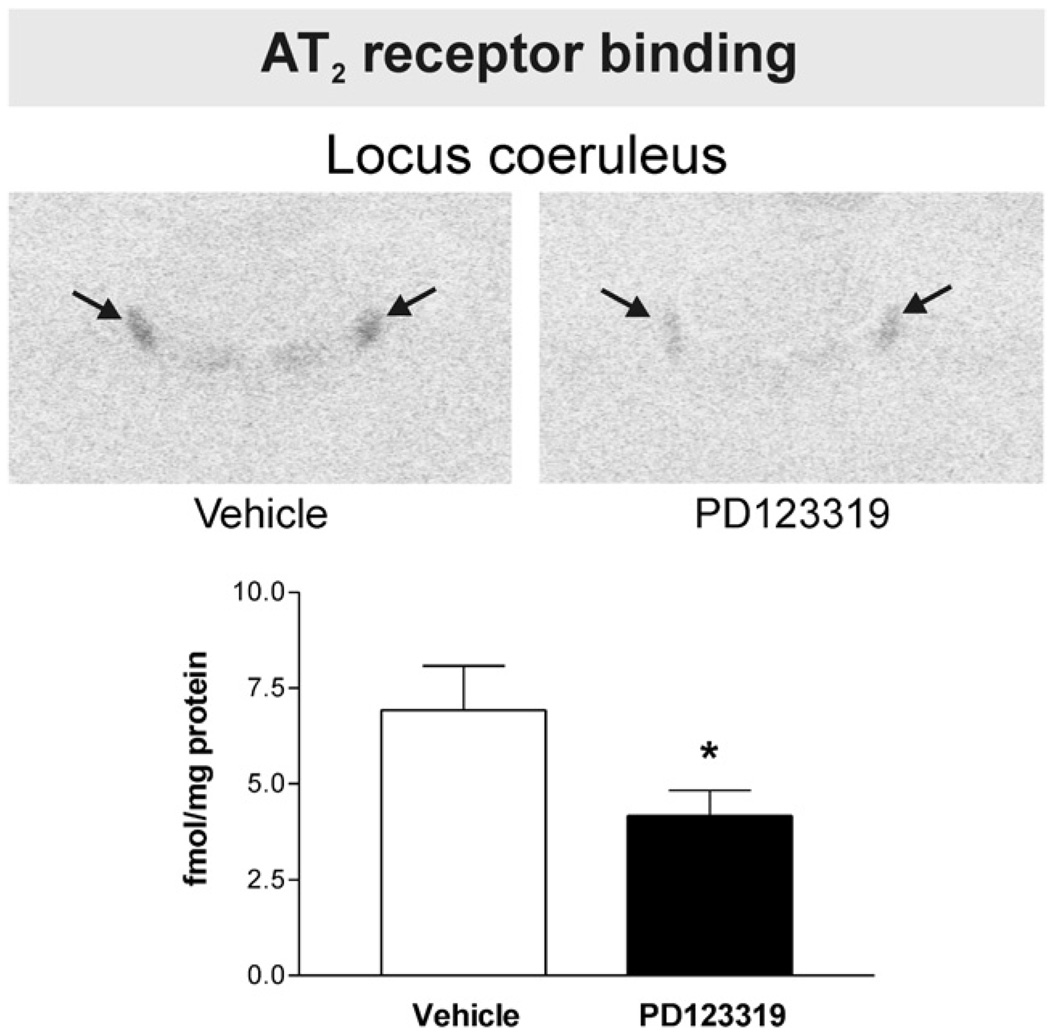
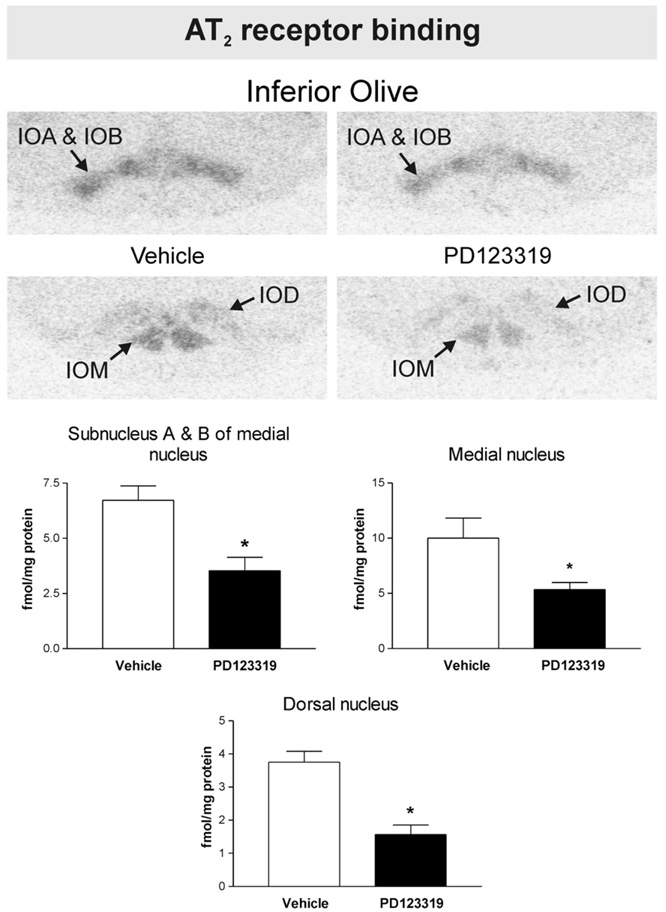
We detected AT2 binding in the LC, the inferior olivary complex and the lateral septal nucleus of vehicle-treated rats. No AT2 binding was expressed in the SFO, PVN, median eminence (ME), piriform cortex, basolateral amygdaloid nucleus or in the nucleus of the solitary tract and area postrema. Peripheral administration of PD123319 significantly decreased AT2 receptor binding in the LC by about 40% (Fig. 1) and in the olivary complex (dorsal and medial nuclei and subnucleus A and B of the medial nucleus) between 50 and 60% (Fig. 2). No significant changes in AT2 binding were noted in the lateral septal nucleus (0.7±0.2 and 0.6±0.3 fmol/mg protein for vehicle-treated and PD123319-treated, respectively, P>0.05 for groups of six to eight animals).
