Abstract
The impact of ostensibly aversive social stresses on triggering, amplifying and prolonging intensely rewarding drug taking is an apparent contradiction in need of resolution. Social stress encompasses various types of significant life events ranging from maternal separation stress, brief episodes of social confrontations in adolescence and adulthood, to continuous subordination stress, each with its own behavioral and physiological profile. The neural circuit comprising the VTA–accumbens–PFC–amygdala is activated by brief episodes of social stress, which is critical for the DA-mediated behavioral sensitization and increased stimulant consumption. A second neural circuit comprising the raphe–PFC–hippocampus is activated by continuous subordination stress and other types of uncontrollable stress. In terms of the development of therapeutics, brief maternal separation stress has proven useful in characterizing compounds acting on subtypes of GABA, glutamate, serotonin and opioid receptors with anxiolytic potential. While large increases in alcohol and cocaine intake during adulthood have been seen after prolonged maternal separation experiences during the first two weeks of rodent life, these effects may be modulated by additional yet to be identified factors. Brief episodes of defeat stress can engender behavioral sensitization that is relevant to escalated and prolonged self-administration of stimulants and possibly opioids, whereas continuous subordination stress leads to anhedonia-like effects. Understanding the intracellular cascade of events for the transition from episodic to continuous social stress in infancy and adulthood may provide insight into the modulation of basic reward processes that are critical for addictive and affective disorders.
Keywords: Defeat, Subordination, Maternal separation, Sensitization, Serotonin, Dopamine, Cocaine, Heroin
1. Why social stress?
Social life is often quite intricate, full of rewards and also intense stresses. The apparent pharmacological contradiction in need of resolution is that ostensibly aversive stress experiences during social conflict interact directly with powerfully rewarding drug consumption. The present discussion focuses on how brief social stresses can serve as triggers for drug seeking, on how other stresses increase the rate of behavior that is reinforced by drug deliveries, and yet other stress conditions prolong and intensify compulsive drug use. In contrast to these stress effects that promote different aspects of drug seeking and taking, still other social stresses severely depress drug intake, hypothesized as being due to a loss of the sense of pleasure, typically referred to as an anhedonic state. Do the rewarding effects of drugs that are blunted by stress experiences prompt an individual to compensate for the lessened effects by self-administering drugs faster? Alternatively, do aversive stressors intensify the rewarding effects of drugs and thereby support more drug-reinforced behavioral exertion? Of course, administration of psychoactive drugs in itself constitutes a stressor (Barry & Buckley, 1966), and the behavior leading to drug delivery is accompanied by endocrine markers of stress. That the delivery of stress hormones can be rewarding seems to be a contradiction eo ipso. Yet, it is quite feasible to arrange conditions under which a rat or monkey will work for the opportunity to self-administer a glucocorticoid (Piazza et al., 1993; Broadbear, Winger, & Woods, 1999). Clinical accounts of sensation-seeking individuals document the stressful nature of seeking out highly challenging situations and the thrill of engaging in high-risk behavior (Zuckerman, 1984).
When defining social stress and the response to stress, the pressures, loads and strains, as studied and characterized in the physical and engineering sciences, serve as metaphors for physiologic, cellular and molecular events. In biological systems, stress is intimately related to the concepts of homeostasis and allostasis, the former relying on a fixed set point and narrow maximal and minimal limits, and the latter on constantly changing boundaries. Importantly, the allostatic model accommodates anticipatory behavioral and physiological responses (Cannon, 1953; McEwen & Chattarji, 2007). When defining stress, it is useful to characterize some critical features of stressful events and responses to those challenges. When a challenging event prompts a response to exceed the minima or maxima of the characteristic range (i.e. constitutes an overload), this event is considered a stressor and the resulting biological change a stress response. Challenges to regulation by actual or expected environmental events, including social challenges, result in a cascade of fast and slow neural, immune, autonomic, and endocrine responses in an effort to adjust to the novel challenges. Hans Selye (Selye, 1936; Selye, 1946) conceptualized a general stress response that encompassed a common adaptation to various noxious stressors ranging from intraperitoneal saline injections to heat and inflammation. Selye's three phases comprise the alarm response, resistance and exhaustion stage. In addition to this generalized stress adaptation, specific stressors such as cold, blood loss, hypoglycemia or immobilization recruit discrete anatomical, immunological and hypothalamic-pituitary-adrenal and sympathetic systems that reveal mechanisms that are specific to each stressor (Pacak & Palkovits, 2001). Social stress is of particular interest, since it is of fundamental and enduring significance in species that rely on parental care and social organization throughout the course of the individual's life. Maternal separation stress and stress arising from social conflict in adulthood incorporate sympathetic and hypothalamic-pituitary-adrenal (HPA) responses that conform to the Selye proposal, and still many other features that are unique to the specific social stress.
Social stress and the acute response to social stress share many of the same characteristics that are seen in reaction to other types of environmental stress. Prominently, the rapid sympathetic activation is readily detected by the tachycardic, hypertensive and hyperthermic responses during social conflict in rodents and primates (Henry & Stephens, 1977; Von Holst, 1985; Fokkema et al., 1988; Meerlo, de Boer, et al, 1996; Sgoifo et al., 2001). Even in socially stable groups of animals, the resting levels of heart rate and blood pressure reflect the social status and prepare the individual for daily challenges. Subordinate rats that lived for several months in stable groups and interacted with the dominant most often, were characterized by the highest resting blood pressure (Fokkema & Koolhaas, 1985). In an acute aggressive confrontation, the intruder rat is characterized by a large and sustained rise in heart rate and blood pressure, which are only initially concurrent with defensive and submissive acts and postures (Tornatzky & Miczek, 1993). This elevated heart rate, blood pressure and core temperature outlasts the brief confrontation and takes hours to return to the resting level. In preparation of anticipated social stress, cardiovascular activation and hyperthermia may precede the actual performance of vigorous behavioral coping activity, providing indirect evidence for neural mechanisms that are activated by temporal cues and other reminders.
The stress of being defeated in an aggressive encounter stimulates adrenocorticotropic hormone (ACTH) and glucocorticoids, while inhibiting androgens (Brain, 1972b; Bronson, 1973; Raab et al., 1986; see Table 1). In fact, following an aggressive episode, both the eventual winner and loser show elevated corticosterone levels, but the recovery to baseline levels is more rapid in winners than in losers (Brain, 1980; Schuurman, 1980; Covington & Miczek, 2005). Glucocorticoid secretion in socially stressed animals evidently fulfills multiple roles. The sympathoadrenal and HPA activation follow each other in rapid succession and represent critical components of the initial reaction to social stress as demonstrated in infants separated from the dam and littermates, or in intruders confronting a resident rodent (Palka & Coyer, 1969;Korte et al., 1990; Covington&Miczek, 2005;Knuth&Etgen, 2005). This immediate glucocorticoid response activates energy metabolism and an immune response that are instrumental in coping with these stressors, and in this sense the protective and restorative role of glucocorticoid activation is evident (Dallman et al., 1993; Sapolsky, 2005). A second role of the glucocorticoid responses becomes evident in anticipation of social stress following repeated predictable episodes of stress (Pardon et al., 2004). If glucocorticoid stimulation is frequent, prolonged and does not terminate, the allostatic load increases and leads to serious pathophysiological consequences in the cardiovascular, metabolic, and immune systems and also in hippocampus-mediated cognitive functions, most prominently in vulnerable individuals (McEwen, 1998).
Table 1.
Behavioral and endocrine effects of various social stressors
| Manipulation | Behavioral impact | Endocrine impact | Key references |
|---|---|---|---|
| Social isolation | Hyperactivity | Sensitization of pituitary response to CRH | Valzelli (1973), Flannelly and Lore (1977), Brain and Benton (1979),Serra etal. (2005) |
| Social crowding | Decreased reproduction; Increased activity and aggression; Agg regate toxicity | Adrenal hypertrophy | Chance (1947), Christian (1950),Henry and Stephens (1977) |
| Social instability | Reduces amphetamine self-administration and amphetamine-induced locomotion | HPA hyperactivity | Bronson (1979), Maccari et al. (1991), Lemaire et al. (1994), Lemaire et al. (1997) |
| Brief maternal separation | Improved adult Morris water-maze performance; More active in a novel environment | Increased glucocorticoid receptor mRNA; Protects against hypercorticosterone secretion to later stressors | Levine (1957), Rosenfeld et al. (1992), Plotsky and Meaney (1993b), Meaney et al. (1996), Ladd et al. (1996), Zaharia et al. (1996), Caldji et al. (2000) |
| Prolonged maternal separation | Decreased adult Morris water-maze performance; Decreased activity in a novel environment | Deceased glucocorticoid receptor mRNA; Increased basal and stress-induced corticosterone levels; Gastric ulcers and hypothermic response to environmental stressors | Ackerman et al. (1978), Meaney et al. (1996), Caldji et al. (2000) |
| Brief or episodic social defeats | Induces tolerance to analgesic effects of opiates; Induces behavioral cross-sensitization to psychomotor stimulants | Dopamine release in mesocorticolimbic structures; Non-habituating HPA activation | Schuurman (1980), Miczek et al. (1982), Tidey and Miczek (1996), Covington and Miczek (2001) |
| Chronic subordination | Suppresses immune response; Lowers activity, fewer motivated behaviors | Adrenal hypertrophy | Davis and Christian (1957), Brain (1972a), Raab et al. (1986), Bohus et al. (1993), Blanchard et al. (1995), Rygula et al. (2005) |
While an exponential decay curve describes the decline in glucocorticoid activation upon repeated exposure to stressors such as novelty or startle stress (File, 1982), no such habituation is detected when social stress is encountered intermittently in infants or adults. Instead of a gradually diminishing response to the stress, intermittent episodes of social stress consistently evoke large sympathetic and HPA responses. In order for maternal separation distress to be effective in impacting vulnerability to alcohol drinking in adulthood, the stress experiences need to occur repeatedly during an early critical period for several hours every day (Huot et al., 2001). In adults, persistent behavioral and neural sensitization results from intermittent exposure to social defeat stress episodes, and this intermittency engenders glucocorticoid and sympathetic responses that remain comparably large from the first to the last stress experience (Tornatzky & Miczek, 1994; Bartolomucci et al., 2003; Covington et al., 2005; Sgoifo et al., 2005).
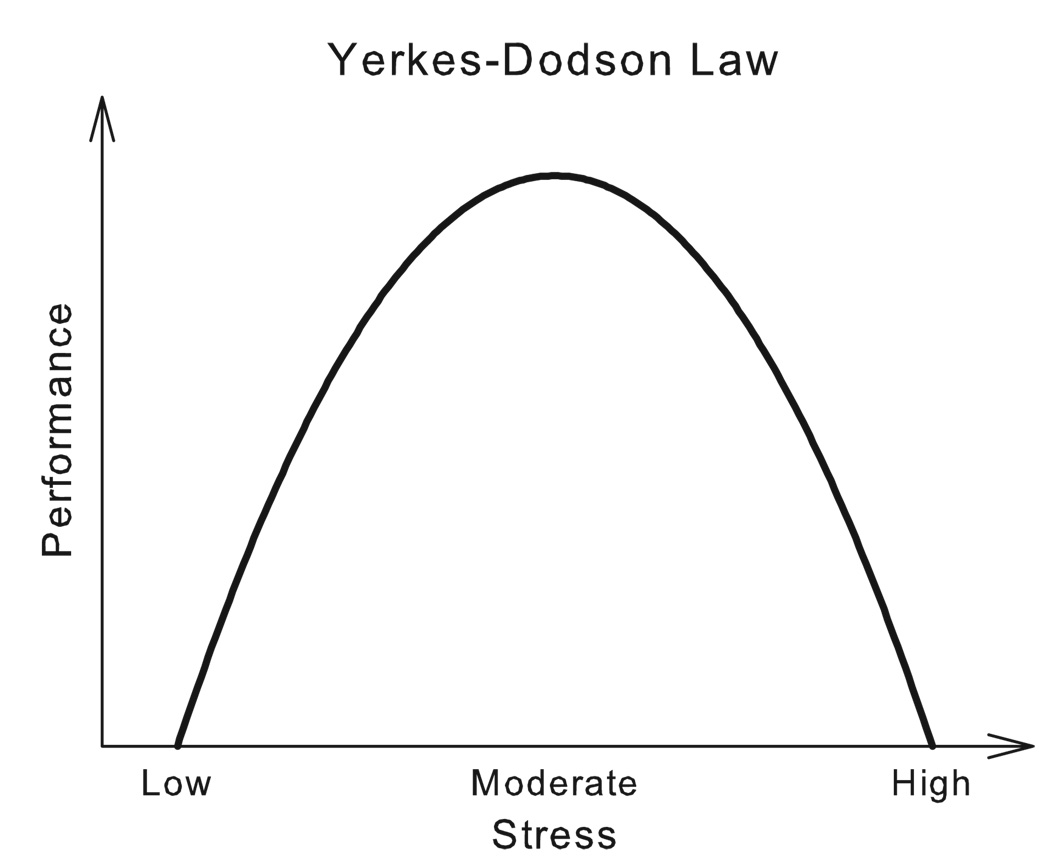
The relationship between stress and drug taking is biphasic, following an inverted U-shaped curve according to the Yerkes–Dodson Law (Yerkes & Dodson, 1908). The ascending limb of the curve depicts how brief and moderate social stress activates, energizes and arouses, whereas the descending limb shows how more intense, frequent, lengthy, inescapable and uncontrollable stress debilitates and suppresses behavior, and also profoundly impairs drug taking (Fig. 1). An example of this biphasic effect of stress pertains to alcohol consumption. Most experimentally controlled stress manipulations suppress alcohol drinking, although mostly anecdotal accounts link stress to increased alcohol consumption (Pohorecky, 1981; Breslin et al., 1995). Like most drugs of abuse, social stress is also linked to increased activation of aminergic cells in the brain stem, among which are the mesocorticolimbic dopamine pathways that terminate in the nucleus accumbens and the prefrontal cortex (e.g., (Louilot et al., 1986; DiChiara & Imperato, 1988; Tidey & Miczek, 1996). Here, we address one of the perplexing issues, namely how dopaminergic activity in the mesocorticolimbic system is essential for both intensely rewarding drug seeking and consumption as well as for coping with salient social stress.
Fig. 1.
Changes in behavioral performance as a function of stress level, as originally proposed by Yerkes and Dodson (1908).
2. Which types of social stress?
The appeal of Selye's proposed general adaptation syndrome has yielded to the accumulating evidence for stressor-specific neural and endocrine mechanisms that detail how various stressors activate a distinct cascade of cellular events in anatomically separate pathways (Pacak & Palkovits, 2001). Information on the endocrine responses and neural pathways characterizing different types of social stress has only recently emerged compared to more frequently studied stressors such as heat, cold, immobilization, inflammation and hypoglycemia (Kollack-Walker et al., 1997; Meaney, 2001; Martinez et al., 2002; Miczek et al., 2004).
2.1. Species-appropriate social housing
Many features of social life have been manipulated, starting with simply removing an individual from social contact to crowding many individuals in a confined space (Table 1). An early study of crowding in small mammals attributed the collapse of the population to adreno-cortical exhaustion, increased susceptibility to disease and decreased reproduction (Christian, 1950). In socially cohesive species such as rats and most primate species, social isolation is stressful (Brain & Benton, 1979; Levine, 1993), whereas in mice and other rodents that disperse after puberty, single housing corresponds to the life of territorial males (Brain, 1975). Of course, most strains of mice or rats that are bred for laboratory research purposes have only rudimentary features of the social characteristics of their feral counterparts (Barnett, 1975). The pharmacological relevance of crowded housing was early on demonstrated by the greatly increased amphetamine toxicity in aggregated mice relative to singly housed counterparts (Chance, 1946).
When small groups of laboratory rats are housed periodically with novel partners, behavioral signs of social instability and HPA activation become evident (Mormede et al., 1990). Most often, laboratory rats habituate rapidly to novel partners without long-term detrimental sequelae, whereas in socially intolerant species such as tree shrews (Tupaia belangeri) a new male will have to be rescued from a morbid course when continuously exposed to a resident, even though the new animal is protected behind a screen (Raab & Oswald, 1980; Von Holst, 1985). In conservation biology, translocating animals from their established habitat to a novel site is extremely stressful andmost often remains unsuccessful in terms of survival of the individuals (Wolf et al., 1998).
Even otherwise placid laboratory rats can develop social hierarchies when housed in small groups with priority of access to essential resources serving as quantitative index of their individual rank. However, the stability and reliability of dominance indices in laboratory animals that are freely provisioned and live in single-sex groups without predatory pressure has been problematic (Baenninger, 1970; Spigel et al., 1972; Taylor& Moore, 1975; Price et al., 1976; Benton, 1982; Lundberg, 1986; Drews, 1993). Dominance in one situation does not transfer readily to another test, and multiple types of dominance have been proposed to exist in parallel. Dominant rats in a triad , as determined by priority access to food, differ in terms of sensitivity to selected behavioral effects of psychomotor stimulants, anxiolytics, alcohol, antipsychotics, antidepressants relative to subordinate members (Gentsch et al., 1990; Bartolomucci et al., 2004; Pohorecky, 2006).
2.2. Repeated maternal separation
The significance of maternal separation is evident by its life-long impact on many biological and behavioral functions, ranging from endocrine and immune responses to stress in adulthood and to cognitive deficits in older age (Meaney, 2001; Levine, 2001; Anisman & Matheson, 2005). Particularly in mammals, the separation of an infant from its dam results in a stress response pattern that is characterized by distinct phases, comprising an initial catecholaminergic activation, followed by glucocorticoid release coincident with explosive motor reactions that are followed by a despair-like state (Smotherman et al., 1987). For example, infant macaque monkeys initially react to being separated from their mother by an intense protest response that is followed eventually by behaviors that have been termed “despair” (Hinde et al., 1966; Kaufman & Rosenblum, 1967; McKinney, & Bunney, 1969). The transition from the initial protest phase to the subsequent despair-like pattern is quite apparent in chicks separated from the clutch (Panksepp, Herman et al., 1978), with the behavior being responsive initially to anxiolytics and later to antidepressants (Insel et al., 1986; Lehr, 1989).
A critical feature of the stress by repeated maternal separation pertains to its precise timing and duration (see Table 1). First, there is the issue of when exactly in the development the infant is separated from the dam and the litter mates. Second, how often are the infants separated from the dam and during the course of which developmental period? Third, the duration of each separation is a critical variable, with short separation periods resulting in a buffered stress response in adulthood (Levine, 1957, 2001), and more prolonged separation periods prompting amplified stress responses (Meaney et al., 1996; Ladd et al., 1996).
The stress response to repeated maternal separation or handling procedures in rats needs to be evaluated in the context of the so-called stress hyporesponsive period that starts about 3–4 days post-partum and extends for 10 days (Sapolsky & Meaney, 1986;Walker et al., 1986). During this period the pup has low basal blood titres of ACTH and corticosterone and an attenuated responsiveness of the pituitary–adrenal system to physical events, such as exposure to ether or saline injection, that elicit marked ACTH and corticosterone stress responses later in life (Walker et al., 1986). Briefmother–pup separations for 15min per day can have protective effects, rendering the animal resistant to the behavioral and physiological effects of stress experienced in adulthood (Levine, 1957; Anisman et al., 1998; Meaney, 2001). Longer separations from the dam for 180 min per day have the opposite effect, enhancing HPA responses to stressors (Plotsky & Meaney, 1993; Ladd et al., 2000; Liu et al., 2000). The brief separations involving early handling result in behavioral and neurobiological changes that have been interpreted to relate to anxiety responses (Pryce et al., 2005), whereas the longer separations from the dam have been studied for their relevance to anhedonia, a core symptom of depression (Matthews & Robbins, 2003).
The behavioral consequences of repeated maternal separation stress that extend into adulthood are of paramount significance. However, it should be noted that while maternal separation has been shown to affect HPA axis responsiveness and other measures in adulthood in Long–Evans, Sprague–Dawley, and Wistar strains of rat (Plotsky & Meaney, 1993; King & Edwards, 1999; Ladd et al., 2000; Lehmann et al., 2000), other rat strains may be resistant. Similarly, studies of maternal separation on thermal and inflammation antinociception indicate that these effects are strain-dependent (Amkraut et al., 1971; Smythe et al., 1994; D'Amato et al., 1999; Ellenbroek & Cools, 2000; Stephan et al., 2002; Lariviere et al., 2006).
2.3. Social stress during adolescence
Hormonal events that trigger dynamic changes in sexual development indicate the progression through the stages of puberty and adolescence (Forest, 1983). While physical attributes and cognitive functioning are useful for determining the later transition from adolescence to adulthood, social and cultural influences engender variations in defining the precise duration of adolescence. Moving away from parental dependencies and confronting social challenges are both necessary for achieving adult status (Primus & Kellogg, 1990b). Adolescents are well suited for accommodating diverse social challenges, as illustrated by modest physiological responses to a broad range of environmental and social stressors (Adriani et al., 1998). Mammalian adolescents participate in discrete forms of social behavior, as both rodents and primates allogroom, cuddle and play fight amongst familiar and similar aged conspecifics, in addition to frequent bouts of social defeat by an older and larger adult (Vanderschuren et al., 1997). For some adolescents, episodes of stress can be too severe and can negatively impact on neural development, including the maturation of neuronal processes (Brunson et al., 2003). A vulnerability to social defeat stress in some adult individuals has been identified (Krishnan et al., 2007), but the question of whether or not certain adolescents are more or less resilient to stress has not been adequately answered. One major concern regarding an adolescent's exposure to social stress is the later acquisition of compulsive drug taking that can occur during adulthood (Laviola et al., 2003; Spear & Varlinskaya, 2005).
Adolescent rats spend significantly more time than adults in social interactions and play behavior (Panksepp, 1981; Brown, 1990). Similarly, adolescents are more behaviorally active in a novel environment, revealed by prolonged periods of exploration (Bronstein, 1972; Caza & Spear, 1980). Experimental work with various animal species has highlighted the significance of social interactions during adolescence on the subsequent expression of adult behaviors (Kabbaj et al., 2002; McCormick et al., 2004). Most primates, for example, follow a course of social development similar to human adolescents, engaging in frequent conflicts with the mother and spending significantly more time with similarly aged peers (Steinberg, 1989; Pereira & Fairbanks, 1993). In most rodent species, adolescence captures broadly postnatal days 20–60, and play fighting emerges after pups develop sufficient motor coordination, most clearly after weaning (Spear & Brake, 1983). In mice and rats, periadolescence characterizes postnatal days 30–40 when most time is spent interacting with other adolescents (Spear & Brake, 1983; Van Den Berg et al., 1999).
Adolescent social behavior, such as play fighting, is a key determinant for facilitating successful adult social behavior, including offensive aggression. Manipulations of the social environment in the rat (i.e. social isolation stress) during the time of periadolescence brings about depressive-like behavioral responses later on, at least when measured by social interactions in adulthood (Van Den Berg et al., 1999; Howes et al., 2000). Similarly, when hamsters are defeated during puberty, i.e. at a time when juvenile play fighting shifts to adult fighting, the type and amount of agonistic behavior in latter adulthood is ultimately determined (Delville et al., 1998). As periadolescence transitions to later stages of adolescence, rodent behavior grows increasingly more aggressive, with a rise in fighting (Vanderschuren et al., 1997). Frequent exposure to environmental stressors during adolescence, including social stress, can promote memory deficits and increase the risk for developing many affective diseases (Hellemans et al., 2004).
