Abstract
5,6-Dimethylxanthenone-4-acetic acid (DMXAA) acts through tumor vascular disruption and cytokine production and is the first of its class to enter phase 3 trials. We characterized leukocytes and cytokines in murine Colon 38 tumors before and after DMXAA treatment. Tumor mass declined 50% 24 hours after DMXAA administration, but the leukocyte count per gram of tumor increased threefold owing to a large influx of Ly6G+CD11b+F4/80- cells with the morphology of neutrophils. However, B and T lymphocytes, natural killer cells, and macrophages in the tumor all decreased in numbers. Seven chemokines were substantially induced in the tumor, spleen, and serum 4 hours after DMXAA administration. Using cultured spleen cell subpopulations, CD11b+ cells (largely monocytes and macrophages) were shown to be the primary producers of tumor necrosis factor α, interleukin 6 (IL-6), and macrophage inflammatory 1α (MIP-1α). CD49b+ natural killer cells produced IP-10, whereas CD45R+ B lymphocytes produced regulated upon activation normal T cell express sequence. T lymphocytes were not major producers of cytokines in the response to DMXAA. Murine peripheral blood leukocytes (PBLs) produced a similar panel of cytokines in culture to that detected in mouse serum after DMXAA treatment. Cytokines in human PBL cultures were subsequently measured with the aim of identifying potential serum markers of the human response to DMXAA. IP-10 (P < .001), monocyte chemoattractant protein 1 (P < .001), and sCD40L (P < .01) were decreased, whereas IL-8 (P < .001) and MIP-1α (P = .03) were increased in DMXAA-treated compared with untreated PBL cultures from a group of 12 donors.
Background
The leukocyte infiltrate, which comprises a major component of the tumor stroma, is recognized as an important contributor to the cytokine milieu that controls tumor growth. Current anticancer strategies have seen a need to include approaches that target the tumor stroma, and 5,6-dimethylxanthenone-4-acetic acid (DMXAA; ASA404) is an example of such an agent. DMXAA was developed at the Auckland Cancer Society Research Centre [1] as a more potent derivative of flavone acetic acid [2], and it is currently in phase 3 (Novartis, ATTRACT-1 trial) clinical development for the treatment of non-small cell lung carcinoma in combination with chemotherapy. A hallmark of the activity of these agents is the induction of hemorrhagic necrosis in murine tumors, which resembles that induced with tumor necrosis factor α (TNFα) [3]. Indeed, TNFα was produced after DMXAA treatment [4–6]. The antitumor activity in TNFα knockout and TNFα receptor 1 knockout mice was attenuated but not completely abolished, however, indicating that whereas TNFα plays a role, other factors are also important [7]. Up-regulation of a number of cytokine genes after DMXAA treatment has been demonstrated [8–11]. Among these, interferons are abundantly produced [8,10] and have been suggested to be responsible for dendritic cell activation and increases in tumor-specific CD8+ T cells that are seen in DMXAA-treated mice [12]. We hypothesized that the induced cytokine cascade will lead to an altered tumor microenvironment and stromal cell infiltration. In this report, we examined changes in the leukocyte infiltrate and cytokine concentrations in murine Colon 38 tumor before and after DMXAA treatment. Because cytokine induction seems an essential component of DMXAA's activity in mice, we also compared the in vitro response of cultured peripheral blood leukocytes (PBLs) from mice and a group of 12 healthy donors to examine for interspecies differences and the variability between donors in the response to DMXAA.
Materials and Methods
5,6-Dimethylxanthenone-4-Acetic Acid
DMXAA was synthesized as the sodium salt at the Auckland Cancer Society Research Centre [1] and dissolved fresh for each experiment in saline. DMXAA was administered to mice by intraperitoneal injection at 25 mg/kg. For in vitro experiments, DMXAA was dissolved in culture medium, which was α-modified essential medium (Gibco BRL, Grand Island, NY), supplemented with fetal calf serum (10%), antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin), and 2-mercaptoethanol (50 µM).
Mice and Tumor Implants
C57Bl/6 mice were bred at the Vernon Jansen Unit, University of Auckland, and were housed under conditions of constant temperature, lighting, and humidity. All experiments conformed to local institutional guidelines. Murine Colon 38 tumors are maintained by serial transfer into syngeneic C57Bl/6 mice. Colon 38 tumors were removed from donor mice and minced, and 1-mm2 fragments were transferred into a subcutaneous pocket made in the left flank of anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine) recipient mice. Tumors were used for experiments when they were approximately 8 mm in diameter.
Characterization of Tumor-Infiltrating Leukocytes
Colon 38 tumors, excised at various times after DMXAA treatment, were pressed through a stainless steel mesh into 20 ml of culture medium and aspirated to break up the large clumps. The leukocytes were isolated by Ficoll-Paque PLUS (Pharmacia, Uppsala, Sweden) density centrifugation. Cells in the leukocyte layer were incubated with allophycocyanin-conjugated anti-CD45 antibodies to label all leukocytes. Leukocyte subsets were identified by labeling with two additional cell-type-specific antibodies; one of which would be fluorescein isothiocyanate (FITC)-conjugated and the other would be phycoerythrin (PE)-conjugated to allow triple staining of each subset. The macrophage subpopulation of CD45+ leukocytes was identified by colabeling with FITC-anti-CD11b and PE-anti-F4/80 antibodies, that of natural killer (NK) cells was identified by colabeling with FITC-anti-CD49b antibodies, that of B lymphocytes was identified by colabeling with FITC-anti-CD45R and PE-anti-CD19 antibodies, and that of CD4+ and CD8+ T lymphocytes was identified by colabeling with PE-anti-CD3ε and FITC-anti-CD4 or FITC-anti-CD8a antibodies, respectively. Antibodies were purchased from Miltenyi Biotec (Bergish Gladbach, Germany) and Serotec, Inc (Raleigh, NC). The cell populations were analyzed using FACS Vantage cell sorter (BD Biosciences, NSW, Australia) and CellQuest Pro software (BD Biosciences). The histologic diagnosis of each population was examined by hematoxylin and eosin staining of a cytospot of 2 x 105 cells of each fraction. Generally, groups of 6 to 10 tumors were used for each labeling procedure.
