Abstract
Major Depressive Disorder (MDD) begins frequently in adolescence and is associated with severe outcomes, but the developmental neurobiology of MDD is not well understood. Research in adults has implicated fronto-limbic neural networks in the pathophysiology of MDD, particularly in relation to the subgenual anterior cingulate cortex (ACC). Developmental changes in brain networks during adolescence highlight the need to examine MDD-related circuitry in teens separately from adults. Using resting state functional magnetic resonance imaging (fMRI), this study examined functional connectivity in adolescents with MDD (n=12) and healthy adolescents (n=14). Seed-based connectivity analysis revealed that adolescents with MDD have decreased functional connectivity in a subgenual ACC-based neural network that includes the supragenual ACC (BA 32), the right medial frontal cortex (BA 10), the left inferior (BA 47) and superior frontal cortex (BA 22), superior temporal gyrus (BA 22), and the insular cortex (BA 13). These preliminary data suggest that MDD in adolescence is associated with abnormal connectivity within neural circuits that mediate emotion processing. Future research in larger, un-medicated samples will be necessary to confirm this finding. We conclude that hypothesis-driven, seed-based analyses of resting state fMRI data hold promise for advancing our current understanding of abnormal development of neural circuitry in adolescents with MDD.
Keywords: adolescence, brain imaging, depression, functional connectivity, resting-state functional MRI, subgenual anterior cingulate cortex
Introduction
Adolescent major depressive disorder (MDD) is associated with severe consequences including suicide, the third leading cause of death in adolescence [4]. Further, adolescent MDD is a strong predictor of MDD in adulthood, which carries its own burden of disadvantage [38]. Because of the substantial brain maturation that takes place during adolescence within the neural networks that mediate emotion processing [11, 39], pathophysiology in adolescents should be examined separately. However, such research has been scarce.
Fronto-limbic neural circuitry has been implicated in MDD [29, 34]. Within this network, converging lines of evidence suggest that the subgenual ACC (ACC) region, plays a critical central role in the neural circuitry that underlies MDD [14, 31]. A core function of subgenual ACC is to regulate amygdala activity, preventing excessive emotional reactivity and stress responses [33]. In a recent investigation of functional connectivity in healthy adults, Stein and colleagues delineated a circuit linking subgenual ACC ventrally to amygdala and rostrally to supragenual ACC (Brodmann area (BA) 32) [35]. Disruption of this regulatory circuit is thought to underlie depression [13], and provides a useful starting point for examination of neural circuitry in adolescents with MDD.
Resting state functional magnetic resonance imaging (fMRI) has emerged as a novel approach to examine neural connections in vivo. This method measures the spontaneous, slow-wave (0.01-0.1 Hz) fluctuations in blood oxygen level dependent (BOLD) signal that are observed at rest; “functional connectivity” is indicated by interregional correlations of these temporal patterns [19]. Resting state fMRI has now been used to map connectivity patterns in healthy adults [12, 27], normative development [17, 24], and disease processes [6]. Resting state fMRI techniques in adults with depression have begun to identify group differences in functional connectivity within brain networks, although analysis strategies have varied [1, 21]. No prior studies have reported using resting state fMRI to examine neural circuitry in adolescents with MDD.
The purpose of this study was to measure functional connectivity in adolescents with depression compared with healthy participants. Based on the model and region specifications proposed by Stein and colleagues [35], we analyzed functional connectivity using seeds in subgenual ACC, amygdala, and supragenual ACC. We hypothesized that depressed adolescents would demonstrate decreased functional connectivity within this neural circuit compared to healthy adolescents.
