Abstract
Early in mitochondria-mediated apoptosis, the mitochondrial outer membrane becomes permeable to proteins that, when released into the cytosol, initiate the execution phase of apoptosis. Proteins in the Bcl-2 family regulate this permeabilization, but the molecular composition of the mitochondrial outer membrane pore is under debate. We reported previously that at physiologically relevant levels, ceramides form stable channels in mitochondrial outer membranes capable of passing the largest proteins known to exit mitochondria during apoptosis (Siskind, L. J., Kolesnick, R. N., and Colombini, M. (2006) Mitochondrion 6, 118–125). Here we show that Bcl-2 proteins are not required for ceramide to form protein-permeable channels in mitochondrial outer membranes. However, both recombinant human Bcl-xL and CED-9, the Caenorhabditis elegans Bcl-2 homologue, disassemble ceramide channels in the mitochondrial outer membranes of isolated mitochondria from rat liver and yeast. Importantly, Bcl-xL and CED-9 disassemble ceramide channels in the defined system of solvent-free planar phospholipid membranes. Thus, ceramide channel disassembly likely results from direct interaction with these anti-apoptotic proteins. Mutants of Bcl-xL act on ceramide channels as expected from their ability to be anti-apoptotic. Thus, ceramide channels may be one mechanism for releasing pro-apoptotic proteins from mitochondria during the induction phase of apoptosis.
Apoptosis is required for normal development and tissue homeostasis in multicellular organisms. Deregulation of apoptosis is fundamental to many diseases, such as cancer, stroke, heart disease, neurodegenerative disorders, autoimmune disorders, and viral diseases. During apoptosis, DNA fragments and other contents of the cell are packaged into apoptotic bodies that are consumed by phagocytosis. There are two main pathways for apoptosis, namely the extrinsic receptor-mediated pathway and an intrinsic mitochondria-mediated one. There is also cross-talk between these two pathways. The intrinsic pathway is initiated when one or more of a multitude of signals converge on mitochondria that ultimately result in an increase in the permeability of the mitochondrial outer membrane (MOM).2 This permeabilization leads to the release of intermembrane space proteins, including cytochrome c, procaspases, apoptosis-inducing factor, heat shock proteins, Smac/Diablo, and endonuclease G (1). In the cytosol, these proteins activate caspases and DNases that carry out the execution phase of apoptosis.
The mechanism for the increased permeability of the MOM during the induction phase of apoptosis is currently highly debated. Several mechanisms have been proposed to explain how mitochondrial intermembrane space proteins are released into the cytosol to facilitate apoptotic cell death. Some involve direct pore formation in the MOM. Several candidate pores exist, but most involve activated multidomain pro-apoptotic Bcl-2 family proteins, Bax and Bak (for example see Refs. 2–5). Another model for MOM permeabilization involves channels formed by ceramide; ceramide can self-associate in membranes to form extremely large channels (6–10 nm in diameter) able to pass proteins (6).
Ceramide (N-acylated sphingosine) is a sphingolipid found in membranes. Ceramide is generated by de novo synthesis, sphingomyelin hydrolysis, and recycling of sphingolipids. Ceramide is known to be involved in the regulation of several cellular processes, including differentiation, growth suppression, cell senescence, and apoptosis. Of these, the role of ceramide in mitochondria-mediated apoptosis has attracted much attention in recent years. Increases in cellular ceramide levels during apoptosis have been shown to occur prior to MOM permeabilization (7–12) indicating that it could be involved in initiating the permeabilization. Mitochondria enriched fractions contain enzymes responsible for ceramide synthesis and hydrolysis, namely ceramide synthase and ceramidase (13–15), and both mitochondrial outer and inner membranes have been shown in vitro to be capable of generating ceramide (15). Apoptosis induced by CD95, tumor necrosis factor-α, ionizing radiation, and ultraviolet radiation have all been shown to occur at least in part via an increase in ceramide levels in the mitochondrial fraction (16–20). In fact, inhibitors of sphingolipid metabolism that prevented ceramide synthesis after UV irradiation also prevented apoptosis (20).
MOM permeabilization and apoptosis occurred in MCF7 breast cancer cells when the bacterial sphingomyelinase protein was targeted to mitochondria and ceramide generated specifically in mitochondria but not when it was targeted to all other intracellular locations (21). Thus, ceramide-induced apoptosis occurs at least in part at the level of mitochondria.
Previous studies show that ceramide possesses the ability to form large protein-permeable channels in planar phospholipid as well as MOMs of isolated mitochondria (6, 22–25). Although channel formation by ceramide occurs in the MOM, it does not occur in the plasma membrane and thus is somehow influenced by the membrane environment (24). Remarkably, channel formation depends on the presence of the 4–5-trans double bond of the sphingoid base backbone of ceramide. Dihydroceramide, the precursor to ceramide in the de novo synthesis pathway, differs only from ceramide by lacking this double bond. This deficiency results in its inability to form channels (22, 23) and an inability to induce apoptosis (26). Thus, the ceramides that can induce apoptosis are also those that can form channels.
Ceramide-induced permeabilization of the MOM shows the characteristics of an organized channel (23, 24). As expected for a channel, ceramide allows the bi-directional flux of cytochrome c across the MOM and not just its release (23). Ceramide, when added to isolated mitochondrial suspensions, allows the release of other intermembrane space proteins, such as adenylate kinase (23). In addition, unlike a detergent-like effect, there is a cutoff for the size of the mitochondrial intermembrane space proteins that are released through ceramide channels. This cutoff under the denaturing conditions of SDS-PAGE is about 60 kDa (23), but preliminary data indicate that it may be higher under nondenaturing conditions. 3 Unlike detergents, which irreversibly dissolve membranes, depletion of ceramide from the MOM restores its permeability barrier (23, 24). Studies with C16-ceramide indicate that less than 5% of the ceramide added to suspensions of isolated mitochondria actually inserts into the mitochondrial membranes (24), and the permeability of the MOM is very sensitive to the amount of ceramide that inserts into mitochondria. Importantly, the formation of protein-permeable ceramide channels in MOMs occurs at physiologically relevant membrane concentrations (24). Only 4–8 pmol of ceramide/nmol of lipid phosphate is required for the formation of large protein-permeable channels in MOMs of isolated rat liver mitochondria (24), a level consistent with those reported to occur in mitochondria-enriched fractions during the early stages of apoptosis (16, 18, 27). The ability of ceramide to form large protein-permeable channels in planar phospholipid membranes and MOMs (23–25) could explain many of the reported effects of ceramide on isolated mitochondria, namely release of intermembrane space proteins, alteration of calcium homeostasis of mitochondria and endoplasmic reticule, collapse in the inner mitochondrial membrane potential and ATP depletion, and enhanced generation of reactive oxygen species (18, 23, 28–34).
Ceramide-induced mitochondria-mediated apoptosis is inhibited by the expression of anti-apoptotic proteins Bcl-2 and Bcl-xL (28, 35–37). However, a key question is whether protein release occurs through ceramide channels and, if so, whether or not these channels are regulated directly or indirectly by anti-apoptotic proteins in the MOM, such as Bcl-xL and Bcl-2. We address these questions here and find evidence that suggests that ceramide channels are good candidate pathways for protein release from mitochondria during the early stages of apoptosis.
EXPERIMENTAL PROCEDURES
Materials
Phospholipids and ceramide were purchased from Avanti Polar Lipids, Alabaster, AL. The ceramide was more than 99% pure. Cytochrome c, enzymes, and fatty acid-depleted bovine serum albumin were purchased from Sigma. Other materials used were reagent grade or as indicated below.
