Abstract
The classical studies of nicotine by Langley at the turn of the 20th century introduced the concept of a “receptive substance,” from which the idea of a “receptor” came to light. Subsequent studies aided by the Torpedo electric organ, a rich source of muscle-type nicotinic receptors (nAChRs), and the discovery of α-bungarotoxin, a snake toxin that binds pseudo-irreversibly to the muscle nAChR, resulted in the muscle nAChR being the best characterized ligand-gated ion channel hitherto. With the advancement of functional and genetic studies in the late 1980s, the existence of nAChRs in the mammalian brain was confirmed and the realization that the numerous nAChR subtypes contribute to the psychoactive properties of nicotine and other drugs of abuse and to the neuropathology of various diseases, including Alzheimer’s, Parkinson’s, and schizophrenia, has since emerged. This review provides a comprehensive overview of these findings and the more recent revelations of the impact that the rich diversity in function and expression of this receptor family has on neuronal and nonneuronal cells throughout the body. Despite these numerous developments, our understanding of the contributions of specific neuronal nAChR subtypes to the many facets of physiology throughout the body remains in its infancy.
I. ACETYLCHOLINE RECEPTORS
Acetylcholine receptors (AChRs), like many other ligand-activated neurotransmitter receptors, consist of two major subtypes: the metabotropic muscarinic receptors and the ionotropic nicotinic receptors. Both share the property of being activated by the endogenous neurotransmitter acetylcholine (ACh), and they are expressed by both neuronal and nonneuronal cells throughout the body (8, 113, 142, 184). The metabotropic receptors are second messenger, G protein-coupled seven-transmembrane proteins. They are classically defined as being activated by muscarine, a toxin from the mushroom Amanita muscaria, and inhibited by atropine, a toxin from Atropa belladonna, a member of the nightshade family. Both toxins cross the blood-brain barrier poorly and were discovered primarily from their influences on postganglionic parasympathetic nervous system functions. Activation of muscarinic AChRs is relatively slow (milliseconds to seconds) and, depending on the subtypes present (M1–M5), they directly alter cellular homeostasis of phospholipase C, inositol trisphosphate, cAMP, and free calcium. A recent review of these receptors is recommended (142).
The other subtype of AChR is the fast ionotropic cationic nicotinic receptor channel (nAChR). These receptors are sensitive to activation by nicotine and have ion channels whose activity is induced in the micro- to submicrosecond range. Our knowledge about nAChRs originated through the combination of two natural oddities (see Refs. 8, 229, 276, 342, 343, 382 for extensive reviews). The first was the finding that the electric organ of a fish that produces an electric pulse to stun its prey, such as Torpedo, expresses nAChRs at densities that approach a crystalline array (245, 438). This provided an unprecedented source of starting material for receptor purification since nAChRs comprise ~40% of the protein from this organ. The second was the discovery of α-bungarotoxin (α-BGT), a component of krait snake venom that binds muscle-type nAChRs with near covalent affinity to inhibit their function and promote debilitating paralysis at the neuromuscular junction (6, 50, 149, 264). The integration of these diverse findings resulted in the use of α-BGT affinity columns to separate nAChRs from other proteins in detergent-solubilized electric organs (reviewed in Ref. 125). The NH2-terminal protein sequence was obtained from the purified nAChR protein, and the newly emerging methods of reverse genetics led to the identification, cloning, and sequencing of genes responsible for encoding these receptors. Studies that combined genetic, protein, immunological, microscopic, and functional assays have provided a consensus view of the muscle nAChR as a heteropentamer consisting of four related, but genetically and immunologically distinct, subunits organized around a central pore in the membrane in the stoichiometry of two α subunits and one each of β, δ, and γ (Fig. 1). The subsequent use of these subunits as probes for low-stringency screening of brain cDNA libraries led to the discovery of a diverse family of distinct nAChR subunits. Collectively, these subunits interact in defined ways to produce a spectrum of nAChRs that are expressed by various cell types extending from muscle to other nonneuronal cells in skin, pancreas, and lung to neurons in the central and peripheral nervous systems. The unique functional properties of distinct nAChR subtypes also customize their role in regulating physiological processes ranging from maintenance of metabolic tone, to control of inflammatory processes, to their widely studied influence over inhibitory and excitatory transmissions in the nervous system.
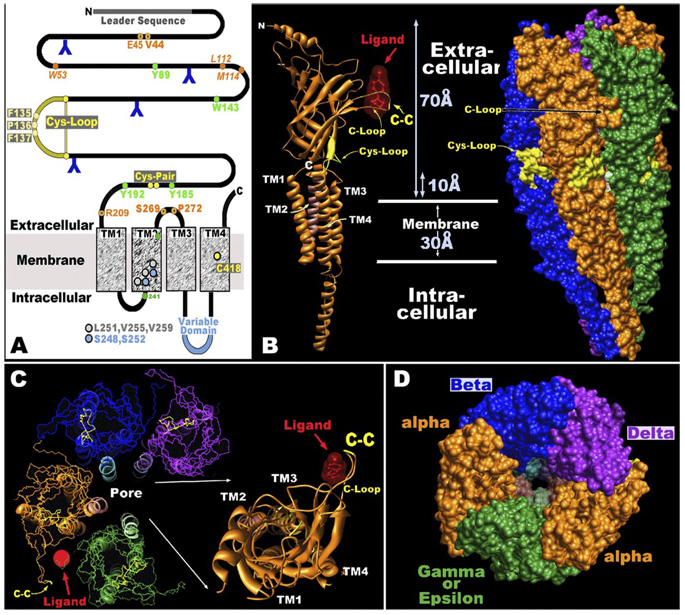
FIG. 1.
Basic structure of nicotinic acetylcholine receptors (nAChRs). A: the basic linear sequence of all nAChR subunits appears as a large extracellular domain, four transmembrane domains, and a cytoplasmic domain of variable size that resides between TM3 and TM4. This produces the classic “3+1” designation that describes this structure. Also characterizing the superfamily of receptor to which nAChRs belong is the Cys-loop that is composed of two disulfide-linked cystines separated by 13 amino acids that are highly conserved. Subunits that have the Cys-Cys pair are designated as α subunits (see text). Amino acids conserved in most nAChRs are identified using the Torpedo α subunit numbering system (476). Residues in green are important to the α subunit contribution to the agonist-binding pocket and orange residues are important to the β or negative face of the agonist-binding site. Orange residues with black dots are required for gating the channel. Amino acids in TM2 important to establishing the channel gate are in gray, and those important to relieving the gate are in blue. Residues lining the pore (green) are important to determine ion selectivity and conductance such as E241 that in part determines the permeability to Ca2+. The lone cysteine418 in TM4 contributes to measuring the response of nAChRs dependent on the lipid environment. The blue “Y” are N-linked glycosylation sites whose relative locations (except near the Cys-loop) vary among subunits. B: the EM structure of the Torpedo nAChR is from Unwin (476), and images were generated using the UCSF-chimera program with coordinates obtained from the Protein Data Bank ID 1OED.pdb. The approximate dimensions of the intact Torpedo receptor are given. An α subunit is shown where ribbons designate the secondary structures of the primary sequence. The extracellular domain is largely β-sheets and all TMs are α-helices. Note that the TM domains are believed to extend ~ 10 Å beyond the membrane. The cytoplasmic domain is depicted as a large α-helix, although this is likely to vary in size and complexity of structure between subunits (see text). This is an α subunit as designated by the C-loop harboring the Cys-Cys pair that projects from the extracellular domain core-β structure to surround an agonist ligand. The Cys-loop position near the extracellular end is noted. The entire receptor complex with a solid surface is shown to the right. Note the cone shape of the receptor and that the subunits are tilted relative to the 90° plane of the membrane. Also, the projection of the C-loop towards the adjacent subunit in the counterclockwise direction is apparent. C: looking down on the receptor from the extracellular side reveals the arrangement of 5 subunits around the central pore, which is lined by the TM2 from each subunit. Note that the agonist-binding site is contained in a pocket between the α and adjacent non-α subunit defined on its outer face by the Cys-Cys pair. One α subunit is removed from the complex and ribbons are added to the structure to designate secondary structure as in B. The arrangement of β strands in a barrel-like portion directly over the TM domains is seen. Also, the extension of the C-loop around ligand is evident. D: similar to B, the receptor surface is added to show the relative positioning of each subunit (as labeled) and to look directly down the pore. The extracellular domains form the mouth of the pore, which is strongly constricted by a residue in TM2 that forms the gate and reduces the diameter of the non-ligand bound receptor to ~3 Å.
II. NICOTINIC RECEPTOR SUBUNIT STRUCTURE AND DIVERSITY AND RECEPTOR SPECIALIZATION
The significance of nAChRs to modulate biological function rests in their ability to translate the binding of an endogenous agonist, such as ACh, to receptor motion that will gate the channel to favor ion flow and induce a cellular response. From the time of its discovery in 1914 by Henry H. Dale (109) and Otto Loewi (283) (the two shared the Nobel Prize in Physiology and Medicine in 1936) as an agent that decreases heart rate, ACh was recognized as an endogenous signaling compound, synthesized from choline and acetyl-CoA, through the action of choline acetyltransferase, that alters cell function. Notably, preceding this discovery was the seminal report from Claude Bernard that skeletal muscle contraction could not be produced by stimulation of nerves in curarized frogs (56). His historical finding was followed by the initial description of the neuromuscular synapse in the early 1860s by his former student W. F. Kühne (1837–1900) and by W. Krause (1833–1910) (251). Finally, in 1905, John Langley reported that a plant alkaloid, nicotine, produced effects consistent with the requirement of a receptor-mediated response on the nerve endings in the autonomic system (259). One of the lasting contributions from Langley’s studies was his proposal that the pharmacological agents being tested worked through receptors. Although this concept was immediately grasped and extended by the immunologist Paul Ehrlich, we now know this insight was a pivotal intellectual jump in how a ligand could initiate and modulate a physiological process (see Ref. 54 for an extensive and insightful discussion).
The fundamental functional studies of Sir Bernard Katz, Sir John C. Eccles, and Stephen Kuffler laid the groundwork for much of our current knowledge of cholinergic synaptic transmission at the neuromuscular junction (137, 138, 230, 231). Earlier seminal contributions to the field of synaptic transmission were the discovery of the quantal nature of acetylcholine release while studying the neuromuscular transmission (103, 117, 307). Following the initial extensive and elegant work on the transmitter release process, Katz and colleagues (116, 144, 230–232) turned their attention to the postsynaptic mechanism by which ACh activates its receptors. Notably, in the mid 1950s, del Castillo and Katz (116) reported that receptor activation and receptor occupation were separate steps. Indeed, Katz and Thesleff (232) and Fatt (144) demonstrated that the rate of development of desensitization increases markedly with drug concentration. The use of microiontophoresis, first developed and used by Nastuk (338), enabled Katz and Thesleff (232) to measure with more reliability the fast events, revealing the kinetics of the process, assuming that the receptor molecules can change from an “effective” to a “refractory” state, and showing that the dose-effect relationship, when agonist is applied iontophoretically, has an S-shape, rather than a linear, start (232).
All ligand-activated ion channels share a similar architecture and function. First, the constituent proteins are, by necessity, transmembrane to create a hydrated receptor channel that is also permeable to selected ions. This basic structural plan subjects the protein to meeting the regulatory demands placed on it by the extracellular, intracellular, and transmembrane compartments that simultaneously impact upon receptor expression and function. Second, ion-channel receptors reside in a constant equilibrium between open and closed states. Therefore, these receptors must contain primary structural components that are responsive to and regulated by the presence of external compounds such as activators (agonists), inhibitors (antagonists), or compounds that modify the efficacy of these agents. Furthermore, modifications of the cytoplasmic domain by phosphorylation, membrane fluidity, or redox state ensure proper receptor placement and magnitude of signaling consistent with the cellular demands. Understanding the molecular mechanisms contributing to these fundamental aspects of nAChR biology has proceeded rapidly in the last several years as reflected by the dynamic growth in our knowledge of how they work and how they participate in normal as well as abnormal physiology (see Ref. 83).
A. Receptor Structure Overview
The cloning explosion of the mid 1980s revealed that the Torpedo nAChR subunits are closely related to an extended family of cDNAs that in mammals encode 16 structurally homologous subunits with primary structural identity (Table 1). As shown in Figure 1A, all subunits have the following: 1) a conserved extracellular large NH2-terminal domain of ~200 amino acids; 2) prominent and conserved, three transmembrane (TM) domains; 3) a cytoplasmic loop of variable size and amino acid sequence; and 4) a fourth TM domain with a relatively short and variable extracellular COOH-terminal sequence. This arrangement forms the basis for the classic designation of a 3+1 configuration based on the location of TM domains relative to each other. Also common to all subunits of this extended family of ligand-gated ion channels is the occurrence in the first extracellular domain of a cysteine-loop (Cys-loop) defined by two cysteines (Cys) that in the mammalian subunits are separated by 13 intervening amino acids. Subunits are next classified into α- and non-a subunits based on the presence of a Cys-Cys pair (residues 191–192 in Torpedo α1) near the entrance to TM1. The Cys-Cys pair is required for agonist binding (229) and its presence designates the subunit as an α-subtype (287). Based on their major site of expression, nAChRs are subdivided into muscle or neuronal subtypes.
TABLE 1.
Chromosomal location and genetic features that distinguish human nicotinic ACh receptor subunits
| Receptor NCBI | Chromosome | Exons | mRNA | Protein | |
|---|---|---|---|---|---|
| Name/Subunit | Number/Band | Gene, kb | (Coding) | (Coding bp) | (Amino Acid) |
| CHRNA1 | 2q31.1 | 16.64 | 9 | 1816 | 482 |
| CHRNA2 | 8p21.2 | 18.51 | 0 | 2684 | 529 |
| CHRNA3 | 15q25.1 | 28.24 | 6 | 2321 | 622 |
| CHRNA4 | 20q13.33 | 14.75 | 6 | 2206 | 627 |
| CHRNA5 | 15q25.1 | 29.71 | 6 | 3578 | 515 |
| CHRNA6 | 8p11.21 | 15.93 | 6 | 2164 | 494 |
| CHRNA7 | 15q13.2 | 142.25 | 10 | 6162 | 534 |
| CHRNA9 | 4p14 | 19.63 | 5 | 2015 | 479 |
| CHRNA10 | 11p15.4 | 5.8 | 5 | 1945 | 450 |
| CHRNB1 | 17p13.1 | 12.65 | 11 | 2557 | 501 |
| CHRNB2 | 1q21.3 | 12.25 | 6 | 5866 | 502 |
| CHRNB3 | 8p11.21 | 39.99 | 6 | 2293 | 458 |
| CHRNB4 | 15q25.1 | 17.48 | 6 | 2972 | 498 |
| CHRND | 2q37.1 | 10.48 | 12 | 2941 | 517 |
| CHRNG | 2q37.1 | 6.6 | 12 | 2187 | 517 |
| CHRNE | 17p13.2 | 5.3 | 12 | 3030 | 496 |
Characteristics for the human neuronal nicotinic ACh receptor family are given.
Muscle nAChRs consist of five subunits: α1 and 4 non-a subunits named β1, δ, γ, and ε. Only two receptors are constructed from this complex subunit pool; one of the subunit composition α1, β1, δ, and γ or α1, β1, δ, and ε, each in the stoichiometry of 2:1:1:1. The relative level of expression of these receptors is based on muscle innervation (below). Neuronal nAChRs can be homopentamers or heteropentamers. To date, seven α-like subunits, termed α2, α3, α4, α5, α6, α7, α9, and α10 (α8 was identified from avian libraries and has not been found in mammals; Refs. 113, 184, 215) and 3 non-α subunits (termed β2, β3, and β4) have been cloned from neuronal tissues. These receptor subunits were so named because they were cloned from neuronal-like cells such as the pheochromocytoma cell line, PC12, or brain-derived cDNA libraries. While most are indeed expressed by neurons of the central and peripheral nervous systems, such a designation can be misleading. There is now ample evidence that many of these nAChR subunits are expressed by many nonneuronal cell types throughout the body (95, 168, 234, 255, 426). In fact, some receptors (such as α7, α9, and α10) have highly specialized functions including those pertaining to regulation of signaling mechanisms used by sensory epithelia and other nonneuronal cell types (see below).
The early studies of the Torpedo nAChR established the first structural definitions that are very much relevant to all subsequently identified nAChR subunits. All functional members of the Cys-loop family of ligand-gated channels are formed from a pentameric arrangement of subunits to create a central pore. Because nAChR subunits exhibit a high degree of evolutionary conservation, studies of high-resolution X-ray crystallographic and electron microscopic analyses of proteins related to nAChRs have provided considerable insight into how structure imparts functional similarities and differences among all nAChRs. Such studies led to the detailed characterization of the 4.6-Å electron microscopic structure of the Torpedo nAChR (Fig. 1) and of the high-resolution X-ray crystallographic structures of the ACh-binding proteins (AChBP) from the snail Lymnaea stagnalis (2.7 Å; Refs. 67, 434), the sea snail Aplysia californica (1.96–3.4 Å; Refs. 205, 474), and freshwater snail Bulinustruncatus (2.0 Å; Ref. 79). These AChBP are secreted as homopentamers that resemble nAChR-like complexes but lack the transmembrane and cytoplasmic domains. For this review we have omitted differences in the detailed structures of these models, which are described in detail in the original studies, to focus on receptor features that are in general applicable to most subunits.
The Torpedo nAChR, as shown in Figure 1B, appears as a conelike structure that traverses the lipid bilayer. The prominent extracellular domain is composed of β-strands that align in a configuration termed a β-barrel. The four TM domains are α-helices neatly packed around the central hydrophilic ion pore. TM helix 2 lines the pore (Fig. 1C). TM4 is away from the pore and mostly interactive with the lipid bilayer. TM helices 1 and 3 complete this helix bundle by positioning opposite to each other and rotated by 90° relative to TM2 and TM4. As suggested by their name, the TM helices traverse the membrane completely (Fig. 1B), although ~25% of the helix of each TM segment extends beyond the extracellular membrane surface (475, 476). The largest intracellular domain, which is located between TM3 and TM4, is depicted as a single large α-helix in the Torpedo nAChR subunits (Fig. 1B and Ref. 476). However, this is not as likely to be generalized to the structure of other nAChR cytoplasmic domains. Rather, a mix of α-helical and β-strand structures is expected. The exact folding pattern of the large cytoplasmic domain reflects both the novelty of the primary structure of specific subunits and the demands placed on the domain for providing its specific cellular function. The COOH-terminal domain of varied length follows TM4 on the extracellular surface.
When looking down the receptor from the outside towards the pore (Fig. 1C), the overall “β-barrel” configuration of the extracellular domain is evident. Also visible is the extended β-loop that contains the Cys-Cys pair of the agonist-binding site. This extended loop appears to partially “wrap” around the outside of the adjacent subunit in the counterclockwise position. This loop and a cleft formed at the interface between the neighboring subunits create the agonist-binding region and are essential to the agonist-induced receptor motion that gates the ion channel, as returned to below. The highly conserved Cys-loop is located adjacent to the membrane where it forms a modified loop structure whose distal amino acids are positioned in close proximity with the extracellular membrane surface and extended portions of TM helices 1 and 3 (Fig. 1). The second TM domain lines the hydrated pore. The outward face of the fourth TM domain is mostly in contact with the lipid bilayer where it forms a receptor-lipid interface (Fig. 1, B and C). When the protein surface is added to the nAChR model (Fig. 1D), the pore itself is relatively large at the mouth of the receptor consisting of the circled extracellular domains, and it becomes strongly constricted by the TM2 ring. This produces the ion gate in the closed receptor. Also evident is that, as with most proteins, the location of the Cys-loop and Cys-Cys pair in the primary structure (Fig. 1A) is not predictive of their relative location in the three-dimensional structure of the nAChR α subunits, nor does it predict easily how these highly conserved amino acids participate in receptor activation and function.
B. Ligand-Binding Site
Ligand-binding and functional assays in combination with site-directed mutagenesis, Cys-replacement scanning mutagenesis, and chemical modification were the first approaches used to define how ligand bound to the receptor and transmitted a signal for channel activation or gating (59, 84, 228, 229). More recently, these methods have been complemented and extended by advancements in defining receptor structure at atomic resolution through the high-resolution electron microscopy visualization of the Torpedo receptor by the Unwin group (476) and X-ray studies of the crystallized AChBP from mollusks as noted above. Basically, when a ligand such as nicotine binds, it does so in a pocket formed at the interface between the α subunit and “back” face of the adjacent subunit (Fig. 2A). This produces a rotational force in the β-barrel that produces torque on TM2 to rotate it from a hydrophobic-based, channel closed configuration to a more open hydrophilic channel that favors the ion passage. How this is accomplished is a masterpiece of structure and motion (Fig. 2B).
FIG. 2.
The ligand binding site and the proposed mechanism for gating the ion pore. A: in this depiction, an agonist-binding α subunit (dark blue) and a structural β subunit (in light blue) are shown with a solid surface looking from the extracellular side with the subunit pair slightly tipped away from the pore. When agonist is bound (as shown for nicotine, red), the α C-loop is moved towards the structural subunit to cap the agonist-binding site and effectively encase the ligand in the deep cleft formed between the subunits. The α Cys-Cys pair (187–188) is in yellow. Other residues interacting with the ligand from the α subunit are colored green and from the β subunit are colored in orange. The circled region is enlarged and the surface removed to reveal in B the amino acids within the agonist-binding site that interact with nicotine. The same color scheme is used, and the residues interacting to form the agonist-binding site are named and numbered. The arrows indicate β-strand structure. The weak lines interacting with nicotine (whose electrostatic surface is in light red) are hydrogen bonds. Certain key residues include tryptophan 143 (W143) from the α subunit which contributes to forming the base of the agonist-binding site and α-tyrosine 185 (Y185), which is important to stabilize the ligand within the pocket upon entry. In the α5 nAChR subunit, this residue is an aspartic acid that introduces a potentially negatively charged group into the pocket to inhibit ligand binding. As indicated by the extent of the molecular surface of nicotine (shown in transparent red), these hydrophobic residues from both subunit faces further stabilize the ligand in the pocket through van der Waals interactions, and other residues not shown (including D85, located near W143) also contribute to ligand binding through stabilizing the position of pocket residues. [Adapted from the 2.7-Å resolution X-ray structure of the AChBP (Protein Data Bank ID 1I9B.pdb) and the images generated in UCSF Chimera by Pettersen et al. (375).] B: upon binding of agonist and capping of the ligand-binding site (1), rotational motion in the β-strands is transmitted through the subunit (2) to residues that are near the TM domain-membrane interface. At this point, the rotational motion imparts two important interactions. The first is to move the loop between β-strands β1 and β2 towards the linking sequence of TM2 and TM3. This positions an invariant valine (V44) into the hydrophobic pocket that is created by the proximity of proline-272 (P272) and serine-269 (S269). These amino acids, or conservative changes, are present in most nAChRs. At the same time, the β10 strand moves counterclockwise to position arginine-209 (R209) towards glutamic acid-45 (E45; also β1 strand) to form an ionic (salt) bond. These interactions result in the rotation of TM4 ~15° to move the hydrophobic gating residues [valines (V255) and (V259) and leucine (L251)] away from the pore and the polar S248 and S252 toward the widened channel. The relief of the gate allows the channel to completely hydrate and conduct ions (5). Residues at the extracellular and intracellular faces (e.g., E241) ring the channel. These residues vary among subunits and receptors as polar and/or charged and contribute to determining the relative ion current through the pore. Also, highly charged rings of amino acids such as E241 enhance certain ion permeability such as by Ca2+. [Model shown is based on the original study of Unwin (475) taken from electron microscopy studies of channel gating from the Torpedo nAChR (Protein Data Bank code 2BG9) and from high-resolution studies of the AChBPs (see text for details).]
The agonist-binding site is a hydrophobic pocket formed at the interface between adjacent subunits (Fig. 2A). In all cases, the “front” or “positive” side of the binding site is produced by an α subunit (α1, α2, α3, α4, α6, α7, or α9) where the Cys-Cys pair is required. The “back” or “negative” face of the agonist-binding site is composed by at least three amino acids of each the α10, β2, β4, δ, γ, or ε subunit. The α5, β1, and β3 subunits assemble in the receptor complex in the fifth subunit position; they do not directly participate in the formation of the agonist-binding site. The α5 and a10 subunits do not bind agonists despite their definition as α subunits, because key residues (see below) required for agonist binding are not conserved in these α subunits.
The majority of the binding pocket (“positive” face) is contributed by a loop in the α subunit (termed the C-loop; see Fig. 1B and Fig. 2A) that at its apex contains the Cys-Cys pair (Torpedo α subunit residues 191–192). This loop extends like an interlocking finger around the face of the adjacent subunit. In addition to the Cys-Cys pair, other residues required for ligand binding are predominantly hydrophobic aromatic amino acids, including aTyr 93, αTrp 149, αTyr 190, and αTyr 198 (80, 229, 431). Notably, the inability of α5 to bind nicotinic agonists is due to the substitution of an aspartic acid for the Tyr198 residue. On the “negative” face, the major residues that contribute to ligand binding are L112, M114, and Trp53 (also Torpedo numbering). In general, the identity of the positive-side hydrophobic residues determines ligand affinity, whereas the residues contributed by the negative face determine ligand selectivity. Analysis of the nAChR structure also reveals that the ligand is well buried in this pocket where it becomes nearly engulfed by the surrounding protein structure of the subunit interface (Fig. 2A). Because of this tight interaction, the identity of amino acids tolerated in this region is limited and often imparts highly local physical constraints on the protein movement or agonist binding.
C. Channel Gating
Ligand binding is converted by the receptor structure into channel opening within microseconds, suggesting that the entire protein structure is well tuned to convey (or at least accommodate) rapid conformational change. This also explains the need to conserve the sequences in these portions of the receptor, and the failure to do so is now linked to several diseases including inherited myasthenia syndromes and some forms of epilepsy (143, 214, 300, 446, 447). Before three-dimensional structural models produced a more unified picture of the nAChRs, early mutagenesis studies placed residues important to the gating motion of the receptor throughout the extracellular domain (84, 99, 348). These studies and those noted previously also defined that going from ligand binding to channel gating is a process requiring several distinct changes in the protein structure. Now we can rationalize how seemingly small deviations in sequence play an important role in receptor specialization as reflected in channel gating.
How ligand binding is converted into motion to open the nAChR channel has been suggested largely through methods of computer simulation. The emerging model (Fig. 2B) indicates that when ACh or nicotine binds to the nAChR, there is a significant rearrangement of hydrogen bonds among invariant amino acids near the binding pocket, including aspartic acid-85, polar groups of the main chain, and even a trapped water molecule (65, 171, 204). In particular, there is a convergence of side chains of invariant aromatic residues towards the ligand from both the α subunit (positive) and negative subunit faces which interact through hydrophobic (van der Waal) interactions. Finally, the C-loop moves a considerable distance (~11 Å) towards the receptor core, allowing the Cys-Cys pair to interact with the ligand and residues in the “F-loop” of the negative subunit face. This in effect caps the ligand binding site to trap the ligand deep inside as seen in Figure 2A (65, 171, 205). When this occurs at both ligand binding sites, sufficient torque is generated through the receptor, via alterations in the relative position of the β-barrel-like loops, to rotate the extracellular surface of the pentamer and, in turn, influence the relative position of residues near the extracellular segment of TM2 and relocate residues critical to channel gating.
The ion pore created by TM2 is critical to establishing the ion gate, selectivity, and channel conductivity. Therefore, the means by which this is mechanically accomplished provides considerable insight into how subunit-specific nAChR function is imparted (Fig. 2B). Because TM2 lines the pore, it also harbors amino acids that contribute to the channel gate. In the non-ligand-bound receptor, the TM2 helices from the five subunits form a barrier to ion flow due to placement of hydrophobic residues near the midpoint to slightly off-center towards the cytoplasmic side of the channel. They project into the putative channel pore to form a narrow (~3 Å) constriction (Fig. 1D). The importance of maintaining the fidelity of these amino acids is demonstrated when more hydrophilic amino acids are substituted either by mutagenic methods or in certain epilepsies. These mutations produce a partial relief of the gate and increase channel permeability nonspecifically (254, 269). To open the channel to ion flow, ligand binding induces rotation of the extracellular domain, and this is translated into rotation of the TM2 helices. Basically, this has three important consequences, including the transient removal of hydrophobic barrier residues from the pore, an increase in the pore diameter to ~8 Å, and movement of hydrophilic residues into the channel to support ion flow (Fig. 2B).
In the Unwin model (476), the rotational torque being generated in the extracellular domain from ligand binding is transferred to TM2 through interactions between residues of the extracellular domain, including the Cys-loop and the linker region between TM2 and TM3 (Fig. 2B). In early models, the Cys-loop was largely thought to perform the gating function. While it does lie within 5 Å of the “gating complex” near the TM2-TM3 linker, it now appears that the Cys-loop facilitates rapid movement (Fig. 2B) through interaction with conserved amino acids of this linker region. In particular, the interaction of residues from the Cys-loop with the TM2-TM3 linker acts as a fixed pivot around which TM2 rotates. In this model, the rotation of the extracellular domain moves the valine-44 in the turn linking the β1 and β2 strands towards the TM2-TM3 linker sequence where this residue fits into a hydrophobic pocket formed by proline-272 and serine-269. Notably, the proline residue is required in this position since its ability to isomerize into the cis-conformation appears to facilitate the TM2 rotation into the open channel conformation. Also, as revealed in structures of greater resolution, a second interaction occurs when a salt bridge between glutamate-45 and arginine-209 at the end of the β10 strand moves into proximity. The importance of this salt bridge has also been confirmed through site-directed mutagenesis, where disrupting its formation interferes with receptor gating (265). This salt bridge is conserved in all Cysloop family members, and valine-44 is present in the pocket between TM2-TM3 in most nAChR subunits. Although the proposal that a kink in TM2 is a component of the gating mechanism, evidence currently available from direct measurement and computer simulation suggests that the predominant gating motion is the 15° clockwise rotation of TM2. There appears to be no major alteration in secondary structure such as alteration of the α-helical TM structure (107, 209, 261, 325, 467). The rigidity of the Cys-loop appears to be a critical determinant of how far and how fast TM2 rotates in response to ligand-induced motion (196). In studies of chimeras between the Cysloop of human glycine receptors and chicken α7 nAChRs (195), the Cys-loop was observed to be required for coupling the allosteric effect of binding to channel opening via accelerating the rate of gating. This detailed study demonstrated that the Cys-loop plays a central role in fine-tuning the speed of the signal transduction and is required for accelerating nAChR activation kinetics. Therefore, subunit-specific differences in the Cys-loop and interacting sequences impart slightly distinct kinetics to the ligand-binding response.
Finally, in simulations of the receptor motion during gating (261), TM4 undergoes the greatest structural change relative to the other TMs during relief of the gate, including the significant outward bending of the helix at the extracellular face. This movement is in part due to the location of TM4 in the lipid environment where it has relatively few contacts with the protein relative to other TMs. This movement may be of additional functional significance, since TM4 contains a highly conserved cysteine residue that projects into the bilayer near the membrane-water interface (52). This conserved cysteine residue appears to be involved in receptor aggregation (including α7 nAChRs into so-called membrane lipid rafts; Refs. 72, 525) and interaction with cholesterol and other lipid-related molecules such as sterols (51, 304, 305). Consequently, manipulation of the membrane lipid content or the degree of receptor aggregation has the potential to modify the gating mechanism.