Materials and Methods
Participants
We enrolled 28 adolescents (14 depressed, 14 healthy) ranging from 15 to 19 years of age in this study. Exclusion criteria for all participants consisted of intelligence quotient (IQ) < 80 on the Wechsler Abbreviated Scale of Intelligence [37], significant medical or neurological disorders, and positive urine pregnancy test in females. For the depressed sample, adolescents with a primary diagnosis of MDD were recruited from the psychiatric inpatient unit, day hospital, and clinics at the University of Minnesota, as well as through community postings. Exclusionary psychiatric disorders were: (i) bipolar disorder, (ii) schizophrenia, (iii) a pervasive developmental disorder, (iv) an eating disorder with active symptoms in the past 12 months, and (v) a substance-related disorder with history of use in the past 60 days. Healthy participants with no major current or past DSM-IV diagnosis were recruited via community postings. The study was approved by the institutional review board of the University of Minnesota. Participants age 18 and over provided signed informed consent; those under age 18 provided assent, and a parent or guardian provided signed informed consent.
Clinical Assessment
For each participant, DSM-IV Axis I diagnoses were established based on a consensus between independent parent and child interviews conducted by a child and adolescent psychiatrist and a developmental clinical psychologist using the Schedule for Affective Disorders and Schizophrenia for Children – Present and Lifetime Version [23]. Severity was assessed using (a) the Beck Depression Inventory-II (BDI-II) self-report scale [2], (b) the Global Assessment of Functioning (GAF) scale, and (c) duration of current illness.
Neuroimaging procedures
All participants were scanned using a research-dedicated Siemens Trio 3 Tesla scanner located at the Center for Magnetic Resonance Research at the University of Minnesota. We obtained a 6 min ‘resting-state’ scan, comprising 180 contiguous echo planar imaging (EPI) whole-brain functional volumes (TR = 2000ms; TE = 30ms; flip angle = 90; 34 contiguous AC-PC aligned axial slices; matrix = 64×64; FOV = 22cm; acquisition voxel size = 3.4×3.4×4mm). During this scan participants were instructed to rest without moving. To increase scanning tolerability, participants listened to their choice of music during the acquisition of all neuroimaging data. Given concerns about the potential impact of this baseline, we limited our examination to positive connections, which show less sensitivity to choice of baselines than negative connections [36]. A high-resolution T1-weighted anatomical image was also acquired for purposes of anatomical localization.
Image preprocessing
AFNI [9] was used for image preprocessing (http://afni.nimh.gov/afni), performing slice timing correction for interleaved acquisition (using Fourier interpolation), motion correction (by aligning each volume to a “base” image [middle volume] using Fourier interpolation) and de-spiking (detection and reduction of extreme time series outliers using a hyperbolic tangent function). All other data processing was carried out using FSL (www.fmrib.ox.ac.uk), including spatial smoothing (FWHM = 6mm), mean-based intensity normalization of all volumes by the same factor, temporal bandpass filtering (highpass temporal filtering: Gaussian-weighted least-squares straight line fitting, with sigma = 100.0s; Gaussian lowpass temporal filtering HWHM 2.8s) and pre-whitening. Each individual's time series was spatially normalized by registration to the MNI152 (Montreal Neurological Institute) template with 1mm3 resolution, using a 12 degree of freedom affine transformation.
Functional connectivity analyses
Following the methods of Castellanos et al. [6], we examined functional connectivity using a seed-based approach. Using coordinates recently validated in a path-analysis of amygdala circuitry [35], spherical seeds with a radius of 8 mm (257 voxels) were created in 2×2×2mm space for subgenual ACC (Montreal Neuroimaging Institute (MNI) coordinates 0, 15, -14), amygdala (-26, 0, -20) and supragenual ACC (0,34, 30). For each participant, we calculated the mean time series of each seed by averaging across all voxels within the seed. Individual participant analyses were carried out for each seed using the general linear model implemented in FSL program FEAT. Nine nuisance covariates (time series for global signal intensity, white matter, cerebrospinal fluid, and six motion parameters) were included in regression analyses to minimize the contributions of artifactual physiological signals (e.g., cardiac and respiratory cycles).
