Abstract
The nuclear envelope (NE) provides a selective barrier between the nuclear interior and the cytoplasm and constitutes a central component of intracellular architecture. During mitosis in metazoa, the NE breaks down leading to the complete mixing of the nuclear content with the cytosol. Interestingly, many NE components actively participate in mitotic progression. After chromosome segregation, the NE is reassembled around decondensing chromatin and the nuclear compartment is reestablished in the daughter cells. Here, we summarize recent progress in deciphering the molecular mechanisms underlying NE dynamics during cell division.
1. Introduction
The nuclear envelope (NE) is composed of two adjacent membranes of distinct protein composition. The outer nuclear membrane (ONM) is continuous with the rough endoplasmic reticulum (rER) and both share a similar protein composition. The inner nuclear membrane (INM) contains a different set of membrane proteins, which establish connections to chromatin and, in metazoan cells, to the nuclear lamina. INM and ONM are fused at numerous sites, generating pores that are occupied by nuclear pore complexes (NPCs) – macromolecular channels assembled from proteins called nucleoporins. At the onset of mitosis, changes in NE architecture occur, which range from subtle alterations in lower eukaryotes with a closed mitosis to the complete disassembly of the NE during nuclear envelope breakdown (NEBD) in vertebrate cells. At the end of mitosis, NE components are recruited back to chromatin in a coordinated fashion to reestablish the nuclear boundary. Here, we will summarize recent insights into the topological and molecular reorganization of the nuclear membrane during cell division.
2. The NE at the entry into mitosis
Onset of mitosis is marked by the formation of microtubule (MT) asters from centrosomes and their migration around the NE. Centrosome migration requires dynein, a cytoplasmic minus-end directed MT motor [1], which associates with the NE at the end of G2 [2]. MT interactions with the NE aid later events in mitotic entry such as a dynein-dependent tearing process, which leads to a rupturing of the NE during prophase and facilitates NEBD and the movement of NE components towards centrosomes [3–5]. However, the mechanism by which dynein is tethered to the ONM is not yet clear. A subpopulation of the INM protein emerin, which resides in the ONM, has recently been proposed to interact with tubulin and facilitate the attachment of centrosomes to the NE in interphase cells [6]. Whether centrosome association with emerin or other NE components are used for centrosome migration remains to be seen.
Notably, NE components have also been implicated in other dynamic nuclear changes occurring early during cell division, like telomere attachment to the INM during prophase I of meiosis. In this case, the INM proteins SUN1 and SUN2, help pairing of homologous chromosomes in the nuclear periphery to facilitate homologous synapsis and recombination [7–9]. SUN1 and SUN2 are members of the evolutionary conserved LINC complexes, which establish interactions between the NE and the cytoskeleton [10].
3. NEBD
One of the most dramatic changes in cell organization during mitosis is the loss of nuclear compartmentalization induced by the breakdown of the NE at the transition from prophase to prometaphase (Figure 1). NEBD is marked by an increase in the permeability of the NE accompanied by the initiation of NPC disassembly [11]. In mammalian somatic cells, NPC dissociation is completed within minutes and is initiated by the loss of the peripheral nucleoporin Nup98, which is followed by a wave of synchronous nucleoporin dissociation [12]. Many nucleoporins are released from the NPC in the form of stable nucleoporin subcomplexes. Subsequent steps of NEBD involve the depolymerization of the nuclear lamina [13], and the detachment and removal of the nuclear membranes from chromatin [3,4]. As a consequence, nuclear membrane proteins are redistributed into the membrane system of the mitotic ER (see below). In C. elegans, this process requires the transmembrane nucleoporin GP210 [14], the GTPase Rab5 and reticulons [15]. The latter family of integral membrane proteins, together with a distinct class of membrane-bending proteins including mammalian DP1 and its yeast homolog Yop1p, has also been shown to shape the tubular ER [16]. This suggests that tubulation of disassembling nuclear membrane sheets might assist the dispersal of NE components into the ER. In vitro experiments using Xenopus egg extracts have also implicated the COPI coatomer complex in NEBD [17], but its contribution to NEBD in vivo still needs to be characterized.
Figure 1.
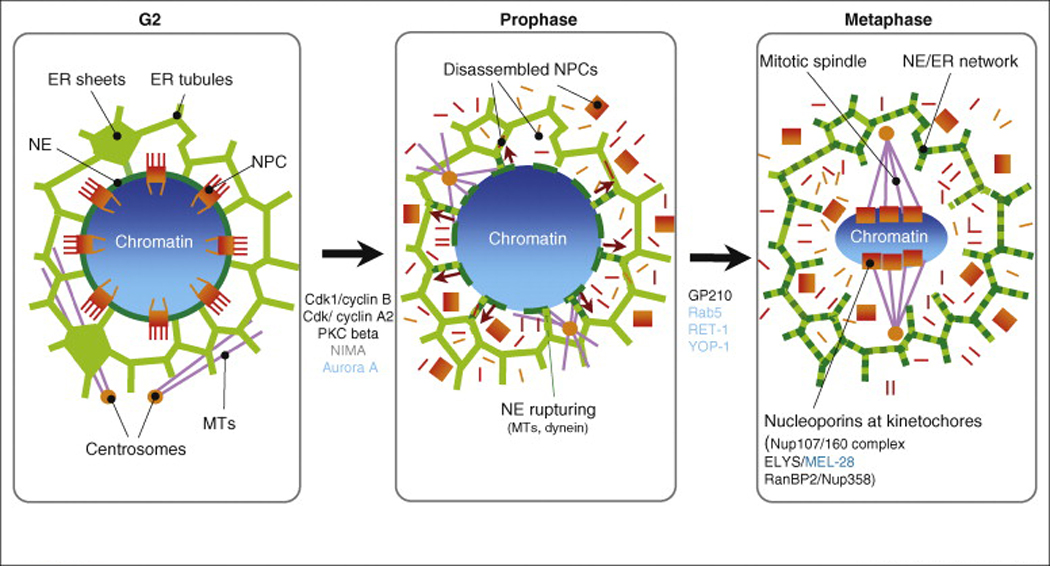
Schematic illustration of NEBD. In G2, the cell nucleus has completed DNA replication and NPC duplication. The NE (dark green), which is continuous with the ER network (green), encloses the chromosomes (blue). NPCs (red) mediate nuclear transport. At this stage, the ER is composed of tubules and sheets. When cells enter mitosis, NPCs disassemble and the NE gets reabsorbed into the ER, which at this stage is composed of tubules. NPC components are dispersed into the cytoplasm, except for three transmembrane nucleoporins, which together with other NE proteins are distributed into the ER (dashed lines). Centrosomes move to the NE (orange dots) and microtubules (purple) participate in the rupturing of the NE. In metaphase, a subset of NPC components has associated with kinetochores and the spindle is established. At this stage, chromosomes at the metaphase plate are devoid of membranes.
