Abstract
In Xenopus, the animal cap is very sensitive to BMP antagonists, which result in neuralization. In chick, however, only cells at the border of the neural plate can be neuralized by BMP inhibition. Here we compare the two systems. BMP antagonists can induce neural plate border markers in both ventral Xenopus epidermis and non-neural chick epiblast. However, BMP antagonism can only neuralize ectodermal cells when the BMP-inhibited cells form a continuous trail connecting them to the neural plate or its border, suggesting that homeogenetic neuralizing factors can only travel between BMP-inhibited cells. Xenopus animal cap explants contain cells fated to contribute to the neural plate border and even to the anterior neural plate, explaining why they are so easily neuralized by BMP-inhibition. Furthermore, chick explants isolated from embryonic epiblast behave like Xenopus animal caps and express border markers. We propose that the animal cap assay in Xenopus and explant assays in the chick are unsuitable for studying instructive signals in neural induction.
Keywords: Xenopus, chick, neural induction, default model, neural plate border, homeogenetic induction, neural crest, pre-placodal region, animal cap assay, BMP signaling, GATA
Introduction
Since the discovery of neural induction by Spemann and Mangold in 1924 (Spemann and Mangold, 1924), there has been considerable interest in identifying the signals responsible. Relatively little progress was made until about a decade ago, when the “default model” was proposed (Hemmati-Brivanlou and Melton, 1997a, 1997b; Harland, 2000; Muñoz-Sanjuán and Brivanlou, 2002). This model states that Bone Morphogenetic Proteins (BMP) are initially active throughout the entire ectoderm. As gastrulation starts, the organizer and dorsal mesoderm secrete BMP antagonists generating a dorso-ventral gradient of BMP activity. Consequently neural tissue, neural crest and epidermis arise in the ectoderm at progressively higher levels of BMP activity as they are situated further away from the dorsal mesoderm. Since the default model was first proposed there has been considerable controversy concerning whether or not it provides an adequate explanation for neural induction. Recent experiments in chicken and Xenopus embryos indicate more complexity to the establishment of a functional neural plate (Streit et al., 1998; Streit and Stern, 1999c, 1999b; Streit et al., 2000; Linker and Stern, 2004; De Almeida et al., 2008). In particular, one set of experiments in the chick raised the possibility that not all of the ectoderm, as the default model predicts, but only cells close to the neural/epidermal border are sensitive to BMP and its antagonists (Streit et al., 1998; Streit and Stern, 1999b). We therefore re-examined this issue in Xenopus and chick to determine whether the two systems behave in a comparable way. In both, we find that non-neural ectoderm can be neuralized by BMP inhibition only when the BMP-inhibited cells form a continuous trail from the neural plate or its border. This suggests that homeogenetic (induction of like by like – in this case induction by the neural plate; (Mangold and Spemann, 1927; Mangold, 1929, 1933; Nieuwkoop et al., 1952; Servetnick and Grainger, 1991) inducing signals from the neural plate can only travel between BMP inhibited cells. We wondered whether the animal cap, which is easily neuralized by BMP inhibitors, might be equivalent to the neural-epidermal border. Detailed fate maps reveal that even the smallest caps contain cells fated to contribute to this border. Finally we show that chick epiblast explants express markers consistent with a border-like identity and behave like Xenopus animal caps.
Materials and methods
Xenopus embryology
Fertilization, staging, injections, lineage tracing, animal cap assays and in situ hybridisation were performed as described (Linker and Stern, 2004). mRNA was transcribed from Smad6-pCS2+ (Linker and Stern, 2004). CerberusShort-pCS2+ was kindly provided by E. de Robertis (Piccolo et al., 1999), ΔSmad7-pCS2+, TEV2GR-pCS2+ by M. Whitman (Wawersik et al., 2005), FGF8a-pCS2+ by R. Harland (Fletcher et al., 2006) and eFGF-pCS2+ (Xenopus FGF4) by J. Slack (Isaacs et al., 1994). Nuclear-LacZ mRNA or 5-10ng lysine-fixable-fluorescein (FDX, 40,000 Mr; Molecular Probes) were used as lineage tracers. Where noted, dexamethasone (DEX) was added (final: 10μM).
Animal caps of different sizes were transplanted from FDX-injected embryos into uninjected hosts (stages 8.5-9; (Nieuwkoop and Faber, 1967). Embryos were allowed to heal in ¾ Normal Amphibian Medium (NAM) for 1 hour and grown overnight (to stage 19) in 1/10 NAM at 14°C. After healing, fluorescent and bright-field pictures of animal views of the embryos were taken. From these, the projected surface area of the transplanted tissue was calculated using ImageJ. Transplants were categorized as smaller or larger than a “typical” animal cap (Sive et al., 2000) and fate maps generated for each of these. Standardized outlines of embryos at stages 9 and 19 were created by averaging the outlines of 10 embryos at each stage. Fluorescence and bright-field photographs were taken after transplantation, just before fixation and after processing for Sox3 expression. Images of the embryos were then morphed to the standard outline and the overlap between transplanted areas in different embryos calculated.
Chick experiments
Fertilized hens' eggs (Brown Bovan Gold; Henry Stewart) were incubated at 38°C. Factors were delivered at stage 3+/4 (Hamburger and Hamilton, 1951) by electroporation, by grafting transfected COS cells or as proteins adsorbed to heparin-coated acrylic beads. Electroporation was performed (Sheng et al., 2003) using the following cloned into pCAβ: XSmad7 (Casellas and Brivanlou, 1998; De Almeida et al., 2008), cSmad6 (Yamada et al., 1999; Linker and Stern, 2004), cChordin (Streit et al., 1998), Xenopus truncated BMP receptor (tBR; (Suzuki et al., 1994) and cCerberus (Zhu et al., 1999; Bertocchini et al., 2004). Expression plasmids (pCDNAII) encoding Noggin (Streit and Stern, 1999b), Dkk1 (gift of E. Laufer; (Foley et al., 2000), Crescent (gift of P. Pfeffer and J.C. Izpisua-Belmonte; (Pfeffer et al., 1997) or soluble NFz8 (Deardorff et al., 1998) were used to transfect COS cells (Streit et al., 1998; Linker and Stern, 2004; De Almeida et al., 2008). FGF8 (R&D systems, 50μg/ml) was delivered on heparin beads (Streit et al., 2000). Movies of cultured embryos (New, 1955) were made as described (Foley et al., 2000).
In situ hybridisation and whole mount immunocytochemistry were performed as described (Stern, 1998). Sox2 produces background staining in grafted cell pellets; expression of the markers in the host was therefore assessed in histological sections.
A fluorescein-labelled morpholino (MO) against GATA2 was designed to target the first splicing site: GGGATGCTCATTTACCGTGTGCCTG. Fluorescent GATA3-MO targeted the initial ATG: AGACCTCCATCTTCCGCG. They were co-electroporated as described (Voiculescu et al., 2008).
Tissues from stage XII embryos were dissected using tungsten needles and cultured for 42 hours in collagen gels in medium-199 containing N2 supplement (Streit et al., 1997). Alternate wax sections were processed for in situ hybridization (Etchevers et al., 2001).