Media and Growth Conditions
Wild-type and M22-2 (VDAC1 knock-out) yeast from frozen stocks were grown at 30 °C in lactic acid medium until they reached an OD600 of 0.6–0.8 (on HACH Co. spectrophotometer) at which point they were harvested for mitochondrial isolation as described previously (38). BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) Saccharomyces cerevisiae was transformed with either plasmid pAD4, a multicopy yeast expression vector carrying the LEU2 selectable marker, or pAD4-bcl-2, the same vector carrying the human BCL-2 gene under transcriptional control of the alcohol dehydrogenase (ADH1) promoter (39). Strains maintained on leucine-free dextrose (YPD) agar plates were grown on lactic acid/leucine medium at 30 °C until an OD600 of 0.6–0.8 was reached. Mitochondria were isolated as described previously (38). All yeast mitochondria were finally resuspended in H-medium (10 mm HEPES, 0.6 m mannitol, 0.1 mm EGTA, pH 7.2). Mitochondrial protein content and intactness of the MOM were measured as described previously (24). The presence of Bcl-2 in mitochondria was confirmed via Western blot analysis utilizing rabbit anti-Bcl-2 polyclonal antibody (Chemicon International; Temecula, CA) and peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson Immuno-Research, West Grove, PA).
Isolation of Rat Liver Mitochondria
Rat liver mitochondria were isolated from Sprague-Dawley males (150–300 g) by differential centrifugation; protein content was measured, and intactness of the MOM was determined as described previously (24). Isolated mitochondria were suspended in rH-buffer (0.28 m mannitol, 5 mm HEPES, 0.1 mm EGTA, pH 7.4).
Isolation of Mitochondria from Bax−/− Bak−/− Baby Mouse Kidney Epithelial (DKO BMK) Cells
DKO BMK cells, a kind gift from Dr. E. White (The Cancer Institute of New Jersey, Center for Advanced Biotechnology and Medicine), were grown in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum and 100 units/ml of both penicillin and streptomycin, 0.3 mg/ml glutamine (Invitrogen) at 37 °C and humidified CO2 (5%). Cells were harvested by trypsinization, washed twice in phosphate-buffered saline, and resuspended in 600 µl of Buffer A (68 mm sucrose, 1 mm EGTA, 10 mm HEPES, pH 7.4, 10mm KCl, 0.05% (w/v) fatty-acid depleted bovine serum albumin, 50 µg/ml Pefabloc, and 15 µg/ml leupeptin, aprotinin, and pepstatin). Cells were immediately lysed via 10 passages through a 28-guage insulin syringe, and the buffer was immediately made isotonic by the addition of 200µl of 880 mm mannitol in Buffer A. Mitochondria were then isolated via differential centrifugation as described (24) in homogenization medium (0.28 m mannitol, 5 mm HEPES, 0.1 mm EGTA, pH 7.4).
Protein Purification
All proteins were purified in the complete absence of detergents. Full-length human Bcl-xL was purified using two different protocols as described (40, 41), except omitting all detergents. Bcl-xL mutants (ΔN76, lacking amino acids 2–76; Δloop, lacking amino acids 26–83; ΔTM, lacking C-terminal amino acids 213–233 (42) and full-length Bcl-xL (aa1–233)) were produced as N-terminal glutathione S-transferase fusion proteins from pGEX vectors using Escherichia coli DH1α as the host strain and isolated as described previously (40), except omitting all detergents. pHis-GB1-CED-9-(1–251) fusion protein was expressed and purified in a manner similar to Bcl-xL using Ni+2 affinity and size-exclusion chromatographies (41).
Measurement of the Permeability of the MOM Using the Complex IV Accessibility Assay
The permeability of the MOM to proteins was assessed by measuring the initial linear rate of oxidation of exogenously added reduced cytochrome c (24) at room temperature (generally 23 °C). Mitochondria (80 µg total) were incubated at room temperature in 0.74 ml of either H-buffer (for yeast mitochondria) or rH-buffer (for rat liver mitochondria and those isolated from DKO BMK cells) supplemented with 0.1 mm carbonyl cyanide p-chlorophenylhydrazone and 5 µm antimycin A for 5 min prior to the incubation with a given test agent. When C16-ceramide (1 mg/ml isopropyl alcohol) was added to the mitochondrial suspension, it was added while vortexing to ensure rapid dispersion. At the end of the incubation period, the mitochondrial suspension was transferred to a cuvette, reduced cytochrome c added, and the absorbance at 550 nm monitored for 3 min. The initial linear portion of the rate (the first 30 s) was used as a measure of the permeability of the MOM to cytochrome c. This is expressed as a percentage of the rate measured in mitochondria whose outer membranes were lysed by hypotonic shock. These rates of cytochrome c oxidation in mitochondria with lysed outer membranes were as follows (in change in A550/s/µmol reduced cytochrome c added initially ± S.D. for at least three independent experiments): 11.1 ± 2.7, rat liver mitochondria; 5.6 ± 0.4, wild type yeast mitochondria; 4.9 ± 0.6, ΔVDAC1 mitochondria; 5.0 ± 0.7, BY4741 pAD4 mitochondria; 5.3 ± 0.9, BY4741 pAD4-Bcl-2 mitochondria; 3.3 ± 0.1 and Bax−/− Bak−/− mitochondria. This normalization allowed us to compare results from mitochondria isolated from different sources and combine data from different mitochondrial isolations. In addition, this normalization puts the data in a context where one can evaluate the percent MOM permeabilization. All vehicle controls were performed and found to have no effect on either MOM permeability or complex IV activity (tested by measuring the rate of cytochrome c oxidation in mitochondria with damaged outer membranes). In addition, all test agents (ceramide alone, protein alone, and ceramide and protein combined) were first tested on mitochondria whose outer membranes were damaged via hypotonic shock to ensure that there were no effects on complex IV activity. All experiments were produced in triplicate from three separate mitochondrial preparations.
Measurement of MOM Permeability Using the Adenylate Kinase Release Assay
The release of adenylate kinase from the intermembrane space of isolated rat liver mitochondria was monitored as described previously (43). C16-ceramide (1 mg/ml isopropanol) was added to the mitochondrial suspension while vortexing to ensure rapid dispersion. The release of adenylate kinase indicates that the MOM was permeabilized at some point and therefore the % release is a measure of the percent permeabilization. All vehicle controls were performed and found to have no effect. The addition of CED-9 alone also had no significant effect on release of adenylate kinase. Experiments were performed in triplicate from three separate mitochondrial preparations.
Electrophysiological Recordings
Solvent-free planar phospholipid membranes were produced by the monolayer method (44) as modified (45) across a 100-µm diameter hole in a Saran partition. Monolayers were produced using a solution of 0.5% (w/v) 1,2-diphytanoyl-sn-glycero-3-phosphocholine, 0.5% (w/v) asolectin (polar extract of soybean phospholipids), 0.05% (w/v) cholesterol in hexane. The aqueous buffer bathing the planar phospholipid membranes consisted of 1.0 m KCl, 1 mm MgCl2, 5 mm PIPES, pH 6.95. The transmembrane voltage was electronically clamped at −10 mV, and the current through the membrane was recorded. C16-ceramide was added to the cis side of the membrane while stirring from a stock solution of either 1 or 0.1 mg/ml in isopropyl alcohol. (The lower concentration is actually more effective in delivering ceramide to the membrane rather than aggregating in solution.) 5-, 10-, or 15-µl aliquots of the 0.1 mg/ml solution were stirred into 5 ml of solution on one side of the membrane depending on the vicissitudes of the experimental set up or the operator. Stirring was stopped after 15 s. Often multiple additions were needed to obtain the initial conductance increase. Typically this was followed by channel enlargement and stabilization at a particular conductance level. The solution was stirred again to ensure that the channel was indeed stable. Test proteins were then added while stirring to the aqueous solutions on both sides of the membrane.