Many, if not all, of these events are triggered by the activation of mitotic kinases, which phosphorylate NE proteins, including nucleoporins, lamins, INM proteins and chromatin-associated factors. Clearly, Cdk1/cyclin B plays a major role in nuclear disassembly and it directly causes lamina depolymerization. Early in vitro studies also implicated the PKC isoform βII in dissolving lamin interactions [18]. A recent study using light-activatable kinase sensors in living cells supports the involvement of PKCβ in NEBD [19].
In addition, other kinases play a role in the nuclear disassembly process in various species, including NIMA [20], Aurora A [21,22] and a cyclin A2/Cdk complex [23]. The role of NIMA in NEBD has been established in the filamentous fungus A. nidulans, which uses partial NPC disassembly during its semi-open mitosis to allow for Cdk1/cyclinB and tubulin influx into nuclei and intranuclear spindle formation. NPC disintegration in A. nidulans is dependent on CDK1 and NIMA and involves the dispersal of a subset of nucleoporins, including Nup98 which is phosphorylated in a NIMA-dependent manner [20]. If NIMA-related kinases play a role in NPC disassembly in higher eukaryotes has not been addressed so far.
The mechanism by which Aurora A affects NEBD is not yet clear. Aurora A depletion in C. elegans delays NEBD relative to the completion of chromatin condensation [21,22]. Future experiments will tell whether Aurora A directly phosphorylates NE components and/or activates factors required for NEBD like Cdk1/cyclin B. Surprisingly, in human cells, there is little effect on the timing of NEBD upon depletion of B-type cyclins, whereas the reduction of cyclin A2 causes a significant delay. A constitutively nuclear cyclin B1 can rescue the loss of cyclin A2, suggesting that at least a part of the function of Cdk/cyclinA complexes in NEBD lies in controlling nuclear accumulation of Cdk1/cyclinB1 [23].
Although several kinases are promoting nuclear disassembly, little is known about how their action is linked to the disassembly of NPCs and subsequent events. An increasing number of mitotic phosphorylation sites are being identified in nucleoporins and other NE proteins through large-scale approaches and individual analyses [24–29]. NPC disassembly is likely a point of no return in nuclear disintegration and therefore must be tightly linked to signaling events controlling mitotic entry. To understand the nature of this connection will require deciphering the molecular events underlying the disruption of the NE permeability barrier. A valuable tool for this task is provided by a recently developed NEBD in vitro system, which allows for dissecting the contribution of factors to individual steps in this process [5].
4. Role of disassembled nucleoporins in mitosis
A number of proteins required for nucleocytoplasmic transport in interphase have important functions during mitosis. For instance, the RanGTPase system in conjunction with the transport receptor importin β plays a pivotal role in controlling NEBD [5], spindle assembly and NE assembly (for review see [30,31]). In addition, certain nucleoporins have been implicated in mitotic events independent of their function in mediating transport through the NPC.
RanBP2/Nup358 is a large, multifunctional component of the cytoplasmic filaments of the NPC, which has SUMO E3 ligase activity and tethers SUMOmodified RanGAP to the NPC during interphase [32,33]. After NPC disassembly, a fraction of RanBP2/SUMO-RanGAP complexes associates with spindle microtubules and, importantly, the MT-bound kinetochores [34,35]. Targeting of RanBP2 to kinetochores is dependent on MT, SUMO-1 and the nuclear export receptor CRM1 [36]. Depletion of RanBP2 by RNAi affects proper chromosome alignment and bipolar spindle formation [34,35]. Recently, an elegant study revealed severe aneuploidy and tumor formation in mice expressing low levels of this essential nucleoporin [37]. The tumorgenicity was linked to defects in the resolution of sister chromatids at the meta-anaphase transition, which requires the recruitment of topoisomerase IIα to the inner centromeres. Centromere targeting of Topo IIα was shown to be dependent on its sumoylation mediated by RanBP2 and compromised in mice with low RanBP2 expression. These data suggest that RanBP2 functions as tumor suppressor by ensuring faithful chromosome segregation during mitosis.