Group-level analyses were carried out using a mixed-effects model implemented in the FSL program FLAME. We included age and sex as statistical covariates to ensure that observed group effects would be independent of age-related changes or differential gender risk. IQ was also included as a covariate to account for group differences (t(24) =2.03, p= 0.054) (see Table 1). Corrections for multiple comparisons were carried out at the cluster level for the statistical map produced with each seed ROI using Gaussian random field theory (min Z > 2.3; cluster significance: p < 0.05, corrected). For each seed, this group-level analysis produced thresholded Z-score maps for functional connectivity observed in the direct comparison of MDD versus controls. Correlational analyses were conducted to assess the impact of clinical variables on functional connectivity. Additionally, data was examined to evaluate whether individuals with specific clinical variables would represent outliers.
Table 1. Demographic and Clinical Characteristics of Adolescents with Major Depressive Disorder (MDD) and Healthy Control Participants.
| Characteristic | MDD n=12 |
Healthy n=14 |
|---|---|---|
| Age (mean years ±SD) | 16.5±0.95 | 16.8±1.5 |
| Gender (male/female, n (%) | 3/9 (25/75%) | 6/8 (43/57%) |
| IQ (mean ±SD) a | 106.0±10.5 | 114.4±10.4 |
| Ethnicity – n (%) | ||
| Caucasian | 10 (83%) | 9(64%) |
| African American | 2 (16%) | 2 (14%) |
| Hispanic | 0 | 0 |
| Asian | 0 | 2 (14%) |
| Other | 0 | 1 (7%) |
| Illness History | ||
| Duration of Illness (mean months ± SD; range) | 26.5±25.9 (1-96) | 0 |
| Medication-naïve/ Medication-free/ Medicated, n | 1/1/10 | 15/0/0 |
| Global Assessment of Functioning (mean ± SD) | 45.42±9.7 | No information |
| Medication class – n (%) | ||
| c Selective serotonin reuptake inhibitors | 9 (75%) | 0 |
| d Atypical antidepressants | 1 (8%) | 0 |
| e Mood stabilizers | 2 (16%) | 0 |
| f Atypical Antipsychotics | 2 (16%) | 0 |
| g Stimulants | 2 (16%) | 0 |
| h Selective Norepinephrine Reuptake Inhibitor | 1 (8%) | 0 |
| 1BDI-II(mean±SD) | 27.7±11.3 b (15-44) | No information |
| Current Comorbidity – n (%) | ||
| j ADHD | 3 (25%) | 0 |
| k Any Anxiety Disorder | 10 (83%) | 0 |
| Past Comorbidity | ||
| Substance Use Disorder, partial or sustained remission, no use for at least 2 months | 4(32%) | 0 |
p=0.054.
Range;
Selective Serotonin Reuptake Inhibitors included fluoxetine (n=3), sertraline (n=1), citalopram (n=4), escitalopram n=1);
venlafaxine (n=1);
mood stabilizers included lamotrigine (n=1) and oxcarbazepine (n=1);
atypical antipsychotics included aripiprazole (n=1) and quetiapine (n=1);
stimulant medications included amphetamine/dextroamphetamine (Adderall) (n=2);
atomoxetine (n=1);
Beck Depression Inventory-II;
Attention Deficit Hyperactivity Disorder;
Current Anxiety Disorders included Generalized Anxiety Disorder (n=7), Social Phobia (n=3), and Post Traumatic Stress Disorder (n=2).
Results
Participants
Two participants with MDD were excluded from analyses due to unusable data leaving 12 depressed participants (9 females) and 14 healthy control participants (8 females) for analyses. This sample of depressed adolescents had moderate to severe illness, with mean duration of illness over 2 years, and mean GAF and BDI-II scores of 45 and 27, respectively. Most (83%) of the MDD group had current comorbid anxiety disorders, including Generalized Anxiety Disorder (n=7), Social Phobia (n=3), and Post Traumatic Stress Disorder (PTSD) (n=2). A fourth of the sample had comorbid Attention Deficit Hyperactivity Disorder (ADHD), and approximately a third had a past diagnosis of a substance use disorder but were free of any use for at least two months. Most depressed participants were being treated with medication; only one subject was medication-naïve, and another was medication-free for one month prior to the scan. Group characteristics are detailed in Table 1.