D. Importance of Subunit Diversity and Expression
The diversity of nAChR subunits is a major determinant of the specialized properties and functions of the mature receptors. For example, the subunit composition imparts a remarkable array of customized pharmacology and functions (e.g., Table 2). Receptor pentamers can be constructed from various combinations of α, β, and other structural subunits that do not participate in ligand binding. The mammalian high-affinity nicotine-binding receptor consists of at least α4 and β2 nAChR subunits (150, 311). The increased expression of this receptor (termed upregulation, see below) accounts for the majority of new binding sites following nicotine administration (150). However, this generalization is complicated by the fact that receptor stoichiometry can impact on the regulation of this receptor subtype function and upregulation. For instance, α4β2-containing nAChRs can be constructed to the final stoichiometry of (α4)2(β2)3, (α4)3(β2)2, and (α4)2(β2)2(α5) (339, 524). While all of these nAChRs bind nicotine with high affinity, it is the (α4)2(β2)3 nAChR that is most sensitive to upregulation by nicotine as measured by differences in conductivity and desensitization. The assembly of nAChRs of different stoichiometry adds to the potential receptor diversity as evidenced by the finding that interneurons of the hippocampus versus those of the thalamus appear to express either mixed or predominantly subtypes of one stoichiometry (185, 339). Furthermore, these differences in stoichiometry appear to also impart specificity of pharmacological agents (530) and even sensitivity to modulation by zinc (331). Modifications to the properties of this basic receptor subtype are also facilitated by the inclusion of the α5 subunit into α4β2 complexes. The inclusion of this subunit appears to enhance receptor assembly and expression, reduce the relative magnitude of ligand-mediated upregulation, and facilitate receptor channel closure (298, 388). Of note are recent findings showing that proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin-1β modify nAChR assembly in HEK293 cells transfected with cDNAs expressing various nAChR subunit combinations (161). Furthermore, TNF-α strongly promotes ligand-mediated upregulation of α4β2-nAChRs through a mechanism that requires p38 mitogen-activated protein kinase (MAPK) signaling (163). Consequently, the importance of assembly and interaction between inflammatory and cholinergic systems appears to be more complicated than previously expected.
TABLE 2.
Pharmacological evidence for distinct functional nicotinic ACh receptor subtypes in brain slices
| nAChR Current Types | Putative Subunits Involved | Neuron Types Expressing nAChRs | Choline as Agonist | Cytisine as Agonist | Choline as Antagonist | MLA Inhibition at 10 nM | DHβE Block at 10 µM | MEC Block at 1 µM | Bupropion Block at 1 µM | Nicotine Inhibition | Reference Nos. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type IA | α7 | Hippocampal interneurons; midbrain dopamine neurons; neurons from olfactory bulb and cortex | Full agonist | Yes, IC50 = 37 µM | Full agonist | >95% | 40% | <10% | 0% | IC50 = 100–1,500 nM | 16, 18, 25, 27, 145, 154, 316, 504 |
| Type II | α4β2 | Hippocampal SLM interneurons; midbrain dopamine neurons; neurons from thalamus, IPn, and catecholaminergic nucleus | Not an agonist Agonist | Yes, IC50 = 370 µM | Partial agonist | 0% | >95% | ND | 14% | IC50 = 20–125 nM | 16, 18, 504 |
| Type III | α3β4β2 | Glutamate axons to hippocampal interneurons; neurons from MHb, IPn, and dorsal medulla | Partial agonist | Yes, IC50 = 15 µM | Full agonist | 0% | 33% | 80% | 54% | IC50 = 13 nM | 16, 18, 527 |
| Type IV | α2β4/α4β4 | Neurons from MHb and IPn | ND | ND | Full gonist | 0% | 0% in MHb; 36% at 1 µM | ND | ND | ND | 527 |
Pharmacological classification of nAChRs in the rodent brain slices is based on patch-clamp analysis. ND, not determined; MHb, medial habenula; IPn, interpeduncular nucleus.
In some brain regions, additional subunits participate in formation of high-affinity nAChRs. In the basal ganglia, including the ventral tegmental area (VTA) and substantia nigra, the α6 and possibly the β3 nAChR subunits are included in α4β2 nAChR complexes to generate high-affinity receptors. At present, this is the only brain area identified where α6 and β3 are coexpressed with α4 and (32 nAChR subunits. This finding is highly relevant for Parkinson’s disease (385, 386). The outcome of expressing these subunits in different brain regions or subjecting them to different conditions, such as prolonged exposure to nicotine, can vary significantly and could account in part for the specific role these receptors play in the progression of this disease.
Receptor assembly from different subunits contributes to differences in other significant aspects of nAChR properties such as ion permeability and desensitization. Receptors composed of α7 subunits are known to desensitize rapidly and to have a high Ca2+:Na+ permeability ratio that exceeds that of the glutamate NMDA receptor, and the 3–4:1 ratio of most other nAChRs (8, 68, 78, 387). As a result, quite distinctly from other nAChRs and even other ligand-activated ion channels, the opening of α7 nAChR channels can impact on several Ca2+-dependent mechanisms, including activation of second messenger pathways (328, 456).
The means by which specific nAChR subunits determine the relative permeability to Ca2+ can be rationalized in recent structural models. Ion selectivity of the pore is in part determined by amino acids that line the ends of TM2 to form either a cytoplasmic ring and/or an extracellular ring (e.g., Fig. 2B). These residues are always hydrophilic, and their charges determine which ions pass through the pore. When polar, uncharged residues comprise this ring, as in the muscle nAChRs and nAChRs harboring α3 subunits, the Ca2+ permeability relative to Na+ is low. In homomeric α7 nAChRs, on the other hand, this ring is composed of glutamic acid residues (e.g., shown as E241 in the Fig. 2B diagram) that impart the remarkably high Ca2+ permeability to this channel. This was demonstrated when alteration of these residues to other hydrophilic amino acids reduced the Ca2+ permeability to levels expected of other nAChRs (100). Of note is that a ring of glutamates in the extracellular milieu is likely to be mostly protonated, whereas the same residues lining the intracellular face are more likely to be ionized to various extents depending on the metabolic state of the cell (1, 408). The selection filter also determines which ions pass through the receptor. For example, when residues lining the GABAA receptor channel are substituted for those in the nAChR, the resulting channel conducts anions rather than cations (170).
Coexpression and assembly of α7 nAChR subunits with other nAChR subunits influence the ion permeability of the resulting receptors. For example, nAChRs made up of α7 and β2 nAChR subunits have pharmacological properties distinct from those of homomeric α7 nAChRs (240). Coassembly of α7 with α5 nAChR subunits results in receptors with distinct desensitization properties and ion permeability relative to the homomeric α7 nAChR (see Refs. 179, 515). This is also true of other nAChR subtypes. The channel kinetics of nAChRs made up of α5, α3, and (32 nAChR subunits are slightly different from those of α3β2 nAChRs (488, 491). More dramatic changes in nAChR channel kinetics are observed when the α5 nAChR subunit incorporates into receptors with the α3 and β4 nAChR subunits; the burst duration of α3α5β2 nAChR channels is almost threefold longer than that of α3β4 nAChRs (488, 491). Notably, the α3β4 nAChR is already very different in function from α3β2 receptors. Although these are only a few of the increasing examples of impact of subunit heterogeneity on functional and pharmacological properties of mature nAChRs, the important message is that local regulation of subunit assembly dictates the properties of the mature channel.
E. Antiquity of nAChRs and Coevolution of Predator-Prey Relationships
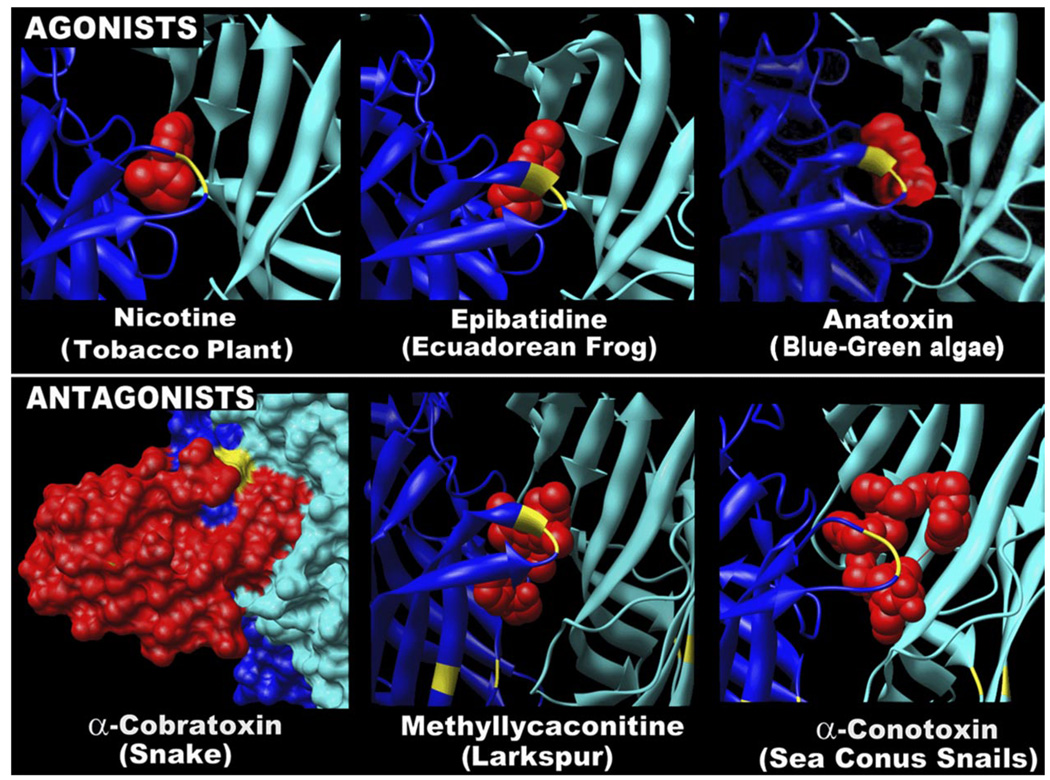
The antiquity of a biological system and its importance to survival can in part be assessed by how many predators use it as a target for capturing prey or as a means for protection against predation. The nAChR system is an excellent target for toxins because it plays a central role in regulating functions important to life and escape from predation (e.g., muscle contraction and autonomic nervous system function). Furthermore, the basic structure of the ligand binding site of nAChRs has been retained with remarkably little variability throughout evolution, making it an excellent structural target for a toxin. This also means that toxins can function as either potent agonists or antagonists. There are abundant examples (e.g., see Fig. 3) of compounds that target nAChRs and are used both as predatory weapons and defensive measures against predation (110).
FIG. 3.
Toxins that have coevolved to interact with nAChRs can be agonists or antagonists. A strong force in driving evolutionary success is the interrelationship between predator-prey strategies. Because the origin of nAChR dates to the earliest of organisms, and these receptors have acquired important roles in animal motility and nervous system function, they are excellent targets both for predation and defense. Shown above are structural models of binding between nAChRs and a variety of toxins. Toxins are in red, the α subunit is in dark blue, and the structural β subunit is in light blue. For α-cobratoxin, the protein surface was added to show the very tight fit between the toxin and the nAChR binding site. Several points are made. 1) The toxins come in a variety of forms. This includes the elaborate proteins produced in snake venoms to the simple molecules of plants used for defense against predation. 2) The toxins can function as either agonists or antagonists. 3) Note the interaction between toxin and receptor that is in general centered at the ligand binding site (note the yellow Cys-Cys pair that usually wraps the toxin at the site of ligand interaction). The exquisite refinement of toxin structure to bind the nAChR also indicates that this site in the nAChR has remained relatively invariant through its evolutionary history.
Probably the most notable nAChR-targeted toxin, nicotine, is produced by plants as a defense to predation (Fig. 3). While we know nicotine as the active ingredient in tobacco, its evolutionary origin was as a potent natural pesticide produced by the tobacco plant to ward off predatory insects. This role is so effective that it found use as a pesticide throughout the world (including the United States) until the mid 1960s when it was sprayed on agricultural as well as ornamental plants. One insect has escaped the ill effects of nicotine, Manduca sextans or the tobacco horn worm. While nicotine binds the nAChR to activate and subsequently desensitize it, this insect eats the tobacco plant without ill effects. Manduca exhibits two adaptations to tolerate the effects of nicotine. The first is altered nAChR amino acid sequences that limit the affinity of nicotine for the nAChR (136). The second is the development of the functional equivalent to a blood-brain barrier. In this case, astrocytes that wrap neurons also express nicotine-binding proteins that function to scavenger nicotine and release it back into the surrounding hemolymph away from the neurons (48).
Like insects, humans have several adaptations that allow the use of nicotine to be tolerated. The most prevalent neuronal nAChR is ~50-fold more sensitive to nicotine than is the muscle nAChR. This differential potency allows nicotine to stimulate neuronal nAChRs preferentially and ensures the success of the tobacco industry in general. Metabolic degradation of nicotine and rapid clearance is a mechanism that protects neurons from greater nicotine concentrations, since nicotine readily crosses the mammalian blood-brain barrier and accumulates in the lipophilic brain environment to concentrations that may exceed plasma concentrations by one order of magnitude. Nevertheless, neurotoxicity to nicotine is not uncommon, as attested to by the recent increase in hospital emergency room visits by smokers who concurrently use the transdermal nicotine patch (503).
Toxins that target nAChRs do so with considerable receptor subtype selectivity, and they are produced by an extensive range of plants, bacteria, fungi, and animals. For the most part, there is a recurring convergent strategy to produce toxins that bind nAChRs at the agonist-binding pocket to modify receptor function (Fig. 3). The most valuable of these toxins to researchers proved to be α-BGT from the snake Bungarus multicinctus. Because this toxin binds to the muscle nAChR with great specificity and a near-covalent affinity, it was an invaluable tool in the purification of the first nAChRs (discussed above). Additional examples of snake toxins include α-cobratoxin (Fig. 3), which binds to the agonist binding site of the receptor and blocks receptor activation. Such toxins are not limited to the muscle receptor as seen in the Taiwanese krate snake. This snake produces “neuronal bungarotoxin” (also referred to as 3.1 toxin or K-bungarotoxin; Ref. 286), which preferentially binds to and inactivates. neuronal nAChRs that contain the α3 and β4 subunits. In this case, the specificity of the toxin appears to in part be controlled by the subtype of β nAChR subunit; β2-containing nAChRs are less sensitive than β4-containing nAChRs to inhibition by neuronal BGT.
Other nAChRs of diverse subunit composition can be targeted by the conotoxins that are present in extracts derived from poisonous cone snails from the south Pacific (351). The origin of the conotoxins extends at least to the Eocene period ~60 million years ago (351). Conotoxins comprise an extensive family of related, but distinct, peptides and proteins that produce paralysis when injected into their prey. Not unlike snake toxins, conotoxins can disrupt multiple components of neurotransmission including voltage-gated Na+ and K+ channels in addition to nAChRs (132, 351). α-Conotoxins include snail toxins that target muscle nAChRs and others that favor neuronal nAChRs (reviewed in Ref. 314). All α-conotoxins share a common structure of a fold comprising a short helix that is stabilized by a disulfide bond harboring a highly conserved proline important to ligand-binding site recognition. Other surrounding sequences in these toxins are highly divergent and impart specificity towards key receptor subtypes such as those composed of the α7 or the α3/α6 nAChR subunits. These toxins are now being widely examined for their therapeutic usefulness and as markers to identify the various nAChR subtypes.
In addition to nicotine, an nAChR agonist of considerable commercial importance is anatoxin-a (Fig. 3). This toxin is a product of the blue-green algae, Anabaena, and can reach high concentrations during algal blooms common to ponds that serve as the summer water source of livestock. While this toxin exerts much of its effect through targeting muscle nAChRs, it was recognized over two decades ago to also interact with nAChRs expressed by ganglionic receptors (38). Its ability to activate in central nervous system (CNS) neurons nicotinic currents sensitive to α-BGT was among the first indicators that functional α7 nAChRs could be distinguished from other nAChRs in neurons of the mammalian brain (38).
More recently, epibatidine, an alkaloid from the skin of the Ecuadorain tree frog Epipedobates tricolor, revealed another example of how a nicotinic agonist can produce toxic effects (111, 130). In addition to being a potent analgesic, when injected into mice at a relatively low dose (0.4 µg/mouse), this compound produced straub tail reaction. The major target of epibatidine is the α4β2 high-affinity nAChR, although other nAChRs are targeted with various affinities (e.g., Ref. 507). Derivatives of this toxin are now under investigation as a new class of phamaceutical agents for treatment of numerous diseases, including Alzheimer’s disease (AD) (135).
Finally, the alkaloid methyllycaconitine (MLA) emerged as a potent and specific competitive antagonist that inhibits muscle, α7-, α6-, and α3-containing nAChRs (30, 326, 445). The alkaloid is derived from the larkspur (genus Delphinium), which is of great economic interest since estimates of its cost to ranchers in poisoned livestock exceeds many millions of dollars annually. Similar to most nAChR poisons, MLA binds to the receptor agonist-binding site (Fig. 3) in a manner similar to that of α-BGT to block agonist binding and receptor activation.
III. REGULATING NICOTINIC RECEPTOR EXPRESSION
A. Transcriptional Regulation
The first level of regulating the regional specificity of nAChR expression is through transcriptional control of subunit expression. Cell-specific regulation of nAChR transcription was observed in early studies of cultured cells including muscle cell lines and others such as the bovine chromaffin cell line PC12 (62–64, 118, 397) whose respective nAChR subunit composition (and corresponding functional and pharmacological properties) differed both qualitatively as well as quantitatively during in vitro development. Similar observations were made on tissues at various states of differentiation in vivo (e.g., Refs. 250, 500). However, the advent of cloning of individual nAChR subunit cDNAs coupled with methods of in situ hybridization provided the necessary components to map nAChR subunit expression in the mammalian nervous system.
The autonomic nervous system is characterized by abundant expression of α3 and β4 nAChR transcripts, whereas α4 and β2 nAChR subunit expression dominates in the CNS. Some brain regions, including the medial habenula and the hippocampus, express multiple transcripts where many subunits (α3, α4, α5, β2, and exceptionally abundant β4) are colocalized. Other brain regions (e.g., VTA) exhibit highly restricted expression of certain subunits such as α6 and β3. Depending on the nAChR subunits coexpressed in different neuronal types, such as hippocampal excitatory versus inhibitory interneurons (below), the resulting receptors can assume distinct (and what may appear to be contradictory) modulatory roles within the same circuits (8, 15, 184, 501).
The coordinate expression of key subunits is strongly regulated in the brain during development (271, 398) and in injury models (238, 272). For instance, the α3 nAChR transcript generally dominates in the prenatal brain or in injured neurons, whereas its expression tends to be downregulated in the adult or healthy neuron, and α4 transcription is increased. Exogenous agents and trophic factors can also influence the relative expression of certain nAChR transcripts to alter the pattern of receptor expression and assembly. Therefore, understanding the regulation of nAChR subunit transcription has important implications to both developmental and regional differences in cholinergic functions in the mammalian brain.
Gene duplication and the resulting clustering of certain subunits in closely linked genomic regions has been an important contributor to the evolution of diversity in nAChRs (returned to in detail below). Therefore, it is not surprising that some of these transcripts are retained in functional units whose regulation is highly coordinated. This is particularly true of the highly conserved gene cluster consisting of the α3, α5, and β4 subunits that together form the dominant nAChR subtype in the peripheral nervous system (93, 94), and whose coordinate transcriptional regulation has been examined in detail by several groups (66, 217, 317, 510). In cell lines, this interaction of trans-activating components is also under the regulation of the Ras-dependent MAPK and pathways related to phosphoinositide-3-kinase (PI3K) and MEK activation whose response to trophic factors such as nerve growth factor (NGF) contributes to regulating transcript initiation. Subsequent studies have revealed that the DNA-binding Sp-1 transcriptional factor interacts in response to NGF with the c-Jun coactivator (317) to increase β4 transcription. Also central to restricting (or at least limiting) the expression of these transcripts to predominantly neuronal-like cell lines (Neuro2A and NGF-treated PC12) are interactions among other factors including SCIP/Tst-1/Oct-6 and transactivation by Sox10 (66, 268, 317, 513). These factors are absent in fibroblast and muscle cells and are only active at very low levels in PC12 cells not treated with NGF. Notably, in PC12 cells, these transcription initiation pathways may actually differ due to culture conditions or the origin of the PC12 line. For example, in the original PC12 line (194), NGF is a potent inducer of β4 transcription (217), but in PC12 lines that are defective in the expression of functional α7 nAChRs, NGF decreases (β4 nAChR subunit transcription (60, 397). Consequently, in addition to the direct regulation of promoter activation through identified factors, the cell status or possibly the coincident expression of other nAChR subtypes may be important components in determining the outcome of signaling cascades and the individuality of a cell’s transcriptional response.
The transcriptional regulation of the α3/α5/β4 gene cluster has been examined in studies using artificial chromosomes. These studies revealed long-range effects of promoter elements on coordinating expression of nAChR transcripts (510). Transgenic animals were constructed harboring a 132-kb artificial chromosome (PAC) that was isolated from a rat genomic library because it included the α3/α5/β4 gene cluster. In addition to the cluster, this PAC had a 26-kb sequence upstream of the β4 gene and a 38-kb sequence upstream of the α5 gene. A particular advantage of this approach is that regulation of expression could be measured within the normal context of the mouse, which includes components of the endrocrine and neuronal en vironments. Several E26 transformation-specific sequence (ETS) factor binding sites were identified that upon deletion led to substantially diminished expression of both α3 and β4, and to direct transgene expression of the reporter gene, LacZ, to major sites of gene cluster expression in multiple brain regions, ganglia, and peripheral systems. Thus these transcripts form a functional unit whose expression is in part regulated through the activation of long-range ETS binding sites. The likelihood of finding such master control elements for other nAChRs seems likely because gene groups referred to as “locus control regions” have been shown to regulate at a distance the expression of mammalian gene clusters in a cell- and tissue-specific manner during normal development (274).
B. Receptor Assembly
The assembly of a pentameric structure, unlike that of an even-numbered structure such as a tetramer, requires multiple mechanisms to overcome issues pertaining to assembly fidelity. Of utmost importance are mechanisms that screen for imprecise assembly or do not allow the number of functional receptors at the cell surface to exceed optimal numbers. While the most obvious method the cell uses to ensure correct subunit association is related to limiting the expression of individual subunits (returned to below), other signals must also be present in the receptors themselves to direct assembly when the expressed mixture of subunits is more complex. This problem is particularly relevant to the nAChR family where subunits expressed in heterologous systems such as Xenopus oocytes or HEK293 cells can interact in almost unlimited combinations to form functional receptors. For example, while the α7 nAChR is primarily a homomeric receptor in neurons (127), combinations of α7 nAChR subunits with α5, β2, or β3 nAChR subunits have been reported to form functional heteromeric receptors in some systems (240, 360, 515). If indeed all subunits can interact to form functional receptors and assembly through stochastic mechanisms dominates, the substantial number of possible receptors does not match the relatively few subtypes found and the consistency of the native subunit combinations across species. Therefore, consistent with pressures of natural selection acting to ensure the nervous systems maintain precise control over the components regulating neurotransmission, rules limiting assembly and expression of nAChRs are likely to be operative and tightly regulated.
Understanding the rules that govern assembly of nAChR subunits into functional receptors is at its infancy. What is clear is that cells employ multiple mechanisms to ensure nAChR assembly fidelity as is evident in the muscle nAChR system. First, the number of possible subunit combinations is limited by the regional and cell-type spe cific expression of subunit transcripts (250, 500). In the muscle, for example, despite the coexpression of as many as five distinct subunits, only receptors of well-defined stoichiometries are expressed: (α1)2β1δγ in noninnervated muscle and (α1)2β1δε at mature neuromuscular synapses. Several mechanisms including regulation of transcript expression and intrinsic properties of the primary structure converge to ensure this proper stoichiometry, and developmental regulation is achieved. In the immature muscle α1, β1, δ and γ nAChR subunit transcripts are made and receptors from these subunits are synthesized and transported to the cell surface. In receptors harboring the γ subunit, agonist-induced receptor activation results in a long-lasting open channel time. The large agonist-induced current in turn leads to local intermittent depolarization and adjustments to protein-protein interactions that favor receptor clustering. As the depolarization increases, transcription of the ε subunit is increased dramatically (183). The ε subunit protein outcompetes the γ subunit for assembly into the receptor. The receptors assembled with the ε subunit are more stable to degradation, aggregate at the neuromuscular junction to greater density and exhibit a more rapid response to agonist (96, 275, 324). This elegant coordination of regulatory mechanisms between transcription and assembly that is responsive to changes in the external environment appears to be a common feature of nAChR biology as will be returned to below.
Appropriate nAChR assembly requires the correct number of subunits to combine in the correct order. Studies of muscle nAChR assembly are the most complete, and these lead to two possible models. Green and colleagues (191, 365, 485) report that nAChR assembly proceeds in the endoplasmic reticulum where specific subunits are added sequentially to the receptor complex according to the conformations the complex assumes. In this model, nAChR subunits are synthesized, and initial polypeptide folding favors the rapid recognition and interaction between α-β-γ subunits to produce trimers that in turn form a structure favorable to the addition of the δ subunit and finally the second α subunit. In another model, a somewhat different route to assembly is proposed (59, 435, 493). In this scenario, dimers between α-γ and α-δ subunits are formed before these paired subunits subsequently interact with the β subunit to assemble the mature pentamer. Although these differences may be ascribed to the poorly defined impact of detergent solubilization on membrane multimeric proteins (485), these studies do share findings that are relevant to all nAChR assembly.
In addition to the extracellular NH2-terminal domain, the variable and large cytoplasmic domain between TM3 and TM4 contributes to defining the more subtle and conditional features that determine receptor expression and function. As described below, this intracellular do main, in addition to contributing to protein-protein interactions involved in nAChR assembly, subcellular localization, and stability, regulates nAChR desensitization (247). No less than 12 distinct functional binding motifs are present in the large intracellular domain of the δ nAChR subunit, and each has the potential to regulate assembly and expression of nAChRs at the neuromuscular junction (252).
A similar level of fidelity in nAChR assembly is achieved by cells of the brain. For example, the α4, α7, and β2 nAChR interact with each other to form functional receptors in heterologous systems such as oocytes. However, in hippocampal neurons expressing the α7, α4, and β2 nAChR subunits, the vast majority of functional nAChRs are pharmacologically identified as being distinctly α4β2 and α7 nAChRs (12). This is also true of α3, α4, β2, and β4 nAChR subunits, which can freely interact to form receptors but appear to exhibit considerable preference in the brain as well as ganglia to form mostly receptors of α3β4 and α4β2 subunit composition (150, 471). Nevertheless, considerable subunit promiscuity is possible as demonstrated in mice lacking the β2 subunit. When this major subunit is absent, a multitude of novel nAChR subtypes appears, suggesting much greater promiscuity when a major subunit is absent (527). While it is possible that the absence of the dominating β2 nAChR subunit unveils these minor activities, it seems more likely that subunit assembly into functional receptors follows favored pathways. Therefore, the control of subunit assembly into distinct receptor subtypes is likely to follow a diverse set of rules whose importance will vary according to the cell type and the combination of subunit expression.
Among the earliest indications that nAChRs are subject to significant cell-specific regulation of expression emerged from studies of different sublines of PC12 cells (60, 217, 397). As was noted above, in different laboratories, these cells were reported to regulate nAChR mRNA expression differently in response to nerve growth factor, and to exhibit dramatically different expression of α7 nAChRs. Careful comparative studies indicated that each of these PC12 lines differed in their ability to fundamentally assemble and express these nAChRs (60). This result has been extended substantially to suggest a more generalized importance of this mechanism to regulation of nAChR expression. Even though HEK293 cells are an overall excellent host for the transient and stable expression of most transfected nAChR subunit pairs (507, 508), functional expression of α7 nAChRs is not easily achieved in these cells (128). Upon transfection of the cDNA encoding α7 nAChR subunits, HEK293 cells reportedly express the corresponding transcripts and even make considerable protein. Yet, the number of functional receptors expressed on the cell surface was low and could vary by three orders of magnitude. These and other mechanisms are operative in a variety of cell subtypes and apply to different receptors to varying degrees. For example, Loring and colleagues (458) compared the relative expression of α4β2 versus α7 nAChRs transfected into five different cell lines (GH4C1, SH-EP1, CV1, SN-56, and CHOCAR). Each cell line expressed appropriate mRNAs (indicating successful transfection); however, the relative levels of expression of each receptor subtype varied significantly among the various cell lines. Only two of these cell lines expressed α7 nAChRs: GH4C1 cells expressed substantially greater numbers of surface receptors than did SH-EP1 cells, which exhibited poor assembly efficiency. All cell lines appeared to produce α4β2 nAChRs, although at considerably variable levels relative to each other. Therefore, cell and receptor identity combine to collectively determine the efficiency of nAChR expression on the cell surface.
C. Posttranslational Regulation
Posttranslational modifications that control the subcellular localization of the mature nAChR and its expression on the cell surface are of particular importance to regulating receptor function. Several subcellular checkpoints are in place to ensure only properly assembled receptors are expressed. One of these is that nAChR subunits harbor unique primary structures that ensure proper folding and preferential interactions between subunits. Studies of recombinant chimeric subunits containing sequences of the NH2-terminal domains of the α7 and the α3 (M1-S232) nAChR subunits indicated that a 23-amino acid region (glycine-23 to asparagine-46) contained residues required for correct association of the α7 subunit into a homopentameric receptor. Not surprisingly, the Cys-loop is required for proper domain folding and receptor expression (131, 485). This might also be conditional, since reducing agents such as dithiothreitol (176) can disrupt the role of this structure in receptor assembly and expression. Although the extracellular domain of the nAChR subunits harbors many of the key signals for receptor assembly, other regions of the proteins are also important. This includes sequences in the TM domains that if deleted from assembly mixtures reduce or abolish much of the assembly into mature receptors (493). Also, chimeric subunits that are constructed from the δ subunit NH2-terminal domain fused to the rest of the γ subunit can substitute for the δ, but not the γ subunits during AChR assembly. This suggests that regions within the COOH-terminal half of the chimera are required for complete assembly (140, 141).
Another significant assembly checkpoint to ensure only correctly assembled nAChRs are transported to the cell surface is the endoplasmic reticulum. Most nAChRs are not constitutively sent to lysosomes. Instead, they are retained in intracellular pools that range from ~65 to 85% of the total receptor number in a cell (147, 359, 397, 496). At least a portion of the intracellularly retained nAChRs can be transported to the surface if conditions permit (221). Protein degradation seems to be an important contributor to regulating concentrations of assembling receptor pools. This level of control is also necessary due to inefficient receptor assembly and transport. In fact, 80% of the synthesized subunits appear to improperly assemble or never leave the endoplasmic reticulum where they are then degraded (485). The process of retaining subunits and possibly fully assembled receptors and then degrading them may be an important component of regulating receptor number. For instance, decreasing the degradation of precursor subunits in the endoplasmic reticulum results in increased nAChR expression at the cell membrane (88). Also, the continuous exposure of cells to nicotine increases nAChR surface expression by reducing degradation of the intracellular pool of receptors (367, 394). This is an attractive mechanism for nicotine-induced receptor upregulation, even though there is no evidence that nAChRs once internalized can recycle back to the membrane (69, 70). Thus inhibitors of proteasome function block endoplasmic reticulum-associated degradation of unassembled AChR subunits, which in turn increases the availability of subunits for assembly into mature receptors that are trafficked to the cell surface.
Additional posttranslational modifications differentially influence the expression of nAChRs as revealed by studies conducted using heterologous transfection systems where receptor complexity can be controlled, in part, by the use of the desired cRNAs or cDNAs. When cRNAs encoding specific nAChR subunits are introduced into Xenopus oocytes, simple (α3β4) as well as more complex (muscle α1β1δγ) heteromeric receptors are assembled and expressed on the cell surface (341). In Xenopus oocytes, these heteromeric nAChRs are assembled and expressed with almost equivalent efficiencies as the homomeric 5HT3A receptor (341). However, when cRNAs coding the α7 nAChR subunit are introduced into oocytes, a variety of assembly intermediates ranging from monomers to nonproductive aggregates develop in the endoplasmic reticulum, and relatively few functional homomeric pentamers are transported to the surface (341). This dramatic difference in receptor assembly and expression indicates that different nAChRs are subject to mechanisms of regulation independent of receptor subunit complexity. Instead, receptor expression appears to be regulated by a combination of intrinsic structural features of the respective receptor and the ability of the cell to recognize and modify the structural sequence in a manner favorable to subsequent receptor expression at the surface.
