Abstract
Because evasion of apoptosis can cause radioresistance of glioblastoma, there is a need to design rational strategies that counter apoptosis resistance. In the present study, we investigated the potential of targeting the antiapoptotic protein XIAP for the radiosensitization of glioblastoma. Here, we report that small-molecule XIAP inhibitors significantly enhance γ-irradiation-induced loss of viability and apoptosis and cooperate with γ-irradiation to suppress clonogenic survival of glioblastoma cells. Analysis of molecular mechanisms reveals that XIAP inhibitors act in concert with γ-irradiation to cause mitochondrial outer membrane permeabilization, caspase activation, and caspase-dependent apoptosis. Importantly, XIAP inhibitors also sensitize primary cultured glioblastoma cells derived from surgical specimens as well as glioblastoma-initiating stemlike cancer stem cells for γ-irradiation. In contrast, they do not increase the toxicity of γ-irradiation on some nonmalignant cells of the central nervous system, including rat neurons or glial cells, pointing to some tumor selectivity. In conclusion, by demonstrating for the first time that small-molecule XIAP inhibitors increase the radiosensitivity of glioblastoma cells while sparing normal cells of the central nervous system, our findings build the rationale for further (pre)clinical development of XIAP inhibitors in combination with γ-irradiation in glioblastoma.
Introduction
Glioblastoma is the most common primary brain tumor and a highly aggressive malignancy with a very poor prognosis [1]. Despite intensive treatment protocols, the resistance of glioblastoma to current regimens including radiotherapy represents an ongoing challenge [2]. This highlights the need to develop novel approaches to overcome radioresistance of glioblastoma to improve the dismal prognosis of this cancer [3].
Apoptosis is the cell's intrinsic death program that controls normal tissue homeostasis [4]. Apoptosis pathways can be initiated through death receptors or mitochondria and usually results in activation of caspases as common effector molecules [4]. The mitochondrial pathway of apoptosis is engaged by the release of cytochrome c and second mitochondria-derived activator of caspase (Smac)/direct IAP binding protein with low pI (DIABLO) from mitochondria into the cytosol [5,6]. Cytochrome c triggers caspase-3 activation through the formation of the apoptosome complex, whereas Smac/DIABLO promotes apoptosis by neutralizing inhibitor of apoptosis (IAP) proteins [5].
Evasion of apoptosis is one of the hallmarks of human cancers including glioblastoma [7]. Also, defects in apoptosis pathways contribute to chemoresistance or radioresistance because therapy-induced cytotoxicity is mediated to a large extent by the induction of cell death including apoptosis in cancer cells [8]. Apoptosis signaling may be disrupted by the aberrant expression of antiapoptotic proteins [9]. For example, most human cancers harbor high levels of IAP proteins including XIAP [10]. Aberrant expression of IAPs in tumor cells has been associated with treatment resistance and dismal prognosis [10]. Therefore, therapeutic targeting of IAPs such as XIAP may offer new possibilities to bypass resistance, for example, resistance to radiation-induced cell death.
In a proof-of-concept study, we previously demonstrated that Smac peptides, which antagonize XIAP, sensitize glioblastoma cells for TRAIL-induced apoptosis in vitro and in vivo [11]. Further, we reported that genetic inactivation of XIAP increases radiation-induced apoptosis in neuroblastoma and pancreatic carcinoma cells [12,13]. To translate the concept of targeting XIAP for radiosensitization into a clinically applicable approach to improve the efficacy of radiotherapy in glioblastoma, in the present study, we evaluated the therapeutic potential of small-molecule XIAP inhibitors for the radiosensitization of glioblastoma.
Materials and Methods
Cell Culture and Reagents
Glioblastoma cell lines were obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM) or RPMI 1640 (Life Technologies, Inc, Eggenstein, Germany) supplemented with 10% fetal calf serum (FCS; Biochrom, Berlin, Germany), 1 mM glutamine (Biochrom), 1% penicillin/streptavidin (Biochrom), and 25 mM HEPES (Biochrom) as described [14]. Primary cultured glioblastoma cells and glioblastoma-initiating cells were cultured as described [14,15]. The study was approved by the Ethics Committee, Medical Faculty, University of Ulm. Hippocampal rat neurons were prepared and cultured as described [16], seeded at 5 x 104 cells/cm2 in 24-well plates and irradiated on day 7. Rat glial cells from the cerebral cortex were prepared and cultured as described [17] and seeded at 5 x 104 cells/cm2 in 96-well plates after irradiation. Animal experiments were performed in accordance with institutional and national regulations; research protocols were approved by relevant authorities. XIAP inhibitor 1, XIAP inhibitor 2, and control compound correspond to compounds 2, 11, and 15, respectively, as described by Oost et al. [18] and were kindly provided by IDUN Pharmaceuticals now Pfizer, Inc (Groton, CT). XIAP inhibitors are capped tripeptides consisting of unnatural amino acids that were designed on the basis of the nuclear magnetic resonance structure of a Smac peptide bound to the BIR3 domain of XIAP and bound to XIAP BIR3 with high nanomolar affinities [18]. A close structural analog that weakly binds to XIAP served as control [18]. All chemicals were purchased by Sigma (Deisenhofen, Germany) unless indicated otherwise.