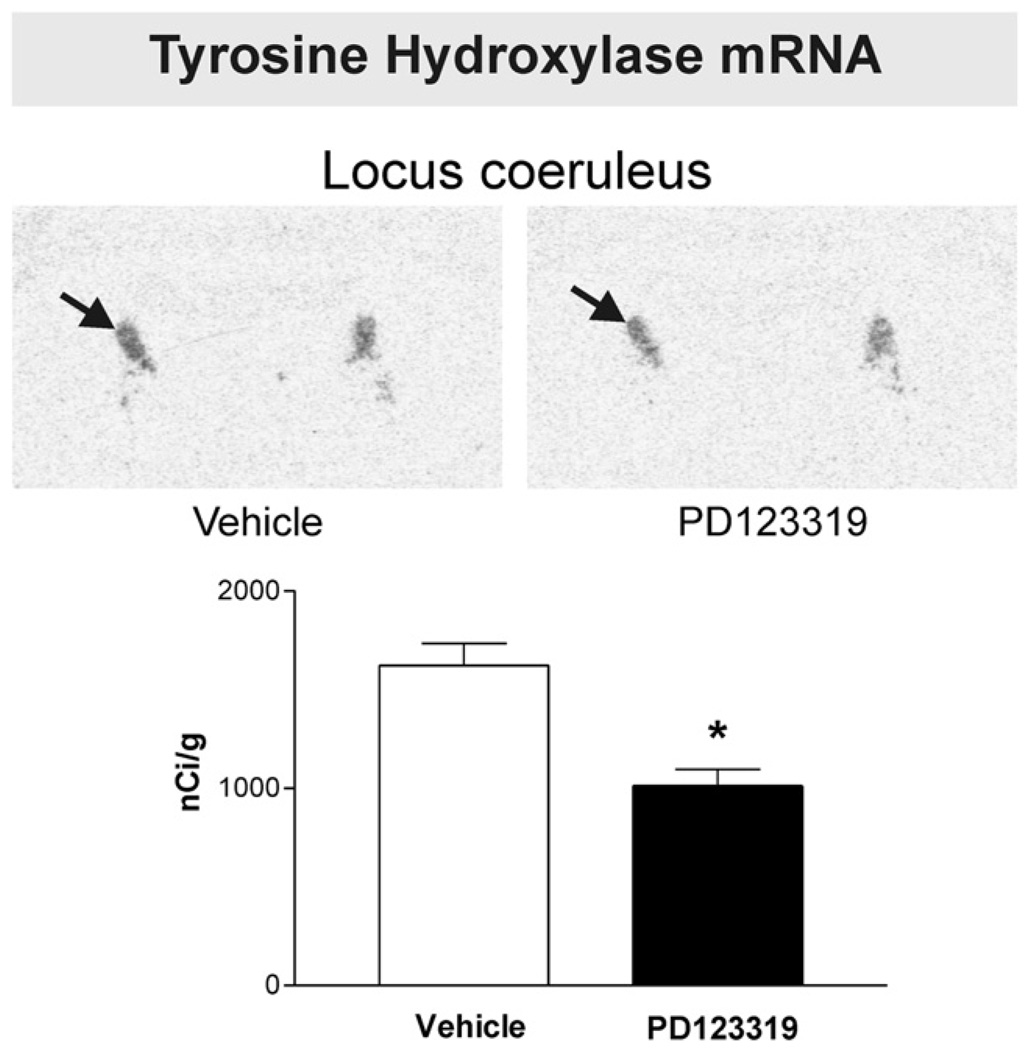
Fig. 1.
PD123319 decreases brain Angiotensin II AT2 receptor binding in the locus coeruleus. Figures are from images obtained from brain sections including the locus coeruleus after peripheral administration of vehicle or PD123319. Figures represent in vitro binding to AT2 receptors as described under Experimental procedures. Arrows point to the locus coeruleus. Note decreased AT2 binding in the locus coeruleus after PD123319 administration, when compared with vehicle-treated control. Bars aremeans±S.E.M. of groups of six to eight animals measured individually. *P<0.05 vs. vehicle treated rats.
Fig. 2.
PD123319 decreases brain Angiotensin II AT2 receptor binding in the inferior olive. Figures are from images obtained from brain sections including the inferior olive after peripheral administration of vehicle or PD123319. Figures represent in vitro binding to AT2 receptors as described under Experimental procedures. Arrows point to the inferior olive. IOM: inferior olive, medial nucleus. IOD: inferior olive, dorsal nucleus. IOA: inferior olive, subnucleus A of medial nucleus. IOB: inferior olive, subnucleus B of medial nucleus. Note decreased AT2 binding in the inferior olive, subnucleus A and B, in the medial and in the dorsal inferior olive nuclei after PD123319 administration, when compared with vehicle-treated control. Bars are means±S.E.M. of groups of six to eight animals measured individually. *P<0.05 vs. vehicle treated rats.
2.2. Peripheral administration of an AT2 receptor antagonist increases AT1 binding and AT1A mRNA expression in specific brain nuclei
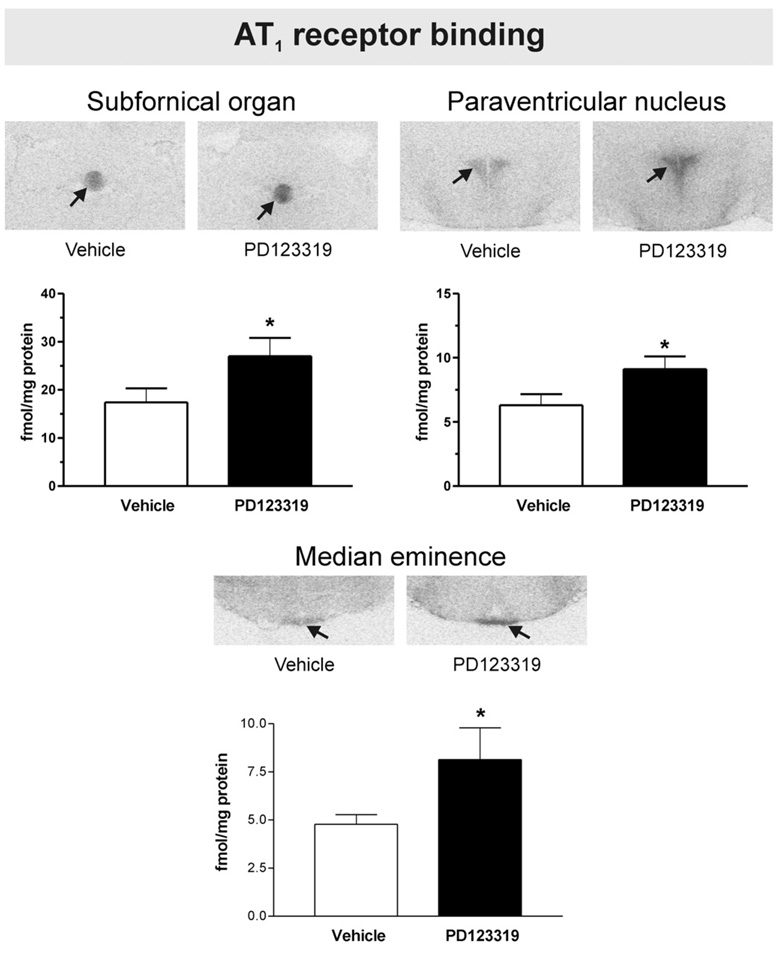
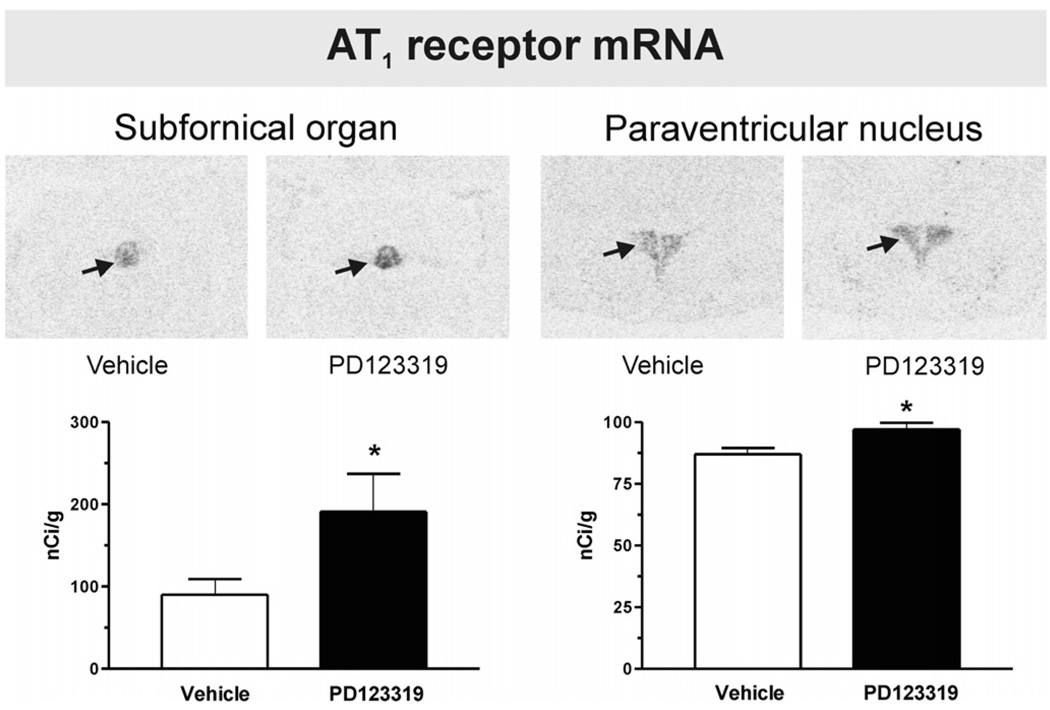
We found AT1 binding in the SFO, PVN, ME, piriform cortex, basolateral amygdaloid nucleus, the nucleus of the solitary tract and the area postrema. No AT1 binding was found in the lateral septal nucleus, the LC or the inferior olivary complex. Peripheral administration of PD123319 significantly increased the AT1 receptor expression by 40 to 60% in the SFO, the PVN and the ME (Fig. 3). In addition, PD123319 increased AT1A mRNA expression in both the SFO and the PVN (Fig. 4).
Fig. 3.