Part of this regulation is achieved through the efficient N-linked glycosylation, and subsequent modification and trimming of these carbohydrate trees are well-recognized mechanisms regulating protein expression (59, 341, 486, 487). Multiple sites in the NH2-terminal domain of nAChR subunits are glycosylated. Some of these sites, including adjacent to the second Cys residue of the Cysloop structure, are highly conserved among different nAChR subunits. In general, studies of the muscle nAChR show that glycosylation is not required for subunit association, receptor assembly, association with calnexin, or formation and function of the Cys-loop (193). However, once the receptors leave the endoplasmic reticulum, proper glycosylation is required for their subsequent insertion into the plasma membrane (175, 455). Furthermore, glycosylation influences correct disulfide formation and participates in favoring proline isomerization of the Cys-loop structure (395).
The evidence also suggests that signals regulating nAChR expression are intrinsic to the receptor. One of these studies of α7 expression has shown that signals regulating expression are contained in portions of the receptor subsequent to the first extracellular domain. In these experiments genetic chimeras were constructed between cDNA regions encoding the large extracellular domain of the α7 nAChR subunit with the transmembrane and intracellular domains of the 5HT3A receptor subunit. Expression of the chimeras in heterologous systems revealed high-efficiency surface expression of a receptor that had most pharmacological properties of the α7 nAChR, including α-BGT binding (101). One mechanism to explain cell-specific expression of α7 nAChRs is now known. An elegant and detailed study revealed that palmitoylation of the α7 nAChR subunit is involved. Palmitoylation is a reversible, posttranslational process that takes place in the endoplasmic reticulum where palmitate is covalently attached to Cys residues to regulate the transport and function of many proteins (399). How this process is regulated remains to be clearly determined. However, it is likely to be dictated at least in part by local primary or secondary structures of the modifiable protein, since the α7:5HT3A chimera is ubiquitously and efficiently palmitoylated, while palmitoylation of α7 homomeric proteins can be rather variable and possibly related to local oxidation state. The extent to which this posttranslational system is operative on nAChRs in general is not yet experimentally determined. These considerations urge caution when nAChR expression is inferred from methods that rely solely on RNA detection or measurements of total protein levels.
Another mechanism emerging as an important modulator of nAChR expression involves association with chaperone proteins that transport receptors away from the endoplasmic reticulum. Among the chaperones shown to associate with nAChRs are calnexin, rapsyn, ERp75 and Bip (muscle or muscle-like receptors; Ref. 219), 14-3-3 β-protein (222), and RIC-3 (260). These chaperones associate with nAChR precursor subunits to enhance and favor the subunits’ folding into complete complexes as well as monitor the glycosylated state. Certain amines that have for many years been reported to enhance receptor expression, particularly nicotine, may also act as chaperones (102, 477). When compounds such as nicotine reach the endoplasmic reticulum, they are thought to interact with assembling receptor subunits to limit conformational changes (possibly through locking them into the desensitized state) and favor assembly. Finally, the idea that slowing assembly increases nAChR expression is also found to be true when cultured cells that express nAChRs are placed at 30°C (97).
Additional functional attributes can be assigned to the large cytoplasmic domain of nAChR subunits. First, this domain is important for regulating receptor assembly. In the early days of molecular manipulation (348), different studies demonstrated that while nAChRs could assemble from subunits where the cytoplasmic domain was largely deleted, efficiency of assembly was extremely poor. More recent reports indicate that assembly tolerates substantial deletions of the cytoplasmic domain because other sequences play key roles in receptor assembly. In one study (253), the assembly of α4β2 nAChRs was conducted in the presence of extensive sequence substitutions and/or chimeric protein construction. That study revealed that functional expression of α4β2 nAChRs depends on proximal, but not nested, sequences in the cytoplasmic domain and on specific sequences in TM3 and TM4. Pharmacological and functional properties of the α4β2 nAChRs were also modified by mutations of the large intracellular domain of the β2 subunit; the chimera and mutated nAChRs had altered sensitivity to agonists and antagonists and increased rates desensitization compared with the wild-type receptors. Highly conserved hydrophobic residues (leucines) within the cytoplasmic domain of the α4 and the β2 nAChR subunits have been identified as critical determinants of endoplasmic reticulum export and surface receptor expression (392). Phosphorylation of specific residues within the cytoplasmic domain of different nAChR subunits is another mechanism that regulates the efficiency of receptor assembly, expression, and function (192, 201).
The large cytoplasmic domain of the nAChR subunits also harbors sequences important to the distribution of receptors on the cell surface. For instance, sequences in the major cytoplasmic loop of the α3 subunit target (α3-containing nAChRs to the synapse of the chicken ciliary ganglion. In contrast, sequences within the cytoplasmic domain of the α7 nAChR subunits exclude α7-containing nAChRs from the synapse and favor their perisynaptic localization (465). Nonsynaptic localization of α7 nAChRs in the chick ciliary ganglion has been shown to contribute to ectopic neurotransmission (90). In addition, colocalization of α7 nAChRs with so-called “lipid rafts” may have specialized signaling impli cations related to regulating nonneurotransmitter systems (355, 525). In PC12 cells, for instance, lipid rafts are essential for the colocalization of α7 nAChRs and adenylyl cyclase within the plasma membrane and for regulation of activities via Ca2+ influx through the α7 nAChRs (355).
Although nAChRs are known to interact with postsynaptic PDZ complexes, specific sequences that facilitate this interaction have not yet been reported. Nevertheless, subunit specificity in these interactions is suggested by numerous findings. Postsynaptic density (PSD)-95 was shown to associate with α3- and α5-containing nAChRs, but not α4β2, α7, or muscle nAChRs. In contrast, PSD-93a associates with most neuronal, but not muscle, nAChRs. The soluble N-ethylmaleimide-sensitive factor (NSF) attachment receptor (SNARE) complex, generally assigned to the trafficking of glutamate receptors, also interacts with α7 nAChRs to enhance clustering of this receptor subtype (282). The cell-specific expression of dominant transport signals may account for the differential expression of a given nAChR subtype on the cell surface in the various brain regions. It could explain why in most CNS neurons β4 nAChR subunits are strongly present on axons, whereas in hippocampal inhibitory interneurons when present these subunits are mostly expressed on dendrites (165, 167). Consequently, nAChRs are likely to be localized to defined compartments on the cell surface based on a combination of their subunit composition and the presence of intracellular proteins that localize them to their final destination.
D. Upregulation
One of the earliest nAChR characteristics to be discovered was the rather curious property of these receptors to increase their expression (termed “upregulation”) when exposed chronically to nicotine (55, 373). In the smoker’s brain, upregulation can increase high-affinity nicotine binding by nearly fourfold relative to age- and gender-matched controls that have not been exposed to nicotine (373, 421). The mechanism by which nicotine increases the total number of high-affinity nAChRs, though poorly defined, is highly conserved among species.
The receptor that exhibits the greatest upregulation when exposed to nicotine is the α4β2 nAChR. Receptors assembled from this subunit combination form the highaffinity nicotine binding site (151, 215) and account for the vast majority of upregulated sites in the brain of smokers (55). As will be returned to below, it is also the first nAChR subtype to exhibit measurable decline in expression in the aged mammalian brain and especially in neurodegenerative disorders such as AD (236, 374). Genetic deletion of the α4 or the β2 nAChR subunit abolishes essentially all high-affinity nicotine binding to brain tissue and upregulation in response to chronic exposure to nicotine (151, 311). Furthe more, transfection of cells with the α4 and β2 nAChR subunits or expression of these in Xenopus oocytes leads to high-affinity nicotine-binding receptors that upregulate in response to prolonged exposure to nicotine (113, 184, 215).
Not all nAChRs upregulate in response to nicotine, or they do so to varying degrees. Measurements of the effects of nicotine on the expression of nAChRs assembled from defined, but varied, subunit combinations stably transfected into HEK293 cells revealed a dramatic contribution of both α and β receptor subunits to upregulation (508). For instance, prolonged exposure of HEK293 cells to saturating nicotine concentrations increased by 6- and 1.5-fold, respectively, the expression of α3β2 and α3β4 nAChRs. Similarly, while α4β2 nAChRs upregulate strongly, α4β4 nAChRs upregulate poorly in response to continuous exposure to nicotine. The systematic construction of chimeric β2/β4 nAChR subunits that contained divergent sequences of the opposite subunit and retained function revealed two regions in the extracellular domain that modulate nicotine-induced upregulation (403). Most notably, when the amino acid sequences 74–89 and 106–115 of the β2 nAChR were substituted in the β4 nAChR subunit, β4-containing nAChRs became highly sensitive to upregulation by nicotine.
Some nAChR subtypes may even be downregulated when exposed to nicotine. Prolonged treatment of rodents and monkeys with nicotine downregulates the expression of α6β3-containing nAChRs in the brain (257, 311, 332). However, in heterologous culture systems, nicotine appears to upregulate the expression of receptors assembled from α4/α6/β2/β3 input cDNA (363), and this may depend on numerous factors including ligand concentrations (483). Differences in relative subunit association and final receptor assembly have been proposed to explain these apparently conflicting results. The experimental resolution of the roles played by the α6 and the β3 nAChR subunits will shed light onto novel mechanisms that regulate nAChR assembly, transport, surface expression, and upregulation (or downregulation) by nicotine.
IV. FUNCTIONAL NICOTINIC RECEPTORS IN THE BRAIN: ELECTROPHYSIOLOGICAL STUDIES
Electrophysiological recordings from brain neurons provided a wealth of knowledge regarding the existence of multiple subtypes of functional nAChRs on the neuronal surface. Patch-clamp studies, in particular, contributed to understanding the properties of native neuronal nAChRs and led to the introduction of pharmacological tools for their identification (see Table 2). Furthermore, such studies assisted in the localization of native nAChRs on discrete neuronal compartments and enhanced our understanding of the role of various nAChRs in mediating or modulating synaptic transmission. Evidence from a multitude of studies converge to the conclusion that nAChRs are located at one of five primary locations: the cell soma, dendrites, preterminal axon regions, axon terminals, and myelinated axons on the neurons (e.g., Fig. 4 and Fig. 5 and Refs. 8, 15, 186, 214, 288, 502).
FIG. 4.
Nicotinic receptor modulation of hippocampal inhibitory circuitry. A: choline (10 mM) induces type IA currents in hippocampal interneurons of different species of animals and in human cortical interneurons. Type IA current results from activation of α7 nAChRs because it is sensitive to blockade by nanomolar concentrations of methyllycaconitine (MLA) or α-bungarotoxin (α-BGT). Type IA current is not blocked by bupropion (1 µM) or nicotine (100 nM), but is partially inhibited by DHβE (10 µM) or choline (50 µM). Nicotinic responses with these characteristics are not detected in neurons of mice with a null mutation in the gene that encodes the α7 nAChR subunit. In the presence of MLA (10 nM), ACh but not choline induces type II current in the interneurons. The poor efficacy of cytisine to induce type II current and the blockade of this current by DHβE (10 µM) indicate that it results from activation of α4β2 nAChRs. A low degree of activation of these nAChRs by ACh (10 µM) fails to induce action potentials; however, it triggers GABAergic PSCs in the interneurons, suggesting that the nAChRs are located on preterminal regions. Type II current is not sensitive to blockade by α-BGT (100 nM) or choline (100 µM) but is partially inhibited by bupropion (1 µM) or nicotine (100 nM). In the presence of MLA (10 nM), nicotinic agonists induce type III responses (AMPA EPSCs at −68 mV and NMDA EPSCs at +40 mV); the order of agonist efficacy is cytisine > ACh > choline. At 1 µM, mecamylamine inhibits type III nAChR responses. Furthermore, type III responses are blocked by nicotine (100 nM), bupropion (1 µM), or choline (30 µM). The pharmacological profile of type III responses suggests that they result from activation of α3β4/β2 nAChRs. B: choline-induced type IA current results in action potentials in interneurons and IPSCs in pyramidal neurons. As expected, MLA and tetrodotoxin blocked both types of events. C: concentration-response relationships for choline- and nicotine-induced inhibition of type IA, II, and III responses recorded from CA1 SR interneurons in rat hippocampal slices (16). D: a diagram of the major neurons in the CA1 field of the hippocampus and how the different nAChR subtypes modulate various aspects of inhibitory circuitry. In the pyramidal layer (py) there are the excitatory pyramidal neurons that are glutamatergic (GLU; green) and pyramidal associated interneurons that are GABAergic (GABA; dark blue). These interneurons extend dendrites both in the direction of the stratum radiatum (SR) where they interact with Schaffer collaterals (Schaf. Col.) and terminate in the stratum lacunosum moleculare (SLM) to interact with perforant path fibers. The majority of nAChRs on these neurons are of the type I (α7) subtype, which can also be located on some principal excitatory neurons. Axons, which also express type II (α4β2) nAChRs, extend from interneurons to interact with many excitatory neurons and other interneurons. In some cases, they can extend to other hippocampal fields via the alveus (alv). Other inhibitory interneurons expressing nAChRs (light blue) are located in the SR and stratum oriens (SO). The SR interneurons often express nAChRs of the types I and II. Type III (α3β4β2) nAChRs are present on glutamate axons innervating SR interneurons and possibly other interneurons. To the right, immunolocalization of nAChR expression in a coronal section of the mouse hippocampus CA1 that is matched approximately to the diagram is also shown. Colored arrows identify examples of the interneurons diagrammed in their respective region.
FIG. 5.
A diagram of nAChR control of dopamine neurotransmission of the basal ganglia system. This diagram shows the complex regulation of dopamine release by excitatory (Glu), inhibitory (GABA), and cholinergic (ACh) neurons. A complex variety of nAChRs participate in regulating these circuits as indicated by their differential subunit composition and location on neurons of different types. While subunit composition is indicated, this is not strictly defined and additional subtypes, especially those incorporating α5, are likely to participate in modulating this circuit. [Adapted from Gotti and Clementi (184) and Wonnacott (502).]
A. Somatodendritic nAChRs
Even though the psychological effects of nicotine have been recognized for centuries, it was not until the late 1980s that the existence of functional nAChRs in various brain regions was demonstrated (38, 335, 389). The development of drug-delivery devices that allowed fast delivery and removal of agonists was essential for accurate and reliable recording of nicotinic responses from CNS neurons, because most neuronal nAChRs, particularly those bearing the α7 subunits, quickly desensitize when exposed to nicotinic agonists. These devices include focal pressure ejection from patch pipettes filled with agonist, using a combination of gravity-driven agonist flow and a micro-solenoid computer-driven system regulating the performance of the U-tube, particularly if the aperture is less than 50 µm. The U-tube was used by Kristal et al. (248, 249) to study proton-activated conductance and also by Fenwick et al. (148) to study chromaffin cells, and was adapted by Albuquerque to study the α7 nAChR function (11–13, 320) with the inclusion of special ejection and uptake valves. The U-tube system has several advantages over other systems in studying various nAChR currents in CNS neurons (5, 7, 11–13, 319). First, it allows for fast exchange of solutions in the cells’ surroundings. Second, it prevents leak of agonists onto the nAChR-expressing cells. Third, it enables controlled application of agonists to a large field including and surrounding the cells under study. For even faster exchanges one can use a dual U-tube system (for further details, see Ref. 320). Also critical for the studies of neuronal nAChRs in their natural environment was the recognition, following cloning and expression studies, that most neuronal nAChR subtypes are not sensitive to blockade by α-BGT.
The first evidence that functional, native α7 nAChRs are expressed in cultured hippocampal neurons was provided in a study in which the nicotinic ligand anatoxin-a (see above) induced α-cobratoxin-sensitive, fast-inactivating whole cell currents (11). These currents resembled pharmacologically and kinetically the response induced by activation of chicken α7 nAChRs expressed artificially in Xenopus oocytes (104). Subsequent studies confirmed that rat hippocampal neurons in culture respond to nicotinic agonists with currents that are sensitive to inhibition by the α7 nAChR antagonists α-BGT, MLA, and α-conotoxin-ImI (368); these currents have been popularly referred to as type IA currents and cannot be detected in mice with a null mutation in the gene that encodes the α7 nAChR subunit (Table 2 and Fig. 4A) (12, 13, 25, 30, 352, 529).
The biophysical properties of the α7 nAChRs that mediate type IA currents in CNS neurons are rather unique compared with those of other nAChR subtypes. Thus, like heterologously expressed homomeric α7 nAChRs, native α7 nAChR channels have a brief open time (~ 100 µs), a large conductance (ranging from 71 to 105 pS; Refs. 9, 77, 78), a high permeability to Ca2+ relative to Na+ (78), and low affinity for agonists (12, 77, 78). In addition, native α7 nAChRs, similarly to ectopically expressed α7 nAChRs, are activated with full efficacy by the ACh metabolite and precursor choline (27, 319, 361).
Although ACh and choline activate α7 nAChR channels with similar single-channel open time and conductance, choline dissociates from the receptor more rapidly and, consequently, induces a less stable state of desensitization than ACh does (319). These findings led to the suggestion that a well-regulated balance between the two closely related endogenous agonists is essential to maintain the functionality of α7 nAChRs. There is evidence in the literature that cholinesterase inhibitors have differential effects on nicotinic cholinergic transmission depending on whether the transmission is mediated by fast-inactivating α7 nAChRs or slowly inactivating non-α7 nAChRs. Thus nicotinic synaptic currents have been recorded from chick ciliary ganglion neurons in response to stimulation of the presynaptic oculomotor nerve root with a suction electrode. The fast component of these synaptic currents is subserved by α7 nAChRs, whereas the slow component is mediated by non-α7 nAChRs (522). The cholinesterase inhibitor phospholine increased the amplitude and prolonged the decay-time constant of the slowly decaying component while having no significant effect on the rapidly decaying current (522). It appears that the termination of synaptic α7 nAChR activity is dictated by the kinetics of receptor desensitization rather than by the hydrolysis of ACh. One could then speculate that upon high-frequency stimulation of cholinergic inputs, accumulation of choline in the synaptic cleft would prevent ACh from inducing a more stable desensitization of α7 nAChRs.
It is yet to be determined whether under any circumstances choline rather than ACh serves as the endogenous neurotransmitter to activate α7 nAChRs. It is tempting to speculate, however, that during maturation of the nervous system choline acts as the primary endogenous α7 nAChR agonist, because expression of the ACh-synthesizing enzyme choline acetyltransferase lags behind the appearance of nAChRs in developing neurons (45, 87, 511). There is also the possibility that primitive organisms may use choline as the neurotransmitter given that α7 nAChRs are the oldest member of the nAChR family (262).
The location of functional α7 nAChRs on the somata of hippocampal neurons is supported by evidence from electrophysiological studies of outside-out somatic patch membranes (77, 319). The dendritic localization of these receptors, on the other hand, was demonstrated in direct and indirect experiments. For example, when the dendrites of cultured hippocampal neurons were reduced in length and number by treating the cultures with the microtubule-destabilizing agent colchicine, the peak amplitude of type IA currents decreased to 10% of the level found in control cultures (20). Furthermore, focal ACh application to small dendritic segments of hippocampal neurons in culture elicited type IA currents of variable amplitude that could be recorded from the cell body (20). Recently, activation of dendritic patches of nAChRs via caged carbachol photolysis demonstrated the presence of dendritic nAChRs in interneurons of rat hippocampal slices (239). In addition, expression of epitope-tagged subunits confirmed that α7 nAChRs are targeted to dendrites in cultured hippocampal neurons (509). Of interest, proteins that are ubiquitously distributed such as CD4 and interleukin-2 receptor α subunit (IL2RA), when fused to the M3–M4 intracellular loop from the α7 nAChR subunit, had their expression confined to dendrites of cultured hippocampal neurons (509).
In the mid 1990s, an explosion of research findings on native brain nAChRs followed initial scanty reports, most using brain slice preparations. Several laboratories confirmed the presence of α7 nAChR-subserved currents (type IA currents) in CA1 interneurons of the rat hippocampus (26, 28, 155, 225, 239, 316), and in midbrain dopaminergic and nondopaminergic neurons (379, 504). At nanomolar concentrations, the α7 nAChR-selective antagonists MLA (1–10 nM) and α-BGT (50–100 nM) were shown to inhibit agonist-evoked type IA currents recorded from interneurons of hippocampal slices (Fig. 4A) obtained from mice (31), rats (25, 28, 29), and human cerebral cortex (21).
The functional and pharmacological properties of nAChRs have been studied largely in the brains of shortgestation rodents, specifically mice and rats. In shortgestation species, the brain is very immature at birth, and the perinatal period, particularly encompassing the first three postnatal weeks, represents a critical time window during which the cholinergic system develops (263). Electrophysiological studies have demonstrated that the peak amplitude and net charge of type IA currents recorded from CA1 stratum radiatum (SR) interneurons in the rat hippocampus increases in an age-dependent manner during postnatal days 5–60, suggesting an increase in the density of functional α7 nAChRs from early postnatal ages to adulthood (32). In long-gestation species, including humans, non-human primates, and guinea pigs, the brain has a high degree of neurological maturity at birth (393). Hence, it may not be valid to extrapolate data from the cholinergic system of rats and mice to all mammals. A recent study carried out in hippocampal slices from guinea pigs revealed the presence of functional nAChRs in CA1 SR interneurons (23). The amplitude of type IA currents recorded from these interneurons increased with the age of the guinea pigs from postnatal day 8 to postnatal day 25 (23). At the end of the third postnatal week, the mean peak amplitude of type IA currents recorded from hippocampal CA1 SR interneurons was observed to be in the following order: mice < rats < guinea pigs (see typical recordings in Fig. 4A). It is, therefore, tempting to speculate that CA1 SR interneurons in the human hippocampus may be enriched with high density of functional α7 nAChRs. It remains to be determined whether the difference in the magnitude of type IA currents recorded from neurons of different species of animals represents variations in α7 nAChR density or in dendritic length. The observation that neurons in hippocampal slices from guinea pigs compared with rats and mice have more extensive dendritic branches (23) supports the secnd possibility.
Application of the α7 nAChR agonist choline to CA1 SR interneurons in rat hippocampal slices triggers sufficient depolarization at the soma and dendrites of these neurons to recruit Na+ channels and initiate action potentials (28). Choline-triggered action potentials result from direct activation of somatodendritic α7 nAChRs in the interneurons because they can be effectively inhibited by nanomolar concentrations of α-BGT and MLA and occur when glutamatergic transmission is inhibited by glutamate receptor antagonists (28). Accordingly, application of ACh or choline to the CA1 field of rat hippocampal slices triggers tetrodotoxin-, MLA-, and α-BGT-sensitive inhibitory postsynaptic currents (IPSCs) that can be recorded from CA1 pyramidal neurons (Fig. 4B; Refs. 14, 223). α7 nAChR-mediated fast synaptic currents have been successfully recorded from CA1 SR interneurons of the rat hippocampus (22, 154). Therefore, cholinergic stimuli, by solely activating synaptic α7 nAChRs in CA1 SR interneurons, can decrease the excitability of CA1 pyramidal neurons, thereby creating a third pathway of inhibition in addition to the known feed-forward and feedback glutamate-dependent inhibition (see scheme in Fig. 4D). The finding that α-BGT decreases GABAergic synaptic activity impinging onto CA1 pyramidal neurons in the kynurenine aminotransferase II knockout mice (31) lends further support to this concept.
In most brain regions, including the hippocampus, somatodendritic α7 nAChRs are not confined to cholinergic synapses. In general, they are in extrasynaptic sites. The rapid and pronounced desensitization of α7 nAChRs and their low affinity for agonists, including ACh and choline (27), raised the question as to whether ambient levels of the endogenous agonists could maintain any physiologically relevant degree of tonic α7 nAChR activity. The use of α7 nAChR-selective antagonists provided the initial evidence that, indeed, in rat hippocampal slices GABAergic synaptic activity is tightly regulated by a tonic degree of α7 nAChR activity (28). Thus perfusion of rat hippocampal slices with physiological solution containing α-BGT results in an increase of the frequency of IPSCs impinging onto CA1 SR interneurons. Analyses of the concentration dependence of α7 nAChR activation and desensitization in cultured hippocampal neurons revealed that desensitization was proportional to channel opening at low, but not high, agonist concentrations (above 100 µM ACh and 600 µM choline). At the high agonist concentrations, desensitization was more pronounced than expected for the probability of channel opening (319). Considering the cumulative charge carried through the α7 nAChR channel, the relative efficacy of ACh or choline was higher at concentrations below the EC50 estimated from the peak of the agonist-evoked currents (319). These findings can explain how agonist concentrations that trigger small, but long-lasting, responses can sustain a physiologically significant degree of α7 nAChR activation that may be particularly important for the regulation of Ca2+-mediated responses in α7 nAChR-expressing neurons.
In the early 1990s, investigators using patch-clamp analysis revealed the existence of functional non-α7 nAChRs on the somatodendritic regions of neurons located in the medial habenula (MHb) and interpeduncular system (267, 270, 333, 335) and in the hypophysial intermediate lobe cells (523). The initial studies in primary neuronal cultures were pivotal for the elaboration of profiles that facilitated the subsequent pharmacological identification of nAChR subtypes subserving responses recorded from neurons in brain slices.
Two pharmacologically distinct, slowly inactivating nicotinic responses were first characterized in an electrophysiological study of cultured hippocampal neurons (12). One of these responses, referred to as type II, could be recorded from ~10% of the neurons exposed to nictinic agonists in culture. Type II nicotinic currents have the pharmacological profile of responses mediated by α4β2 nAChRs ectopically expressed in different systems. Thus these receptors 1) are sensitive to inhibition by nanomolar to low micromolar concentrations of dihydro-β-erythroidine (DHβE); 2) are insensitive to inhibition by nanomolar concentrations of α-BGT or MLA; 3) recognize ACh, nicotine, and other nicotinic agonists with high affinity; 4) cannot be activated by choline; and 5) are partially activated by cytisine (5, 12). The other response, recorded from no more than 2% of the neurons in culture, was referred to as type III. The pharmacological profile of type III responses resembled that of nicotinic responses arising from activation of α3β4 nAChRs heterologously expressed in Xenopus oocytes or mammalian cell lines. These receptors are 1) fully blocked by low micromolar concentrations of mecamylamine, 2) insensitive to inhibition by α-BGT, and 3) partially activated by choline (27, 361). This receptor classification was subsequently expanded to include a fourth nAChR type (527), the type IV representing either α4β4 or α2β4 nAChRs that were identified in neurons of the MHb and interpeduncular nucleus.
Several groups have now confirmed the presence of somatodendritic α4β2 nAChRs in CA1 interneurons in hippocampal slices from rats and mice (15, 28, 316, 453). Accordingly, U-tube application of ACh to these neurons induces a whole cell current that 1) is inhibited by 10 µM DHβE, but not by 10 nM MLA; 2) cannot be evoked by choline; and 3) is weakly activated by cytisine (Fig. 4A). The finding that α4β2 nAChRs can be effectively activated by low micromolar concentrations of ACh (1–10 µM) (Fig. 4A; see also Ref. 16) suggests that these receptors are tonically activated by an ambient levels of ACh in the brain. This has been demonstrated experimentally by the application of desensitizing concentrations of nicotine in dopaminergic neurons in midbrain slices (380). For example, the long-lasting decrease in the sIPSC frequency in dopaminergic neurons is consistent with nicotine desensitizing cholinergic afferents that particularly drive the GABAergic activity in the slices. Dopaminergic neurons in the midbrain slices express α7, α4β2, and to some extent α6(α4)β2 nAChR subtypes on the soma membrane (81).
B. Axon Terminal nAChRs
The presence of α7 nAChRs at neuronal axon terminals was demonstrated by the finding that in neurons of hippocampal slices or in olfactory bulb cultures continuously perfused with physiological solution containing tetrodotoxin, a nicotinic agonist is able to increase the frequency of miniature excitatory postsynaptic currents (mEPSCs) in an α-BGT-sensitive manner (33, 190, 312). Activation of terminal (also referred to as presynaptic) α7 nAChRs results in enhancement of field stimulationevoked glutamatergic transmission and forms the basis for the involvement of these nAChRs in synaptic plasticity in different brain regions (190, 296). In the immature rat hippocampus, activation of presynaptic α7 nAChRs stimulates silent glutamate synapses impinging onto CA1 pyramidal neurons (292).
Numerous reports have also provided evidence that α7 nAChRs are present on glutamatergic terminals in the human neocortex (299) and in the rat olfactory bulb (33), striatum (299), VTA (224, 297, 412), MHb (180), and frontal cortex (400). Finally, functional α7 nAChRs have been detected in axon terminals of dopaminergic neurons in the rat striatum (439). In general, activation of these receptors facilitates transmitter release via a Ca2+-dependent, tetrodotoxin-insensitive, and α-BGT-sensitive mechanism.
Other nAChR subtypes are also reported to be expressed in axon terminals of various neurons in different brain areas, whereby their activation increases the tetrodotoxin-insensitive release of neurotransmitter. For instance, α6 nAChRs present at the dopaminergic striatal nerve terminals contribute to 50% of the synaptosomal dopamine release (81). In mouse striatal synaptosomes, nicotinic agonists acting via two major classes of nAChRs trigger dopamine release. One class consists of a-conotoxin MII-sensitive nAChRs that are likely α6β3β2 and/or a6a4β3β2. The other class includes α-conotoxin MII-resistant nAChRs that are probably α4β2 and/or α4α5β2 (404). Other pharmacological profiles compatible with that of α4β2 nAChRs are present on cholinergic terminals in the human neocortex (299). Activation of these autoreceptors has been shown to facilitate ACh release from human neocortical synaptosomes (299).
C. Preterminal nAChRs
In addition to being expressed on the somatodendritic and presynaptic terminals of various neurons throughout the brain, different nAChR subtypes are expressed on axonal preterminal regions. Activation of these receptors facilitates action potential-dependent, tetrodotoxin-sensitive release of neurotransmitters. For instance, application of nicotinic agonists to neurons that were acutely dissociated from the interpeduncular nucleus and retained synaptophysin-stained terminals triggered IPSCs that could not be observed in the presence of tetrodotoxin (267). Likewise, tetrodotoxin-sensitive IPSCs can be triggered by application of nicotinic agonists to chick lateral spiriform nucleus (315), rat hippocampal neurons in culture(10), and CA1 interneurons in rat hippocampal slices (28).
Pharmacological tools were pivotal for the identification of the nAChR subtypes that regulate action potential-dependent transmitter release in different brain regions. For instance, the finding that focal application of ACh, but not choline, to the somatodendritic region of CA1 SR interneurons triggered tetrodotoxin-sensitive IPSCs supported the notion that nAChRs located on preterminal regions of GABAergic axons regulate GABAergic transmission in the CA1 field of hippocampal slices. Given that this response was blocked by DHβE, while being insensitive to blockade by MLA or α-BGT, indicated that it was subserved by α4β2 (type II) nAChRs. The activation of these receptors by low concentrations of ACh depolarizes preterminal axonal segments and causes GABA release at the synapses without causing a generalized firing of interneurons. This mechanism helps implement segmental inhibition rather than generalized inhibition at all innervated sites. Although the functional significance of preterminal nAChRs is currently not clear, it is likely that a sequential activation of different nAChR subtypes may converge to a desired action at the neurons (Fig. 4D).
Preterminal nAChRs are critical regulators of a number of neurotransmitter systems in different areas of the brain. For instance, receptors with pharmacological properties compatible with those of α3β2 nAChRs have been shown to regulate norepinephrine release from rat hippocampal slices (424). As reported in that study, application of nicotinic agonists, including nicotine, dimethyl-phenylpiperazinium, anatoxin-a, epibatidine, and lobeline, [3H]norepinephrine-preloaded hippocampal slices triggered the tetrodotoxin-sensitive release of norepinephrine. It was suggested that nicotinic agonists, acting on nAChRs at the preterminal area of noradrenergic neurons, caused local depolarization and subsequent generation of action potentials that subsequently triggered the release of norepinephrine. However, the possibility could not be ruled out that these nAChRs were located on an interneuronal circuitry that regulated the activity of noradrenergic neurons rather than directly on the noradrenergic terminals. Likewise, action potential-dependent glutamatergic transmission impinging onto CA1 interneurons is regulated by preterminal nAChRs on glutamatergic axons (17, 24). Application of nicotinic agonists to CA1 interneurons in rat hippocampal slices evokes tetrodotoxin-sensitive AMPA and NMDA EPSCs. Compared with ACh, choline is a weak agonist, whereas cytisine is a strong agonist to induce this nicotinic response (Fig. 4A). Nicotinic agonist-evoked AMPA and NMDA EPSCs recorded from CA1 interneurons, similarly to type III nicotinic responses recorded from cultured hippocampal neurons, are potently blocked by mecamylamine and bupropion (16). The pharmacological profile of these responses is comparable to that of nicotinic responses resulting from activation of α3β4 nAChRs ectopically expressed in heterologous systems. However, the findings that agonist-evoked EPSCs are exquisitely sensitive to the desensitizing action of nicotine and choline (Fig. 4C; Refs. 16, 18) suggest that type III nAChRs could be composed of α3β4β2 subunits. The glutamatergic activity impinging onto CA1 interneurons is effectively regulated by functional type III nAChRs present on glutamate axons/neurons during the first postnatal week, when choline uptake mechanisms are not fully developed (246). Thus, considering the sensitivity of type III nAChRs to desensitization by choline, it can be speculated that the degree to which tonic α3β4β2 nAChR activity regulates glutamatergic synaptic transmission in the rat hippocampus changes along with age.