Determination of Apoptosis, Cell Viability, and Clonogenic Survival
Cells were treated with γ-irradiation (Cs-137, 44 Tbq, 4 Gy/min; Nuclear Data, Frankfurt, Germany) at indicated doses and incubated for the indicated times in the presence of XIAP inhibitors, control compound, or solvent without replenishment of medium or XIAP inhibitors. Apoptosis was determined by fluorescence-activated cell-sorting analysis (FACScan; BD Biosciences, Heidelberg, Germany) of DNA fragmentation of propidium iodide-stained nuclei as described [19]. The percentage of specific apoptosis is calculated as follows: 100 x [(experimental apoptosis - spontaneous apoptosis)/(100 - spontaneous apoptosis)]. Spontaneous apoptosis refers to the rate of apoptosis of untreated cells. Cell viability was assessed by MTT assay according to the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany). For clonogenic assay, cells were seeded at 0.017 x 103 cells/cm2 after irradiation, and colony formation after 3 weeks was assessed by crystal violet staining (0.75% crystal violet, 50% ethanol, 0.25% NaCl, 1.57% formaldehyde).
Determination of Mitochondrial Membrane Potential and Cytochrome c Release
CMXRos (1 µM; Molecular Probes, Karlsruhe, Germany) was used to measure the mitochondrial transmembrane potential. Cells were incubated for 30 minutes at 37°C in the presence of the fluorochrome and immediately analyzed by flow cytometry. Cytochrome c release was determined in permeabilized cells using mouse anti-cytochrome c monoclonal antibody (BD Biosciences) as described [20].
Western Blot Analysis
Western blot analysis was performed as described previously [14] using the following antibodies: mouse anti-caspase-8 (ApoTech Corp, Epalinges, Switzerland); rabbit anti-caspase-3 (Cell Signaling, Beverly, MA); rabbit anti-caspase-9 and mouse anti-XIAP (BD Biosciences); rabbit anti-cIAP2, goat anti-cIAP1, and rabbit antisurvivin (R&D Systems, Inc, Wiesbaden, Germany); or mouse anti-β-actin (Sigma) followed by goat-antimouse or goat-antirabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). Enhanced chemiluminescence was used for detection (Amersham Bioscience, Freiburg, Germany).
Caspase Activity
Caspase activity was determined in living nonfixed nonlysed cells as described [13] using a caspase-3 substrate conjugated to rhodamine R110 (N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone-R110 [zDEVD-R110]) from Molecular Probes. The broad-range caspase inhibitor zVAD.fmk (Bachem,Heidelberg, Germany) was used for the inhibition of caspases.
Immunohistochemistry
For immunohistochemical staining, cells were seeded on poly-l-lysine-coated coverslips (Karl Hecht KG, Sondheim, Germany), incubated for 3 hours at 37°C, and stained with mouse anti-CD133/1 antibody (1:10; Miltneyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. For nestin staining, cells were fixed for 10 minutes with 4% paraformaldehyde, incubated for 30 minutes with blocking buffer (2% BSA, 0.1% horse serum, and 0.1% Triton X-100), and stained with mouse anti-nestin antibody (1:100; Chemicon, Schwalbach, Germany) overnight at 4°C followed by staining with goat-antimouse IgG-fluorescein isothiocyanate antibody (1:1000; Chemicon) and 4′-6-diamidino-2-phenylindole. Slides were mounted using Vecta shield mounting medium (Vector Laboratories, Burlingame, CA). Negative controls were performed by omitting the first step with primary antibody and yielded negative results. Slides were analyzed using an immunofluorescence microscope (Olympus, Hamburg, Germany).
Statistical Analysis
Statistical significance was assessed by Student's t test using Winstat software (R. Fitch Software, Bad Krozingen, Germany).
Results
Sensitization of Glioblastoma Cells for γ-Irradiation-Induced Apoptosis by XIAP Inhibitors
To explore the therapeutic potential of small-molecule XIAP inhibitors for the radiosensitization of glioblastoma, we selected the glioblastoma cell lines U87MG, A172, T98G, and U118MG, which all harbor PTEN mutation and are p53 wild type (U87MG and A172) or p53 mutant (T98G and U118MG) [21]. We used small-molecule XIAP inhibitors that were designed against the BIR3 domain of XIAP based on the nuclear magnetic resonance structure of a Smac peptide bound to XIAP BIR3 [18]. A close structural analog that weakly binds to XIAP served as a control [18]. First, we investigated the effects of XIAP inhibitors as single agents on cell viability of glioblastoma cells. XIAP inhibitors exerted no detectable effect on cell viability of glioblastoma cells even upon prolonged treatment for 144 hours (Figure W1).