PD123319 selectively increases brain Angiotensin II AT1 receptor binding in the subfornical organ, hypothalamic paraventricular nucleus and median eminence. Figures are from images obtained from brain sections including the SFO, PVN and ME after peripheral administration of vehicle or PD123319. Figures represent in vitro binding to AT1 receptors as described under Experimental procedures. Arrows point to the SFO, PVN and ME. Note increased AT1 binding in the SFO, PVN and ME after PD123319 administration, when compared with vehicle-treated control. Bars are means±S.E.M. of groups of six to eight animals measured individually. *P<0.05 vs. vehicle treated rats.
Fig. 4.
PD123319 increases Angiotensin II AT1A receptor mRNA expression in the paraventricular nucleus and the subfornical organ. Figures are from images obtained from brain sections including the SFO and PVN after peripheral administration of vehicle or PD123319. Figures represent AT1A receptor mRNA expression as described under Experimental procedures. Arrows point to the SFO and PVN. Note increased AT1A mRNA expression in the SFO and PVN after PD123319 administration, when compared with vehicle-treated control. Bars are means±S.E.M. of groups of six to eight animals, assayed individually. *P<0.05 vs. vehicle-treated group.
Conversely, no significant changes in AT1 receptor expression after PD123319 administration were noted in the piriform cortex, the basolateral amygdaloid nucleus, the nucleus of the solitary tract or the area postrema. Values were 8.2±2.1 and 9.1±2.3 for the piriform cortex, 2.4±0.7 and 1.8±0.2 for the basolateral amygdaloid nucleus, 11.0±3.0 and 16.4±3.5 for the nucleus of the solitary tract, and 12.9±2.4 and 13.8±2.4 for the area postrema, fmol/mg protein for vehicle-treated and PD123319-treated, respectively, P>0.05, for groups of 6 to 8 animals.
2.3. Peripheral administration of an AT2 receptor antagonist decreased tyrosine hydroxylase mRNA expression in the locus coeruleus
PD123319 administration produced a significant decrease in TH mRNA expression in the locus coeruleus (Fig. 5).
Fig. 5.
PD123319 decreases tyrosine hydroxylase mRNA expression in the locus coeruleus. Figures are from images obtained from brain sections including the locus coeruleus after peripheral administration of vehicle or PD123319. Arrows point to the locus coeruleus. Note the significant decrease in tyrosine hydroxylase mRNA after treatment with the AT2 receptor antagonist. Bars are means±S.E.M. of groups of six to eight animals, assayed individually. *P<0.05 vs. vehicle-treated group.
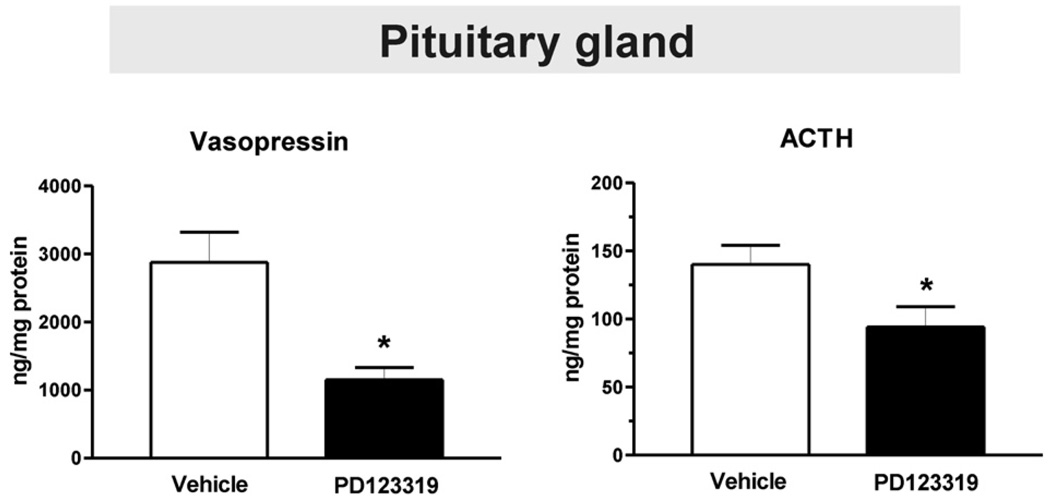
2.4. Peripheral administration of an AT2 receptor antagonist decreases pituitary ACTH and vasopressin content
Treatment with PD123319 resulted in a pronounced decrease in the content of pituitary ACTH and AVP (Fig. 6).