Nicotinic regulation of action potential-dependent glutamatergic transmission is not confined to the hippocampus. There are reports that ACh and nicotine trigger tetrodotoxin-sensitive glutamate release as measured by an increase in the frequency of spontaneous EPSCs recorded from layer V pyramidal neurons of prefrontal cortical slices (258). It was suggested that ACh- and nicotine-triggered glutamate release resulted from activation of preterminal β2-containing nAChRs on thalamocortical axons that synapse onto the cerebral cortical neurons (258).
D. Myelinated Axon nAChRs
The demonstration, in the early 1960s, that ACh can depolarize nonmyelinated vagus nerves in rabbits led to the suggestion that axons express receptors that directly regulate axonal excitability (41). Histological studies from the 1980s revealed that nAChRs expressed in the retinal ganglion cells of rodents are transported along the optic nerve (457). At the time it was unclear whether these receptors were simply destined for insertion in nerve terminals or were indeed inserted in the membrane along the axonal length. In the 1990s, a report that nicotine induces Ca2+ influx in axonal segments of the developing frog optic tectum strongly indicated that nAChRs are indeed expressed in myelinated axons (139). A later study of rat and mouse optic nerves isolated from synaptic elements and the ganglion cell bodies demonstrated the presence of nAChRs on the axon proper and suggested that these receptors play a role in regulation of axonal guidance, branching, and excitability (520).
Positron emission tomography studies in humans revealed that nAChRs are also present in the white matter of sensory thalamocortical pathways (124). In vitro and in vivo studies of the effects of nicotinic agonists on the excitability of thalamocortical axons and of nicotinic antagonists on sound-evoked cortical responses in vivo supported the contention that nAChRs are expressed in myelinated thalamocortical axons (233). Thus application of nicotine to thalamocortical slices in vitro enhanced and synchronized action potential discharges along thalamocortical axons. In vivo, blockade of endogenous nAChRs by thalamic microinjections of DHβE reduced soundevoked cortical responses. Altogether, the results of Kawai et al. (233) demonstrated that α4β2 nAChRs in thalamocortical axons modulate neurotransmission in the brain via changes in axon excitability.
Recent studies have shown that some nAChR subtypes do have motifs that target them to be expressed on myelinated axons. For instance, transfection of primary hippocampal cultures with hemagglutinin- or green fluorescent protein-tagged nAChR subunits revealed that α4β2 nAChRs are targeted to both dendrites and axons of hippocampal neurons (509). The axonal targeting sequence was identified as a 25-residue leucine motif located in the M3–M4 loop of the α4 nAChR subunits (509). It remains an intriguing possibility that the preterminal nAChRs are the same entity as the myelinated axon nAChRs.
E. Presence of Diverse Functional nAChR Subtypes: A Redundant Function or a Specific Functional Design?
There is strong evidence indicating that a single nAChR subtype is present at multiple locations in a neuron or that more than one nAChR subtype is found at a single neuronal domain. For example, functional α7 and α4β2 nAChRs have been found to be differentially expressed on the somata, dendrites, and preterminal axonal regions of different CA1 interneurons (Fig. 4D). α7 nAChRs present on the somatodendritic region of hippocampal interneurons can subserve both synaptic and nonsynaptic functions. When activated by synaptically released ACh, somatodendritic α7 nAChRs can induce a short-lasting depolarization of the interneurons that is of sufficient magnitude to induce action potential and transmit inhibition or disinhibition to the pyramidal neurons, depending on the interneuron that expresses the α7 nAChRs. Likewise, activation of somatodendritic α4β2 nAChRs by synaptic ACh can induce a long-lasting excitation of the interneurons resulting in prolonged inhibition of the pyramidal neurons. Diffusing or ambient levels of ACh or choline can trigger a small, though long-lasting activation of α7 nAChRs at the interneurons that is sufficient to trigger a cascade of Ca2+-dependent events, including induction of gene transcription. In the CA1 interneurons, activation of preterminal α4β2 nAChRs by diffusing ACh can impart segmental inhibition at the pyramidal neurons.
As mentioned in the section above, in the developed hippocampus nAChRs are primarily, though not exclusively, expressed on interneurons in the various strata. These neurons are heterogeneous in type, location, dendritic placement, and axonal termination zone, and, depending on the stratum they are in, they express different levels of specific nAChRs (Fig. 4D). Considering that the anatomical diversity of the CA1 interneurons in the hippocampus provides a lamina-specific control of the activity of CA1 pyramidal neurons (158), it has been proposed that nAChRs can alter the function of CA1 pyramidal neurons in at least three distinct ways. First, activation in the interneurons of either somatodendritic nAChRs or presynaptic/preterminal may facilitate GABAergic transmission to pyramidal neurons and, thereby, exert an in hibitory effect during cholinergic neuron firing. Nicotinic cholinergic inhibition has the potential to suppress weak excitatory signals arriving at the pyramidal neuron dendrites and allow propagation of only strong signals. This could be a mechanism by which nicotinic agonists filter extraneous signals (364) and increase attention (449). On the basis of the differential level of expression of nAChRs by the CA1 interneurons synapsing directly onto pyramidal neurons, α4β2 nAChRs would have a greater role than α7 nAChRs in this process (14). Second, nAChR-mediated GABA release may disinhibit CA1 pyramidal neurons via inhibition of the interneurons. When α7 nAChRs are activated, stratum lacunosum moleculare (SLM) interneurons are inhibited more than other interneurons, resulting in a selective disinhibition of the dendritic segments of pyramidal neurons innervated by SLM axon terminals (Fig. 4D). On the other hand, when α4β2 nAChRs are activated, both SR and SLM interneurons are inhibited, resulting in disinhibition of dendritic areas innervated by both neuron types. Disinhibition would be less prominent in dendritic compartments innervated by stratum oriens (SO) and stratum pyramidale (SP) interneurons, because these interneurons receive the least GABAergic input from nAChR-expressing interneurons (14). Thus nAChRs appear to disinhibit feed-forward inhibitory zones (i.e., SR and SLM interneuron target zones) more than feedback inhibitory zones (i.e., SO and SP interneuron target zones) at the pyramidal neuron dendrites (Fig. 4D). Third, nAChR-mediated GABA release can cause neuronal hyperpolarization, which in turn affects neuronal function via several mechanisms, including removal of inactivation of inward currents (89). It is noteworthy that, via such mechanisms, α7 nAChR activation could trigger rebound burst firing in SLM interneurons even in the absence of excitation (256). Burst firing in SLM interneurons suppresses spikes in pyramidal neurons evoked by stimulation of Schaffer collaterals (134), and, thereby allows selective activation of the pyramidal cells via the perforant pathway. Such selective regulation of intrinsic (e.g., Schaffer collateral) and extrinsic (e.g., perforant path) afferent inputs is considered important in switching between encoding and retrieval modes of associative memory systems (208, 364, 482).
In CA1 and CA3 pyramidal neurons of the developed hippocampus, α7 nAChRs are expressed primarily on axon terminals whereby their activation modulates the efficacy of glutamate synaptic transmission. Glutamatergic axons/neurons that innervate CA1 interneurons have been shown to express functional α3β4β2 nAChRs, and activation of these receptors by diffusing and/or ambient levels of ACh releases glutamate which in turn activates AMPA/NMDA receptors in the interneurons (16, 24). Because some of the CA1 SR interneurons contain both somatodendritic α7 and α4β2 nAChRs and are innervated by glutamate axons carrying α3β4/β2 nAChRs and GABAergic axons carrying α4β2 nAChRs (15), it is likely that one or more of the following interactions could occur in these neurons (Fig. 6). For example, during a low degree of activation of α7 and α3β4 nAChRs, Ca2+ can enter the cells through nAChRs or NMDA receptors and favor activation (i.e., phosphorylation) of the transcription factor CREB, which in turn modifies gene expression (82). If there is intense stimulation of all three nAChRs, the resulting depolarization can trigger activation of voltage-gated Ca2+ channels (VGCC), which in turn would activate the calcineurin pathway and prevent CREB activation. A concurrent activation of preterminal α4β2 nAChRs would hyperpolarize the neuron via GABAergic inhibition and prevent activation of the VGCC. Such a sequential interplay between nicotinic and GABAergic signaling has been shown to guide neuronal development in the hippocampus and other regions (281). It is interesting to note that a single neurotransmitter, in this case ACh, uses the diversity of the nAChR pathways to regulate a specific function in different neurons.
FIG. 6.
Schematic representation of intracellular signaling resulting from activation of nAChRs, glutamate ionotropic receptors, and GABAA receptors. In the CA1 field of the hippocampus, a single interneuron can express somatodendritic α7 and α4β2 nAChRs and receive α3β4/β2 nAChR-regulated glutamatergic inputs. Thus there is the potential that intracellular signaling is regulated by the cross-talk of the various transmitter systems. During a low degree of activation of α7 nAChRs and α3β4β2 nAChRs, for instance, Ca2+ may enter the cells through nAChRs or NMDA receptors and favor phosphorylation of the transcription factor CREB, which in turn modifies gene expression (82). If there is intense stimulation of all three nAChRs, the resulting depolarization can trigger activation of VGCC, which in turn would activate the calcineurin pathway and prevent CREB activation. A concurrent activation of preterminal α4β2 nAChRs would hyperpolarize the neuron via GABAergic inhibition and prevent activation of the VGCC. Such a sequential interplay between nicotinic and GABAergic signaling has been shown to guide neuronal development in the hippocampus and other regions (281).
In numerous other areas of the brain, a single neuron expresses various nAChR subtypes at multiple sites and that the apparent redundancy of the system within a single cell leads to a convergent action among the AChRs. In the basal ganglia, for instance, dopaminergic transmission is ultimately regulated by the activity of specific nAChR subtypes in different neurons and neuronal compartments (Fig. 5). Thus evidence exists that in the VTA, α6- and α4-containing nAChRs are mainly located on dopaminergic nerve terminals, whereas α7 nAChRs are primarily expressed on the soma of dopaminergic neurons (Fig. 5). Activation of somatodendritic α7 nAChRs increases the action potential-dependent release of dopamine, while activation of presynaptic α6 and/or α4 nAChRs increases action potential-independent dopamine release. Other levels of regulation of dopaminergic transmission arise from α7 nAChRs located on cortical glutamatergic terminals; activation of these receptors increases glutamate release onto dopaminergic neurons in the VTA and, consequently, increases the their firing (344). Activation of α4β2 nAChRs on GABAergic interneurons in the VTA relieves the inhibitory control they exert on dopaminergic neurons (295, 380). Considering the relevance of the dopaminergic rewarding systems to drug addiction, studies of mice with null mutations in the genes that code for specific nAChR subunits have shed light onto the contribution of the different nAChRs to nicotine addiction (discussed in the next section).
V. EMERGING VIEWS OF NICOTINIC RECEPTOR FUNCTION
Neuronal nAChRs are not expressed exclusively in neurons. Instead, they are expressed by multiple cell). types of diverse origins and functions including glia (165, 167, 425), keratinocytes (44, 86, 95, 426), endothelial cells (290, 495), and multiple cell types of the digestive system, lungs, and immune system (e.g., Refs. 95, 309, 492, 495). Many of these cells synthesize and release acetylcholine, which in nonneuronal cells of the periphery is often referred to as a “cytotransmitter” (323). Unique functional and pharmacological properties of the nAChRs are likely to contribute to highly specific local and often tissuespecific responses to circulating levels of nicotinic ligands in the non-blood-brain barrier-buffered peripheral environment. In turn, the chronic use of a nonselective nAChR agent such as nicotine can imbalance these systems and establish less desirable physiological setpoints. Some of these emerging areas of brain-peripheral nAChR function and interaction are discussed in this section, which will also explore the more unconventional “metabotropic” functions of the nAChRs and the less traditional ligands that modify nAChR activity as they are becoming increasingly more relevant for the development of therapeutic strategies for neurological disorders in which nAChR activity and/or expression is known to be altered.
A. Nontraditional Ligands
There are several ligands that interact with nAChRs at sites other than the agonist-binding domains, yielding either potentiation or depression of receptor activity. This section will focus on studies of exogenous nAChR modulators that found their way to the clinics and on studies of endogenous ligands that physiologically regulate the activity of specific nAChR subtypes.
At the neuromuscular junction, nicotinic function is enhanced by inhibition of acetylcholinesterase (AChE), the enzyme that metabolizes the endogenous neurotransmitter ACh. However, unlike muscle nAChRs, some neuronal nAChRs, particularly the α7 nAChRs, recognize both ACh and its metabolite choline as full agonists (371). Therefore, AChE inhibition may not necessarily enhance functions mediated by these nAChRs. In fact, as described above, AChE inhibitors do not affect α7 nAChR-mediated synaptic transmission evoked by low-frequency stimulation of cholinergic fibers in chick ciliary ganglia (522).
An alternative means to increase nicotinic functions in the brain is to sensitize the nAChRs to activation by the endogenous agonist(s) using the so-called nicotinic allosteric potentiating ligands (APLs), which include drugs such as physostigmine and galantamine, a drug currently approved for the treatment of AD. Studies from the early 1980s provided evidence that the cholinesterase (ChE) inhibitor physostigmine could interact directly with nAChRs at the frog neuromuscular junction and induce nicotinic single-channel currents (428, 429). In the early 1990s, galantamine, an alkaloid originally extracted from the bulbs and flowers of the wild Caucasian snowdrop Galanthus nivalis and other related Amaryllidacea species, was found to act like physostigmine on muscle and neuronal nAChRs (370, 372). Surprisingly, however, activation of nAChRs by galantamine or physostigmine was insensitive to blockade by competitive nAChR antagonists, was detected even when the receptors were desensitized by high agonist concentrations, and was inhibited by the monoclonal antibody FK1 (350, 370, 372, 413, 428, 429). The agonistic activity of physostigmine and galantamine, initially referred to as noncompetitive agonists (NCAs; Ref. 450), was found to result from their binding to a site close to, but distinct from, the ACh-binding site on nAChR α subunits (4, 369, 372, 413). The region flanking the amino acid Lys-125 on the nAChR α subunits contains essential elements of the physostigmine site and is highly conserved across species (372, 413, 415).
The nicotinic NCA action is not common to all ChE inhibitors, since, for example, the ChE inhibitor pyridostigmine is unable to induce nicotinic single-channel currents by directly interacting with nAChRs (39). Conversely, a drug does not have to be a ChE inhibitor to be a nicotinic NCA. For instance, studies carried out in PC12 cells demonstrated that codeine, a drug with no significant effect on ChE, can activate nicotinic single-channel currents and that this nicotinic agonist effect is sensitive to inhibition by FK1 while unaffected by classical nAChR antagonists (450).
Even though NCAs induce opening of nAChR single channels in numerous neuronal and nonneuronal preparations, the probability of channel openings by these compounds is so low that the single-channel currents they activate do not give rise to macroscopic responses (4, 370, 414, 450). In different systems, however, NCAs have been shown to potentiate the activation of most nAChRs by subsaturating concentrations of classical nAChR agonists. The nicotinic potentiating action of these drugs is also sensitive to inhibition by the FK1 antibody, and, thereby, likely to result from their interactions with the physostigmine-binding site on nAChRs (414). A recent study performed in HEK293 cells stably expressing muscle nAChRs, however, revealed that galantamine acts as a nicotinic NCA but not as a nicotinic APL (4). Thus the possibility cannot be ruled out that the NCA and the APL sites share some common elements, but are in fact distinct from one another in the nAChRs. As described below, a recent study using the AChBP isolated from the mollusk Aplysia californica shed some light onto this puzzle.
As mentioned in section I, the AChBP is a soluble homopentamer that resembles the extracellular domain of the nAChRs and has the ligand-binding elements that make up the sites for classical agonists and competitive antagonists (206). Crystallographic analysis of the AChBP-galantamine complex revealed that galantamine associates with elements present at the interface between two AChBPs (207). As expected from the pharmacological studies described above, no significant interaction was observed between galantamine and the vicinal cysteine residues that are essential for binding of classical nicotinic agonists and competitive antagonists (207; see also Fig. 7). Elements that appear essential for binding of galantamine to the AChBP include the tryptophan residues 147 and 149, the tyrosine residue 55 or 93, and to a lesser extent the tyrosine residue 195. It has also been proposed that the dipole between the carbonyl group of the tryptophan residues and the protonated nitrogen of galantamine may be strengthened by the anionic side chain of the residue aspartate-89 (207). These findings are in agreement with our earlier suggestion that the region including and surrounding the residue Lys-125 on the nAChR α subunits, which spans the nAChR epitope against which the antibody FK1 was raised, contains elements that are essential for the binding of galantamine to the nAChRs (372, 413). However, the crystallographic study of the AChBP-galantamine complex also revealed that some of the residues that contact galantamine in the complex are conserved among non-α nAChR subunits, suggesting that galantamine may bind to both α- and non-α interfaces (207). Therefore, it is tempting to speculate that, depending on the subunit composition of the nAChRs, differential interactions of galantamine with α- or non-α interfaces can favor its action as an NCA or an APL.
FIG. 7.
Regulation of nAChRs by nontraditional ligands. In this illustration, an agonist-binding α subunit (light blue) and a structural β subunit (dark blue) are shown with a solid surface looking from the extracellular side with either nicotine alone (left) or nicotine and galantamine (right). Photoaffinity labeling studies carried out using [3H]physostigmine and mapping of the epitope of the monoclonal antibody FK1 revealed that the region flanking the amino acid Lys-125 on the nAChR α subunit contains essential elements of the physostigmine-binding site and is highly conserved among different α subunits and across species (372, 413, 415). The galantamine-binding region is close to, but distinct from, the classical agonist-binding region. The galantamine-binding region is highly hydrophobic. As described in the text, elements that appear essential for binding of galantamine to the AChBP include the tryptophan residues 147 and 149, the tyrosine residue 93 or 55, and to a lesser extent the tyrosine residue 195. It has also been proposed that the dipole between the carbonyl group of the tryptophan residues and the protonated nitrogen of galantamine may be strengthened by the anionic side chain of the residue aspartate 89 (207). The crystallographic study of the AChBP-galantamine complex also revealed that some of the residues that contact galantamine in the complex are conserved among non-α nAChR subunits, suggesting that galantamine may bind to both α- and non-α interfaces (207).
The exact mechanism by which nicotinic APLs sensitize nAChRs to activation by classical agonists is still poorly understood. There are reports that nicotinic APLs increase the probability of nAChR channel openings in duced by ACh in outside-out patches from PC12 cells and enhance the apparent potency, but not the efficacy of nicotinic agonists in activating different nAChR subtypes (406, 450). These results support the notion that APLs enhance the binding affinity of agonists and/or the frequency of channel openings for a given level of receptor occupancy as long as receptor activation is still submaximal.
The nicotinic APL action is not common to all ChE inhibitors; for instance, donepezil and rivastigmine are devoid of this action (405). To date, all compounds characterized as nicotinic APLs have a nitrogen that is cationic at physiological pH and is located at a fixed distance from a phenolic group (372, 450). The few drugs identified so far as nicotinic APLs increase with similar potencies the activity of different nAChR subtypes and have a bimodal effect on these receptors (406). Therefore, it has so far not been possible to pinpoint the pharmacophore that will make a compound to act exclusively as a nicotinic APL in a given nAChR subtype.
The discovery of galantamine as a nonconventional nicotinic ligand of exogenous origin led to the suggestion that an endogenous galantamine-like ligand might exist. Initial attempts to identify such endogenous compound(s) were focused on the concept that a given substance can control synaptic activity in the brain by acting as the primary agonist in one neurotransmitter system and as a modulator in another system. Glycine is a classical example of such an endogenous substance. Whereas in glycin-ergic synapses glycine activates glycine-gated channels, in the glutamatergic system glycine acts as a coagonist at the NMDA-receptor channels. Since some studies have indi cated that indolamines can interact with the ChEs found in the plaques of patients with AD (505), and since some ChE inhibitors, including galantamine, act as nicotinic APLs, the neurotransmitter serotonin (5-HT) was tested for its ability to modulate ACh-evoked nicotinic currents in PC12 cells (414). As consistently reported in other systems (e.g., Refs. 172, 218), 5-HT at micromolar concentrations was also found to inhibit agonist-induced nAChR activity in PC12 cells (414). However, at submicromolar concentrations, 5-HT sensitized the nAChRs to classical agonists, an effect that could be blocked by FK1 (414). Thus the possibility exists that 5-HT acts as an endogenous nicotinic APL.
It remains unclear whether under normal physiological conditions, endogenous galantamine-like modulators of nAChR activity would be stored together with ACh in the cholinergic terminals or would have a paracrine action. Considering that in many CNS areas, tryptaminergic and cholinergic synapses are colocalized (327), it is tempting to speculate that 5-HT released from its terminal could diffuse away and act as a nicotinic APL on closely located nAChRs. The finding that submicromolar concentrations of 5-HT are sufficient to potentiate nicotinic responses is in agreement with a paracrine action of 5-HT on nAChRs. It also lends support to the concept that brain functions are regulated by complex neuronal and chemical networks.
Reduced nAChR function/expression in the brain has been associated with the pathophysiology of catastrophic disorders, including AD and schizophrenia (discussed in later sections, and see Refs. 277, 432). In particular, the association of the α7 nAChR gene with a sensory gating deficit that is similar to attention deficits in patients with schizophrenia (157), and the degree of α4β2 nAChR loss and altered α7 expresson correlate well with the magnitude of progressive cognitive decline in mild-to-moderate AD patients (46). The nicotinic APL action of galantamine appears to be an important determinant of its clinical effectiveness (reviewed in Refs. 98, 291, 371). Acting primarily as a nicotinic APL, galantamine improves synaptic transmission and decreases neurodegeneration, two effects essential for its cognitive-enhancing properties (40, 108, 241, 409, 521). Of note is that in both of these catastrophic disorders, reduced nAChR activity/expression is accompanied by increased levels of kynurenic acid (KYNA), a tryptophan metabolite that in the brain is primarily produced and released by astrocytes (244, 419). The neuroactive properties of KYNA have long been attributed to the inhibition of NMDA receptors (329). Electrophysiological studies, however, have demonstrated that physiologically relevant concentrations of KYNA block α7 nAChR activity noncompetitively and voltage independently (210).
Biosynthesis and disposition of KYNA in the mammalian brain have been extensively investigated. KYNA is formed enzymatically by the irreversible transamination of l-kynurenine, a major peripheral tryptophan metabolite with ready access to the brain. Immunohistochemical and lesion studies demonstrated that cerebral KYNA synthesis takes place almost exclusively in astrocytes (129, 187, 199). Newly formed KYNA is rapidly liberated into the extracellular compartment for possible interaction with neurotransmitter receptors, including the α7 nAChRs and NMDA receptors (472). Because of the absence of reuptake or degradation mechanisms, subsequent KYNA removal is accomplished exclusively by probenecid-sensitive brain efflux (330, 473). Interestingly, astrocytic KYNA production is regulated by neuronal activity (187) and cellular energy metabolism (213). This dependence of extracellular KYNA concentrations on the functional interplay between neurons and astrocytes is in line with the postulated neuromodulatory role of KYNA (418) and adds to the complexity of the neurochemical networks in the brain. In the normal brain, >70% of KYNA formation is catalyzed by KAT II, one of the three cerebral KATs (199, 200). Systemic treatment of rats and mice with kynurenine leads to an elevation of brain levels of several neuroactive intermediates, including KYNA, the free radical generator 3-hydroxykynurenine, and the excitotoxic quinolinic acid (419).
Because the overall effects of α7 nAChR and NMDA receptor antagonists on neuronal plasticity and viability are similar and resemble those of KYNA, a review of the neuroactive properties of KYNA in vivo and in vitro does not adequately resolve the question of whether the metabolite acts in vivo through α7 nAChRs or NMDA receptors. Mice with a null mutation in the gene that encodes KAT II became a unique tool to resolve this issue (31, 410, 516). Low levels of KYNA in these mutant mice lead to α7 nAChR disinhibition in hippocampal CA1 SR interneurons, thereby increasing the activity of GABAergic interneurons impinging onto CA1 pyramidal neurons (31). It is noteworthy that NMDA receptor activity in CA1 SR interneurons in hippocampal slices of mKat-2−/− mice is not significantly different from that recorded from wild-type interneurons (31). This constituted the first evidence that in the hippocampus endogenous levels of KYNA are sufficient to directly modulate the activity of α7 nAChRs, but not that of NMDA receptors (31). Potential developmental and age-dependent adaptations to the elimination of KAT II, however, limit the usefulness of the mKat-2−/− mice to the understanding of the pathological effects of KYNA in the mature brain. Thus brain levels of KYNA in 60-day-old mKat-2−/− mice become comparable to those of agematched wild-type mice, and no phenotypic differences in hippocampal α7 nAChR activity or GABAergic transmission exist in these older animals (31). Changes in the mechanisms that regulate the expression of KATs other than KAT-2 in the brain could represent adaptative responses to the elimination of the mKat-2 gene (517).Therefore, a better understanding of how abnormal levels of brain KYNA contribute to the pathophysiology of disorders such as AD and schizophrenia will depend on pharmacological manipulations that induce selective fluctuations in brain KYNA levels at specific ages.
Acting as an endogenous regulator of the α7 nAChR activity, astrocyte-derived KYNA can modulate synaptic transmission, synaptic plasticity, neuronal viability, and neuronal connectivity in different areas of the brain (Fig. 8). Activation of α7 nAChRs in somatodendritic and preterminal/terminal areas of interneurons in various strata of the CA1 region and in the dentate gyrus facilitates spontaneous quantal release of GABA (14, 25). Glutamate release from mossy fibers onto CA3 pyramidal neurons is also modulated by α7 nAChRs present in the mossy fiber terminals (190). Furthermore, α7 nAChRs have been im plicated in “inhibitory” and “disinhibitory” circuits in the CA1 field of the hippocampus (19, 26, 28, 223; see also Fig. 4D). As mentioned above, under normal physiological conditions, endogenous levels of KYNA are sufficient to maintain a degree of inhibition of α7 nAChRs in CA1 SR interneurons that tunes down the intensity of the GABAergic transmission impinging onto CA1 glutamatergic neurons (15).
FIG. 8.
Role of astrocyte-derived kynurenic acid (KYNA) in regulating the activity of dopaminergic neurons in the ventral tegmental area. This simplified scheme illustrates the role of astrocyte-derived KYNA in modulating synaptic transmission between a cortical glutamatergic axon and a dopaminergic neuron in the ventral tegmental area (VTA). The VTA supplies dopaminergic inputs to several nuclei in the so-called reward circuit. This circuit, which is centered around the nucleus accumbens and is critical for animals to display goal-directed behaviors, has been shown to be strategically positioned to relay information about motivation, drive, and affective state to motor systems. Dopaminergic activity in the nucleus accumbens has an essential role in the functioning of this circuit. The reinforcing properties of drugs of abuse, including nicotine, are associated with increased dopaminergic activity in the nucleus accumbens, which receives dopaminergic inputs from the VTA. The nucleus accumbens receives input from several limbic structures, including the amygdala, the hippocampus, and the medial prefrontal cortex and innervates the ventral pallidum, subpallidal area, and substantia nigra, which provide inputs to motor structures. The dopaminergic activity in the nucleus accumbens is, thus, regulated by local integration of various neurotransmitter systems originating in different areas of the brain. However, it is also controlled by the cortical glutamatergic input to the dopaminergic neurons in the VTA. Local infusion in the rat striatum of a kynurenine hydroxylase inhibitor has been shown to increase extracellular levels of dopamine, which were decreased by the addition of KYNA to the perfusate (390, 391). The association between low levels of KYNA and increased levels of dopamine has also been observed in mice with a null mutation in the gene that encodes the KATII enzyme (516). Thus it is tempting to speculate that dopaminergic transmission in the striatum is regulated by astrocyte-derived KYNA. VTA dopaminergic neurons are known to express somatodendritic α7 nAChRs and to receive excitatory inputs from cortical glutamatergic terminals that express α7 nAChRs. Activation of these receptors stimulates dopamine release into the nucleus accumbens within the striatum. It is plausible to hypothesize that endogenous KYNA released from astrocytes may inhibit tonically active α7 nAChRs and, thereby, decrease dopamine levels in the striatum. This emphasizes the concept of tripartite synapses in the brain, whereby synaptic activity is tuned by astrocyte-derived regulatory signals (480).
Activation of α7 nAChRs is known to contribute to the regulation of extracellular dopamine levels in the rat striatum (81). Application via microdialysis of KYNA or α-BGT to the rat striatum significantly reduces the extracellular levels of dopamine, and the magnitude of the effect of either antagonist alone is comparable to that of both antagonists together (285). In contrast, the NMDA receptor antagonist 7-chloro-KYNA has no significant ef fect on the extracellular levels of dopamine in the rat striatum (391). As illustrated in Figure 8, KYNA-induced reduction of extracellular dopamine levels can be explained by the inhibition of tonically active α7 nAChRs in the dopaminergic neurons within the VTA and/or in cortical glutamatergic terminals that synapse onto striatal neurons. VTA dopaminergic neurons represent the major dopaminergic input to the nucleus accumbens.
Disruption of reciprocal glia-neuron signaling mechanisms involving KYNA and nAChRs may be causally related to diseases such as AD and schizophrenia. Chronic α7 nAChR inhibition in the hippocampus by elevated levels of KYNA can contribute to auditory gating deficits, which appear to be associated with the development of schizophrenia (156). It is also feasible that KYNA-induced inhibition of α7 nAChRs contributes to the cognitive impairment observed in patients with AD and schizophrenia (273). Finally, the finding that KYNA, acting via α7 nAChRs, regulates striatal dopamine levels (Fig. 8; Refs. 285, 391) suggests that the interplay between astrocyte-derived KYNA and synaptic transmission can modify reward mechanisms implicated in the pathophysiology of drug abuse and neuropsychological disorders such as schizophrenia. Detailed knowledge of how KYNA, acting via α7 nAChRs, regulates synaptic transmission throughout the brain at different ages is essential for the understanding of the involvement of KYNA and α7 nAChRs in specific disease states.
The exact amino acids required for binding of KYNA to α7 nAChRs are yet to be identified. However, recent electrophysiological experiments have demonstrated a competitive interaction of galantamine and KYNA with α7 nAChRs in hippocampal neurons (285). The finding suggested that KYNA-induced inhibition of α7 nAChRs is dependent on the interactions of the metabolite with the region on nAChRs that binds galantamine. Two questions were then raised: 1) Why are the actions of KYNA and galantamine on α7 nAChRs opposite? 2) Why does KYNA inhibit α7 nAChRs selectively, while galantamine acts more promiscuously as an APL on most nAChRs?
Superimposition of the lowest energy conformers of galantamine and KYNA shed some light on structural differences that could explain the opposite actions that result from the interactions of the two compounds with the APL-binding region on α7 nAChRs. Like galantamine, KYNA has an aromatic ring with a phenolic hydroxyl group. This group, which bears the same spatial orientation as the phenol group in galantamine, is located at a fixed distance from a pyridinic nitrogen. However, this nitrogen is largely unionized at physiological pH and is at a shorter distance from the phenolic group than the tertiary nitrogen is from the corresponding phenolic group in galantamine. The previous report that 7-chloro-KYNA does not inhibit α7 nAChRs (210) suggests that the car-boxyl group contributes to interactions of KYNA with specific residues in the APL-binding region. The introduction of the electron-withdrawing chlorine in position 7 of the phenolic ring creates a dipole in the molecule that can weaken its potential interactions with positively charged residues in the APL region. The nAChR α7 subunit is the only mammalian nAChR α subunit that has a positively charged residue within the segment α118–140 of the putative APL-binding region. It is, therefore, tempting to speculate that the selectivity of KYNA for α7 nAChRs is encoded in the carboxyl group in position 2 of the pyridine ring.