Results
BMP inhibition induces neural plate border markers in chick
It was previously shown that BMP inhibition does not induce neural markers (Sox3, Sox2) in chick ectoderm (Streit et al., 1998; Streit and Stern, 1999b; Linker and Stern, 2004). However it has not been determined whether this treatment induces neural plate border markers (prospective neural crest/placodes). Electroporation of Smad6 or Smad7 into the area opaca epiblast induces Pax7 (13/14; Fig. 1 A-C), Dlx5 (9/9; Fig. 1 D-F), Msx1 (9/10; not shown) and Slug (13/14; not shown) but not neural plate (Sox2: 0/23; Fig.1 A-F) or mesoderm (Brachyury: 0/37; Supplementary Fig. 1 A-L; (Linker and Stern, 2004). It is possible that Smad6 or -7 alone do not inhibit enough BMP-activity for full neural induction. However, even a combination of Smad6 + Smad7 + dominant-negative-BMP-receptor (dnBMPR) + Noggin + Chordin + Cerberus, together with FGF and Wnt inhibitors, fails to induce neural markers (Sox2: 0/11; Supplementary Fig. 1 J-L). Thus, although BMP inhibition is insufficient for neural induction, it does induce neural plate border markers.
Fig. 1. BMP inhibitors induce neural plate border markers in chick.
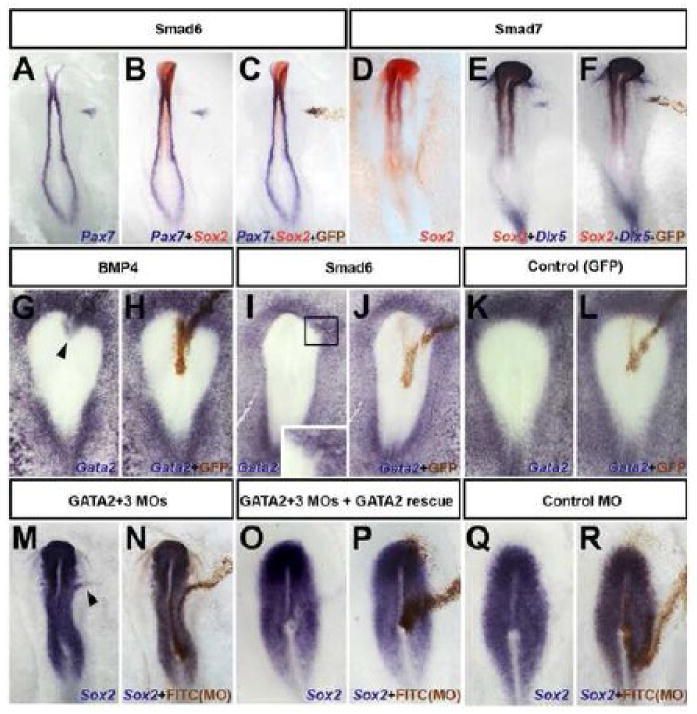
A-L. Electroporation of Smad6 or Smad7 in prospective epidermis induces Pax7 (A-C) and Dlx5 (E-F) in the absence of Sox2 (B-C and D-F). Electroporation of BMP4 induces Gata2 in the neural plate (G-H). Inhibition of BMP by Smad6 inhibits Gata2 at the neural border (I-J). GFP (control) does not affect Gata2 (K-L). M-R. Gata-2/-3 morpholinos expand Sox2 into the non-neural territory (M-N) (arrowhead), which is rescued by Gata2 (O-P), the slight down-regulation of Sox2 in the neural plate is an electroporation artefact; control morpholino has no effect (Q-R). Electroporated cells were stained with anti-GFP antibody (C,F,H,J,L, for the embryos to their left) or with anti-FITC antibody (N,P,R).
GATA2/3 plays a role in positioning the border
GATA2/3 are targets of BMP signaling (Maeno et al., 1996; Benchabane and Wrana, 2003; Kobielak et al., 2003; Dalgin et al., 2007; Dee et al., 2007) and also induce BMP4 expression (Sykes et al., 1998). Their expression abuts the anterior/lateral neural plate, partially overlapping with Sox2 and Sox3 at late primitive streak stages (Sheng and Stern, 1999). These observations implicate GATA2/3 as candidates to position the neural plate border, perhaps as mediators of BMP activity. To test this, we first confirmed that GATA2 is activated by BMP4 and inhibited by Smad6 in chick epiblast (BMP4: 5/6, Smad6: 7/8, Control: 0/5; Fig. 1 G-L). To test whether GATA2/3 function is required to define the lateral limits of the neural plate or its border, GATA2- and GATA3-morpholinos (MO) were co-electroporated as a line. This causes lateral expansion of Sox2 expression (6/7; Fig. 1 M-N), but the effect is much less dramatic than misexpression of BMP antagonists near the border of the neural plate (c.f. Fig. 2 A-D). Control-MO (0/7; Fig. 1 Q-R) had no effect and co-electroporation of GATA2 (lacking the GATA2-MO recognition sequence) rescued the consequences of MO electroporation (8/9; Fig. 1 O-P). These findings are consistent with work in Xenopus showing that although inhibition of GATA can mimic some effects of BMP-inhibition, it is not sufficient for neuralization (Sykes et al., 1998). Together, these results implicate GATA2/3 in positioning the neural border, where it may act as a mediator of BMP activity. However, GATA2/3 activity does not completely account for all BMP effects.
Fig. 2. Only the border of the neural plate is sensitive to BMP in chick.
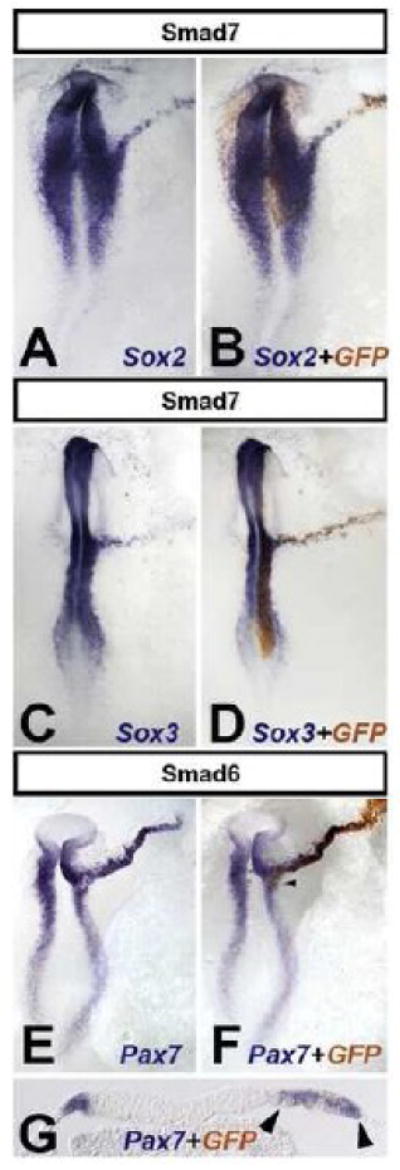
Electroporation of Smad6 or Smad7 as a line extending out from the neural plate induce an expansion in the expression of Sox2 (A-B), Sox3 (C-D) and Pax7 (E-G). G is a section through the embryo in F (arrowhead), showing non-cell-autonomous expansion of Pax7 (arrowheads). Electroporated cells were stained with anti-GFP antibody (B, D, F and G for the embryos to their left).