Mitotic functions have also been attributed to the multimeric Nup107/160 complex and its associated partner ELYS/MEL-28. A fraction of the Nup107/160 complex and ELYS/MEL-28 localize to kinetochores [38–40]. Kinetochore targeting of the Nup107 complex depends primarily on the Ndc80 complex [41], which is part of the outer kinetochore layer and involved in establishing MTkinetochore attachments. In addition, the Nup107/160 complex is also found on spindle poles and proximal microtubules during prometaphase [42]. In vitro, depletion of the Nup107/160 complex impairs correct bipolar spindle formation, likely by affecting the maintenance of MT fibers between spindle poles and chromosomes [42]. If recruitment of the Nup107/160 complex to kinetochores is compromised in living cells, a striking prolongation of mitosis is induced [41]. This mitotic delay is caused by an extended prometaphase and accompanied by failures in chromosome congression, originating from defects in stable MT-kinetochore interactions. Strikingly, loss of the Nup107/160 complex at kinetochores also affects the recruitment of RanBP2/RanGAP. Although the defects upon loss of the Nup107/160 complex or RanBP2/RanGAP resemble each other, the Nup107/160 complex may harbor an independent function at kinetochores, as RanBP2/RanGAP fail to target to kinetochores in the Xenopus in vitro spindle assembly system [36]. Interestingly, such a function might be linked to CENP-F, an outer kinetochore protein that associates with the dynein partners Nde1 and Nde1l as well as with Nup133 [41,43].
Rae1 (Gle2; Mnrp41) is a nucleoporin involved in mRNA export during interphase, which symmetrically associates with the NPC through binding to its partner subunit Nup98. One important mitotic role of the Rae1/Nup98 complex is the inhibition of securin degradation during prometaphase/metaphase, until the spindle assembly checkpoint (SAC) has been satisfied [44]. The SAC prevents onset of anaphase until chromosome alignment at the metaphase plate is completed. Securin is an inhibitor of separase that cleaves cohesin complexes to allow for sister chromatid separation [45]. Binding of Rae1/Nup98 to the E3 ubiquitin ligase APC/CCdh1 inhibits securin polyubiquitinylation and degradation [44]. Accordingly, cells of double heterozygous mice (Rae1+/−/Nup98+/−) expressing reduced levels of both Nup98 and Rae1 display severe aneuploidy caused by premature securin degradation and separation of sister chromatids. It is, however, currently unclear by which mechanism Rae1/Nup98 are dissociated from APC/CCdh1 to allow its timely activation once all kinetochores are correctly attached to microtubules at the metaphase plate.
In addition, Rae1 can directly bind to MTs and supports spindle assembly in vitro [46]. Surprisingly, the Rae1 activity was linked to a Rae1-containing ribonucleoprotein complex promoting MT nucleation/stabilization. Its activity is impaired by RNase treatment, suggesting that RNA plays a role in mitotic spindle assembly. However, it remains to be seen if Nup98 contributes to the function of Rae1 in spindle assembly and if any of the various RNA-binding proteins associated with Rae1 promotes this effect. The function of Rae1 in spindle formation is supported by the observation that RNAi against Rae1 in HeLa cells results in a twofold increase in the mitotic index, accompanied by failures in chromosome alignment and a high incidence of multipolar spindles [46,47]. Recently, NuMA, a microtubule-associated protein involved in spindle assembly, has also been identified as an interaction partner of Rae1 in mitotic HeLa cell extracts [47]. Codepletion of NuMA partially rescued spindle bipolarity in Rae1 depleted cells, suggesting that the proper balance of NuMA and Rae1 is a critical determinant for proper spindle formation.
Taken together, many nucleoporins affect the progression or control of mitosis and it becomes obvious that there is an important connection between nucleoporins and spindle assembly. The strong link between kinetochores and NPCs suggests that a possible outer pre-kinetochore complex may be a constituent of the NPC during interphase, which is recruited to kinetochores during mitosis. In evolutionary terms, it is interesting to consider whether this is a recent invention or a remnant of an old mechanism. Intriguingly, dinoflagellates, which are unicellular protists derived from typical eukaryotes by early divergence, divide through a closed mitosis using a cytoplasmic spindle [48]. During ‘dinomitosis’, kinetochore-like chromatin regions are closely associated with the nuclear envelope (perhaps NPCs), which forms channel-like structures that allow for penetration of microtubules.
Mitotic ER
During NEBD, INM proteins are redistributed into the membrane system of the mitotic ER [49,50]. This process not only requires the phosphorylation of INM proteins [51] but recent studies in C. elegans also revealed an involvement of two factors that control the formation of ER tubules, namely the GTPase Rab5 and YOP-1/RET-1 [15]. YOP-1/RET-1 belong to a class of evolutionary conserved integral membrane proteins [52], which can be grouped into two distinct families that structurally shape ER tubules [16]. The first group of proteins, the reticulons, includes two genes in yeast (Rtn1, Rtn2) and four in mammals (Rtn1–4). The second group consists of proteins related to DP1 (Yop1p in yeast) [16]. These proteins localize to the tubular ER and are excluded from low-curvature membranes such as the NE and peripheral ER sheets. Reticulons share with DP1 a conserved domain of 200 aa containing two hydrophobic segments, each thought to form a hairpin in form of a wedge within a lipid bilayer [53]. These wedge-like domains are thought to increase the surface area of the outer leaflet to create asymmetry in the lipid bilayers and reticulons have been shown to be sufficient to form tubules in vitro [54]. The involvement of YOP-1/RET-1 in NE dynamics during C. elegans mitosis [15] suggests that the intrinsic propensity of the ER to oscillate between tubules and sheets is utilized during mitosis and affects the fate of the NE. Indeed, consistent with the idea of ER restructuring during mitosis, three-dimensional EM reconstruction demonstrated that the ER is entirely composed of tubules in metaphase and no sheets are observed [55]. Since the NE is also reabsorbed in the ER during mitosis, NE reformation must likely occur from tubular ER [56].
NE formation from tubular ER
Consistent with such a mechanism, in vitro analyses suggest that an intact tubular ER is required for NE formation at the end of mitosis [50]. Live imaging revealed that the ER is targeted to chromosomes via tubule-end binding and subsequently immobilized on the chromatin surface. This chromatin-bound network then flattens and seals into a closed NE. In vitro data further suggests that targeting of membranes to chromatin is at least partially regulated by NEspecific transmembrane proteins binding to DNA and/or chromatin [50,57].