Drugs currently approved to treat mild-to-moderate AD, including galantamine, donepezil, and rivastigmine, all inhibit AChE, the enzyme that hydrolyzes ACh (462). As mentioned above, galantamine is unique in that it also acts as a nicotinic APL. Recently, these drugs have been evaluated as adjuvant therapies to decrease the cognitive impairment and negative symptoms of patients with schizophrenia. Data are still sparse and so far derived from small samples in open uncontrolled studies. However, a small randomized, double-blind trial showed positive outcomes when galantamine was administered as an add-on therapy to antipsychotics (417). To date, no similarly promising clinical effects have been observed with donepezil or rivastigmine (310, 427). Since KYNA levels are significantly elevated in the brain of individuals with AD (49) and schizophrenia (420), it is possible that the antagonism of KYNA-induced inhibition of α7 nAChRs may be causally related to the effectiveness of galantamine in these catastrophic disorders.
Other endogenous ligands that impact on the activity of nAChRs noncompetitively and voltage independently include the amyloid β peptide 1–42 (Aβ1–42; Refs. 123, 376) and the canabinoid anandamide (356, 442). The Aβ1–42 peptide is one of the breakdown products of the proteolytic cleavage of the amyloid precursor protein by β- and γ-secretases. In biopsy samples of human brain tissue obtained from AD patients and in ectopic systems overexpressing either α7 nAChRs or APP, Aβ1–42 coimmunoprecipitates with α7 nAChRs (490). The Aβ1–42 peptide also displaces binding of [3H]MLA from α7 nAChRs in cerebral cortical and hippocampal synaptosomes (490). More functional studies reported that while at picomolar concentrations Aβ1–42 activates α7 nAChRs ectopically expressed in Xenopus oocytes (123, 126), at nanomolar concentrations it inhibits α7 nAChRs present in different preparations (278, 376). The α7 nAChR inhibition by Aβ1–42 is noncompetitive with respect to the agonist, is voltage independent, and is therefore likely to be mediated by the interaction of the peptide with a site different from that for ACh on the nAChRs. Other studies have reported that α4β2 nAChRs are more sensitive than α7 nAChRs to inhibition by nanomolar concentrations of Aβ1–42 (506). Factors that confer Aβ sensitivity to nAChRs include, but are not restricted to, nAChR subunit composition and stoichiometry, regional distribution of specific nAChR subtypes, neuronal compartmentalization of different nAChR subtypes, as well as neuronal and nonneuronal nAChR expression (122). It is noteworthy that the α7 nAChR activity increases intracellular accumulation of Aβ in neurons (336), and Aβ peptides, in addition to modulating nAChR activity, downregulate the expression of nAChRs (197). Though poorly understood, reciprocal relationships might exist in vivo between endogenous levels of Aβ peptides and nAChR activity that are essential to the pathophysiology of AD.
Anandamide, a compound originally isolated from porcine brain extracts, is known to interact with canabinoid receptors 1 and 2 in the brain (120, 159). However, anandamide interacts with numerous other receptors, including voltage-gated Ca2+ channels (357), voltage-gated K+ channels (293), 5-HT3 receptors (358), kainate receptors (3), and nAChRs (356). At nanomolar concentrations, anandamine blocks noncompetitively and voltage independently the activation of α7 nAChRs ectopically expressed in Xenopus oocytes (356). It also inhibits the activity of α4β2 nAChRs expressed in SH-EP1 cells (443). There is evidence that anandamide is produced by postsynaptic neurons in response to elevated intracellular Ca2+ levels. For instance, concomitant activation of α7 nAChRs and NMDA receptors triggers the production of anandamine in postsynaptic neurons (448). Anandamine, then, functions as a retrograde messenger and regulates synaptic transmission by interacting with specific receptors in the presynaptic neurons/terminals (498). It has been suggested that nAChRs may serve as potential targets for modulation of synaptic transmission by anandamide (356). The mutual interactions between the endocannabinoid system and the nAChRs have led to the recent discovery of α7 nAChRs as potential targets for development of medical therapies for the treatment of cannabis addiction (440).
Finally, bupropion (16, 294, 433) and UCI-30002 (514) are examples of synthetic compounds that act as noncompetitive inhibitors of different nAChRs, including those made up of the subunits α7, α4β2, or α3β4. Both compounds effectively decrease nicotine self-administration in rats (280, 514). Bupropion is presently approved as an adjunct therapy for smoking cessation. The sites that contribute to the inhibitory actions of these compounds are completely unknown.
B. Receptor Signaling
It has long been recognized that nAChR activation in mammalian sympathetic neurons induces the opening of a nonselective cation channel that leads to Na+ influx, membrane depolarization, and consequently activation of voltage-gated Ca2+ channels (92, 119). Long before the identification of the high Ca2+ permeability of α7 nAChR channels, different studies reported significant Ca2+ influx through nAChRs in muscle, parasympathetic neurons, pheochromocytoma cells, and human neuroblastoma cells (115, 321, 347, 407, 411, 459, 468). Subsequent studies also reported significant Ca2+ influx through nAChRs in neurons isolated from the CNS (78, 334) and oocytes transfected with different nAChR subunits (423, 479). It was then recognized that Ca2+ flux directly through nAChR channels or indirectly via voltage-gated Ca2+ channels is relevant for nicotinic modulation of transmitter release, synaptic plasticity, as well as neuronal viability, differentiation, and migration.
An ever-growing body of evidence indicates that in CNS and parasympathetic nervous system neurons and in heterologous systems expressing specific nAChR subtypes, nicotine stimulates several Ca2+-dependent kinases, including PI3K, protein kinase C (PKC), protein kinase A (PKA), calmodulin-dependent protein kinase II (CAM kinase II), and extracellular signal-regulated kinases (ERKs; Refs. 108, 112, 146, 318, 469). Downstream from the nicotine-stimulated kinases, a number of transcription factors have been shown to be activated. Among these factors are the cAMP response element binding protein (CREB) and the activating transcription factor 2 (ATF-2) in PC12 cells (211, 337, 460), the Ets-like transcription factor Elk-1 in the rat hippocampus (349), and the signal transducer and activator of transcription (STAT3) in macrophages and skin cells (114, 354). Recent studies have supported a role for ERK and CREB activity in neural plasticity associated with nicotine addiction (71, 381, 484). It has also been proposed that the ERK and JAK-2/STAT-3 signaling pathways contribute to the toxic effects of nicotine in skin cells (42), and other pathways contribute to the effects of nicotine and other nicotinic ligands on inflammatory responses as described below. It appears that the placement of relatively small numbers of nAChRs at key regulatory sites can lead to multiple outcomes in terms of normal cell performance and susceptibility to exogenous challenges or participation in processes ranging from neurodegeneration to inflammation.
C. Nicotine Effects in Peripheral Systems and Inflammation
While the effects of nicotine in the CNS, including its addictive effects, remain a central focus of nAChR studies, as Langley and colleagues demonstrated over 100 years ago (259), the alkaloid has dramatic effects on peripheral systems. This includes the ability of high nicotine concentrations to act on muscle receptors as well as to impart often more subtle effects through preganglionic receptors of the autonomic nervous system. Recent stud ies have identified nAChRs present in numerous nonneuronal cell types outside the nervous systems and investigated how these receptors participate in modifying a range of physiological processes. In fact, the relationship between tobacco abuse (including smokeless) and difficulty in healing, increased susceptibility to infection (especially oral), enhanced expression of indicators of skin aging, and increased cancer risk are all well-documented (383, 452). The recognition of the expression of nAChRs in adipose tissue (36, 37) provides a mechanistic rationalization for the long-standing observation that on average smokers appear thinner, and, yet, more prone to metabolic syndromes such as type II diabetes.
Probably the first written report of an interaction between nicotine and inflammation emerged over 150 years ago when the German physician Rudolph Virchow recognized that smoking could in some cases provide acute, and even long-term therapeutic relief to the symptoms of severe asthma. Modulation by nicotine of inflammatory responses in the intestines is much better reported. Early studies found that patients with ulcerative colitis who stopped smoking tobacco developed the disease or exhibited more severe disease progression, which was ameliorated by either returning to smoking (58, 401, 466), or, in some cases, administering nicotine through transdermal patches (313). In contrast, patients with Crohn’s disease experience much more severe disease when smoking (401). Complicating this finding is that not all human subjects respond in this way. Recent studies of mice suggest that this may in part be related to specific nAChRs and their interactions with distinct inflammatory pathways (353, 366, 489), which in turn are subject to individual genetics. Notably, mice with a null mutation in the gene that encodes the α5 nAChR subunit exhibit enhanced sensitivity to induction of inflammatory bowel disease relative to controls (353). Despite increased sensitivity to disease initiation, administration of transdermal nicotine remains effective in attenuating the disease process. Therefore, again nicotine appears to impact on inflammatory processes with considerable specificity and tissue dependency. Understanding how these interactions proceed to pathology will require a much greater and detailed examination of the interaction between specific nAChR subtypes and inflammatory cytokines in different cell types, within the context of individual genetics.
There is current evidence that nAChRs present in skin cells modulate the responses triggered by inflammatory stimuli applied to the skin (354). Smoking is a welldefined risk factor in delayed wound healing and possibly the development of premature facial wrinkling (226). Distinct nAChRs are expressed in diverse cells that compose the skin (95, 189, 255, 323, 354, 526). For example, epithelial keratinocytes express functional nAChRs and, importantly, they also are capable of synthesizing the so-called cytotransmitter ACh (526). Mechanistically, nicotine, acting through nAChRs, decreases keratinocyte migration (188, 189) and modifies the activity of PI3K/Akt, ERK, MEK, and JAK signaling pathways. Furthermore, pharmacological dissection of nicotine’s influence on cell cycle progression, apoptosis, and differentiation (43) indicate that α7 nAChRs expressed in keratynocytes are important. Other receptors are clearly involved in this process, since atropine, a muscarinic and sometimes nAChR inhibitor (531, 532), reduces cell adhesion through decreasing desmoligein expression.
A relationship also exists between nAChRs and the normal physiology of adipose tissue. It has long been known that smokers tend to be leaner, and yet approximately four times more likely to become insulin resistant and develop type I diabetes (497), a condition that is more commonly observed in obese patients. This correlation is of general biological relevance, because it also extends to certain mouse strains. For example, weight loss is observed when C57BL/6 and AKR mice, but not A/J, SJL, and NZW mice are exposed to cigarette smoking for 6 mo (198). There is a genetic predisposition that may be linked to variable expression of nAChRs and individualizes the effect of nicotine on body weight. Although nAChRs are expressed in adipose tissue, their role in normal metabolism is not presently understood. Notably, nicotine pretreatment of rat adipocytes (279) reduces the release of TNF-α as well as free fatty acids and the adipokine adiponectin (whose function is not known, although its levels change in metabolic syndrome). It remains to be determined whether the effects of nicotine on metabolism result from its direct interactions with specific nAChR subtypes in adipocytes controlling levels of proinflammatory cytokines and adipokines.
D. Genetic Influences on nAChR Expression
Mice have been extensively used to identify the influences of genetic background on the responsiveness to nicotine (105). Mice are particularly well-defined for their strain-specific complex genetic traits related to the effects of nicotine (105, 302) and morphological variations in the brain (e.g., Refs. 166, 167, 169). As noted above, because each nAChR subunit is expressed in unique, but overlapping cell and tissue-specific patterns, this can impart remarkable specialization of their function. However, as demonstrated by the extensive studies of the Collins group (105, 302), the responses of mice to nicotine depends on still ill-defined components of the genetic background.
Mouse strains exhibit differences in their respective level of nAChR expression and the morphological context of the neuronal circuitries in which they are expressed. For instance, substantial strain-specific variability in nAChR expression has been observed in the striatum (34), retina (227), cerebellum (471), and dorsal hippocampus (164, 165, 167, 169) of mice. It is noteworthy that isogenic mouse strains differ in gross measures of hippocampal architecture including volume, shape, and neuronal number that are nevertheless determined genetically (169, 499). The dorsal hippocampus shows exquisitely different morphological features among isogenic mouse strains. In addition, within the dorsal hippocampus, immunostaining for the α4 nAChR subunit varies dramatically between CA1 inhibitory interneurons and astrocytes of adult mice of differing strains (169). For example, the expression of nAChRs by inhibitory CA1 interneurons in the hippocampus of C3H mice outnumbers that observed in astrocytes by ~3:1, whereas these values are reversed in C57BL/6 (B6) mice (165, 169). Taking into account that basic hippocampal architecture and nAChR expression in hippocampal neurons and astrocytes differ among mouse strains, it remains to be elucidated whether nontraditional nicotinic modulators that are produced and released by astrocytes contribute to the strain-specific responses of mice to nicotine administration.
Strain-dependent variations in nAChR density in regions of the rat brain have also been reported. For instance, numbers of α-BGT- and cytisine-binding sites, which represent primarily α7 and α4β2 nAChRs, respectively, are significantly higher in specific regions of the brains of Wistar normotensive rats compared with spontaneously hypertensive rats (174). It has been suggested that the poorer cognitive performance of spontaneously hypertensive compared with Wistar normotensive rats relates to their differential expression of nAChRs in the brain (174). A more recent study reported that α3β4 nAChR activity/expression is higher in the hippocampus of August Copenhagen Irish (ACI) than in the hippocampus of Sprague-Dawley (SD) rats (29). The ACI rat, an inbred strain, is well-known for its higher propensity to develop estrogen-dependent mammary and prostate cancers compared with the outbred SD rat (220, 430, 441). The brain of ACI rats is also highly sensitive to the actions of estradiol (444), a sex hormone that appears to have a neuroprotective function in schizophrenia (202, 422) and to prevent disruption of prepulse inhibition (PPI) in laboratory animals and healthy women subjected to different treatments (182, 478). The question is, therefore, posed as to whether the differential expression/function of α3β4 nAChRs in the hippocampus of ACI and SD rats contributes to differences in neurocognitive functions in these animals. Studies aimed at addressing this question could prove extremely relevant for the understanding of the contribution of specific nAChRs and differences in genetic background to the diverse susceptibility and penetrance of neuropathological disorders inclusive of disorders such as schizophrenia (see next section).
VI. NICOTINIC RECEPTORS AND DISEASE
A. Changes in nAChRs With Age and Alzheimer’s Disease
One measure of normal age-related decline in the CNS is the diminishment and eventual dysfunction of the limbic cholinergic system that, in its most severe form, contributes to the neuropathologies of dementias including AD. AD is the most common form of dementia in the elderly population. The histopathology of this disease is well known to have at least four components: 1) loss of cholinergic neurotransmission, 2) deposition of extracellular Aβ peptides into plaques, 3) hyperphosphorylation of the τ protein that leads to excessive formation of neurofibrillar tangles, and 4) increased local inflammation. Studies that examine the state of cholinergic neurotransmission in aging and dementia often focus on muscarinic receptor expression. However, loss of brain nAChRs precedes that of muscarinic receptors during normal aging, and it is often much more extensive in human brains afflicted with AD relative to age-matched controls (236, 308, 373, 374, 416, 519). In fact, α4 nAChR expression can decrease by >80% in the AD brain (306, 374).
The importance of retaining the high-affinity nicotine binding sites to brain integrity has been demonstrated in studies of mice with a null mutation in the gene that encodes the β2 nAChR subunit, a structural subunit of the high-affinity nicotine binding site (150, 184, 215, 311); these mice experience early onset neurodegeneration (528). Therefore, arresting or slowing age-related decline in nAChRs is predicted to have therapeutic benefit. The simplest of these interventions with suggested efficacy to slow down age-related losses of nAChRs is the long-term use of nicotine (160, 177, 340, 345, 377, 519). In human trials, nicotine showed little efficacy in ameliorating AD symptoms (437). However, treatment was initiated after diagnosis of symptoms, and there is both epidemiological data and direct evidence from animal models that this is too late (106, 346, 396).
To identify the age- and strain-dependent effects of long-term or acute exposure to nicotine on nAChR subunit expression in the mouse brain, levels of α4, α5, α7, β2, and β4 nAChR subunits were measured in the dorsal hippocampus of both adult (10–14 mo) and aged (22–26 mo) CBA/J or B6 mice (164, 165, 396). First, age-related nAChR subunit expression decline was observed in both strains, and this was dominated by diminished α4 nAChR expression. Second, long-term (12 mo) oral nicotine failed to reduce the age-related decline in the number of neurons expressing α4 nAChR subunits, although the neurons that remained exhibited larger processes with more varicosities than age-matched controls (165, 396). Acute nic otine treatment (~6 wk of oral nicotine) of aged mice had no measurable influence on nAChR expression, neuronal viability, or dendritic complexity (e.g., Ref. 396). Third, CBA/J mice exhibited greater overall neuronal loss of α4 relative to β6 nAChR subunit expression. Fourth, a significant component underlying the relative severity of strainspecific diminished nAChR expression in the dorsal hippocampus appears related to differences in cytoarchitecture between these strains (165, 169). Coincident with neuronal loss of α4 nAChR subunit expression, astrocyte expression of this subunit increased substantially in aged CBA mice relative to adults (~10–12 mo old), but to a much lesser extent between adult and aged B6 mice (166). It is noteworthy that nAChR expression by astrocytes in brains afflicted with AD is increased (463, 518), and astrocytes in general have been reported to be more plentiful in the hippocampus of some rat strains with age (35, 284). Fifth, possible impacts of selective nAChR loss on the aging brain were provided by evidence that in primary cultures α4-containing nAChRs protect neurons against toxic fragments of the amyloid β peptides while α7 nAChRs protect against excitotoxic challenges (e.g., NMDA) (162, 242, 243, 278, 519). However, this appealing scenario is complicated by recent findings that β-amyloid peptides directly modify α7 nAChR function (242, 278).
Mouse strains, like humans, also exhibit a striking age-related decline in nAChR expression. For instance, in the hippocampus of aged CBA and B6 mice, expression of α4 and α7 nAChR subunits decreases with age (166). However, while α4 nAChR loss is more severe in aged B6 mice, α7 nAChR loss is more prominent in aged CBA/J (166). Coincident with the loss of neuronal α4 nAChR expression in the hippocampus of CBA/J strain is a significant age-related increase in α7 nAChR staining of astrocytes, which has also been reported in cases with AD (463). These results suggest that mouse strains of different genetic backgrounds undergo dissimilar age-related changes in the expression of nAChR subunits. They also imply that the responses of aging animals to any given toxic insult will be largely dependent on their genetic backgrounds. This leads to the speculation that the loss of α4-containing nAChRs could significantly increase susceptibility to age-associated insults by β-amyloid peptides, while loss of α7 nAChRs would enhance susceptibility to excitotoxic challenges such as those associated with ischemic damage or the presence of TNF-α (75, 76). One could infer that early genetic predispositions are important determinants of the life-long dynamics of nAChR function and that therapeutic interventions will have widely differing effects consistent with the individual genetic backgrounds of the patients.
Also of relevance are recent studies of an interaction in mice between nAChRs and long-term use of anti-inflammatories such as nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs may impart therapeutic benefit in neurodegenerative diseases of aging, including reduced risk of age-related dementia (2, 173, 178, 216, 289, 454, 470). NSAIDs [e.g., drugs such as ibuprofen and NS398 (celecoxib or Celebrex)] antagonize to varying degrees two related cyclooxygenase (Cox) enzymes, Cox1 and Cox2 (also termed, prostaglandin-endoperoxide synthase 2), that are rate-limiting in converting arachidonic acid to prostaglandin H2, a precursor to many additional prostaglandins (for review, see Ref. 436). The two Cox forms are known to be differentially regulated; Cox1 is often expressed constitutively while Cox2 expression is induced by proinflammatory conditions. In the brain, however, Cox2 is constitutively expressed by neurons (212, 512), participates in modulating synaptic plasticity (53, 464), and conditionally can either inhibit or promote cell death (74, 85, 237, 322, 451). A link between α4 nAChRs and Cox2 was suggested by the observation that interneurons in the hippocampus coexpress both proteins (165). A mechanistic connection was inferred when long-term treatment of aged animals with NS398 promoted retention of α4 nAChR expression in the brain, an effect that was antagonized by the coadministration of nicotine. It was then proposed that NSAIDs could impart age-related therapeutic benefit in the nAChR system, although how this effect was imparted and antagonized by nicotine remains unclear. There is the possibility that a compensatory mechanism changes nAChR expression among interneuron classes in animals given NS398, but does so in a way that maintains the appearance of the adult phenotype (164–166, 169). These relationships are particularly intriguing in light of the interaction between the nAChR and proinflammatory cytokine systems noted above. In fact, the nAChR interaction with inflammatory regulation may prove to have a more generalized contribution in pathologies.
B. Nicotine Effects on Parkinson’s Disease
Parkinson’s disease (PD) is characterized by selective damage to dopaminergic nigrostriatal neurons and is clinically revealed by motor deficits, including rigidity, tremor, and bradykinesia. Dopamine replacement therapy (usually with l-dopa) is the most common treatment, although this drug loses efficacy over time. The etiology of this disease remains unclear. However, epidemiological studies have reported that heavy smokers are less likely to experience PD (see reviews in Refs. 384, 385). Furthermore, like AD, it is apparent that this protection is real and not related to selective diminishment of the smoking population through early death related to other side effects of smoking. More direct evidence of the protective effects of nicotine in this disease process comes from studies in primates, where oral nicotine reduces the nigrostriatal neuronal loss observed in chemically induced PD (384, 385).
As in AD, clinical studies using nicotine therapies such as the patch to treat PD have provided inconsistent results (266). In particular, the need to start nicotine therapy before neuronal loss is not practical in the design of these studies, and the use of the nicotine patch for delivery of drug may also be inefficient for therapeutic value. However, in rodent and nonprimate animal models, nicotine has been shown to enhance striatal dopamine release and to prevent toxin-induced degeneration of dopaminergic neurons (384, 385). It has also been proposed that, as in other diseases, in PD nicotine favorably influences otherwise neurodegenerative influences of astrocytes and inflammatory processes (362). A key future direction in this field of research is to examine the timing of drug administration towards optimizing therapeutic efficacy and to develop drugs that, unlike nicotine, specifically target receptors that play a more critical role in regulating dopamine release in the striatum, such as those harboring a6 subunits (see above).
C. Addiction
Nicotine is perhaps the most addictive drug that is widely used; 95% or more of its users with a strong desire to stop using it relapse within 1 yr (47, 203). Chronic nicotine use and the phenotypes of addiction are closely associated in humans and other animals with concurrent physiological changes in nAChR function and expression. In particular, repeated self-administration produces the upregulation of high-affinity (α4β2) nAChR expression, reduces receptor function due to desensitization and, in most cases, imparts developmental tolerance. Additional changes imposed by nicotine abuse range from reinforcement to physical discomfort associated with withdrawal including craving, anxiety, and a multitude of other less than desirable sensations of autonomic dysfunction when use is stopped. In some rarer cases, the cessation of nicotine use can have more curious physiological consequences such as promoting the onset of “flares” in certain inflammatory diseases such as ulcerative colitis as noted above. Consequently, because addiction to nicotine and the physiological consequences of long-term self-administration vary greatly among individuals, the interaction and signaling of nicotine through nAChRs must be highly influenced, and possibly determined by the genetic background (91).
The mouse is particularly amenable to well-defined genetic and pharmacological experimental manipulations. This animal model has successfully been used to reveal key nAChRs that contribute to specific effects of nicotine. For example, the measurement of acute and chronic influence of nicotine administration on at least 19 mouse strains has established a remarkable database that quantitatively describes the genetic influence on multiple acute and chronic physiological and behavioral effects of treatments with nicotine. A principle component of genetic analysis of the contribution of α7 and α4β2 nAChRs to the effects of nicotine was reported 15 years ago. The number of α-BGT binding sites (presumably α7 nAChRs) was shown to be highly correlated with sensitivity to nicotinic-induced seizures (105, 301, 303). In contrast, the effect of nicotine on physiologically diverse behaviors such as altering body temperature or performance in Y-maze was more closely related to the high-affinity nicotine binding sites related to α4β2 nAChRs (105). Recent genetic manipulations of the expression of nAChR subunits in mice in conjunction with pharmacological, morphological, and functional studies of neuronal functions in the brains of these mice are paving the way for a better understanding of the complex trait of nicotine addiction.
Targeted genetic manipulation of mouse models is offering considerable insights into the role of specific nAChRs in behaviors related to nicotine administration such as reinforcement, upregulation, and tolerance. Mice with a null mutation in the gene that encodes the β2 nAChR subunit were among the first to be employed. The concept that β2-containing nAChRs are involved in the reinforcing effects of nicotine was supported by the findings that these mice lacked the high-affinity nicotine binding site, exhibited poor nicotine self-administration, and failed to develop behaviors related to reinforcement (378). The demonstration that these mice developed symptoms of the nicotine withdrawal syndrome similar to those observed in wild-type mice led to the conclusion that β2-containing nAChRs do not contribute to the physical dependence on nicotine (57). With the use of numerous genetic approaches, subsequent studies examined the role of different nAChR α subunits in nicotine addiction.
Direct evidence of the participation of α4 nAChR subunits in several components of nicotine addiction came from elegant experiments where the sensitivity of this subunit to nicotine was increased through genetic manipulation (461). In these experiments, a knock-in mouse was generated through directed homologous recombination that exchanged a highly conserved leucine in TM2 with an alanine in the α4 nAChR subunit. The mutant subunit reduced the concentration of nicotine required to gate the receptor (mostly α4β2 nAChRs). Mice with this mutation were also susceptible to epilepsy and other neurological disorders that required the subunit expression to be reduced through genetic means to ensure animal viability (152, 461). Extensive measurements of these animals revealed that the α4(L:A) nAChR subunit mutation and enhanced receptor activation alone can account for nicotine reinforcement, sensitization, and the development of tolerance (461).
There has been a long-standing suspicion that nAChR upregulation and tolerance are closely related in establishing mechanisms contributing to nicotine addiction susceptibility (55, 73, 373). However, discrepancies in this correlation have also been experimentally tested through the use of genetically modified mice. Among the earliest findings that upregulation and the development of tolerance could be genetically separated was seen in C3H mice, where chronic nicotine administration robustly upregulated high-affinity nicotine binding sites but failed to induce tolerance (105, 301). Several possibilities exist for the identity of the nAChR important to tolerance development. The strong positive correlation between α-BGT site number and sensitivity to nicotine-induced seizures among multiple mouse strains led to the suggestion that α7 nAChRs are critical to limit oral nicotine self-administration in mice (105, 301). However, mice with a null mutation in the gene that codes the α7 nAChR subunit remain sensitive to nicotine-induced seizures and limit their nicotine intake as much as wild-type mice do (153), suggesting that more complex genetic traits underlie these effects. While development of tolerance does not seem to be regulated by α7 nAChRs, a recent study of α7 nAChR-null mice indicates that these receptors control the severity of the nicotine withdrawal syndrome (402). Other nAChRs that appear as good candidates to underlie the ability of nicotine to induce tolerance are those bearing the β3 and/or β4 subunits. First, the expression of the β3 nAChR subunit is highly restricted in the brain, and null mutants of this subunit appear relatively normal except for decreased anxiety-like behavior (61, 494). While the β4 nAChR subunit has also been proposed to be strongly restricted in its expression in the CNS (133), more recent studies suggest a broader distribution (121, 167, 481). For example, more sensitive methods of in situ hybridization and PCR have revealed β4 expression in many brain regions and cell types, including subpopulations of hippocampal inhibitory interneurons, not previously observed to express this subunit. The β4 nAChR-null mouse is coincidently less sensitive to nicotine-induced seizure (235), which, as noted above, correlates with limiting nicotine consumption. Furthermore, the possibility that α3β4 nAChRs or other β4-containing receptors contributes to nicotine reward has been reported (181). In summary, while nicotine-induced upregulation requires at least the β2 nAChR subunit, development of tolerance to nicotine requires neither the β2 nor the α7 nAChR subunit; instead, it appears to be modulated by a β4-containing nAChR and to require an α4-containing nAChR.
Studies using mice with specific mutations in selected nAChR subunits have accurately complemented pharmacological and functional studies, helped to clarify key issues related to nicotinic cholinergic functions in neuronal and nonneuronal tissues, and added considerable linkage between the gene and both physiological and behavioral components of nicotine biology. More importantly, they have offered compelling evidence that possibly minor nAChRs are important to significant aspects of the biology of nicotine and the effectiveness of nicotinicbased therapies, opening novel avenues for examining underlying mechanisms of nAChR regulation of cell function and viability. There is no doubt that the development of conditional nAChR knockout and knock-in mice will be essential for the understanding of the differential roles of specific nAChR subtypes in neuronal and nonneuronal functions throughout life.
VII. FUTURE PERSPECTIVES
Though the past 20 years have experienced a significant growth on nicotinic research, we are still facing a number of challenges. For instance, it is imperative to answer the question of why there are two natural agonists (ACh and choline) for α7 nAChRs and to identify the conditions under which each agonist would play a major role. Is this a way by which selective activation or inactivation of a particular nAChR subtype is achieved? Or is it a way by which α7 nAChR signaling can change its frequency by using different endogenous agonists? Crosstalk among various nAChRs and between nAChRs and other receptors needs to be investigated in detail. It is crucial to identify how specific nAChR subtypes are compartmentalized on the cell surface and how such segregation targets their signaling to given intracellular mechanisms. Likewise, development of new pharmacological tools will be necessary to better identify the native nAChR subtypes expressed in neuronal and nonneuronal cells throughout life. Revealing how glia-neuron interactions shape nAChR functions, and vice versa, in the brain will be essential for the understanding of the involvement of specific nAChR subtypes in normal physiology and in disease states. Determining how AChE inhibitors and nicotinic APLs affect the activity of different nAChRs in vivo, and, accordingly, developing ligands that selectively enhance the activity of a given nAChR subtype will be a crucial step for future drug development to treat a number of catastrophic disorders. Mapping how the genetic background, sex, and age shape the responses to nicotinic ligands has to be prioritized should these ligands receive recommendation for treatment of diseases that afflict children and the elderly. As important will be understanding the role that nAChRs play in regulating immunological responses within and outside the CNS under normal physiological conditions and in numerous diseases. Developing conditional knockout and knock-in mice for individual nAChR subunits will be a sine-qua-non step to identify how neuronal and nonneuronal functions are regulated by specific nAChR subtypes at various ages. These are only a few of the many, highly exciting challenges for future research in this field.
ACKNOWLEDGMENTS
We are indebted to Dr. Lorise Gahring (Univ. of Utah School of Medicine), Dr. William Randall (Univ. of Maryland School of Medicine), Dr. Robert Schwarcz (Univ. of Maryland School of Medicine), and Daniel Nagode (Univ. of Maryland School of Medicine) for their insightful comments and discussions as well as Mabel A. Zelle for superb assistance.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant NS-25296, the National Institutes of Health CounterACT Program through NINDS Award U01-NS-59344, and the United States Army Research Office Contract W911NF-06-1-0098 (to E. X. Albuquerque) as well as National Institute of Aging Grant AG-17517 and National Heart, Lung, and Blood Institute Grant PO1-HL-72903 (to S. W. Rogers). The contents of this work are solely the responsibility of the authors and do not necessarily reflect the official views of the federal government.