Expansion of the neural plate by BMP-inhibition requires cellular continuity of BMP-inhibited cells to the neural plate or its border
Studies using grafts of Chordin- or Noggin-secreting cells have shown that inhibition of BMP affects neural/epidermal choice only at the neural plate border (Streit et al., 1998; Streit and Stern, 1999b; Linker and Stern, 2004). To test whether cell-autonomous BMP antagonists can reproduce this effect, we electroporated Smad7, Smad6 or dominant-negative BMP-receptor (dnBMPR) as a line extending outwards from the prospective neural plate. These treatments cause a marked extension in the expression of Sox2 and Sox3 into the prospective epidermis and even into the extraembryonic area opaca (Sox2: 11/14 [Smad7; Fig. 2 A-B], 20/21 [Smad6; not shown], 4/5 [dnBMPR; not shown], 0/25 [GFP control; not shown]; Sox3: 7/7 [Smad7; Fig. 2 C-D], 8/8 [Smad6; not shown], 0/21 [GFP control; not shown]). Expression of neural plate border markers is also dramatically extended (Pax7: 18/18 [Fig. 2 E-G]; Slug: 11/12 non shown). Surprisingly, Pax7 is not restricted to the Smad-electroporated cells (Fig. 2 G) but is also seen in neighboring, non-electroporated cells.
This last observation raises the possibility that cells from the host neural plate are stimulated to migrate laterally when BMP is inhibited. To test this, we compared cell movements between the electroporated side and the contralateral side (marked with DiI). No differences were observed between the two sides (Supplementary Movie 1), showing that the expansion of neural plate and border markers by misexpression of cell-autonomous BMP antagonists is due to induction rather than cell recruitment. Together, these results suggest that chick non-neural ectoderm cells can only be induced to express neural markers by BMP-inhibition when these cells form a continuous trail to the neural plate or its border. Without such continuity, only border markers are induced.
Cellular continuity with the neural plate or its border is necessary for neural induction by BMP-inhibition in Xenopus
Does Xenopus ectoderm respond in a similar way? It has been shown that BMP-inhibition is not sufficient to induce neural markers in prospective epidermis (descendants of the A4 blastomeres) and that neural markers are only induced in ventral epidermis by BMP-antagonists when eFGF is also supplied (Linker and Stern, 2004; Delaune et al., 2005). A similar combination (FGF4+Smad6 or Smad7) in chick induces mesodermal markers (Linker and Stern, 2004), raising the possibility that the neural induction by this combination in Xenopus is indirect.
First, we confirmed our previous results: inhibition of BMP by injection of Smad6 (1ng) or ΔSmad7 (10pg) (Wawersik et al., 2005) does not induce neural markers when injected into the A4 blastomeres (Sox3 [Smad6 0/70; ΔSmad7 0/237] or Sox2 [Smad6 0/60; ΔSmad7 0/324] Fig. 3 A-D and Supplementary Fig. 2 A-D). Injection of a combination of Smad6 (1ng) or ΔSmad7 (10pg) and eFGF (0.16pg) in these blastomeres is now able to induce neural markers (Sox3 [Smad6 108/120; ΔSmad7 85/103] Sox2 [Smad6 46/71; ΔSmad7 81/102] Fig. 3 E-H, N-O for Smad6 and Supplementary Fig 2. E-H for ΔSmad7).
Fig. 3. BMP inhibition together with eFGF activation induces neural marker expression indirectly in Xenopus.
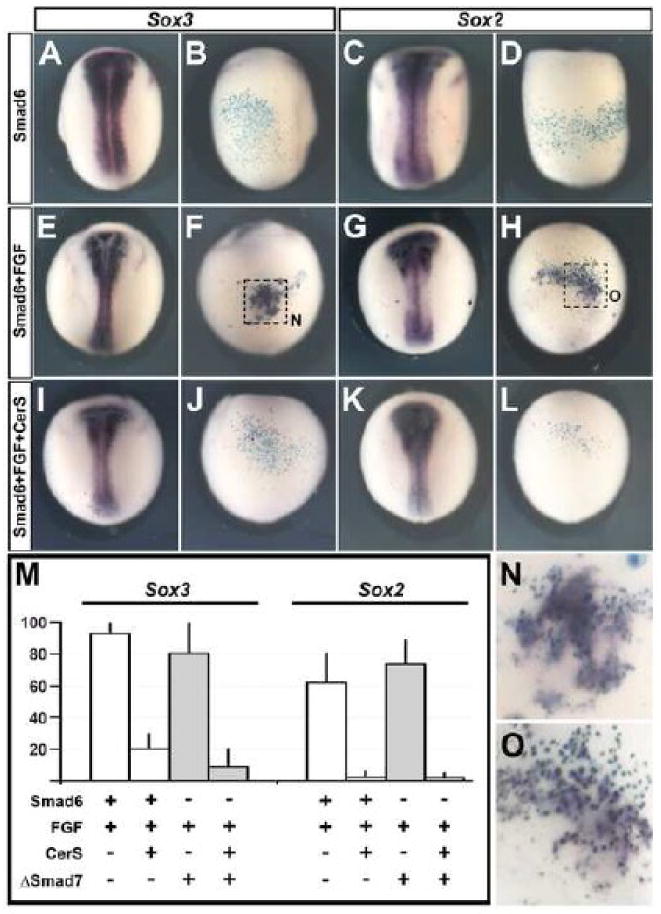
A-L. Inhibition of BMP by injection of Smad6 into the A4 blastomere does not induce either Sox3 (A-B) or Sox2 (C-D) expression. eFGF together with BMP inhibition into the A4 blastomere induces Sox3 (E-F, N) and Sox2 (G-H, O). Neural induction by the former combination is inhibited when Nodal signaling is blocked: injection of Smad6 + eFGF together with CerS no longer induces Sox3 (I-J) or Sox2 (K-L). M. Quantification of Sox3 and Sox2 expression in the different experiments described above. A, C, E, G, I and K dorsal views. B,D,F,H,J and L ventral views of the embryos to their left. N and O are enlargements of the areas enclosed by a square in F and H, respectively.
Next, we analysed whether neural induction by BMP inhibition and FGF activation requires mesoderm. We co-injected Smad6 (1ng) or ΔSmad7 (10pg) and eFGF (0.16pg) together with the nodal inhibitor CerS. To test the effectiveness of CerS, we injected CerS in the whole embryo (4 cells at the 4 cell stage, 1.5-2ng). This inhibits the formation of mesoderm (MyoD 0/90, chordin 0/91, brachyury 0/102; not shown) and completely prevents gastrulation, as previously reported (Piccolo et al., 1999). We then tested whether inhibition of Nodal signaling and mesendoderm formation by CerS affects the induction of neural markers by BMP-inhibition+eFGF. Strikingly, co-injection of CerS + eFGF + Smad6 or ΔSmad7 into one A4 blastomere strongly reduces the induction of Sox3 (Smad6 from 93% to 20.4%; n=212, Fig. 3 I-J and M or ΔSmad7 from 82.5% to 6.5%; n=195, Supplementary Fig. 2 I-J) and virtually abolishes induction of Sox2 (Smad6 from 62% to 2.7%; n=152, Fig. 3 K-M; ΔSmad7 from 79.4% to 1.4%; n=174, Supplementary Fig. 2 K-L). Together, these data suggest that in Xenopus embryos, as in the chick, the induction of neural markers by eFGF and BMP antagonism is indirect, due to either a prior induction of mesendoderm or to cooperation with Nodal signaling (see also (De Almeida et al., 2008).