RESULTS
Ceramide-induced Permeabilization of the Mitochondrial Outer Membrane Is Inhibited by Bcl-xL and CED-9
To test whether ceramide channels are regulated by the anti-apoptotic proteins in the Bcl-2 family, ceramide-induced permeabilization of the outer membrane of isolated mitochondria was examined in the presence and absence of Bcl-xL. The complex IV accessibility assay was utilized as a means of measuring the permeability of the MOM. This assay measures the oxidation of exogenously added reduced cytochrome c by cytochrome c oxidase, located in the inner membrane. The initial rate, expressed as a percentage of that measured in mitochondria whose outer membranes were damaged by hypotonic shock, is a good measure of the permeability of the outer membrane to cytochrome c. One advantage of using this assay over the direct measurement of cytochrome c release is that it allows one to quantitate the permeability and do so as a function of time of treatment. It also allows one to determine both increases and decreases in permeability.
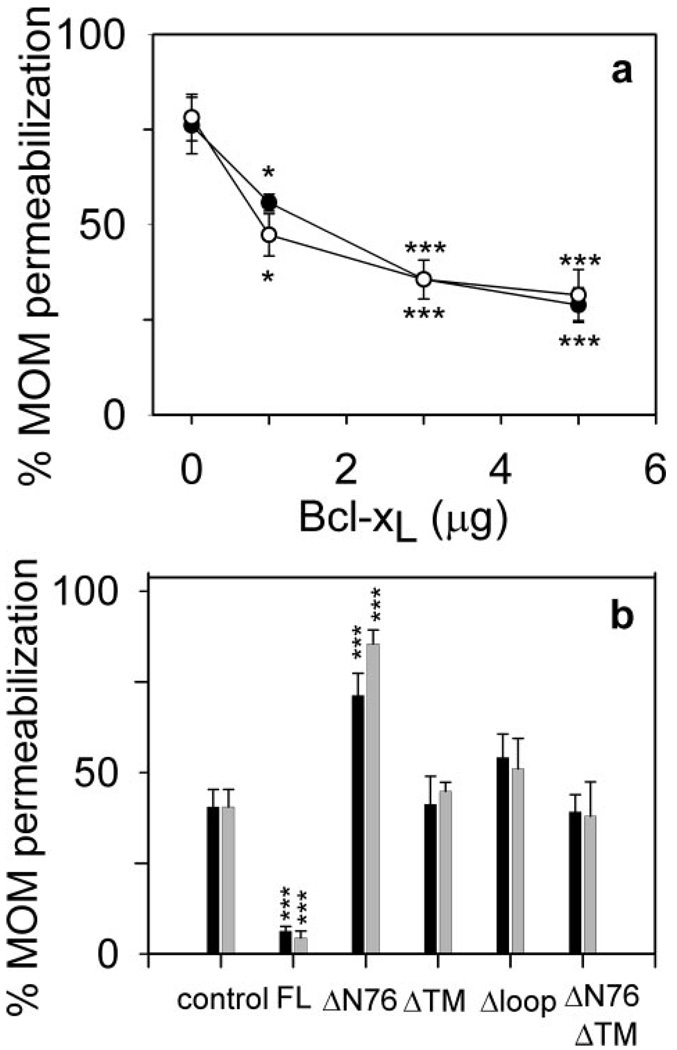
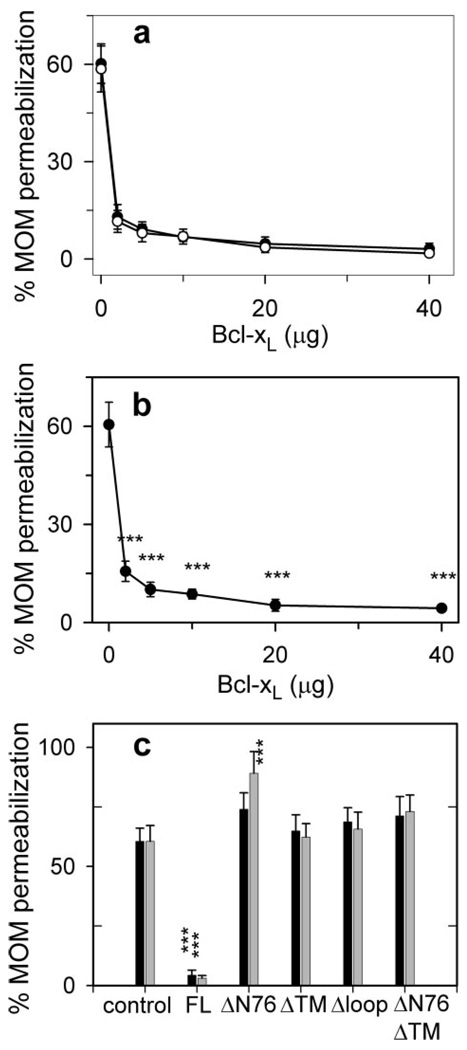
Healthy mitochondria with intact outer membranes do not oxidize exogenously added reduced cytochrome c. However, we have previously shown (23) that incubation of rat liver mitochondria with C16-ceramide (N-palmitoyl-sphingosine) permeabilizes the outer membrane to cytochrome c. This permeabilization is dose-dependent (23). In Fig. 1a, the rate of cytochrome c oxidation without added Bcl-xL is 75% of the rate achieved by damaging the outer membrane. Previous studies indicate that this permeabilization is likely because of the formation of ceramide channels in the MOM (23, 24). To determine whether this channel formation is regulated by anti-apoptotic Bcl-2 proteins, mitochondria were preincubated with purified Bcl-xL prior to incubation with C16-ceramide. This experiment is termed “prevention” because we are testing whether Bcl-xL prevents the formation of ceramide channels. Indeed, Bcl-xL pretreatment decreased the MOM permeability (Fig. 1a, open circles) to cytochrome c to less than half that observed for C16-ceramide alone (28.8 ± 4.5% and 35.6 ± 1.1% for 5 µg and 3 µg Bcl-xL, respectively). To determine whether Bcl-xL could block or disassemble preformed ceramide channels, mitochondria were incubated with C16-ceramide first to allow it to form channels (verified by performing the complex IV accessibility assay) prior to the addition of Bcl-xL. The Bcl-xL treatment again reduced the permeability of the MOM to cytochrome c to less than half that observed for C16-ceramide alone (31.5 ± 6.7 and 35.6 ± 5.1% for 5 and 3 µg of Bcl-xL, respectively; Fig. 1a, filled circles). These results indicate that Bcl-xL can both prevent the formation of ceramide channels and block or disassemble preformed ceramide channels. Both channel prevention and blockage/disassembly displayed a Hill coefficient of 1 (0.95 ± 0.07 and 0.87 ± 0.1 for prevention and block-age/disassembly, respectively), indicating a lack of cooperativity and suggesting a 1:1 complex is formed between the monomeric form of Bcl-xL and a ceramide channel. The inhibition of ceramide-induced permeabilization of the MOM by Bcl-xL is in agreement with its ability to protect whole cells from ceramide-induced mitochondria-mediated apoptosis (46).