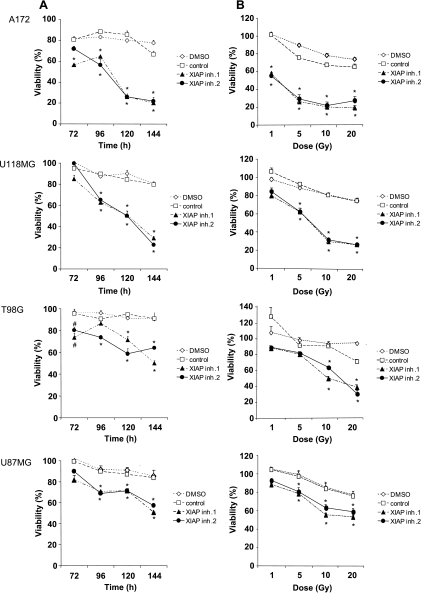
Next, we tested the effect of XIAP inhibitors in combination with γ-irradiation in glioblastoma cells. Importantly, XIAP inhibitors significantly enhanced γ-irradiation-induced loss of viability in a time- and dose-dependent manner (Figure 1, A and B). By comparison, glioblastoma cells displayed low sensitivity to γ-irradiation alone, in line with previous findings [22]. These results demonstrate that inhibition of XIAP by small-molecule inhibitors radiosensitizes glioblastoma cells.
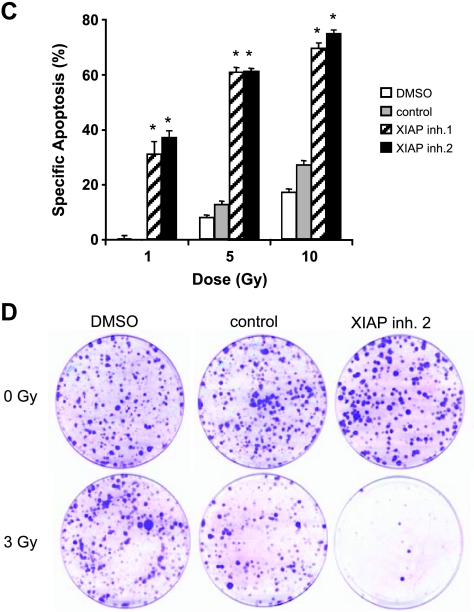
Figure 1.
XIAP inhibitors enhance γ-irradiation-induced apoptosis in glioblastoma cells. (A and B) Glioblastoma cells were treated for the indicated times with 10 Gy of γ-irradiation (A) or for 144 hours with indicated doses of γ-irradiation and/or 10 µM XIAP inhibitors, control compound, or solvent (B). Cell viability was determined by MTT assay and expressed as a percentage of untreated controls. (C) A172 cells were treated for 144 hours with indicated doses of γ-irradiation and/or 10 µM XIAP inhibitors, control compound, or solvent. Apoptosis was determined by FACS analysis of DNA fragmentation of propidium iodide-stained nuclei. (D) Clonogenic survival after treatment with 3 Gy of γ-irradiation and/or 10 µM XIAP inhibitor 2, control compound, or DMSO was assessed by crystal violet staining. A representative experiment of three independent experiments is shown. In A to C, mean ± SEM of three independent experiments performed in triplicate are shown; #P < .05, *P < .01, comparing XIAP inhibitors with solvent (signs above graphs are for XIAP inhibitor 1, signs below graphs are for XIAP inhibitor 2).
To examine radiosensitization of glioblastoma cells by XIAP inhibitors in more detail, we selected A172 glioblastoma cells because γ-irradiation-induced cytotoxicity was strongly enhanced in these cells in the presence of XIAP inhibitors. Analysis of DNA fragmentation as a characteristic feature of apoptotic cell death showed that XIAP inhibitors significantly increased γ-irradiation-induced apoptosis (Figure 1C). The control compound slightly enhanced γ-irradiation-induced apoptosis (Figure 1C), consistent with its strongly reduced ability to bind to the BIR3 domain of XIAP [18]. To investigate whether inhibition of XIAP also affects long-term survival after γ-irradiation, we performed colony assays. Notably, XIAP inhibitors cooperated with γ-irradiation to suppress colony formation showing that they also act in concert with γ-irradiation to reduce long-term survival (Figure 1D). Together, this set of experiments demonstrates that XIAP inhibitors cooperate with γ-irradiation to induce apoptosis and to suppress clonogenic growth of glioblastoma cells.