2.4. Social defeat stress
In most species, aggressive confrontations are often motivated by access to reproductive partners and limited resources; these fights result in the defeat of one opponent, particularly when escape is barred (Ginsburg & Allee, 1942; Roches & Leshner, 1979;Miczek et al., 1982). In many mammalian and avian species, these aggressive episodes occur often during the formation of dominance hierarchies and during the breeding season (Michael & Zumpe, 1978; Michael & Zumpe, 1981; Winslow & Miczek, 1988); aggressive confrontations are considerably less frequent during territorial conflicts and in established social groups (Abbott et al., 2003), although in some species such as the tree shrew fighting is part of daily life (Von Holst, 1969).
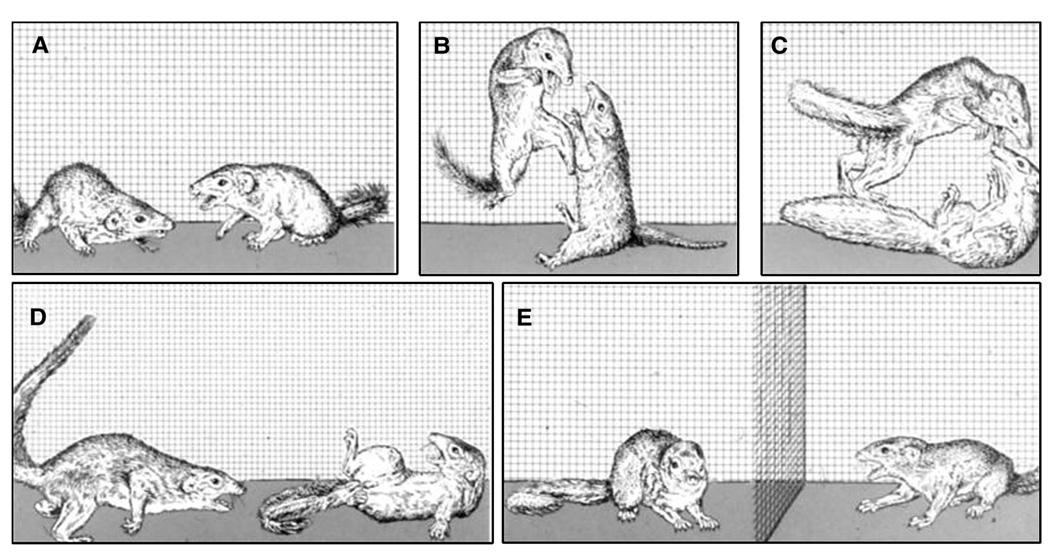
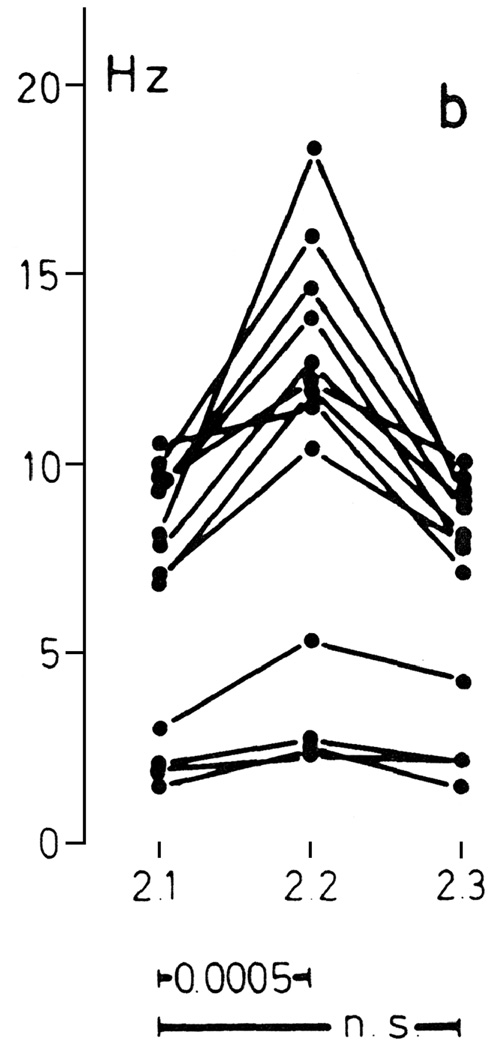
The salient behavioral characteristics of social defeat are clearly identified, operationally defined and illustrated for various animal species ranging from invertebrates to hominids (Grant & Mackintosh, 1963; Sade, 1967; Huber & Kravitz, 1995). For example in common laboratory animals, an upright posture with limp forearms, head angled upward and audible vocal signals characterizes the defeat response in murine species, whereas the display of a supine posture with limp extremities and the emission of loud and frequent ultrasonic 22-kHz vocalizations is typical of defeated rats (Fig. 2; Miczek et al., 1982; Tornatzky & Miczek, 1993).
Fig. 2.
(A) Display of an upright defeat posture by a mouse and (B) display of submissive supine posture by a defeated male rat of the Long–Evans strain. (From Miczek et al., 1982, 2004).
Socially-defeated animals interact less with unfamiliar animals (Frischknecht et al., 1982; Kudryavtseva et al., 1991; Kudryavtseva, 1994; Meerlo, Overkamp, Daan, et al., 1996; Huhman et al., 2003), engage in few reproductive activities (Kahn, 1961; D'Amato, 1988; Blanchard&Blanchard, 1989;Yoshimura&Kimura, 1991; Foxet al., 1997) and readily display defensive and submissive postures and vocal signals (Van de Poll et al., 1982; Siegfried et al., 1984; Puglisi-Allegra & Cabib, 1988; van der Poel & Miczek, 1991; Potegal et al., 1993). In general, defeated animals are less active, and explore, forage, eat and drink less (Raab et al., 1986; Meerlo, Overkamp, Benning, et al., 1996; Meerlo, Overkamp, Daan et al., 1996; Flügge et al., 1998). Defeated rats explore open and brightly lit spaces less, which has been interpreted as increased anxiety-like responses (Heinrichs et al., 1992; Rodgers & Cole, 1993; Avgustinovich et al., 1997; Fendt et al., 1997). Rats that have been conditioned to recognize pentylenetetrazol as a cue in a drug discrimination task substitute a brief defeat experience for the PTZ cue, presumably based on similar anxiogenic-like effects of both pentylenetetrazol and defeat experience (Vellucci et al., 1988; Vivian et al., 1994).
Even a single episode of social defeat stress can have persistent behavioral and neurobiological effects, such as for example on circadian activity, nociception, motor activity, and cellular activation in corticolimbic structures (Krugers et al., 1993; Tornatzky & Miczek, 1993; Meerlo, Overkamp, Benning et al., 1996; Meerlo, Sgoifo et al., 1999; Nikulina et al., 1999; Miczek, Nikulina et al., 1999; Marini et al., 2006). Repeated episodes of social defeat, particularly if they are unpredictable and uncontrollable, amplify and prolong these behavioral and neurobiological consequences (Tornatzky & Miczek, 1993; Yap et al., 2006). Repeated defeat followed by individual housing results in long-term impaired social memory, decreased social interaction and diminished anticipation for a sucrose reward up to 3 months after the last defeat experience, as well as attenuated induction of long-term potentiation and suppressed facilitation of long-term depression in the CA1 region of the hippocampus (Von Frijtag et al., 2001). Animals learn rapidly when to be submissive (Siegfried et al., 1982) and display submissive behavior more readily in successive confrontations (Ginsburg & Allee, 1942; Scott & Marston, 1953; Leshner & Nock, 1976).
2.5. Subordination stress
It is important to distinguish between brief episodes of social defeat stress and continuous subordination stress. In colonial animal species, the position or rank within social hierarchies of varying complexity determines the intercourse among individuals and determines a cascade of many behavioral and neurobiological events. For example, the social rank of the mother significantly influences the offspring's trajectory of social development as illustrated in primate species such as in baboons, rhesus macaques and vervet monkeys (Walters & Seyfarth, 1986). Offspring from low-ranking female bonnet macaques are abused by other females more often (Silk, 1980). Dominance hierarchies may be despotic, linear or more complex (Wilson, 1974), with low-ranking animals yielding to higher ranking individuals in terms of access to specific commodities, although an intriguing proposal inverts the definition of dominance and refers to a hierarchy of submission (Rowell, 1974). Considerable debate led to the realization that dominance is limited to a specific behavioral category. For example, prevailing in food competition does not necessarily generalize to transmitting more genes into the next generation (Baenninger, 1970; Benton, 1982; Drews, 1993), the latter being the ultimate criterion of dominance. Under controlled laboratory tests, females of several mammalian species prefer dominant mates relative to subordinates (Carr et al., 1982; Keddy, 1986; Shapiro & Dewsbury, 1986). For example, dominant male deer mice copulate more frequently than subordinates and sire more offspring (Dewsbury, 1984).
Subordination stress is characterized by the display of a behavioral repertoire that consists of species-typical acts and postures during conflict with many long-term implications for physiological and immunological functions (Miczek et al., 1991; Von Holst, 1998). Displays of behavioral, vocal and pheromonal signs of submission appear to have adaptive significance; they may prevent further attacks by the dominant opponent (Eibl-Eibesfeldt & Munster, 1957; Blanchard & Blanchard, 1988). Overall, the behavior of subordinate animals is restricted as manifested by less exploration, prolonged display of inactive crouching postures and physiological functions are often severely compromised, in extreme cases such as tree shrews leading to a morbid course (see Fig. 3; (Barnett et al., 1975; Spencer & Cameron, 1983; Blanchard et al., 1985; Von Holst, 1985; Stefanski, 2001; Sgoifo et al., 2001). Not only the prevalence of scars or wounds characterizes subordinate animals, but also increased release of ACTH and glucocorticoids, decrease in circulating androgens, and eventually testicular regression and adrenal hypertrophy (Davis & Christian, 1957; Beer et al., 1972; Brain, 1972a; Raab et al., 1986; Jasnow et al., 2001). If subordinate mice do copulate, they ejaculate after fewer intromissions and may not inseminate females successfully (Dewsbury, 1984). Lower androgen production and less androgen-dependent emission of pheromones renders subordinate animals less likely to be attacked (Bronson & Marsden, 1973; Jones & Nowell, 1973). One of the cardinal implications of the persistent behavioral, immunologic and endocrine characteristics of a subordinate animal is its significantly lowered success in transmitting genes into the next generation and ensuring the survival of its offspring.
Fig. 3.
Social conflict in Tupaia belangeri (from von Holst).
2.6. Controllability and predictability of social stress
Future work needs to uncover the mechanisms via which an individual initiates potentially stressful social interactions and controls their outcome. A common feature of several types of social stress ranging from prolonged maternal separation during infancy to persistent subordination in adults is the loss of control and predictability of the stress-inducing conditions. Controllability and predictability have emerged as highly significant characteristics of several stressors. These characteristics are particularly important risk factors for engendering pathologies, as identified early on using rats that were exposed to inescapable electric shock pulses (Weiss, 1972; Maier et al., 1982). The preceding discussion emphasized the stress of loosing contact with social partners or the sudden stress of potential confrontations as most uncontrollable threats to the individual. It will be important to learn how controllability and predictability of specific social stressors can be traced to neurobiological mechanisms.
3. Neurobiological characteristics, therapeutics and drug abuse
3.1. Maternal separation
The transient and involuntary separation of an attachment bond between the infant and the mother with its uncertainties and loss of control triggers a cascade of neurobiological events with life-long consequences. Several GABAergic, glutamatergic, monoaminergic and peptidergic mechanisms are activated by this separation response, and they represent important targets for pharmacological interventions and appear to be important in altering vulnerability to abuse of alcohol and other drugs.
3.1.1. Neurobiology
Maternal separation in early life can lead to very long-lasting and persistent effects on GABA receptors in adulthood. Adult rats that experienced as few as two episodes of neonatal handling with brief maternal separation as pups show an immature GABA receptor phenotype, with permanent molecular and functional differences in the GABA receptors within hippocampal dentate granule neurons, as characterized by single-cell recordings and antisense mRNA amplification. These rats also show increased activity in response to swim stress (Hsu et al., 2003). However, in terms of GABAA receptor changes in adulthood, both non-handling and repeated maternal separation during postnatal days 1–14 led to: (1) reduced GABAA receptor levels in the locus coeruleus and the n. tractus solitarius, (2) reduced central benzodiazepine receptor sites in the central and lateral n. of the amygdala, the frontal cortex and in the locus coeruleus and n. tractus solitarius, and (3) reduced levels of mRNA for the gamma 2 subunit of the GABAA receptor complex, compared to handled pups (Caldji et al., 2000). Daily handling during this 14-day period had protective effects, leading to reduced startle responsivity, increased exploration in a novel open field and decreased novelty-induced suppression of feeding in adulthood, compared to non-handled and animals that were separated for longer time from the dam and litter mates.
Expression of corticotropin-releasing hormone (CRH) mRNA is affected differentially, depending on the duration of the individual stress experiences (i.e., short, 15 min separations vs, long, 180 min separations). Rat pups that were exposed to either brief maternal separation (15 min of separation from mother and home cage per day) during 2–14 days after birth, prolonged maternal separation (180 min per day) or were non-handled, differed in hypothalamic CRH mRNA levels in adulthood (Plotsky & Meaney, 1993). Pups that were maternally separated for 180 min showed increased CRH mRNA relative to non-handled rats, while brief maternal separation was protective, resulting in significantly lower CRH mRNA levels compared to both 180-min maternal separation and non-handling. Environmental enrichment during the peripubertal period completely reversed the effects of maternal separation on both HPA and behavioral responses to stress, with no effect on CRH mRNA expression (Francis et al., 2002). In the paraventricular nucleus, central nucleus of the amygdala, bed nucleus of the stria terminalis, and locus coeruleus, CRH-like immunoreactivity and CRH mRNA levels were significantly elevated in non-handled rats separated for 180 min. Neonatal maternal separation for 180 min was associated with regionally specific alterations in CRH receptor type 1 mRNA density, while no differences due to rearing were seen in CRH-2 alpha binding (Plotsky et al., 2005). Long separations (180 min) on postnatal days 2–14 sensitized the adult limbic hypothalamo-pituitary-adrenal (LHPA) axis to air-puff startle (Ladd et al., 2005), while chronic variable stress decreased pituitary–adrenal reactivity and central amygdala CRH mRNA density in rats previously separated for 180 min from their dam.
Glutamate receptor expression levels are affected by repeated prolonged maternal separation (i.e. 360 min). In the hippocampus, mRNA expression of N-methyl-d-aspartate (NMDA) NR2B and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) GluR1 and GluR2 receptors were significantly lower in the 360-min maternal separation group relative to rats separated for 15 min a day during postnatal days 1–21. In addition, expression of the glutamate receptor transporter GLAST was increased in the 360-min group relative to the 15 min group. In the prefrontal cortex, no differences in mRNA expression were observed for NMDA NR2A and NR2B or AMPA GluR1 and GluR2, suggesting that while prolonged maternal separation produces neuroadaptive changes in the hippocampus, these changes may partially account for the behavioral deficits observed after prolonged maternal separation (Pickering et al., 2006).
Maternal separation stress can induce neural changes that can persist into adulthood, inducing molecular and functional changes in GABA receptors, even after only 2 brief separations, suggesting the extreme sensitivity of the GABAergic system during this postnatal development period. Alterations in CRH mRNA expression also result from maternal separation episodes, and the direction in which the changes occur is highly dependent on the intensity and duration of the stress experience, with daily 180 min separations over postnatal days 2–14 increasing hypothalamic CRH mRNA in adulthood, while brief, 15 min separations had protective effects, resulting in significantly lower CRH mRNA levels. Finally, adaptations in hippocampal glutamate receptor mRNA expression are seen in adulthood following repeated, 360-min maternal separation episodes. These studies demonstrate that neural adaptations that occur as a consequence of maternal separation during the “stress hyporesponsive period” are very much dependent on how long the repeated separations were, with short separations having protective effects, while long separations are more deleterious.
3.1.2. Therapeutics
Maternal separation stress is relevant to pharmacotherapy and drug abuse in two ways. First, the acute response during separation from the dam is studied. 35 to 70 kHz ultrasonic vocalizations (USVs) are emitted by neonatal rodents that are separated from the dam and littermates (Gardner, 1985; Mos & Olivier, 1989;Winslow & Insel, 1991a). These distress calls are further potentiated under stressful environmental conditions such as social isolation, reduced ambient temperature, hunger, rough handling, novelty and threat, and renewed separation (Okon, 1970; Allin & Banks, 1971; Bell, 1979; Shair et al., 1997), and the emission of these calls is not limited to rodents; they are also displayed by neonatal birds (Panksepp, Vilberg et al., 1978), dogs (Panksepp, Herman, et al., 1978) and primates (Kalin et al., 1987; Miczek et al., 1995). Because these vocalizations can be clearly potentiated by very discrete stimuli, they are an attractive measure in the evaluation of anxiolytic compounds (Winslow & Insel, 1991b).
Second, the effects of repeated maternal separation are studied in adulthood in order to observe the persistent consequences of early life stress. Rodent pups repeatedly separated from the dam and littermates can show depressive-like changes in behavior and brain function, and antidepressant treatment can prevent some of these changes (Lee et al., 2001; MacQueen et al., 2003; Mirescu et al., 2004). Moreover, the maternal separation experiences profoundly alter the effects of drugs of abuse during adulthood and the probability of intense drug self-administration (vide infra).
The first neurobiological studies investigating the maternal separation response focused on neuroendocrine factors governing activity of opioid peptides and their receptors (Panksepp, Vilberg, et al., 1978; Insel et al., 1984). These early studies demonstrated that blockade of opioid receptors, using antagonists with relatively non-selective receptor subtype action, was effective in increasing distress vocalizations in chicks that were removed from the clutch. This evidence extends to mammals, as injections with morphine and oxymorphone effectively suppressed distress vocalization in guinea pig pups (Herman & Panksepp, 1978; Panksepp, Herman, et al., 1978), and vocalizations were reliably increased with the mu-opioid receptor antagonist naloxone. Also, enkephalin analogues injected into the fourth ventricle reduced distress vocalizations in socially isolated chicks (Panksepp, Herman, et al., 1978). Intracisternal injections of mu and delta receptor agonists effectively reduced the calling rate in 10-day-old rat pups, whereas intracisternal injections of a kappa agonist had the opposite effect (Carden et al., 1991). Systemic administration of the kappa opioid agonist U50,488 increased the rate of USVs in 3-,10-, and 18-day-old rat pups that were in contact with their littermates in their home cage, an environment where USVs are seldom emitted (Carden et al., 1993; Carden et al., 1994). Methionine enkephalin (Met-ENK) immunoreactivity studies in 10-day-old pups revealed higher striatal peptide levels when pups were briefly separated (5 min) than when separated for 60 min, perhaps reflecting depletion of the peptide following an initial release during the period when the pups are most vociferous (Carden et al., 1996). Mouse pups with a mu-opioid receptor gene deletion (Orpm−/−, mu-opioid “knockouts”) emit fewer USVs than their wild-type controls (Orpm+/+)when removed from their mothers but not when exposed to male mice odors or cold stress (Moles et al., 2004). Moreover, these knockout pups do not show a preference toward their mothers' cues and do not exhibit the typical potentiation of USVs after brief maternal exposure. Receptor autoradiography in brains of rat pups that experienced repeated brief separations (15 min/day during postnatal days 1–21) from the dam shows long-term changes in delta receptor density in the basomedial amygdala compared to animal facility-reared rats (handled only when home cage was changed once a week) 2 months after repeated maternal separation (Ploj et al., 2003). These studies highlight the important role of opioid peptides in modulating the frequency of distress vocalizations in rodent pups, with kappa receptor activation intensifying the separation calls, while mu and delta receptor agonist action mimics the calming influence of the dam.
Administration of exogenous peptides can have anxiolytic-like effects on rat pup USVs induced bymaternal separation. Intraventricular administration of oxytocin, a nonapeptide produced primarily in the paraventricular and supraoptic nuclei of the hypothalamus (Gainer & Wray 1994), decreased distress calling in these pups (Insel & Winslow, 1991). However, when separation distress was measured in pups with a deletion of the first exon of the oxytocin precursor gene (i.e., oxytocin (OT) knockout mice), these pups showed a significant decrease in distress calling compared to wild-type pups (Winslow et al., 2000). While this finding is seemingly paradoxical to the pharmacological studies with administration of oxytocin, one interpretation is that OT-deficient pups fail to form social attachments in life and are therefore less distressed by the separation. Oxytocin is an important peptide in the modulation of social behaviors, such as affiliation (Witts et al., 1992; Insel, 1992), maternal behavior (Insel 1990) and species-specific pair bonding in monogamous species (Insel, 1992; Insel & Hulihan, 1995). Shapiro and Insel (1990) found that pups from themore sociable prairie voles were much more vocal during social separation than pups of the less socially attached montane vole. These differences in social bonding were associated with remarkable differences in oxytocin receptor binding patterns (Shapiro & Insel, 1992). Therefore decreased sensitivity to maternal separation in OT knockout pups may be attributed to deficiencies in social bonding of these pups to their mothers.
Arginine-vasopressin is a closely related peptide known tomodulate USVs. Arginine-vasopressin is found in the rat brain very early in development. Fully processed arginine-vasopressin can be detected by embryonic day 17, with hypothalamic levels at birth being comparable to adult levels (Alstein et al., 1988). Central administration of this neuropeptide decreased the number of rat pup vocalizations, and co-administration of arginine-vasopressin and V1 or V2 receptor antagonists suggested that changes in vocal behaviorwere mediated by the V1 receptor subtype (Winslow & Insel, 1993). These data point to a role of these exogenous peptides in modulating anxiety-like behavior in pups separated from the dam.
Anxiety-like behavior in the form of USVs can be modulated by serotonergic ligands. Agonists at serotonin 5-HT1A receptors decrease potently the emission of USVs. The 5-HT1A antagonist WAY 100,135 also may reduce USVs when given at higher doses that target DA D4 receptors (Olivier et al., 1998). 5-HT uptake inhibitors are also effective in reducing maternal separation-induced vocalizations in rat and mouse pups (Olivier et al., 1998; Fish et al., 2004). Escitalopram, citalopram, fluoxetine, R-citalopramand venlafaxine all reduced USV emission (Fish et al., 2004). Recently, the selective 5-HT1B antagonist SB-616234-A was shown to reduce maternal separation-induced calling in both rat and guinea pig pups (Dawson et al., 2006). Several serotonergic compounds are clinically used to alleviate anxiety in humans and have been shown to effectively reduce anxiety-like behaviors in rodent pups. These anxiolytic-like effects can be dissociated from the effects of serotonergic drugs on motor and physiological activities. It remains to be determined whether or not the anxiolytic-like effects of 5-HT1A and 5-HT1B receptor agonists on USVs in pups are mediated via action on somatodendritic autoreceptors, on presynaptic autoreceptors or on post-synaptic heteroreceptors.