Figure 1. Bcl-xL inhibits C16-ceramide channels in isolated rat liver mitochondria.
a, mitochondria were incubated with the indicated amount of Bcl-xL and 16 nmol of C16-ceramide but in two different ways in separate experiments. Open circles indicate that the mitochondria were first incubated with C16-ceramide for 10 min immediately followed by the addition of Bcl-xL and incubation for an additional 10 min. Then the permeability of the MOM was measured by using the complex IV accessibility assay (see “Experimental Procedures”). Filled circles indicate that mitochondria were first incubated with Bcl-xL for 10 min followed immediately by the addition of C16-ceramide and a further incubation for 10 min. Again this was immediately followed by the measurement of the MOM permeability. Data are presented as means ± S.D. from three independent experiments. A two-tailed t test was performed, and * and *** indicate a p value of ≤0.05 and 0.005, respectively, when comparing the indicated results with 16 nmol of C16-ceramide with no Bcl-xL addition. b, experiments were performed as in a. Control indicates that mitochondria were incubated with 8 nmol of C16-ceramide only for 20 min prior to measuring the MOM permeability. Black bars indicate that the mitochondria were incubated with 5 µg of the specified Bcl-xL or analogue protein for 10 min followed by the addition of 8 nmol of C16-ceramide and incubation for an additional 10 min. Gray bars indicate that the mitochondria were incubated with 8 nmol of C16-ceramide for 10 min followed by the addition of 5µg of the specified Bcl-xL or analogue protein and incubation for an additional 10 min. MOM permeability was then measured as above. Data are presented as mean ± S.D. from three independent experiments. A two-tailed t test was performed, and *** indicates a p value ≤0.005 when comparing the indicated results with the control.
To eliminate the possibility that ceramide channel inhibition is a nonspecific effect of Bcl-xL, several mutant recombinant Bcl-xL proteins were also analyzed for their ability to inhibit ceramide channel formation in the MOM of isolated rat liver mitochondria. The mutants are as follows: Δloop Bcl-xL (deletion of the flexible loop region between the BH4 and BH3 domains; amino acids 26–83), ΔTM Bcl-xL (deletion of the C-terminal transmembrane domain; amino acids 213–233), ΔN76 Bcl-xL (deletion of the N-terminal 76 residues; amino acids 1–76), and ΔTMΔN76 Bcl-xL (deletion of both the C-terminal transmembrane domain and the N-terminal 76 residues; amino acids 1–76 and 213–233). Isolated mitochondria were incubated with 5 µg of mutant protein either prior to (Fig. 1b, black bars) or following incubation with C16-ceramide (light bars), and the permeability of the MOM to cytochrome c was measured. In Fig. 1b, the “control” represents mitochondria treated only with ceramide. As shown in Fig. 1a, full-length Bcl-xL (designated as FL in Fig. 1b) both prevented and blocked/disassembled ceramide-induced MOM permeabilization. Δloop Bcl-xL and ΔTM Bcl-xL were unable to influence the permeability induced by ceramide (Fig. 1b). Previous studies showed that the deletion of the N-terminal 76 amino acids of Bcl-xL (ΔN76 Bcl-xL) not only eliminates its anti-apoptotic functions but also renders it pro-apoptotic (40, 47). In these experiments, ΔN76 Bcl-xL actually favored C16-ceramide permeabilization of the MOM(Fig. 1b). In the absence of ceramide, ΔN76 Bcl-xL had no effect on the permeability of the MOM to cytochrome c unless ΔN76 Bcl-xL was purified using detergents as reported previously (40) (data not shown). The structure without use of detergents is believed to be the native structure. Furthermore, deletion of the transmembrane region at the C terminus abolished the ability of ΔN76 Bcl-xL to enhance ceramide channel activity (Fig. 1b). These results demonstrate that inhibition of ceramide-induced permeabilization of the MOM by Bcl-xL or its mutants requires that the protein be functionally anti-apoptotic.
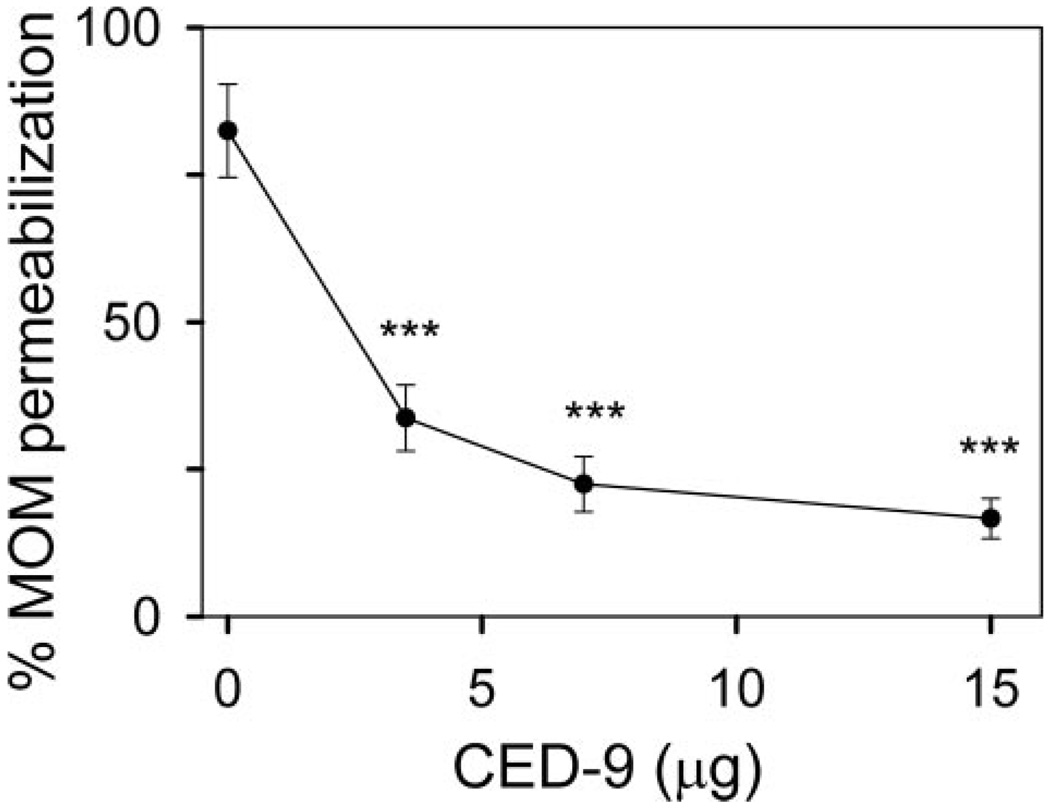
To determine whether ceramide channel inhibition by anti-apoptotic Bcl-2 proteins is an evolutionarily conserved function, the effect of the Caenorhabditis elegans Bcl-2 homologue CED-9 on ceramide channel formation was also tested. The purified CED-9 protein requires the presence of the thiol-reducing agent, dithiothreitol, to remain soluble and in its monomeric form. However, dithiothreitol interferes with the complex IV accessibility assay. Therefore, the release of adenylate kinase from the mitochondrial intermembrane space was used to measure the permeability of the MOM to proteins. When using an assay that monitors protein release from the mitochondrial intermembrane space, channel prevention can be examined but not blockage/disassembly of preformed channels. Mitochondria were incubated with CED-9 prior to incubation with C16-ceramide. CED-9 inhibited C16-ceramide channel formation in a dose-dependent manner (Fig. 2). These results demonstrate that anti-apoptotic proteins from both mammals and worms are capable of inhibiting ceramide-induced permeabilization of the MOM.
Figure 2. CED-9 inhibits C16-ceramide channels in isolated rat liver mitochondria.
Mitochondria were incubated for 10 min with the indicated amounts of purified CED-9 followed by addition of 16 nmol of C16-ceramide and a further 10-min incubation. The MOM permeability was assessed by measuring the release of adenylate kinase (see “Experimental Procedures”). Data are presented as mean ± S.D. from three independent experiments. A two-tailed t test was performed and *** indicates a p value ≤0.005 when comparing the indicated results with 16 nmol of C16-ceramide with no CED-9 addition.