XIAP Inhibitors Enhance γ-Irradiation-Induced Caspase Activation and Mitochondrial Perturbations
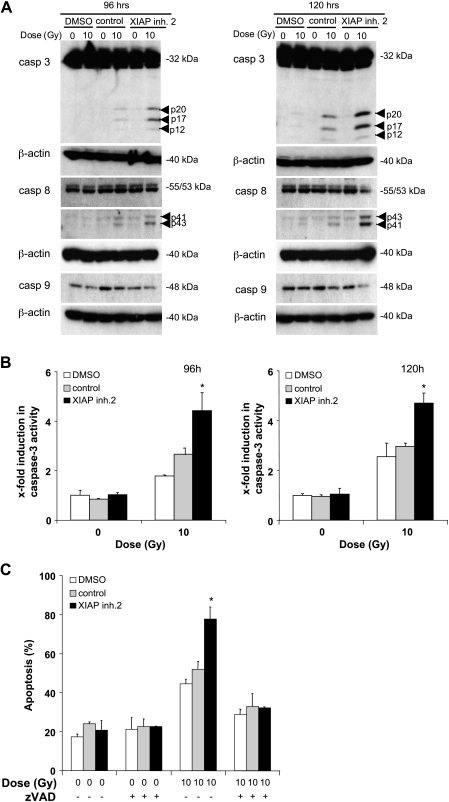
Next, we investigated possible molecular mechanisms that mediate the synergistic action of XIAP inhibitors and γ-irradiation. Because XIAP inhibitors 1 and 2 showed similar activities to enhance γ-irradiation-induced apoptosis (Figure 1), we used XIAP inhibitor 2 for these studies. To explore the involvement of caspases, we assessed activation of the caspase cascade by Western blot analysis. XIAP inhibitor 2 enhanced γ-irradiation-induced cleavage of caspase-3 and caspase-8 into active cleavage fragments (Figure 2A). To examine whether the increased cleavage of caspase-3 also translates into enhanced enzymatic caspase activity, we performed caspase activity assays. Similarly, XIAP inhibitor 2 significantly increased γ-irradiation-induced caspase-3 activity compared with cells that were exposed to γ-irradiation in the absence of the XIAP inhibitor (Figure 2B). To test whether caspase activity was required for apoptosis induction, we used the broad-range caspase inhibitor zVAD.fmk. Importantly, apoptosis in response to the combination treatment with XIAP inhibitor 2 and γ-irradiation was significantly reduced in the presence of zVAD.fmk, demonstrating that apoptosis occurred in a caspase-dependent manner (Figure 2 C).
Figure 2.
XIAP inhibitor enhances γ-irradiation-induced activation of caspases. (A and B) A172 cells were treated with 10 Gy of γ-irradiation and/or 10 µM XIAP inhibitor 2, control compound, or DMSO for the indicated times. Caspase activation was assessed by Western blot analysis (A) and caspase activity was assessed by FACS analysis using rhodamine-conjugated caspase-3 substrate zDEVD-R110 (B); fold induction in caspase-3 activity is shown. (C) A172 cells were treated with 10 Gy of γ-irradiation and/or 10 µM XIAP inhibitor 2, control compound, or DMSO for 144 hours in the presence or absence of 25 µM zVAD.fmk. Apoptosis was determined by FACS analysis of DNA fragmentation of propidium iodide-stained nuclei. Means ± SEM of three independent experiments performed in triplicate are shown; *P < .01 comparing XIAP inhibitor with solvent.
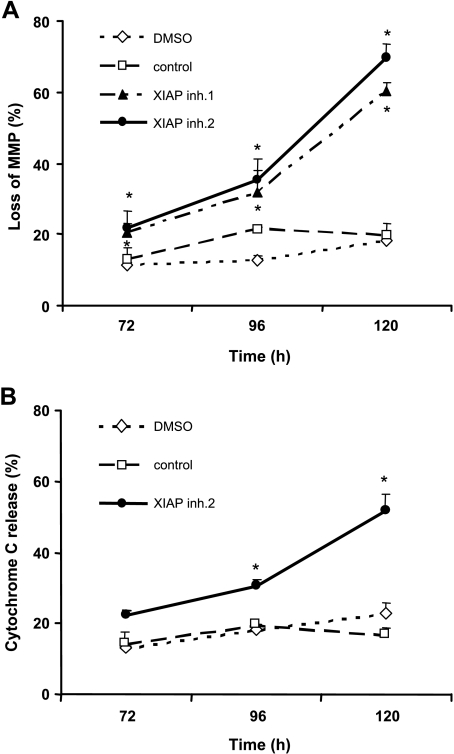
To investigate whether inhibition of XIAP affects the mitochondrial pathway of apoptosis upon γ-irradiation, we assessed mitochondrial membrane potential and cytochrome c release. Interestingly, XIAP inhibitor 2 significantly increased γ-irradiation-induced loss of mitochondrial membrane potential and cytochrome c release from mitochondria in a time-dependent manner (Figure 3). Together, these findings show that inhibition of XIAP enhances γ-irradiation-induced caspase activation and mitochondrial perturbations in glioblastoma cells.
Figure 3.
XIAP inhibitor enhances γ-irradiation-induced mitochondrial perturbations. Glioblastoma cells were treated with 10 Gy of γ-irradiation and/or 10 µM XIAP inhibitor 2, control compound, or DMSO for the indicated times. Mitochondrial membrane potential (MMP; A) and cytochrome c release (B) were assessed by FACS analysis. Means ± SEM of three independent experiments performed in triplicate are shown; *P < .01 comparing XIAP inhibitor with solvent (signs above graphs are for XIAP inhibitor 1, signs below graphs are for XIAP inhibitor 2).