Rat pup USVs are modulated by clinically effective anxiolytics and other compounds acting at the GABAA receptor complex. GABAA receptor agonists, neurosteroids and benzodiazepines acting as positive allosteric modulators of the GABAA receptor complex were effective in decreasing maternal separation-induced USVs (Gardner, 1985; Gardner & Budhram, 1987; Insel et al., 1989; Vivian et al., 1997; Fish et al., 2000; Takahashi et al., unpublished data). Benzodiazepine receptor inverse agonists increased the production of USVs and antagonized the USV-suppressive effects of diazepam (Insel et al., 1986; Gardner & Budhram, 1987; Nastiti at el., 1991). The barbiturate pentobarbital produced biphasic effects of USVs. The neurosteroid 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) dose-dependently decreased USVs and when given in combination with alprazolam and diazepam, resulted in a leftward shift in the dose–effect curves of these two benzodiazepines and a lesser shift for pentobarbital (Vivian et al., 1997). Separation-induced USVs are sensitive to the effects of compounds that influence GABAergic transmission through the modulatory sites for the benzodiapepines and barbiturates on the GABAA receptor complex.
Glutamate has received scant attention as a neurotransmitter involved in anxiety disorders until relatively recently (Mathew et al., 2001; Bergink et al., 2004; Swanson et al., 2005; Mathew, 2005). The noncompetitive antagonist of the NMDA receptor, dizocilpine (MK-801), dose-dependently reduced distress calling in maternally separated pups (Takahashi et al., unpublished data). Interestingly, at low doses, memantine and neramexane (low-to-moderate affinity, uncompetitive NMDA receptor antagonists) potentiated calling frequency, while suppressing it at higher doses Takahashi et al., unpublished data). These data suggest a glutamatergic influence on anxiety-like behavior and deserve further examination.
Rat pups subjected to repeated daily maternal separation for 180min from postnatal days 2–14 showed an enhancement of the inhibitory effect of citalopram on serotonergic cell firing in the dorsal raphe in adulthood compared to rats that were briefly handled as pups (Arborelius et al., 2004). Interestingly, repeated maternal separation has minimal effects on the serotonin transporter and 5-HT1A receptor in adulthood. Rat pups that remained non-handled during postnatal days 2–14 showed significantly lower serotonin transporter and 5-HT1A densities in the hypothalamus as well as in the basolateral amygdala, compared to rats that were repeatedly separated from their mothers as pups (Vicentic et al., 2006). Using this same separation protocol (180 min vs, 15 min) or animal facility rearing, changes in distribution of tryptophan hydroxylase and c-Fos-like immunoreactivity following exposure to social defeat experience in adulthood have been analyzed. Independent of early life experience, rats exposed to social defeat showed an increase in the number of c-Fos-like immunoreactive nuclei in serotonergic neurons in the middle and caudal parts of the dorsal raphe nucleus and caudal part of the ventral dorsal raphe nucleus, regions known to contain serotonergic neurons projecting to central autonomic and emotional motor control centers (Gardner et al., 2005). Rats exposed to 180 min of maternal separation displayedmore passive–submissive behaviors and less proactive coping behaviors, showing that the effects of social defeat stresswere equally effective in modulating the physiological and neurochemical response, despite their treatments as young pups (Gardner et al., 2005).
Maternal deprivation can produce profound changes in the hippocampus associated with depression. A decrease in cell proliferation and immature neuron production were observed in the dentate gyrus of adult rats that are maternally separated as pups for 180 min/day from postnatal days 1–14 (Mirescu et al., 2004). Treatment with fluoxetine during maternal separation (postnatal days 14–20, 24 h/day) enhanced cell proliferation and prevented apoptosis in the dentate gyrus, with the number of bromodeoxyuridine (BrdU) positive cells and terminal dUTP nick-end labeling (TUNEL) positive cells reaching control levels (Lee et al., 2001). Desipramine treatment after maternal separation (postnatal days 4–22, 180 min/day) prevented both maternal separation stress-induced reductions in active swim times during the forced swim test and the reduction of brain-derived neurotrophic factor (BDNF) levels in the dentate gyrus and CA3 regions of the hippocampus (MacQueen et al., 2003). However, in another study rats maternally separated for 180 min a day from postnatal days 2–14 showed increased adult hippocampal BDNF protein levels, while hippocampal BDNF mRNA levels as well as levels of neurogenesis in the hippocampus were unaffected by maternal separation stress (Greisen et al., 2005). Opposing cellular changes in hippocampus may be due to the use of different separation and control protocols. Using a line of rats that has been used as a model for the study of antidepressant compounds, the Flinders Sensitive Line, it has been shown that maternal separation from the dam for 180 min/day (postnatal days 2–14), led to further decreased active swim duration during the forced swim test, while the Flinders Resistant Line (control line) was unaffected by repeated maternal separation (El Khoury et al., 2006). Treatment with escitalopram had no effect in the Flinders Resistant Line but increased swim duration in both maternally separated and non-separated Flinders Sensitive Line rats, demonstrating the reversal of behavioral abnormalities caused by either genetic factors (rat line) or environmental factors (maternal separation). While most studies point to a depressive-like behavioral and neurobiological profile resulting from long, repeated maternal separations, further investigation is necessary to fully characterize the long-term behavioral and neuronal consequences of this form of early life stress.
There are two general ways in which maternal separation stress is a valuable tool in the study and development of therapeutics. First, acute maternal separation stress can induce anxiety-like behavior in the form of distress calling by separated pups, and this has proven useful in the evaluation of anxiolytic compounds. Early work had shown the importance of opioid peptide receptors in modulating the calling rate of pups briefly separated from the dam, with kappa receptor activation intensifying the separation calls and mu and delta receptor activation mimicking the calming presence of the dam. Later work pointed to the role of the endogenous neuropeptides oxytocin and arginine-vasopressin in the formation of social bonds between dam and pup and the calming effect they have on separation calling. Serotonergic agonists and reuptake inhibitors, as well as drugs activating GABAA and NMDA receptors decrease calling frequency, highlighting the importance of these neurotransmitter systems in regulating this anxiety-like response. Measurement of USVs following acute separation from the dam can be used in the examination of novel experimental therapeutics targeting a reduction in anxiety. However, since data are collected from pups who lack fully developed transmitter and receptor systems, the findings should be treated with caution.
Second, it provides away to study the influence of early life stress on the consequences in adulthood. Repeated maternal separation can induce neural changes related to depression-like phenomena, such as decreased cell proliferation and neurogenesis in the hippocampus, and this can be reversed with chronic administration of an antidepressant. Further study in this direction is important in uncovering the long-term maladaptations these early life manipulations have in adulthood.
3.1.3. Drugs of abuse
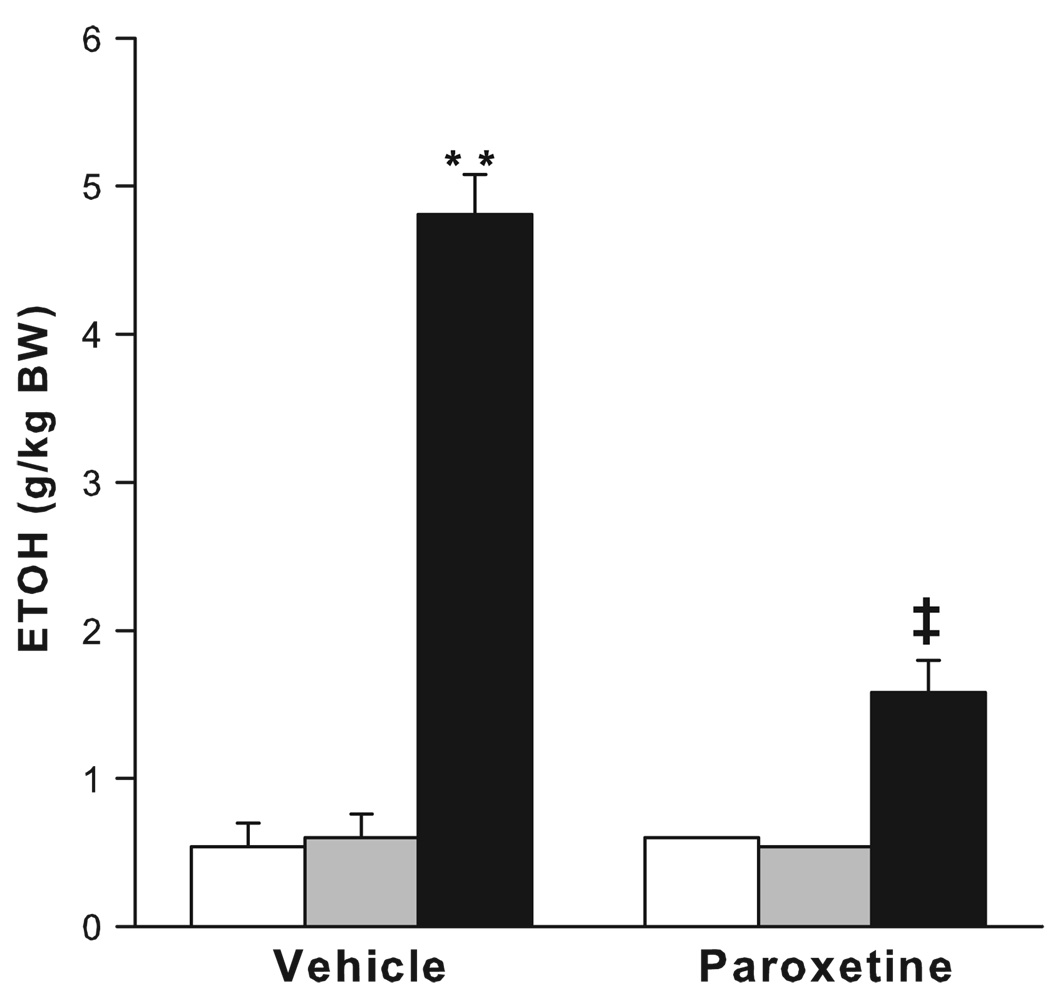
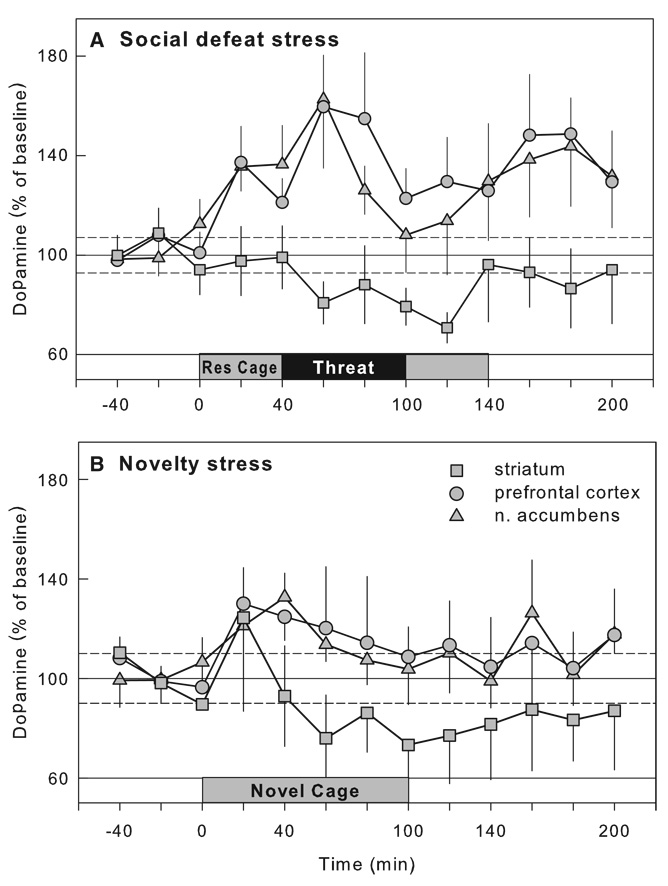
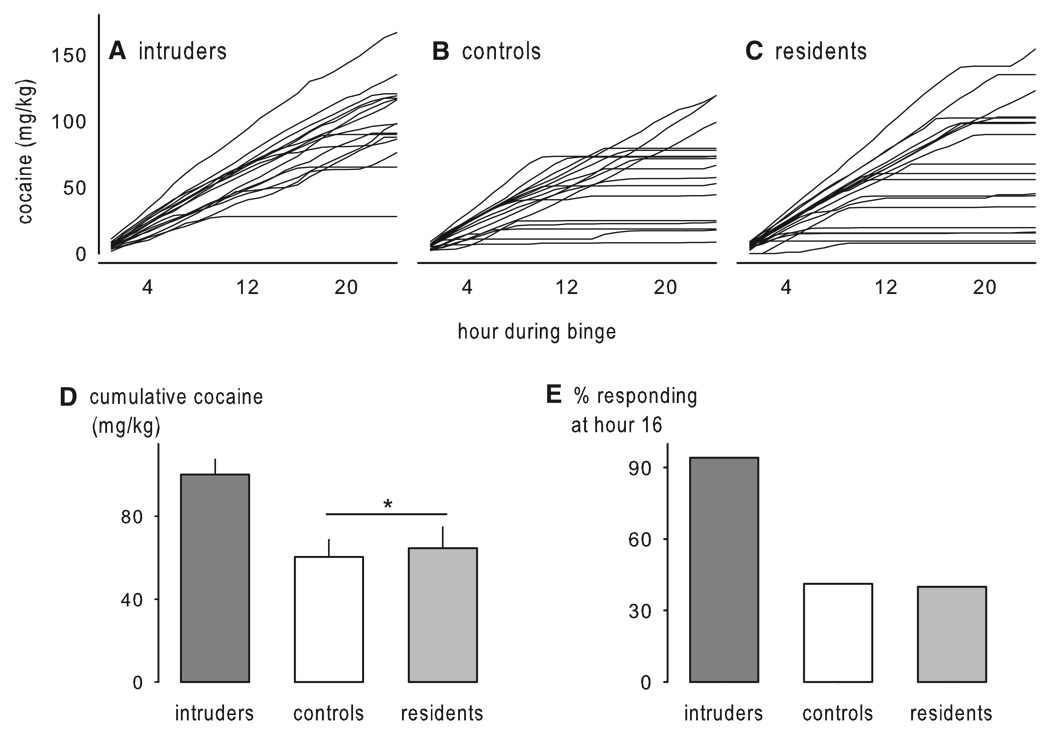
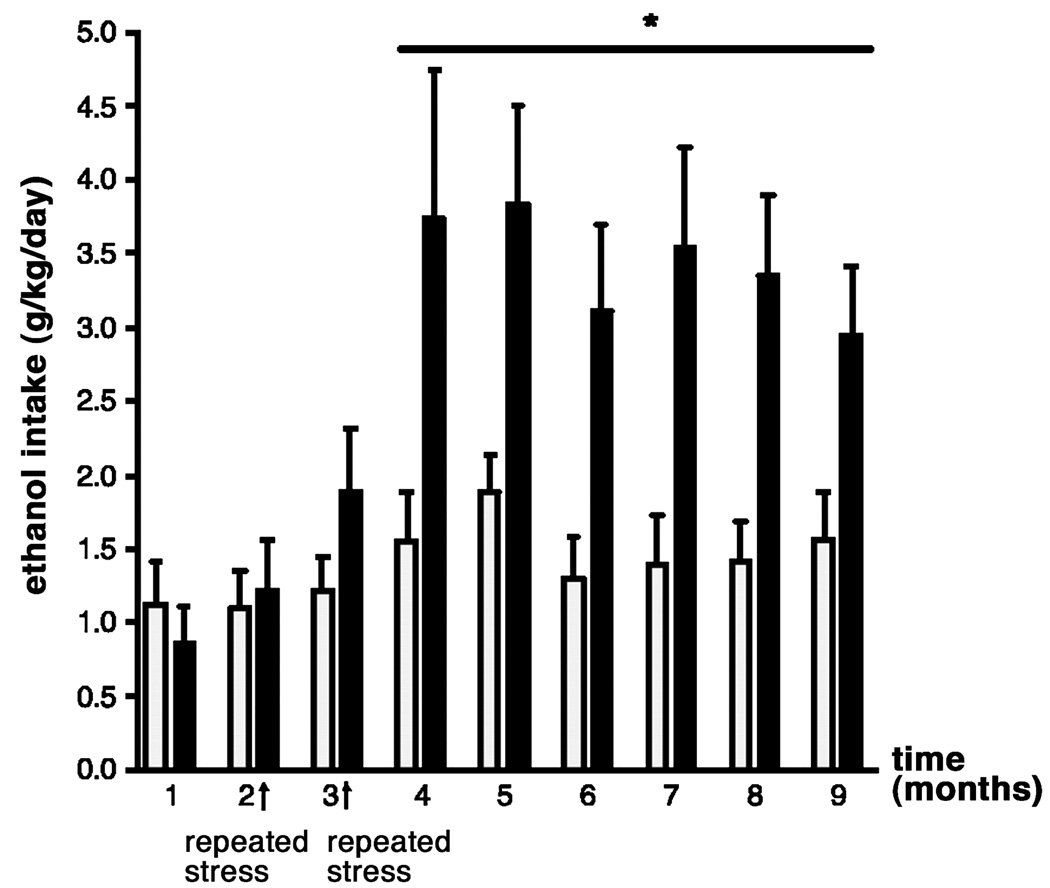
Prolonged maternal separation has been shown to greatly increase ethanol consumption to more than 4 g/kg per day in adulthood (see Table 2; Fig. 4; Huot et al., 2001). Rat pups separated from their dams for 180 min from postnatal days 2–14 show a significant preference for alcohol over a water–sucrose solution in a two-bottle, free-choice task during adulthood. Treatment with the selective serotonin reuptake inhibitor, paroxetine, for 21 days led to a reduction in ethanol drinking in maternally separated rats. However, conflicting results have also been reported, with non-handled animals consuming the highest level of ethanol compared to rats that were maternally separated for 15 or 180 min (Jaworski et al., 2005). It is puzzling that while the methodologies used in these two studies are closely similar, the results are virtually opposite. In a third study by Ploj et al. (2003), rats that were either separated from the dam for 360 min or were animal facility-reared controls showed similar levels of ethanol drinking, while separations for 15 min a day led to the consumption of the least amount of ethanol, reminiscent of an early experiment by Levine (1957). It was only after experiencing additional restraint stress and after access to a higher ethanol concentration (8% ethanol) that the rats repeatedly separated for 360 min showed the highest levels of ethanol consumption. The differential ethanol intake between groups has been attributed to the greater densities of delta opioid receptors in the pontine nuclei of rats separated for 15 and 360 min compared to the animal facility-reared group, or to increased density of hippocampal DA D1 receptors in the rats separated for 15 min compared to the rats separated for 360 min. Increases in DA D2 receptors in the ventral tegmental area (VTA) of rats separated for 15 minmay also contribute to the increased ethanol intake (Ploj et al., 2003). Most recently, mouse pups separated daily for 180 min during postnatal days 1–14 or were animal facility-reared (only handled during weekly cage cleaning) show large increases in ethanol intake, in both a bottle choice procedure and in operant alcohol self-administration (Fig. 5; Cruz et al., 2008). The critical determinants for the differential outcome of these studies are only beginning to be identified.
Table 2.
Effects of maternal separation on drug intake and behavioral sensitization
| Maternal stress manipulation | Control group | Reference | ||
|---|---|---|---|---|
| Short | Long | Animal Facility Reared (AFR), Non-handled (NH), or Moved but not separated (MS0) |
||
| 15 min/day | 180 min/day (MS180) or 360 min/day (MS360) |
|||
| Ethanol intake | <1 g/kg/day | MS180: >4 g/kg/day | AFR: <1 g/kg/day | Huot et al. (2001) |
| <0.5 g/kg/day | MS180: <1.5 g/kg/day | NH: >N2 g/kg/day | Jaworski et al. (2005a) | |
| AFR: <1 g/kg/day | ||||
| MS0: <1 g/kg/day | ||||
| <1 g/kg/day | MS360: >1 g/kg/day | AFR: >1 g/kg/day | Ploj et al. (2003) | |
| (None) | MS180: >2 g/kg/day | AFR: <1.25 g/kg/day | Cruz et al. (2008) | |
| (bottle choice) MS180:>3 g/kg/day (operant self-administration) |
(bottle choice and operant self-administration) | |||
| Cocaine intake | (None) | MS360 males: ↓ intake | Controls separated for 5 min/day |
Matthews et al. (1999) |
| MS360 females: ↑ intake | ||||
| MS15: no acquisition of cocaine self-administration at any doses tested (0.0625–1.0 mg/kg/inf); rate of self-administration same for cocaine and saline | MS180: acquisition at lowest doses tested (0.0625 and 0.125 mg/kg/inf) | NH: acquisition at most doses tested (0.125–1.0 mg/kg/inf); ↑ intake at highest doses (0.5 and 1.0 mg/kg/inf) MS0: no acquisition of cocaine self-administration at any doses tested (0.0625–1.0 mg/kg/inf); slight inverted U dose-response curve | ||
| Cocaine sensitization | No sensitization | MS180: cross-sensitization to cocaine challenge | NH: cross-sensitization to cocaine challenge | Meaney et al. (2002) |
| Attenuated sensitization to cocaine, no cross-sensitization to cocaine in animals treated with saline during induction phase | MS180: attenuated sensitization to cocaine, no cross-sensitization to cocaine in animals treated with saline during induction phase | AFR: highest level of sensitization to cocaine, but no cross-sensitization to cocaine in animals treated with saline during induction phase | Li et al. (2003) | |
| (None) | MS60 (pups separated for 60 min/day): ↑ sensitized response to repeated injections of cocaine; cross-sensitization to cocaine in males | NH: repeated cocaine injections led to sensitization | Kikusui et al. (2004) | |
| Morphine sensitization | (None) | MS180: highest level of sensitization to morphine | Both NH and Handled controls sensitized to repeated morphine | Kalinichev et al. (2002) |
AFR – Standard procedure was to transfer animals to new cages (new bedding and water) twice per week; NH – Dams and pups untouched and left alone in home cage; MS0 – Dams and pups touched to move to other side of home cage but not separated.
Fig. 4.
The effects of paroxetine (7 mg/kg/day) or 20% polyethelyne glycol vehicle on average intake over three 24-h periods of 8% ethanol in 2.5% sucrose by adult rats reared in the animal facility (AFR; white bars), those handled and separated from the mother for 15 min (HMS 15; gray bars) and those handled and separated from the mother for 180 min (HMS 180; black bars). Bars are means ± SEM, ** p <0.01 compared with AFR and HMS 15 groups, ‡p <0.01 compared to vehicle-treated HMS 180. (From Huot et al., 2001).
Fig. 5.
10% ethanol intake by AFR (white bars) and MS (gray bars) mice using a 3-bottle choice procedure and during a 60-min session with unlimited dosage, using operant self-administration panels inserted into the home cage. Alcohol solutions were diluted in 0.05% (w/v) saccharin. Alcohol intake (g/kg) is presented as group averages (±SEM) over 10 days during the bottle choice procedure and as data from a single 60-min self-administration session. *p < 0.05. (From Cruz et al., 2008).