Fig. 6.
PD123319 decreases pituitary adrenocorticotropic hormone and vasopressin content. Bars are means±S.E.M. of groups of six to eight animals, assayed individually. *P<0.05 vs. vehicle-treated rats.
3. Discussion
In an effort to further clarify the role of brain Ang II AT2 receptors, we determined to what extent sustained administration of an AT2 receptor antagonist blocked central AT2 receptor binding. Ligand selectivity for AT1 and AT2 receptors is relative, because very high concentration of AT1 or AT2 receptor antagonists can displace AT2 or AT1 receptor binding, respectively. We chose PD123319, an effective and widely used AT2 receptor antagonist, administered peripherally at a dose of 1 mg/kg/day, significantly decreasing AT2 receptor binding in peripheral organs without interference with AT1 receptor binding (Jezova et al., 2003). Similar doses of PD123319 have been used by other investigators to test the role of AT2 receptors in vivo (Nagai et al., 2007; Lukkarinen et al., 2005). There are no literature reports suggesting that PD123319 exerts important effects in addition to its AT2 receptor-blocking effects. Peripheral administration was selected to avoid artifacts resulting from infusion of large doses of PD123319 into the brain. For our initial experiments, we prolonged PD123319 treatment for two weeks to determine the effects of sustained receptor blockade beyond the initial acute response. We also studied the effect of the AT2 receptor antagonist on AT1 receptor transcription and expression, because absence of AT2 receptor function in AT2−/− mice is accompanied by enhanced AT1 receptor expression in selected brain areas (Armando et al., 2002).
We selected brain areas with a well-characterized receptor presence, AT1 receptors in hypothalamic and brain stem nuclei, and AT2 receptors in the inferior olive, LC and septal nuclei (Tsutsumi and Saavedra, 1991a). We found significant AT2 receptor blockade in all areas of the inferior olivary complex and in the LC, of amagnitude similar to that observed for peripheral AT2 receptors (Jezova et al., 2003), indicating that this compound crosses the blood brain barrier and may be used as a tool for the study of the regulation and role of brain AT2 receptors. Binding to AT2 receptors was not significantly decreased only in the lateral septal nucleus, suggesting that some brain areas where AT2 binding is low may be more resistant to PD123319 blockade. Since PD123319 does not bind tightly to AT2 receptors some of the compound may be washed out during the incubation procedure. For this reason, the reported degree of reduction in receptor binding may underestimate the actual proportion of receptors occupied in vivo. Additionally, chronic drug treatment can decrease receptor binding without actually blocking the receptor, by affecting receptor transcription and translation. However, we have found that a similar treatment with the AT2 receptor antagonist did not modify the expression of AT2 receptor protein in the adrenal gland (Jezova et al., 2003). Nevertheless, we considered that receptor inhibition sustained for two weeks might be sufficient to determine the consequences of this partial, but significant AT2 receptor blockade.
In the SFO and PVN, AT1 receptors are of the AT1A receptor subtype (Leong et al., 2002). A partial inhibition of AT2 receptor binding, interpreted as a possible decrease in receptor activation, significantly up-regulated AT1A receptor transcription and AT1 receptor expression in the SFO and PVN, and AT1 receptor expression in the ME. There were no significant changes in AT1 receptor expression in brain stem areas indicated that the influence of AT2 receptor activation was anatomically selective. Our observations are in line with previous reports of enhanced AT1 receptor expression in the PVN, ME, kidney and adrenal gland of animals with life-long absence of AT2 receptor function, the AT2−/− mice (Armando et al., 2002; Saavedra et al., 2001a,b).We found up-regulation of AT1 receptor expression in two different rodent models, the AT2−/− mice where the receptor activity does not develop (Armando et al., 2002, Saavedra et al., 2001a,b), and adult rats with pharmacological AT2 receptor blockade (present results). In addition, in normotensive adult rats, knock down of AT2 receptors in peripheral tissues and in brain increases blood pressure, indirectly suggesting enhanced AT1 receptor activation (Wang et al., 2004).
The conclusion is that AT2 receptor activity appears to limit AT1 receptor expression, since either decreased or absent AT2 receptor function increases the number of AT1 receptors in some brain areas and peripheral tissues. Conversely, in normal rats, overexpression of AT2 receptors in the rostral ventrolateral medulla decreased blood pressure and urinary norepinephrine secretion (Gao et al., 2008), a finding compatible with decreased AT1 receptor function. Additionally, there is indirect evidence that over expressing AT2 receptors in the heart decreases AT1 receptor activity (Masaki et al., 1998).
The enhanced AT1 receptor number has consequences expressed in the mouse AT2−/− phenotype and in the response of adult rats to pharmacological AT2 receptor blockade. AT2−/− mice exhibit an exaggerated HPA axis activity with increased ACTH and corticosterone release (Armando et al., 2002), adrenomedullary activity (Armando et al., 2002), AVP secretion (Li et al., 2003), and increased locomotion and anxiety (Ichiki et al., 1995; Okuyama et al., 1999). This phenotype may be best interpreted as an indirect consequence of AT1 receptor up-regulation. Similarly, we may interpret our current findings of decreased ACTH and AVP pituitary stores after AT2 receptor blockade not as a direct result of decreased AT2 receptor function, because AT2 receptors are not expressed in brain areas directly controlling the HPA axis or in the pituitary gland (Tsutsumi and Saavedra, 1991b). In addition, AT1, but not AT2, receptors are a major factor controlling pituitary ACTH and AVP formation and release (Becú-Villalobos et al., 1994; Hogarty et al., 1992). For these reasons the most logical explanation is that increased hormone secretion and utilization is an indirect consequence of increased AT1 receptor function in the SFO, PVN and ME, leading to enhanced HPA axis activity. AT2−/− mice exhibit enhanced blood pressure increase as a result of peripheral or central administration of Ang II (Li et al., 2003), indication of increased AT1 receptor activation. However, administration of PD123319 to normal animals and to humans does not significantly affect blood pressure (Welch et al., 2007; Chappellaz and Smith, 2007; Brillante et al., 2005; Duke et al., 2005). This suggests a differential influence of AT2 receptor activity on blood pressure control when comparing the mouse AT2−/− model and pharmacological blockade of AT2 receptors in adult subjects.