Immunofluorescence Staining of Tumor Sections
Excised tumors in OCT (Tissue-Tek, Sakura Finetechnical, Tokyo, Japan) were snap frozen in liquid nitrogen and stored at -80°C until sectioning. Tumor sections of 7-µm thickness were mounted onto glass slides and immunostained as previously described [13–15]. Primary rat antimouse antibodies used in these studies were as follows: FITC-labeled anti-CD11b (BD Pharmingen, San Diego, CA), unconjugated anti-F4/80 (Serotec), and anti-Ly6G (BD Pharmingen). Secondary antibodies used were Alexa Fluor 488-anti-FITC and Alexa Fluor 555-antirat immunoglobulin (IgG) from Molecular Probes (Eugene, OR). All antibodies were diluted with 1% goat serum in Tris-buffered saline. When two primary antibodies raised in the same species were applied to the same tumor section, they were applied sequentially. Initially, sections were incubated with rat anti-F4/80 (10 µg/ml) or anti-Ly6G (10 µg/ml) and detected with antirat Alexa Fluor 555. Tumor sections were then blocked with 5% rat serum to bind any free sites on the antirat IgG secondary antibody. The section was then probed with FITC-labeled anti-CD11b, which was subsequently detected with an anti-FITC-Alexa Fluor 488 secondary antibody. Nuclei of cells were detected using 4′,6-diamidino-2-phenylindole stain. After the final wash in Tris-buffered saline, sections were mounted with Prolong Gold (Invitrogen, Christchurch, New Zealand) and visualized sequentially using the 350 nm (blue), 470 to 490 nm (green), and 515 to 560 nm (red) excitation filters on a Leica DMRE microscope and photographed using a Leica DC500 camera (Leica, Solms, Germany). Sequential images were processed using Portia (CytoCode, Auckland, New Zealand; www.cytocode.com). Negative control sections that were unstained or stained only with secondary antibodies were used to determine the amount of autofluorescence and to identify any potential nonspecific binding of the secondary antibodies. These sections were also used to set the input levels for each color such that the background autofluorescence was reduced to zero, and this setting was applied to every image. Three individual tumors per group were stained, and representative images of each group are presented.
Preparation of Tumor, Spleen, and Serum Samples for Cytokine Measurements
Mice with tumors, without treatment, or 2 to 6 hours after injection of DMXAA (25 mg/kg) were bled through the ocular sinus while under isoflurane anesthesia. Tumors and spleens were excised after cervical dislocation. Blood was allowed to clot overnight at 4°C and was then centrifuged (2000g for 30 minutes at 4°C). The layer of serum was transferred into fresh tubes and stored at -80°C until assay. Tumors and spleens were weighed and homogenized in phosphate-buffered saline with protease inhibitors (Sigma, St. Louis, MO). The homogenates were centrifuged, and the supernatants were transferred to fresh tubes, which were recentrifuged before the supernatants were transferred and stored at -80°C until assay. Groups of three mice were used for each treatment group. Highest concentrations were detected 4 hours after DMXAA injection. Only the data for the 4-hour time point have been presented and are consistent with unpublished data for cytokine induction by DMXAA in mice of different strains and with different tumors models that have been carried out for other studies.
Murine Splenocyte Cultures
Spleens from mice (three to six per experiment) were removed, the cells were squeezed out into culture medium and aspirated to form a single cell suspension, and red blood cells were removed by osmotic lysis. Cells (3 x 106 cells per well) were cultured with DMXAA in flat-bottomed 96-well plates in a total volume of 200 µl of culture medium in a humidified incubator at 37°C with an atmosphere of 5% carbon dioxide in air. The supernatant from each well was removed 4 hours after treatment and stored at -20°C until assay for cytokines. Triplicate cultures per group were assayed.
MidiMACS separator cell isolation kits (Miltenyi Biotec) were used following the manufacturer's instructions to positively select for different splenocyte subpopulations for culture. Magnetically labeled antibodies to CD11b, CD45R, and CD49b antigens, purchased from Miltenyi Biotec, were used to isolate populations that were enriched for macrophages, B lymphocytes, and NK cells, respectively, whereas magnetically labeled anti-CD4 and anti-CD8a antibodies were used to fractionate out the two subsets of T lymphocytes. The purity of each fraction was determined by flow cytometry following labeling of the positively selected subpopulation with FITC-conjugated antibodies to the antigen used for selection. Only fractions that were greater than 95% pure were used. The positively selected cells were cultured as described above for the unfractionated splenocytes.
Cells from 10 spleens were pooled for the isolation of each cell type in the first experiment. Generally, 10 spleens provided 6 x 108 nucleated cells after osmotic lysis, from which 3 x 108 CD11b+, 6 to 9 x 107 CD4+, 4 to 6 x 107 CD8+, and 1 to 2 x 106 CD49b+ cells could be obtained. In a second experiment, CD11b and CD8 and/or CD 4 cells were isolated from the one pool of 10 spleens, and CD49b and CD45R and/or CD4 were isolated from a second pool of 10 spleens.
Peripheral Blood Leukocyte Cultures
Blood from halothane-anesthetized C57Bl/6 mice was collected aseptically by cardiac puncture into heparinized tubes. Blood from 50 mice were pooled for the first experiment and from 30 mice for the second experiment. Blood from healthy human donors were obtained from NZ Blood Services. Blood from a total number of 12 donors were processed in batches of two to three per setup. Mononuclear cells from murine or human blood were isolated using Ficoll-Paque density centrifugation and were cultured in flat-bottomed 96-well plates (106 cells per well) with 10 or 300 µg/ml DMXAA in a final volume of 200 µl of culture medium. Supernatants from human and mouse PBL cultures were harvested after 16 and 4 hours, respectively, and stored at -20°C until assay.
Multiplex Cytokine Assays
Multiplex cytokine kits; murine 22-plex and 32-plex; and human 7-plex, 30-plex, and 42-plex (Linco Research, St Charles, MO) were used following the manufacturer's instructions. Serum samples were diluted 1:5, and tumor and spleen samples were diluted 1:10 with matrix diluent supplied with the kits, and culture supernatants were assayed undiluted. The concentration of each cytokine in the samples was read using the Luminex 100 instrument (Luminex Corporation, Austin, TX). Each sample was assayed in duplicate, and results were expressed as mean ± SEM from three mice per group or triplicate cultures per experimental group. Data between untreated and DMXAA-treated groups were compared using Student's t tests or one-way analysis of variance if multiple comparisons were made. Paired t tests were carried out comparing cytokine concentrations in treated and untreated cultures for all 12 donors (n = 36; triplicate cultures from 12 donors). Data were considered significant when P ≤ .05.