To determine whether the activity of eFGF is due to its mesendoderm-inducing ability, we examined whether FGF8a (an isoform without mesoderm inducing activity; (Fletcher et al., 2006) can induce neural markers when injected in combination with BMP inhibitors into ventral epidermis. First, to test the effectiveness of FGF8a, 10-50pg were injected into one cell at the two-cell stage. This did not affect the expression of a mesodermal marker (Brachyury 0/60; Fig 4 A), but did expand neural markers (Sox3 18/23 not shown, β-tubulin 25/28; Fig 4 B), as expected (Fletcher et al., 2006). Next, we tested the effects of injection of FGF8a (10-50pg) into the A4 blastomere: neither mesodermal nor neural markers were induced (Chordin 0/40, β-tubulin 0/17, Sox2 0/6, not shown, Sox3 0/30; Fig 4 C-D), as was reported for eFGF (Linker and Stern, 2004; Delaune et al., 2005). We then tested if co-injection of FGF8a (10-50pg) + Smad6 (1ng) can induce neural markers in ventral epidermis: neither neural (Sox2 0/24 not shown, Sox3 0/23; Fig 4 E-F), nor mesodermal markers (Chordin 0/31, not shown) were induced. These results strengthen our previous suggestion that induction of neural markers by FGF activation and BMP antagonism an indirect consequence of mesendoderm induction.
Fig. 4. BMP inhibition together with FGF8a does not induce neural marker expression in Xenopus.
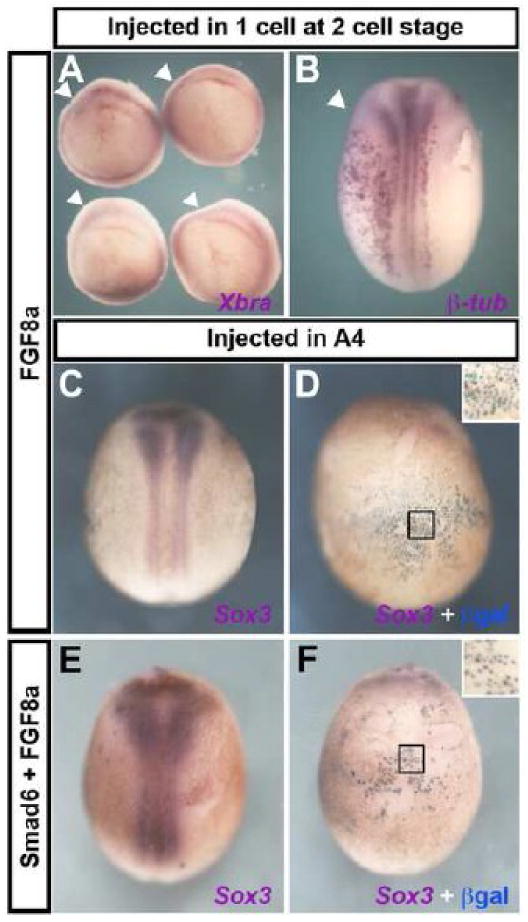
A-B. Injection of FGF8a into one cell at the two-cell stage does not alter Brachyury expression at the gastrula stage (A) but does expand β-tubulin expression at the neurula stage (B); arrowheads indicate the injected side. C-F. Injection of FGF8a into an A4 blastomere, alone (C-D) or in combination with the BMP inhibitor Smad6 (E-F) does not induce Sox3 expression in ventral epidermis. A: vegetal view; B, C and E are dorsal views; D and F are ventral views of the embryos to their left. Black squares show the area enlarged in the inset in panels D and F.
We then analyzed whether BMP-antagonists induce border markers in ventral epidermis in Xenopus, as shown above for chick embryos. Indeed, injection of Smad6 into the A4 blastomere induces the neural border markers Pax3 (20/26; Fig. 5 A-C), Slug (62/73; Fig. 5 D-F), Hairy2A (22/33; Fig. 5 G-I) and Xiro1 (17/19; not shown), but not neural markers (Sox2, Sox3; Fig. 3 A-D). Thus, as in chick, BMP inhibition in Xenopus ventral epidermis induces neural plate border markers.
Fig. 5. Only the border of the neural plate is sensitive to BMP inhibition in Xenopus.
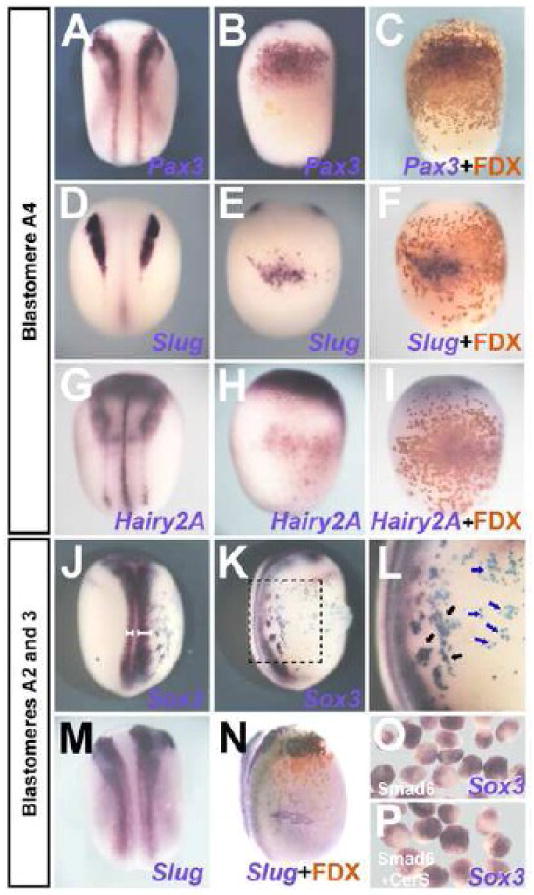
A-I. Smad6 (1ng) injection into the A4 blastomere induces Pax3 (A-C), Slug (D-F) and Hairy2A (G-I). (A, D, and G: dorsal view; B, C, E, F, H and I: ventral view of the embryo to their left). J-N. Injection into blastomere A2/3 expands Sox3 (J-L) and Slug (M, N). J, M: dorsal view; K, L and N are lateral views of the embryos to their left. White brackets in J show the extension of the neural plate in the injected and non-injected sides of the embryo. The black square in K indicates the area enlarged in L. Black arrows in L point to injected cells adjacent to the endogenous neural plate, expressing Sox3; the blue arrows point to injected cells distant from the endogenous neural plate, which do not express Sox3. Injected cells were recognized by FDX or LacZ (C, F, I, K, L and N, for embryos to their left). O-P. Animal caps from Smad6-injected embryos at the 2-cell stage express Sox3 (O), which is not inhibited by CerS (P).
Finally, we examined if the border of the Xenopus neural plate is especially sensitive to BMP-inhibition, as it is in chick. Injection of Smad6 into the prospective neural plate border (blastomeres A2/3) causes lateral expansion of Sox3 (43/45; Fig. 5 J-L; white brackets in J and black arrows in L) and Slug (38/42; Fig. 5 M-N). These results in chick and Xenopus show that although border markers can be induced by BMP-inhibition in lateral/ventral epidermis, neural induction in the same cells requires the BMP-inhibited cells to form a continuous trail to the neural plate and/or its border.
The Xenopus animal cap behaves like a neural plate border and contains prospective border cells
The above results are at odds with the widely reported finding that Xenopus animal caps, thought to contain cells destined to contribute to epidermis but not neural tissue, can be neuralized easily by BMP antagonists (Harland, 2000; Muñoz-Sanjuán and Brivanlou, 2002; De Robertis and Kuroda, 2004; Vonica and Brivanlou, 2006). We therefore performed animal cap assays: animal caps were isolated at stage-8 from embryos injected with Smad6 in the animal pole at the 2-cell-stage. Unlike injections into A4, animal pole injections of Smad6 induce Sox3 (Fig. 5O; 38/38). Moreover, co-injection of Smad6+CerS does not inhibit Sox3 induction in animal caps (Fig. 5P; 50/53). This confirms that animal caps can be neuralized by BMP-antagonism and that this is insensitive to Nodal signaling.