Functional Connectivity
Adolescents with MDD exhibited decreased functional connectivity between subgenual ACC and a network of cortical areas including supragenual ACC (BA 32), right medial frontal cortex (BA 10), left superior (BA 8) and inferior (BA 47) frontal cortex, left superior temporal cortex (BA 22), and insular cortex (BA 13) (Z>2.3, p < 0.05, corrected). No significant group differences were found in the analyses using amygdala or supragenual ACC seeds.
Clinical Correlations
Within the depressed group, no significant relationships were identified between measures of functional connectivity and symptom severity (BDI-II scores), duration of illnesss, medication status, or presence of an anxiety disorder. Individuals without comorbid anxiety (n=2) and those not taking medications (n=2) did not represent outliers in the analysis.
Discussion
In this preliminary study we identified decreased functional connectivity within a key neural circuit arising from the subgenual ACC in adolescents with MDD in comparison to healthy participants using resting state functional neuroimaging. This finding adds to converging lines of evidence implicating subgenual ACC as a central component of altered brain networks in depression. Structural and functional imaging studies have demonstrated abnormalities in subgenual ACC volume and metabolism, possibly mediated by reduced glial cell density that has been shown in post-mortem studies of adults with MDD [reviewed in 15]. When successful, deep-brain stimulation targeting this region in adults with treatment-resistant depression leads to altered functional activity within fronto-limbic networks [31]. Emerging research using diffusion tensor imaging in healthy adults has begun to delineate the anatomical circuitry connected with this key area [i.e., 22]. However, to our knowledge this represents the first study using seed-based resting state fMRI techniques to specifically measure functional connectivity of subgenual ACC in depressed patients of any age.
The findings presented here add to a previous report from a seed-based analysis of resting state fMRI data in adults with MDD [1]. In a study that examined functional connectivity between supragenual ACC (BA 32) and three limbic targets, Anand and colleagues reported decreased functional connectivity between supragenual ACC and thalamus in MDD adults [5]. In contrast, our analysis found no group difference in functional connectivity using the supragenual ACC seed. The divergence may reflect methodological differences (i.e., use of targets versus brain-wide search). or may reflect developmental differences.
The refinement of neural connections that transpires in adolescence is the backdrop for the current findings. Preclinical data has documented postnatal maturation of hemodynamic response characteristics in rats, which correlated with maturation of cortical connectivity from infancy to adulthood [7]. Emerging human fMRI research has documented maturation in functional connectivity of neural networks through adolescence [16, 17]. One study reported that ACC functional connectivity, especially in networks associated with social and emotional functions, undergoes development across adolescence from diffuse patterns in childhood to focal patterns in adulthood [24]. This evidence depicting functional connectivity during adolescence as a “moving target” emphasizes the need for future research to carefully delineate, across multiple developmental time points, how the trajectory of neurodevelopment may be abnormal in depressed individuals.
The subgenual ACC-based network identified in the present study maps on to a set of connections identified in a recent meta-analysis of cortical-subcortical interactions in emotion processing [25]. This analysis identified the “medial PFC group” as “the interface between cognitive context and core affect” [10]. Thus, decreased connectivity in this network may underlie the dysregulation of emotion that occurs in adolescents with MDD. In addition, the network identified here includes the left insular cortex (BA 13). The insula is thought to mediate interpretation of sensory information received from the body (interoception) that contributes to emotional states [18]. Therefore, it is plausible that decreased connectivity in this circuit may underlie depressive symptoms such as somatic complaints and negative bias in interpreting interpersonal feedback. Finally, this network included the superior temporal lobe (BA 22). Although not an area commonly emphasized in MDD research, a recent meta-analysis of MDD fMRI studies noted that this region shows decreased activation during induction of negative affect in MDD patients [28]. We speculate that our finding of reduced connectivity with subgenual ACC may relate to the reported diminished responses of superior temporal cortex in other MDD studies; future studies are needed to further understand this circuit in MDD pathophysiology.