Results
Effect of DMXAA on Leukocytes in the Colon 38 Tumor
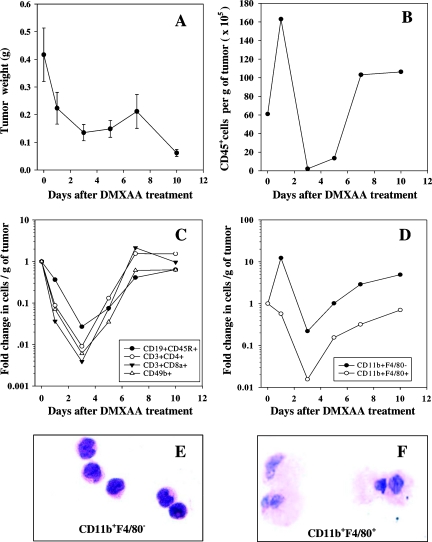
The CD45+ leukocyte infiltrate in Colon 38 tumors was shown by FACS analysis to comprise 43% CD3+CD8a+ cells (T lymphocytes mainly), 20% CD3+CD4+ T cells, 12% CD19+CD45R+ B lymphocytes, 14% CD11b+F4/80- immature macrophages/monocytes, 11% CD11b+F4/80+ mature macrophages, and 12% CD49b+ NK cells. The weight and changes in the leukocyte content of groups of Colon 38 tumors before and 1, 3, 5, 7, and 10 days after a single injection of DMXAA at its maximum tolerated dose of 25 mg/kg was monitored (Figure 1). Tumor weights dropped nearly 70% during the first 3 days, then increased slightly during the next 4 days before a second phase of tumor shrinkage was observed on day 7 (Figure 1A). The number of CD45+ leukocytes per gram of tumor increased three-fold during the first 24 hours after treatment when the tumor's size was decreasing. CD45+ leukocytes then dropped from 16 x 106 to a nadir of 3 x 103 cells per gram tumor weight on day 3 (Figure 1B), before increasing and stabilizing at 10 x 106 cells after 7 days. The increase in leukocyte content during the first 24 hours was not due to an influx of lymphoid cells. CD19+CD45R+ B lymphocytes, CD49b+ NK cells, and CD3+CD8a+ and CD3+CD4+ subsets (mainly T lymphocytes) all decreased in number during the first 3 days, then increased to pretreatment levels after 7 days, and then stabilized (Figure 1C). CD11b+F4/80+ myeloid cells followed a similar pattern of change to that of the lymphocytes (Figure 1D). CD11b+F4/80+ from untreated tumors have the appearance of mature macrophages (Figure 1F). Strikingly, the number of CD11b+F4/80- cells increased by 10-fold during the first 24 hours (Figure 1D), and these cells from untreated tumors have the appearance of immature monocytes (Figure 1E).
Figure 1.
Change in tumor weight (A) and number of CD45+ leukocytes (B), lymphoid populations (C), and myeloid cell populations (D) isolated per gram of Colon 38 tumor after treatment with DMXAA at 25 mg/kg. Histologic diagnosis of hematoxylin and eosin-stained CD11b+F4/80- (E) and CD11b+F4/80+ (F) cells extracted from untreated tumors (original magnification, x400).
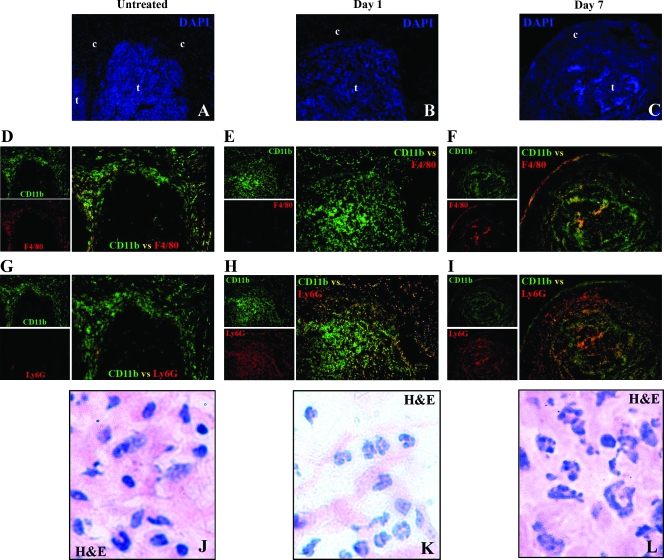
The influx of CD11b+F4/80- cells was confirmed by immunofluorescence staining of Colon 38 cryosections with FITC-anti-CD11b antibodies (green fluorescent) plus anti-F4/80 antibodies detected with Alexa Fluor 555 (red fluorescent)-conjugated secondary antibodies. In untreated tumors, a mixed population of CD11b+F4/80- (green) and CD11b+F4/80+ (yellow) cells was seen in the tumor capsule (Figure 2D). A large influx of CD11b+F4/80- (green) cells was seen in the parenchyma of the tumor 24 hours after treatment (Figure 2E). Tumors 7 days after treatment showed a mixture of CD11b+F4/80- and CD11b+F4/80+ cells (Figure 2F).
Figure 2.
Sequential sections of Colon 38 tumors from mice untreated (A, D, G, J) or 1 day (B, E, H, K) or 7 days (C, F, I, L) after treatment with DMXAA (25 mg/kg) and immunostained with DAPI to locate the nuclei of cells in the tumor (t) and capsule (c) (A, B, C); immunostained for CD11b+ (green), F4/80+ (red), and CD11b+ F4/80+ double positive (yellow/orange) cells (D, E, F); or immunostained for CD11b+ (green), Ly6G+ (red), and CD11b+Ly6G+ double-positive (yellow/orange) cells (G, H, I). Images were acquired at original magnifications of x5 (C, F, I) and x10 (A, B, D, E, G, H). Histologic diagnosis of cells in the capsular region of hematoxylin and eosin (H&E)-stained sections (J, K, L) were acquired at an original magnification of x100.
The CD11b+F4/80- cells from untreated tumors had the appearance of monocytes (Figure 1E), but neutrophils and a minor subset of dendritic cells also share this phenotype. We therefore colabeled sequential sections of tumors with the neutrophil-specific Ly6G marker (Figure 2, G–I), and hematoxylin and eosin-stained another set for histologic examinations (Figure 2, J–L). In untreated tumors, most CD11b+ cells did not express Ly6G (green) and were seen mainly in the capsule (Figure 2G). One-day-treated tumors, conversely, show a large number of CD11b+Ly6G+ (yellow) cells in the tumor parenchyma (Figure 2H), and 7-day-treated tumors show a mixture of CD11b+Ly6G+ and CD11b+Ly6G- cells (Figure 2I). The cells in the capsule of untreated tumors seem to be a mix of monocytes and macrophages (Figure 2J). Most cells seen in 1-day-treated tumors, however, have the distinct twisted ring-shaped nuclei of murine neutrophils (Figure 2K), and these cells remain the dominant cell-type seen in 7-day-treated tumors (Figure 2L).
Cytokines Induced with DMXAA in Tumor-Bearing Mice
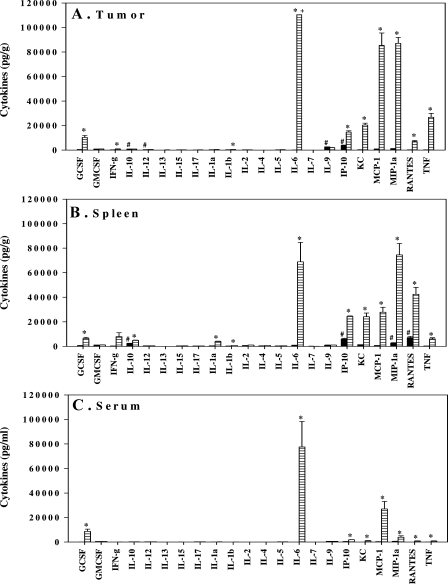
We next investigated the production of chemokines that may have influenced the influx of neutrophils into the tumor after treatment with DMXAA. Highest concentrations of cytokines were detected after 4 hours, and of the panel of 22 cytokines assayed, granulocyte-colony-stimulating factor (G-CSF), interleukin 6 (IL-6), interferon-inducible protein 10 (IP-10), keratinocyte-derived chemokine (KC), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), regulated upon activation normal T-cell express sequence (RANTES), and TNFα were highly induced in the tumor (Figure 3A). These same eight cytokines were also detected in spleen (Figure 3B) and in serum (Figure 3C). Low but statistically significant increases in IL-10, IL-1α, and IL-1β were additionally detected in the spleen. Although interferon γ (IFN-γ) levels increased 31-fold (250 pg/g in untreated compared with 7904 pg/g in DMXAA-treated) in the spleen, it was not significant (P = .07). Cytokine concentrations in the serum were lower than those in the spleen, which were lower than those in the tumor.