REFERENCES
- 1.Adcock C, Smith GR, Sansom MS. The nicotinic acetylcholine receptor: from molecular model to single-channel conductance. Eur Biophys J. 2000;29:29–37. doi: 10.1007/s002490050248. [DOI] [PubMed] [Google Scholar]
- 2.Aisen PS. Evaluation of selective COX-2 inhibitors for the treatment of Alzheimer’s disease. J Pain Symptom Manage. 2002;23:S35–S40. doi: 10.1016/s0885-3924(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 3.Akinshola BE, Taylor RE, Ogunseitan AB, Onaivi ES. Anandamide inhibition of recombinant AMPA receptor subunits in Xenopus oocytes is increased by forskolin and 8-bromo-cyclic AMP. Naunyn-Schmiedebergs Arch Pharmacol. 1999;360:242–248. doi: 10.1007/s002109900078. [DOI] [PubMed] [Google Scholar]
- 4.Akk G, Steinbach JH. Galantamine activates muscle-type nicotinic acetylcholine receptors without binding to the acetylcholinebinding site. J Neurosci. 2005;25:1992–2001. doi: 10.1523/JNEUROSCI.4985-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albuquerque EX, Alkondon M, Pereira EFR, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280:1117–1136. [PubMed] [Google Scholar]
- 6.Albuquerque EX, Barnard EA, Porter CW, Warnick JE. The density of acetylcholine receptors and their sensitivity in the postsynaptic membrane of muscle endplates. Proc Natl Acad Sci USA. 1974;71:2818–2822. doi: 10.1073/pnas.71.7.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albuquerque EX, Pereira EFR, Alkondon M, Schrattenholz A, Maelicke A. Nicotinic acetylcholine receptors on hippocampal neurons: distribution on the neuronal surface and modulation of receptor activity. J Recept Signal Transduct Res. 1997;17:243–266. doi: 10.3109/10799899709036607. [DOI] [PubMed] [Google Scholar]
- 8.Albuquerque EX, Pereira EFR, Castro NG, Alkondon M, Reinhardt S, Schroder H, Maelicke A. Nicotinic receptor function in the mammalian central nervous system. Ann NY Acad Sci. 1995;757:48–72. doi: 10.1111/j.1749-6632.1995.tb17464.x. [DOI] [PubMed] [Google Scholar]
- 9.Albuquerque EX, Pereira EFR, Mike A, Eisenberg HM, Maelicke A, Alkondon M. Neuronal nicotinic receptors in synaptic functions in humans and rats: physiological and clinical relevance. Behav Brain Res. 2000;113:131–141. doi: 10.1016/s0166-4328(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 10.Albuquerque EX, Pereira ERF, Braga MF, Matsubayashi H, Alkondon M. Neuronal nicotinic receptors modulate synaptic function in the hippocampus and are sensitive to blockade by the convulsant strychnine and by the anti-Parkinson drug amantadine. Toxicol Lett. 1998;102–103:211–218. doi: 10.1016/s0378-4274(98)00309-9. [DOI] [PubMed] [Google Scholar]
- 11.Alkondon M, Albuquerque EX. α-Cobratoxin blocks the nicotinic acetylcholine receptor in rat hippocampal neurons. Eur J Pharmacol. 1990;191:505–506. doi: 10.1016/0014-2999(90)94190-9. [DOI] [PubMed] [Google Scholar]
- 12.Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- 13.Alkondon M, Albuquerque EX. Initial characterization of the nicotinic acetylcholine receptors in rat hippocampal neurons. J Recept Res. 1991;11:1001–1021. doi: 10.3109/10799899109064693. [DOI] [PubMed] [Google Scholar]
- 14.Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor α7 and α4β2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- 15.Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- 16.Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–750. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- 17.Alkondon M, Albuquerque EX. A non-α7 nicotinic acetylcholine receptor modulates excitatory input to hippocampal CA1 interneurons. J Neurophysiol. 2002;87:1651–1654. doi: 10.1152/jn.00708.2001. [DOI] [PubMed] [Google Scholar]
- 18.Alkondon M, Albuquerque EX. Subtype-specific inhibition of nicotinic acetylcholine receptors by choline: a regulatory pathway. J Pharmacol Exp Ther. 2006;318:268–275. doi: 10.1124/jpet.106.103135. [DOI] [PubMed] [Google Scholar]
- 19.Alkondon M, Braga MF, Pereira EFR, Maelicke A, Albuquerque EX. α7 Nicotinic acetylcholine receptors and modulation of GABAergic synaptic transmission in the hippocampus. Eur J Pharmacol. 2000;393:59–67. doi: 10.1016/s0014-2999(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 20.Alkondon M, Pereira EF, Albuquerque EX. Mapping the location of functional nicotinic and gamma-aminobutyric acidA receptors on hippocampal neurons. J Pharmacol Exp Ther. 1996;279:1491–1506. [PubMed] [Google Scholar]
- 21.Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkondon M, Pereira EFR, Albuquerque EX. α-Bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- 23.Alkondon M, Aracava Y, Pereira EFR, Albuquerque EX. A single in vivo application of cholinesterase inhibitors has neuron type-specific effects on nicotinic receptor activity in guinea pig hippocampus. J Pharmacol Exp Ther. In press doi: 10.1124/jpet.108.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkondon M, Pereira EFR, Albuquerque EX. NMDA and AMPA receptors contribute to the nicotinic cholinergic excitation of CA1 interneurons in the rat hippocampus. J Neurophysiol. 2003;90:1613–1625. doi: 10.1152/jn.00214.2003. [DOI] [PubMed] [Google Scholar]
- 25.Alkondon M, Pereira EFR, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 26.Alkondon M, Pereira EFR, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates y-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- 27.Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 28.Alkondon M, Pereira EFR, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alkondon M, Pereira EFR, Potter MC, Kauffman FC, Schwarcz R, Albuquerque EX. Strain-specific nicotinic modulation of glutamatergic transmission in the CA1 field of the rat hippocampus: August Copenhagen Irish versus Sprague-Dawley. J Neurophysiol. 2007;97:1163–1170. doi: 10.1152/jn.01119.2006. [DOI] [PubMed] [Google Scholar]
- 30.Alkondon M, Pereira EFR, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992;41:802–808. [PubMed] [Google Scholar]
- 31.Alkondon M, Pereira EFR, Yu P, Arruda EZ, Almeida LE, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via α7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkondon M, Pereira ERF, Albuquerque EX. Age-dependent changes in the functional expression of two nicotinic receptor subtypes in CA1 stratum radiatum interneurons in the rat hippocampus. Biochem Pharmacol. 2007;74:1134–1144. doi: 10.1016/j.bcp.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Alkondon M, Rocha ES, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat brain. V. α-Bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther. 1996;278:1460–1471. [PubMed] [Google Scholar]
- 34.Altavista MC, Gozzo S, Iacopino C, Albanese A. A genetic study of neostriatal cholinergic neurones in C57BL/6 and DBA/2 mice. Funct Neurol. 1987;2:273–279. [PubMed] [Google Scholar]
- 35.Amenta F, Bronzetti E, Sabbatini M, Vega JA. Astrocyte changes in aging cerebral cortex and hippocampus: a quantitative immunohistochemical study. Microsc Res Tech. 1998;43:29–33. doi: 10.1002/(SICI)1097-0029(19981001)43:1<29::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Andersson K, Arner P. Cholinoceptor-mediated effects on glycerol output from human adipose tissue using in situ microdialysis. Br J Pharmacol. 1995;115:1155–1162. doi: 10.1111/j.1476-5381.1995.tb15018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersson K, Arner P. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. Int J Obes Relat Metab Disord. 2001;25:1225–1232. doi: 10.1038/sj.ijo.0801654. [DOI] [PubMed] [Google Scholar]
- 38.Aracava Y, Deshpande SS, Swanson KL, Rapoport H, Wonnacott S, Lunt G, Albuquerque EX. Nicotinic acetylcholine receptors in cultured neurons from the hippocampus and brain stem of the rat characterized by single channel recording. FEBS Lett. 1987;222:63–70. doi: 10.1016/0014-5793(87)80192-8. [DOI] [PubMed] [Google Scholar]
- 39.Aracava Y, Ikeda SR, Daly JW, Brookes N, Albuquerque EX. Interactions of bupivacaine with ionic channels of the nicotinic receptor. Analysis of single-channel currents. Mol Pharmacol. 1984;26:304–313. [PubMed] [Google Scholar]
- 40.Arias E, Ales E, Gabilan NH, Cano-Abad MF, Villarroya M, Garcia AG, Lopez MG. Galantamine prevents apoptosis induced by β-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology. 2004;46:103–114. doi: 10.1016/s0028-3908(03)00317-4. [DOI] [PubMed] [Google Scholar]
- 41.Armett CJ, Ritchie JM. The action of acetylcholine and some related substances on conduction in mammalian non-myelinated nerve fibres. J Physiol. 1961;155:372–384. doi: 10.1113/jphysiol.1961.sp006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: alterations of the NF-κB expression and activity downstream of α7 nicotinic receptor in oral keratinocytes. Life Sci. 2007;80:2191–2194. doi: 10.1016/j.lfs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arredondo J, Nguyen VT, Chernyavsky AI, Bercovich D, Orr-Urtreger A, Kummer W, Lips K, Vetter DE, Grando SA. Central role of α7 nicotinic receptor in differentiation of the stratified squamous epithelium. J Cell Biol. 2002;159:325–336. doi: 10.1083/jcb.200206096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arredondo J, Nguyen VT, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. A receptor-mediated mechanism of nicotine toxicity in oral keratinocytes. Lab Invest. 2001;81:1653–1668. doi: 10.1038/labinvest.3780379. [DOI] [PubMed] [Google Scholar]
- 45.Aubert I, Cecyre D, Gauthier S, Quirion R. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J Comp Neurol. 1996;369:31–55. doi: 10.1002/(SICI)1096-9861(19960520)369:1<31::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 47.Balfour DJ. The neurobiology of tobacco dependence: a commentary. Respiration. 2002;69:7–11. doi: 10.1159/000049362. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer’s disease. J Neural Transm. 1999;106:165–181. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- 50.Barnard EA, Coates V, Dolly JO, Mallick B. Binding of α-bungarotoxin and cholinergic ligands to acetylcholine receptors in the membrane of skeletal muscle. Cell Biol Int Rep. 1977;1:99–106. doi: 10.1016/0309-1651(77)90016-9. [DOI] [PubMed] [Google Scholar]
- 51.Barrantes FJ. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Res. 2004;47:71–95. doi: 10.1016/j.brainresrev.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Barrantes FJ, Antollini SS, Blanton MP, Prieto M. Topography of nicotinic acetylcholine receptor membrane-embedded domains. J Biol Chem. 2000;275:37333–37339. doi: 10.1074/jbc.M005246200. [DOI] [PubMed] [Google Scholar]
- 53.Bazan NG. COX-2 as a multifunctional neuronal modulator. Nat Med. 2001;7:414–415. doi: 10.1038/86477. [DOI] [PubMed] [Google Scholar]
- 54.Bennett MR. History of the Synapse. London: Harwood Academic; 2001. [Google Scholar]
- 55.Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (—)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 56.Bernard C. Leçons sur Les Effets des Substances Toxiques et Medicamenteuses. Paris: Bailliere; 1857. [PubMed] [Google Scholar]
- 57.Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: β-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology. 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- 58.Birtwistle J, Hall K. Does nicotine have beneficial effects in the treatment of certain diseases? Br J Nurs. 1996;5:1195–1202. doi: 10.12968/bjon.1996.5.19.1195. [DOI] [PubMed] [Google Scholar]
- 59.Blount P, Merlie JP. Mutational analysis of muscle nicotinic acetylcholine receptor subunit assembly. J Cell Biol. 1990;111:2613–2622. doi: 10.1083/jcb.111.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blumenthal EM, Conroy WG, Romano SJ, Kassner PD, Berg DK. Detection of functional nicotinic receptors blocked by α-bungarotoxin on PC12 cells and dependence of their expression on post-translational events. J Neurosci. 1997;17:6094–6104. doi: 10.1523/JNEUROSCI.17-16-06094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Booker TK, Butt CM, Wehner JM, Heinemann SF, Collins AC. Decreased anxiety-like behavior in β3 nicotinic receptor subunit knockout mice. Pharmacol Biochem Behav. 2007;87:146–157. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Boulter J, Evans K, Goldman D, Martin G, Treco D, Heinemann S, Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor α-subunit. Nature. 1986;319:368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- 63.Boulter J, Luyten W, Evans K, Mason P, Ballivet M, Goldman D, Stengelin S, Martin G, Heinemann S, Patrick J. Isolation of a clone coding for the α-subunit of a mouse acetylcholine receptor. J Neurosci. 1985;5:2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D. α3, α5, β4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- 65.Bourne Y, Talley TT, Hansen SB, Taylor P, Marchot P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake α-neurotoxins and nicotinic receptors. EMBO J. 2005;24:1512–1522. doi: 10.1038/sj.emboj.7600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyd RT. Transcriptional regulation and cell specificity determinants of the rat nicotinic acetylcholine receptor α 3 gene. Neurosci Lett. 1996;208:73–76. doi: 10.1016/0304-3940(96)12561-1. [DOI] [PubMed] [Google Scholar]
- 67.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 68.Broide RS, Leslie FM. The α7 nicotinic acetylcholine receptor in neuronal plasticity. Mol Neurobiol. 1999;20:1–16. doi: 10.1007/BF02741361. [DOI] [PubMed] [Google Scholar]
- 69.Bruneau E, Sutter D, Hume RI, Akaaboune M. Identification of nicotinic acetylcholine receptor recycling and its role in maintaining receptor density at the neuromuscular junction in vivo. J Neurosci. 2005;25:9949–9959. doi: 10.1523/JNEUROSCI.3169-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruneau EG, Akaaboune M. The dynamics of recycled acetylcholine receptors at the neuromuscular junction in vivo. Development. 2006;133:4485–4493. doi: 10.1242/dev.02619. [DOI] [PubMed] [Google Scholar]
- 71.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 72.Bruses JL, Chauvet N, Rutishauser U. Membrane lipid rafts are necessary for the maintenance of the (α)7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J Neurosci. 2001;21:504–512. doi: 10.1523/JNEUROSCI.21-02-00504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- 74.Carlson NG. Neuroprotection of cultured cortical neurons mediated by the cyclooxygenase-2 inhibitor APHS can be reversed by a prostanoid. J Neurosci Res. 2003;71:79–88. doi: 10.1002/jnr.10465. [DOI] [PubMed] [Google Scholar]
- 75.Carlson NG, Bacchi A, Rogers SW, Gahring LC. Nicotine blocks TNF-α-mediated neuroprotection to NMDA by an α-bungarotoxin-sensitive pathway. J Neurobiol. 1998;35:29–36. doi: 10.1002/(sici)1097-4695(199804)35:1<29::aid-neu3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 76.Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC. Inflammatory cytokines IL-1α, IL-1β, IL-6, TNF-α impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163:3963–3968. [PubMed] [Google Scholar]
- 77.Castro NG, Albuquerque EX. Brief-lifetime, fast-inactivating ion channels account for the α-bungarotoxin-sensitive nicotinic response in hippocampal neurons. Neurosci Lett. 1993;164:137–140. doi: 10.1016/0304-3940(93)90876-m. [DOI] [PubMed] [Google Scholar]
- 78.Castro NG, Albuquerque EX. α-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Celie PH, Kasheverov IE, Mordvintsev DY, Hogg RC, van Nierop P, van Elk R, van Rossum-Fikkert SE, Zhmak MN, Bertrand D, Tsetlin V, Sixma TK, Smit AB. Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an a-conotoxin PnIA variant. Nat Struct Mol Biol. 2005;12:582–588. doi: 10.1038/nsmb951. [DOI] [PubMed] [Google Scholar]
- 80.Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–914. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 81.Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–865. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- 83.Changeux JP. Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition. New York: Odile Jacob; 2003. [DOI] [PubMed] [Google Scholar]
- 84.Changeux JP, Galzi JL, Devillers-Thiery A, Bertrand D. The functional architecture of the acetylcholine nicotinic receptor explored by affinity labelling and site-directed mutagenesis. Q Rev Biophys. 1992;25:395–432. doi: 10.1017/s0033583500004352. [DOI] [PubMed] [Google Scholar]
- 85.Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- 86.Chernyavsky AI, Arredondo J, Marubio LM, Grando SA. Differential regulation of keratinocyte chemokinesis and chemotaxis through distinct nicotinic receptor subtypes. J Cell Sci. 2004;117:5665–5679. doi: 10.1242/jcs.01492. [DOI] [PubMed] [Google Scholar]
- 87.Chiappinelli VA, Giacobini E. Time course of appearance of α-bungarotoxin binding sites during development of chick ciliary ganglion and iris. Neurochem Res. 1978;3:465–478. doi: 10.1007/BF00966328. [DOI] [PubMed] [Google Scholar]
- 88.Christianson JC, Green WN. Regulation of nicotinic receptor expression by the ubiquitin-proteasome system. EMBO J. 2004;23:4156–4165. doi: 10.1038/sj.emboj.7600436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 90.Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, Berg DK, Ellisman MH, Sejnowski TJ. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309:446–451. doi: 10.1126/science.1108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collins AC, Marks MJ. Progress towards the development of animal models of smoking-related behaviors. J Addict Dis. 1991;10:109–126. doi: 10.1300/J069v10n01_08. [DOI] [PubMed] [Google Scholar]
- 92.Colquhoun D. Molecular neurobiology. A new type of ion-channel block. Nature. 1987;329:204–205. doi: 10.1038/329204a0. [DOI] [PubMed] [Google Scholar]
- 93.Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- 94.Conroy WG, Vernallis AB, Berg DK. The α5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron. 1992;9:679–691. doi: 10.1016/0896-6273(92)90031-8. [DOI] [PubMed] [Google Scholar]
- 95.Conti-Fine BM, Navaneetham D, Lei S, Maus AD. Neuronal nicotinic receptors in non-neuronal cells: new mediators of to bacco toxicity? Eur J Pharmacol. 2000;393:279–294. doi: 10.1016/s0014-2999(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 96.Conti-Tronconi BM, McLane KE, Raftery MA, Grando SA, Protti MP. The nicotinic acetylcholine receptor: structure and autoimmune pathology. Crit Rev Biochem Mol Biol. 1994;29:69–123. doi: 10.3109/10409239409086798. [DOI] [PubMed] [Google Scholar]
- 97.Cooper ST, Harkness PC, Baker ER, Millar NS. Up-regulation of cell-surface α4β2 neuronal nicotinic receptors by lower temperature and expression of chimeric subunits. J Biol Chem. 1999;274:27145–27152. doi: 10.1074/jbc.274.38.27145. [DOI] [PubMed] [Google Scholar]
- 98.Corey-Bloom J. Galantamine: a review of its use in Alzheimer’s disease and vascular dementia. Int J Clin Pract. 2003;57:219–223. [PubMed] [Google Scholar]
- 99.Corringer PJ, Bertrand S, Bohler S, Edelstein SJ, Changeux JP, Bertrand D. Critical elements determining diversity in agonist binding and desensitization of neuronal nicotinic acetylcholine receptors. J Neurosci. 1998;18:648–657. doi: 10.1523/JNEUROSCI.18-02-00648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corringer PJ, Bertrand S, Galzi JL, Devillers-Thiery A, Changeux JP, Bertrand D. Mutational analysis of the charge selectivity filter of the α7 nicotinic acetylcholine receptor. Neuron. 1999;22:831–843. doi: 10.1016/s0896-6273(00)80741-2. [DOI] [PubMed] [Google Scholar]
- 101.Corringer PJ, Galzi JL, Eisele JL, Bertrand S, Changeux JP, Bertrand D. Identification of a new component of the agonist binding site of the nicotinic α7 homooligomeric receptor. J Biol Chem. 1995;270:11749–11752. doi: 10.1074/jbc.270.20.11749. [DOI] [PubMed] [Google Scholar]
- 102.Corringer PJ, Sallette J, Changeux JP. Nicotine enhances intracellular nicotinic receptor maturation: a novel mechanism of neural plasticity? J Physiol Paris. 2006;99:162–171. doi: 10.1016/j.jphysparis.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 103.Couteaux R. The differentiation of synaptic areas. Proc R Soc Lond B Biol Sci. 1963;158:457–480. doi: 10.1098/rspb.1963.0058. [DOI] [PubMed] [Google Scholar]
- 104.Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 105.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 106.Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2003;58:P249–P255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- 107.Cymes GD, Grosman C, Auerbach A. Structure of the transition state of gating in the acetylcholine receptor channel pore: a phi-value analysis. Biochemistry. 2002;41:5548–5555. doi: 10.1021/bi011864f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dajas-Bailador FA, Heimala K, Wonnacott S. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine is transduced into cellular responses in neurons: Ca2+ signals and neurotransmitter release. Mol Pharmacol. 2003;64:1217–1226. doi: 10.1124/mol.64.5.1217. [DOI] [PubMed] [Google Scholar]
- 109.Dale HH. The action of certain esters of choline and their relation to muscarine. J Pharmacol Exp Ther. 1914;6:147–190. [Google Scholar]
- 110.Daly JW. Nicotinic agonists, antagonists, and modulators from natural sources. Cell Mol Neurobiol. 2005;25:513–552. doi: 10.1007/s10571-005-3968-4. [DOI] [PubMed] [Google Scholar]
- 111.Daly JW, Garraffo HM, Spande TF, Decker MW, Sullivan JP, Williams M. Alkaloids from frog skin: the discovery of epibatidine and the potential for developing novel non-opioid analgesics. Nat Prod Rep. 2000;17:131–135. doi: 10.1039/a900728h. [DOI] [PubMed] [Google Scholar]
- 112.Damaj MI. The involvement of spinal Ca2+/calmodulin-protein kinase II in nicotine-induced antinociception in mice. Eur J Pharmacol. 2000;404:103–110. doi: 10.1016/s0014-2999(00)00579-3. [DOI] [PubMed] [Google Scholar]
- 113.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 114.De Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 115.Decker ER, Dani JA. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990;10:3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Del Castillo J, Katz B. On the localization of acetylchoine receptors. J Physiol. 1955;128:157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deneris ES, Boulter J, Connolly J, Wada E, Wada K, Goldman D, Swanson LW, Patrick J, Heinemann S. Genes encoding neuronal nicotinic acetylcholine receptors. Clin Chem. 1989;35:731–737. [PubMed] [Google Scholar]
- 119.Derkach VA, Selyanko AA, Skok VI. Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurones. J Physiol. 1983;336:511–526. doi: 10.1113/jphysiol.1983.sp014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 121.Dineley-Miller K, Patrick J. Gene transcripts for the nicotinic acetylcholine receptor subunit, β4, are distributed in multiple areas of the rat central nervous system. Brain Res. 1992;16:339–344. doi: 10.1016/0169-328x(92)90244-6. [DOI] [PubMed] [Google Scholar]
- 122.Dineley KT. β-Amyloid peptide–nicotinic acetylcholine receptor interaction: the two faces of health and disease. Front Biosci. 2007;12:5030–5038. doi: 10.2741/2445. [DOI] [PubMed] [Google Scholar]
- 123.Dineley KT, Bell KA, Bui D, Sweatt JD. β-Amyloid peptide activates α7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 124.Ding YS, Fowler JS, Logan J, Wang GJ, Telang F, Garza V, Biegon A, Pareto D, Rooney W, Shea C, Alexoff D, Volkow ND, Vocci F. 6-[18F]Fluoro-A-85380, a new PET tracer for the nicotinic acetylcholine receptor: studies in the human brain and in vivo demonstration of specific binding in white matter. Synapse. 2004;53:184–189. doi: 10.1002/syn.20051. [DOI] [PubMed] [Google Scholar]
- 125.Dolly JO, Barnard EA. Nicotinic acetylcholine receptors: an overview. Biochem Pharmacol. 1984;33:841–858. doi: 10.1016/0006-2952(84)90437-4. [DOI] [PubMed] [Google Scholar]
- 126.Dougherty JJ, Wu J, Nichols RA. β-Amyloid regulation of presynaptpic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drisdel RC, Green WN. Neuronal α-bungarotoxin receptors are α7 subunit homomers. J Neurosci. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drisdel RC, Manzana E, Green WN. The role of palmitoylation in functional expression of nicotinic α7 receptors. J Neurosci. 2004;24:10502–10510. doi: 10.1523/JNEUROSCI.3315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Du F, Schmidt W, Okuno E, Kido R, Kohler C, Schwarcz R. Localization of kynurenine aminotransferase immunoreactivity in the rat hippocampus. J Comp Neurol. 1992;321:477–487. doi: 10.1002/cne.903210313. [DOI] [PubMed] [Google Scholar]
- 130.Dukat M, Glennon RA. Epibatidine: impact on nicotinic receptor research. Cell Mol Neurobiol. 2003;23:365–378. doi: 10.1023/a:1023692705700. [DOI] [PubMed] [Google Scholar]
- 131.Dunckley T, Wu J, Zhao L, Lukas RJ. Mutational analysis of roles for extracellular cysteine residues in the assembly and function of human α7-nicotinic acetylcholine receptors. Biochemistry. 2003;42:870–876. doi: 10.1021/bi020586x. [DOI] [PubMed] [Google Scholar]
- 132.Dutertre S, Lewis RJ. Toxin insights into nicotinic acetylcholine receptors. Biochem Pharmacol. 2006;72:661–670. doi: 10.1016/j.bcp.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 133.Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: β4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- 134.Dvorak-Carbone H, Schuman EM. Patterned activity in stratum lacunosum moleculare inhibits CA1 pyramidal neuron firing. J Neurophysiol. 1999;82:3213–3222. doi: 10.1152/jn.1999.82.6.3213. [DOI] [PubMed] [Google Scholar]
- 135.Dwoskin LP, Crooks PA. Competitive neuronal nicotinic receptor antagonists: a new direction for drug discovery. J Pharmacol Exp Ther. 2001;298:395–402. [PubMed] [Google Scholar]
- 136.Eastham HM, Lind RJ, Eastlake JL, Clarke BS, Towner P, Reynolds SE, Wolstenholme AJ, Wonnacott S. Characterization of a nicotinic acetylcholine receptor from the insect Manduca sexta. Eur J Neurosci. 1998;10:879–889. doi: 10.1046/j.1460-9568.1998.00095.x. [DOI] [PubMed] [Google Scholar]
- 137.Eccles JC, Katz B, Kuffler SW. Effect of eserine on neuromuscular transmission. J Neurophysiol. 1942;5:211–230. [Google Scholar]
- 138.Eccles JC, Katz B, Kuffler SW. Nature of the “end-plate potential” in curarized muscle. J Neurophysiol. 1941;4:362–387. [Google Scholar]
- 139.Edwards JA, Cline HT. Light-induced calcium influx into retinal axons is regulated by presynaptic nicotinic acetylcholine receptor activity in vivo. J Neurophysiol. 1999;81:895–907. doi: 10.1152/jn.1999.81.2.895. [DOI] [PubMed] [Google Scholar]
- 140.Eertmoed AL, Green WN. Nicotinic receptor assembly requires multiple regions throughout the gamma subunit. J Neurosci. 1999;19:6298–6308. doi: 10.1523/JNEUROSCI.19-15-06298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eertmoed AL, Vallejo YF, Green WN. Transient expression of heteromeric ion channels. Methods Enzymol. 1998;293:564–585. doi: 10.1016/s0076-6879(98)93034-8. [DOI] [PubMed] [Google Scholar]
- 142.Eglen RM. Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem. 2005;43:105–136. doi: 10.1016/S0079-6468(05)43004-0. [DOI] [PubMed] [Google Scholar]
- 143.Engel AG. Congenital myasthenic syndromes. Neurol Clin. 1994;12:401–437. [PubMed] [Google Scholar]
- 144.Fatt P. The electromotive action of acetylcholine at the motor end-plate. J Physiol. 1950;111:408–422. doi: 10.1113/jphysiol.1950.sp004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharmacol. 2004;66:658–666. doi: 10.1124/mol.104.000042. [DOI] [PubMed] [Google Scholar]
- 146.Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, Lester RA. Regulation of α4/32 nicotinic receptor desensitization by calcium and protein kinase C. Mol Pharmacol. 1999;55:432–443. [PubMed] [Google Scholar]
- 147.Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fertuck HC, Salpeter MM. Localization of acetylcholine receptor by 125I-labeled α-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci USA. 1974;71:1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Flores CM, DeCamp RM, Kilo S, Rogers SW, Hargreaves KM. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: demonstration of α3β4, a novel subtype in the mammalian nervous system. J Neurosci. 1996;16:7892–7901. doi: 10.1523/JNEUROSCI.16-24-07892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 152.Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, Deshpande P, McKinney S, Kwoh S, Munoz J, Labarca C, Collins AC, Marks MJ, Lester HA. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive α4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franceschini D, Paylor R, Broide R, Salas R, Bassetto L, Gotti C, De Biasi M. Absence of α7-containing neuronal nicotinic acetylcholine receptors does not prevent nicotine-induced seizures. Brain Res. 2002;98:29–40. doi: 10.1016/s0169-328x(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 154.Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Freedman R, Adams CE, Leonard S. The α7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 157.Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, Tsuang MT, Farone SV, Malaspina D, Svrakic DM, Sanders A, Gejman P. Linkage disequilibrium for schizophrenia at the chromosome 15q13–14 locus of the α7-nicotinic acetylcholine receptor subunit gene (CHRNA7) Am J Med Genet. 2001;105:20–22. [PubMed] [Google Scholar]
- 158.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 159.Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- 160.Fujii S, Sumikawa K. Acute and chronic nicotine exposure reverse age-related declines in the induction of long-term potentiation in the rat hippocampus. Brain Res. 2001;894:347–353. doi: 10.1016/s0006-8993(01)02057-1. [DOI] [PubMed] [Google Scholar]
- 161.Gahring LC, Days EL, Kaasch T, Gonzalez de Mendoza M, Owen L, Persiyanov K, Rogers SW. Pro-inflammatory cytokines modify neuronal nicotinic acetylcholine receptor assembly. J Neuroimmunol. 2005;166:88–101. doi: 10.1016/j.jneuroim.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 162.Gahring LC, Meyer EL, Rogers SW. Nicotine-induced neuroprotection against N-methyl-D-aspartic acid or β-amyloid peptide occur through independent mechanisms distinguished by pro-inflammatory cytokines. J Neurochem. 2003;87:1125–1136. doi: 10.1046/j.1471-4159.2003.02074.x. [DOI] [PubMed] [Google Scholar]
- 163.Gahring LC, Osborne-Hereford AV, Vasquez-Opazo GA, Rogers SW. Tumor necrosis factor α enhances nicotinic receptor up-regulation via a p38MAPK-dependent pathway. J Biol Chem. 2008;283:693–699. doi: 10.1074/jbc.M707330200. [DOI] [PubMed] [Google Scholar]
- 164.Gahring LC, Persiyanov K, Days EL, Rogers SW. Age-related loss of neuronal nicotinic receptor expression in the aging mouse hippocampus corresponds with cyclooxygenase-2 and PPAR gamma expression and is altered by long-term NS398 administration. J Neurobiol. 2005;62:453–468. doi: 10.1002/neu.20106. [DOI] [PubMed] [Google Scholar]
- 165.Gahring LC, Persiyanov K, Dunn D, Weiss R, Meyer EL, Rogers SW. Mouse strain-specific nicotinic acetylcholine receptor expression by inhibitory interneurons and astrocytes in the dorsal hippocampus. J Comp Neurol. 2004;468:334–346. doi: 10.1002/cne.10943. [DOI] [PubMed] [Google Scholar]
- 166.Gahring LC, Persiyanov K, Rogers SW. Mouse strain-specific changes in nicotinic receptor expression with age. Neurobiol Aging. 2005;26:973–980. doi: 10.1016/j.neurobiolaging.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 167.Gahring LC, Persiyanov K, Rogers SW. Neuronal and astrocyte expression of nicotinic receptor subunit β4 in the adult mouse brain. J Comp Neurol. 2004;468:322–333. doi: 10.1002/cne.10942. [DOI] [PubMed] [Google Scholar]
- 168.Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on non-neuronal cells. AAPS J. 2005;7:E885–E894. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gahring LC, Rogers SW. Nicotinic acetylcholine receptor expression in the hippocampus of 27 mouse strains reveals novel inhibitory circuitry. Hippocampus. In press doi: 10.1002/hipo.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Galzi JL, Devillers-Thiery A, Hussy N, Bertrand S, Changeux JP, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- 171.Gao F, Bren N, Burghardt TP, Hansen S, Henchman RH, Taylor P, McCammon JA, Sine SM. Agonist-mediated conformational changes in acetylcholine-binding protein revealed by simulation and intrinsic tryptophan fluorescence. J Biol Chem. 2005;280:8443–8451. doi: 10.1074/jbc.M412389200. [DOI] [PubMed] [Google Scholar]
- 172.Garcia-Colunga J, Miledi R. Effects of serotonergic agents on neuronal nicotinic acetylcholine receptors. Proc Natl Acad Sci USA. 1995;92:2919–2923. doi: 10.1073/pnas.92.7.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gasparini L, Ongini E, Wenk G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J Neurochem. 2004;91:521–536. doi: 10.1111/j.1471-4159.2004.02743.x. [DOI] [PubMed] [Google Scholar]
- 174.Gattu M, Pauly JR, Boss KL, Summers JB, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: role of central nicotinic receptors. Brain Res. 1997;771:89–103. doi: 10.1016/s0006-8993(97)00793-2. [DOI] [PubMed] [Google Scholar]