Contrary to predictions, we did not observe a group difference in connectivity between subgenual ACC and amygdala. Prior studies using fMRI data that used emotion-valenced tasks have documented a disconnect or “uncoupling” between amygdala and supragenual ACC or ventromedial prefrontal cortex in depressed patients [1]. Notably, Anand and colleagues also failed to identify a disconnect between the ACC and amygdala in their resting state fMRI study of adults with MDD [30]. The failure to identify a group difference in amygdala circuitry in both studies may reflect the specific location used for creating the seeds. Alternatively, detecting group differences in functional connectivity in this circuit may require the use of fear-processing tasks. Future research studies combining resting state and task-based approaches will advance understanding of this circuitry in MDD.
Results from this exploratory study must be interpreted cautiously in light of study limitations. First, most patients were receiving psychotropic medications, a potential confound. However, since successful antidepressant medication treatment in adults has been found to reverse abnormalities of subgenual ACC metabolism [8], the high prevalence of medication treatment in our sample likely diminished group differences rather than representing a source of type I error. Further, this sample of depressed adolescents had moderate to severe levels of depression at the time of the scan despite treatment, indicating poor response. Thus, regardless of medication status, the neural data from this sample is pertinent to understanding the underlying neurobiology of active illness. Future research with treatment-naive adolescents with MDD is needed to confirm these findings.
Given high rates of comorbidity in this sample of depressed adolescents, future efforts will need to clarify the specificity of the reported findings. Although MDD was the primary diagnosis, most patients in this sample had one or more secondary comorbid diagnoses, most frequently anxiety. Inclusion of anxiety disorders in MDD studies is standard because depression and anxiety are so highly comorbid in adolescents [3] that exclusion of all anxiety disorders would result in a highly atypical sample. Similarly, ADHD precedes depression in many adolescents [i.e., 26], and inclusion of patients with ADHD is common in adolescent MDD studies [i.e., 20]. Finally, although subjects denied all use of substances for 60 days prior to the scan, our sample included 4 subjects with a history of a substance use disorder. Substance use disorders commonly co-occur with depression [36], arguably making our sample more representative. However, the long-term effects of substance use on brain connectivity are poorly studied. While the inclusion of these subjects is a limitation, it was noted that these subjects did not represent outliers in the analysis. Continued efforts to investigate the specific impact of depressive illness versus each of the co-morbid secondary disorders on functional connectivity are warranted but will require substantially larger samples.
A methodological limitation of this pilot study pertains to participants continuing to listen to their choice of music during the resting BOLD scan. Anecdotally, it appeared that this approach enhanced relaxation, increased scan tolerability and minimized motion artifacts. However, this approach introduced the potential confound of uncontrolled state-related changes in connectivity. Although spontaneous BOLD fluctuations remain easily detectable across distinct baselines [32], different types of music (happy, sad, neutral) activate distinct neural networks as observed using task-based fMRI. We did not assess the emotional import of participants' music selections which would have been difficult without limiting choices. Thus we cannot rule out the possibility that systematic between-group differences in music choice may have contributed to our finding of decreased functional connectivity in the subgenual ACC network in depressed adolescents. Replication with independent samples in which data is acquired during varying controlled resting states will be useful in determining the extent to which emotionally meaningful stimuli like music modulate brain connectivity in emotion regulation networks.
In conclusion, the data presented here document decreased functional connectivity in a key network implicated in emotion regulation in adolescents with MDD, and add to converging evidence highlighting the central role of the subgenual ACC in the neural circuitry of depression. These findings in adolescents with MDD are hypothesized to reflect an aberrant developmental process in the formation of these neural connections. Future research is needed in treatment-naïve adolescents (1) to confirm the implication of this circuitry in adolescents with MDD, and (2) to examine the effects of treatment on functional connectivity within this network. Given the presumed increased neuroplasticity during adolescent development, confirmation of our findings should lead to efforts directed toward altering risk trajectories and improving outcomes for youth with MDD.
Figure 1.