Figure 3.
Cytokine concentrations in Colon 38 tumors (A), spleen (B), and serum (C) from mice untreated (black bars) or 4 hours after DMXAA (25 mg/kg) treatment (lined bars). #Above the minimum detection limit. *Significantly different from untreated controls (P < .05, Student's t test). +Above maximum detection limit.
Cell Type Responsible for Producing the Cytokines
To investigate if different types were involved in producing the various cytokines, splenocytes were fractionated, and the different subsets were each cultured with DMXAA. The supernatants were assayed for a panel of cytokines. Two different concentrations of DMXAA were used: 10 µg/ml, which induces maximal production TNFα, and 300 µg/ml, which induces maximal production of IFN-γ in splenocyte cultures [16].
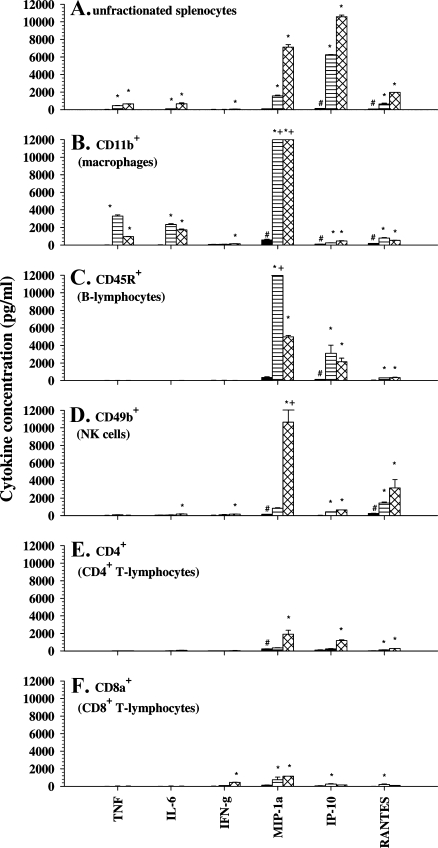
In cultures of unfractionated spleen cells, IL-6, IP-10, MIP-1α, RANTES, and TNFα were shown to be induced with higher levels obtained with 300 µg/ml DMXAA. IFN-γ was induced with 300 µg/ml but not with 10 µg/ml of DMXAA (Figure 4A). G-CSF, KC, and MCP-1 produced in vivo in the spleen (Figure 3B) were not detected in culture. IP-10 was the most abundant cytokine produced in culture (Figure 4A) compared with IL-6, which is the most abundant cytokine detected in vivo (Figure 3B).
Figure 4.
Cytokine concentrations in supernatants from cultures of unfractionated murine splenocytes (A), CD11b+ macrophage-enriched (B), CD45R+ B-lymphocyte-enriched (C), CD49b+ NK cell-enriched (D), CD4+ T-lymphocyte-enriched (E), and CD8a+ T-lymphocyte-enriched (F) spleen cell subsets; untreated (black bars) or treated for 4 hours with DMXAA at 10 µg/ml (lined bars) or 300 µg/ml (hatched bars). #Above minimum detection limit. *Statistically different from untreated controls (P < .05, one-way analysis of variance). +Above maximum detection limit.
Spleen cells were then fractionated using cell-type-specific antibodies linked to magnetic beads. The purity of each positively selected fraction was determined by examining an aliquot by FACS analysis and used only if greater than 95% pure. In addition, the histologic diagnosis of the cells in each fraction was examined. Of note, and consistent with the lower side scatter profile obtained with FACS of that fraction, the CD11b+ fraction was shown to be mainly macrophages with less than 4% granulocytes. The various fractions were each cultured at the same cell concentration with DMXAA at 10 and 300 µg/ml, and the supernatants were assayed for cytokines compared with untreated cultures. The macrophage-enriched CD11b+ subset (Figure 4B) and the B-lymphocyte-enriched CD45R+ subset (Figure 4C) both responded better to DMXAA at 10 µg/ml. However, the CD49b+ NK cell population (Figure 4D) and the CD4+ and CD8+ T-lymphocyte-enriched subsets (Figure 4, E and F) produced higher levels of cytokines at 300 µg/ml DMXAA. The CD11b+ macrophage-enriched fraction was the primary producer of TNFα and IL-6 (Figure 4B). This fraction also produced high amounts of MIP-1α to either concentration of DMXAA, as did the CD45R+ B-lymphocyte fraction at 10 µg/ml (Figure 4C), or the CD49b+ NK cell-enriched fraction at 300 µg/ml (Figure 4D). The CD45R+ B lymphocytes were the main producers of IP-10, whereas the CD49b+ NK cells were the main producers of RANTES. The CD8a+ T-lymphocyte-enriched fraction seem the best in producing IFN-γ (Figure 4F). Low but significant IFN-γ production was observed in the CD49b+ and CD11b+ cell fractions (Figure 4, B and C). However, because a small proportion of NK cells also express the CD11b antigen, we carried out an experiment to determine whether the IFN-γ detected in the CD11b+ fraction was due to the NK cells. Firstly, we depleted CD49b+ cells and then selected for CD11b+ cells in the CD49b- fraction. The CD11b+ fraction that was devoid of CD49b+ NK cells was subsequently tested for IFN-γ production and was shown not to produce IFN-γ in response to DMXAA at 300 µg/ml (34 ± 16 pg/ml compared with 27 ± 14 pg/ml in untreated controls). IFN-γ was produced, however, by the CD11b+ fraction that did not have the CD49b NK cells removed (137 ± 20 pg/ml) and by the CD49b+ fraction (150 ± 17 pg/ml). This result indicated that the IFN-γ was most likely produced by CD11b+CD49b+ NK cells. Overall, the results in Figure 4 establish that multiple cell types contribute to the cytokine response induced with DMXAA. Both the dose dependency of each cell type to DMXAA and the panel of cytokines induced differed.