Recent proteomic studies have revealed a large number of INM proteins [58] and many of them can bind chromatin and even DNA directly [50,57]. This property might help to ensure that they reassociate with decondensing chromatin to aid chromatin enclosure in a concerted fashion – possibly triggered by their dephosphorylation as well as by changes of chromatin.
One chromatin-associated protein implicated in the process of NE assembly is the barrier-to-autointegration factor (BAF) [59]. BAF is known to bind a class of INM proteins sharing a common sequence motif referred to as LEM domain (based on its presence in Lamina-associated polypeptide (LAP2), Emerin and MAN1). In C. elegans, BAF depletion leads to defects in post-mitotic NE assembly and mislocalization of lamin, emerin and MAN1 [60]. The chromatin association of BAF during mitosis is controlled through its phosphorylation by vaccinia-related kinase 1 (VRK-1), which promotes the release of BAF from DNA after mitotic entry. Depletion of VRK-1 leads to defects in NE formation, likely caused by a failure to dissociate BAF and its partner INM proteins from chromatin in first place. NE reformation requires chromatin reassociation of BAF, presumably in its dephosphorylated form. These data suggest that the dynamic localization of BAF to chromatin is required for proper NE assembly.
In mammalian cells, oligomerized complexes of BAF and LAP2α, a soluble LEM protein, have been suggested to serve as a nucleation site for nuclear membrane assembly by recruiting membrane-anchored LEM proteins such as emerin and LAP2β to specific chromatin regions in late anaphase [61].
While the formation of flat membrane sheets on chromatin could in principal be initiated by the recruitment of DNA-binding INM proteins [57], it is difficult to imagine how they can mediate the final sealing of patches to form a fully closed NE. However, considering the fact that a typical mammalian cell nucleus contains thousands of pores, the formation of a completely closed membrane sphere is not necessary. Instead, membranes might expand across the chromatin surface with the last remaining holes being stabilized and occupied by newly forming NPCs (Figure 2). This step might involve interactions between chromatinassociated nucleoporins [62] and transmembrane nucleoporins like POM121 and NDC1 [63–65], the latter being delivered from the ER network. Such a mechanism would not require a final closing step of the membrane network and therefore could occur without specific NE fusion machinery. Previously observed inhibition of NE formation by non-hydrolyzable GTP analogues [66] and the dependence on SNARE-mediated membrane fusion [67] could be explained by defects in ER reconstitution due to a block in ER fusion.
Figure 2.
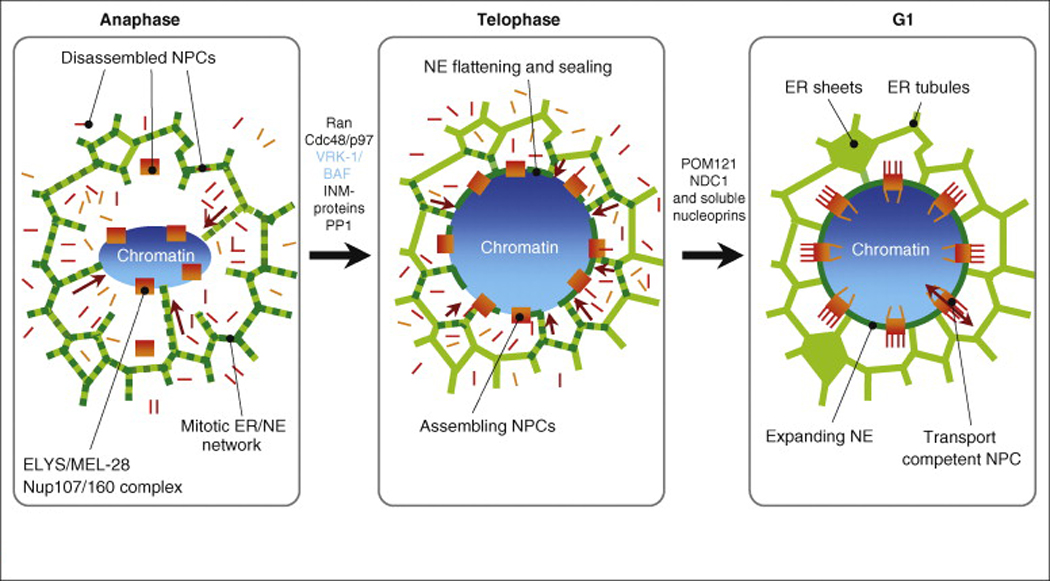
NE reformation around segregated chromosomes in one of the two daughter cells. In anaphase ER tubules associate with chromatin (purple arrows) and a subset of nucleoporins associates with chromatin. This step involves the small GTPase Ran and phosphatases such as PP1 [79,84,85]. Additional membrane tubules and NE proteins are recruited to the chromatin surface and mediate NE flattening. At this stage most NE proteins are cleared form the ER network. In late anaphase/early telophase a closed NE has formed with pores being assembled in a step-wise manner. Once pores become transport competent, the NE expands and cells move into G1.
NPC assembly: filling gaps and making holes in the NE
New insights have uncovered that chromatin compaction is highest in midanaphase just before NE formation occurs [68]. Interestingly, Aurora B kinase has to be removed from chromatin to allow decondensation to occur. This step is mediated by Cdc48/p97, an AAA-ATPase and ubiquitin-dependent chaperone that promotes NE formation by extracting ubiquitinylated Aurora B from chromatin [69]. This novel function of Cdc48/p97 provides the first mechanistic link between chromatin coordination and NE formation.