No Cytotoxicity of XIAP Inhibitors in Combination with γ-Irradiation on Nonmalignant Central Nervous System Cells
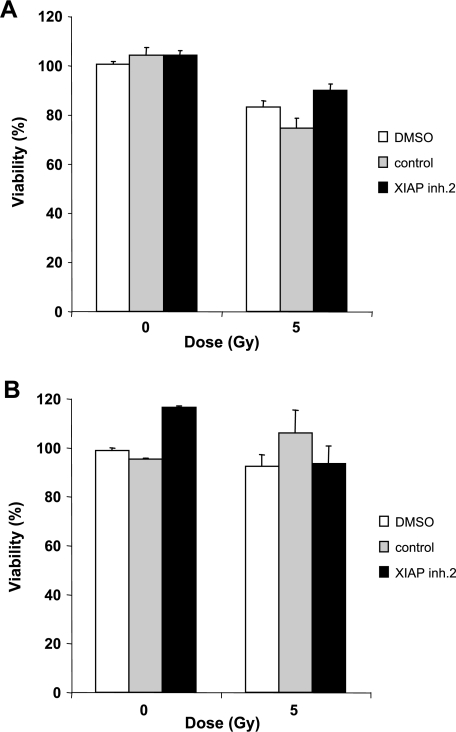
Furthermore, we examined the effect of XIAP inhibitors on normal cells of the central nervous system to test for potential toxic effects. Notably, treatment with XIAP inhibitors as single agents did not alter cell viability of rat neurons or rat glial cells (Figures 4 and W3). In addition, XIAP inhibitors did not increase the sensitivity of these cells toward γ-irradiation (Figures 4 and W3). By comparison, XIAP inhibitors and γ-irradiation showed cross-species activity against rat glioblastoma cells (data not shown), further supporting the notion that XIAP inhibitors preferentially sensitize malignant over nonmalignant cells to γ-irradiation. These data demonstrate that XIAP inhibitors do not exert detectable toxicity on some normal cells of the central nervous system and also do not enhance the toxicity of γ-irradiation on these nonmalignant cells at concentrations that sensitize glioblastoma cells for γ-irradiation.
Figure 4.
Effect of XIAP inhibitors in combination with γ-irradiation on nonmalignant central nervous system cells. Rat neurons (A) and rat glial cells (B) were treated with 5 Gy of γ-irradiation and/or 10 µM XIAP inhibitor 2, control compound, or DMSO for 144 hours. Cell viability was determined by MTT assay and expressed as a percentage of untreated controls. Means ± SEM of three independent experiments performed in triplicate are shown.
XIAP Inhibitors Sensitize Primary Cultured Glioblastoma Cells and Glioblastoma-Initiating Cells for γ-Irradiation
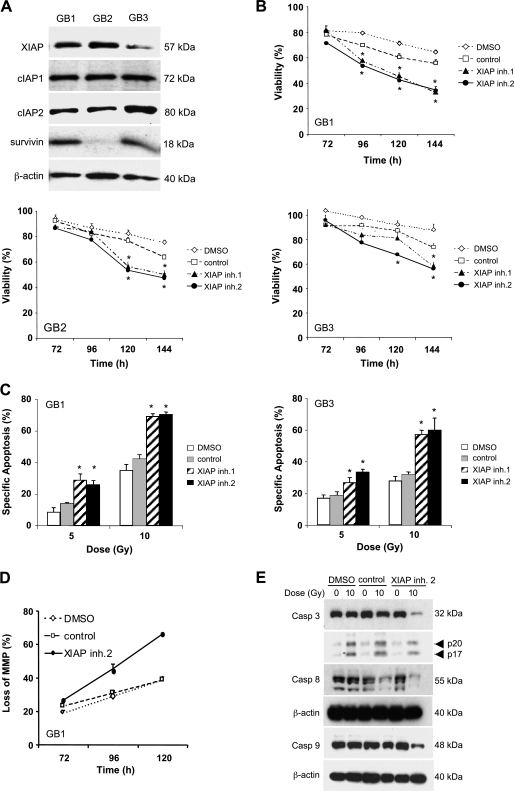
To test the potential clinical relevance of our findings, we extended our studies to primary cultured glioblastoma samples obtained from surgical specimens, which we previously characterized [14]. Primary cultured glioblastoma cells expressed considerable levels of IAP proteins, including XIAP, cIAP1, cIAP2, and survivin, with the exception of GB2, which displayed low levels of survivin (Figure 5A). Importantly, XIAP inhibitors significantly increased γ-irradiation-induced loss of viability also in primary cultured glioblastoma cells (Figure 5B). In addition, XIAP inhibitors acted in concert with γ-irradiation to trigger apoptosis, loss of mitochondrial membrane potential, and caspase activation in these cells (Figure 5, C–E).