The observation that BMP inhibited cells can express neural markers if they form a continuous trail to the neural plate or its border, together with the fact that animal caps are easily neuralized by BMP antagonists, prompted us to test whether animal caps contain prospective neural plate or border cells. To this end, we assessed the contribution of animal cap cells to the neural plate and the neural/epidermal border by fate mapping animal caps. Donor embryos were injected with fluorescein-lysine dextran (FDX) in both cells at the 2 cell stage, and the animal cap excised from these embryos at stage 8. The excised tissue was grafted into an identical region of unlabeled host embryos at the same stage and analyzed at stage-19, examining both fluorescence as a lineage tracer and expression of the neural marker Sox3 (Fig. 6 A-C). The outlines of all small and all large transplants, at stage 8 and stage 19, were drawn in separate model embryo outlines (see Materials and Methods; Fig. 6 D-E and H-I). In Fig. 6 F and J (stage 8) and G and K (stage 19), the areas that receive a cellular contribution from 60%, 80% and 93% of the transplants are shown in red, orange and yellow, respectively. At stage 19, the region expressing Sox3 is also shown (grey; Fig. 6 G, K). Surprisingly, 60% of even the smallest caps (Fig. 6 D-G) contribute to the anterior neural plate itself and virtually all caps (>80%) contribute to the anterior neural/epidermal border (prospective placodes; Fig. 6 D-K). These data show that nearly all animal caps dissected at stage 8 contain neural plate and/or neural plate border cells.
Fig. 6. The Xenopus animal cap contains cells fated as anterior neural border.
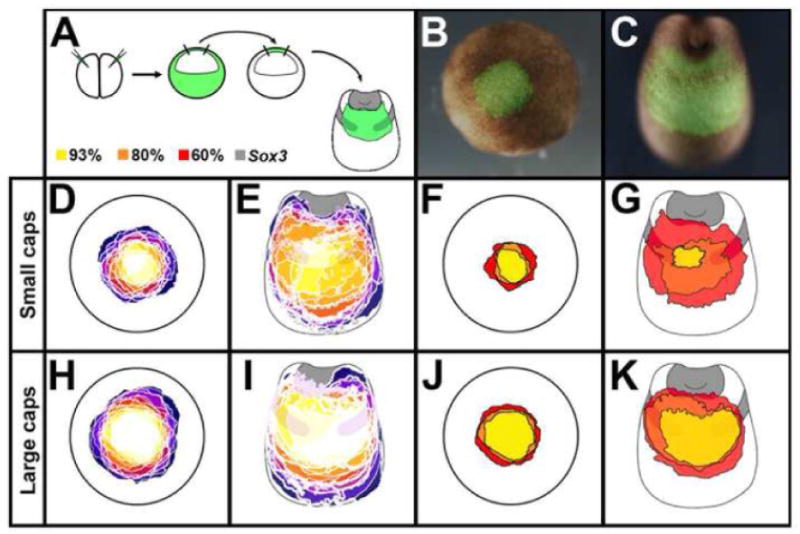
A. Caps from FDX-injected embryos were obtained at stage 8, transplanted to uninjected hosts and analysed for Sox3 at stage 19. B. Example of a transplant at st.8 after 1.5h healing, the same embryo at st.19 (C). D-K. Results of all small (D-G; n=14) and large (H-K; n=15) transplants, each in a different colour, at stages 9 (D,H,F,J) and 19 (E,G,I,K; including Sox3 expression). In D-E and H-I, the regions of overlap are shown in progressively lighter shades, with white indicating a region where all transplanted caps overlap. In F-G and J-K, the areas that receive a cellular contribution from the transplant are in Yellow: 93%; Orange: 80%; Red: 60%.
Chick epiblast explants behave like the Xenopus animal cap
It has been reported that explants of “lateral” chick epiblast (mainly prospective non-neural ectoderm) can be induced to express neural markers in response to BMP antagonists in culture (Wilson et al., 2000; Wilson et al., 2001). The above results raise the possibility that chick explants are equivalent to Xenopus animal caps and are specified as border cells. To assess this we dissected “medial” and “lateral” epiblast (Wilson et al., 2000; Wilson et al., 2001) from stage-XII (Eyal-Giladi and Kochav, 1976) chicken embryos and assessed expression of neural and neural border markers after 42 hours' culture (Fig. 7A). Both medial and lateral explants express neural (Sox3: medial 10/11, lateral 8/8; Sox2: medial 10/10, lateral 6/6; Fig. 7 B-E) and neural border markers (Pax7: medial 9/9, lateral 8/8; Slug: medial 8/11, lateral 9/10; Msx1: medial 5/9, lateral 8/8; Fig. 7 F-K). These results suggest that under these conditions, epiblast explants from any embryonic region are specified as neural plate and its border, explaining the discrepancy between the results of BMP inhibition in vivo (Streit et al., 1998; Streit and Stern, 1999b; Linker and Stern, 2004; De Almeida et al., 2008) and in vitro (Wilson et al., 2000; Wilson et al., 2001).
Fig. 7. Chick epiblast explants express neural plate and border markers.
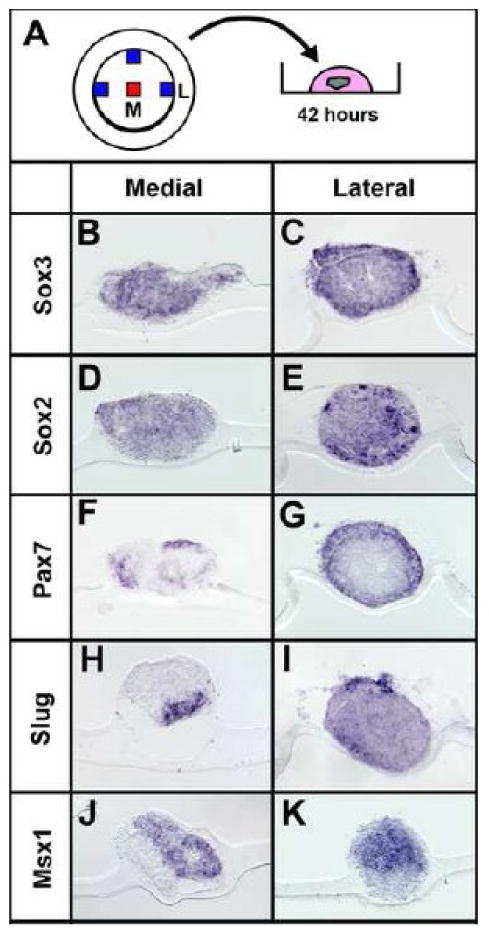
A-K. Medial (M) or lateral (L) stage-XII epiblast explants were analysed for neural plate/border markers in alternate sections. All express Sox3 (B-C), Sox2 (D-E), Pax7 (F-G), Slug (H-I) and Msx1 (J-K).