Recent studies suggest that NPC assembly and NE reformation are coordinated processes initiated from chromatin-bound nucleoporins [12,40,62,70]. The Nup107/160 complex is essential for pore assembly since its depletion results in pore-free NEs [62,71]. It is targeted to chromatin by ELYS/MEL-28, a process that can occur independently of membranes in vitro [70,72]. ELYS binding to chromatin occurs via its AT hooks, small DNA binding motifs [73]. Two additional nucleoporins, Nup153 and Nup50, associate with chromatin prior to recruitment of membranes [12]. However, since most of both proteins bind to the intact NE only when nuclear import is reinitiated, they are probably not involved in NE formation.
Known spatial regulators for the deposition of nucleoporins on chromatin are the small GTPase Ran and its regulator, the transport receptor importin β [74]. Ran, when bound to GTP, dissociates importin β from its binding partners and thereby regulates nucleocytoplasmic transport in interphase cells [75], as well as spindle formation and nuclear assembly in mitosis [31]. Several nucleoporins including Nup153, Nup358 and the Nup107/160 complex bind to importin β during mitosis, an interaction that negatively regulates NPC assembly [76,77]. RanGTP is generated on chromatin by its exchange factor RCC1. This produces a local gradient of high RanGTP concentration around chromatin [78], which triggers the local release of importin β from nucleoporins and consequently assembly into NPCs. In addition to Ran, recruitment of nucleoporins is likely controlled by their timely dephosphorylation, which also may control their ability to bind to chromatin and to each other upon anaphase onset, although clear data is lacking. A potential candidate for nucleoporin dephosphorlyation is PP1, which is recruited to the NE by AKAP149 and has been shown to remove mitotic phosphorylation marks from lamin B in telophase to allow lamina assembly [79].
After the initial recruitment of some nucleoporins to chromatin, a crucial yet poorly understood step in NPC reassembly is the establishment of contacts to the NE membrane, presumably to the transmembrane nucleoporins POM121 and NDC1 [63,65]. Interestingly, POM121 and the Nup107/160 complex may have a checkpoint function in coordinating the assembly of NPCs and nuclear membranes [65]. How this postulated checkpoint functions in molecular terms is still unknown.
The incorporation of Nup107/160, POM121 and NDC1 most likely mark the formation of a pore spanning the INM and ONM. In order to stabilize the highly curved fusion pore membrane (i.e. connections of INM and ONM) additional nucleoporins have to be attached. Examples are the Nup93 and Nup62 subcomplexes, which combined add almost a third of all known NPC components [12]. The members of the Nup62 complex and several other nucleoporins contain phenylalanine-glycine (FG) repeats, which establish transport capability and the permeability barrier. Import activity of NPCs starts concomitantly with the association of the Nup93 and Nup62 subcomplexes [12]. Nup53, a member of the Nup93 complex, interacts with the transmembrane nucleoporin NDC1, establishing a link between the pore membrane and the soluble nucleoporins [63,80]. Nup155, another subunit of the Nup93 complex, is required for NPC and NE formation in Xenopus egg extract and C. elegans [81]. In addition, the Nup93 complex contributes significantly to the exclusion limit of the NPC [82] and may play a major structural role in the assembly and formation of NPCs from chromatin-bound NPC intermediates.
The final step of NPC assembly is the addition of peripheral nucleoporins such as Nup214, Nup153, Tpr and Nup50 as well as the membrane nucleoporin gp210 [12].
In summary, NPC assembly at the end of mitosis is a highly complex multi-step process that involves molecular interactions between chromatin, nuclear membrane and soluble nucleoporins. It is important to note that new pores are also formed during interphase [83], when the total number of NPCs doubles. Whether mitotic and interphase assembly of pores exhibit different molecular requirements remains to be determined.
Outlook
The dynamic reorganization of the NE during mitosis is of fundamental importance for chromosome segregation and re-establishment of the nuclear compartment in the daughter cells. Many open questions and technical challenges in studying NEBD and nuclear assembly remain. For instance, membrane reorganization events such as NEBD occur rapidly, making it difficult to dissect and analyze individual steps of this process. In addition, membrane intermediates are difficult to preserve by fixation protocols, requiring detailed studies in living cells. Another obstacle is the complexity of nuclear disassembly and reformation, which involves molecular interactions between chromatin, membranes and soluble components. Importantly, only a small fraction of proteins that associate with the NE have been characterized. Since many of these NE-specific proteins can interact with chromatin or the cytoskeleton, it can be expected that the NE will become of central interest for our understanding of eukaryotic chromatin and cell organization. Recent advances in the structural analysis of nucleoporins, characterization of the NE proteome, advances in fluorescence microscopy and cell-free reconstitution systems hold the promise of exciting new insight into the biology of the NE in the near future.
Acknowledgements
We thank Stephan Güttinger, Eva Laurell, Roberta Schulte and Daniel Anderson for critically reading the manuscript. UK is supported by the Swiss National Science Foundation. MH is supported by NIH grant R01 GM073994.
Contributor Information
Ulrike Kutay, Email: ulrike.kutay@bc.biol.ethz.ch, Institute of Biochemistry, ETH Zurich, HPM F11.1, Schafmattstr.18, 8093 Zurich, Switzerland, Phone +41-44-632 3013, Fax: +41-44-632 1591.
Martin W. Hetzer, Email: hetzer@salk.edu, Salk Institute for Biological Studies, Molecular and Cell Biology Laboratory, 10010 N. Torrey Pines Road, La Jolla, 92037 CA, United States, Phone (858) 453-4100 x1419, Fax: (858) 457-4765.