Figure 5.
XIAP inhibitors sensitize primary cultured glioblastoma cells for γ-irradiation. (A) Protein expression of XIAP, cIAP1, cIAP2, and survivin were assessed in primary cultured glioblastoma cells (GB1, GB2, and GB3) by Western blot analysis; β-actin was used as loading control. (B) Primary cultured glioblastoma cells were treated with 10 Gy of γ-irradiation and/or 10 µM XIAP inhibitors, control compound, or DMSO for the indicated times. Cell viability was determined by MTT assay and expressed as a percentage of untreated controls. (C–E) Primary cultured glioblastoma cells were treated with indicated doses of γ-irradiation and/or 10 µM XIAP inhibitors, control compound, or DMSO for 144 hours (C), 96 hours (E), or indicated time points (D). Apoptosis was determined by FACS analysis of DNA fragmentation of propidium iodide-stained nuclei (C), loss of mitochondrial membrane potential by flow cytometry (E), and caspase activation by Western blot analysis (D). In B and C, mean ± SEM of three independent experiments performed in triplicate are shown; *P < .01 comparing XIAP inhibitor with solvent (signs above graphs are for XIAP inhibitor 1, signs below graphs are for XIAP inhibitor 2).
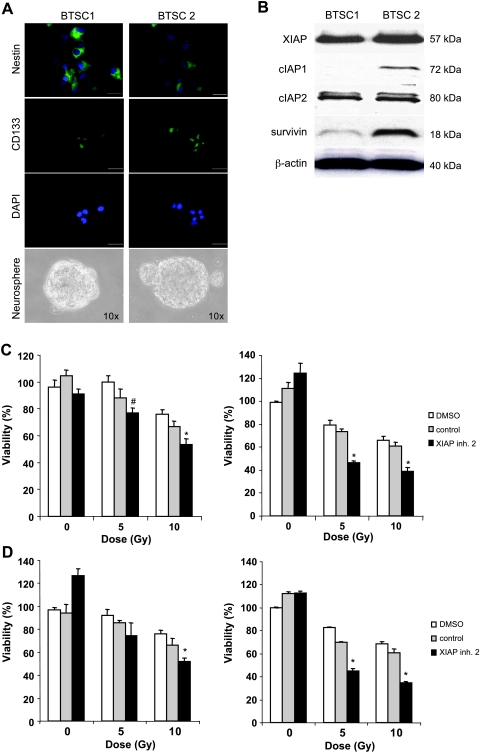
Because glioblastoma stem cells have recently been linked to radioresistance [23,24], we then explored whether XIAP inhibitors modulate the sensitivity of these tumor-initiating cancer stem cells toward γ-irradiation. Glioblastoma-initiating cells were isolated from clinical samples and have been previously described and characterized [15]. Expression of the stem cell markers nestin and CD133, neurosphere formation, and differentiation into neuronal and glial lineages as parameters of glioblastoma-initiating cells are shown (Figures 6A and W2). Analysis of IAP expression showed that cIAP2 and XIAP protein were expressed in both glioblastoma-initiating cell lines, whereas cIAP1 and survivin were detectable at much higher levels in one of the glioblastoma-initiating cell lines (Figure 6B). Intriguingly, XIAP inhibitor 2, but not the control compound, significantly reduced cell viability of glioblastoma-initiating cells upon γ-irradiation (Figure 6, C and D). This set of experiments demonstrates that XIAP inhibitors sensitize primary cultured glioblastoma cells as well as glioblastoma-initiating cells for γ-irradiation.
Figure 6.
XIAP inhibitors sensitize glioblastoma-initiating cells for γ-irradiation. (A and B) Glioblastoma-initiating cells (BTSC1 and BTSC2) were analyzed for nestin and CD133 expression by immunohistochemistry (A, original magnifications: upper panels, x60; middle and lower panels, x40). Sphere-forming ability was analyzed by culture in neural stem cell medium (A, original magnification, x10). The expression of XIAP, cIAP1, cIAP2, and survivin was determined by Western blot analysis (B). (C and D) Glioblastoma-initiating cells (C, BTSC1; D, BTSC2) were treated with the indicated doses of γ-irradiation and/or 10 µM XIAP inhibitors, control compound, or DMSO for 120 hours (left panels) or 168 hours (right panels). Cell viability was determined by MTT assay and expressed as a percentage of untreated controls. Means ± SEM of three independent experiments performed in triplicate are shown; *P < .01 comparing XIAP inhibitor with solvent.