There is a reciprocal influence between Ang II receptor types and limitations in AT1 receptor activity enhance AT2 receptor expression in brain and peripheral tissues. For example, AT1 receptor blockers increase AT2 binding in the LC, inferior olive and cerebral microvessels and prevent the isolation-stress AT2 decrease in expression in LC and inferior olive (Seltzer et al., 2004; Zhou et al., 2006; Bregonzio et al., 2008), adipocytes (Zorad et al., 2006) and in the muscular gastric layer during cold-restraint stress (Bregonzio et al., 2003). Because AT1 receptor blockade enhances renin production and release, increasing Ang II levels, their use is expected to result in over-stimulation of AT2 receptors.
Our observations and those of the literature confirm the hypothesis of an interaction between AT1 and AT2 receptors. We have found no evidence of same cell AT1 and AT2 receptor co-localization in the brain. In addition, the reciprocal influence between AT1 and AT2 receptor activity appears to be anatomically selective, with receptor expression modulated in some brain areas but not in others. The conclusion is that the reciprocal AT1/AT2 receptor influence may be indirect and dependent on differential regulatory mechanisms, since one receptor changes in one area and the other in an anatomically distinct and distant location.
A good example of such indirect interaction is the role of AT1 and AT2 receptors on central norepinephrine formation. TH transcription in the locus coeruleus is a major factor in the regulation of central norepinephrine activity, and increase in TH transcription is an important part of the brain reaction to stress (Sabban and Serova, 2007). It is well-established that brain Ang II contributes to regulate brain norepinephrine activity through AT1 receptor stimulation (Toney and Porter, 1993). Intracerebral Ang II injection, isolation stress and cold-restraint stress increase TH transcription in the LC, effects prevented by AT1 receptor blockade (Seltzer et al., 2004; Saavedra et al., 2006; Bregonzio et al., 2008). However, the rat LC expresses only AT2 receptors (Tsutsumi and Saavedra, 1991a), and we demonstrate here that AT2 receptor blockade in the LC decreases TH transcription in this area. We may conclude from our results that AT2 receptor activity is also important to maintain TH transcription in the LC, and therefore to achieve a normal level of central norepinephrine activation. On the other hand, AT1 receptor blockade also decreases TH transcription while increasing AT2 receptor expression in the LC (Seltzer et al., 2004), isolation stress enhances TH transcription while decreasing AT2 receptor expression in the LC (Saavedra et al., 2006), and cold-restraint stress increases TH transcription without modification in AT2 receptor expression (Bregonzio et al., 2008). Thus, the level of TH transcription does not necessarily correlate with the degree of AT2 receptor expression. Consideration of indirect mechanisms is necessary to explain this apparent contradiction. TH transcription in the LC is controlled by multiple systems, including direct corticotrophin-releasing hormone (CRH) projections originated in the PVN and distinct from those regulating the HPA axis (Reyes et al., 2005). AT1 receptor blockade prevents the extra-hypothalamic stimulation of the CRH system during stress (Saavedra et al., 2006). We may speculate that PVN CRH projections to the LC stimulate TH transcription, and that these projections are under AT1 receptor control. Stress increases PVN AT1 receptor function, resulting in enhanced TH transcription in the LC, effects prevented by AT1 receptor blockade. In this case, increased AT2 receptor expression in the LC resulting from AT1 receptor blockade may be considered a compensatory mechanism to maintain TH activity. When the AT2 receptors in LC are blocked, as reported here, TH transcription decreases in spite of enhanced PVN AT1 receptor expression. We may conclude that TH transcription in the LC is under the indirect influence of the activity of both Ang II receptor types, and that AT1 and AT2 receptors may act in synchrony through similar, and not opposing, mechanisms. A similar effect was noted in the adrenal medulla, where TH transcription, previously considered exclusively under AT1 receptor control (Wong et al., 1990), has been shown to be decreased to a similar extent by either AT1 or AT2 receptor antagonists (Jezova et al., 2003).
In conclusion, our results demonstrate that brain AT2 receptor binding can be decreased by peripheral administration of an AT2 receptor antagonist, selectively enhancing AT1 receptor transcription, stimulating the HPA axis, and decreasing central norepinephrine activity. Revised, the AT1/AT2 receptor cross-talk hypothesis may be formulated as a series of differentinter-cellular relationships, with receptor activities sometimes in opposition and sometimes in synergy, regulated in a complex, selective and indirect series of mechanisms.
4. Experimental procedures
4.1. Animals and tissue preparation
Wistar Hanover male rats (8 wk old) were purchased from Taconic Farms, Inc. (Germantown, NY), were kept at 22 °C under a 12-h dark, 12-h light cycle with lights on at 07.00 h and were given free access to normal rat diet and tap water. The National Institute of Mental Health (NIMH) Animal Care and Use Committee approved all procedures. Rats were anesthetized with pentobarbital (30 mg/kg) and Alzet osmotic minipumps (Alza Scientific Products, Palo Alto, CA) were implanted subcutaneously. Groups of eight animals (treated) received minipumps containing vehicle or the AT2 receptor antagonist PD123319 (Sigma-Aldrich Corp., St. Louis, MO; 1 mg/kg/day), respectively. Since the half-life of PD123319 is short (Levy et al., 1996), we chose subcutaneous administration for two weeks to provide continuous receptor blockade (Jezova et al., 2003). After minipump implantation the rats were kept in their cages in groups of three to four under standard conditions with regular rat food and water ad libitum for 14 days. At the end of the experiment the animals were killed by decapitation and the brains and pituitary glands were removed, frozen in isopentane at −30 °C on dry ice, and stored at −80 °C until used.
Consecutive, 16-µmthick coronal brain sections were cut at −20 °C in a cryostat. Alternate brain sections were collected for receptor binding and in situ hybridization. For receptor autoradiography, sections were thaw-mounted on gelatin-coated glass slides, dried overnight in a desiccator at 4 °C, and stored at −80 °C. For in situ hybridization, sections were thaw-mounted on silanated glass slides (Digene Diagnostics, Beltsville, MD), dried at 50 °C for 5 min on a slide warmer and stored at −80 °C until use. For anatomical localization of Ang II receptor binding or mRNA, sections were stained with Toluidine Blue, and brain regions were identified and designated according to a rat brain atlas (Paxinos and Watson, 1986). Each animal was quantified independently, and four sections per brain region were studied for each animal and for each procedure.