Discussion
BMP-inhibited cells express neural markers only when they form a continuous trail to the neural plate or its border
The default model proposes that BMP inhibition is the only necessary signal for neural induction. Although its simplicity made it very attractive, there has been considerable debate about whether this mechanism is sufficient to explain neural induction (for reviews see (Streit and Stern, 1999a; Stern, 2005, 2006). The current prevailing view is that additional factors are required, and in particular that FGF signaling is important. At least in the chick, BMP inhibitors alone have not been shown to induce any markers (neural or otherwise) in vivo to date. Here we show that BMP inhibition induces border markers in non-neural ectoderm of both chick and Xenopus embryos, but neural induction in the same cells only occurs if the BMP-inhibited cells form a continuous trail connecting them to the neural plate and/or its border. These findings suggest that neuralizing factors emanating from the neural plate spread through the ectoderm (“homeogenetic induction”, or induction of neural plate by neural plate; (Mangold and Spemann, 1927; Mangold, 1929, 1933; Nieuwkoop et al., 1952; Servetnick and Grainger, 1991), but only between BMP-inhibited cells.
We propose a relay mechanism by which homeogenetic signals can spread from the neural plate only through cells in which BMP signaling is inhibited. Overexpression of BMP inhibitors in cells adjacent to the neural plate or its border allows these cells to respond to homeogenetic neural inducers emanating from the neural plate, resulting in an expansion of the neural territory. In contrast, cells distant from the endogenous neural plate (e.g. the progeny of the A4 blastomere in Xenopus or distant epiblast cells in chick, which are competent to make neural tissue in response to an organizer graft or to mesoderm generated by co-injection of eFGF and Samd6) cannot receive homeogenetic inducing signals unless they are connected to it by a continuous trail of BMP-inhibited cells. This provides an explanation for why not all cells injected with BMP antagonist express neural markers (Fig. 5L). Only those cells that are adjacent to the neural plate (black arrows, Fig. 5 L) express neural markers, while cells distant from it (blue arrows, Fig. 5 L) do not.
However, these results also generate a paradox. BMP inhibition in prospective epidermis induces border markers but not neural markers, while inhibition of BMP at the border does induce neural markers. Despite this, increasing the amount of BMP inhibition (in the case of chick even by a combination of Smad6 + Smad7 + dnBMPR + Noggin + Chordin + Cerberus, together with FGF and Wnt inhibitors) outside the border is not sufficient to induce neural markers. This suggests that the border markers induced by BMP inhibitors alone (Slug, Pax7, Dlx5, Msx1) are not indicative of induction of a full-fledged border, and that other factors must also be important. This is consistent with one model of neural crest induction proposing that signals from the underlying mesoderm are required along with BMP-inhibition for neural crest to be specified (Streit and Stern, 1999b; Meulemans and Bronner-Fraser, 2004; Steventon et al., 2005).
What could be the missing signals, present in the neural plate, which can only travel between BMP-inhibited cells? A possible candidate is the Notch pathway, which has been implicated in establishing the border of the neural plate (Kintner, 1992; Cornell and Eisen, 2002; Endo et al., 2002; Glavic et al., 2004) as well as generating boundaries between adjacent domains in many other systems (Bray, 1998; Sanson, 2001; Bray, 2006). Preliminary experiments with Notch inhibitors (DAPT) or NICD overexpression did not produce clear results (unpublished observations), but do not exclude this possibility, which requires further investigation.
The animal cap behaves like the neural plate border and contributes cells to it
The above results prompted us to explore whether animal caps, which are so easily neuralized by misexpression of BMP antagonists, might contain some neural plate and/or border cells. Systematic fate mapping of animal caps of a wide range of sizes revealed that even the smallest caps contribute cells to the anterior neural plate itself in as many as 60% of cases, and nearly all caps contribute to the prospective placodal domain at the border of the anterior neural plate. Furthermore, isolated animal caps express both anterior neural (Otx2) and border (XAG1/XCG1) markers (Lamb et al., 1993; Knecht et al., 1995; Lamb and Harland, 1995). Although these data are consistent with previous fate maps made at the 32 cell stage (Dale and Slack, 1987; Moody, 1987a, 1987b), our results provide the first demonstration that virtually all animal caps excised at stage 8 contain cells fated to became neural plate border. These findings explain why animal caps can be neuralized so easily by BMP-antagonists.
Thus, animal cap explants contain prospective border cells (as well as prospective neural plate in many cases). This implies that when animal cap assays from BMP-antagonist-injected embryos are used for assessing neural induction, the animal cap preserves cellular continuity between the prospective neural plate/neural plate border and prospective epidermis, through which neural inducing signals can spread (see above). This may also explain why neural marker expression is always restricted to a subset of cells in animal caps excised from BMP-inhibited embryos (e.g.: Fig. 5 O-P). We (Fig. 7) and others (Wilson et al., 2000; Wilson et al., 2001) have made similar observations in explants of chick epiblast.
The animal cap assay was designed by Nieuwkoop to study mesoderm induction (Nieuwkoop, 1969b; Nieuwkoop, 1969a), because the animal pole does not contain prospective mesoderm. It is indisputable that, in addition to providing an understanding of mesodermal induction (e.g.: (Slack et al., 1987; Green et al., 1990; Green and Smith, 1990; Kimelman and Bjornson, 2004), this assay has also identified a number of important functions of BMP signaling (Smith and Harland, 1992; Sasai et al., 1994; Zimmerman et al., 1996). However, our finding that animal caps excised from stage 8 embryos contain prospective neural plate and border cells suggests that this assay is not suitable for studying neural induction because it cannot distinguish between “permissive” (stabilising) and true “instructive” induction (Gurdon, 1987; Streit and Stern, 1999a; Stern, 2001). We propose that experiments targeting the A4 blastomere in Xenopus and peripheral misexpression in the chick, provided that the cells are not contiguous to the neural plate, are more rigorous assays for neural inducing signals than animal cap assays or chick epiblast explants.
Interestingly, fate and specification maps of pre-primitive-streak-stage chick embryos reveal that almost the entire epiblast contributes cells to the neural plate and/or its border (Rudnick, 1935, 1938; Hatada and Stern, 1994). One difference between the Xenopus animal cap and the chick explant assays is that neural markers are only expressed in the latter. One possible reason for this difference is that chick explants are grown in the presence of complex culture medium that includes N2 supplement (containing a number of factors including insulin, transferrin and others, intended to promote neural differentiation) whereas Xenopus animal caps are cultured in simple saline. Together, our results suggest that candidate neural inducing signals revealed by chick epiblast explants or Xenopus animal cap assays need to be validated in ectodermal cells distant from the endogenous neural plate and its border.
Supplementary Material
Supplementary Fig. 1. Inhibition of BMP and Wnt together with activation of FGF signaling does not induce neural or mesodermal tissue in the chick.
None of the following combinations induces either Brachyury (37/37 light blue in A, D, G, and J) or Sox2 (0/30; dark blue in B, E, H and K, the same embryo to the left): FGF + Smad6 + Smad7 (0/11; J-L); FGF + Smad7 + Wnt antagonists (Dkk1+NFz8+Crescent) + Noggin + dnBMPR + Cerberus + Chordin (0/7; D-F); FGF + Smad6 + Smad7 + Wnt antagonists (0/9; G-I); FGF + Smad6 + Smad7 + Wnt antagonists + Noggin + dnBMPR + Cerberus + Chordin (0/10; J-L). Electroporated cells were recognized by GFP expression (C, F, I and L, in the embryo shown to their left). In D-L, pellets of a mixture of COS cells transfected with Wnt antagonists (Dkk1+NFz8+Crescent, 1000 cells per factor) were grafted. In situ hibridization with Sox2 always produces background staining in the grafted cell pellets; therefore expression of this marker in the host was assessed in histological sections. The plane of section is indicated by a black line.