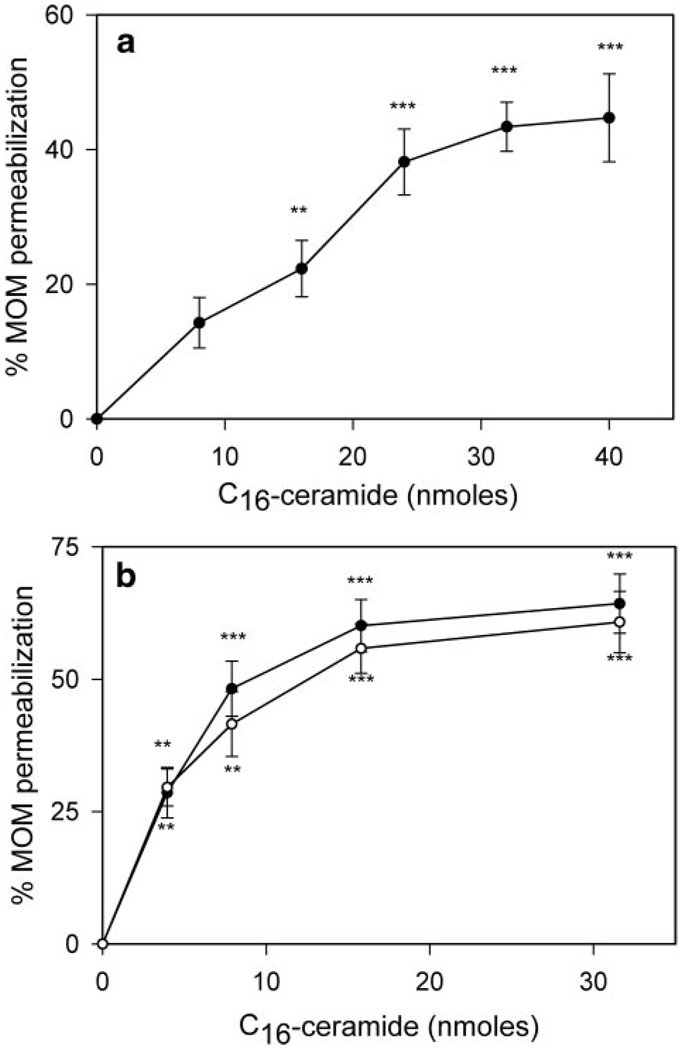
Ceramide permeabilizes the outer membrane of mitochondria lacking Bcl-2 proteins. The inhibition of the ceramide-induced permeabilization of mitochondria by Bcl-xL and CED-9 could be through a direct action on ceramide channels or through an indirect action (for example, interaction with another mitochondrial protein). Mammalian mitochondria are rich with a variety of proteins that have been shown to interact with Bcl-xL, including pro-apoptotic Bcl-2 family proteins (48) and the voltage-dependent anion channel (49). Indeed, it is possible that ceramide permeabilization of the MOM could be due to an interaction between ceramide and pro-apoptotic Bcl-2 family members, which is inhibited in the presence of anti-apoptotic Bcl-2 proteins. There may be several pro-death Bcl-2-related proteins present in isolated rat liver mitochondria (e.g. Bax, Bak, and Bok). Therefore, mitochondria were isolated from baby mouse kidney (BMK) epithelial cells lacking both Bax and Bak, and the sensitivity of the MOM to ceramide was tested. In the absence of both Bax and Bak, mitochondria are still sensitive to ceramide (Fig. 3a). Ceramide permeabilizes the outer membrane of these mitochondria in a dose-dependent manner. This indicates that Bax and Bak are not required for ceramide-induced permeabilization of the MOM.
Figure 3. Pro-apoptotic Bcl-2 proteins and VDAC are not required for ceramide-induced permeabilization of the mitochondrial outer membrane.
a, mitochondria isolated from Bax−/− Bak−/− BMK cells (80 µg of mitochondrial protein) were incubated for 10 min with C16-ceramide at the indicated levels. The permeability of the MOM was measured by the complex IV accessibility assay. Data are a representative experiment (from three independent experiments) and are presented as means ± S.D. A two-tailed t test was performed, and ** and *** indicate p values ≤0.01 and 0.005, respectively, when comparing the indicated results with the control (no ceramide addition). b, mitochondria (80µg) isolated from either wild-type (filled circles) or VDAC1 KO (open circles) yeast were incubated for 10 min with the indicated amounts of C16-ceramide, and the permeability of MOM was measured by the complex IV accessibility assay. Data are presented as means ± S.D. from three independent experiments. A two-tailed t test was performed, and ** and *** indicate p values ≤0.01 and 0.005, respectively, when comparing the indicated results with the control (no ceramide addition).
Bax−/− Bak −/− BMK cells still contain BH3-only pro-apoptotic Bcl-2 proteins that could be present in the MOM. It is possible that one or more of these proteins is required for ceramide-induced permeabilization of the MOM. Thus, mitochondria were purified from the yeast, S. cerevisiae, as they lack any known Bcl-2 family members. Using the complex IV accessibility assay, C16-ceramide forms channels in the MOM of isolated yeast mitochondria in a dose-dependent manner (Fig. 3b, filled circles). As VDAC has been implicated in playing a role in mitochondria-mediated apoptosis (49, 50), we determined whether C16-ceramide could form channels in the MOM of mitochondria isolated from a strain of yeast in which the POR1 gene that encodes VDAC1 has been deleted (ΔVDAC1 yeast). C16-ceramide forms channels to the same extent in mitochondria isolated from ΔVDAC1 yeast as it does in mitochondria isolated from wild-type (Fig. 3b, open circles). Thus, the ability of C16-ceramide to form protein-permeable channels in the MOM is not dependent on the presence of proteins in the Bcl-2 family nor a functional voltage-dependent anion channel.
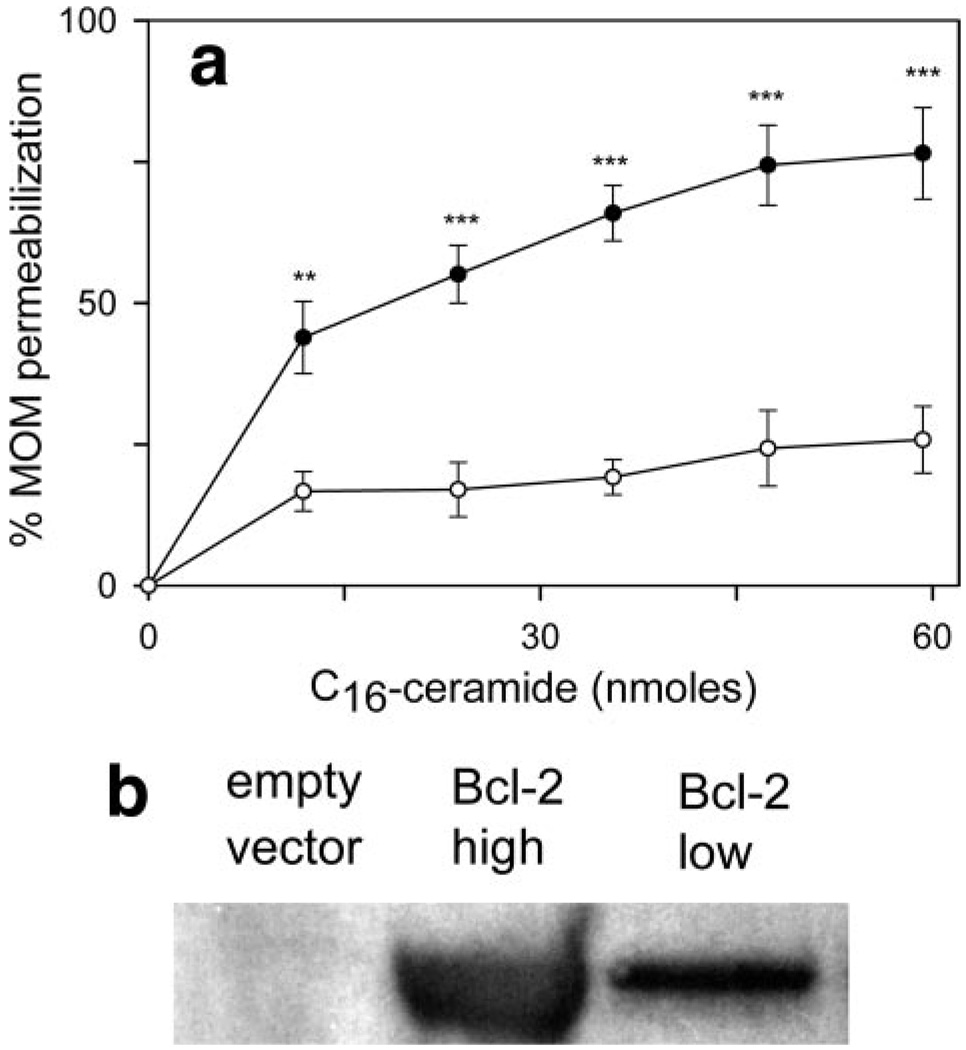
If anti-apoptotic proteins act directly on ceramide channels, rather than indirectly through other mitochondrial Bcl-2 proteins, then they should influence ceramide channel formation even in yeast mitochondria, where no endogenous Bcl-2 proteins would be expected to be present. Mitochondria isolated from Bcl-2-expressing yeast cells (Fig. 4a, open circles) are resistant to C16-ceramide channel formation as compared with mitochondria isolated from empty vector control cells (filled circles). Western blot analysis confirms that human Bcl-2 is present in the mitochondria-enriched fraction of Bcl-2-expressing yeast cells (Fig. 4b). Mitochondria isolated from either wild-type (Fig. 5, closed circles) or ΔVDAC1 (open circles) yeast become resistant to ceramide channel formation in the MOM when they are preincubated with Bcl-xL. Alternatively, when ceramide was added to these yeast mitochondria and allowed to form channels (verified via the complex IV accessibility assay), the subsequent addition of Bcl-xL blocks/disassembles the ceramide-induced permeabilization of the MOM(Fig. 5b). As was the case in rat liver mitochondria, Bcl-xL inhibition of ceramide channels in yeast mitochondria (for wild type and ΔVDAC1) exhibited a Hill coefficient of 1.