4.2. Quantitative autoradiography of Angiotensin II AT1 and AT2 receptors
Brain coronal sections containing the SFO, the PVN, the ME, the piriform cortex, the basolateral amygdaloid nucleus, LC, the nucleus of the solitary tract, the area postrema and the inferior olivary complex were preincubated for 15 min at 22 °C in 10 mM Na phosphate buffer, pH 7.4, containing 120 mM NaCl, 5 mM Na2EDTA, 0.005% bacitracin (Sigma Chemical, St. Louis, MO), and 0.2% proteinase-free bovine serum albumin (Sigma Chemical), followed by incubation for 120 min in fresh buffer containing 0.5 nM of [125I]Sarcosine1–Ang II ([125I]Sar1–Ang II, Peninsula Laboratories, Belmont, CA; iodinated by the Peptide Radioiodination Service Center, School of Pharmacy, University of Mississippi, to a specific activity of 2176 Ci/mmol). We determined total binding by incubating the sections as described above (Tsutsumi and Saavedra, 1991a). Nonspecific binding was determined in consecutive sections incubated as above in the presence of 1 µM unlabeled Ang II (Peninsula), and was defined as the binding remaining in the presence of excess unlabeled agonist. To determine selective binding to the Ang II AT1 and AT2 receptors, we incubated consecutive sections with 0.5 nM of [125I]Sar1–Ang II in the presence of 10 µM of the selective AT1 receptor antagonist losartan (DuPont-Merck, Wilmington, DE, USA) or 10 µM of the selective AT2 receptor antagonist PD123319 (Sigma Aldrich), respectively, to give maximum specific displacement. The number of AT1 and AT2 receptors was defined as the binding displaced by the AT1 and AT2 receptor antagonists, respectively (Tsutsumi and Saavedra, 1991a).
After incubation, slides were rinsed four consecutive times, for 1 min each, in fresh ice-cold 50 mM Tris–(hydroxymethyl) aminomethane) HCl buffer, pH 7.6, followed by a dip in ice-cold distilled water, and the sections were dried under air. Sections were exposed to Kodak Biomax MR film (Eastman Kodak Company, Rochester, NY) together with 14C-labeled microscales (American Radiolabeled Chemicals, St Louis, MO). Films were developed in ice-cold GBX developer (Eastman Kodak) for 4 min, fixed in Kodak GBX fixer for 4 min at 22 °C, and rinsed in water for 15 min. Optical densities of auto-radiograms generated by incubation with the 125I-labeled ligands were quantified by computerized densitometry using the Image 1.6 Program (National Institute of Mental Health, Bethesda, MD) after calibration with 14C-labeled standards as described (Miller and Zahniser, 1987). The films were exposed for different times to obtain film images within the linear portion of the standard curve and transformed to corresponding values of fmol per mg protein (Nazarali et al., 1989 and Miller and Zahniser, 1987).
4.3. In situ hybridization histochemistry
To obtain rat AT1A receptor-specific riboprobes, partial fragments of full-length cDNA were subcloned into the polylinker site of the pBluescript KS (+) vector (Stratagene, La Jolla, Calif., USA) as described (Jöhren et al., 1995). The rAT1A cDNA was restricted with HaeIII. The restriction fragment of 368 bp (from nt 1317 through 1684) was prepared and ligated into the EcoRV site of the vector. The fragment corresponded to the 3′-untranslated region (3′-UTR) of the gene with a 35-bp ORF region that did not show significant homology with rAT1B cDNA ORF. The subclone, named rAT1A-S2, was confirmed by DNA sequencing.
For in vitro transcription of the 35S-labeled antisense and sense (control) riboprobes, the subclones were linearized with HindIII or EcoRI for the rAT1A-S2 and then they were treated with T7 or T3 RNA polymerase. In vitro transcription was performed using the RNA transcription kit (Pharmacia Biotech, Piscataway, NJ) as described (Jöhren et al., 1995).The quality of riboprobes was monitored with liquid scintillation counting, polyacrylamide gel electrophoresis by a preliminary experiment using adrenal gland as a positive control and subsequent autoradiography (Jöhren et al., 1995).
To perform in situ hybridization histochemistry, sections were fixed for 10 min in 4% paraformaldehyde, and rinsed twice in phosphate-buffered saline. After acetylation in 0.1 M triethanolamine HCl, pH 8.0, containing 0.25% acetic anhydride for 10 min, sections were dehydrated through graded ethanol and air-dried. In situ hybridization was performed as described earlier (Jöhren et al., 1995). Each section was covered with 150 µl hybridization buffer [containing 50% formamide, 0.3 M NaCl, 2 mM EDTA, 20 mM tris(hydroxymethyl)aminomethane (Tris), pH 8.0, 1× Denhardt’s solution, 10% dextran sulfate, 100 pg/ml salmon sperm DNA, 250 pg/ml yeast RNA, 250 pg/ml yeast tRNA, 100 mM dithiothreitol, 0.1% sodium dodecyl sulfate, and 2×l07 cpm/ml labeled sense or antisense probes] applied to each slide, and sections were incubated for 16–18 h at 54 °C. After hybridization, sections were rinsed several times in 4× standard saline citrate (SSC) buffer to remove coverslips and excess riboprobes (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate). Nonhybridized riboprobes were digested by incubation with 40 pg/ml ribonuclease A (Sigma Chemical, St. Louis, MO) for 30 min at room temperature. After a final high-stringency wash in 0.1× SSC at 65 °C for 60 min, sections were dehydrated in graded ethanol containing 0.3 M ammonium acetate and air-dried. Sections were exposed to Hyperfilm-3H (Amersham, Arlington Heights, IL) for 14 days. Films were developed in D-19 developer (Eastman Kodak, Rochester, NY) for 4 min at 0 °C and fixed in Kodak rapid fixer for 4 min at 22 °C. The intensities of hybridization signals were quantified as nCi/g tissue equivalent (Wisden and Morris, 1994) by measuring optical film densities using the NIH Image 1.61 program after calibration with the [14C] micro-scales. Sense probes were considered as control to determine nonspecific hybridization.
4.4. In situ hybridization of tyrosine hydroxylase mRNA
One antisense (TH-AS) oligonucleotide of 48-mer for the rat TH cDNA sequence (Grima et al., 1985), localized in nt 1562–1609, synthesized by Lofstrand Labs Ltd (Gaithersburg, Md., USA) was labeled with a 3′-end labeling kit (Amersham) using terminal deoxynucleotidyl transferase to a specific activity of 3–4×108 dpm/µg. Each reaction was performed with 70 pmol of oligonucleotides in the presence of 70 µCi of [35S] ATP (SJ 1334) (Amersham). The labeled oligonucleotides were separated from unincorporated nucleotides using MicroSpin G-25 columns (Amersham). In situ hybridization of rat LC sections and post-hybridization washings were performed as described (Wisden and Morris, 1994). In situ hybridization was performed in consecutive LC sections, one with the TH-AS antisense oligonucleotide and another with excess unlabelled TH-AS probe (157 pmol/ml). After the washing, sections were dehydrated in alcohol gradient containing 0.3 M ammonium acetate, air-dried and exposed to Hyperfilm-3H (Amersham). The films were developed and quantified as described above by comparison with 14C standards (American Radiolabeled Chemicals, Inc., St. Louis, Mo., USA).