Supplementary Fig. 2. BMP inhibition by ΔSmad7 together with eFGF activation induces neural marker expression indirectly in Xenopus.
A-D. Inhibition of BMP by injection of ΔSmad7 into one A4 blastomere does not induce either Sox3 (A-B) or Sox2 (C-D) expression. E-F. Injection of eFGF together with the BMP inhibitor ΔSmad7 into an A4 blastomere induces Sox3 (E-F) and Sox2 (G-H, O). I-L. Neural induction by the former combination is inhibited when Nodal signaling is blocked: injection of ΔSmad7 + eFGF together with CerS no longer induces Sox3 (I-J) or Sox2 (K-L). A, C, E, G, I and K are dorsal views; B, D, F, H, J and L are ventral views of the embryos to their left. White squares mark enlargements of the area in the inserts in panels F and H.
Supplementary Movie 1: Cell-autonomous inhibition of BMP does not affect cell movements of epiblast cells in the chick.
On the left, a line of cells (extending out from the neural plate border) was labelled with DiI. On the right, a similar line of cells was electroporated with Smad6 using a vector also containing GFP. The fluorescence of DiI and GFP are both pseudo-coloured red. Time-lapse sequence (1 frame every 10 min). No significant difference in the patterns of movement is seen between the control and experimental sides.
Acknowledgments
We are indebted to Sharon Boast for technical support, Octavian Voiculescu for help with data analysis, to Federica Bertocchini for useful discussions and to the members of the Mayor and Dale laboratory for their invaluable help. This study was funded by MRC, BBSRC, NIH (GM60156) and the EU Network-of-Excellence “Cells into Organs”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Claudia Linker, Department of Cell and Developmental Biology, University College London, Gower Street (Anatomy Building), London WC1E 6BT, U.K..
Irene de Almeida, Department of Cell and Developmental Biology, University College London, Gower Street (Anatomy Building), London WC1E 6BT, U.K..
Costis Papanayotou, Department of Cell and Developmental Biology, University College London, Gower Street (Anatomy Building), London WC1E 6BT, U.K..
Matthew Stower, Department of Cell and Developmental Biology, University College London, Gower Street (Anatomy Building), London WC1E 6BT, U.K..
Virginie Sabado, Department of Craniofacial Development, King's College London, Guy's Tower, London SE1 9RT, U.K..
Ehsan Ghorani, Department of Cell and Developmental Biology, University College London, Gower Street (Anatomy Building), London WC1E 6BT, U.K..
Andrea Streit, Department of Craniofacial Development, King's College London, Guy's Tower, London SE1 9RT, U.K..
Roberto Mayor, Department of Cell and Developmental Biology, University College London, Gower Street (Anatomy Building), London WC1E 6BT, U.K..
Claudio D. Stern, Department of Cell and Developmental Biology, University College London, Gower Street (Anatomy Building), London WC1E 6BT, U.K..
References
- Benchabane H, Wrana JL. GATA- and Smad1-dependent enhancers in the Smad7 gene differentially interpret bone morphogenetic protein concentrations. Mol Cell Biol. 2003;23:6646–6661. doi: 10.1128/MCB.23.18.6646-6661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchini F, Skromne I, Wolpert L, Stern CD. Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development. 2004;131:3381–3390. doi: 10.1242/dev.01178. [DOI] [PubMed] [Google Scholar]
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin Cell Dev Biol. 1998;9:591–597. doi: 10.1006/scdb.1998.0262. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Casellas R, Brivanlou AH. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev Biol. 1998;198:1–12. doi: 10.1006/dbio.1998.8893. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Eisen JS. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing Neurogenin 1 function. Development. 2002;129:2639–2648. doi: 10.1242/dev.129.11.2639. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Dalgin G, Goldman DC, Donley N, Ahmed R, Eide CA, Christian JL. GATA-2 functions downstream of BMPs and CaM KIV in ectodermal cells during primitive hematopoiesis. Dev Biol. 2007;310:454–469. doi: 10.1016/j.ydbio.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida I, Rolo A, Batut J, Hill C, Stern CD, Linker C. Unexpected activities of Smad7 in Xenopus mesodermal and neural induction. Mech Dev. 2008 doi: 10.1016/j.mod.2008.02.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Tan C, Conrad LJ, Klein PS. Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development. 1998;125:2687–2700. doi: 10.1242/dev.125.14.2687. [DOI] [PubMed] [Google Scholar]
- Dee CT, Gibson A, Rengifo A, Sun SK, Patient RK, Scotting PJ. A change in response to Bmp signalling precedes ectodermal fate choice. Int J Dev Biol. 2007;51:79–84. doi: 10.1387/ijdb.062204cd. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Endo Y, Osumi N, Wakamatsu Y. Bimodal functions of Notch-mediated signaling are involved in neural crest formation during avian ectoderm development. Development. 2002;129:863–873. doi: 10.1242/dev.129.4.863. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H, Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Foley AC, Skromne I, Stern CD. Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development. 2000;127:3839–3854. doi: 10.1242/dev.127.17.3839. [DOI] [PubMed] [Google Scholar]
- Glavic A, Silva F, Aybar MJ, Bastidas F, Mayor R. Interplay between Notch signaling and the homeoprotein Xiro1 is required for neural crest induction in Xenopus embryos. Development. 2004;131:347–359. doi: 10.1242/dev.00945. [DOI] [PubMed] [Google Scholar]
- Green JB, Howes G, Symes K, Cooke J, Smith JC. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990;108:173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Gurdon JB. Embryonic induction - molecular prospects. Development. 1987;99:285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harland R. Neural induction. Curr Opin Genet Dev. 2000;10:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Hatada Y, Stern CD. A fate map of the epiblast of the early chick embryo. Development. 1994;120:2879–2889. doi: 10.1242/dev.120.10.2879. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997a;88:13–17. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate neural induction. Annu Rev Neurosci. 1997b;20:43–60. doi: 10.1146/annurev.neuro.20.1.43. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. Embo J. 1994;13:4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D, Bjornson C. Vertebrate mesoderm induction. In: Stern CD, editor. Gastrulation: from cells to embryo. Cold Spring Harbor Press; New York: 2004. pp. 363–372. [Google Scholar]
- Kintner C. Molecular bases of early neural development in Xenopus embryos. Annu Rev Neurosci. 1992;15:251–284. doi: 10.1146/annurev.ne.15.030192.001343. [DOI] [PubMed] [Google Scholar]
- Knecht A, Good PJ, Dawid IB, Harland RM. Dorsal-ventral patterning and differentiation of noggin-induced neural tissue in the absence of mesoderm. Development. 1995;121:1927–1936. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–5681. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Maeno M, Mead PE, Kelley C, Xu RH, Kung HF, Suzuki A, Ueno N, Zon LI. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood. 1996;88:1965–1972. [PubMed] [Google Scholar]
- Mangold O, Spemann H. Über Induktion von Medullarplatte durch Medullarplatte im jüngeren Keim, ein Beispiel homöogenetischer oder assimilatorischer Induktion. Wilhem Roux Arch EntwMech Org. 1927;111:341–422. doi: 10.1007/BF02080953. [DOI] [PubMed] [Google Scholar]
- Mangold O. Experimente zur Analyse der determination und induktion der Medullarplatte. W Roux Arch EntwMech Org. 1929;117:586–696. doi: 10.1007/BF02110974. [DOI] [PubMed] [Google Scholar]