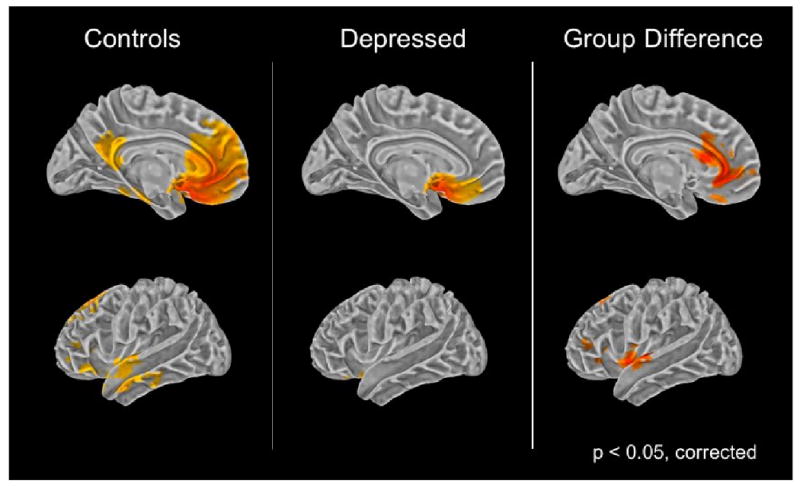
Group comparison of subgenual cingulate functional connectivity.
Left and middle panel: Colored areas indicate functional connectivity with the subgenual ACC in the control group and the depressed group.
Right Panel: Colored areas depict where functional connectivity is greater in the healthy group in comparison to the depressed group.
Acknowledgments
The authors gratefully acknowledge support from the National Institute of Mental Health (Cullen: T32 MH073129; Gabbay: MH077072; Kumra: MH 073150; Lim: MH060662), the Center for Neurobehavioral Development and the Center for Magnetic Resonance Research (BTPRR-P41 RR008079 and P30 NS057091) at the University of Minnesota, the Minnesota Medical Foundation, the Stavros S. Niarchos Foundation, Linda and Richard Schaps, and Jill and Bob Smith.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT, Steer RA, Brown KB. Beck Depression Inventory - Revised. Harcourt Brace; San Antonio, Texas: 1996. [Google Scholar]
- 3.Biederman J, Faraone S, Mick E, Lelon E. Psychiatric comorbidity among referred juveniles with major depression: fact or artifact? J Am Acad Child Adolesc Psychiatry. 1995;34:579–90. doi: 10.1097/00004583-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J. Childhood and adolescent depression: a review of the past 10 years. Part II. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1575–1583. doi: 10.1097/00004583-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Carhart-Harris RL, Mayberg HS, Malizia AL, Nutt D. Mourning and melancholia revisited: correspondences between principles of Freudian metapsychology and empirical findings in neuropsychiatry. Ann Gen Psychiatry. 2008;7:9. doi: 10.1186/1744-859X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonnese MT, Phillips MA, Constantine-Paton M, Kaila K, Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci. 2008;11:72–9. doi: 10.1038/nn2017. [DOI] [PubMed] [Google Scholar]
- 8.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–44. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 9.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- 12.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional Connectivity of Human Striatum: A Resting State fMRI Study. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 13.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 14.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 15.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 20.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 21.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276–82. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of Anterior Cingulate Functional Connectivity from Late Childhood to Early Adulthood. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 25.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. Jama. 2004;292:807–20. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 27.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 30.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 31.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Mitterschiffthaler MT, Fu CH, Dalton JA, Andrew CM, Williams SC. A functional MRI study of happy and sad affective states induced by classical music. Hum Brain Mapp. 2007;28:1150–62. doi: 10.1002/hbm.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 34.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 35.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Tian L, Jiang T, Liu Y, Yu C, Wang K, Zhou Y, Song M, Li K. The relationship within and between the extrinsic and intrinsic systems indicated by resting state correlational patterns of sensory cortices. Neuroimage. 2007;36:684–90. doi: 10.1016/j.neuroimage.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 37.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) American Psychological Association; San Antonio, Texas: 1999. [Google Scholar]
- 38.Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Klier CM, Ryan ND, Dahl RE, Wickramaratne P. Depressed adolescents grown up. Jama. 1999;281:1707–13. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- 39.Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci Lett. 2006;406:194–9. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]