Discussion
Because defects in apoptosis programs, for example, high expression of antiapoptotic molecules, can cause resistance to treatment regimens including radiotherapy [25,26], current attempts to improve the outcome of glioblastoma patients depend on strategies to overcome apoptosis resistance. In the present study, for the first time, we provide evidence that inhibition of the antiapoptotic protein XIAP by small-molecule inhibitors is a novel and effective approach for the radiosensitization of glioblastoma. This conclusion is supported by several independent pieces of evidence. First, small-molecule XIAP inhibitors significantly enhance γ-irradiation-induced cytotoxicity and apoptosis and cooperate with γ-irradiation to suppress clonogenic survival of glioblastoma cell lines. Mechanistic studies reveal that XIAP inhibitors act in concert with γ-irradiation to enhance caspase activation and mitochondrial outer membrane permeabilization. Second, XIAP inhibitors also increase the sensitivity to γ-irradiation in glioblastoma-initiating cells and in primary cultured glioblastoma cells derived from surgical specimens, further underscoring the clinical relevance of the findings. Third, XIAP inhibitors do not enhance the toxicity of γ-irradiation on some nonmalignant cells of the central nervous system, including neurons and glial cells, at concentrations that sensitize glioblastoma cells to γ-irradiation. Together, these findings indicate that inhibition of XIAP by small-molecule inhibitors is a promising strategy to enhance the radiosensitivity of glioblastoma cells with little effect on normal cells of the central nervous system, thus pointing to a therapeutic window.
Our findings have several important implications. First, novel approaches to increase the responsiveness of glioblastoma to current treatment modalities such as radiation open new perspectives for more effective therapeutic options for glioblastoma patients and may ultimately affect patients' survival. Although radiotherapy is one mainstay of glioma therapy, resistance to radiotherapy still presents an unsolved clinical problem [3]. This highlights the need to design innovative strategies to potentiate the efficacy of radiotherapy. Increasing evidence suggests that high expression levels of XIAP are associated with a reduced response to irradiation in several types of cancers, for example, neuroblastoma, pancreatic carcinoma, and lung cancer [12,13,27,28]. Thus, one approach to increase the efficacy of radiotherapy is based on the concept that intact apoptosis signaling pathways are crucial for mediating the antitumor effects of radiotherapy. In line with this notion, our results demonstrate that the neutralization of the antiapoptotic function of XIAP by small-molecule inhibitors enhances the antitumor cytotoxicity of γ-irradiation. The clinical relevance of this strategy is highlighted by the fact that XIAP inhibitors cooperate with γ-irradiation to induce apoptosis not only in glioblastoma cell lines but also in primary cultured glioblastoma cells. Although our study is the first to demonstrate that small-molecule XIAP inhibitors cooperate with γ-irradiation in glioblastoma, our group and other investigators have previously reported that pharmacological inhibition of XIAP enhance radiosensitivity in pancreatic, lung, and breast carcinoma [13,27,29,30]. This points to a broader relevance of the concept to use XIAP inhibitors for the radiosensitization of human cancers.
Second, XIAP inhibitors are also effective to increase the radiosensitivity of glioblastoma-initiating cells. Because it is in particular the cancer stem cell population that is considered to confer radioresistance in glioblastoma [16,23,24], strategies that also target this rare yet highly malignant population in glioblastoma are of foremost relevance. Whereas the preferential activation of the DNA damage response in glioblastoma stem cells has been implied in the resistance to irradiation [23], the molecular mechanisms of radioresistance of glioblastoma stem cells have not exactly been identified. Irrespective of the primary mechanisms of radioresistance of glioblastoma stem cells, our findings demonstrate that enhanced activation of apoptosis effector programs upon irradiation by inhibition of XIAP can increase the radiosensitivity of glioblastoma-initiating cells.
Third, it is feasible that our approach can be translated into clinical application because small-molecule XIAP inhibitors have recently entered clinical trials and are currently evaluated as single agents [10,31]. Therefore, it is a timely question to develop rational XIAP inhibitor-based combination regimens with cooperative antitumor activity for the future clinical development of XIAP inhibitors.
Fourth, the apparent lack of toxicity of XIAP inhibitors alone and in combination with γ-irradiation on some normal cells of the central nervous system at concentrations of XIAP inhibitors that sensitize glioblastoma cells to γ-irradiation points to a therapeutic index that could be exploited for preferential radiosensitization of malignant versus nonmalignant cells. The identification of the underlying mechanism(s) that are responsible for this differential sensitivity to XIAP inhibitors remains subject to future investigations.
Clinically, resistance to apoptosis is a major cause of treatment failure in glioblastoma [32]. In a clinical perspective, our findings provide the rationale for further (pre)clinical development of XIAP inhibitors in combination with γ-irradiation to increase the radiosensitivity of glioblastoma.
Supplementary Material
Acknowledgments
The authors thank C.Hulford and M. Luzzio (Pfizer, Inc, Groton, CT) for providing XIAP inhibitors and Dr. R. Pallini and R. De Maria for glioblastoma-initiating cells.
Footnotes
This work has been partially supported by grants from the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe, the Else-Kröner-Fresenius Stiftung, IAP6/18, and the European Community (ApopTrain, APO-SYS; to S.F.).
This article refers to supplementary material, which is designated by Figures W1 to W3 and is available online at www.neoplasia.com.