4.5. Hormone determinations
Pituitary hormones were determined by RIA using commercially available kits [ICN Biomedicals, Inc. (Costa Mesa, CA) for ACTH and Phoenix Pharmaceuticals, Inc. (Belmont, CA), for pituitary vasopressin].
4.6. Statistics
Data are means±SEM, for groups of 6 to 8 animals measured individually. For quantitative autoradiography and in situ hybridization methods, values for 2 to 3 consecutive sections were averaged for each animal. We used Student’s t test to assess the significance of differences between groups. P<0.05 was considered statistically significant.
Acknowledgments
This study was supported by the Division of Intramural Research Programs, National Institute of Mental Health, Department of Health and Human Services, USA.
Abbreviations
- Ang II
Angiotensin II
- LC
locus coeruleus
- PVN
hypothalamic paraventricular nucleus
- SFO
subfornical organ
- ME
median eminence
- IOM
inferior olive, medial nucleus
- IOD
inferior olive, dorsal nucleus
- IOA
inferior olive, subnucleus A of medial nucleus
- IOB
inferior olive, subnucleus B of medial nucleus
- ACTH
Adrenocorticotropic hormone
- AVP
vasopressin
- TH
tyrosine hydroxylase
- HPA
axis, hypothalamic–pituitary–adrenal axis
- CRH
corticotropin-releasing hormone
REFERENCES
- Armando I, Carranza A, Nishimura Y, Hoe K-L, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral-administration of an angiotensin IIAT1 receptor antagonist decreases the hypothalamic–pituitary–adrenal response to isolation stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- Armando I, Terrón JA, Falcón-Neri A, Takeshi I, Häuser W, Inagami T, Saavedra JM. Increased angiotensin II AT(1) receptor expression in paraventricular nucleus and hypothalamic–pituitary–adrenal axis stimulation in AT(2) receptor gene disrupted mice. Neuroendocrinology. 2002;76:137–147. doi: 10.1159/000064525. [DOI] [PubMed] [Google Scholar]
- Becú-Villalobos D, Lacau-Mengido IM, Thyssen SM, Díaz-Torga GS, Libertun C. Effects of LHRH and ANG II on prolactin stimulation are mediated by hypophysial AT1 receptor subtype. Am. J. Physiol. 1994;266:E274–E278. doi: 10.1152/ajpendo.1994.266.2.E274. [DOI] [PubMed] [Google Scholar]
- Bleuel A, de Gasparo M, Whitebread S, Püttner I, Monard D. Regulation of protease nexin-1 expression in cultured Schwann cells is mediated by angiotensin II receptors. J. Neurosci. 1995;15:750–761. doi: 10.1523/JNEUROSCI.15-01-00750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am. J. Physiol.: Gasterointest. Liver Physiol. 2003;285:G414–G423. doi: 10.1152/ajpgi.00058.2003. [DOI] [PubMed] [Google Scholar]
- Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM. Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold-restraint stress-induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress. 2008 Jan Jun;13 doi: 10.1080/10253890801892040. [Electronic Publication ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillante DG, Johnstone MT, Howes LG. Effects of intravenous PD123319 on haemodynamic and arterial stiffness indices in healthy volunteers. J. Renin. Angiotensin. Aldosterone Syst. 2005;6:102–106. doi: 10.3317/jraas.2005.007. [DOI] [PubMed] [Google Scholar]
- Chappellaz ML, Smith FG. Systemic and renal hemodynamic effects of the AT1 receptor antagonist, ZD 7155, and the AT2 receptor antagonist, PD123319, in conscious lambs. Pflugers Arch. 2007;453:477–486. doi: 10.1007/s00424-006-0148-4. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Siragy HM. The AT2 receptor: fact, fancy and fantasy. Regul. Pept. 1999;81:11–24. doi: 10.1016/s0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Duke LM, Evans RG, Widdop RE. AT2 receptors contribute to acute blood pressure-lowering and vasodilator effects of AT1 receptor antagonism in conscious normotensive but not hypertensive rats. Am. J. Physiol, Heart Circ. Physiol. 2005;288:H2289–H2297. doi: 10.1152/ajpheart.01096.2004. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband CH, Zhu M, Lu D, Reagan LP, Fluharty SJ, Posner P, Raizada MK, Sumners C. Functional interactions between neuronal AT1 and AT2 receptors. Endocrinology. 1997;138:2195–2198. doi: 10.1210/endo.138.5.5236. [DOI] [PubMed] [Google Scholar]
- Grima B, Lamouroux A, Blanot F, Biguet NF, Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc. Natl. Acad. Sci. U. S. A. 1985;82:617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarty DC, Speakman EA, Puig V, Phillips MI. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res. 1992;586:289–294. doi: 10.1016/0006-8993(92)91638-u. [DOI] [PubMed] [Google Scholar]
- Inagami T, Eguchi S, Numaguchi K, Motley ED, Tang H, Matsumoto T, Yamakawa T. Cross-talk between angiotensin II receptors and the tyrosine kinases and phosphatases. J. Am. Soc. Nephrol. 1999 Suppl. 11:S57–S61. [PubMed] [Google Scholar]
- Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimur F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and explorative behavior of mice lacking Angiotensin II type 2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- Jezova M, Armando I, Bregonzio C, Yu ZX, Qian S, Ferrans VJ, Imboden H, Saavedra JM. Angiotensin II AT(1) and AT(2) receptors contribute to maintain basal adrenomedullary norepinephrine synthesis and tyrosine hydroxylase transcription. Endocrinology. 2003;144:2092–2101. doi: 10.1210/en.2002-0019. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Inagami T, Saavedra JM. AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. NeuroReport. 1995;15:2549–2552. doi: 10.1097/00001756-199512150-00024. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim SJ, Song EC, Kim EH, Park DK, Sinn DI, Kim JM, Kim M, Roh JK. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J. Pharmacol. Exp. Ther. 2007;322:1051–1058. doi: 10.1124/jpet.107.120097. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Bevilaqua LR, Bonini JS, Rossato JI, Köhler CA, Medina JH, Izquierdo I, Cammarota M. Angiotensin II blocks memory consolidation through an AT2 receptor-dependent mechanism. Psychopharmacology. 2005;179:529–535. doi: 10.1007/s00213-004-2074-5. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin II type-2 mRNA expression in the adult rat brain. J. Comp. Neurol. 1996;373:322–339. doi: 10.1002/(SICI)1096-9861(19960923)373:3<322::AID-CNE2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience. 1998;82:827–841. doi: 10.1016/s0306-4522(97)00328-x. [DOI] [PubMed] [Google Scholar]
- Leong DS, Terrón JA, Falcón-Neri A, Armando I, Ito T, Jöhren O, Tonelli LH, Hoe K-L, Saavedra JM. Restraint stress modulates brain, pituitary and adrenal expression of Angiotensin II AT1A, AT1B and AT2 receptors. Neuroendocrinology. 2002;75:227–240. doi: 10.1159/000054714. [DOI] [PubMed] [Google Scholar]