References and recommended reading
Paper of particular interest, published within the annual period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Vaisberg EA, Koonce MP, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- 3.Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- 4.Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 5.Muhlhausser P, Kutay U. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J Cell Biol. 2007;178:595–610. doi: 10.1083/jcb.200703002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol. 2007;178:897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12:863–872. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Penkner A, Tang L, Novatchkova M, Ladurner M, Fridkin A, Gruenbaum Y, Schweizer D, Loidl J, Jantsch V. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev Cell. 2007;12:873–885. doi: 10.1016/j.devcel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9. Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci U S A. 2007;104:7426–7431. doi: 10.1073/pnas.0609198104. These three references (7•,8•,9•) identify a role of mammalian SUN proteins in telomere attachment to the NE and homologous chromosome pairing during meiosis.
- 10.Tzur YB, Wilson KL, Gruenbaum Y. SUN-domain proteins: 'Velcro' that links the nucleoskeleton to the cytoskeleton. Nat Rev Mol Cell Biol. 2006;7:782–788. doi: 10.1038/nrm2003. [DOI] [PubMed] [Google Scholar]
- 11.Lenart P, Rabut G, Daigle N, Hand AR, Terasaki M, Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J Cell Biol. 2003;160:1055–1068. doi: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dultz E, Zanin E, Wurzenberger C, Braun M, Rabut G, Sironi L, Ellenberg J. Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J Cell Biol. 2008;180:857–865. doi: 10.1083/jcb.200707026. This is an impressive kinetic analysis of NPC disassembly and reassembly by time-lapse microscopy revealing that both processes are not the reversal of each other.
- 13.Gerace L, Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980;19:277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- 14.Galy V, Antonin W, Jaedicke A, Sachse M, Santarella R, Haselmann U, Mattaj I. A role for gp210 in mitotic nuclear-envelope breakdown. J Cell Sci. 2008;121:317–328. doi: 10.1242/jcs.022525. [DOI] [PubMed] [Google Scholar]
- 15.Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol. 2007;178:43–56. doi: 10.1083/jcb.200701139. This paper reports the surprising finding that the endosome-associated GTPase Rab5 has a mitotic function in ER remodelling, which affects NEBD.
- 16. Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. This study together with reference [54] answered the long-standing question how ER tubules are formed and identified reticulons and DP1 as ER-shaping proteins.
- 17.Liu J, Prunuske AJ, Fager AM, Ullman KS. The COPI complex functions in nuclear envelope breakdown and is recruited by the nucleoporin Nup153. Dev Cell. 2003;5:487–498. doi: 10.1016/s1534-5807(03)00262-4. [DOI] [PubMed] [Google Scholar]
- 18.Buendia B, Courvalin JC, Collas P. Dynamics of the nuclear envelope at mitosis and during apoptosis. Cell Mol Life Sci. 2001;58:1781–1789. doi: 10.1007/PL00000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai Z, Dulyaninova NG, Kumar S, Bresnick AR, Lawrence DS. Visual snapshots of intracellular kinase activity at the onset of mitosis. Chem Biol. 2007;14:1254–1260. doi: 10.1016/j.chembiol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Souza CP, Osmani AH, Hashmi SB, Osmani SA. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr Biol. 2004;14:1973–1984. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Hachet V, Canard C, Gonczy P. Centrosomes promote timely mitotic entry in C. elegans embryos. Dev Cell. 2007;12:531–541. doi: 10.1016/j.devcel.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Portier N, Audhya A, Maddox PS, Green RA, Dammermann A, Desai A, Oegema K. A microtubule-independent role for centrosomes and aurora a in nuclear envelope breakdown. Dev Cell. 2007;12:515–529. doi: 10.1016/j.devcel.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong D, Pomerening JR, Myers JW, Gustavsson C, Jones JT, Hahn AT, Meyer T, Ferrell JE., Jr Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr Biol. 2007;17:85–91. doi: 10.1016/j.cub.2006.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Favreau C, Worman HJ, Wozniak RW, Frappier T, Courvalin JC. Cell cycledependent phosphorylation of nucleoporins and nuclear pore membrane protein Gp210. Biochemistry. 1996;35:8035–8044. doi: 10.1021/bi9600660. [DOI] [PubMed] [Google Scholar]
- 25.Glavy JS, Krutchinsky AN, Cristea IM, Berke IC, Boehmer T, Blobel G, Chait BT. Cell-cycle-dependent phosphorylation of the nuclear pore Nup107–160 subcomplex. Proc Natl Acad Sci U S A. 2007;104:3811–3816. doi: 10.1073/pnas.0700058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blethrow JD, Glavy JS, Morgan DO, Shokat KM. Covalent capture of kinasespecific phosphopeptides reveals Cdk1-cyclin B substrates. Proc Natl Acad Sci U S A. 2008;105:1442–1447. doi: 10.1073/pnas.0708966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nousiainen M, Sillje HH, Sauer G, Nigg EA, Korner R. Phosphoproteome analysis of the human mitotic spindle. Proc Natl Acad Sci U S A. 2006;103:5391–5396. doi: 10.1073/pnas.0507066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Hetzer M, Walther TC, Mattaj IW. Pushing the Envelope: Structure, Function, and Dynamics of the Nuclear Periphery. Annu Rev Cell Dev Biol. 2005 doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- 31.Di Fiore B, Ciciarello M, Lavia P. Mitotic functions of the Ran GTPase network: the importance of being in the right place at the right time. Cell Cycle. 2004;3:305–313. [PubMed] [Google Scholar]
- 32.Joseph J, Tan SH, Karpova TS, McNally JG, Dasso M. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J Cell Biol. 2002;156:595–602. doi: 10.1083/jcb.200110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–120. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 34.Salina D, Enarson P, Rattner JB, Burke B. Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J Cell Biol. 2003;162:991–1001. doi: 10.1083/jcb.200304080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M. The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr Biol. 2004;14:611–617. doi: 10.1016/j.cub.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Arnaoutov A, Dasso M. Ran-GTP regulates kinetochore attachment in somatic cells. Cell Cycle. 2005;4:1161–1165. doi: 10.4161/cc.4.9.1979. [DOI] [PubMed] [Google Scholar]
- 37. Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, Shuai K, Grosschedl R, van Deursen JM. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008;133:103–115. doi: 10.1016/j.cell.2008.01.045. This remarkable study demonstrates a mitotic function of the nucleoporin and SUMO E3 ligase RanBP2 in vivo. It identifies topoisomerase IIα as sumoylation substrate of RanBP2 and reveals a role for RanBP2 in tumor suppression.