- 175.Gehle VM, Walcott EC, Nishizaki T, Sumikawa K. N-glycosylation at the conserved sites ensures the expression of properly folded functional ACh receptors. Brain Res. 1997;45:219–229. doi: 10.1016/s0169-328x(96)00256-2. [DOI] [PubMed] [Google Scholar]
- 176.Gelman MS, Prives JM. Arrest of subunit folding and assembly of nicotinic acetylcholine receptors in cultured muscle cells by dithiothreitol. J Biol Chem. 1996;271:10709–10714. doi: 10.1074/jbc.271.18.10709. [DOI] [PubMed] [Google Scholar]
- 177.Giacobini E. Nicotine acetylcholine receptors in human cortex: aging and Alzheimer’s disease. In: Lippiello PM, Collins AC, Gray AC, Robinson JH, editors. Biology of Nicotine. New York: Raven; 1992. pp. 183–215. [Google Scholar]
- 178.Giovannini MG, Scali C, Prosperi C, Bellucci A, Pepeu G, Casamenti F. Experimental brain inflammation and neurodegeneration as model of Alzheimer’s disease: protective effects of selective COX-2 inhibitors. Int J Immunopathol Pharmacol. 2003;16:31–40. [PubMed] [Google Scholar]
- 179.Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, Role L. Heteromeric complexes of α5 and/or α7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann NY Acad Sci. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- 180.Girod R, Role LW. Long-lasting enhancement of glutamatergic synaptic transmission by acetylcholine contrasts with response adaptation after exposure to low-level nicotine. J Neurosci. 2001;21:5182–5190. doi: 10.1523/JNEUROSCI.21-14-05182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking α3 β4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- 182.Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27:226–233. doi: 10.1016/j.tips.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 183.Goldman D, Brenner HR, Heinemann S. Acetylcholine receptor α-, β-, γ-, δ-subunit mRNA levels are regulated by muscle activity. Neuron. 1988;1:329–333. doi: 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- 184.Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 185.Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor α4 or β2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb + efflux in cortex and thalamus and alters relative expression of α4 and β2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- 186.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 187.Gramsbergen JBP, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R. Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem. 1997;69:290–298. doi: 10.1046/j.1471-4159.1997.69010290.x. [DOI] [PubMed] [Google Scholar]
- 188.Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol. 2006;15:265–282. doi: 10.1111/j.0906-6705.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 189.Grando SA, Horton RM, Pereira EFR, Diethelm-Okita BM, George PM, Albuquerque EX, Conti-Fine BM. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol. 1995;105:774–781. doi: 10.1111/1523-1747.ep12325606. [DOI] [PubMed] [Google Scholar]
- 190.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 191.Green WN, Claudio T. Acetylcholine receptor assembly: subunit folding and oligomerization occur sequentially. Cell. 1993;74:57–69. doi: 10.1016/0092-8674(93)90294-z. [DOI] [PubMed] [Google Scholar]
- 192.Green WN, Ross AF, Claudio T. Acetylcholine receptor assembly is stimulated by phosphorylation of its gamma subunit. Neuron. 1991;191:659–666. doi: 10.1016/0896-6273(91)90378-d. [DOI] [PubMed] [Google Scholar]
- 193.Green WN, Wanamaker CP. The role of the cystine loop in acetylcholine receptor assembly. J Biol Chem. 1997;272:20945–20953. doi: 10.1074/jbc.272.33.20945. [DOI] [PubMed] [Google Scholar]
- 194.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grutter T, de Carvalho LP, Dufresne V, Taly A, Changeux JP. Identification of two critical residues within the Cys-loop sequence that determine fast-gating kinetics in a pentameric ligand-gated ion channel. J Mol Neurosci. 2006;30:63–64. doi: 10.1385/JMN:30:1:63. [DOI] [PubMed] [Google Scholar]
- 196.Grutter T, de Carvalho LP, Dufresne V, Taly A, Edelstein SJ, Changeux JP. Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc Natl Acad Sci USA. 2005;102:18207–18212. doi: 10.1073/pnas.0509024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. J Neurochem. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- 198.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med. 2004;170:974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 199.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 200.Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J Neurosci Res. 1997;50:457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 201.Guo X, Wecker L. Identification of three cAMP-dependent protein kinase (PKA) phosphorylation sites within the major intracellular domain of neuronal nicotinic receptor α4 subunits. J Neurochem. 2002;82:439–447. doi: 10.1046/j.1471-4159.2002.01027.x. [DOI] [PubMed] [Google Scholar]
- 202.Hafner H, Maurer K, Loffler W, Riecher-Rossler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry. 1993;162:80–86. doi: 10.1192/bjp.162.1.80. [DOI] [PubMed] [Google Scholar]
- 203.Hajek P, Stead LF, West R, Jarvis M. Relapse Prevention Interventions for Smoking Cessation. 2005 doi: 10.1002/14651858.CD003999.pub2. Cochrane Database Syst Rev CD003999. [DOI] [PubMed] [Google Scholar]
- 204.Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Bourne Y, Taylor P. Structural characterization of agonist and antagonist-bound acetylcholine-binding protein from Aplysia californica. J Mol Neurosci. 2006;30:101–102. doi: 10.1385/JMN:30:1:101. [DOI] [PubMed] [Google Scholar]
- 205.Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hansen SB, Talley TT, Radic Z, Taylor P. Structural and ligand recognition characteristics of an acetylcholine-binding protein from Aplysia californica. J Biol Chem. 2004;279:24197–24202. doi: 10.1074/jbc.M402452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hansen SB, Taylor P. Galanthamine and non-competitive inhibitor binding to ACh-binding protein: evidence for a binding site on non-α-subunit interfaces of heteromeric neuronal nicotinic receptors. J Mol Biol. 2007;369:895–901. doi: 10.1016/j.jmb.2007.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hill DG, Baenziger JE. The net orientation of nicotinic receptor transmembrane α-helices in the resting and desensitized states. Biophys J. 2006;91:705–714. doi: 10.1529/biophysj.106.082693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hiremagalur B, Sabban EL. Nicotine elicits changes in expression of adrenal catecholamine biosynthetic enzymes, neuropeptide Y and immediate early genes by injection but not continuous administration. Brain Res. 1995;32:109–115. doi: 10.1016/0169-328x(95)00068-4. [DOI] [PubMed] [Google Scholar]
- 212.Ho L, Pieroni C, Winger D, Purohit DP, Aisen PS, Pasinetti GM. Regional distribution of cyclooxygenase-2 in the hippocampal formation in Alzheimer’s disease. J Neurosci Res. 1999;57:295–303. doi: 10.1002/(SICI)1097-4547(19990801)57:3<295::AID-JNR1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 213.Hodgkins PS, Schwarcz R. Interference with cellular energy metabolism reduces kynurenic acid formation in rat brain slices: reversal by lactate and pyruvate. Eur J Neurosci. 1998;10:1986–1994. doi: 10.1046/j.1460-9568.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 214.Hogg RC, Bertrand D. Regulating the regulators: the role of nicotinic acetylcholine receptors in human epilepsy. Drug News Perspect. 2003;16:261–266. doi: 10.1358/dnp.2003.16.5.829313. [DOI] [PubMed] [Google Scholar]
- 215.Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 216.Hoozemans JJ, Veerhuis R, Rozemuller AJ, Eikelenboom P. Non-steroidal anti-inflammatory drugs and cyclooxygenase in Alzheimer’s disease. Curr Drug Targets. 2003;4:461–468. doi: 10.2174/1389450033490902. [DOI] [PubMed] [Google Scholar]
- 217.Hu M, Whiting Theobald NL, Gardner PD. Nerve growth factor increases the transcriptional activity of the rat neuronal nicotinic acetylcholine receptor beta 4 subunit promoter in transfected PC12 cells. J Neurochem. 1994;62:392–395. doi: 10.1046/j.1471-4159.1994.62010392.x. [DOI] [PubMed] [Google Scholar]
- 218.Hu WP, Ma SY, Wu JL, Li ZW. 5-Hydroxytryptamine directly inhibits neuronal nicotinic acetylcholine receptors in rat trigeminal ganglion neurons. Eur J Pharmacol. 2007;574:120–126. doi: 10.1016/j.ejphar.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 219.Huebsch KA, Maimone MM. Rapsyn-mediated clustering of acetylcholine receptor subunits requires the major cytoplasmic loop of the receptor subunits. J Neurobiol. 2003;54:486–501. doi: 10.1002/neu.10177. [DOI] [PubMed] [Google Scholar]
- 220.Isaacs JT. The aging ACI/Seg versus Copenhagen male rat as a model system for the study of prostatic carcinogenesis. Cancer Res. 1984;44:5785–5796. [PubMed] [Google Scholar]
- 221.Jacob MH, Lindstrom JM, Berg DK. Surface and intracellular distribution of a putative neuronal nicotinic acetylcholine receptor. J Cell Biol. 1986;103:205–214. doi: 10.1083/jcb.103.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. The chaperone protein 14–3–3eta interacts with the nicotinic acetylcholine receptor α4 subunit. Evidence for a dynamic role in subunit stabilization. J Biol Chem. 2001;276:28281–28290. doi: 10.1074/jbc.M011549200. [DOI] [PubMed] [Google Scholar]
- 223.Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- 224.Jones IW, Wonnacott S. Precise localization of α7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kadunce DP, Burr R, Gress R, Kanner R, Lyon JL, Zone JJ. Cigarette smoking: risk factor for premature facial wrinkling. Ann Intern Med. 1991;114:840–844. doi: 10.7326/0003-4819-114-10-840. [DOI] [PubMed] [Google Scholar]
- 227.Kang TH, Ryu YH, Kim IB, Oh GT, Chun MH. Comparative study of cholinergic cells in retinas of various mouse strains. Cell Tissue Res. 2004;317:109–115. doi: 10.1007/s00441-004-0907-5. [DOI] [PubMed] [Google Scholar]
- 228.Kao PN, Dwork AJ, Kaldany RR, Silver ML, Wideman J, Stein S, Karlin A. Identification of the α subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J Biol Chem. 1984;259:11662–11665. [PubMed] [Google Scholar]
- 229.Karlin A, Cox RN, Dipaola M, Holtzman E, Kao PN, Lobel P, Wang L, Yodh N. Functional domains of the nicotinic acetylcholine receptor. Ann NY Acad Sci. 1986;463:53–69. doi: 10.1111/j.1749-6632.1986.tb21503.x. [DOI] [PubMed] [Google Scholar]
- 230.Katz B. The Croonian Lecture. The transmission of impulses from nerve to muscle, the subcellular unit of synaptic action. Proc R Soc Lond B. 1961;155:455–477. [Google Scholar]
- 231.Katz B. The Release of Neural Transmitter Substances. Springfield, IL: Thomas; 1969. [Google Scholar]
- 232.Katz B, Thesleff S. A study of the “desensitization” produced by acetylcholine at the motor end-plate. J Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10:1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- 234.Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 235.Kedmi M, Beaudet AL, Orr-Urtreger A. Mice lacking neuronal nicotinic acetylcholine receptor β4-subunit and mice lacking both α5-and β4-subunits are highly resistant to nicotine-induced seizures. Physiol Gen. 2004;17:221–229. doi: 10.1152/physiolgenomics.00202.2003. [DOI] [PubMed] [Google Scholar]
- 236.Kellar KJ, Whitehouse PJ, Martino-Barrows AM, Marcus K, Price DL. Muscarinic and nicotinic cholinergic binding sites in Alzheimer’s disease cerebral cortex. Brain Res. 1987;436:62–68. doi: 10.1016/0006-8993(87)91556-3. [DOI] [PubMed] [Google Scholar]
- 237.Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, Pasinetti GM. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am J Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kelso ML, Wehner JM, Collins AC, Scheff SW, Pauly JR. The pathophysiology of traumatic brain injury in α7 nicotinic cholinergic receptor knockout mice. Brain Res. 2006;1083:204–210. doi: 10.1016/j.brainres.2006.01.127. [DOI] [PubMed] [Google Scholar]
- 239.Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–9031. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor α7 and β2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kihara T, Sawada H, Nakamizo T, Kanki R, Yamashita H, Maelicke A, Shimohama S. Galantamine modulates nicotinic receptor and blocks Abeta-enhanced glutamate toxicity. Biochem Biophys Res Commun. 2004;325:976–982. doi: 10.1016/j.bbrc.2004.10.132. [DOI] [PubMed] [Google Scholar]
- 242.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A. α7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A β-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 243.Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T, Maeda T, Akaike A. Stimulation of α4β2 nicotinic acetylcholine receptors inhibits β-amyloid toxicity. Brain Res. 1998;792:331–334. doi: 10.1016/s0006-8993(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 244.Kiss C, Ceresoli-Borroni G, Guidetti P, Zielke CL, Zielke HR, Schwarcz R. Kynurenate production by cultured human astrocytes. J Neural Transm. 2003;110:1–14. doi: 10.1007/s00702-002-0770-z. [DOI] [PubMed] [Google Scholar]
- 245.Kistler J, Stroud RM. Crystalline arrays of membrane-bound acetylcholine receptor. Proc Natl Acad Sci USA. 1981;78:3678–3682. doi: 10.1073/pnas.78.6.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Klein J, Weichel O, Ruhr J, Dvorak C, Loffelholz K. A homeostatic mechanism counteracting K+-evoked choline release in adult brain. J Neurochem. 2002;80:843–849. doi: 10.1046/j.0022-3042.2001.00754.x. [DOI] [PubMed] [Google Scholar]
- 247.Kracun S, Harkness PC, Gibb AJ, Millar NS. Influence of the M3–M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br J Pharmacol. In press doi: 10.1038/sj.bjp.0707676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- 249.Krishtal OA, Pidoplichko VI, Shakhovlov YA. Conductance of the calcium channel in the membrane of snail neurones. J Physiol. 1981;310:423–434. doi: 10.1113/jphysiol.1981.sp013558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kues WA, Sakmann B, Witzemann V. Differential expression patterns of five acetylcholine receptor subunit genes in rat muscle during development. Eur J Neurosci. 1995;7:1376–1385. doi: 10.1111/j.1460-9568.1995.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 251.Kühne W. On the origin and the causation of vital movement. Proc R Soc Lond B. 1888;4 [Google Scholar]
- 252.Kukhtina V, Kottwitz D, Strauss H, Heise B, Chebotareva N, Tsetlin V, Hucho F. Intracellular domain of nicotinic acetylcholine receptor: the importance of being unfolded. J Neurochem. 2006;97 Suppl 1:63–67. doi: 10.1111/j.1471-4159.2005.03468.x. [DOI] [PubMed] [Google Scholar]
- 253.Kuo YP, Xu L, Eaton JB, Zhao L, Wu J, Lukas RJ. Roles for nicotinic acetylcholine receptor subunit large cytoplasmic loop sequences in receptor expression and function. J Pharmacol Exp Ther. 2005;314:455–466. doi: 10.1124/jpet.105.084954. [DOI] [PubMed] [Google Scholar]
- 254.Kuryatov A, Gerzanich V, Nelson M, Olale F, Lindstrom J. Mutation causing autosomal dominant nocturnal frontal lobe epilepsy alters Ca2+ permeability, conductance, gating of human α4β2 nicotinic acetylcholine receptors. J Neurosci. 1997;17:9035–9047. doi: 10.1523/JNEUROSCI.17-23-09035.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- 256.Lacaille JC, Schwartzkroin PA. Intracellular responses of rat hippocampal granule cells in vitro to discrete applications of norepinephrine. Neurosci Lett. 1988;89:176–181. doi: 10.1016/0304-3940(88)90377-1. [DOI] [PubMed] [Google Scholar]
- 257.Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment decreases striatal α6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol. 2005;67:1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- 258.Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- 259.Langley JN. On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curare. J Physiol. 1905;33:374–413. doi: 10.1113/jphysiol.1905.sp001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lansdell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, Gibb AJ, Millar NS. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharmacol. 2005;68:1431–1438. doi: 10.1124/mol.105.017459. [DOI] [PubMed] [Google Scholar]
- 261.Law RJ, Henchman RH, McCammon JA. A gating mechanism proposed from a simulation of a human α7 nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 2005;102:6813–6818. doi: 10.1073/pnas.0407739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Le Novere N, Changeux JP. The ligand gated ion channel database. Nucleic Acids Res. 1999;27:340–342. doi: 10.1093/nar/27.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Leanza G, Nilsson OG, Nikkhah G, Wiley RG, Bjorklund A. Effects of neonatal lesions of the basal forebrain cholinergic system by 192 immunoglobulin G-saporin: biochemical, behavioural and morphological characterization. Neuroscience. 1996;74:119–141. doi: 10.1016/0306-4522(96)00095-4. [DOI] [PubMed] [Google Scholar]
- 264.Lee CY. Recent advances in chemistry and pharmacology of snake toxins. Adv Cytopharmacol. 1979;3:1–16. [PubMed] [Google Scholar]
- 265.Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- 266.Lemay S, Chouinard S, Blanchet P, Masson H, Soland V, Beuter A, Bedard MA. Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:31–39. doi: 10.1016/S0278-5846(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 267.Lena C, Changeux JP, Mulle C. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Leppa S, Eriksson M, Saffrich R, Ansorge W, Bohmann D. Complex functions of AP-1 transcription factors in differentiation and survival of PC12 cells. Mol Cell Biol. 2001;21:4369–4378. doi: 10.1128/MCB.21.13.4369-4378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lester HA, Fonck C, Tapper AR, McKinney S, Damaj MI, Balogh S, Owens J, Wehner JM, Collins AC, Labarca C. Hypersensitive knockin mouse strains identify receptors and pathways for nicotine action. Curr Opin Drug Discov Dev. 2003;6:633–639. [PubMed] [Google Scholar]
- 270.Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- 271.Levey MS, Brumwell CL, Dryer SE, Jacob MH. Innervation and target tissue interactions differentially regulate acetylcholine receptor subunit mRNA levels in developing neurons in situ. Neuron. 1995;14:153–162. doi: 10.1016/0896-6273(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 272.Levey MS, Jacob MH. Changes in the regulatory effects of cell cell interactions on neuronal AChR subunit transcript levels after synapse formation. J Neurosci. 1996;16:6878–6885. doi: 10.1523/JNEUROSCI.16-21-06878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 274.Li Q, Peterson KR, Fang X, Stamatoyannopoulos G. Locus control regions. Blood. 2002;100:3077–3086. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- 276.Lindstrom J, Merlie J, Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979;18:4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- 277.Lindstrom JM. Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann NY Acad Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [DOI] [PubMed] [Google Scholar]
- 278.Liu Q, Kawai H, Berg DK. β-Amyloid peptide blocks the response of α7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci USA. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liu RH, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- 280.Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology. 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- 282.Liu Z, Tearle AW, Nai Q, Berg DK. Rapid activity-driven SNARE-dependent trafficking of nicotinic receptors on somatic spines. J Neurosci. 2005;25:1159–1168. doi: 10.1523/JNEUROSCI.3953-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Loewi O. Uber humorale Ubertragbarkeit der Herznervenwirkung. Pflügers. 1921;189:239–242. [Google Scholar]
- 284.Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 285.Lopes C, Pereira EFR, Wu HQ, Purushottamachar P, Njar V, Schwarcz R, Albuquerque EX. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at α7* nicotinic receptors. J Pharmacol Exp Ther. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- 286.Loring RH, Schulz DW, Zigmond RE. Characterization of neuronal nicotinic receptors using neuronal bungarotoxin. Prog Brain Res. 1989;79:109–116. doi: 10.1016/s0079-6123(08)62470-x. [DOI] [PubMed] [Google Scholar]
- 287.Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- 288.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 289.Mackenzie IR, Munoz DG. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology. 1998;50:986–990. doi: 10.1212/wnl.50.4.986. [DOI] [PubMed] [Google Scholar]
- 290.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–439. [PubMed] [Google Scholar]
- 291.Maelicke A. Nicotinic receptors of the vertebrate CNS: introductory remarks. Prog Brain Res. 1996;109:107–110. doi: 10.1016/s0079-6123(08)62092-0. [DOI] [PubMed] [Google Scholar]
- 292.Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature “silent” connections in the developing hippocampus. Proc Natl Acad Sci USA. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mansvelder HD, Fagen ZM, Chang B, Mitchum R, McGehee DS. Bupropion inhibits the cellular effects of nicotine in the ventral tegmental area. Biochem Pharmacol. 2007;74:1283–1291. doi: 10.1016/j.bcp.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 296.Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- 297.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 298.Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- 299.Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 300.Marini C, Guerrini R. The role of the nicotinic acetylcholine receptors in sleep-related epilepsy. Biochem Pharmacol. 2007;74:1308–1314. doi: 10.1016/j.bcp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 301.Marks MJ, Stitzel JA, Collins AC. Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. J Pharmacol Exp Ther. 1986;239:358–364. [PubMed] [Google Scholar]
- 302.Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- 303.Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- 304.Marsh D, Barrantes FJ. Immobilized lipid in acetylcholine receptor-rich membranes from Torpedo marmorata. Proc Natl Acad Sci USA. 1978;75:4329–4333. doi: 10.1073/pnas.75.9.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Marsh D, Watts A, Barrantes FJ. Phospholipid chain immobilization and steroid rotational immobilization in acetylcholine receptor-rich membranes from Torpedo marmorata. Biochim Biophys Acta. 1981;645:97–101. doi: 10.1016/0005-2736(81)90516-2. [DOI] [PubMed] [Google Scholar]
- 306.Martin-Ruiz CM, Court JA, Molnar E, Lee M, Gotti C, Mamalaki A, Tsouloufis T, Tzartos S, Ballard C, Perry RH, Perry EK. α4 but not α3 and α7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in Alzheimer’s disease. J Neurochem. 1999;73:1635–1640. doi: 10.1046/j.1471-4159.1999.0731635.x. [DOI] [PubMed] [Google Scholar]
- 307.Martin AR. A further study of the statistical composition of the end-plate potential. J Physiol. 1955;130:114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Marutle A, Warpman U, Bogdanovic N, Nordberg A. Regional distribution of subtypes of nicotinic receptors in human brain and effect of aging studied by (+/−)-[3H]epibatidine. Brain Res. 1998;801:143–149. doi: 10.1016/s0006-8993(98)00558-7. [DOI] [PubMed] [Google Scholar]
- 309.Maus AD, Pereira EFR, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol. 1998;54:779–788. doi: 10.1124/mol.54.5.779. [DOI] [PubMed] [Google Scholar]
- 310.Mazeh D, Zemishlani H, Barak Y, Mirecki I, Paleacu D. Donepezil for negative signs in elderly patients with schizophrenia: an add-on, double-blind, crossover, placebo-controlled study. Int Psychogeriatr. 2006;18:429–436. doi: 10.1017/S1041610205003017. [DOI] [PubMed] [Google Scholar]
- 311.McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the β2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology. 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- 312.McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 313.McGrath J, McDonald J, Macdonald J. Transdermal Nicotine for Induction of Remission in Ulcerative Colitis. 2004 doi: 10.1002/14651858.CD004722.pub2. Cochrane Database Syst Rev CD004722. [DOI] [PubMed] [Google Scholar]
- 314.McIntosh JM, Santos AD, Olivera BM. Conus peptides targeted to specific nicotinic acetylcholine receptor subtypes. Annu Rev Biochem. 1999;68:59–88. doi: 10.1146/annurev.biochem.68.1.59. [DOI] [PubMed] [Google Scholar]
- 315.McMahon LL, Yoon KW, Chiappinelli VA. Nicotinic receptor activation facilitates GABAergic neurotransmission in the avian lateral spiriform nucleus. Neuroscience. 1994;59:689–698. doi: 10.1016/0306-4522(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 316.McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Melnikova IN, Gardner PD. The signal transduction pathway underlying ion channel gene regulation by SP1-C-Jun interactions. J Biol Chem. 2001;276:19040–19045. doi: 10.1074/jbc.M010735200. [DOI] [PubMed] [Google Scholar]
- 318.Messing RO, Stevens AM, Kiyasu E, Sneade AB. Nicotinic and muscarinic agonists stimulate rapid protein kinase C translocation in PC12 cells. J Neurosci. 1989;9:507–512. doi: 10.1523/JNEUROSCI.09-02-00507.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Mike A, Castro NG, Albuquerque EX. Choline and acetylcholine have similar kinetic properties of activation and desensitization on the α7-nicotinic receptors in rat hippocampal neurons. Brain Res. 2000;882:155–168. doi: 10.1016/s0006-8993(00)02863-8. [DOI] [PubMed] [Google Scholar]
- 320.Mike A, Pereira EFR, Albuquerque EX. Ca2+-sensitive inhibition by Pb2+ of α7-containing nicotinic acetylcholine receptors in hippocampal neurons. Brain Res. 2000;873:112–123. doi: 10.1016/s0006-8993(00)02533-6. [DOI] [PubMed] [Google Scholar]
- 321.Miledi R, Molenaar PC, Polak RL. The effect of lanthanum ions on acetylcholine in frog muscle. J Physiol. 1980;309:199–214. doi: 10.1113/jphysiol.1980.sp013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Mirjany M, Ho L, Pasinetti GM. Role of cyclooxygenase-2 in neuronal cell cycle activity and glutamate-mediated excitotoxicity. J Pharmacol Exp Ther. 2002;301:494–500. doi: 10.1124/jpet.301.2.494. [DOI] [PubMed] [Google Scholar]
- 323.Misery L. Nicotine effects on skin: are they positive or negative? Exp Dermatol. 2004;13:665–670. doi: 10.1111/j.0906-6705.2004.00274.x. [DOI] [PubMed] [Google Scholar]
- 324.Missias AC, Chu GC, Klocke BJ, Sanes JR, Merlie JP. Maturation of the acetylcholine receptor in skeletal muscle: regulation of the AChR γ-to-ε switch. Dev Biol. 1996;179:223–238. doi: 10.1006/dbio.1996.0253. [DOI] [PubMed] [Google Scholar]
- 325.Mitra A, Cymes GD, Auerbach A. Dynamics of the acetylcholine receptor pore at the gating transition state. Proc Natl Acad Sci USA. 2005;102:15069–15074. doi: 10.1073/pnas.0505090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- 327.Moliver M. Computerization helps reduce human error in patient dosing. Contemp Longterm Care. 1987;10:56–58. [PubMed] [Google Scholar]
- 328.Morley BJ, Happe HK. Cholinergic receptors: dual roles in transduction and plasticity. Hear Res. 2000;147:104–112. doi: 10.1016/s0378-5955(00)00124-6. [DOI] [PubMed] [Google Scholar]
- 329.Moroni F, Alesiani M, Facci L, Fadda E, Skaper SD, Galli A, Lombardi G, Mori F, Ciuffi M, Natalini B. Thiokynurenates prevent excitotoxic neuronal death in vitro and in vivo by acting as glycine antagonists and as inhibitors of lipid peroxidation. Eur J Pharmacol. 1992;218:145–151. doi: 10.1016/0014-2999(92)90158-z. [DOI] [PubMed] [Google Scholar]
- 330.Moroni F, Russi P, Carla V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci Lett. 1988;94:145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- 331.Moroni M, Vijayan R, Carbone A, Zwart R, Biggin PC, Bermudez I. Non-agonist-binding subunit interfaces confer distinct functional signatures to the alternate stoichiometries of the α4β2 nicotinic receptor: an α4-α4 interface is required for Zn2+ potentiation. J Neurosci. 2008;28:6884–6894. doi: 10.1523/JNEUROSCI.1228-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mugnaini M, Garzotti M, Sartori I, Pilla M, Repeto P, Heidbreder CA, Tessari M. Selective down-regulation of [125I]Y0-α-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137:565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 333.Mulle C, Changeux JP. A novel type of nicotinic receptor in the rat central nervous system characterized by patch-clamp techniques. J Neurosci. 1990;10:169–175. doi: 10.1523/JNEUROSCI.10-01-00169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mulle C, Choquet D, Korn H, Changeux JP. Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron. 1992;8:135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- 335.Mulle C, Vidal C, Benoit P, Changeux JP. Existence of different subtypes of nicotinic acetylcholine receptors in the rat habenulo-interpeduncular system. J Neurosci. 1991;11:2588–2597. doi: 10.1523/JNEUROSCI.11-08-02588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Nagele RG, D’Andrea MR, Anderson WJ, Wang HY. Intracellular accumulation of β-amyloid(1–42) in neurons is facilitated by the α 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience. 2002;110:199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 337.Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–498. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- 338.Nastuk WL. Membrane potential changes at a single muscle endplate produced by transitory application of acetylcholine with an electrically controlled microjet. Federation Proc. 1953;12:102. [Google Scholar]
- 339.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- 340.Newhouse PA, Potter A, Levin ED. Nicotinic system involvement in Alzheimer’s and Parkinson’s diseases. Implications for therapeutics. Drugs Aging. 1997;11:206–228. doi: 10.2165/00002512-199711030-00005. [DOI] [PubMed] [Google Scholar]
- 341.Nicke A, Thurau H, Sadtler S, Rettinger J, Schmalzing G. Assembly of nicotinic α7 subunits in Xenopus oocytes is partially blocked at the tetramer level. FEBS Lett. 2004;575:52–58. doi: 10.1016/j.febslet.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 342.Noda M, Takahashi H, Tanabe T, Toyosato M, Furutani Y, Hirose T, Asai M, Inayama S, Miyata T, Numa S. Primary structure of α-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982;299:793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- 343.Noda M, Takahashi H, Tanabe T, Toyosato M, Kikyotani S, Furutani Y, Hirose T, Takashima H, Inayama S, Miyata T, Numa S. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983;302:528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- 344.Nomikos GG, Schilstrom B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of α7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav Brain Res. 2000;113:97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- 345.Nordberg A. Pharmacological treatment of cognitive dysfunction in dementia disorders. Acta Neurol Scand Suppl. 1996;168:87–92. doi: 10.1111/j.1600-0404.1996.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 346.Nordberg A. Toward an early diagnosis and treatment of Alzheimer’s disease. Int Psychogeriatr. 2003;15:223–237. doi: 10.1017/s1041610203009499. [DOI] [PubMed] [Google Scholar]
- 347.Noronha-Blob L, Gover R, Baumgold J. Calcium influx mediated by nicotinic receptors and voltage sensitive calcium channels in SK-N-SH human neuroblastoma cells. Biochem Biophys Res Commun. 1989;162:1230–1235. doi: 10.1016/0006-291x(89)90805-x. [DOI] [PubMed] [Google Scholar]
- 348.Numa S. Molecular basis for the function of ionic channels. Biochem Soc Symp. 1986;52:119–143. [PubMed] [Google Scholar]
- 349.Nuutinen S, Barik J, Jones IW, Wonnacott S. Differential effects of acute and chronic nicotine on Elk-1 in rat hippocampus. Neuroreport. 2007;18:121–126. doi: 10.1097/WNR.0b013e328010a1ff. [DOI] [PubMed] [Google Scholar]
- 350.Okonjo KO, Kuhlmann J, Maelicke A. A second pathway of activation of the Torpedo acetylcholine receptor channel. Eur J Biochem. 1991;200:671–677. doi: 10.1111/j.1432-1033.1991.tb16231.x. [DOI] [PubMed] [Google Scholar]
- 351.Olivera BO. Conus venom peptides: reflections from the biology of clades and species. Annu Rev Ecol Syst. 2002;33:25–47. [Google Scholar]
- 352.Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungaro-toxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Orr-Urtreger A, Kedmi M, Rosner S, Karmeli F, Rachmilewitz D. Increased severity of experimental colitis in α5 nicotinic acetylcholine receptor subunit-deficient mice. Neuroreport. 2005;16:1123–1127. doi: 10.1097/00001756-200507130-00018. [DOI] [PubMed] [Google Scholar]