- Mangold O. Über die Induktionsfähighkeit der verschiedenen Bezirke der Neurula von Urodelen. Naturwissenshaften. 1933;21:761–766. [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol. 1987a;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987b;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Muñoz-Sanjuán I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- New DAT. A new technique for the cultivation of the chick embryo in vitro. J Embryol exp Morph. 1955;3:326–331. [Google Scholar]
- Nieuwkoop PD, Botternenbrood EC, Kremer A, Bloesma FFSN, Hoessels ELMJ, Meyer G, Verheyen FJ. Activation and organization of the Central Nervous System in Amphibians. J Exp Zool. 1952;120:1–108. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) North-Holland Publ. Co.; Amsterdam: 1967. [Google Scholar]
- Nieuwkoop PD. The formation of mesoderm in urodelean amphibians I. Induction by the endoderm. W Roux Arch EntwMech Org. 1969a;162:341–373. doi: 10.1007/BF00578701. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD. The formation of mesoderm in Urodelean amphibians. II. The origin of the dorso-ventral polarity of the mesoderm. Wilh Roux' Arch EntwMech Organ. 1969b;163:298–315. doi: 10.1007/BF00577017. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, De Robertis EM, Izpisua-Belmonte JC. Crescent, a novel chick gene encoding a Frizzled-like cysteine-rich domain, is expressed in anterior regions during early embryogenesis. Int J Dev Biol. 1997;41:449–458. [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick D. Regional restriction of potencies in the chick during embryogenesis. Journal of Experimental Zoology. 1935;71:83–99. [Google Scholar]
- Rudnick D. Differentiation in culture of pieces of the early chick blastoderm. The Anatomical Record. 1938;70:351–368. [Google Scholar]
- Sanson B. Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep. 2001;2:1083–1088. doi: 10.1093/embo-reports/kve255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servetnick M, Grainger RM. Homeogenetic neural induction in Xenopus. Dev Biol. 1991;147:73–82. doi: 10.1016/s0012-1606(05)80008-9. [DOI] [PubMed] [Google Scholar]
- Sheng G, Stern CD. Gata2 and Gata3: novel markers for early embryonic polarity and for non-neural ectoderm in the chick embryo. Mech Dev. 1999;87:213–216. doi: 10.1016/s0925-4773(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- Sive H, Grainger RM, Harland RM. Early development of Xenopus laevis: A laboratory Manual. Cold Spring Harbor Press; New York: 2000. [Google Scholar]
- Slack JMW, Darlington BG, Heath JK, Godsave SF. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Roux' Arch EntwMech Org. 1924;100:599–638. [Google Scholar]
- Stern CD. Detection of multiple gene products simultaneously by in situ hybridization and immunohistochemistry in whole mounts of avian embryos. In: de Pablo F, Ferrus A, Stern CD, editors. Cellular and Molecular Procedures in Developmental Biology. Vol. 36. Academic Press; San Diego: 1998. pp. 223–244. [DOI] [PubMed] [Google Scholar]
- Stern CD. Initial patterning of the central nervous system: how many organizers? Nat Rev Neurosci. 2001;2:92–98. doi: 10.1038/35053563. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: 10 years on since the ‘default model’. Curr Opin Cell Biol. 2006;18:692–697. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin Cell Dev Biol. 2005;16:647–654. doi: 10.1016/j.semcdb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Streit A, Sockanathan S, Perez L, Rex M, Scotting PJ, Sharpe PT, Lovell-Badge R, Stern CD. Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development. 1997;124:1191–1202. doi: 10.1242/dev.124.6.1191. [DOI] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Neural induction. A bird's eye view. Trends Genet. 1999a;15:20–24. doi: 10.1016/s0168-9525(98)01620-5. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999b;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev. 1999c;85:85–96. doi: 10.1016/s0925-4773(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes TG, Rodaway AR, Walmsley ME, Patient RK. Suppression of GATA factor activity causes axis duplication in Xenopus. Development. 1998;125:4595–4605. doi: 10.1242/dev.125.23.4595. [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Papanayotou C, Stern CD. Spatially and temporally controlled electroporation of early chick embryos. Nature Protocols. 2008;3:419–426. doi: 10.1038/nprot.2008.10. [DOI] [PubMed] [Google Scholar]
- Vonica A, Brivanlou AH. An obligatory caravanserai stop on the silk road to neural induction: inhibition of BMP/GDF signaling. Semin Cell Dev Biol. 2006;17:117–132. doi: 10.1016/j.semcdb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Evola C, Whitman M. Conditional BMP inhibition in Xenopus reveals stage-specific roles for BMPs in neural and neural crest induction. Dev Biol. 2005;277:425–442. doi: 10.1016/j.ydbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Yamada M, Szendro PI, Prokscha A, Schwartz RJ, Eichele G. Evidence for a role of Smad6 in chick cardiac development. Dev Biol. 1999;215:48–61. doi: 10.1006/dbio.1999.9419. [DOI] [PubMed] [Google Scholar]
- Zhu L, Marvin MJ, Gardiner A, Lassar AB, Mercola M, Stern CD, Levin M. Cerberus regulates left-right asymmetry of the embryonic head and heart. Current Biology. 1999;9:931–938. doi: 10.1016/s0960-9822(99)80419-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Inhibition of BMP and Wnt together with activation of FGF signaling does not induce neural or mesodermal tissue in the chick.
None of the following combinations induces either Brachyury (37/37 light blue in A, D, G, and J) or Sox2 (0/30; dark blue in B, E, H and K, the same embryo to the left): FGF + Smad6 + Smad7 (0/11; J-L); FGF + Smad7 + Wnt antagonists (Dkk1+NFz8+Crescent) + Noggin + dnBMPR + Cerberus + Chordin (0/7; D-F); FGF + Smad6 + Smad7 + Wnt antagonists (0/9; G-I); FGF + Smad6 + Smad7 + Wnt antagonists + Noggin + dnBMPR + Cerberus + Chordin (0/10; J-L). Electroporated cells were recognized by GFP expression (C, F, I and L, in the embryo shown to their left). In D-L, pellets of a mixture of COS cells transfected with Wnt antagonists (Dkk1+NFz8+Crescent, 1000 cells per factor) were grafted. In situ hibridization with Sox2 always produces background staining in the grafted cell pellets; therefore expression of this marker in the host was assessed in histological sections. The plane of section is indicated by a black line.
Supplementary Fig. 2. BMP inhibition by ΔSmad7 together with eFGF activation induces neural marker expression indirectly in Xenopus.
A-D. Inhibition of BMP by injection of ΔSmad7 into one A4 blastomere does not induce either Sox3 (A-B) or Sox2 (C-D) expression. E-F. Injection of eFGF together with the BMP inhibitor ΔSmad7 into an A4 blastomere induces Sox3 (E-F) and Sox2 (G-H, O). I-L. Neural induction by the former combination is inhibited when Nodal signaling is blocked: injection of ΔSmad7 + eFGF together with CerS no longer induces Sox3 (I-J) or Sox2 (K-L). A, C, E, G, I and K are dorsal views; B, D, F, H, J and L are ventral views of the embryos to their left. White squares mark enlargements of the area in the inserts in panels F and H.
Supplementary Movie 1: Cell-autonomous inhibition of BMP does not affect cell movements of epiblast cells in the chick.
On the left, a line of cells (extending out from the neural plate border) was labelled with DiI. On the right, a similar line of cells was electroporated with Smad6 using a vector also containing GFP. The fluorescence of DiI and GFP are both pseudo-coloured red. Time-lapse sequence (1 frame every 10 min). No significant difference in the patterns of movement is seen between the control and experimental sides.