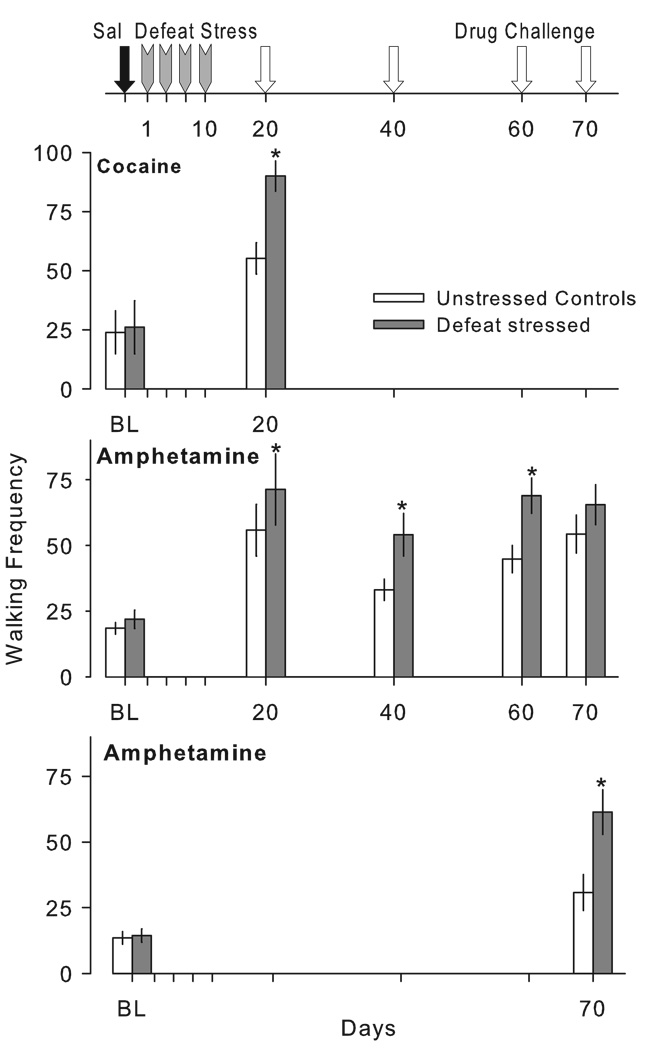
Studies of the behavioral effects of cocaine in adulthood after maternal separation in infancy also show large but disparate effects (Table 2). Repeated long separations led to a sensitized locomotor response to cocaine when compared to rats handled only for 15 min a day on postnatal days 2–14 (Meaney et al., 2002); however, the non-handled rats also showed the same level of behavioral sensitization as the rats that experienced the long separations from the dam. In further work, control (animal facility-reared) rats that were pretreated with cocaine during the induction or development phase — the phase during which repeated administration of cocaine leads to a progressive augmentation in the locomotor response to the drug — showed the highest level of sensitization when challenged with cocaine 7 days later, while rats that were maternally separated for 15 or 180 per day during the first 21 days of life showed an attenuated sensitized response (Li et al., 2003). Maternal separation blunted sensitization to cocaine but did not alter the effect of repeated treatment with saline control injections. By contrast, in adult mice, repeated administration of cocaine to previously maternally separated animals (1 h/day from PND 1–13) led to an increased sensitized response to a later cocaine challenge compared to non-separated male and female mice (Kikusui et al., 2005). Male mice, but not female mice, treated with saline during the induction phase of sensitization showed cross-sensitization to a later challenge with 7.5 mg/kg of cocaine. The effects of maternal separation on behavioral sensitization to morphine are more consistent, with maternally separated rats showing greater locomotor sensitization to a 1.0 mg/kg of morphine challenge relative to handled and non-handled controls (Table 2; Kalinichev et al., 2002).
Maternal separation has also been shown to influence acquired food-conditioned responses in adulthood. Rats that experienced prolonged repeated maternal separation (360 min on 10 occasions between PND 5 and 20)were less responsive to the activity-enhancing effects of low dose d-amphetamine in the fasted state, and their conditioned locomotor activity was further suppressed by prefeeding, which may be related to neuroadaptations in the mesocorticolimbic dopamine system (Matthews et al., 1996). Repeated maternal separation (1 h/day on PND 2–9) resulted in enhanced cocaine-induced increases in ventral striatal dopamine levels of 10-day-old rat pups relative to non-handled pups, while baseline dopamine levels remained unaffected (Kosten et al., 2003). Early life stress effects endure into adulthood as demonstrated by enhanced amphetamine-induced increases in dopamine levels in the nucleus accumbens of adult rats with 6 h/day maternal separation experience on postnatal days 5–20 (Hall et al., 1999).
Divergent effects of maternal separation on intravenous cocaine self-administration have emerged so far. On the one hand, male rats that were separated from dam and littermates for 360 min on 10 occasions spaced randomly between postnatal days 5 and 20 showed as adults a downward shift in the dose–response curve for the rate of intravenous cocaine self-administration, while their female counterparts self-administered more cocaine, compared to their respective controls (Matthews et al., 1999). On the other hand, rats separated for 180 min daily during the hyporesponsive period acquired cocaine self-administration at the lowest dose, whereas briefly separated (15 min/day) rats did not (Moffett et al., 2006). However, just as with the ethanol study discussed above (Jaworski et al., 2005), the non-handled rats self-administered the most cocaine at the highest dose tested (1 mg/kg/infusion) (Moffett et al., 2006). Overall, the results from these experiments thus far have been quite perplexing. Clearly, short- and long-duration separations during the first two weeks of life can result in contrasting effects on self-administration of alcohol and psychomotor stimulants. It is unclear why under some conditions, and not others, these long, repeated maternal separations increase cocaine and alcohol self-administration.
3.2. Social stress during adolescence
The adolescent's brain undergoes well-regulated and dramatic morphological adaptations, as well as receptor-mediated endocrine and neural changes. The orderly progression of these neural adaptations throughout adolescence is critical for facilitating appropriate social behaviors in adulthood (Andersen, 2003). The impact of social stressors during adolescence can dramatically influence each phase of neural development. Interestingly, the phase of neural development most impacted by stress during adolescence may predict the expression of particular affective disorders (Andersen & Teicher, 2008).
3.2.1. Neurobiology
Adolescence is considered to be a time of dynamic synaptic organization and formation of neural circuitry (Spear, 2000). During adolescence, social stressors significantly impact later behavioral responses, particularly in females, but neuronal activation in response to stress during adolescence ismuch less dramatic than that of the adult (Bardo et al., 1995; Kellogg et al., 1998; Maslova et al., 2002; Isgor et al., 2004; McCormick et al., 2005). While the adolescent HPA axis appears functionally similar to adults (Choi & Kellogg, 1996), c-Fos expression is not induced to the same degree by environmental stressors in adolescents relative to adults within many brain areas, including the hypothalamus, olfactory nucleus, amygdala, pyriform cortex and the tenia tecta (Kellogg et al., 1998). Low levels of neuronal activation during adolescence are potentially due to an underdeveloped, yet emerging, response to catecholamines, particularly norepinepherine, in hypothalamic and cortical areas expressing GABAA receptors (Choi et al., 1997). Interestingly, 6-hydroxydopamine lesions of the paraventricular nucleus of the hypothalamus in adult rats prompt social behaviors that are similar to those expressed during adolescence (Kellogg, Inglefield, et al., 1993).
The mesocorticolimbic dopamine systemis critical for the facilitation of behavioral responses to novel stimuli, including social interactions (Hooks et al., 1994; Berton et al., 2006). Ascending mesocorticolimbic dopamine projections begin to undergo a significant maturational process early on during adolescence (Campbell et al., 2000). The maturation of ascending dopaminergic projections in cortical areas, as well as the nucleus accumbens, is an ongoing process that continues into adulthood (Kalsbeek et al., 1988). Maturation of mesocorticolimbic circuitry is critical for the appropriate formation of descending cortical projections, and maladaptations within this circuitry have been experimentally linked to the expression of psychotic-like behaviors, as well as early onset drug taking (Laviola et al., 1999, 2001; Lesting et al., 2005). Also of note is a “pruning” of D1 dopamine receptors at the onset of periadolescence (Teicher et al., 1995). Adolescents appear to have a subtly but significantly lower tonic level of striatal dopamine release and reuptake when compared to adults, as detected by voltammetric methods (Stamford, 1989). In addition, adolescents have more dopaminergic synapses in the prefrontal cortex than adults (Seeman et al., 1987), allowing for a diverse range of contextual and social stimuli to be imprinted.
3.2.2. Therapeutics
Diazepam administration significantly increases the number of social interactions in adults (File & Hyde, 1978), but under similar experimental conditions adolescents are insensitive to repeated diazepam treatment in terms of the time they spend interacting with a novel counterpart (Primus & Kellogg, 1990a). Interestingly, administration of diazepam to an adolescent does increase social interactions later in adulthood, an effect dependent on intact gonadal hormones (Primus & Kellogg, 1990a). In vitro, the cortical GABAA receptor complex is responsive to benzodiazepines similarly across adolescence and adulthood (Kellogg, Inglefield, et al., 1993; Kellogg, Taylor et al., 1993). Together, these data indicate that there may not be a functional difference at the level of the GABAA receptor, but the divergent activation of neural circuits across developmental periods in response to environmental challenges is potentially responsible for the adolescent's lack of sensitivity to anxiolytics.
Initial experiences of clinical depression can arise as early as the time of adolescence (Kessler et al., 2001). Depressive symptoms such as anhedonia, anxiety, cognitive disturbances and abnormal sleep patterns occur frequently in adolescents with this disorder, and may be severe (Brozina & Abela, 2006). Suicidalities are highest amongst depressed adolescents, as compared to any other age group, which underscores the societal impact of depression during this time and the significant need for more understanding of its etiology (Spirito & Esposito-Smythers, 2006). Interactions between a range of genetic (e.g. two short alleles for the gene encoding the serotonin transporter) and environmental (e.g. physical or sexual abuse) factors are potential triggers for the emergence of adolescent depression (Brown & Harris, 2008). Likewise, social isolation in rats during periadolescence decreases social interactions and sexual behavior later in adulthood, without increasing anxiety-like behavioral responses (Van Den Berg et al., 1999; Hol et al., 1999). Conventional pharmacotherapies for treating adult depression have proven to be controversial when considered for the treatment of depressed adolescents, as it remains unclear as to how chronic administrations of antidepressants affect the developing brain (Kutcher & Gardner, 2008). In addition, acute elevations of serotonergic activity or genetic ablation of the serotonin transporter decrease the expression of important adolescent social behaviors (i.e. play) in the rat (Homberg et al., 2007). Alternatively, manipulations of opioid and cannabinoid systems can increase social play in adolescent rats, and studies such as these provide valuable insight regarding neural mechanisms critical to adolescent neural development (Trezza & Vanderschuren, 2008).
3.2.3. Drugs of abuse
The HPA axis is a prominent mediator of the stress response, and the reactivity of this system to stressors and drugs of abuse is positively correlated with the emergence of depressive-like behaviors (Rao, 2006). Acutely, systemic environmental stressors may directly activate the paraventricular nucleus in response to the release of monoamines, whereas “processive” stressors (i.e. requiring interpretation via cortical processing) appear to activate the HPA axis via cortical innervations of the PVN (Herman & Cullinan, 1997). Adolescents with a dysregulation of circadian LHPA axis activity are particularly vulnerable to drugs of abuse (Bruijnzeel et al., 2004). Depressive-like adolescents with elevated cortisol at the time of sleep-onset are most likely to engage in compulsive drug taking, as compared to other depressed adolescents with normal LHPA activity at the beginning of sleep (Rao et al., 1999). Reducing the acute LHPA response to stress and maintaining integrity over its circadian activity is hypothesized to be a promising method for preventing escalations in drug taking (Holsboer, 2001). Likewise, administering alcohol or cocaine to rats significantly lowers plasma ACTH and corticosteroid responses to stress or CRH challenges (Rivier & Vale, 1988; Goeders, 1997) which is consistent with a self-medication hypothesis. Conversely, modulating corticosteroid secretion or CRH antagonism attenuates the reinforcing effects of psychomotor stimulants, and alleviates some of the affective symptoms that emerge during withdrawal from drug taking (Piazza et al., 1994; Fahlke et al., 1994; Heinrichs et al., 1995; Zobel et al., 2000).
An obvious interaction between social stressors and drug-induced behavioral responses occurs during adolescence. Social behavior in adolescent rats is readily increased after an acute injection of alcohol (Spear & Varlinskaya, 2005). Adolescents exposed to alcohol under chronic conditions decrease their social preference during periods of withdrawal, and this effect is reversed by renewed administration of alcohol (Spear&Varlinskaya, 2005). In terms of psychomotor stimulants, adolescent rats experimentally deprived of social interactions will self-administer more cocaine during a fixed ratio schedule of reinforcements than non-isolated rats (Ding et al., 2005). Social isolation during adolescence also increases the acquisition of intravenous cocaine self-administration at low unit doses and decreases the acquisition of high unit doses of cocaine, which points to a leftward shift in the dose–response curve for cocaine responding (Howes et al., 2000). It will be instructive to gain further insight regarding the effects of psychomotor stimulant and social challenges experienced during adolescence on drug self-administration behaviors in adulthood, particularly cocaine taking, as these stimuli experienced in adulthood can significantly intensify cocaine intake (Tidey & Miczek, 1997; Schenk & Partridge, 2000; Covington, & Miczek, 2001).
The behavioral effects of an acute administration of the psychomotor stimulants cocaine or amphetamine in adult rats are typically far greater than the effects produced in adolescents (Lanier & Isaacson, 1977; Bolanos et al., 1998). Hyporesponsiveness to psychomotor stimulants during adolescence is linked to the elevated expression of cortical DA D1 receptors and this observation may partly explain significant elevations in cocaine taking that occur during this developmental phase (Estroff et al., 1989). Interestingly, the behavioral and underlying neuronal effects of psychomotor stimulants in adolescents also diverge from their adult counterpart. In adult rodents, repeated intermittent exposures to a psychomotor stimulant progressively augment its behavioral effects (i.e. behavioral sensitization), whereas adolescents are far less sensitive to the progressively increasing behavioral effects of repeated cocaine or amphetamine (Bolanos et al., 1998; Collins & Izenwasser, 2002). Adolescent rodents that are socially defeated become even less sensitive to the immediate behavioral effects of psychomotor stimulants. For example, the induction of behavioral sensitization to amphetamine in adult rats is attenuated by the repeated experience of social defeat by an older and larger aggressor during adolescence (Kabbaj et al., 2002). Social defeat stress attenuates the induction of behavioral sensitization to a subsequent cocaine challenge in adolescent hamsters (Trzcinska et al., 2002). Conversely, experimental approaches have examined the effects of cocaine administered during adolescence on subsequent social behaviors and neuronal circuitry. Repeated administrations of cocaine during a hamster's adolescence substantially increase their aggressive behavior toward a similarly aged male of the same size and weight, and this behavioral effect coincides with a persistent increase in functional activation of the anterior and lateral hypothalamus, lateral septum, nucleus circularis, and medial amygdala as measured by the number of Fos positive cells (Knyshevski et al., 2005). The long-lasting cocaine-induced pattern of activation in adolescent neuronal circuits is strikingly similar to that of chronic social defeat stress observed in the adult hamster and mouse (Matsuda et al., 1996; Kollack-Walker et al., 1999).
Given that the adolescent brain undergoes a series of continual changes, including the maturation of limbic and striatal circuitry, there is potential for identifying times of vulnerability to social stress that later potentiate the incidence of affective disorders (Andersen & Teicher, 2008), as well as drug abuse. Likewise, understanding the impact of social stress on the development of particular neural systems during adolescence will lead to better treatment strategies for adolescents, and also for affective or behavioral disturbances emerging later during adulthood.
3.3. Social defeat and subordination stress in adults
3.3.1. Neurobiology
The first insights into the neural circuitry for social stress in adult animals originated with the neurophysiological studies of defensive cats (Hess&Brügger, 1943; Fernandez DeMolina&Hunsperger, 1962);. Using various neuroanatomical tracing techniques (e.g., silver impregnation, 2-deoxyglucose, horseradish peroxidase), the circuit mediating the feline defense response has been systematically characterized, consisting of central and basolateral amygdala, stria terminalis and its bed nucleus, anterior and medial hypothalamus and the periaqueductal grey area (PAG) (Chi & Flynn, 1971; Bandler & McCulloch, 1984; Fuchs et al., 1985). Early on, a significant role for excitatory and inhibitory amino acid transmitters emerged in the synapses of this neural circuitry subserving the expression of defensive behavior, particularly in the hypothalamus and PAG (Bandler & Carrive, 1988; Cheu & Siegel, 1998).
As for many other types of stress responses, the first neurochemical system linked to defeat and subordination was the catecholamine system. For example, Hendley et al. (1973) measured more uptake sites and reduced affinity for reuptake of norepinephrine (NE) in the cortex of scarred and wounded group-housed mice that were defeated by a despotic dominant. More recently, chronic subordination stress in tree shrews was found to persistently upregulate alpha 2C receptors in discrete brain stem nuclei, but not in the locus coeruleus or prefrontal cortex (Flügge et al., 1997, 2003). Such findings need to be seen in the context of similar observations in animals that engaged in attack behavior (Welch et al., 1974; Tizabi et al., 1980) and point to a much more general role of noradrenergic activity during social conflict that is not specific to defeat. It will be instructive to learn how activation of norepinephrine and its receptor and transporter molecules are linked with a specific type of social stress, and how this noradrenergic activity is coordinated with those of excitatory amino acids and other amines and peptides.
Early postmortem tissue analysis demonstrated increases in mesolimbic, but not striatal DA activity in rats and mice that were attacked by an opponent — an ostensibly and intensely aversive experience (Mos & Van Valkenburg, 1979; Haney et al., 1990; Puglisi-Allegra & Cabib, 1990). These findings and similar ones with non-social stressors are difficult to reconcile with the prevailing view that assigned a special role to the mesolimbic DA activity in reward processes (Wise & Rompre, 1989). The early postmortem studies were extended and confirmed with in vivo microdialysis methodology showing an increase in DA in prefrontal cortex and nucleus accumbens, but not striatum, when an intruder rat was beginning to be threatened by an aggressive resident (Fig. 6 and Fig 7; Tidey & Miczek, 1996; Miczek, Mutschler et al., 1999). Moreover, repeated social defeat experiences engendered a larger DA response to an amphetamine challenge than is seen in non-defeated rats (Miczek, Mutschler et al., 1999). It is, however, noteworthy that the rise in accumbal DA does not appear to be specific to the experience of defeat and the display of defensive responses, but characterizes also those individuals who engage in offensive threat and attack behavior (Van Erp & Miczek, 2000; Ferrari et al., 2003). As highlighted in Fig. 6, activity in the DAergic projections from the ventral tegmental area to the n. accumbens and to the medial prefrontal cortex is intimately regulated by glutamatergic feedback (Murase et al., 1993; Taber & Fibiger, 1995; Taber et al., 1996; Vorel et al., 2001). A brief episode of social defeat stress leads to increased NMDA receptor binding specifically in hippocampal CA3 neurons of intruder rats (Krugers et al., 1993), and the functional significance of glutamatergic input from the hippocampus to the VTA has been demonstrated (Vorel et al., 2001).
Fig. 6.
Neural circuits for brief social defeat stress and continuous subordination stress. The ascending DA pathway originating in the ventral tegmental area (VTA) and projecting to themedial prefrontal cortex is inhibited by GABA interneurons which in turn receive input from opioid peptides and CRH, among others. The glutamatergic feedback from PFC and amygdala modulates the DA pathway either directly or by acting on the GABA interneurons. This pathway may be rendered hyperactive as a result of brief social defeat episodes. By contrast, continuous uncontrollable subordination stress activates the serotonergic DRN cells that project to the forebrain, including the PFC. Glutamatergic feedback fromPFC and limbic forebrain modulates the ascending 5-HT projections and it has been proposed that this feedback prevents dysregulation of the 5-HT system.
Fig. 7.
Extracellular DA in prefrontal cortex, n. accumbens or dorsal striatum of (A) socially-defeated rats, before, during and after threat of social defeat, and (B) non-defeated rats, before, during and after being placed in a novel cage. Dopamine concentrations are expressed as percent of baseline (home cage). (From Tidey and Miczek, 1996).
A very large increase in firing of single dorsal raphe cells was recorded in tree shrews that assume a defensive posture when approached by a dominant conspecific or by a human handler (Walletschek & Raab, 1982), presumably reflecting serotonergic activation (Fig. 8). Tissue analysis had earlier demonstrated that 5-HT and its primary acid metabolite 5-hydroxyindoleacetic acid (5-HIAA) were elevated in several forebrain regions (e.g., septum, hippocampus, hypothalamus) of tree shrews that were attacked and threatened by a dominant opponent (Raab, 1971). More recently, elevations in hippocampal 5-HT were measured via in vivo microdialysis in mice that had experienced repeated defeat and were threatened by an aggressive opponent (Keeney et al., 2006). It is noteworthy that increases in hippocampal 5-HT in repeatedly defeated mice contrast with decreased cortical 5-HT in aggressive rats (Van Erp &Miczek, 2000), although both attacking dominant and defeated animals show elevations in corticosterone, even during and after repeated confrontations (Schuurman, 1980; Covington &Miczek, 2005). It is apparent that glucocorticoid and CRH on the one hand and forebrain 5-HT on the other interact in multiple ways in the mechanisms governing the initial stress response and its long-term consequences, involving also GABA interneurons (Valentino & Commons, 2005). While the expression of various genes associated with neurotransmission and neuroplasticity was upregulated after daily social defeat stress in intruder rats, no differential expression of genes for serotonin transporter (SERT), tryptophan hydroxylase or the 5-HT1A autoreceptor was detected (Abumaria et al., 2006), pointing to non-serotonergic neurons as sites of neuroplastic changes resulting from social defeat stress. By contrast, in CBA/Lac mice the levels of SERT and MAOA mRNA are significantly elevated in the raphe nucleus of repeatedly defeated mice relative to aggressors and controls (Filipenko et al., 2002). This latter observation is concordant with the increased serotonin efflux in themedial prefrontal cortex after inescapable electric shock (Bland et al., 2003). One resolution of these seemingly conflicting findings is that the latter two social stress experiences were episodic, whereas the former involved continuous subordination stress. The brief sensitizing social defeat stress predominantly involves the VTA–mPFC–amygdala circuit, whereas continuous uncontrollable subordination stress relies on glutamatergic modulation of the serotonergic dorsal raphe nucleus (DRN) corticolimbic projections (Fig. 6).
Fig. 8.
Firing rates of the dorsal raphe neurons during 13 defensive encounters of a male tree shrew with a conspecific resident. The numbers 2.1 and 2.3 correspond to the experimental animal at rest before and after the encounter, while 2.2 corresponds to the animal showing a defensive posture after being approached by the resident. (From Walletschek and Raab, 1982).
Receptor binding and functional assessments have identified changes in 5-HT1A , mineralocorticoid and glucocorticoid receptors, particularly in the hippocampus, after acute and chronic social defeat stress (Buwalda et al., 2001, 2005). Chronic social subordination stress in rats and tree shrews, but not in house mice, leads to reduced 5-HT1A receptor binding in hippocampus (Flügge, 1995;McKittrick et al., 1995), and an attenuated hypothermic and HPA response to 5-HT1A agonist challenge (Korte et al., 1995; Buwalda et al., 2005). These latter findings can be interpreted as evidence for 5-HT1A receptor desensitization, comparable to that seen in depressed patients (Lesch et al., 1990).