- Levy BI, Benessiano J, Henrion D, Caputo L, Heymes C, Duriez M, Poitevin P, Samuel JL. Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J. Clin. Invest. 1996;98:418–425. doi: 10.1172/JCI118807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Culman J, Hörtnagl H, Zhao Y, Gerova N, Timm M, Blume A, Zimmermann M, Seidel K, Dirnagl U, Unger T. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 2005;6:617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui T-X, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am. J. Physiol, Heart Circ. Physiol. 2003;284:H116–H121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- Lukkarinen HP, Laine J, Aho H, Zagariya A, Vidyasagar D, Kääpä PO. Angiotensin II receptor inhibition prevents pneumocyte apoptosis in surfactant-depleted rat lungs. Pediatr. Pulmonol. 2005;39:349–358. doi: 10.1002/ppul.20187. [DOI] [PubMed] [Google Scholar]
- Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J. Clin. Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin. Exp. Pharmacol. Physiol. 1996 Suppl. 3:S99–S104. [PubMed] [Google Scholar]
- Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc. Natl. Acad. Sci. U. S. A. 1991;88:11440–11444. doi: 10.1073/pnas.88.24.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Zahniser NR. The use of 14C-labelled tissue paste standards for the calibration of 125I-labelled ligands in quantitative autoradiography. Neurosci. Lett. 1987;81:345–350. doi: 10.1016/0304-3940(87)90408-3. [DOI] [PubMed] [Google Scholar]
- Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, Yamashiro K, Inoue M, Tsubota K, Umezawa K, Ishida S. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest. Ophthalmol. Vis. Sci. 2007;48:4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- Nazarali AJ, Gutkind JS, Saavedra JM. Calibration of [125I]-polymer standards with [125I]-brain paste standards for use in quantitative receptor autoradiography. J. Neurosci. Methods. 1989;30:247–253. doi: 10.1016/0165-0270(89)90135-0. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Sakagawa T, Chaki S, Imagawa Y, Ichiki T, Inagami T. Anxiety-like behavior in mice lacking the angiotensin II type-2 receptor. Brain Res. 1999;821:150–159. doi: 10.1016/s0006-8993(99)01098-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. pp. 1–262. [Google Scholar]
- Reagan LP, Flanagan-Cato LM, Yee DK, Ma LY, Sakai RR, Fluharty SJ. Immunohistochemical mapping of angiotensin type 2 (AT2) receptors in rat brain. Brain Res. 1994;662:45–59. doi: 10.1016/0006-8993(94)90794-3. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur. J. Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- Rowe BP, Saylor DL, Speth RC. Analysis of angiotensin II receptor subtypes in individual rat brain nuclei. Neuroendocrinology. 1992;55:563–573. doi: 10.1159/000126177. [DOI] [PubMed] [Google Scholar]
- Saad WA, Camargo LA, Antunes-Rodríguez J, Saad WA, Guarda IF, Guarda RS. Interaction between arginine vasopressin and angiotensin II receptors in the central regulation of sodium balance. Regul. Pept. 2005;132:53–58. doi: 10.1016/j.regpep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell. Mol. Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Häuser W, Ciuffo G, Egidy G, Hoe KL, Jöhren O, Sembonmatsu T, Inagami T, Armando I. Increased AT(1) receptor expression and mRNA in kidney glomeruli of AT(2) receptor gene-disrupted mice. Am. J. Physiol. Renal. Physiol. 2001a;280:F71–F78. doi: 10.1152/ajprenal.2001.280.1.F71. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Terrón JA, Falcón-Neri A, Jöhren O, Häuser W, Inagami T. Increased AT(1) receptors in adrenal gland of AT(2) receptor gene-disrupted mice. Regul. Pept. 2001b;102:41–47. doi: 10.1016/s0167-0115(01)00303-2. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sánchez-Lemus E. A centrally acting, anxiolytic Angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharm. 2006;31:1123–1134. doi: 10.1038/sj.npp.1300921. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Serova LI. Influence of prior experience with homotypic or heterotypic stressor on stress reactivity in catecholaminergic systems. Stress. 2007;10:137–143. doi: 10.1080/10253890701404078. [DOI] [PubMed] [Google Scholar]
- Seltzer A, Bregonzio C, Armando I, Baiardi G, Saavedra JM. Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res. 2004;1028:9–18. doi: 10.1016/j.brainres.2004.06.079. [DOI] [PubMed] [Google Scholar]
- Toney GM, Porter JP. Effects of blockade of AT1 and AT2 receptors in brain on the central angiotensin II pressor response in conscious spontaneously hypertensive rats. Neuropharmacology. 1993;32:581–592. doi: 10.1016/0028-3908(93)90054-7. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptors subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 1991a;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K, Saavedra JM. Angiotensin-II receptor subtypes in median eminence and basal forebrain areas involved in regulation of pituitary function. Endocrinology. 1991b;129:3001–3008. doi: 10.1210/endo-129-6-3001. [DOI] [PubMed] [Google Scholar]
- Wang H, Gallinat S, Li HW, Sumners C, Raizada MK, Katovich MJ. Elevated blood pressure in normotensive rats produced by ‘knockdown’ of the angiotensin type 2 receptor. Exp. Physiol. 2004;89:313–322. doi: 10.1113/expphysiol.2004.027359. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Patel K, Modlinger P, Mendonca M, Kawada N, Dennehy K, Aslam S, Wilcox CS. Roles of vasoconstrictor prostaglandins, COX-1 and -2, and AT1, AT2, and TP receptors in a rat model of early 2K,1C hypertension. Am. J. Physiol, Heart Circ. Physiol. 2007;293:H2644–H2649. doi: 10.1152/ajpheart.00748.2007. [DOI] [PubMed] [Google Scholar]
- Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. In Situ Hybridization Protocols for the Brain. Academic: San Diego; 1994. pp. 9–34. [Google Scholar]
- Wong PC, Hart SD, Zaspel AM, Chiu AT, Ardecky RJ, Smith RD, Timmermans PB. Functional studies of nonpeptide Angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2) J. Pharm. Exp. Ther. 1990;255:584–592. [PubMed] [Google Scholar]
- Yiu AK, Wong PF, Yeung SY, Lam SM, Luk SK, Cheung WT. Immunohistochemical localization of type-II (AT2) angiotensin receptors with a polyclonal antibody against a peptide from the C-terminal tail. Regul. Pept. 1997;70:15–21. doi: 10.1016/s0167-0115(97)00010-4. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, Nishioku T, Dou J, Delgiacco E, Saavedra JM. AT1 receptor blockade regulates the local Angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARγ. Eur. J. Pharm. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]