Cytokine Response to DMXAA by Murine and Human PBLs in Culture
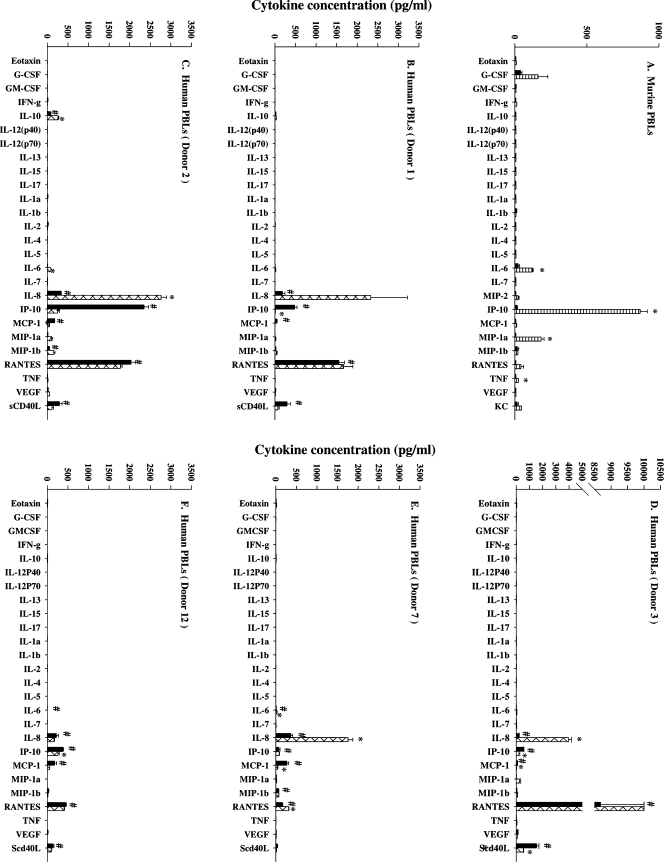
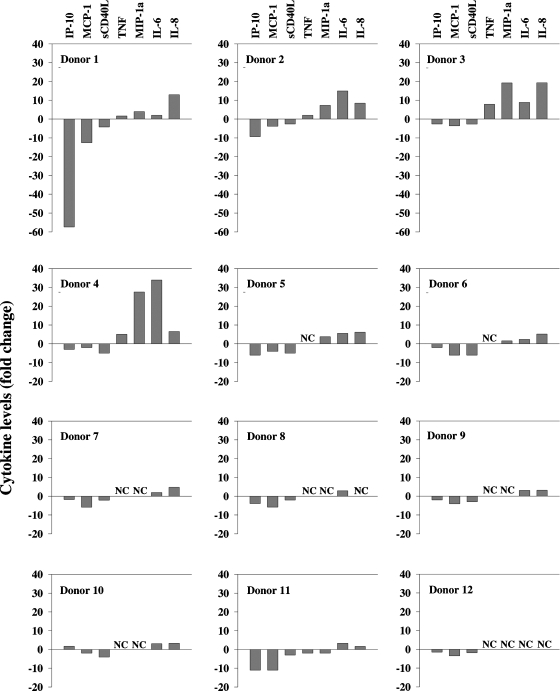
The spectrum of cytokines induced in vitro by cultured murine PBLs was next examined and compared with that detected in serum of DMXAA-treated mice. The purpose for the comparison was to establish if the in vitro response reflected the in vivo response. DMXAA induced IP-10, MIP-1α, G-CSF, RANTES, IL-6, and TNFα in murine PBL cultures in descending order of abundance (Figure 5A). Although the relative abundances differed, the panel of cytokines detected in culture was identical to that detected in serum (Figure 4A). The response of human PBLs in culture was subsequently examined to provide insights into the human cytokine response to DMXAA. Multiplex cytokine profiles for five individual PBL donors ranging from the highest (Figure 5B) to the lowest responder (Figure 5F) in the cohort of 12 donors are shown in Figure 5, B–F. Unlike murine PBLs (Figure 5A), human PBLs in culture constitutively produced IL-10, IL-8, IP-10, MCP-1, RANTES, and sCD40L without treatment. The addition of DMXAA had no significant effect on RANTES concentrations but significantly decreased levels of IP-10, MCP-1, and sCD40L (Table 1). Conversely, concentrations of IL-8 and MIP-1α were significantly increased (Table 1). Tumor necrosis factor α and IL-6 were not constitutively produced, and DMXAA did not induce their production in human PBL cultures (Table 1), although the induction of these two cytokines provides a strong determinant of the cytokine response to DMXAA in mice. The fold change in the concentrations of IP-10, sCD40L, MCP-1, MIP-1α, IL-8, as well as that of TNFα and IL-6 for each donor is presented in Figure 6. They show the trend of decreased production of IP-10, MCP-1, and sCD40L in response to DMXAA in most donors. Whereas TNFα, MIP-1α, IL-6, and IL-8 show a trend of being increased with DMXAA treatment in some of the donor PBL cultures, only the increases in IL-8 and MIP-1α concentrations reached statistical significance in the cohort (Table 1).
Figure 5.
Cytokine concentrations in culture supernatants from murine PBLs (A), untreated (black bars) or treated for 4 hours with 10 µg/ml DMXAA, and PBLs from a human donor (B, C, D, E, F) untreated (black bars) or 16 hours after treatment with DMXAA at 300 µg/ml (hatched bars). #Above minimum detection limit. *Statistically different from untreated controls (P < .05, Student's t test).
Table 1.
Cytokine Concentrations in Untreated and DMXAA-Treated Cultures of Human PBLs.
| Donor | Cytokine Concentration (pg/ml) | |||||||||||||
| IP-10 | MCP-1 | sCD40L | TNFα | MIP-1α | IL-6 | IL-8 | ||||||||
| Untreated | Treated | Untreated | Treated | Untreated | Treated | Untreated | Treated | Untreated | Treated | Untreated | Treated | Untreated | Treated | |
| 1 | 481 ± 58 | 8 ± 5* | 45 ± 10 | 4 ± 0* | 295 ± 80 | 71 ± 36 | 4 ± 0 | 7 ± 4 | 4 ± 1 | 17 ± 10 | 6 ± 3 | 12 ± 8 | 180 ± 62 | 2327 ± 892 |
| 2 | 2352 ± 105 | 252 ± 39* | 175 ± 5 | 45 ± 8* | 294 ± 64 | 111 ± 40 | 7 ± 0 | 14 ± 3* | 14 ± 1 | 105 ± 27* | 6 ± 0 | 85 ± 2* | 326 ± 13 | 2763 ± 127* |
| 3 | 536 ± 49 | 206 ± 58* | 100 ± 12 | 28 ± 3* | 1539 ± 173 | 529 ± 31* | 5 ± 0 | 37 ± 17 | 12 ± 2 | 239 ± 103 | 4 ± 1 | 39 ± 19 | 205 ± 21 | 3961 ± 218* |
| 4 | 429 ± 102 | 159 ± 27 | 245 ± 37 | 136 ± 66 | 133 ± 11 | 27 ± 3* | 5 ± 0 | 26 ± 22 | 7 ± 2 | 188 ± 176 | 14 ± 1 | 489 ± 467 | 328 ± 22 | 2156 ± 374* |
| 5 | 322 ± 97 | 55 ± 19* | 150 ± 24 | 37 ± 7* | 156 ± 7 | 30 ± 1* | <LLD | <LLD | 5 ± 1 | 18 ± 3* | 9 ± 1 | 52 ± 6* | 373 ± 19 | 2301 ± 113* |
| 6 | 174 ± 7 | 77 ± 2* | 79 ± 43 | 14 ± 1 | 151 ± 16 | 25 ± 0* | 7 ± 2 | 5 ± 0 | 11 ± 8 | 17 ± 1 | 17 ± 2 | 40 ± 3* | 357 ± 82 | 1859 ± 63* |
| 7 | 74 ± 30 | 82 ± 8 | 267 ± 44 | 45 ± 6* | 32 ± 12 | 13 ± 5 | 5 ± 0 | 4 ± 1 | 18 ± 1 | 18 ± 3 | 13 ± 1 | 27 ± 3* | 362 ± 50 | 1751 ± 116* |
| 8 | 1235 ± 197 | 314 ± 27* | 99 ± 6 | 17 ± 5* | 1047 ± 111 | 518 ± 38* | 4 ± 0 | <LLD | 13 ± 2 | 12 ± 1 | 7 ± 0 | 19 ± 1* | 153 ± 6 | 174 ± 18 |
| 9 | 812 ± 134 | 355 ± 48* | 350 ± 51 | 86 ± 23* | 178 ± 17 | 55 ± 19* | 4 ± 0 | <LLD | 6 ± 0 | 4 ± 1 | <LLD | 6 ± 2 | 351 ± 55 | 1115 ± 125* |
| 10 | 128 ± 39 | 220 ± 41 | 679 ± 167 | 316 ± 66 | 70 ± 10 | 20 ± 3* | 4 ± 1 | <LLD | 4 ± 1 | 5 ± 1 | 3 ± 2 | 10 ± 4 | 464 ± 81 | 1529 ± 62* |
| 11 | 550 ± 43 | 49 ± 13* | 349 ± 36 | 33 ± 11* | 15 ± 8 | 35 ± 2* | 9 ± 0 | 4 ± 1* | 12 ± 1 | 6 ± 1* | 9 ± 1 | 30 ± 4* | 340 ± 25 | 542 ± 17* |
| 12 | 382 ± 14 | 262 ± 30* | 177 ± 49 | 58 ± 2 | 142 ± 22 | 94 ± 17 | 4 ± 0 | <LLD | 5 ± 2 | 5 ± 2 | 4 ± 0 | <LLD | 222 ± 49 | 160 ± 20 |
| Mean ± SEM† | 623 ± 104 | 170 ± 20 | 226 ± 31 | 68 ± 15 | 346 ± 76 | 127 ± 31 | 5 ± 0 | 9 ± 3 | 9 ± 1 | 53 ± 19 | 8 ± 1 | 68 ± 39 | 305 ± 19 | 1720 ± 194 |
| P | <.001 | <.001 | <.010 | =.106 | =.030 | =.132 | <.001 | |||||||