Figure 4. Mitochondria isolated from yeast expressing human Bcl-2 are resistant to ceramide.
a, mitochondria isolated from yeast expressing human full-length Bcl-2 (open circles) or empty vector controls (closed circles) were studied as described in the legend to Fig. 3b. Data are presented as means ± S.D. from three independent experiments. A two-tailed t test was performed, and ** and *** indicate p values ≤0.01 and 0.005, respectively, when comparing the indicated results with the control (no ceramide addition). b, Western blot of mitochondria isolated from empty vector control yeast and Bcl-2-expressing yeast. High is a 30-µg total protein run on the gel, whereas low is 15 µg.
Figure 5. Bcl-xL inhibition of C16-ceramide-induced permeabilization of the MOM requires neither other Bcl-2 proteins nor VDAC.
a, MOM permeability was measured by the complex IV accessibility assay in isolated wild type (closed circles) or VDAC1 knock-out (open circles) yeast mitochondria incubated with the indicated amount of full-length Bcl-xL for 10 min followed by the addition of 16 nmol of C16-ceramide and preincubation for an additional 10 min. b, MOM permeability was assessed by the complex IV accessibility assay in isolated wild type yeast incubated for 10 min with 16 nmol of C16-ceramide followed the addition of the indicated amount of Bcl-xL and incubation for an additional 10 min. c, permeability of the MOM was measured in isolated wild type yeast mitochondria. Control indicates that mitochondria were incubated with 16 nmol of C16-ceramide only for 20 min. Black bars indicate that the mitochondria were incubated with 20 µg of the specified Bcl-xL or analogue protein for 10 min followed by the addition of 16 nmol of C16-ceramide and incubation for an additional 10 min. Gray bars indicate that the mitochondria were incubated with 16 nmol of C16-ceramide for 10 min followed by the addition of 20 µg of the specified Bcl-xL or analogue protein and incubation for an additional 10 min. MOM permeability was then measured via the complex IV accessibility assay. Data are presented as the means ± S.D. from three independent experiments. A two-tailed t test was performed between the indicated results and the control. *** indicates a p value ≤0.005.
Prevention and blockage/disassembly of ceramide channel formation by Bcl-xL was dependent on the full-length version of the protein. Similar to rat liver mitochondria, all the deletion mutants tested failed to prevent (Fig. 5c, black bars) or reverse (gray bars) the formation of ceramide channels in the MOM of isolated yeast mitochondria. Similarly, the pro-apoptotic N-terminally truncated Bcl-xL (ΔN76) augmented ceramide-induced permeabilization (Fig. 5c). All Bcl-xL deletion mutants also had no activity when tested at pH 6.0 (data not shown), where they are thought to more effectively insert into membranes.
Bcl-xL and CED-9 Inhibit Ceramide Channels in Planar Phospholipid Membranes
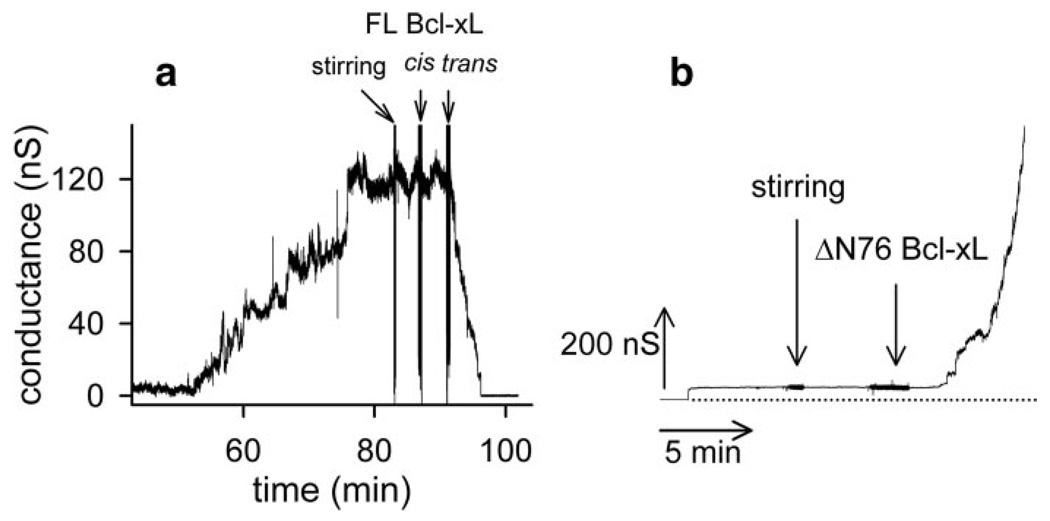
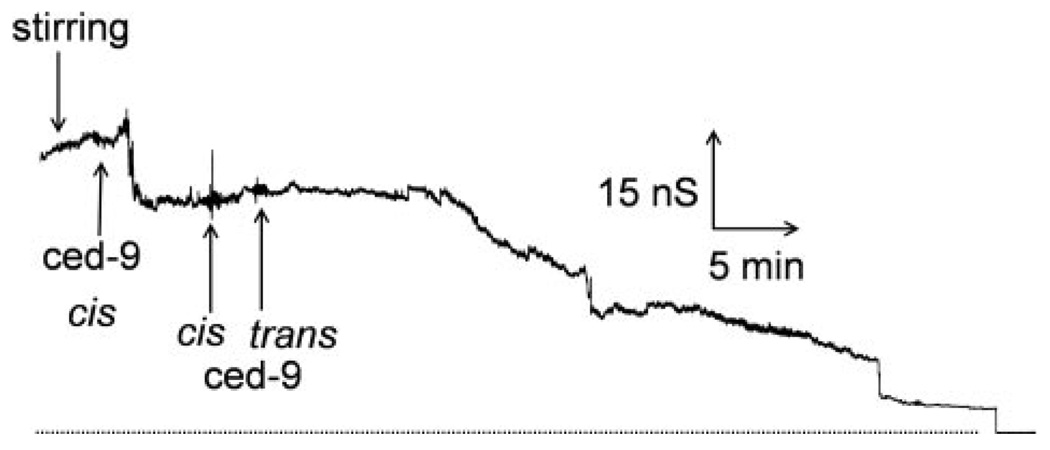
Although the results in isolated yeast mitochondria suggest that neither pro-apoptotic Bcl-2 proteins nor a functional voltage-dependent anion channel are required for Bcl-2 and Bcl-xL inhibition of ceramide-induced permeabilization of the MOM, they do not rule out other possibilities. Perhaps the inhibition is because of stimulation of ceramide catabolism. Perhaps other proteins mediate the inhibition. Thus, we tested the inhibition in solvent-free planar phospholipid membranes. This is a defined system free of other proteins. These membranes are phospholipids bilayers made from phospholipid monolayers, and these membranes separate two aqueous compartments. The transmembrane voltage is controlled; the ionic current flowing through the membrane is recorded, and the conductance is calculated, and this is a measure of the permeability of the membrane to ions. When C16-ceramide is added to the aqueous solution bathing one side of a planar phospholipid membrane, some of the ceramide inserts into the membrane. The ceramide forms a channel that allows ions to cross the membrane. This is recorded as a series of discontinuous increases in conductance under voltage-clamp conditions (6, 25). Extensive characterization of ceramide channel formation in planar phospholipid membranes in a previous study (25) indicates that these conductances arise from one ceramide channel enlarging in size rather than the formation of multiple ceramide channels in parallel (25). In this study, ceramide was added to the aqueous phase bathing a planar phospholipid membrane (Fig. 6a), and after a lag, the conductance increased finally reaching a fairly steady value. This increase in conductance is because of the formation and enlargement in size of a ceramide channel. Bcl-xL addition to this stable ceramide channel resulted in a dramatic decrease in the conductance all the way to base line (Fig. 6a). The decrease was not one event, not a sudden discontinuous drop to zero conductance, as would be expected from a physical block of the aqueous pathway through the membrane, but occurred in multiple stepwise conductance decrements. This is consistent with channel disassembly. Similar results were obtained with CED-9, albeit at a slower rate of ceramide channel disassembly (Fig. 7). Vehicle controls resulted in no conductance change. Addition of the pro-apoptotic ΔN76 