Morphological and molecular changes in the hippocampus have been documented after many types of stress, including social stress (McEwen & Chattarji, 2007). The reduction in hippocampal volume appears to be of clinical significance, since imaging studies point to a selectively decreased left hippocampal volume in depressed patients (Bremner et al., 2000). Several weeks of chronic subordination stress in tree shrews was associated with a lower hippocampal volume, no change in the density of pyramidal neurons, but remodeling of apical dendrites (Fuchs et al., 1995; Magarinos et al., 1996; Czeh et al., 2001). Retraction of apical dendrites can also be seen in subordinate and in dominant alpha rats in a captive colony (McKittrick et al., 2000). In intruder rats even one or two defeats rapidly produced dendrite retraction in the apical tree that later shifts to changes in basal dendrites (Kole et al., 2004). One possible mechanism for the hippocampal changes in socially stressed animals may be the inhibition of neurogenesis in the subgranular zone of the dentate gyrus (Gould et al., 1998). A reduction in cell proliferation is already seen after a few episodes of social defeat stress in intruder mice (Yap et al., 2006). In addition to the glutamatergic and GABAergic feedback from prefrontal cortex to the cell groups that give rise to ascending monoamine pathways, evidence is emerging that glutamatergic feedback fromthe hippocampus to the VTA modulates dopaminergic cells (Vorel et al., 2001).
In addition to hippocampal cell activity, social defeat stress, experienced either acutely or repeatedly, is associated with increased cellular activity in structures along the core of the neuroaxis (Fig. 9). Evidence from studies in mice, hamsters and rats shows that acute social defeat results in increased c-fos expression in the prefrontal cortex, lateral septum, medial and central amygdala, several hypothalamic nuclei and several brainstem nuclei such as the central and periaqueductal grey areas, locus coeruleus, dorsal raphe nucleus, ventral tegmental area, and the nucleus of the solitary tract (Matsuda et al., 1996; Kollack-Walker et al., 1997; Martinez et al., 1998; Nikulina et al., 1998; Miczek, Nikulina et al., 1999). A critical determinant of cfos activation in socially stressed intruder animals is their history of social intercourse and the social context after their defeat experience (Kollack-Walker & Newman, 1995). For example, in hamsters prior social experiences reduce the differences in cellular activation between resident and intruder animals after a confrontation. Further work will have to assess which of the fos-labeled cells reflect cellular activation that is common to many kinds of stress, that is specific to social defeat stress, and that is specific to a particular animal species.
Fig. 9.
Schematic presentation of the sagittal rodent brain depicting cellular activation by c-fos in mice, hamsters and rats that were attacked by a resident in an aggressive episode (data from Martinez et al., 1998; Nikulina et al., 1998; Kollack-Walker et al., 1999).
The neuroadaptations that result from repeated experiences with social defeat are of particular interest, since they mediate behavioral changes in rodents that appear relevant to increased drug taking and affective disturbances. Under these conditions, intruder animals are exposed to repeated episodes of social defeat ranging from about one week to one month. These repeated defeats, often referred to as “chronic,” differ from continuous subordination stress, the latter requiring cohabitation, albeit protected, with a dominant opponent. After repeated confrontations with an aggressive opponent, rats and hamsters show continued expression of c-fos in the anterior and ventromedial hypothalamus, medial amygdala, central grey, dorsal and medial raphe nuclei (Martinez et al., 1998; Kollack-Walker et al., 1999). Cells in the paraventricular nucleus of the hypothalamus and the lateral septum appear to habituate rapidly to stimulation by repeated stressors, including also social defeat stress. Evidence of persistent changes in cellular activity after repeated social defeat experiences is of particular interest. When challenged with a moderate 1.0 mg/kg amphetamine dose, c-fos expression remains augmented in the VTA and prefrontal cortex even more than two months after four episodes of social defeat (Nikulina et al., 2004; Covington et al., 2005). As discussed below, this kind of sensitized cellular activity may reflect a dysregulated cascade of intracellular processes that may promote stress-related drug taking. So far, the pattern of cellular activity in prefrontal cortical, amygdaloid, septal, hypothalamic, tegmental structures remains to be characterized for individuals who experience continuous subordination stress.
3.3.2. Therapeutics
While the evolutionary origin of affect and mood has been studied ever since Darwin's proposal (Darwin, 1872), it is difficult to map an animal's emotional response pattern precisely onto the diagnostic criteria that are promulgated and periodically revised by clinical experts. For example, the subordinate tree shrew is varyingly referred to as a model of anxiety-like physiology and behavior (Von Holst, 1985) or as model of depressive-like symptoms (Fuchs et al., 2004). Similarly, the socially-defeated intruder rats are considered to model anxiety-like features (Becker et al., 2001, 2004), whereas the loss in social status as a result of defeat by an opponent has been argued to reflect depressive-like features (Willner et al., 1995). While it is apparent that social defeat can engender behavioral features indicative of profound emotion, these responses may relate to symptoms in anxiety and affective disorders.
3.3.2.1. Antidepressants
The study of social defeat stress has begun to contribute significantly to characterizing the effects of prototypic antidepressant drugs and compounds with therapeutic potential. The most attractive feature of social defeat stress procedures is the modeling of some cardinal features of affective disorders. In particular, the loss of experiencing pleasure (“anhedonia”), mainly measured by the preference for sweet tastes, is evident after exposure to uncontrollable, unpredictable and chronic defeat stress, particularly in continuously subordinate rats (Rygula et al., 2005, Rygula, Abumaria, Flügge, et al. 2006; Miczek et al., in preparation) and mice (Berton et al., 2006), but not always in all animals (e.g. Von Frijtag et al., 2002; Strekalova et al., 2006).
So far, tricyclic antidepressants such as imipramine, clomipramine and tianeptine as well as SSRIs such as citalopram and fluoxetine have been found to attenuate, at least in part, several salient behavioral and endocrine defects that were engendered by repeated social stress experiences. Gradual increases in the preference for or consumption of a sucrose solution after its suppression by social stress has been highlighted as the validating effect of daily imipramine, citalopram or fluoxetine in rats and mice (Willner et al., 1995; Strekalova et al., 2006; Rygula, Abumaria, Domenici, et al., 2006; Rygula, Abumaria, Flügge, et al., 2006; Miczek et al., in preparation), although this effect is limited to very specific concentrations of the sucrose or saccharin. Additionally, low psychomotor activity and suppressed social contact due to repeated social stress experiences can be restored by daily imipramine, citalopram, fluoxetine or clomipramine in mice, rats and tree shrews (Kudryavtseva, 1991; Fuchs et al., 1996; Berton et al., 1999; Von Frijtag et al., 2002; Rygula, Abumaria, Domenici, et al., 2006; Rygula, Abumaria, Flügge, et al., 2006).
This behavioral evidence is complemented by chronic tianeptine and imipramine treatment effects that reverse HPA axis activation and the suppression of hippocampal neurogenesis and cellular activity after repeated social stress (Czeh et al., 2001; Von Frijtag et al., 2001). It has been proposed that the beneficial effects of antidepressant drug treatment in animals that experienced repeated social defeat stress and that were continuously exposed to a potential aggressor may involve the intracellular cascade of events leading to the expression of BDNF (Tsankova et al., 2006). Viral delivery of BDNF can achieve restorative effects on the social stress-suppressed sexual dysfunction, social interactions and sucrose preference similar to those of SSRIs in mice (Berton et al., 2006).
3.3.2.2. Anxiolytics
In addition to its potential relevance for the study of antidepressant drugs, repeated social defeat stress in mice, rats and tree shrews induces behavioral and neurobiological changes that appear relevant to anxiety disorders (Von Holst, 1985; Kudryavtseva & Avgustinovich, 1998). For example, a single social defeat experience decreases the time spent in the open arm of an elevated plus maze, a traditional measure that is sensitive to the actions of anxiolytic drugs, both in rats and mice (Rodgers & Cole, 1993; Berton et al., 1999; Buwalda et al., 2005). In repeatedly defeated mice, the anxiolytic-like effects of ethanol consumption can be detected by increased social contact (Kudryavtseva & Avgustinovich, 1998).
When cues signal the imminent occurrence of social defeat stress, the anticipatory physiological and behavioral responses appear particularly sensitive to anxiolytic-like compounds (Tornatzky & Miczek, 1994; Tornatzky & Miczek, 1995; Becker et al., 2001, 2004). For example, the prototypic benzodiazepines diazepam and chlordiazepoxide prevented anxiogenic-like behaviors such as ultrasonic distress calls, immobility, and defensive and submissive acts and postures in intruder rats in a flumazenil-reversible manner; at the same time, diazepam protected against the increased release of cortical cholecystokinin (CCK)-like material during the anticipation of social defeat stress (Becker et al., 2001; Andre et al., 2005). Similar anxiolytic-like effects were obtained with melatonin and synthetic agonists of MT1/2 receptors in the same situation, using identical measures of behavior and CCK-like material (Becker et al., 2004). By contrast, reactive rather than anticipatory measures of repeated social defeat stress are not alleviated by chronic diazepam, as indicated by persistent elevation of glucocorticoid activity and behavioral disruption (van Kampen et al., 2000). The distinction between anticipatory and reactive responses to social defeat stress is consistent with the preferential effects of anxiolytic drugs that act as positive modulators of the GABAA receptor and as agonists at 5-HT1A receptors on ultrasonic distress calls and hyperthermia in anticipation of a confrontation. Diazepam, gepirone and also alcohol attenuate anticipatory hyperthermia and USVs, but did not affect the defensive reactions (Vivian & Miczek, 1993; Tornatzky & Miczek, 1994; Tornatzky & Miczek, 1995).
3.3.3. Drugs of abuse
Epidemiological links between the propensity to drug abuse and stress, particularly social stress, are well documented (Rhodes & Jason, 1990; Sinha, 2001; Sarnyai et al., 2001; Goeders, 2003). These correlational data allow for several scenarios, and it must also be recognized that drug taking itself is stressful (Sarnyai et al., 2001). Nonetheless, unpredictable social stresses have been assigned to contribute to the initiation of drug abuse and to trigger relapse (Funk et al., 2005). Experimental data fromlaboratory studies in rodents and non-human primates have begun to manipulate the exposure to social stress in a precise and controlled manner and assess the impact of these experiences on the initiation of drug taking, its maintenance and also relapse to drug taking (Miczek et al., 2004).
3.3.3.1. Psychomotor stimulants
As few as four brief episodes of social defeat stress over the course of one week were sufficient to increase the rate of acquiring intravenous cocaine self-administration (0.32 mg/kg/infusion) in both male and female rats (Haney et al., 1995). In this experiment, female intruder rats were defeated by a lactating opponent and male intruders were attacked by a resident male rat during brief encounters separated by 24–48 h. No sex differences in the acquisition of cocaine self-administration at this relatively low unit dose emerged. This evidence was confirmed and extended in male intruder rats that were exposed on four consecutive days to brief episodes of social defeat stress by an aggressive resident (Fig. 10; Tidey & Miczek, 1997); the defeated intruder rats acquired cocaine self-administration (0.25 mg/infusion which correspond to ca. 0.75 mg/kg) twice as fast as the non-defeated control animals. Moreover, extracellular DA was increased in the n. accumbens and the medial prefrontal cortex, but not in dorsal striatum, in repeatedly defeated intruders that were threatened by an aggressive resident behind a protective screen (Tidey & Miczek, 1996). These studies on increased rates of acquiring cocaine self-administration were substantiated in rats that were non-responsive to a novel environment and had experienced four episodes of social defeat stress; by contrast, novelty-responsive rats acquired cocaine self-administration after social defeat stress with significant delays (Kabbaj et al., 2001). In fact, considerable differences in how an individual perceives the anxiogenic and pleasurable effects of cocaine or amphetamine are apparent in the discriminative stimulus and reinforcing effects of these drugs (Ettenberg & Geist, 1991; Miczek, Mutschler, et al., 1999). These individual differences are not surprising, given the often replicated observation that the amount of exploratory behavior predicts the readiness to initiate amphetamine self-administration. Significant predispositions may render some individuals more vulnerable to the social stress-facilitated acquisition of cocaine self-administration, and characterizing these predispositions remains to be accomplished (Piazza & Le Moal, 1998).
Fig. 10.
Cumulative histogram showing rates of acquisition of cocaine self-administration for previously defeated (solid bars) and non-defeated (open bars) rats. Rate data for each group were parsed into 12-h bins. (From Tidey and Miczek, 1997).
Once cocaine self-administration is established during daily limited access conditions, brief episodes of social defeat stress prior to each experimental session can increase the rate of drug intake significantly, particularly at lower unit doses (Miczek & Mutschler, 1996). It is a common feature of self-administration protocols to impose a time-out period when the infusion begins and during this period response rates are typically very low. In animals that experience daily social defeat stress, the rate of responding on the cocaine-reinforced lever is very high during the time-out period, possibly indicating a loss of stimulus control or impulsive cocaine seeking. These activating effects of brief episodes of social defeat stress persist for weeks without signs of habituation, and this enduring effect on cocaine-seeking behavior appears to be the result of neuroplastic changes.
In fact, considerable evidence points to the sensitizing effects of episodic stress as detected by augmented locomotor activity in response to stimulant challenge and by the heightened activity in the mesocorticolimbic neural circuitry. Similar to other laboratory stressors such as tail pinch or mild foot shock pulses (Sorg & Kalivas, 1991), intermittent exposure to brief episodes of social defeat stress induces behavioral sensitization as is evident by an augmented hyperactivity to a challenge with a moderate dose of cocaine, amphetamine, morphine or ethanol (Nikulina et al., 1998; Marrow et al., 1999;Miczek, Nikulina, et al., 1999; Covington, & Miczek, 2001; Covington et al., 2005; Yap et al., 2005; Covington & Miczek, 2005; Yap & Miczek, 2007). The sensitized locomotor activity can be seen transiently after as little as a single episode of social defeat stress, and it persists in intruder rats for at least several months after multiple episodes (Fig. 11; Covington et al., 2005; de Jong et al., 2005).
Fig. 11.
The effect of a cocaine or amphetamine challenge on the frequency of walking behavior for defeat-stressed rats (gray bars) and unstressed controls (open bars). Baseline (BL) measurements of walking behavior were obtained after a saline injection before the first stress exposure. Stressed rats received episodes of social defeat on days 1, 4, 7, and 10. One cohort was challenged with 10.0 mg/kg cocaine on day 20 (top) for the expression of behavioral sensitization. Two additional cohorts were challenged with 1.0 mg/kg amphetamine either on days 20, 40, 60, and 70 (middle) or only on day 70 (bottom). Bars represent averages ±SEM for the sum of walking frequency over 5–10 and 25–30 min postinjection for each group. *Indicates stressed vs. control rats were significantly different from each other (p < 0.05) on that day. (From Miczek et al., 2004).
Evidence from studies in mice, rats and hamsters highlights the cellular activation along the core of the neuroaxis that appears to delineate the neural basis for the behavioral sensitization induced by episodes of social defeat stress (Matsuda et al., 1996; Martinez et al., 1998, 2002; Miczek et al., 2004). Social defeat stress activates cells in the periaqueductal grey area, dorsal raphe, and locus coeruleus that project via hypothalamic structures to accumbal, prefrontal cortical and amygdaloid targets. Of particular significance is the feedback from amygdala, hippocampus and prefrontal cortex to the dopamine cells in the ventral tegmental area, since this chiefly glutamatergic feedback has been hypothesized to be particularly relevant to the induction of neuroadaptations that lead to intense drug taking (Pierce & Kalivas, 1997; Vanderschuren & Kalivas, 2000). Blockade of specific glutamate receptor subtypes prior to each defeat stress episode prevented the expression of cross-sensitized hyperactivity to a stimulant challenge. NMDA receptor antagonism attenuated the sensitized locomotor activity in response to an amphetamine challenge in mice and rats in a dose-dependent manner. Specifically, microinjections of 2-amino-5phosphopentanoic acid (AP-5) into the VTA before each of four episodes of social defeat significantly attenuated the sensitized locomotion in response to an amphetamine challenge 10 days thereafter (Fig.12; Yap et al., 2005; Covington et al., 2008). Blockade of mGlu1 receptors with 2-methyl-6-(phenylethynyl)-pyridine (MPEP) before each episode of social defeat stress also reduced the sensitized locomotor hyperactivity in mice. Further studies with episodically defeated intruder rats pointed to AMPA receptors in the VTA as targets for the sensitizing effect of social defeat stress, since blockade of these receptors with the AMPA receptor antagonist NBQX prevented the augmented response to amphetamine challenge (Covington et al., 2008). Of most recent interest is the role of intracellular signaling pathways in the modulation of the stress-induced sensitized response. One signaling cascade of interest is the extracellular signal-regulated kinase (ERK) pathway, which when activated by repeated cocaine administration, can promote the downstream activation of the transcription factor cAMP response element binding protein (CREB) and enhance immediate early gene expression of c-fos and zif268 (Mattson et al., 2005; Lu et al., 2006). Using the MEK inhibitor U0126 to prevent the phosphorylation of ERK in the VTA before each defeat protected against the development of social stress-sensitization, suggesting enhanced activation of ERK in the VTA due to brief social defeat experiences is critical in the induction of sensitization (Yap et al., 2008).
Fig. 12.
Total self-administered intake during a 24 h cocaine binge in intermittently defeated and non-defeated rats that were “pharmacologically protected” with infusions of the NMDA receptor antagonist AP-5 into the VTA prior to each stress episode. The mean (±SEM) total amount of cocaine (mg/kg) self-administered during the 24-hour bringe is shown for non-stressed (open bar) and episodically defeated (filled bars) rats. The open and dark gray bars represent rats that were infused with artificial cerebrospinal fluid (aCSF) and the light gray bar represents rats that were infused with the NMDA receptor antagonist AP-5 (5 nmol). (From Covington et al., 2008).
Intense cocaine seeking and taking was engendered by prior social defeat stress-induced sensitization in intruder rats that experienced four episodes of social defeat over the course of ten days, subsequently engaged in an augmented locomotor response to a stimulant challenge (i.e. cross-sensitization) and thereafter self-administered cocaine intravenously. When cocaine infusions were delivered contingent upon fulfilling progressively more demanding behavioral requirements (i.e. progressive ratio (PR) schedule of reinforcement), stress-sensitized rats, but not mice, exerted themselves more and were more resistant to extinction, i.e. attained a higher “break point” relative to contemporary control animals. (The break point is defined as the highest completed ratio before responding is insufficient to meet the next highest ratio requirement within a specific interval.) (Covington, & Miczek, 2001; Covington et al., 2005; Covington, & Miczek, 2005; Yap &Miczek, 2007; Covington et al., 2008). Under conditions of unlimited access to cocaine for 24 h, a so-called binge, stress-sensitized rats self-administered cocaine with shorter inter-infusion intervals and accumulated more cocaine, in excess of 100 mg/kg, i.v., whereas most control animals stopped self-administering cocaine after 10–12 h (Fig. 13). During the initial hour of a binge, rats attained a steady level of cocaine self-administration that was maintained for several hours before the first signs of dysregulation began to appear; eventually, after 12–16 h a pattern of bursting and gaps replaced the consistent and regular timing of self-administration (Kreek and Koob, 1998; Covington & Miczek, 2005; Fowler et al., 2007). Stress-sensitized rats self-administered cocaine continuously during the 24 h binge, indicating an abolished circadian-like pattern of drug taking (Fig. 14).
Fig. 13.
The effect of social defeat or offensive aggression on cocaine self-administration behavior during a 24-h cocaine binge (0.3 mg/kg/infusion). Individual cumulative records of cocaine intake across each hour of the binge in (A) previously defeated intruder rats, (B) contemporary handled control rats, and (C) resident rats with a history of offensive aggression. (D) Average total cocaine intake (mg/kg) during the 24-h binge in groups of intruder, resident, and control rats. *Indicates that intruders accumulated significantly more cocaine than controls and residents (p < 0.05). (E) The percentage of intruder, resident, and control rats that continued to respond after hour 16 of the 24-h binge. The difference between groups was statistically significant (p = 0.001) (From Covington and Miczek, 2005).
Fig. 14.
Hourly cocaine intake during a 24-h cocaine binge. Circadian-like cocaine self-administration behavior was maintained in control rats (open circles), whereas stressed rats (filled circles) self-administered cocaine intensely for 24 h, effectively abolishing the circadian pattern of intake (p <0.01). (From Covington et al., 2005).
Transient increases in dopamine D2 receptor binding were measured in those few cynomolgus monkeys that became dominant once being transferred from single housing (Morgan et al., 2002). Within three months of group formation, these changes in DA receptor regulation correlated with a substantially reduced rate of acquiring intravenous cocaine self-administration. Across a range of cocaine unit doses, dominant monkeys self-administered the drug in smaller amounts than subordinate monkeys. After prolonged daily cocaine self-administration, the differences in drug intake and DA receptor regulation between dominant and subordinate monkeys disappeared (Czoty et al., 2004). If indeed the initially upregulated DA D2 receptors reflect DA hyperactivity, as has been hypothesized, then it may be possible to alter the social conditions for the lower ranking monkeys so that their DA activity is increased as seen in acutely stressed intruder rats.
In striking contrast to the intense cocaine taking after episodic social stress, continuously threatened subordinate rats responded to stimulant challenges with a much attenuated motor hyperactivity and their intravenous cocaine intake was greatly reduced. Continuous subordination stress was engendered in male intruder rats that were periodically defeated by an aggressive resident during a brief confrontation and subsequently housed with full provisions in the resident's home cage behind a protective screen. Throughout the five weeks of continuous subordination stress, the intruder rat gained less weight, explored less and did not prefer sucrose as is typical for non-stressed rats. When challenged with an amphetamine injection, continuously subordinate rats showed less hyperactivity than concurrent control animals. In contrast to the sensitized locomotor response to a stimulant challenge that characterizes episodically defeated animals, continuous subordination stress led to a blunted stimulant response.
The pattern of intravenous cocaine self-administration by intruder rats sustaining continuous subordination stress differed profoundly from that of the episodically defeated rats. As discussed above, brief intermittent episodes of unpredictable social defeat stress prompted rats to exert themselves more in order to meet the progressively higher demands for being infused with cocaine (i.e. higher break points and more resistance to extinction). Moreover, these episodically stressed rats accumulated higher amounts of cocaine during a 24-h unlimited access binge. The opposite changes ensued in rats that experienced continuous subordination stress (Miczek et al., 2005); during daily limited access sessions, these rats attained fewer intravenous cocaine infusions, completed fewer ratio requirements for cocaine infusions on the PR schedule of reinforcement, and ceased self-administering sooner when cocaine was available during a 24-h unlimited access binge. Intermittency and controllability appear to be key determinants for social defeat stress to activate or inactivate neuroadaptive processes that are reflected in behavioral sensitization, response to sweet rewards, and ultimately regulate intense cocaine seeking and taking.