PBLs were cultured for 16 hours with DMXAA (300 µg/ml) or without treatment. Cytokine concentrations in supernatants were determined using multiplex cytokine assays. Means ± SEM of triplicate treated and untreated cultures for each cytokine and for each donor were compared using Student's t tests.
LLD indicates lower limits of detection.
P < .05.
Mean ± SEM of all untreated or treated cultures (n = 36, triplicate cultures/donor x 12 donors). P values shown reflect a comparison of treated and untreated cultures using paired t tests.
Figure 6.
Fold change in cytokine concentrations in supernatants from untreated and DMXAA-treated (16 hours, 300 µg/ml) PBL cultures from individual donors. NC indicates no change.
Discussion
The results here are the first to demonstrate a large influx of neutrophils into subcutaneously implanted Colon 38 tumors at a time when T and B lymphocytes, NK cells, and macrophages were all decreasing in numbers after DMXAA treatment (Figure 1). Activated neutrophils have been strongly implicated as mediators of endothelial cell damage and killing during inflammation [17]. Our observations here suggest that neutrophils may play a role in the antivascular effects of DMXAA. Endothelial cell apoptosis is seen in Colon 38 tumors within 30 minutes of DMXAA administration [18], although tumor vascular collapse is not measurable until 4 hours and is maximal after 24 hours [19,20]. The early influx of neutrophils into the tumor could be a response to the endothelial cell damage. Increased myeloperoxidase activity, indicative of increased neutrophil activity, was also reported in murine sarcomas treated with another vascular disrupting agent, combretastatin A-4 phosphate [21]. With DMXAA, however, the production of chemokines that include MCP-1, MIP-1α, KC, RANTES, and IP-10 in the tumor (Figure 3) may amplify the initial influx, creating a more sustained antivascular action.
The results in Figure 3 confirm our previous studies [5–7] stating that higher levels of TNFα are induced by DMXAA in the Colon 38 tumor than in the spleen or serum. In a rat model of chemically induced primary mammary adenocarcinomas, DMXAA also induced a significantly higher production of TNFα in the tumor than in the serum [22]. In addition to its direct antivascular effects, TNFα has been shown to promote adhesion and transmigration of neutrophils into sites of inflammation by up-regulating the expression of cellular adhesion molecules on endothelial cells [23]. Tumor necrosis factor α may also activate neutrophils directly, as antibodies to TNFα applied to cultures of human neutrophils inhibited production of reactive oxygen species [24]. The studies here indicate a potential role of TNFα-activated neutrophils in the antivascular action of DMXAA in rodent models.
Although TNFα has been the most studied, the multiplex assays here show that TNFα concentrations are much lower than those of IL-6, MCP-1, and MIP-1α that have been induced with DMXAA (Figure 3). The role that each cytokine plays in the antitumor action of DMXAA has not been fully investigated. It is likely that they all play a part. Mice deficient in the expression or response to a given cytokine all show restricted or reduced antitumor activity in response to DMXAA. Colon 38 tumors in IFN-γ receptor knockout mice regressed more slowly and required higher doses of DMXAA than in wild-type mice [25]. The antitumor activity in TNFα knockout and TNFα receptor 1 knockoutmice was also attenuated, requiring higher doses of DMXAA to achieve a similar degree of hemorrhagic necrosis and cures in Colon 38 tumors compared with that in wild-type mice [7]. Growth inhibition against Lewis lung carcinomas was not observed in IFN-β knockout mice at a dose of DMXAA that produced a modest growth delay in wild-type mice [26]. IFN-β is abundantly produced by murine macrophages in response to DMXAA [8,27], but this cytokine is unfortunately not available in the multiplex cytokine assays for inclusion into these studies. Up-regulation of IFN-β messenger RNA expression was not detected in Colon 38 tumors after DMXAA treatment, however [10].
The central role of B lymphocytes in the host cell infiltrate in chronic inflammation and carcinogenesis has lately been recognized [28]. We show here that B lymphocytes constitute approximately 12% of the leukocyte infiltrate in Colon 38 tumors. B lymphocytes were shown to be the primary producers of IP-10 in the response to DMXAA (Figure 4). Along with macrophages, B lymphocytes also produced high amounts of MIP-1α, one of the more abundantly induced chemokines after DMXAA treatment in mice. Macrophages were the primary source of TNFα and IL-6 (Figure 4). Natural killer cells were the main producers of RANTES, whereas both NK cells and CD8+ T lymphocytes produced IFN-γ in response to DMXAA (Figure 4). T lymphocytes on the whole did not seem to be major contributors to the cytokine response, consistent with the limited detection of T-cell cytokines such as IL-2 in the response to DMXAA.