References
- 1.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarti A, Palanichamy K. Overcoming therapeutic resistance in malignant gliomas: current practices and future directions. Cancer Treat Res. 2008;139:173–189. [PubMed] [Google Scholar]
- 4.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 5.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124:511–515. doi: 10.1002/ijc.24064. [DOI] [PubMed] [Google Scholar]
- 10.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 11.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 12.Giagkousiklidis S, Vogler M, Westhoff MA, Kasperczyk H, Debatin KM, Fulda S. Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res. 2005;65:10502–10513. doi: 10.1158/0008-5472.CAN-05-0866. [DOI] [PubMed] [Google Scholar]
- 13.Giagkousiklidis S, Vellanki SH, Debatin KM, Fulda S. Sensitization of pancreatic carcinoma cells for gamma-irradiation-induced apoptosis by XIAP inhibition. Oncogene. 2007;26:7006–7016. doi: 10.1038/sj.onc.1210502. [DOI] [PubMed] [Google Scholar]
- 14.Opel D, Westhoff MA, Bender A, Braun V, Debatin KM, Fulda S. Phosphatidylinositol 3-kinase inhibition broadly sensitizes glioblastoma cells to death receptor- and drug-induced apoptosis. Cancer Res. 2008;68:6271–6280. doi: 10.1158/0008-5472.CAN-07-6769. [DOI] [PubMed] [Google Scholar]
- 15.Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 16.Proepper C, Johannsen S, Liebau S, Dahl J, Vaida B, Bockmann J, Kreutz MR, Gundelfinger ED, Boeckers TM. Abelson interacting protein 1 (Abi-1) is essential for dendrite morphogenesis and synapse formation. EMBO J. 2007;26:1397–1409. doi: 10.1038/sj.emboj.7601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito V, Beyer C, Kuppers E. BDNF-dependent stimulation of dopamine D5 receptor expression in developing striatal astrocytes involves PI3-kinase signaling. Glia. 2004;46:284–295. doi: 10.1002/glia.10356. [DOI] [PubMed] [Google Scholar]
- 18.Oost TK, Sun C, Armstrong RC, Al-Assaad AS, Betz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP, Olejniczak ET, et al. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem. 2004;47:4417–4426. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]
- 19.Fulda S, Sieverts H, Friesen C, Herr I, Debatin KM. The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res. 1997;57:3823–3829. [PubMed] [Google Scholar]
- 20.Mohr A, Zwacka RM, Debatin KM, Stahnke K. A novel method for the combined flow cytometric analysis of cell cycle and cytochrome c release. Cell Death Differ. 2004;11:1153–1154. doi: 10.1038/sj.cdd.4401480. [DOI] [PubMed] [Google Scholar]
- 21.Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, Van Meir EG. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulda S, Scaffidi C, Pietsch T, Krammer PH, Peter ME, Debatin KM. Activation of the CD95 (APO-1/Fas) pathway in drug- and gamma-irradiation-induced apoptosis of brain tumor cells. Cell Death Differ. 1998;5:884–893. doi: 10.1038/sj.cdd.4400419. [DOI] [PubMed] [Google Scholar]
- 23.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 24.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 25.Fulda S, Debatin KM. Targeting apoptosis pathways in cancer therapy. Curr Cancer Drug Targets. 2004;4:569–576. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- 26.Belka C, Jendrossek V, Pruschy M, Vink S, Verheij M, Budach W. Apoptosis-modulating agents in combination with radiotherapy—current status and outlook. Int J Radiat Oncol Biol Phys. 2004;58:542–554. doi: 10.1016/j.ijrobp.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 27.Cao C, Mu Y, Hallahan DE, Lu B. XIAP and survivin as therapeutic targets for radiation sensitization in preclinical models of lung cancer. Oncogene. 2004;23:7047–7052. doi: 10.1038/sj.onc.1207929. [DOI] [PubMed] [Google Scholar]
- 28.Holcik M, Yeh C, Korneluk RG, Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene. 2000;19:4174–4177. doi: 10.1038/sj.onc.1203765. [DOI] [PubMed] [Google Scholar]
- 29.Karikari CA, Roy I, Tryggestad E, Feldmann G, Pinilla C, Welsh K, Reed JC, Armour EP, Wong J, Herman J, et al. Targeting the apoptotic machinery in pancreatic cancers using small-molecule antagonists of the X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2007;6:957–966. doi: 10.1158/1535-7163.MCT-06-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fandy TE, Shankar S, Srivastava RK. Smac/DIABLO enhances the therapeutic potential of chemotherapeutic drugs and irradiation, and sensitizes TRAIL-resistant breast cancer cells. Mol Cancer. 2008;7:60. doi: 10.1186/1476-4598-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vucic D, Fairbrother WJ. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res. 2007;13:5995–6000. doi: 10.1158/1078-0432.CCR-07-0729. [DOI] [PubMed] [Google Scholar]
- 32.Bogler O, Weller M. Apoptosis in gliomas, and its role in their current and future treatment. Front Biosci. 2002;7:e339–e353. doi: 10.2741/a928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.