Initial mechanistic studies focused on BDNF in the ventral tegmental area, prefrontal cortex and the amygdaloid complex (Miczek et al., in preparation), since suppression of this growth factor in hippocampal cells is a key consequence of exposure to stress, including social defeat stress (Chao & McEwen, 1994; Pizarro et al., 2004). A large decrease in BDNF-labeled cells in the VTA and subsections of the prefrontal cortex (anterior cingulate, prelimbic and infralimbic areas) is seen in intruder rats that had undergone continuous subordination stress (Fig. 15). By contrast, rats that had displayed behavioral sensitization after four episodes of social defeat stress show elevated BDNF-labeled cells in the VTA and the infralimbic area of the prefrontal cortex. These divergent adaptations in BDNF cells after episodic relative to continuous social stress appear critical to the escalated vs. suppressed patterns of cocaine taking that follow these different schedules of stress. It is likely that the controllability of the social stress experience is an important determinant for the BDNF activation in the medial prefrontal cortex, as has been demonstrated for escapable vs. inescapable tail shock (Amat et al., 2005).
Fig. 15.
Effect of 36 days of chronic subordination or intermittent social defeat stress episodes on BDNF protein levels in the VTA. Continuous subordination stress leads to significantly decreased levels of BDNF protein in the VTA compared to control levels, whereas 4 acute defeats over 10 days result in increased BDNF protein levels. Bars represent average number of BDNF-labeled cells in the VTA. *Indicates stressed vs. control rats were significantly different from each other (p < 0.05). (From Miczek et al., in preparation).
3.3.3.2. Opioids
Shortly after the discovery of endogenous opioid peptides, their activation by social stress experiences became evident in studies of maternal separation stress and social defeat stress in adults (Panksepp et al., 1980; Miczek et al., 1982). When an intruder mouse is attacked frequently and eventually displays the characteristic defeat response pattern (upright posture, retracted fore limbs, vocalizations), it develops an opioid-like analgesia. This analgesia is blocked by centrally acting mu-opioid receptor antagonists, and is concurrent with elevated beta-endorphin and met-enkephalin levels in several brain regions (Siegfried et al., 1984; Teskey et al., 1984; Rodgers & Randall, 1985; Miczek et al., 1986; Külling et al., 1988; Miczek et al., 1991). Similarly, acutely defeated rats show a potentiated analgesic response to mu and delta opioid receptor agonists ([d-Ala2, N-Me-Phe4Gly5-ol]-enkephalin (DAMGO) and [d-Pen2,d-Pen5]-enkephalin (DPDPE), respectively), and this potentiation is reversed by the receptor-selective antagonists, involving receptor sites in the periaqueductal grey area (Vivian & Miczek, 1998; Vivian & Miczek, 1999). There is also evidence that the kappa opioid receptor antagonist nor-binaltorphimine can block the social defeat stress-induced analgesia in C57Bl/6 mice (McLaughlin et al., 2006).
Daily episodes of social defeat result in cross-tolerance to morphine analgesia and, concurrently, to cross-sensitization to morphine hyperactivity. These contrasting types of neuroadaptation involve mechanisms that appear independent from each other and await adequate characterization. So far, microinjections of opioid antagonists into the periaqueductal grey area and the arcuate nucleus of the hypothalamus have proven effective to block social defeat-induced analgesia and stress-potentiated opioid analgesia, implicating mu-opioid receptors (Miczek et al., 1985; Vivian & Miczek, 1998,1999). By contrast, microinjections of the mu receptor-selective agonist DAMGO into the VTA one week after the last of repeated exposures to social defeat stress resulted in a sensitized motor hyperactivity, while the expression of mu-opioid receptors in the VTA is persistently increased (Nikulina et al., 1999, 2005, 2008). In all likelihood, repeated social defeat stress activates endogenous opioid peptides which act on mu-opioid receptors that are located on GABA neurons in the VTA which normally inhibit DA neurons. The stress-induced opioid peptides such as enkephalin disinhibit DA activity via their action on mu receptors at GABA cells. It is possible that the elevated mu-opioid receptor activity extends over the long-term and contributes to the mechanisms via which episodic social defeat stress engenders cross-sensitization to psychomotor stimulant hyperactivity (Nikulina et al., 2004; Covington et al., 2005).
It is surprising that neither the impact of intermittent episodes of social defeat stress nor of continuous subordination stress on opioid drug seeking or taking has been studied adequately so far. Acutely defeated rats that were previously dominant show less preference for a context in which they had received a small dose of morphine (Coventry et al., 1997). This finding is difficult to reconcile with observations that environmental stressors such as brief pulses of foot shock reinstate opioid-seeking in animals that were previously trained to self-administer heroin and reinstate morphine-induced conditioned place preference (Shaham& Stewart, 1995; Shahamet al., 2000; Lu et al., 2003). In mice that have been conditioned to prefer an environment where they have been injected with morphine and then undergone extinction of this place preference, the experience of a social defeat episode reinstated the conditioned place preference for the morphine-paired environment (Do Couto et al., 2006). Acute social defeat stress, particularly when experienced repeatedly, is expected to enhance heroin self-administration, whereas continuous subordination stress may have the opposite effect. One may hypothesize that acute social defeat stress, due to its activation of endogenous opioid synthesis and receptor activation, adds to and possibly potentiates the seeking and self-administration of opioids. This hypothesis is supported by the findings that inescapable electric shocks potentiates DA and 5-HT efflux in the medial prefrontal cortex and also increases the preference for the context where morphine was injected, an opioid conditioned place preference (Will et al., 1998; Bland et al., 2003).
3.3.3.3. Alcohol
A persistent and large increase in alcohol drinking after three episodes of social defeat stress was seen in mice that lack a functional CRH1 receptor (Sillaber et al., 2002). These mutant mice began to drink more than 3.0 g/kg daily ethanol three weeks after the last exposure to stress, and this increase persisted for at least half a year (Fig. 16). These findings in CRH1 receptor mutant mice are at variance with most studies that have found inconsistent effects of alcohol consumption in genetically intact rodents and primates that are exposed to social stress, either intermittently or continuously. Subordinate rats or monkeys tended to drink more alcohol than dominant animals (Ellison, 1981; Blanchard et al., 1987; Higley et al., 1991; McKenzie-Quirk & Miczek, in press), but none of these intake measurements approximated the intoxication level of the CRH1 receptor mutants. Intruder rats that experienced episodes of social defeat drank actually less alcohol in their home cage or responded less when reinforced with alcohol (Van Erp & Miczek, 2001). Similar decreases in alcohol intake were seen in rats that were exposed to continuous subordination stress for several days (Van Erp et al., 2001). After the discontinuation of social stress, similar to that of other stresses, an increase in alcohol consumption can be seen, possibly a rebound phenomenon (Volpicelli et al., 1982; Bowers et al., 1997). Once drinking is extinguished, a cue that has been associated with social defeat experiences, but not social defeat itself, facilitates alcohol-seeking (Funk et al., 2005).
Fig. 16.
Long-term voluntary alcohol intake of Crhr1−/− (black bar; n = 9) and wild-type (white bar; n = 11) mice. The animals were offered water and an ethanol solution (8% v/v) in a two-bottle free-choice paradigm. The data represent mean ethanol intake per month. Stress events are indicated by arrows. *Indicates Crhr1−/− is significantly different from wild type (p < 0.01). (From Sillaber et al., 2002).
Defining the social conditions under which alcohol drinking is persistently escalated remains challenging. It is apparent that housing conditions and prior social experiences do modulate moderate alcohol consumption significantly. Whether the activating effects of low-intensity social stress experiences or the rebound from intense subordination stress may in fact be more relevant to escalating alcohol consumption remains to be resolved. It is quite feasible that individuals cope with these types of stresses differentially, and individual differences in coping strategies may extend to alcohol consumption.
4. Translational implications of social stress in animals
The term“social stress” encompasses various types of life challenges and responses to them that share the feature of involving at least one more individual, but differ inmany other respects. Divergent behavioral, physiologic, neural and molecular adaptations characterize infants that experience maternal separation stress for short vs. long intervals during a critical developmental period. Similarly, in adulthood, brief episodes of social defeat stress engender adaptations that diverge profoundly from those seen after continuous subordination stress. One key determinant for the propensity to self-administer drugs and for the behavioral effects of therapeutic drugs appears to be the intermittency and duration of the social stress in both infants and adults. Intermittent episodes of brief social stress appears to trigger an intracellular cascade of molecular events in the VTA–accumbens–prefrontal cortex–amygdala circuit that parallel those induced by intermittent injections of psychomotor stimulants, opioids, and possibly alcohol (see Fig. 6). Recovery from intermittent social challenges contrasts with the inescapable and uncontrollable nature of continuous subordination stress which appears to rely primarily on glutamate- and GABA-modulated 5-HT projections from the DRN.
Considerable evidence points to different kinds of social stress as risk factors for initiating, escalating and resuming drug abuse. Specific types of repeated maternal separation stress during critical periods in early development or repeated discrete episodes of social defeat stress in adulthood have been shown to be particularly effective to increase stimulant and alcohol abuse. Individuals differ in their resilience or vulnerability to these risk factors, and it will be important to identify the individual's genotype that enables these risk factors to become effective during discrete developmental periods, often referred to as critical periods (Caspi & Moffitt, 2006).
The simplistic proposal to consider dominance and subordination as equivalent to mania and depression has mostly been discredited (Price, 1967;Gardner, 1982). The hyper-defensive behavior, weight loss, altered sleep and activity patterns, and the profile of activity of the HPA axis of subordinate rats have been compared to similar characteristics of depressed patients (Blanchard & Blanchard, 1988; Koolhaas et al., 1995). Other features in the behavioral repertoire of socially stressed animals such as certain types of distress calls, in rodents in the ultrasonic frequency, may reflect anxiety-like responses. It is often difficult to differentiate with prototypic pharmacological probes the cardinal symptoms. Clearly, socially stressed animals exhibit intense changes in affect, and the study of emotional behavior in these animals requires insight into the species-typical behavioral adaptations (Miczek& deWit, 2008).
While it is feasible to construct a precise life history of social intercourse in laboratory animals, most clinical work relies on verbal recollections of past events, often traumatic in nature. One source for affective disorders, including addiction, depression and anxiety is gradual adaptations that appear in mesocorticolimbic DA and 5-HT circuits which in turn are modulated by other amines, GABA, glutamate and peptides. Understanding the intracellular cascade of events for the transition from episodic to continuous social stress in infancy and adulthood may provide insight into the modulation of basic reward processes that are critical for addictive and affective disorders.
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- ACTH
adrenocorticotropic hormone
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- AP-5
2-amino-5-phosphopentanoic acid
- BDNF
brain-derived neurotrophic factor
- BrdU
bromodeoxyuridine
- CCK
cortical cholecystokinin
- CRH
corticotropin-releasing hormone
- DAMGO
[d-Ala2,N-Me-Phe4-Gly5-ol]-enkephalin
- DPDPE
[d-Pen2,d-Pen5]-enkephalin
- DRN
dorsal raphe nucleus
- GABA
γ-aminobutyric acid
- GLAST
glutamate/aspartate transporter
- HPA
hypothalamic-pituitary-adrenal
- LHPA
limbic hypothalamo-pituitary-adrenal
- MAO
monoamine oxidase
- Met-ENK
methionine enkephalin
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- NE
norepinephrine
- NMDA
N-methyl-d-aspartate
- PAG
periaqueductal grey area
- PND
postnatal day
- PR
progressive ratio schedule
- PVN
paraventricular nucleus of the hypothalamus
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
- TUNEL
terminal dUTP nick-end labeling
- USV
ultrasonic vocalizations
- VTA
ventral tegmental area
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Abumaria N, Rygula R, Havemann-Reinecke U, Ruther E, Bodemer W, Roos C, et al. Identification of genes regulated by chronic social stress in the rat dorsal raphe nucleus. Cell Mol Neurobiol. 2006;26:145–162. doi: 10.1007/s10571-006-9024-1. [DOI] [PubMed] [Google Scholar]
- Ackerman SH, Hofer MA, Weiner H. Early maternal separation increases gastric ulcer risk in rats by producing a latent thermoregulatory disturbance. Science. 1978;201:373–376. doi: 10.1126/science.566471. [DOI] [PubMed] [Google Scholar]
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Allin JT, Banks EM. Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol. 1971;4:149–156. doi: 10.1002/dev.420040206. [DOI] [PubMed] [Google Scholar]
- Alstein M, Whitnall MH, House S, Key S, Gainer H. An immunochemical analysis of oxytocin and vasopressin prohormone processing in vivo. Peptides. 1988;9:87–105. doi: 10.1016/0196-9781(88)90014-9. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amkraut AA, Solomon GF, Kraemer HC. Stress, early experience and adjuvant-induced arthritis in the rat. Psychosom Med. 1971;33:203–214. doi: 10.1097/00006842-197105000-00002. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andre J, Zeau B, Pohl M, Cesselin F, Jean J, Becker C. Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats: Behavioral and biochemical studies. J Neurosci. 2005;25:7896–7904. doi: 10.1523/JNEUROSCI.0743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB. Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology. 2004;176:248–255. doi: 10.1007/s00213-004-1883-x. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Gorbach OV, Kudryavtseva NN. Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol Behav. 1997;61:37–43. doi: 10.1016/s0031-9384(96)00303-4. [DOI] [PubMed] [Google Scholar]
- Baenninger LP. Social dominance orders in the rat: “Spontaneous,” food, and water competition. J Comp Physiol Psychol. 1970;71:202–209. [Google Scholar]
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R, McCulloch T. Afferents to a midbrain periaqueductal grey region involved in the ‘defense reaction’ in the cat as revealed by horseradish peroxidase. II. The diencephalon. Behav Brain Res. 1984;13:279–285. doi: 10.1016/0166-4328(84)90171-2. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Barnett SA. A study in behavior. Chicago: University of Chicago Press; 1975. The Rat. [Google Scholar]
- Barnett SA, Hocking WE, Munro KMH, Walker KZ. Socially induced renal pathology of captive wild rats. Aggress Behav. 1975;1:123–133. [Google Scholar]
- Barry H, III, Buckley JP. Drug effects on animal performance and the stress syndrome. J Pharm Sci. 1966;55:1159–1183. doi: 10.1002/jps.2600551102. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Costoli T, Savani E, Laviola G, Parmigiani S, et al. Chronic psychosocial stress persistently alters autonomic function and physical activity in mice. Physiol Behav. 2003;80:57–67. doi: 10.1016/s0031-9384(03)00209-9. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Becker C, Andre J, Zeau B, Rettori MC, Guardiola-Lemaitre B, Hamon M, et al. Melatonin MT1/2 receptor stimulation reduces cortical overflow of cholecystokinin-like material in a model of anticipation of social defeat in the rat. Neuropharmacology. 2004;46:1158–1167. doi: 10.1016/j.neuropharm.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Becker C, Thiebot MH, Touitou Y, Hamon M, Cesselin F, Benoliel JJ. Enhanced cortical extracellular levels of cholecystokinin-like material in a model of anticipation of social defeat in the rat. J Neurosci. 2001;21:262–269. doi: 10.1523/JNEUROSCI.21-01-00262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer B, Chasin M, Clody DE, Vogel JR, Horovitz ZP. Cyclic adenosine monophosphate phosphodiesterase in brain: Effect on anxiety. Science. 1972;176:428–430. doi: 10.1126/science.176.4033.428. [DOI] [PubMed] [Google Scholar]
- Bell RW. Ultrasonic control of maternal behavior: Developmental implications. Am Zool. 1979;19:413–418. [Google Scholar]
- Benton D. Is the concept of dominance useful in understanding rodent behaviour? Aggress Behav. 1982;9:104–107. [Google Scholar]
- Bergink V, van Megen HJ, Westenberg HG. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14:175–183. doi: 10.1016/S0924-977X(03)00100-7. [DOI] [PubMed] [Google Scholar]
- Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Annu Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Flannelly KJ. Social stress, mortality and aggression in colonies and burrowing habitats. Behav Processes. 1985;11:209–213. doi: 10.1016/0376-6357(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28:437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Parmigiani S, Agullana R, Weiss SM, Blanchard DC. Behaviors of Swiss-Webster and C57/BL/6N Sin mice in fear/defense test battery. Aggress Behav. 1995;21:21–28. [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Bohus B, Koolhaas JM, Heijnen CJ, Deboer O. Immunological responses to social stress-dependence on social environment and coping abilities. Neuropsychobiology. 1993;28:95–99. doi: 10.1159/000119008. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: A behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Sabongui AG, Amit Z. The role of ethanol availability on stress-induced increases in ethanol consumption. Alcohol. 1997;14:551–556. doi: 10.1016/s0741-8329(96)00046-8. [DOI] [PubMed] [Google Scholar]
- Brain P, Benton D. The interpretation of physiological correlates of differential housing in laboratory rats. Life Sci. 1979;24:99–115. doi: 10.1016/0024-3205(79)90119-x. [DOI] [PubMed] [Google Scholar]
- Brain PF. Endocrine and behavioral differences between dominant and subordinate male house mice housed in pairs. Psychon Sci. 1972;28:260–262. [Google Scholar]
- Brain PF. Mammalian behavior and the adrenal cortex — A review. Behav Biol. 1972;7:453–477. doi: 10.1016/s0091-6773(72)80209-8. [DOI] [PubMed] [Google Scholar]
- Brain PF. What does individual housing mean to a mouse? Life Sci. 1975;16:187–200. doi: 10.1016/0024-3205(75)90017-x. [DOI] [PubMed] [Google Scholar]
- Brain PF. Adaptive aspects of hormonal correlates of attack and defence in laboratory mice: A study in ethobiology. In: McConnell PS, editor. Progress in brain research: Adaptive capabilities of the nervous system. Amsterdam: Elsevier North-Holland Biomedical; 1980. pp. 391–414. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Breslin FC, Hayward M, Baum AS. Stress and alcohol: The moderating effect of chronic stress on the acute stress–intoxication relationship. J Stud Alcohol. 1995;56:546–552. doi: 10.15288/jsa.1995.56.546. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Glucocorticoid-reinforced responding in the rhesus monkey. Psychopharmacology. 1999;147:46–55. doi: 10.1007/s002130051141. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Establishment of social rank among grouped male mice: Relative effects on circulating FSH, LH, and corticosterone. Physiol Behav. 1973;10:947–951. doi: 10.1016/0031-9384(73)90065-6. [DOI] [PubMed] [Google Scholar]
- Bronson FH. The reproductive ecology of the house mouse. Q Rev Biol. 1979;54:265–299. doi: 10.1086/411295. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Marsden HM. The preputial gland as an indicator of social dominance in male mice. Behav Biol. 1973;9:625–628. doi: 10.1016/s0091-6773(73)80056-2. [DOI] [PubMed] [Google Scholar]
- Bronstein PM. Repeated trials with the albino rat in the open field as a function of age and deprivation. J Comp Physiol Psychol. 1972;81:84–93. doi: 10.1037/h0033361. [DOI] [PubMed] [Google Scholar]
- Brown BB. Peer groups and peer cultures. In: Feldman SS, Elliot GR, editors. At the threshold: The developing adolescent. Cambridge, MA: Harvard University Press; 1990. pp. 171–196. [Google Scholar]
- Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: A review and a hypothesis concerning gene–environment interaction. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.04.009. [Electronic publication ahead of print]. [DOI] [PubMed] [Google Scholar]
- Brozina K, Abela JR. Symptoms of depression and anxiety in children: Specificity of the hopelessness theory. J Clin Child Adolesc Psychol. 2006;35:515–527. doi: 10.1207/s15374424jccp3504_3. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Repetto M, Gold MS. Neurobiological mechanisms in addictive and psychiatric disorders. Psychiatr Clin North Am. 2004;27:661–674. doi: 10.1016/j.psc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Chen YC, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus. Mol Neurobiol. 2003;27:121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Felszeghy K, Horvath KM, Nyakas C, de Boer SF, Bohus B, et al. Temporal and spatial dynamics of corticosteroid receptor down-regulation in rat brain following social defeat. Physiol Behav. 2001;72:349–354. doi: 10.1016/s0031-9384(00)00414-5. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MHP, Veenema AH, Huininga M, de Boer SF, Korte SM, et al. Long-term effects of social stress on brain and behavior: A focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Cannon WB. In: Bodily changes in pain, hunger, fear and rage. 2nd ed. Charles T, editor. Boston: Branford; 1953. [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential-effects of specific opioid receptor agonists on rat pup isolation calls. Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Carden SE, Bortot AT, Hofer MA. Ultrasonic vocalizations are elicited from rat pups in the home cage by pentylenetetrazol and U50,488, but not naltrexone. Behav Neurosci. 1993;107:851–859. doi: 10.1037//0735-7044.107.5.851. [DOI] [PubMed] [Google Scholar]
- Carden SE, Davachi L, Hofer MA. U50,488 increases ultrasonic vocalizations in 3-, 10-, and 18-day-old rat pups in isolation and the home cage. Dev Psychobiol. 1994;27:65–83. doi: 10.1002/dev.420270107. [DOI] [PubMed] [Google Scholar]
- Carden SE, Tempel A, Hernandez N, Hofer MA. Isolation alters striatal met-enkephalin immunoreactivity in rat pups. Physiol Behav. 1996;60:51–53. doi: 10.1016/0031-9384(95)02243-0. [DOI] [PubMed] [Google Scholar]
- Carr WJ, Kimmel KR, Anthony SL, Schlocker DE. Female rats prefere to mate with dominant rather than subordinate males. Bull Psychon Soc. 1982;20:89–91. [Google Scholar]
- Caspi A, Moffitt TE. Opinion–gene–environment interactions in psychiatry: Joining forces with neuroscience. Nat Rev, Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caza PA, Spear LP. Ontogenesis of morphine-induced behavior in the rat. Pharmacol Biochem Behav. 1980;13:45–50. doi: 10.1016/0091-3057(80)90119-7. [DOI] [PubMed] [Google Scholar]
- Chance MRA. Aggregation as a factor influencing the toxicity of sympathomimetic amines in mice. J Pharmacol Exp Ther. 1946;87:214–219. [PubMed] [Google Scholar]
- Chance MRA. Factors influencing the toxicity of sympathomimetic amines to solitary mice. J Pharm Pharmacol. 1947;89:289–296. [PubMed] [Google Scholar]
- Chao HM, McEwen BS. Glucocorticoids and the expression of messenger-RNAs for neurotrophins, their receptors and GAP-43 in the rat hippocampus. Brain Res Mol Brain Res. 1994;26:271–276. doi: 10.1016/0169-328x(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Cheu JW, Siegel A. GABA receptor mediated suppression of defensive rage behavior elicited from the medial hypothalamus of the cat: Role of the lateral hypothalamus. Brain Res. 1998;783:293–304. doi: 10.1016/s0006-8993(97)01357-7. [DOI] [PubMed] [Google Scholar]
- Chi CC, Flynn JP. Neuroanatomic projections related to biting attack elicited from hypothalamus in cats. Brain Res. 1971;35:49–66. doi: 10.1016/0006-8993(71)90594-4. [DOI] [PubMed] [Google Scholar]
- Choi S, Kellogg CK. Adolescent development influences functional responsiveness of noradrenergic projections to the hypothalamus in male rats. Brain Res Dev Brain Res. 1996;94:144–151. doi: 10.1016/s0165-3806(96)80005-8. [DOI] [PubMed] [Google Scholar]
- Choi S, Weisberg SN, Kellogg CK. Control of endogenous norepinephrine release in the hypothalamus of male rats changes over adolescent development. Brain Res Dev Brain Res. 1997;98:134–141. doi: 10.1016/s0165-3806(96)00179-4. [DOI] [PubMed] [Google Scholar]