Bcl-xL to a stable ceramide channel 50 nS in size did not result in channel disassembly (Fig. 6b) but rather resulted in a rapid and dramatic increase in conductance. The increase occurred as a series of stepwise conductance increments, which is consistent with growth in size of the original channel or the formation of new channels. Control experiments with ΔN76 Bcl-xL alone in the membrane can result in modest increases in conductance, as expected (40), but these conductances, when observed, are an order of magnitude smaller than those observed with ceramide. Ceramide addition to a small ΔN76 Bcl-xL conductance had no effect. This suggests that the dramatic increase in conductance observed when ΔN76 Bcl-xL was added to a preformed C16-ceramide channel is the result of enlargement of C16-ceramide channels by ΔN76 Bcl-xL. The opposing effects of the anti-apoptotic Bcl-xL and the pro-apoptotic ΔN76 Bcl-xL on ceramide channels in planar phospholipid membranes correlates with results obtained in isolated yeast and rat liver mitochondria.
Figure 6. C16-ceramide channels formed in planar phospholipid membranes are disassembled by full-length Bcl-xL and activated by ΔN76Bcl-xL.
a, representative experiment (of four independent experiments) where C16-ceramide was stirred into the aqueous buffer on one side of a phospholipid membrane (a total of 3.7 µM ceramide was added in this experiment). When the ceramide channel conductance reached a plateau, indicating that the ceramide channel had enlarged and stabilized, 0.15 µM Bcl-xL was added to both sides (cis and trans) of the membrane. b, same as in a except that ΔN76 Bcl-xL was used instead of Bcl-xL.ΔN76 Bcl-xL was added when the ceramide channel enlarged and stabilized at 50 nS. The dotted line is the zero conductance level. This is representative of three independent experiments.
Figure 7. CED-9 disassembles C16-ceramide channels in planar phospholipid membranes.
This is a representative experiment (of three independent experiments) where a total of 2.5µM C16-ceramide was stirred into the aqueous buffer on one side of a phospholipid membrane. Following ceramide channel enlargement and stabilization, 0.15 µM CED-9 was added to both sides of the membrane. The dotted line is the zero conductance level.
DISCUSSION
The results presented above indicate that anti-apoptotic Bcl-2 proteins inhibit ceramide channel formation in the MOM of isolated mitochondria and in planar phospholipid membranes. This direct action of anti-apoptotic proteins on ceramide channels indicates that ceramide channels could play a role in the apoptotic process. These results are in harmony with published work demonstrating that anti-apoptotic proteins inhibit ceramide-induced apoptosis in whole cells. Overexpression of Bcl-xL in the B-lymphocyte cell line WEHI 231 protected against ceramide-induced apoptosis without altering the cellular level of ceramide (46). In addition, overexpression of Bcl-2 blocked ceramide-induced apoptosis without inhibiting ceramide generation (36, 51). To further support the notion that anti-apoptotic proteins act downstream of ceramide generation, Ghafourifar et al. (28) showed that ceramide addition to isolated mitochondria induces cytochrome c release, and this cytochrome c release was completely prevented by preincubation with Bcl-2. Although our results would certainly add weight to the argument that the inhibition of ceramide-induced mitochondria-mediated apoptosis by anti-apoptotic Bcl-2 proteins occurs at least in part at the site of ceramide action, they cannot rule out the possibility that in vivo these proteins also regulate ceramide generation. There have been studies that have shown that Bcl-2 and Bcl-xL can inhibit ceramide-induced apoptosis by preventing ceramide accumulation (35, 52, 53). Ceramide is generated in several subcellular locations, and elevated ceramide levels can arise from the activation or inhibition of several different enzymes. Thus, the mechanism that these proteins utilize to inhibit ceramide-induced apoptosis may depend both on the metabolic pathway and the subcellular location in which ceramide is generated. Anti-apoptotic Bcl-2 proteins may have evolved multiple means to keep ceramide-induced apoptosis at bay.
The inhibition of the C16-ceramide channels by Bcl-xL and CED-9 in planar phospholipid membranes suggests a direct interaction between ceramide channels and anti-apoptotic Bcl-2 proteins. In addition, the inhibition in both isolated rat liver and yeast mitochondria displayed a Hill coefficient of 1, indicating that the monomeric form of Bcl-xL is responsible for ceramide channel inhibition. We do not currently know the molecular mechanism by which Bcl-xL and CED-9 induce disassembly of ceramide channels. Perhaps the interaction simply destabilizes the channel structure. Future studies will hopefully elucidate the mechanism of inhibition of the ceramide channels.
Regardless of the molecular mechanism of action, the data indicate that this inhibition is a specific effect. The ceramide channel inhibition requires the full-length Bcl-xL protein. In fact, the N-terminal deletion mutant, ΔN76 Bcl-xL, actually stimulated ceramide-induced permeabilization both in isolated rat liver and yeast mitochondria as well as in planar phospholipid membranes. This is consistent with previously reported pro-apoptotic effects of ΔN76 Bcl-xL (40, 42, 47).