- 354.Osborne-Hereford AV, Rogers SW, Gahring LC. Neuronal nicotinic α7 receptors modulate inflammatory cytokine production in the skin following ultraviolet radiation. J Neuroimmunol. 2008;193:130–139. doi: 10.1016/j.jneuroim.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Oshikawa J, Toya Y, Fujita T, Egawa M, Kawabe J, Umemura S, Ishikawa Y. Nicotinic acetylcholine receptor α7 regulates cAMP signal within lipid rafts. Am J Physiol Cell Physiol. 2003;285:C567–C574. doi: 10.1152/ajpcell.00422.2002. [DOI] [PubMed] [Google Scholar]
- 356.Oz M, Ravindran A, Diaz-Ruiz O, Zhang L, Morales M. The endogenous cannabinoid anandamide inhibits α7 nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. J Pharmacol Exp Ther. 2003;306:1003–1010. doi: 10.1124/jpet.103.049981. [DOI] [PubMed] [Google Scholar]
- 357.Oz M, Tchugunova YB, Dunn SM. Endogenous cannabinoid anandamide directly inhibits voltage-dependent Ca2+ fluxes in rabbit T-tubule membranes. Eur J Pharmacol. 2000;404:13–20. doi: 10.1016/s0014-2999(00)00396-4. [DOI] [PubMed] [Google Scholar]
- 358.Oz M, Zhang L, Morales M. Endogenous cannabinoid, anandamide, acts as a noncompetitive inhibitor on 5-HT3 receptor-mediated responses in Xenopus oocytes. Synapse. 2002;46:150–156. doi: 10.1002/syn.10121. [DOI] [PubMed] [Google Scholar]
- 359.Pakkanen JS, Stenfors J, Jokitalo E, Tuominen RK. Effect of chronic nicotine treatment on localization of neuronal nicotinic acetylcholine receptors at cellular level. Synapse. 2006;59:383–393. doi: 10.1002/syn.20249. [DOI] [PubMed] [Google Scholar]
- 360.Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. J Biol Chem. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- 361.Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α 7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- 362.Park HJ, Lee PH, Ah NYW, Choi YJ, Lee G, Lee DY, Chung ES, Jin BK. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26:79–89. doi: 10.1111/j.1460-9568.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- 363.Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM, Sharp BM. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the α6 subunit. Mol Pharmacol. 2004;65:611–622. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- 364.Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- 365.Paulson HL, Ross AF, Green WN, Claudio T. Analysis of early events in acetylcholine receptor assembly. J Cell Biol. 1991;113:1371–1384. doi: 10.1083/jcb.113.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem Soc Trans. 2006;34:1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 367.Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994;46:523–530. [PubMed] [Google Scholar]
- 368.Pereira EF, Alkondon M, McIntosh JM, Albuquerque EX. α-Conotoxin-ImI: a competitive antagonist at alpha-bungarotoxin-sensitive neuronal nicotinic receptors in hippocampal neurons. J Pharmacol Exp Ther. 1996;278:1472–1483. [PubMed] [Google Scholar]
- 369.Pereira EF, Reinhardt-Maelicke S, Schrattenholz A, Maelicke A, Albuquerque EX. Identification and functional characterization of a new agonist site on nicotinic acetylcholine receptors of cultured hippocampal neurons. J Pharmacol Exp Ther. 1993;265:1474–1491. [PubMed] [Google Scholar]
- 370.Pereira EFR, Alkondon M, Reinhardt S, Maelicke A, Peng X, Lindstrom J, Whiting P, Albuquerque EX. Physostigmine and galanthamine: probes for a novel binding site on the α4β2 subtype of neuronal nicotinic acetylcholine receptors stably expressed in fibroblast cells. J Pharmacol Exp Ther. 1994;270:768–778. [PubMed] [Google Scholar]
- 371.Pereira EFR, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. J Neurobiol. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- 372.Pereira EFR, Reinhardt-Maelicke S, Schrattenholz A, Maelicke A, Albuquerque EX. Identification and functional characterization of a new agonist site on nicotinic acetylcholine receptors of cultured hippocampal neurons. J Pharmacol Exp Ther. 1993;265:1474–1491. [PubMed] [Google Scholar]
- 373.Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- 374.Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, Haroutunian V, Buxbaum JD, Nasland J, Davis K, Gotti C, Clementi F, Tzartos S, Cohen O, Soreq H, Jaros E, Perry R, Ballard C, McKeith I, Court J. Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol. 2000;393:215–222. doi: 10.1016/s0014-2999(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 375.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 376.Pettit DL, Shao Z, Yakel JL. beta-Amyloid(1—42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. J Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- 378.Picciotto MR, Zoli M, Zachariou V, Changeux JP. Contribution of nicotinic acetylcholine receptors containing the β 2-subunit to the behavioural effects of nicotine. Biochem Soc Trans. 1997;25:824–829. doi: 10.1042/bst0250824. [DOI] [PubMed] [Google Scholar]
- 379.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 380.Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Pluzarev O, Pandey SC. Modulation of CREB expression and phosphorylation in the rat nucleus accumbens during nicotine exposure and withdrawal. J Neurosci Res. 2004;77:884–891. doi: 10.1002/jnr.20216. [DOI] [PubMed] [Google Scholar]
- 382.Popot JL, Changeux JP. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev. 1984;64:1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- 383.Proia NK, Paszkiewicz GM, Nasca MA, Franke GE, Pauly JL. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer—a review. Cancer Epidemiol Biomarkers Prev. 2006;15:1061–1077. doi: 10.1158/1055-9965.EPI-05-0983. [DOI] [PubMed] [Google Scholar]
- 384.Quik M. Smoking, nicotine and Parkinson’s disease. Trends Neurosci. 2004;27:561–568. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 385.Quik M, Bordia T, O’Leary K. Nicotinic receptors as CNS targets for Parkinson’s disease. Biochem Pharmacol. 2007;74:1224–1234. doi: 10.1016/j.bcp.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Quik M, McIntosh JM. Striatal α6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. J Pharmacol Exp Ther. 2006;316:481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- 387.Quik M, Philie J, Choremis J. Modulation of α7 nicotinic receptor-mediated calcium influx by nicotinic agonists. Mol Pharmacol. 1997;51:499–506. [PubMed] [Google Scholar]
- 388.Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 389.Ramoa AS, Alkondon M, Aracava Y, Irons J, Lunt GG, Deshpande SS, Wonnacott S, Aronstam RS, Albuquerque EX. The anticonvulsant MK-801 interacts with peripheral and central nicotinic acetylcholine receptor ion channels. J Pharmacol Exp Ther. 1990;254:71–82. [PubMed] [Google Scholar]
- 390.Rassoulpour A, Wu HQ, Albuquerque EX, Schwarcz R. Prolonged nicotine administration results in biphasic, brain-specific changes in kynurenate levels in the rat. Neuropsychopharmacology. 2005;30:697–704. doi: 10.1038/sj.npp.1300583. [DOI] [PubMed] [Google Scholar]
- 391.Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 392.Ren XQ, Cheng SB, Treuil MW, Mukherjee J, Rao J, Braunewell KH, Lindstrom JM, Anand R. Structural determinants of α4β2 nicotinic acetylcholine receptor trafficking. J Neurosci. 2005;25:6676–6686. doi: 10.1523/JNEUROSCI.1079-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cell Mol Neurobiol. 1997;17:627–648. doi: 10.1023/a:1022534019718. [DOI] [PubMed] [Google Scholar]
- 394.Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Rickert KW, Imperiali B. Analysis of the conserved glycosylation site in the nicotinic acetylcholine receptor: potential roles in complex assembly. Chem Biol. 1995;2:751–759. doi: 10.1016/1074-5521(95)90103-5. [DOI] [PubMed] [Google Scholar]
- 396.Rogers SW, Gahring LC, Collins AC, Marks M. Age-related changes in neuronal nicotinic acetylcholine receptor subunit α4 expression are modified by long-term nicotine administration. J Neurosci. 1998;18:4825–4832. doi: 10.1523/JNEUROSCI.18-13-04825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Rogers SW, Mandelzys A, Deneris ES, Cooper E, Heinemann S. The expression of nicotinic acetylcholine receptors by PC12 cells treated with NGF. J Neurosci. 1992;12:4611–4623. doi: 10.1523/JNEUROSCI.12-12-04611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Rosenberg MM, Blitzblau RC, Olsen DP, Jacob MH. Regulatory mechanisms that govern nicotinic synapse formation in neurons. J Neurobiol. 2002;53:542–555. doi: 10.1002/neu.10112. [DOI] [PubMed] [Google Scholar]
- 399.Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, III, Davis NG. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Rousseau SJ, Jones IW, Pullar IA, Wonnacott S. Presynaptic α7 and non-α7 nicotinic acetylcholine receptors modulate [3H]daspartate release from rat frontal cortex in vitro. Neuropharmacology. 2005;49:59–72. doi: 10.1016/j.neuropharm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 401.Rubin DT, Hanauer SB. Smoking and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:855–862. doi: 10.1097/00042737-200012080-00004. [DOI] [PubMed] [Google Scholar]
- 402.Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the α7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Sallette J, Bohler S, Benoit P, Soudant M, Pons S, Le Novere N, Changeux JP, Corringer PJ. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J Biol Chem. 2004;279:18767–18775. doi: 10.1074/jbc.M308260200. [DOI] [PubMed] [Google Scholar]
- 404.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 405.Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EFR, Lubbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- 406.Samochocki M, Zerlin M, Jostock R, Groot Kormelink PJ, Luyten WH, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of the human α4/β2 nAChR. Acta Neurol Scand Suppl. 2000;176:68–73. doi: 10.1034/j.1600-0404.2000.00310.x. [DOI] [PubMed] [Google Scholar]
- 407.Sands SB, Barish ME. Calcium permeability of neuronal nicotinic acetylcholine receptor channels in PC12 cells. Brain Res. 1991;560:38–42. doi: 10.1016/0006-8993(91)91211-i. [DOI] [PubMed] [Google Scholar]
- 408.Sansom MS, Adcock C, Smith GR. Modelling and simulation of ion channels: applications to the nicotinic acetylcholine receptor. J Struct Biol. 1998;121:246–262. doi: 10.1006/jsbi.1997.3950. [DOI] [PubMed] [Google Scholar]
- 409.Santos MD, Alkondon M, Pereira EFR, Aracava Y, Eisenberg HM, Maelicke A, Albuquerque EX. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol Pharmacol. 2002;61:1222–1234. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- 410.Sapko MT, Guidetti P, Yu P, Tagle DA, Pellicciari R, Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington’s disease. Exp Neurol. 2006;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 411.Sasakawa N, Ishii K, Yamamoto S, Kato R. Differential effects of protein kinase C activators on carbamylcholine- and high K+-induced rises in intracellular free calcium concentration in cultured adrenal chromaffin cells. Biochem Biophys Res Commun. 1986;139:903–909. doi: 10.1016/s0006-291x(86)80263-7. [DOI] [PubMed] [Google Scholar]
- 412.Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, Nomikos GG, Svensson TH. Putative role of presynaptic a7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–383. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 413.Schrattenholz A, Coban T, Schroder B, Okonjo KO, Kuhlmann J, Pereira EFR, Albuquerque EX, Maelicke A. Biochemical characterization of a novel channel-activating site on nicotinic acetylcholine receptors. J Recept Res. 1993;13:393–412. doi: 10.3109/10799899309073669. [DOI] [PubMed] [Google Scholar]
- 414.Schrattenholz A, Pereira EF, Roth U, Weber KH, Albuquerque EX, Maelicke A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol Pharmacol. 1996;49:1–6. [PubMed] [Google Scholar]
- 415.Schroder B, Reinhardt-Maelicke S, Schrattenholz A, McLane KE, Kretschmer A, Conti-Tronconi BM, Maelicke A. Monoclonal antibodies FK1 and WF6 define two neighboring ligand binding sites on Torpedo acetylcholine receptor α-polypeptide. J Biol Chem. 1994;269:10407–10416. [PubMed] [Google Scholar]
- 416.Schroder H, Giacobini E, Struble RG, Zilles K, Maelicke A. Nicotinic cholinoceptive neurons of the frontal cortex are reduced in Alzheimer’s disease. Neurobiol Aging. 1991;12:259–262. doi: 10.1016/0197-4580(91)90107-u. [DOI] [PubMed] [Google Scholar]
- 417.Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychiatry. 2006;60:530–533. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 418.Schwarcz R, Ceresoli-Borroni G, Wu HQ, Rassoulpour A, Poeggeler B, Hodgkins PS, Guidetti P. Modulation and function of kynurenic acid in the immature rat brain. Adv Exp Med Biol. 1999;467:113–123. doi: 10.1007/978-1-4615-4709-9_17. [DOI] [PubMed] [Google Scholar]
- 419.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 420.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 421.Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- 422.Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- 423.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Sershen H, Balla A, Lajtha A, Vizi ES. Characterization of nicotinic receptors involved in the release of noradrenaline from the hippocampus. Neuroscience. 1997;77:121–130. doi: 10.1016/s0306-4522(96)00425-3. [DOI] [PubMed] [Google Scholar]
- 425.Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Sharma G, Vijayaraghavan S. Nicotinic receptor signaling in nonexcitable cells. J Neurobiol. 2002;53:524–534. doi: 10.1002/neu.10114. [DOI] [PubMed] [Google Scholar]
- 427.Sharma T, Reed C, Aasen I, Kumari V. Cognitive effects of adjunctive 24-weeks Rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, and double-blind investigation. Schizophr Res. 2006;85:73–83. doi: 10.1016/j.schres.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 428.Shaw KP, Aracava Y, Akaike A, Daly JW, Rickett DL, Albuquerque EX. The reversible cholinesterase inhibitor physostigmine has channel-blocking and agonist effects on the acetylcholine receptor-ion channel complex. Mol Pharmacol. 1985;28:527–538. [PubMed] [Google Scholar]
- 429.Sherby SM, Eldefrawi AT, Albuquerque EX, Eldefrawi ME. Comparison of the actions of carbamate anticholinesterases on the nicotinic acetylcholine receptor. Mol Pharmacol. 1985;27:343–348. [PubMed] [Google Scholar]
- 430.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 431.Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 432.Singh SP, Burns T, Amin S, Jones PB, Harrison G. Acute and transient psychotic disorders: precursors, epidemiology, course and outcome. Br J Psychiatry. 2004;185:452–459. doi: 10.1192/bjp.185.6.452. [DOI] [PubMed] [Google Scholar]
- 433.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- 434.Smit AB, Celie PH, Kasheverov IE, Mordvintsev DY, van Nierop P, Bertrand D, Tsetlin V, Sixma TK. Acetylcholine-binding proteins: functional and structural homologs of nicotinic acetylcholine receptors. J Mol Neurosci. 2006;30:9–10. doi: 10.1385/JMN:30:1:9. [DOI] [PubMed] [Google Scholar]
- 435.Smith MM, Lindstrom J, Merlie JP. Formation of the α-bungarotoxin binding site and assembly of the nicotinic acetylcholine receptor subunits occur in the endoplasmic reticulum. J Biol Chem. 1987;262:4367–4376. [PubMed] [Google Scholar]
- 436.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 437.Snaedal J, Johannesson T, Jonsson JE, Gylfadottir G. The effects of nicotine in dermal plaster on cognitive functions in patients with Alzheimer’s disease. Dementia. 1996;7:47–52. doi: 10.1159/000106852. [DOI] [PubMed] [Google Scholar]
- 438.Sobel A, Hofler J, Heidmann T, Changeux JP. Structural and functional properties of the acetylcholine regulator. Adv Cytopharmacol. 1979;3:191–196. [PubMed] [Google Scholar]
- 439.Soliakov L, Gallagher T, Wonnacott S. Anatoxin-a-evoked [3H]dopamine release from rat striatal synaptosomes. Neuropharmacology. 1995;34:1535–1541. doi: 10.1016/0028-3908(95)00122-m. [DOI] [PubMed] [Google Scholar]
- 440.Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, Fratta W, Goldberg SR. Nicotinic α7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007;27:5615–5620. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Spady TJ, Harvell DM, Snyder MC, Pennington KL, McComb RD, Shull JD. Estrogen-induced tumorigenesis in the Copenhagen rat: disparate susceptibilities to development of prolactin-producing pituitary tumors and mammary carcinomas. Cancer Lett. 1998;124:95–103. doi: 10.1016/s0304-3835(97)00455-2. [DOI] [PubMed] [Google Scholar]
- 442.Spivak CE, Lupica CR, Oz M. The endocannabinoid anandamide inhibits the function of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2007;72:1024–1032. doi: 10.1124/mol.107.036939. [DOI] [PubMed] [Google Scholar]
- 443.Spivak JL. The anaemia of cancer: death by a thousand cuts. Nat Rev Cancer. 2005;5:543–555. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 444.Stakhiv TM, Mesia-Vela S, Kauffman FC. Phase II antioxidant enzyme activities in brain of male and female ACI rats treated chronically with estradiol. Brain Res. 2006;1104:80–91. doi: 10.1016/j.brainres.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 445.Stegelmeier BL, Hall JO, Gardner DR, Panter KE. The toxicity and kinetics of larkspur alkaloid, methyllycaconitine, in mice. J Anim Sci. 2003;81:1237–1241. doi: 10.2527/2003.8151237x. [DOI] [PubMed] [Google Scholar]
- 446.Steinlein O. New functions for nicotinic acetylcholine receptors? Behav Brain Res. 1998;95:31–35. doi: 10.1016/s0166-4328(97)00207-6. [DOI] [PubMed] [Google Scholar]
- 447.Steinlein OK. Channelopathies can cause epilepsy in man. Eur J Pain. 2002;(6) Suppl A:27–34. doi: 10.1053/eujp.2001.0319. [DOI] [PubMed] [Google Scholar]
- 448.Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- 449.Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–154. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- 450.Storch A, Schrattenholz A, Cooper JC, Abdel Ghani EM, Gutbrod O, Weber KH, Reinhardt S, Lobron C, Hermsen B, Soskic V. Physostigmine, galanthamine and codeine act as “non-competitive nicotinic receptor agonists” on clonal rat pheochromocytoma cells. Eur J Pharmacol. 1995;290:207–219. doi: 10.1016/0922-4106(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 451.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 452.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 453.Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Sugaya K, Uz T, Kumar V, Manev H. New anti-inflammatory treatment strategy in Alzheimer’s disease. Jpn J Pharmacol. 2000;82:85–94. doi: 10.1254/jjp.82.85. [DOI] [PubMed] [Google Scholar]
- 455.Sumikawa K, Gehle VM. Assembly of mutant subunits of the nicotinic acetylcholine receptor lacking the conserved disulfide loop structure. J Biol Chem. 1992;267:6286–6290. [PubMed] [Google Scholar]
- 456.Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, Andra M, Matsubayashi H, Sakai N, Kohsaka S, Inoue K, Nakata Y. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- 457.Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987;7:3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Sweileh W, Wenberg K, Xu J, Forsayeth J, Hardy S, Loring RH. Multistep expression and assembly of neuronal nicotinic receptors is both host-cell-and receptor-subtype-dependent. Brain Res. 2000;75:293–302. doi: 10.1016/s0169-328x(99)00302-2. [DOI] [PubMed] [Google Scholar]
- 459.Takeuchi N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Tang K, Wu H, Mahata SK, O’Connor DT. A crucial role for the mitogen-activated protein kinase pathway in nicotinic cholinergic signaling to secretory protein transcription in pheochromocytoma cells. Mol Pharmacol. 1998;54:59–69. doi: 10.1124/mol.54.1.59. [DOI] [PubMed] [Google Scholar]
- 461.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 462.Tariot PN, Schneider L, Katz IR, Mintzer JE, Street J, Copenhaver M, Williams-Hughes C. Quetiapine treatment of psychosis associated with dementia: a double-blind, randomized, placebo-controlled clinical trial. Am J Geriatr Psychiatry. 2006;14:767–776. doi: 10.1097/01.JGP.0000196628.12010.35. [DOI] [PubMed] [Google Scholar]
- 463.Teaktong T, Graham A, Court J, Perry R, Jaros E, Johnson M, Hall R, Perry E. Alzheimer’ disease is associated with a selective increase in α7 nicotinic acetylcholine receptor immunoreactivity in astrocytes. Glia. 2003;41:207–211. doi: 10.1002/glia.10132. [DOI] [PubMed] [Google Scholar]
- 464.Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002;9:41–47. doi: 10.1101/lm.43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Temburni MK, Blitzblau RC, Jacob MH. Receptor targeting and heterogeneity at interneuronal nicotinic cholinergic synapses in vivo. J Physiol. 2000;525:21–29. doi: 10.1111/j.1469-7793.2000.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Thomas GA, Rhodes J, Ingram JR. Mechanisms of disease: nicotine—a review of its actions in the context of gastrointestinal disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:536–544. doi: 10.1038/ncpgasthep0316. [DOI] [PubMed] [Google Scholar]
- 467.Tikhonov DB, Zhorov BS. Kinked-helices model of the nicotinic acetylcholine receptor ion channel and its complexes with blockers: simulation by the Monte Carlo minimization method. Biophys J. 1998;74:242–255. doi: 10.1016/S0006-3495(98)77783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Trouslard J, Mirsky R, Jessen KR, Burnstock G, Brown DA. Intracellular calcium changes associated with cholinergic nicotinic receptor activation in cultured myenteric plexus neurones. Brain Res. 1993;624:103–108. doi: 10.1016/0006-8993(93)90065-u. [DOI] [PubMed] [Google Scholar]
- 469.Tuominen RK, McMillian MK, Ye H, Stachowiak MK, Hudson PM, Hong JS. Long-term activation of protein kinase C by nicotine in bovine adrenal chromaffin cells. J Neurochem. 1992;58:1652–1658. doi: 10.1111/j.1471-4159.1992.tb10037.x. [DOI] [PubMed] [Google Scholar]
- 470.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 471.Turner JR, Kellar KJ. Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci. 2005;25:9258–9265. doi: 10.1523/JNEUROSCI.2112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Turski WA, Gramsbergen JB, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon exposure to l-kynurenine. J Neurochem. 1989;52:1629–1636. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- 473.Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell WO, Jr, Schwarcz R. Identification and quantification of kynurenic acid in human brain tissue. Brain Res. 1988;454:164–169. doi: 10.1016/0006-8993(88)90815-3. [DOI] [PubMed] [Google Scholar]
- 474.Ulens C, Hogg RC, Celie PH, Bertrand D, Tsetlin V, Smit AB, Sixma TK. Structural determinants of selective α-conotoxin binding to a nicotinic acetylcholine receptor homolog AChBP. Proc Natl Acad Sci USA. 2006;103:3615–3620. doi: 10.1073/pnas.0507889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Unwin N. The nicotinic acetylcholine receptor of the Torpedo electric ray. J Struct Biol. 1998;121:181–190. doi: 10.1006/jsbi.1997.3949. [DOI] [PubMed] [Google Scholar]
- 476.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 477.Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Van den Buuse M, Eikelis N. Estrogen increases prepulse inhibition of acoustic startle in rats. Eur J Pharmacol. 2001;425:33–41. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- 479.Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 480.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 481.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of α2, α3, α4, and β2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 482.Wallenstein GV, Hasselmo ME. Functional transitions between epileptiform-like activity and associative memory in hippocampal region CA3. Brain Res Bull. 1997;43:485–493. doi: 10.1016/s0361-9230(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 483.Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. Upregulation of nicotinic receptors by nicotine varies with receptor subtype. J Biol Chem. 2008;283:6022–6032. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- 484.Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 485.Wanamaker CP, Christianson JC, Green WN. Regulation of nicotinic acetylcholine receptor assembly. Ann NY Acad Sci. 2003;998:66–80. doi: 10.1196/annals.1254.009. [DOI] [PubMed] [Google Scholar]
- 486.Wanamaker CP, Green WN. Endoplasmic reticulum chaperones stabilize nicotinic receptor subunits and regulate receptor assembly. J Biol Chem. 2007;282:31113–31123. doi: 10.1074/jbc.M705369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Wanamaker CP, Green WN. N-linked glycosylation is required for nicotinic receptor assembly but not for subunit associations with calnexin. J Biol Chem. 2005;280:33800–33810. doi: 10.1074/jbc.M501813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor α5 subunits with α3, β2, and β4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- 489.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor a7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 490.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. βAmyloid(1—42) binds to α7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 491.Wang N, Orr-Urtreger A, Chapman J, Ergu NY, Rabinowitz R, Korczyn AD. Hidden function of neuronal nicotinic acetylcholine receptor β2 subunits in ganglionic transmission: comparison to α5 and β4 subunits. J Neurol Sci. 2005;228:167–177. doi: 10.1016/j.jns.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 492.Wang Y, Pereira EFR, Maus AD, Ostlie NS, Navaneetham D, Lei S, Albuquerque EX, Conti-Fine BM. Human bronchial epithelial and endothelial cells express α7 nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:1201–1209. doi: 10.1124/mol.60.6.1201. [DOI] [PubMed] [Google Scholar]
- 493.Wang ZZ, Fuhrer C, Shtrom S, Sugiyama JE, Ferns MJ, Hall ZW. The nicotinic acetylcholine receptor at the neuromuscular junction: assembly and tyrosine phosphorylation. Cold Spring Harb Symp Quant Biol. 1996;61:363–371. [PubMed] [Google Scholar]
- 494.Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 495.Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 2003;72:2055–2061. doi: 10.1016/s0024-3205(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 496.Whiteaker P, Sharples CG, Wonnacott S. Agonist-induced upregulation of α4β2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol. 1998;53:950–962. [PubMed] [Google Scholar]
- 497.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 498.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 499.Wimer RE, Wimer CC, Cohen AJ, Alameddine L. Search for a gene that may result in fewer neurons in the adult mouse hippocampus. Brain Res. 1995;701:293–296. doi: 10.1016/0006-8993(95)01046-8. [DOI] [PubMed] [Google Scholar]
- 500.Witzemann V, Barg B, Criado M, Stein E, Sakmann B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett. 1989;242:419–424. doi: 10.1016/0014-5793(89)80514-9. [DOI] [PubMed] [Google Scholar]
- 501.Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- 502.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 503.Woolf A, Burkhart K, Caraccio T, Litovitz T. Self-poisoning among adults using multiple transdermal nicotine patches. J Toxicol Clin Toxicol. 1996;34:691–698. doi: 10.3109/15563659609013830. [DOI] [PubMed] [Google Scholar]
- 504.Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Wright CI, Geula C, Mesulam MM. Protease inhibitors and indolamines selectively inhibit cholinesterases in the histopathologic structures of Alzheimer’s disease. Ann NY Acad Sci. 1993;695:65–68. doi: 10.1111/j.1749-6632.1993.tb23029.x. [DOI] [PubMed] [Google Scholar]
- 506.Wu J, Kuo YP, George AA, Xu L, Hu J, Lukas RJ. beta-Amyloid directly inhibits human α4β2-nicotinic acetylcholine receptors heterologously expressed in human SH-EP1 cells. J Biol Chem. 2004;279:37842–37851. doi: 10.1074/jbc.M400335200. [DOI] [PubMed] [Google Scholar]
- 507.Xiao Y, Baydyuk M, Wang HP, Davis HE, Kellar KJ. Pharmacology of the agonist binding sites of rat neuronal nicotinic receptor subtypes expressed in HEK 293 cells. Bioorg Med Chem Lett. 2004;14:1845–1848. doi: 10.1016/j.bmcl.2003.09.105. [DOI] [PubMed] [Google Scholar]
- 508.Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- 509.Xu J, Zhu Y, Heinemann SF. Identification of sequence motifs that target neuronal nicotinic receptors to dendrites and axons. J Neurosci. 2006;26:9780–9793. doi: 10.1523/JNEUROSCI.0840-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Xu X, Scott MM, Deneris ES. Shared long-range regulatory elements coordinate expression of a gene cluster encoding nicotinic receptor heteromeric subtypes. Mol Cell Biol. 2006;26:5636–5649. doi: 10.1128/MCB.00456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Yamada S, Kagawa Y, Isogai M, Takayanagi N, Hayashi E. Ontogenesis of nicotinic acetylcholine receptors and presynaptic cholinergic neurons in mammalian brain. Life Sci. 1986;38:637–644. doi: 10.1016/0024-3205(86)90057-3. [DOI] [PubMed] [Google Scholar]
- 512.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 513.Yan GZ, Ziff EB. Nerve growth factor induces transcription of the p21 WAF1/CIP1 and cyclin D1 genes in PC12 cells by activating the Sp1 transcription factor. J Neurosci. 1997;17:6122–6132. doi: 10.1523/JNEUROSCI.17-16-06122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, Leslie FM, Gee KW. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self administration in rats. J Pharmacol Exp Ther. 2007;323:907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]
- 515.Yu CR, Role LW. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. J Physiol. 1998;509:667–681. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Yu P, Di Prospero NA, Sapko MT, Cai T, Chen A, Melendez-Ferro M, Du F, Whetsell WO, Jr, Guidetti P, Schwarcz R, Tagle DA. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol Cell Biol. 2004;24:6919–6930. doi: 10.1128/MCB.24.16.6919-6930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Yu P, Li Z, Zhang L, Tagle DA, Cai T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene. 2006;365:111–118. doi: 10.1016/j.gene.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 518.Yu WF, Guan ZZ, Bogdanovic N, Nordberg A. High selective expression of α7 nicotinic receptors on astrocytes in the brains of patients with sporadic Alzheimer’s disease and patients carrying Swedish APP 670/671 mutation: a possible association with neuritic plaques. Exp Neurol. 2005;192:215–225. doi: 10.1016/j.expneurol.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 519.Zanardi A, Leo G, Biagini G, Zoli M. Nicotine and neurodegeneration in ageing. Toxicol Lett. 2002;127:207–215. doi: 10.1016/s0378-4274(01)00502-1. [DOI] [PubMed] [Google Scholar]
- 520.Zhang CL, Verbny Y, Malek SA, Stys PK, Chiu SY. Nicotinic acetylcholine receptors in mouse and rat optic nerves. J Neurophysiol. 2004;91:1025–1035. doi: 10.1152/jn.00769.2003. [DOI] [PubMed] [Google Scholar]
- 521.Zhang L, Zhou FM, Dani JA. Cholinergic drugs for Alzheimer’s disease enhance in vitro dopamine release. Mol Pharmacol. 2004;66:538–544. doi: 10.1124/mol.104.000299. [DOI] [PubMed] [Google Scholar]
- 522.Zhang ZW, Coggan JS, Berg DK. Synaptic currents generated by neuronal acetylcholine receptors sensitive to α-bungarotoxin. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]
- 523.Zhang ZW, Feltz P. Nicotinic acetylcholine receptors in porcine hypophyseal intermediate lobe cells. J Physiol. 1990;422:83–101. doi: 10.1113/jphysiol.1990.sp017974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human α4β2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Zhu D, Xiong WC, Mei L. Lipid rafts serve as a signaling platform for nicotinic acetylcholine receptor clustering. J Neurosci. 2006;26:4841–4851. doi: 10.1523/JNEUROSCI.2807-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Zia S, Ndoye A, Nguyen VT, Grando SA. Nicotine enhances expression of the α3, α4, α5, α7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res Commun Mol Pathol Pharmacol. 1997;97:243–262. [PubMed] [Google Scholar]
- 527.Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using β2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J. 1999;18:1235–1244. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Zorumski CF, Thio LL, Isenberg KE, Clifford DB. Nicotinic acetylcholine currents in cultured postnatal rat hippocampal neurons. Mol Pharmacol. 1992;41:931–936. [PubMed] [Google Scholar]
- 530.Zwart R, Broad LM, Xi Q, Lee M, Moroni M, Bermudez I, Sher E. 5-I A-85380 and TC-2559 differentially activate heterologously expressed α4β2 nicotinic receptors. Eur J Pharmacol. 2006;539:10–17. doi: 10.1016/j.ejphar.2006.03.077. [DOI] [PubMed] [Google Scholar]
- 531.Zwart R, Van Kleef RG, Vijverberg HP. Physostigmine and atropine potentiate and inhibit neuronal α4 β4 nicotinic receptors. Ann NY Acad Sci. 1999;868:636–639. doi: 10.1111/j.1749-6632.1999.tb11339.x. [DOI] [PubMed] [Google Scholar]
- 532.Zwart R, Vijverberg HP. Potentiation and inhibition of neuronal nicotinic receptors by atropine: competitive and noncompetitive effects. Mol Pharmacol. 1997;52:886–895. doi: 10.1124/mol.52.5.886. [DOI] [PubMed] [Google Scholar]