- Christian JJ. The adreno-pituitary system and population cycles inmammals. J Mammal. 1950;31:247–259. [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Coventry TL, D'Aquila PS, Brain P, Willner P. Social influences on morphine conditioned place preference. Behav Phamacol. 1997;8:575–584. doi: 10.1097/00008877-199711000-00015. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Miczek KA. Brief social defeat stress: Long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: Dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: A critical site for social stress to intensify cocaine taking. Psychopharmacology. 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Planeta CS, Miczek KA. Maternal separation stress in mice: Long-term increases in alcohol intake. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1307-4. [Electronic publication ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology. 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- D'Amato FR. Effects of male social status on reproductive success and on behavior in mice (Mus musculus) J Comp Psychol. 1988;102:146–151. doi: 10.1037/0735-7036.102.2.146. [DOI] [PubMed] [Google Scholar]
- D'Amato FR, Mazzacane E, Capone F, Pavone F. Effects of postnatal manipulation on nociception and morphine sensitivity in adult mice. Brain Res Dev Brain Res. 1999;117:15–20. doi: 10.1016/s0165-3806(99)00090-5. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, et al. Feast and famine — Critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. London: John Murray; 1872. [Google Scholar]
- Davis DE, Christian JJ. Relation of adrenal weight to social rank of mice. Proc Soc Exp Biol Med. 1957;94:728–731. doi: 10.3181/00379727-94-23067. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Hughes ZA, Starr KR, Storey JD, Bettelini L, Bacchi F, et al. Characterisation of the selective 5-HT1B receptor antagonist SB-616234-A (1-[6-(cis-3,5-dimethylpiperazin-1-yl)-2,3-dihydro-5-methoxyindol-1-yl]-1-[2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl) biphenyl-4-yl]methanonehydrochloride): In vivo neurochemical and behavioural evidence of anxiolytic/antidepressant activity. Neuropharmacology. 2006;50:975–983. doi: 10.1016/j.neuropharm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- de Jong JG, Wasilewski M, Van Der Vegt BJ, Buwalda B, Koolhaas JM. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiol Behav. 2005;83:805–811. doi: 10.1016/j.physbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Delville Y, Melloni RHJ, Ferris CF. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J Neurosci. 1998;18:2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury DA. Aggression, copulation, and differential reproduction of deer mice (Peromyscus maniculatus) in a semi-natural enclosure. Behaviour. 1984;91:1–23. [Google Scholar]
- DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YJ, Kang L, Li BM, Ma L. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav. 2005;82:673–677. doi: 10.1016/j.pbb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Do Couto BR, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Drews C. The concept and definition of dominance in animal behaviour. Behaviour. 1993;125:283–313. [Google Scholar]
- Eibl-Eibesfeldt I, Munster B. Ausdrucksformen der Säugetiere. In: Keukental W, editor. Handbüch der Zoologie. 1957. pp. 1–26. [Google Scholar]
- El Khoury A, Gruber SH, Mork A, Mathe AA. Adult life behavioral consequences of early maternal separation are alleviated by escitalopramtreatment in a rat model of depression. Prog Neuro-psychopharmacol Biol Psychiatry. 2006;30:535–540. doi: 10.1016/j.pnpbp.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Ellison GD. A novel animal model of alcohol consumption based on the development of extremes of ethanol preference in colony-housed but not isolated rats. Behav Neural Biol. 1981;31:324–330. doi: 10.1016/s0163-1047(81)91364-9. [DOI] [PubMed] [Google Scholar]
- Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse. Addictive potential, behavioral and psychiatric effects. Clin Pediatr (Phila) 1989;28:550–555. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for invesigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Engel JA, Eriksson CJ, Hard E, Soderpalm B. Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994;11:195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Corticotropin-releasing factor in the caudal pontine reticular nucleus mediates the expresion of fear-potentiated startle in the rat. Eur J Neurosci. 1997;9:299–305. doi: 10.1111/j.1460-9568.1997.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Fernandez De Molina A, Hunsperger RW. Organization of the subcortical system governing defense and flight reactions in the cat. J Physiol. 1962;160:200–213. doi: 10.1113/jphysiol.1962.sp006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- File SE. The rat corticosterone response — Habituation and modification by chlordiazepoxide. Physiol Behav. 1982;29:91–95. doi: 10.1016/0031-9384(82)90371-7. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JRG. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Repeated experience of social defeats increases serotonin transporter and monoamine oxidase A mRNA levels in raphe nuclei of male mice. Neurosci Lett. 2002;321:25–28. doi: 10.1016/s0304-3940(01)02495-8. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Gupta S, Miczek KA. Anxiolytic-like effects of escitalopram, citalopram, and R-citalopram in maternally separated mouse pups. J Pharmacol Exp Ther. 2004;308:474–480. doi: 10.1124/jpet.103.058206. [DOI] [PubMed] [Google Scholar]
- Fish EW, Sekinda M, Ferrari PF, Dirks A, Miczek KA. Distress vocalizations in maternally separated mouse pups: Modulation via 5-HT1A, 5-HT1B and GABAA receptors. Psychopharmacology. 2000;149:277–285. doi: 10.1007/s002130000370. [DOI] [PubMed] [Google Scholar]
- Flannelly K, Lore R. Observations of the subterranean activity of domesticated and wild rats (Rattus norvegicus): A descriptive study. Psychol Rec. 1977;2:315–329. [Google Scholar]
- Flügge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge G, Ahrens O, Fuchs E. Monoamine receptors in the prefrontal cortex of Tupaia belangeri during chronic psychosocial stress. Cell Tissue Res. 1997;288:1–10. doi: 10.1007/s004410050787. [DOI] [PubMed] [Google Scholar]
- Flügge G, Kramer M, Rensing S, Fuchs E. 5HT1A receptors and behaviour under chronic stress: Selective counteraction by testosterone. Eur J Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- Flügge G, van Kampen M, Meyer H, Fuchs E. α2A and α 2C-adrenoceptor regulation in the brain: α2A changes persist after chronic stress. Eur JNeurosci. 2003;17:917–928. doi: 10.1046/j.1460-9568.2003.02510.x. [DOI] [PubMed] [Google Scholar]
- Fokkema DS, Koolhaas JM. Acute and conditioned blood pressure changes in relation to social and psychosocial stimui in rats. Physiol Behav. 1985;34:33–38. doi: 10.1016/0031-9384(85)90073-3. [DOI] [PubMed] [Google Scholar]
- Fokkema DS, Smit K, van der Gugten J, Koolhaas JM. A coherent pattern among social behavior, blood pressure, corticosterone and catecholamine measures in individual male rats. Physiol Behavior. 1988;42:485–489. doi: 10.1016/0031-9384(88)90181-3. [DOI] [PubMed] [Google Scholar]
- Forest MG. Role of androgens in fetal and pubertal development. Horm Res. 1983;18:69–83. doi: 10.1159/000179780. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Covington HE, III, Miczek KA. Stereotyped and complex motor routines expressed during cocaine self-administration: Results from a 24-h binge of unlimited cocaine access in rats. Psychopharmacology. 2007;192:465–478. doi: 10.1007/s00213-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Fox HE, White SA, Kao MHF, Fernald RD. Stress and dominance in a social fish. J Neurosci. 1997;17:6463–6469. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht H, Siegfried B, Waser PG. Learning of submissive behavior in mice: A new model. Behav Processes. 1982;7:235–245. doi: 10.1016/0376-6357(82)90038-9. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czeh B, Flügge G. Examining novel concepts of the pathophysiology of depression in the chronic psychosocial stress paradigm in tree shrews. Behav Pharmacol. 2004;15:315–325. doi: 10.1097/00008877-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Kramer M, Hermes B, Netter P, Hiemke C. Psychosocial stress in tree shrews: Clomipramine counteracts behavioral and endocrine changes. Pharmacol Biochem Behav. 1996;54:219–228. doi: 10.1016/0091-3057(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Uno H, Flügge G. Chronic psycosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. Brain Res. 1995;673:275–282. doi: 10.1016/0006-8993(94)01424-g. [DOI] [PubMed] [Google Scholar]
- Fuchs SAG, Edinger HM, Siegel A. The organization of the hypothalamic pathways mediating affective defense behavior in the cat. Brain Res. 1985;330:77–92. doi: 10.1016/0006-8993(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Gainer H, Wray W. Cellular and molecular biology of oxytocin and vasopressin. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 1099–1129. [Google Scholar]
- Gardner R. Mechanisms in manic-depressive disorder — An evolutionary model. Arch Gen Psychiatry. 1982;39:1436–1441. doi: 10.1001/archpsyc.1982.04290120066013. [DOI] [PubMed] [Google Scholar]
- Gardner CR. Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J Pharmacol Methods. 1985;14:181–187. doi: 10.1016/0160-5402(85)90031-2. [DOI] [PubMed] [Google Scholar]
- Gardner CR, Budhram P. Effects of agents which interact with contral benzodiazepine binding sites on stress-induced ultrasounds in rat pups. Eur J Pharmacol. 1987;134:275–283. doi: 10.1016/0014-2999(87)90358-x. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: Focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Feer H. Competition for sucrose-pellets in triads of male Wistar rats: Effects of acute and subchronic chlordiazepoxide. Psychopharmacology. 1990;100:530–534. doi: 10.1007/BF02244007. [DOI] [PubMed] [Google Scholar]
- Ginsburg B, Allee WC. Some effects of conditioning on social dominance and subordination in inbred strains of mice. Physiol Zool. 1942;15:485–506. [Google Scholar]
- Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–295. [Google Scholar]
- Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wortwein G. Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. J Neurosci Res. 2005;79:772–778. doi: 10.1002/jnr.20418. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Haney M, Noda K, Kream R, Miczek KA. Regional serotonin and dopamine activity: Sensitivity to amphetamine and aggressive behavior in mice. Aggress Behav. 1990;16:259–270. [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hendley ED, Moisset B, Welch BL. Catecholamine uptake in cerebral cortex: Adaptive change induced by fighting. Science. 1973;180:1050–1052. doi: 10.1126/science.180.4090.1050. [DOI] [PubMed] [Google Scholar]
- Henry JP, Stephens PM. The social environment and essential hypertension in mice: Possible role of inervation of the adrenal cortex. In: de Jong W, Provoost AP, Shapiro AP, editors. Progress in Brain Research Hypertension and brain mechanisms vol. 47. New York: Elsevier; 1977. pp. 263–273. [DOI] [PubMed] [Google Scholar]
- Herman B, Panksepp J. Effect of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hess WR, Brügger M. Das subkortikale Zentrum der affektiven Abwehrreaktion. Helv Physiol Pharmacol Acta. 1943;1:33–52. [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse — Effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde RA, Spencer-Booth Y, Bruce M. Effects of 6-day maternal deprivation on rhesus monkey infants. Nature. 1966;210:1021–1023. doi: 10.1038/2101021a0. [DOI] [PubMed] [Google Scholar]
- Hol T, Van Den Berg CL, van Ree JM, Spruijt BM. Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res. 1999;100:91–97. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- Holsboer F. CRHR1 antagonists as novel treatment strategies. CNS Spectr. 2001;6:590–594. doi: 10.1017/s1092852900002133. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schiepers OJ, Schoffelmeer AN, Cuppen E, Vanderschuren LJ. Acute and constitutive increases in central serotonin levels reduce social play behaviour in peri-adolescent rats. Psychopharmacology. 2007;195:175–182. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Meiergerd SM, Povlock SL, Schenk JO, et al. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J Neurosci. 1994;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: Relationship to extracellular levels of dopamine, serotonin and glutamate inthe nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology. 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Zhang GJ, Raol YSH, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Kravitz EA. A quantitative analysis of agonistic behavior in juvenile American lobsters (Homarus americanus L.) Brain Behav Evol. 1995;46:72–83. doi: 10.1159/000113260. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, et al. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology. 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Insel TR. Regional changes in brain oxytocin receptor post-partum: Time-course and relationship to maternal behavior. J Neuroendocrinol. 1990;2:539–545. doi: 10.1111/j.1365-2826.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Insel TR. Oxytocin – a neuropeptide for affiliation: Evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard RE, Miller LP. Rat pup isolation distress and the brain benzodiazepine receptor. Dev Psychobiol. 1989;5:509–525. doi: 10.1002/dev.420220508. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hill JL, Mayor RB. Rat pup ultrasonic isolation calls: Possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav. 1986;24:1263–1267. doi: 10.1016/0091-3057(86)90182-6. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mehanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Ninan PT, Aloi J, Jimerson DC, Skolnick P, Paul SM. A benzodiazepine receptor-mediatedmodel of anxiety. Arch Gen Psychiatry. 1984;41:741–750. doi: 10.1001/archpsyc.1984.01790190015002. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT. Central administration of oxytocin modulates the infant rat's response to social isolation. Eur J Pharmacol. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal–juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Drazen DL, Huhman KL, Nelson RJ, Demas GE. Acute and chronic social defeat suppresses humoral immunity of male Syrian hamsters (Mesocricetus auratus) Horm Behav. 2001;40:428–433. doi: 10.1006/hbeh.2001.1708. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology. 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- Jones RB, Nowell NW. Aversive and aggression-promoting properties of urine from dominant and subordinate male mice. Anim Learn Behav. 1973;1:207–210. doi: 10.3758/bf03199141. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: Role of individual differences in cocaine-taking behavior. Psychopharmacology. 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Kahn MW. The effect of socially learned aggression or submission on the mating behavior of C57 mice. J Genet Psychol. 1961;98:211–217. doi: 10.1080/00221325.1961.10534371. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Separation distress in infant rhesus monkeys: Effects of diazepam and Ro 15-1788. Brain Res. 1987;408:192–198. doi: 10.1016/0006-8993(87)90371-4. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long–Evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology. 2002;27:518–533. doi: 10.1016/S0893-133X(02)00326-3. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HBM. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kaufman IC, Rosenblum LA. Depression in infant monkeys separated from their mothers. Science. 1967;155:1030–1031. doi: 10.1126/science.155.3765.1030. [DOI] [PubMed] [Google Scholar]
- Keddy AC. Female mate choice in vervet monkeys (Cercopithecus aethiops sabaeus) Am J Primatol. 1986;10:125–134. doi: 10.1002/ajp.1350100204. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamicpituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neuroscience. 1998;83:681–689. doi: 10.1016/s0306-4522(97)00408-9. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Inglefield JR, Taylor MK, Pleger GL. Importance of hypothalamic function to stressor-induced responsiveness of the GABAA receptor in the cerebral cortex: A non-corticosterone influence. Brain Res. 1993;609:244–252. doi: 10.1016/0006-8993(93)90879-r. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Taylor MK, Rodriguez-Zafra M, Pleger GL. Altered stressor-induced changes in GABAA receptor function in the cerebral cortex of adult rats exposed in utero to diazepam. Pharmacol Biochem Behav. 1993;44:267–273. doi: 10.1016/0091-3057(93)90461-2. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries MK. Mood disorders in children and adolescents: An epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Faccidomo S, Miczek KA. Repeated maternal separation: Differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology. 2005;178:202–210. doi: 10.1007/s00213-004-1989-1. [DOI] [PubMed] [Google Scholar]
- King JA, Edwards E. Early stress and genetic influences on hypothalamic-pituitary-adrenal axis functioning in adulthood. Horm Behav. 1999;36:79–85. doi: 10.1006/hbeh.1999.1525. [DOI] [PubMed] [Google Scholar]
- Knuth ED, Etgen AM. Corticosterone secretion induced by chronic isolation in neonatal rats is sexually dimorphic and accompanied by elevated ACTH. Horm Behav. 2005;47:65–75. doi: 10.1016/j.yhbeh.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Knyshevski I, Connor DF, Harrison RJ, Ricci LA, Melloni RH. Persistent activation of select forebrain regions in aggressive, adolescent cocaine-treated hamsters. Behav Brain Res. 2005;159:277–286. doi: 10.1016/j.bbr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Kole MHP, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinology. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Meerlo P, de Boer SF, Strubbe JH, Bohus B. Social stress in rats: An animal model of depression? Acta Neuropsychiatr. 1995;7:9–11. doi: 10.1017/S0924270800037479. [DOI] [PubMed] [Google Scholar]
- Korte SM, Buwalda B, Meijer O, De Kloet ER, Bohus B. Socially defeated male rats display a blunted adrenocortical response to a low dose of 8-OH-DPAT. Eur J Pharmacol. 1995;272:45–50. doi: 10.1016/0014-2999(94)00621-d. [DOI] [PubMed] [Google Scholar]
- Korte SM, Smit J, Bouws GAH, Koolhaas JM, Bohus B. Behavioral and neuroendocrine response to psychosocial stress in male rats — The effects of the 5-HT1A agonist ipsapirone. Horm Behav. 1990;24:554–567. doi: 10.1016/0018-506x(90)90041-u. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. Chronic neonatal isolation stress enhances cocaine-induced increases in ventral striatal dopamine levels in rat pups. Brain Res Dev Brain Res. 2003;141:109–116. doi: 10.1016/s0165-3806(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Koolhaas JM, Bohus B, Korf J. A single social stress-experience alters glutamate receptor binding in rat hippocampal CA3 area. Neurosci Lett. 1993;154:73–77. doi: 10.1016/0304-3940(93)90174-j. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN. A sensory contact model for the study of aggressive and submissive behavior in male mice. Aggress Behav. 1991;17:285–291. [Google Scholar]
- Kudryavtseva NN. Experience of defeat decreases the behavioural reactivity to conspecifics in the partition test. Behav Processes. 1994;32:297–304. doi: 10.1016/0376-6357(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Avgustinovich DF. Behavioral and physiological markers of experimental depression induced by social conflicts (DISC) Aggress Behav. 1998;24:271–286. [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Külling P, Frischknecht HR, Pasi A, Waser PG, Siegfried B. Social conflict-induced changes in nociception and beta-endorphin-like immunoreactivity in pituitary and discrete brain areas of C57BL/6 and DBA/2 mice. Brain Res. 1988;450:237–246. doi: 10.1016/0006-8993(88)91563-6. [DOI] [PubMed] [Google Scholar]
- Kutcher S, Gardner DM. Use of selective serotonin reuptake inhibitors and youth suicide: Making sense from a confusing story. Curr Opin Psychiatry. 2008;21:65–69. doi: 10.1097/YCO.0b013e3282f29853. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotrophin-releasing factor neuronal systems induced bymaternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–533. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lanier LP, Isaacson RL. Early developmental changes in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Sattar MA, Melzack R. Inflammation-susceptible Lewis rats show less sensitivity than resistant Fischer rats in the formalin inflammatory pain test and with repeated thermal testing. J Neurophysiol. 2006;95:2889–2897. doi: 10.1152/jn.00608.2005. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci Biobehavi Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to d-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6:725–728. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experience and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Lehr E. Distress call reactivation in isolated chicks: A behavioral indicator with high selectivity for antidepressants. Psychopharmacology. 1989;97:145–146. doi: 10.1007/BF00442235. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Deminiere JM, Mormede P. Chronic social stress conditions differentially modify vulnerability to amphetamine self-administration. Brain Res. 1994;649:348–352. doi: 10.1016/0006-8993(94)91086-3. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Taylor GT, Mormede P. Adrenal axis activation by chronic social stress fails to inhibit gonadal function in male rats. Psychoneuroendocrinology. 1997;22:563–573. doi: 10.1016/s0306-4530(97)00051-6. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mayer S, Disselkamp-Tietze J, Hoh A, Schoellnhammer G, Schulte HM. Subsensitivity of the 5-hydroxytryptamine 1A (5-HT 1A) receptor-mediated hypothermic response to ipsapirone in unipolar depression. Life Sci. 1990;46:1271–1277. doi: 10.1016/0024-3205(90)90359-y. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Nock BL. The effects of experience on agonistic responding: An expectancy theory interpretation. Behav Biol. 1976;17:561–566. doi: 10.1016/s0091-6773(76)91009-9. [DOI] [PubMed] [Google Scholar]
- Lesting J, Neddens J, Busche A, Teuchert-Noodt G. Hemisphere-specific effects on serotonin but not dopamine innervation in the nucleus accumbens of gerbils caused by isolated rearing and a single early methamphetamine challenge. Brain Res. 2005;1035:168–176. doi: 10.1016/j.brainres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. The influence of social factors on the response to stress. Psychother Psychosom. 1993;60:33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Robinson TE, Bhatnagar S. Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Res. 2003;960:42–47. doi: 10.1016/s0006-8993(02)03752-6. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Infuence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Louilot A, Le Moal M, Simon H. Differential reactivity of dopaminergic neurons in the nucleus accumbens in response to different behavioral situations. An in vivo voltammetric study in free moving rats. Brain Res. 1986;397:395–400. doi: 10.1016/0006-8993(86)90646-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: A review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lundberg U. Dominanzstrukturen mannlicher Laborratten. Z Versuchstierkd. 1986;28:257–268. [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Deminiere JM, Lemaire V, Mormede P, Simon H, et al. Life events-induced decrease of corticosteroid type I receptors is associated with reduced corticosterone feedback and enhanced vulnerability to amphetamine self-administration. Brain Res. 1991;547:7–12. doi: 10.1016/0006-8993(91)90568-g. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Ratnasingan R, Chen B, Young LT. Desipramine treatment reduces the long-term behavioural and neurochemical sequelae of early-life maternal separation. Int J Neuropsychopharmacol. 2003;6:391–396. doi: 10.1017/S1461145703003729. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Drugan RC, Grau JW. Controllability, coping behavior, and stress-induced analgesia in the rat. Pain. 1982;12:47–56. doi: 10.1016/0304-3959(82)90169-5. [DOI] [PubMed] [Google Scholar]
- Marini F, Pozzato C, Andreetta V, Jansson B, Arban R, Domenici E, et al. Single exposure to social defeat increases corticotropin-releasing factor and glucocorticoid receptor mRNA expression in rat hippocampus. Brain Res. 2006;1067:25–35. doi: 10.1016/j.brainres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Marrow LP, Overton PG, Brain PF, Clark D. Encounters with aggressive conspecifics enhance the locomotor-activating effects of cocaine in the rat. Addict Biol. 1999;4:437–441. doi: 10.1080/13556219971425. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: Immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002;27:549–561. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- Mathew SJ. Exploring glutamate function in mood an anxiety disorders: Rationale and future directions. CNS Spectr. 2005;10:806–807. [Google Scholar]