In summary, the results presented above could have profound implications on multiple levels. First, that Bcl-xL and CED-9 inhibit C16-ceramide channels in planar phospholipid membranes is compelling evidence that the ceramide-induced permeabilization of the MOM, following the addition of C16-ceramide to isolated mitochondria, is because of the formation of ceramide channels. Second, these results indicate that the ability of ceramide to induce mitochondria-mediated apoptosis could be due in part to its ability to form channels. Third, these results show that the inhibition of ceramide channels by anti-apoptotic Bcl-2 proteins could, at least partially, explain their ability to inhibit ceramide-induced apoptosis in whole cell systems. Fourth, these results could help tie ceramide-induced mitochondria-mediated apoptosis to the Bcl-2 system of regulation of these events. Finally, inhibition of ceramide channels by anti-apoptotic Bcl-2 proteins indicates that these channels could be good candidates for a pathway through which pro-apoptotic proteins are released from mitochondria during the induction phase of apoptosis. It is possible that cells possess multiple pathways for inducing the release of pro-apoptotic proteins from mitochondria during the induction phase of apoptosis, and it is logical that proteins like Bcl-2 and Bcl-xL would evolve to act on all pathways.
Supplementary Material
Supplemental Material can be found at: http://www.jbc.org/cgi/content/full/M706115200/DC1
Acknowledgments
We thank Dr. E. White for providing the wild-type and Bax−/− Bak−/− baby mouse kidney cells and Dr. E. B. Gralla for providing the Bcl-2 plasmids.
Footnotes
This work was supported by National Institutes of Health Grants NS42025 (to M. C.), NS37402, GM77875 (to J. M. H.), and GM067180 (to R. B. H.) and National Science Foundation Grant MCB-0641208 (to M. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains a supplemental table.
The abbreviations used are: MOM, mitochondrial outer membrane; BMK, baby mouse kidney epithelial; C16-ceramide, N-palmitoyl-sphingosine; VDAC, voltage-dependent anion-selective channel; nS, nanosiemens; PIPES, 1,4-piperazinediethanesulfonic acid.
J. Stiban and M. Colombini, unpublished observations.
REFERENCES
- 1.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 2.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Dejean LM, Martinez-Caballero S, Kinnally KW. Cell Death Differ. 2006;13:1387–1395. doi: 10.1038/sj.cdd.4401949. [DOI] [PubMed] [Google Scholar]
- 4.Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, Herrmann A, Basanez G. J. Biol. Chem. 2004;279:30081–30091. doi: 10.1074/jbc.M313420200. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalvez F, Pariselli F, Dupaigne P, Budihardjo I, Lutter M, Antonsson B, Diolez P, Manon S, Martinou JC, Goubern M, Wang X, Bernard S, Petit PX. Cell Death Differ. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 6.Siskind LJ. J. Bioenerg. Biomembr. 2005;37:143–153. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witty JP, Bridgham JT, Johnson AL. Endocrinology. 1996;137:5269–5277. doi: 10.1210/endo.137.12.8940345. [DOI] [PubMed] [Google Scholar]
- 8.Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 9.Charles AG, Han TY, Liu YY, Hansen N, Giuliano AE, Cabot MC. Cancer Chemother. Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Lafrasse C, Alphonse G, Broquet P, Aloy MT, Louisot P, Rousson R. Biochem. J. 2001;357:407–416. doi: 10.1042/0264-6021:3570407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroesen BJ, Pettus B, Luberto C, Busman M, Sietsma H, de Leij L, Hannun YA. J. Biol. Chem. 2001;276:13606–13614. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- 12.Thomas RL, Jr, Matsko CM, Lotze MT, Amoscato AA. J. Biol. Chem. 1999;274:30580–30588. doi: 10.1074/jbc.274.43.30580. [DOI] [PubMed] [Google Scholar]
- 13.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. J. Biol. Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 14.Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T. Lipids. 1998;33:601–605. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- 15.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Biochem. J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. Biochem. J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vance JE. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 18.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 19.Matsko CM, Hunter OC, Rabinowich H, Lotze MT, Amoscato AA. Biochem. Biophys. Res. Commun. 2001;287:1112–1120. doi: 10.1006/bbrc.2001.5696. [DOI] [PubMed] [Google Scholar]
- 20.Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Oncogene. 2004;23:3650–3658. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- 21.Birbes H, El Bawab S, Hannun YA, Obeid LM. FASEB J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 22.Siskind LJ, Colombini M. J. Biol. Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siskind LJ, Kolesnick RN, Colombini M. J. Biol. Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siskind LJ, Kolesnick RN, Colombini M. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M. Biophys. J. 2003;85:1560–1575. doi: 10.1016/S0006-3495(03)74588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Lafrasse C, Alphonse G, Aloy MT, Ardail D, Gerard JP, Louisot P, Rousson R. Int. J. Cancer. 2002;101:589–598. doi: 10.1002/ijc.10652. [DOI] [PubMed] [Google Scholar]
- 28.Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. J. Biol. Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- 29.Gudz TI, Tserng KY, Hoppel CL. J. Biol. Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 30.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. J. Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- 32.Di Paola M, Cocco T, Lorusso M. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 33.Di Paola M, Zaccagnino P, Montedoro G, Cocco T, Lorusso M. J. Bioenerg. Biomembr. 2004;36:165–170. doi: 10.1023/b:jobb.0000023619.97392.0c. [DOI] [PubMed] [Google Scholar]
- 34.Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. J. Biol. Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 35.Kawatani M, Uchi M, Simizu S, Osada H, Imoto M. Exp. Cell Res. 2003;286:57–66. doi: 10.1016/s0014-4827(03)00098-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G. J. Exp. Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morizono H, Woolston JE, Colombini M, Tuchman M. Mol. Genet. Metab. 2005;86:431–440. doi: 10.1016/j.ymgme.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. J. Cell Biol. 1997;137:1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basanez G, Zhang J, Chau BN, Maksaev GI, Frolov VA, Brandt TA, Burch J, Hardwick JM, Zimmerberg J. J. Biol. Chem. 2001;276:31083–31091. doi: 10.1074/jbc.M103879200. [DOI] [PubMed] [Google Scholar]
- 41.Thuduppathy GR, Terrones O, Craig JW, Basanez G, Hill RB. Biochemistry. 2006;45:14533–14542. doi: 10.1021/bi0616652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Proc. Natl. Acad. Sci. U. S. A. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elrick MJ, Fluss S, Colombini M. Biophys. J. 2006;91:1749–1756. doi: 10.1529/biophysj.106.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montal M, Mueller P. Proc. Natl. Acad. Sci. U. S. A. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colombini M. Methods Enzymol. 1987;148:465–475. doi: 10.1016/0076-6879(87)48045-2. [DOI] [PubMed] [Google Scholar]
- 46.Wiesner DA, Kilkus JP, Gottschalk AR, Quintans J, Dawson G. J. Biol. Chem. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- 47.Jonas EA, Hickman JA, Chachar M, Polster BM, Brandt TA, Fannjiang Y, Ivanovska I, Basanez G, Kinnally KW, Zimmerberg J, Hardwick JM, Kaczmarek LK. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13590–13595. doi: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. J. Biol. Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 50.Chandra D, Choy G, Daniel PT, Tang DG. J. Biol. Chem. 2005;280:19051–19061. doi: 10.1074/jbc.M501391200. [DOI] [PubMed] [Google Scholar]
- 51.Allouche M, Bettaieb A, Vindis C, Rousse A, Grignon C, Laurent G. Oncogene. 1997;14:1837–1845. doi: 10.1038/sj.onc.1201023. [DOI] [PubMed] [Google Scholar]
- 52.Tepper AD, de Vries E, van Blitterswijk WJ, Borst J. J. Clin. Investig. 1999;103:971–978. doi: 10.1172/JCI5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, Nishimura Y, Sakai N, Nozawa Y. Oncogene. 2000;19:3508–3520. doi: 10.1038/sj.onc.1203699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material can be found at: http://www.jbc.org/cgi/content/full/M706115200/DC1