B lymphocytes and macrophages required lower concentrations of DMXAA than NK and T lymphocytes for maximal cytokine production (Figure 3). These results establish that different cell types exhibit different dose dependencies for DMXAA. They also explain our earlier observations that maximal production of TNFα (by macrophages) was obtained at 10 µg/ml, whereas maximal IFN-γ production (from NK and T cells) was obtained using 300 µg/ml of DMXAA [16]. The differential dose requirements of the various cell types could be due to the differential expression of the yet unidentified receptor for DMXAA. Cytokine induction by DMXAA seems not to involve Toll-like receptors and is MyD88-independent [12,27,29]. Tumor necrosis factor α and IFN-γ production and nuclear factor κB (NF-κB) activation were concomitantly blocked using NF-κB inhibitors salicylate and parthenolide in DMXAA-treated murine splenocyte cultures, implicating the involvement of signaling through NF-κB [9]. Conversely, up-regulation of IFN-β gene transcription by DMXAA in primary murine macrophages was critically dependent on the TANK-binding kinase 1-interferon regulatory factor 3 signaling axis and did not seem to involve NF-κB [27]. Current studies in our laboratory defining the molecular mode of action of DMXAA indicate that multiple targets and signaling pathways may be involved.
The cytokines induced with DMXAA in murine PBL cultures was similar to that obtained in the serum of mice after DMXAA treatment (Figures 3 and 4). This observation suggested that the in vitro activity can be indicative of the in vivo response. With this perspective, the response of cultured human PBLs was examined in an effort to obtain the determinants of the cytokine response to DMXAA in humans. The studies have clearly demonstrated that DMXAA affects cytokine production in human PBLs. They also demonstrate that the pattern of regulation by DMXAA on human and murine PBLs may be considerably different. One major difference is that human PBLs produced high quantities of a number of cytokines in culture without treatment (Figure 5 and Table 1), whereas constitutive cytokine production by murine PBLs without treatment was minimal (Figure 5A). DMXAA was shown to downregulate the production of some of the constitutively produced cytokines, notably IP-10, MCP-1, and sCD40L. At the same time, other cytokines, which include IL-8 and MIP-1α, were upregulated by DMXAA. The inhibitory action of DMXAA is not apparent in studies with murine PBLs because they are not constitutively producing cytokines in culture without an added stimulus. Whether DMXAA would inhibit cytokine production in murine leukocytes if they were constitutively activated is not known. The simultaneous yet seemingly opposing regulatory actions of DMXAA on human PBLs could be explained on the basis that different cell types producing the various cytokines are differentially regulated by DMXAA. Differential responses to DMXAA by different subsets of murine splenocytes were established in the studies shown in Figure 3, and studies with fractionated subpopulations of human PBLs are planned.
Another notable difference between the murine and human response to DMXAA is the modest or insignificant effects on IL-6 and TNFα in human PBLs. The low induction of TNFα seen in this study is compatible with that in previous studies of TNFα induction by DMXAA in human PBLs [30,31] and with data from the clinical trials [32–34]. On the basis of studies on rodents [5–7,22], TNFα only was evaluated as a surrogate marker of activity in the phase 1 and 2 trials of DMXAA [30–32]. The results here show significant increases in IL-8 concentrations in our cohort of 12 donors, and IL-8 may be a more dependable marker than TNFα. However, because of the complexity of the cytokine response and the differential responses of the various cell types in the blood, we suggest that monitoring the effects on a panel of cytokines would be more appropriate. The panel that we have derived from the analysis of the data from the large multiplex screens includes IP-10, MCP-1, sCD40L, IL-8, and MIP-1. Tumor necrosis factor α and IL-6 were also included for comparisons with murine studies and with earlier studies in humans. Presentation of the fold change in the concentration of this panel of cytokines provided a relatively uncomplicated way to compare or rank the responsiveness of the donors. The studies with our small cohort of 12 donors suggest considerable variability between individuals in the response of the PBLs in culture to DMXAA (Figure 6). Determination of whether the responsiveness of their PBLs in culture correlates with a patient's responsiveness to DMXAA treatment is clearly outside the scope of our studies. The phase 3 trials of DMXAA, however, would provide an excellent opportunity for such determinations to be made.
Abbreviations
- DMXAA
5,6-dimethylxanthenone-4-acetic acid
- FITC
fluorescein isothiocyanate
- LLD
lower limits of detection
- NK
natural killer
- PBL
peripheral blood leukocyte
- PE
phycoerythrin
- TNFα
tumor necrosis factor α
Footnotes
This work was funded by a project grant from the Health Research Council of New Zealand and by the Auckland Division of the Cancer Society of New Zealand.
References
- 1.Rewcastle GW, Atwell GJ, Li ZA, Baguley BC, Denny WA. Potential antitumor agents. 61. Structure-activity relationships for in vivo colon 38 activity among disubstituted 9-oxo-9H-xanthene-4-acetic acids. J Med Chem. 1991;34:217–222. doi: 10.1021/jm00105a034. [DOI] [PubMed] [Google Scholar]
- 2.Plowman J, Narayanan VL, Dykes D, Szarvasi E, Briet P, Yoder OC, Paull KD. Flavone acetic acid: a novel agent with preclinical antitumor activity against colon adenocarcinoma 38 in mice. Cancer Treat Rep. 1986;70:631–635. [PubMed] [Google Scholar]
- 3.Baguley BC, Calveley SB, Crowe KK, Fray LM, O'Rourke SA, Smith GP. Comparison of the effects of flavone acetic acid, fostriecin, homoharringtonine and tumour necrosis factor alpha on colon 38 tumours in mice. Eur J Cancer Clin Oncol. 1989;25:263–269. doi: 10.1016/0277-5379(89)90018-7. [DOI] [PubMed] [Google Scholar]
- 4.Ching LM, Goldsmith D, Joseph WR, Korner H, Sedgwick JD, Baguley BC. Induction of intratumoral tumor necrosis factor (TNF) synthesis and hemorrhagic necrosis by 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF knockout mice. Cancer Res. 1999;59:3304–3307. [PubMed] [Google Scholar]
- 5.Cao Z, Joseph WR, Browne WL, Mountjoy KG, Palmer BD, Baguley BC, Ching LM. Thalidomide increases both intra-tumoural tumour necrosis factor-α production and anti-tumour activity in response to 5,6-dimethylxanthenone-4-acetic acid. Br J Cancer. 1999;80:716–723. doi: 10.1038/sj.bjc.6690415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph WR, Cao Z, Mountjoy KG, Marshall ES, Baguley BC, Ching LM. Stimulation of tumors to synthesize tumor necrosis factor-alpha in situ using 5,6-dimethylxanthenone-4-acetic acid: a novel approach to cancer therapy. Cancer Res. 1999;59:633–638. [PubMed] [Google Scholar]