- 38.Loiodice I, Alves A, Rabut G, Van Overbeek M, Ellenberg J, Sibarita JB, Doye V. The entire Nup107–160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol Biol Cell. 2004;15:3333–3344. doi: 10.1091/mbc.E03-12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galy V, Askjaer P, Franz C, Lopez-Iglesias C, Mattaj IW. MEL-28, a novel nuclearenvelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr Biol. 2006;16:1748–1756. doi: 10.1016/j.cub.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 40.Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc Natl Acad Sci U S A. 2006;103:17801–17806. doi: 10.1073/pnas.0608484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuccolo M, Alves A, Galy V, Bolhy S, Formstecher E, Racine V, Sibarita JB, Fukagawa T, Shiekhattar R, Yen T, et al. The human Nup107–160 nuclear pore subcomplex contributes to proper kinetochore functions. EMBO J. 2007;26:1853–1864. doi: 10.1038/sj.emboj.7601642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orjalo AV, Arnaoutov A, Shen Z, Boyarchuk Y, Zeitlin SG, Fontoura B, Briggs S, Dasso M, Forbes DJ. The Nup107–160 nucleoporin complex is required for correct bipolar spindle assembly. Mol Biol Cell. 2006;17:3806–3818. doi: 10.1091/mbc.E05-11-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergnolle MA, Taylor SS. Cenp-F links kinetochores to Ndel1/Nde1/Lis1/dynein microtubule motor complexes. Curr Biol. 2007;17:1173–1179. doi: 10.1016/j.cub.2007.05.077. [DOI] [PubMed] [Google Scholar]
- 44.Jeganathan KB, Malureanu L, van Deursen JM. The Rae1-Nup98 complex prevents aneuploidy by inhibiting securin degradation. Nature. 2005;438:1036–1039. doi: 10.1038/nature04221. [DOI] [PubMed] [Google Scholar]
- 45.Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- 46.Blower MD, Nachury M, Heald R, Weis K. A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell. 2005;121:223–234. doi: 10.1016/j.cell.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Wong RW, Blobel G, Coutavas E. Rae1 interaction with NuMA is required for bipolar spindle formation. Proc Natl Acad Sci U S A. 2006;103:19783–19787. doi: 10.1073/pnas.0609582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costas E, Goyanes V. Architecture and evolution of dinoflagellate chromosomes: an enigmatic origin. Cytogenet Genome Res. 2005;109:268–275. doi: 10.1159/000082409. [DOI] [PubMed] [Google Scholar]
- 49.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol. 2007;9:1160–1166. doi: 10.1038/ncb1636. This article provides the first evidence that NE reformation after mitosis occurs through chromatin-mediated reshaping of the ER.
- 51.Courvalin JC, Segil N, Blobel G, Worman HJ. The lamin B receptor of the inner nuclear membrane undergoes mitosis-specific phosphorylation and is a substrate for p34cdc2-type protein kinase. J Biol Chem. 1992;267:19035–19038. [PubMed] [Google Scholar]
- 52.Yang YS, Strittmatter SM. The reticulons: a family of proteins with diverse functions. Genome Biol. 2007;8:234. doi: 10.1186/gb-2007-8-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibata Y, Voss C, Rist JM, Hu J, Rapoport TA, Prinz WA, Voeltz GK. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem. 2008 doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA. Membrane proteins of the endoplasmic reticulum induce highcurvature tubules. Science. 2008;319:1247–1250. doi: 10.1126/science.1153634. [DOI] [PubMed] [Google Scholar]
- 55.Puhka M, Vihinen H, Joensuu M, Jokitalo E. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J Cell Biol. 2007;179:895–909. doi: 10.1083/jcb.200705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson DJ, Hetzer MW. The life cycle of the metazoan nuclear envelope. Curr Opin Cell Biol. 2008 doi: 10.1016/j.ceb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulbert S, Platani M, Boue S, Mattaj IW. Direct membrane protein-DNA interactions required early in nuclear envelope assembly. J Cell Biol. 2006;173:469–476. doi: 10.1083/jcb.200512078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schirmer EC, Gerace L. The nuclear membrane proteome: extending the envelope. Trends Biochem Sci. 2005;30:551–558. doi: 10.1016/j.tibs.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Segura-Totten M, Wilson KL. BAF: roles in chromatin, nuclear structure and retrovirus integration. Trends Cell Biol. 2004;14:261–266. doi: 10.1016/j.tcb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 60. Gorjanacz M, Klerkx EP, Galy V, Santarella R, Lopez-Iglesias C, Askjaer P, Mattaj IW. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. EMBO J. 2007;26:132–143. doi: 10.1038/sj.emboj.7601470. This paper reveals a novel role for the kinase VRK-1 and its substrate BAF in postmitotic NE assembly in C.elegans early embryos.