- 7.Zhao L, Ching LM, Kestell P, Baguley BC. The antitumour activity of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice. Br J Cancer. 2002;87:465–470. doi: 10.1038/sj.bjc.6600479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera PY, Barber SA, Ching LM, Vogel SN. Activation of LPS-inducible genes by the antitumor agent 5,6-dimethylxanthenone-4-acetic acid in primary murine macrophages. Dissection of signaling pathways leading to gene induction and tyrosine phosphorylation. J Immunol. 1994;153:4684–4693. [PubMed] [Google Scholar]
- 9.Wang L-CS, Woon S-T, Baguley BC, Ching L-M. Inhibition of DMXAA-induced tumor necrosis factor production in murine splenocyte cultures by NF-kappaB inhibitors. Oncol Res. 2006;16:1–14. doi: 10.3727/000000006783981288. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Baguley BC, Ching LM. Interferon-inducible protein 10 induction and inhibition of angiogenesis in vivo by the antitumor agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Cancer Res. 2001;61:1517–1521. [PubMed] [Google Scholar]
- 11.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching L-M, Kaiser LR, et al. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 12.Wallace A, LaRosa DF, Kapoor V, Jing S, Cheng G, Jassar A, Blouin A, Ching L-M, Albelda SM. The vascular disrupting agent, DMXAA, directly activates dendritic cells through a MyD88-independent mechanism and generates antitumor cytotoxic T lymphocytes. Cancer Res. 2007;67:7011–7019. doi: 10.1158/0008-5472.CAN-06-3757. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd CM, Phillips ARJ, Cooper GJS, Dunbar PR. Three-colour fluorescence immunohistochemistry reveals the diversity of cells staining for macrophage markers in murine spleen and liver. J Immunol Methods. 2008;334:70–81. doi: 10.1016/j.jim.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Angel CE, Chen CJ, Horlacher OC, John T, Browning J, MacGregor D, Cebon J, Dunbar PR. Distinctive localization of antigen presenting cells in human lymph nodes. Blood. 2009;113:1257–1267. doi: 10.1182/blood-2008-06-165266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730–5734. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- 16.Wang LC, Reddy CB, Baguley BC, Kestell P, Sutherland R, Ching LM. Induction of tumour necrosis factor and interferon-gamma in cultured murine splenocytes by the antivascular agent DMXAA and its metabolites. Biochem Pharmacol. 2004;67:937–945. doi: 10.1016/j.bcp.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 18.Ching LM, Cao Z, Kieda C, Zwain S, Jameson MB, Baguley BC. Induction of endothelial cell apoptosis by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Br J Cancer. 2002;86:1937–1942. doi: 10.1038/sj.bjc.6600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwi LJ, Baguley BC, Gavin JB, Wilson WR. Correlation between immune and vascular activities of xanthenone acetic acid antitumor agents. Oncol Res. 1994;6:79–85. [PubMed] [Google Scholar]
- 20.Lash CJ, Li AE, Rutland M, Baguley BC, Zwi LJ, Wilson WR. Enhancement of the anti-tumour effects of the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) by combination with 5-hydroxytryptamine and bioreductive drugs. Br J Cancer. 1998;78:439–445. doi: 10.1038/bjc.1998.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkins CS, Holder AL, Hill SA, Chaplin DJ, Tozer GM. Determinants of anti-vascular action by combretastatin A-4 phosphate: role of nitric oxide. Br J Cancer. 2000;83:811–816. doi: 10.1054/bjoc.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lui JJ, Ching L-M, Goldthorpe M, Sutherland R, Baguley BC, Kirker JA, McKeage MJ. Antitumour action of 5,6-dimethylxanthenone-4-acetic acid in rats bearing chemically induced primary mammary tumours. Cancer Chemother Pharmacol. 2007;59:661–669. doi: 10.1007/s00280-006-0321-7. [DOI] [PubMed] [Google Scholar]
- 23.Schall TJ, Bacon KB. Chemokines, leucocyte trafficking, and inflammation. Current Opinion Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 24.Pay S, Musabak U, Erdem H, Simsek I, Pekel A, Sengul A, Dinc A. Chimerical anti-TNF-alpha, infliximab, inhibits neutrophil chemotaxis and production of reactive oxygen species by blocking the priming effect of mononuclear cells on neutrophils. Immunopharmacol Immunotoxicol. 2005;27:187–198. doi: 10.1081/iph-200067702. [DOI] [PubMed] [Google Scholar]
- 25.Pang J-H, Cao Z, Joseph WR, Baguley BC, Ching L-M. Antitumor activity of the novel immune modulator 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in mice lacking the interferon-gamma receptor. Eur J Cancer. 1998;34:1282–1289. doi: 10.1016/s0959-8049(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 26.Roberts ZJ, Ching L-M, Vogel SN. IFN-β-dependent inhibition of tumor growth by the vascular disrupting agent 5,6-dimethyxanthenone-4-acetic acid (DMXAA) J Inteferon Cytokine Res. 2008;28:133–139. doi: 10.1089/jir.2007.0992. [DOI] [PubMed] [Google Scholar]
- 27.Roberts ZJ, Goutagny N, Perera P-Y, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Roberts ZJ, Toshchakov V, Cuesta M, Thomas KE, Polumuri S, Rallabhandi P, Ching LM, Vogel SN. Utilization of known TLR signaling pathways in the activation of murine macrophages by the inflammatory anti-tumor agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) J Leukoc Biol Suppl. 2004;76:32. [abstract 43] [Google Scholar]
- 30.Patel S, Parkin S, Bibby MC. The effect of 5,6-dimethylxanthenone-4-acetic acid on tumour necrosis factor production by human immune cells. Anticancer Res. 1997;17:141–150. [PubMed] [Google Scholar]
- 31.Philpott M, Joseph WR, Crosier KE, Baguley BC, Ching L-M. Production of tumour necrosis factor-α by cultured human peripheral blood leucocytes in response to the anti-tumour agent 5,6-dimethylxanthenone-4-acetic acid (NSC 640488) Br J Cancer. 1997;76:1586–1591. doi: 10.1038/bjc.1997.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKeage MJ, Fong P, Jeffrey M, Baguley BC, Kestell P, Ravic M, Jameson MB. 5,6-Dimethylxanthenone-4-acetic acid in the treatment of refractory tumors: a phase I safety study of a vascular disrupting agent. Clin Cancer Res. 2006;12:1776–1784. doi: 10.1158/1078-0432.CCR-05-1939. [DOI] [PubMed] [Google Scholar]
- 33.Jameson M, Thompson P, Baguley B, Evans B, Harvey V, Porter D, McCrystal M, Kestell P. Phase I pharmacokinetic and pharmacodynamic study of 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent. Proc Am Soc Clin Oncol. 2000;19 [abstract 705] [Google Scholar]
- 34.Rustin GJS, Bradley C, Galbraith S, Stratford M, Loadman P, Waller S, Bellenger K, Gumbrell L, Folkes L, Halbert G. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent: phase I clinical and pharmacokinetic study. Br J Cancer. 2003;88:1160–1167. doi: 10.1038/sj.bjc.6600885. [DOI] [PMC free article] [PubMed] [Google Scholar]