- 61.Dechat T, Gajewski A, Korbei B, Gerlich D, Daigle N, Haraguchi T, Furukawa K, Ellenberg J, Foisner R. LAP2alpha and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly. J Cell Sci. 2004;117:6117–6128. doi: 10.1242/jcs.01529. [DOI] [PubMed] [Google Scholar]
- 62.Walther TC, Alves A, Pickersgill H, Loiodice I, Hetzer M, Galy V, Hulsmann BB, Kocher T, Wilm M, Allen T, et al. The conserved Nup107–160 complex is critical for nuclear pore complex assembly. Cell. 2003;113:195–206. doi: 10.1016/s0092-8674(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 63.Mansfeld J, Guttinger S, Hawryluk-Gara LA, Pante N, Mall M, Galy V, Haselmann U, Muhlhausser P, Wozniak RW, Mattaj IW, et al. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol Cell. 2006;22:93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 64.Stavru F, Hulsmann BB, Spang A, Hartmann E, Cordes VC, Gorlich D. NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes. J Cell Biol. 2006;173:509–519. doi: 10.1083/jcb.200601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell. 2005;17:83–92. doi: 10.1016/j.molcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Boman AL, Delannoy MR, Wilson KL. GTP hydrolysis is required for vesicle fusion during nuclear envelope assembly in vitro. J Cell Biol. 1992;116:281–294. doi: 10.1083/jcb.116.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baur T, Ramadan K, Schlundt A, Kartenbeck J, Meyer HH. NSF- and SNAREmediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J Cell Sci. 2007;120:2895–2903. doi: 10.1242/jcs.010181. [DOI] [PubMed] [Google Scholar]
- 68.Mora-Bermudez F, Gerlich D, Ellenberg J. Maximal chromosome compaction occurs by axial shortening in anaphase and depends on Aurora kinase. Nat Cell Biol. 2007;9:822–831. doi: 10.1038/ncb1606. [DOI] [PubMed] [Google Scholar]
- 69.Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450:1258–1262. doi: 10.1038/nature06388. This study shows that chromatin decondensation and post-mitotic nuclear assembly require the inactivation of chromatin-bound Aurora B, which is promoted by the AAA-ATPase Cdc48/p97 through extraction of ubiquitylated Aurora B from chromatin.
- 70.Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, Galy V, Hetzer M, Mattaj IW, Antonin W. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 2007;8:165–172. doi: 10.1038/sj.embor.7400889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harel A, Orjalo AV, Vincent T, Lachish-Zalait A, Vasu S, Shah S, Zimmerman E, Elbaum M, Forbes DJ. Removal of a single pore subcomplex results in vertebrate nuclei devoid of nuclear pores. Mol Cell. 2003;11:853–864. doi: 10.1016/s1097-2765(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 72.Gillespie PJ, Khoudoli GA, Stewart G, Swedlow JR, Blow JJ. ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing. Curr Biol. 2007;17:1657–1662. doi: 10.1016/j.cub.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura N, Takizawa M, Okita K, Natori O, Igarashi K, Ueno M, Nakashima K, Nobuhisa I, Taga T. Identification of a novel transcription factor, ELYS, expressed predominantly in mouse foetal haematopoietic tissues. Genes Cells. 2002;7:435–446. doi: 10.1046/j.1365-2443.2002.00529.x. [DOI] [PubMed] [Google Scholar]
- 74.Hetzer M, Gruss OJ, Mattaj IW. The Ran GTPase as a marker of chromosome position in spindle formation and nuclear envelope assembly. Nat Cell Biol. 2002;4:E177–E184. doi: 10.1038/ncb0702-e177. [DOI] [PubMed] [Google Scholar]
- 75.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 76.Walther TC, Askjaer P, Gentzel M, Habermann A, Griffiths G, Wilm M, Mattaj IW, Hetzer M. RanGTP mediates nuclear pore complex assembly. Nature. 2003;424:689–694. doi: 10.1038/nature01898. [DOI] [PubMed] [Google Scholar]
- 77.Harel A, Chan RC, Lachish-Zalait A, Zimmerman E, Elbaum M, Forbes DJ. Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol Biol Cell. 2003;14:4387–4396. doi: 10.1091/mbc.E03-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalab P, Weis K, Heald R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science. 2002;295:2452–2456. doi: 10.1126/science.1068798. [DOI] [PubMed] [Google Scholar]
- 79.Steen RL, Martins SB, Tasken K, Collas P. Recruitment of protein phosphatase 1 to the nuclear envelope by A-kinase anchoring protein AKAP149 is a prerequisite for nuclear lamina assembly. J Cell Biol. 2000;150:1251–1262. doi: 10.1083/jcb.150.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hawryluk-Gara LA, Platani M, Santarella R, Wozniak RW, Mattaj IW. Nup53 is required for nuclear envelope and nuclear pore complex assembly. Mol Biol Cell. 2008;19:1753–1762. doi: 10.1091/mbc.E07-08-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Franz C, Askjaer P, Antonin W, Iglesias CL, Haselmann U, Schelder M, de Marco A, Wilm M, Antony C, Mattaj IW. Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. EMBO J. 2005;24:3519–3531. doi: 10.1038/sj.emboj.7600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galy V, Mattaj IW, Askjaer P. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol Biol Cell. 2003;14:5104–5115. doi: 10.1091/mbc.E03-04-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D'Angelo MA, Anderson DJ, Richard E, Hetzer MW. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312:440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- 84.Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemee A. Emerging roles of nuclear protein phosphatases. Nat Rev Mol Cell Biol. 2007;8:234–244. doi: 10.1038/nrm2126. [DOI] [PubMed] [Google Scholar]
- 85.Ito H, Koyama Y, Takano M, Ishii K, Maeno M, Furukawa K, Horigome T. Nuclear envelope precursor vesicle targeting to chromatin is stimulated by protein phosphatase 1 in Xenopus egg extracts. Exp Cell Res. 2007;313:1897–1910. doi: 10.1016/j.yexcr.2007.03.015. [DOI] [PubMed] [Google Scholar]