- Mathew SJ, Coplan JD, Gorman JM. Neurobiological mechanisms of social anxiety disorder. Am J Psychiatry. 2001;158:1558–1567. doi: 10.1176/appi.ajp.158.10.1558. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS, Robbins TW. Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: Effects of prefeeding, d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology. 1996;126:75–84. doi: 10.1007/BF02246414. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology. 1999;141:123–134. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, et al. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm Behav. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S. Neuroendocrinology of stress. In: Blaustein JD, editor. Behavioral neurochemistry, neuroendocrinology and molecular neurobiology. Heidelberg: Springer; 2007. pp. 571–593. [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology. doi: 10.1007/s00213-008-1256-y. (in press). [Electronic publication ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney WT, Jr, Bunney WE., Jr Animal model of depression. I. Review of evidence: Implications for research. Arch Gen Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: A neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meerlo P, de Boer SF, Koolhaas JM, Daan S, van den Hoofdakker RH. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol Behav. 1996;59:735–739q. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJF, Benning MA, Koolhaas JM, van den Hoofdakker RH. Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiol Behav. 1996;60:115–119. doi: 10.1016/0031-9384(95)02271-6. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJF, Daan S, Vandenhoofdakker RH, Koolhaas JM. Changes in behavior and body weight following a single or double social defeat in rats. Stress. 1996;1:21–32. doi: 10.3109/10253899609001093. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, de Boer SF, Koolhaas JM. Long-lasting consequences of a social conflict in rats: Behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci. 1999;113:1283–1290. doi: 10.1037//0735-7044.113.6.1283. [DOI] [PubMed] [Google Scholar]
- Michael RP, Zumpe D. Annual cycles of aggression and plasma testosterone in captive male rhesus monkeys. Psychoneuroendocrinology. 1978;3:217–220. doi: 10.1016/0306-4530(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Michael RP, Zumpe D. Relation between the seasonal changes in aggression, plasma testosterone and the photoperiod in male rhesus monkeys. Psychoneuroendocrinology. 1981;6:145–158. doi: 10.1016/0306-4530(81)90007-x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, Nikulina EA, Hammer RP. Aggression and defeat: Persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, Yap JJ, Nikulina EM. Emergence of anhedonia in laboratory rats during continuous subordination stress: Relevance to antidepressants and stimulant abuse. Neuropsychopharmacology. 2005;30:S182. [Google Scholar]
- Miczek KA, de Wit H. Challenges for translational psychopharmacology research — Some basic principles. Psychopharmacology. 2008;199:291–301. doi: 10.1007/s00213-008-1198-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology. 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, Van Erp AMM, Blank AD, McInerney SC. d-Amphetamine “cue” generalizes to social defeat stress: Sensitization and role of accumbens dopamine. Psychopharmacology. 1999;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Covington HE., III Social stress and cocaine reward: Divergent roles for tegmental BDNF. doi: 10.1523/JNEUROSCI.0637-11.2011. (in preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology. 1999;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Naloxone injections into periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology. 1985;87:39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Analgesia following defeat in an aggressive encounter: Development of tolerance and changes in opioid receptors. In: Kelly DD, editor. Stress-induced analgesiaAnnals of the NY Academy of Science. New York: 1986. pp. 14–29. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Tornatzky W. Subordinate animals: Behavioral and physiological adaptations and opioid tolerance. In: Brown MR, editor. Stress: Neurobiology and neuroendocrinology. New York: Marcel Dekker; 1991. pp. 323–357. [Google Scholar]
- Miczek KA, Weerts EM, Vivian JA, Barros HM. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology. 1995;121:38–56. doi: 10.1007/BF02245590. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther. 2006;317:1210–1218. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the µ-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance inmonkeys: Dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Mormede P, Lemaire V, Castanon N, Dulluc J, Laval M, Lemoal M. Multiple neuroendocrine responses to chronic social stress: Interaction between individual characteristics and situational factors. Physiol Behav. 1990;47:1099–1105. doi: 10.1016/0031-9384(90)90358-b. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B. Ultrasonic vocalizations by rat pups as an animal model for anxiolytic activity: Effects of serotonergic drugs. In: Bevan P, editor. Behavioural pharmacology of 5-HT. Hillsdale: Lawrence Erlbaum Associates; 1989. [Google Scholar]
- Mos J, Van Valkenburg CFM. Specific effect on social stress and aggression on regional dopamine metabolism in rat brain. Neurosci Lett. 1979;15:325–327. doi: 10.1016/0304-3940(79)96134-2. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in-vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Nastiti K, Benton D, Brain PF. The effects of compounds acting at the benzodiazepine receptor complex on the ultrasonic calling of mouse pups. Behav Pharmacol. 1991;2:121–128. [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP., Jr Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: Time course of µ-opioid receptor mRNA and FosB/ΔFosB immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Jr, Miczek KA, Kream RM. Social defeat stress increases expression of µ-opioid receptor mRNA in rat ventral tegmental area. Neuroreport. 1999;10:3015–3019. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP., Jr Prolonged effects of repeated social defeat stress on mRNA expression and function of µ-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- Okon EE. Effect of environmental temperature on production of ultrasounds by isolated non-handled albino mouse pups. J Zool. 1970;162:71–83. [Google Scholar]
- Olivier B, Molewijk HE, van der Heyden JAM, Van Oorschot R, Ronken E, Mos J, et al. Ultrasonic vocalizations in rat pups: Effects of serotonergic ligands. Neurosci Biobehav Rev. 1998;23:215–227. doi: 10.1016/s0149-7634(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Palka Y, Coyer D. Effects of isolation of medial basal hypothalamus on pituitary-adrenal and pituitary-ovarian functions. Neuroendocrinology. 1969;5:333–349. doi: 10.1159/000121893. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: Opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Vilberg T, Bean NJ, Coy DH, Kastin AJ. Reduction of distress vocalization in chicks by opiate-like peptides. Brain Res Bull. 1978;3:663–667. doi: 10.1016/0361-9230(78)90014-x. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Kendall DA, Perez-Diaz F, Duxon MS, Marsden CA. Repeated sensory contact with aggressive mice rapidly leads to an anticipatory increase in core body temperature and physical activity that precedes the onset of aversive responding. Eur J Neurosci. 2004;20:1033–1050. doi: 10.1111/j.1460-9568.2004.03549.x. [DOI] [PubMed] [Google Scholar]
- Pereira M, Fairbanks LA. Juvenile primates. New York, NY: Oxford University Press; 1993. [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, et al. Inhibition of corticosterone synthesis by Metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res. 1994;658:259–264. doi: 10.1016/s0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotrophin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. The interaction of alcohol and stress, a review. Neurosci Biobehav Rev. 1981;5:209–229. doi: 10.1016/0149-7634(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Housing and rank status of male Long-Evans rats modify ethanol's effect on open-field behaviors. Psychopharmacology. 2006;185:289–297. doi: 10.1007/s00213-005-0257-3. [DOI] [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Price EO, Belanger PL, Duncan RA. Competitve dominace of wild and domestic Norway rats (Rattus norvegicus) Anim Behav. 1976;24:589–599. [Google Scholar]
- Price J. Hypothesis: The dominance hierarchy and the evolution of mental illness. Lancet. 1967;2:243–246. [Google Scholar]
- Primus RJ, Kellogg CK. Developmental influence of gonadal function on the anxiolytic effect of diazepam on environment-related social interaction in the male rat. Behav Pharmacol. 1990;1:437–446. [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal-hormones during puberty organize environment-related social-interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. Pharmacological evidence for a role of D2 dopamine receptors in the defensive behavior of the mouse. Behav Neural Biol. 1988;50:98–111. doi: 10.1016/s0163-1047(88)90804-7. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. Effects of defeat experiences on dopamine metabolism in different brain areas of the mouse. Aggress Behav. 1990;16:271–284. [Google Scholar]
- Raab A. Der Serotoninstoffwechsel in einzelnen Hirnteilen von Tupaia (Tupaia belangeri) bei soziopsychischem Stress. Z Vgl Physiol. 1971;72:54–66. [Google Scholar]
- Raab A, Dantzer R, MIchaud B, Mormede P, Taghzouti K, Simon H, et al. Behavioural, physiological and immunological consequences of social status and aggression in chronically coexisting resident-intruder dyads of male rats. Physiol Behav. 1986;36:223–228. doi: 10.1016/0031-9384(86)90007-7. [DOI] [PubMed] [Google Scholar]
- Raab A, Oswald R. Coping with social conflict: Impact on the activity of tyrosine hydroxylase in the limbic system and in the adrenals. Physiol Behavior. 1980;24:387–394. doi: 10.1016/0031-9384(80)90103-1. [DOI] [PubMed] [Google Scholar]
- Rao U. Links between depression and substance abuse in adolescents: Neurobiological mechanisms. Am J Prev Med. 2006;31:S161–S174. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Rao U, Ryan ND, Dahl RE, Birmaher B, Rao R, Williamson DE, et al. Factors associated with the development of substance use disorder in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:1109–1117. doi: 10.1097/00004583-199909000-00014. [DOI] [PubMed] [Google Scholar]
- Rhodes JE, Jason LA. A social stress model of substance-abuse. J Consult Clin Psychol. 1990;58:395–401. doi: 10.1037//0022-006x.58.4.395. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Interaction between ethanol and stress on ACTH and beta-endorphin secretion. Alcohol Clin Exp Res. 1988;12:206–210. doi: 10.1111/j.1530-0277.1988.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Roches KE, Leshner AI. ACTH and vospressin treatments immediately after a defeat increase future submissiveness in male mice. Science. 1979;204:1343–1344. doi: 10.1126/science.221973. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. Anxiety enhancement in the murine elevated plus maze by immediate prior exposure to social stressors. Physiol Behav. 1993;53:383–388. doi: 10.1016/0031-9384(93)90222-2. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Randall JI. Social conflict analgesia: Studies on naloxone antagonism and morphine cross-tolerance in male DBA/2 mice. Pharmacol Biochem Behavior. 1985;23:883–887. doi: 10.1016/0091-3057(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- Rowell TE. The concept of social dominance. Behav Biol. 1974;11:131–154. doi: 10.1016/s0091-6773(74)90289-2. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Hiemke C, Fuchs E, Ruther E, et al. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav Pharmacol. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Sade DS. Determinants of dominance in a group of free-ranging rhesus monkeys. In: Altmann SA, editor. Social communication among primates. Chicago: University of Chicago Press; 1967. pp. 99–114. [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization to cocaine's reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmaco Biochem Behav. 2000;66:765–770. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Schuurman T. Hormonal correlates of agonistic behavior in adult male rats. In: McConnel PS, Boer GJ, Romijn HJ, Van de Poll NE, Corner MA, editors. Progress in brain research Adaptive capabilities of the nervous system vol. 53. Amsterdam: Elsevier Biomedical Press; 1980. pp. 415–420. [DOI] [PubMed] [Google Scholar]
- Scott JP, Marston MV. Nonadaptive behavior resulting from a series of defeats in fighting mice. J Abnorm Soc Psychol. 1953;48:417–428. doi: 10.1037/h0058844. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowef NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse noxious agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Selye H. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol Metab. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress. 2005;8:259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Costoli T, Meerlo P, Buwalda B, Pico'Alfonso MA, de Boer S, et al. Individual differences in cardiovascular response to social challenge. Neurosci Biobehav Rev. 2005;29:59–66. doi: 10.1016/j.neubiorev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Costoli T, Manghi M, Stilli D, Ferrari PF, et al. Cardiac autonomic responses to intermittent social conflict in rats. Physiol Behav. 2001;73:343–349. doi: 10.1016/s0031-9384(01)00455-3. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: A review. Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shair HN, Masmela JR, Brunelli SA, Hofer MA. Potentiation and inhibition of ultrasonic vocalization of rat pups: Regulation by social cues. Dev Psychobiol. 1997;30:195–200. doi: 10.1002/(sici)1098-2302(199704)30:3<195::aid-dev2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Dewsbury DA. Male dominance, female choice and male copulatory in two species of voles (Microtus ochrogaster and Microtus montanus) Behav Ecol Sociobiol. 1986;18:267–274. [Google Scholar]
- Shapiro LE, Insel TR. Infants response to social separation reflects adult differences in affiliative behavior: A comparative developmental study in prairie and montane voles. Dev Psychobiol. 1990;23:375–393. doi: 10.1002/dev.420230502. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Ann NY Acad Sci. 1992;652:448–451. doi: 10.1111/j.1749-6632.1992.tb34380.x. [DOI] [PubMed] [Google Scholar]
- Siegfried B, Frischknecht HR, Waser PG. Defeat, learned submissiveness, and analgesia in mice: Effect of genotype. Behav Neural Biol. 1984;42:91–97. doi: 10.1016/s0163-1047(84)90484-9. [DOI] [PubMed] [Google Scholar]
- Siegfried B, Frischnecht HR, Waser PG. A new learning model for submissive behavior in mice: Effects of naloxone. Aggress Behav. 1982;8:112–115. [Google Scholar]
- Silk JB. Kidnapping and female competition among captive bonnet macaques. Primates. 1980;21:100–110. [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, et al. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR, Lavallee PA, Hennessy MB. Influences of the early olfactory environment on the survival, behavior and pituitary-adrenal activity of cesarean delivered preterm rat pups. Dev Psychobiol. 1987;20:415–423. doi: 10.1002/dev.420200406. [DOI] [PubMed] [Google Scholar]
- Smythe JW, McCormick CM, Rochford J, Meaney MJ. The interaction between prenatal stress and neonatal handling on nociceptive response latencies in male and female rats. Physiol Behav. 1994;55:971–974. doi: 10.1016/0031-9384(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence — Age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. In: Galanter M, editor. Alcohol problems in adolescents and young adults. New York: Springer; 2005. pp. 143–159. [PubMed] [Google Scholar]
- Spencer SR, Cameron GN. Behavioral dominance and its relationship to habitat patch utilization by the hispid cotton rat (Sigmodon hispidus) Behav Ecol Sociobiol. 1983;13:27–36. [Google Scholar]
- Spigel IM, Trivett S, Fraser D. Grooming behavior and competitive dominance in the albino rat. J Comp Physiol Psychol. 1972;78:409–411. [Google Scholar]
- Spirito A, Esposito-Smythers C. Attempted and completed suicide in adolescence. Annu Rev Clin Psychol. 2006;2:237–266. doi: 10.1146/annurev.clinpsy.2.022305.095323. [DOI] [PubMed] [Google Scholar]
- Stamford JA. Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. J Neurochem. 1989;52:1582–1589. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Stefanski V. Social stress in laboratory rats. Behavior, immune function, and tumor metastasis. Physiol Behav. 2001;73:385–391. doi: 10.1016/s0031-9384(01)00495-4. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Pubertal maturation and parent-adolescent distance: An evolutionary perspective. In: Adams GR, Montemayor R, Gulotta CS, editors. Advances in adolescent behaviorand development. Newbury Park, CA: Sage Publications; 1989. pp. 71–97. [Google Scholar]
- Stephan M, Helfritz F, Pabst R, von Horsten S. Postnatally induced differences in adult pain sensitivity depend on genetics, gender and specific experiences: Reversal of maternal deprivation effects by additional postnatal tactile stimulation or chronic imipramine treatment. Behav Brain Res. 2002;133:149–158. doi: 10.1016/s0166-4328(01)00468-5. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17:271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Taber MT, Baker GB, Fibiger HC. Glutamate receptor agonists decrease extracellular dopamine in the rat nucleus accumbens in vivo. Synapse. 1996;24:165–172. doi: 10.1002/(SICI)1098-2396(199610)24:2<165::AID-SYN8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: Modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GT, Moore S. Social position and competition in laboratory rats. J Comp Physiol Psychol. 1975;88:424–430. doi: 10.1037/h0076224. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC. Evidence for dopamine-receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Teskey GC, Kavaliers M, Hirst M. Social conflict activates opioid analgesic and ingestive behaviors in male mice. Life Sci. 1984;35:303–315. doi: 10.1016/0024-3205(84)90114-0. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: An in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: Role of accumbens dopamine. Psychopharmacology. 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Massari VJ, Jacobowitz DM. Isolation-induced aggression and catecholamine variations in discrete brain areas of the mouse. Brain Res Bull. 1980;5:81–86. doi: 10.1016/0361-9230(80)90287-7. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: Differential effects of clonidine and metoprolol. Psychopharmacology. 1994;116:346–356. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Alcohol, anxiolytics and social stress in rats. Psychopharmacology. 1995;121:135–144. doi: 10.1007/BF02245600. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzcinska M, Bergh J, DeLeon K, Stellar JR, Melloni RH. Social stress does not alter the expression of sensitization to cocaine. Physiol Behav. 2002;76:457–463. doi: 10.1016/s0031-9384(02)00727-8. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- Van de Poll NE, de Jonge F, van Oyen HG, van Pelt J. Aggressive behaviour in rats: Effects of winning or losing on subsequent aggressive interactions. Behav Processes. 1982;7:146–155. doi: 10.1016/0376-6357(82)90023-7. [DOI] [PubMed] [Google Scholar]
- Van Den Berg CL, Hol T, van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- van der Poel AM, Miczek KA. Long ultrasonic calls in male rats following mating, defeat and aversive stimulation: Frequency modulation and bout structure. Behaviour. 1991;119:127–142. [Google Scholar]
- Van Erp AMM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp AMM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Tachi N, Miczek KA. Short or continuous social stress: Suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001;12:335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- van Kampen M, Schmitt U, Hiemke C, Fuchs E. Diazepam has no beneficial effects on stress-induced behavioural and endocrine changes in male tree shrews. Pharmacol Biochem Behav. 2000;65:539–546. doi: 10.1016/s0091-3057(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Pee JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vellucci SV, Martin PJ, Everitt BJ. The discriminative stimulus produced by pentylenetetrazol: Effects of systemic anxiolytics and anxiogenics, aggressive defeat and midazolam or muscimol infused into the amygdala. J Psychopharmacol. 1988;2:80–93. doi: 10.1177/026988118800200203. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, et al. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Barros HMT, Manitiu A, Miczek KA. Ultrasonic vocalizations in rat pups: Modulation at the ³-aminobutyric acid-A receptor complex and the neurosteroid recognition site. J Pharmacol Exp Ther. 1997;282:318–325. [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Diazepam and gepirone selectively attenuate either 20–32 or 32–64 kHz ultrasonic vocalizations during aggressive encounters. Psychopharmacology. 1993;112:66–73. doi: 10.1007/BF02247364. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Effects of µ and δ opioid agonists and antagonists on affective vocal and reflexive pain responses during social stress in rats. Psychopharmacology. 1998;139:364–375. doi: 10.1007/s002130050727. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Interactions between social stress and morphine in the periaqueductal grey: Effects on affective vocal and reflexive pain responses in rats. Psychopharmacology. 1999;146:153–161. doi: 10.1007/s002130051101. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Weerts EM, Miczek KA. Defeat engenders pentylenetetrazole-appropriate responding in rats: Antagonism by midazolam. Psychopharmacology. 1994;116:491–498. doi: 10.1007/BF02247483. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Tiven J, Kimmel SC. The relationship between tension reduction and ethanol consumption in rats. Physiol Psychol. 1982;10:114–116. [Google Scholar]
- Von Frijtag JC, Kamal A, Reijmers LGJE, Schrama LH, van den Bos R, Spruijt BM. Chronic imipramine treatment partially reverses the long-term changes of hippocampal synaptic plasticity in socially stressed rats. Neurosci Lett. 2001;309:153–156. doi: 10.1016/s0304-3940(01)02062-6. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, van den Bos R, Spruijt BM. Imipramine restores the long-term impairment of appetitive behavior in socially stressed rats. Psychopharmacology. 2002;162:232–238. doi: 10.1007/s00213-002-1093-3. [DOI] [PubMed] [Google Scholar]
- Von Holst D. Sozialer Stress bei Tupias (Tupaia belangeri) Z Vgl Physiol. 1969;63:1–58. [Google Scholar]
- Von Holst D. Coping behaviour and stress physiology in male tree shrews (Tupaia belangeri) In: Hölldobler B, Lindberg I, editors. Experimental behavioral ecology and sociobiology. Sunderland, MA: Sinauer Associates; 1985. pp. 461–470. [Google Scholar]
- Von Holst D. The concept of stress and its relevance for animal behavior. In: Moller AP, Milinski M, Slater PJB, editors. Advances in the study of behaviorStress and behavior Vol. 27. New York: Academic Press; 1998. pp. 1–131. [Google Scholar]
- Vorel SR, Liu XH, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat — Role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Walletschek H, Raab A. Spontaneous activity of dorsal raphe neurons during defensive and offensive encounters in the tree-shrew. Physiol Behav. 1982;28:697–705. doi: 10.1016/0031-9384(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Walters JR, Seyfarth RM. Conflict and cooperation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago: The University of Chicago Press; 1986. pp. 306–317. [Google Scholar]
- Weiss JM. Psychological factors in stress and disease. Sci Am. 1972;266:104–113. doi: 10.1038/scientificamerican0672-104. [DOI] [PubMed] [Google Scholar]
- Welch BL, Hendley ED, Turek I. Norepinephrine uptake into cerebral cortical synaptosomes after one fight or electroconvulsive shock. Science. 1974;183:220–221. doi: 10.1126/science.183.4121.220. [DOI] [PubMed] [Google Scholar]
- Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine's rewarding properties. Pharmacol Biochem Behav. 1998;60:655–664. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Willner P, Daquila PS, Coventry T, Brain P. Loss of social status: Preliminary evaluation of a novel animal model of depression. J Psychopharmacol. 1995;9:207–213. doi: 10.1177/026988119500900302. [DOI] [PubMed] [Google Scholar]
- Wilson EO. Sociobiology. Cambridge: The Belknap Press; 1974. [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Infant rat separation is a sensitive test for novel anxiolytics. Prog Neuro-psychopharmacol Biol Psychiatry. 1991;15:745–757. doi: 10.1016/0278-5846(91)90003-j. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The infant rat separation paradigm: A novel test for novel anxiolytics. Trends Pharmacol Sci. 1991;12:402–404. doi: 10.1016/0165-6147(91)90616-z. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Effects of central vasopressin administration to infant rats. Eur J Pharmacol. 1993;233:101–107. doi: 10.1016/0014-2999(93)90354-k. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Androgen dependency of alcohol effects on aggressive behavior: A seasonal rhythm in high-ranking squirrel monkeys. Psychopharmacology. 1988;95:92–98. doi: 10.1007/BF00212774. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Witts DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
- Wolf CM, Garland T, Griffith B. Predictors of avian and mammalian translocation success: Reanalysis with phylogenetically independent contrasts. Biol Conserv. 1998;86:243–255. [Google Scholar]
- Yap JJ, Chartoff EH, Hogenelst K, Carlezon WA, Jr, Miczek KA. Social defeat stress-induced sensitization and escalated cocaine self-administration: Role of ERK-CREB signaling in the mesocorticolimbic dopamine system. Abstr Soc Neurosci. 2008;663 663.21/JJ16. [Google Scholar]
- Yap JJ, Covington HE, III, Gale MC, Datta R, Miczek KA. Behavioral sensitization due to social defeat stress in mice: Antagonism at mGluR5 and NMDA receptors. Psychopharmacology. 2005;179:230–239. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, cross-sensitization to d-amphetamine and cocaine self-administration in mice. Psychopharmacology. 2007;192:261–274. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. Repeated brief social defeat episodes in mice: Effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurology Psychol. 1908;18:459–482. [Google Scholar]
- Yoshimura H, Kimura N. Ethopharmacology of copulatory disorder induced by chronic social conflict in male mice. Neurosci Biobehav Rev. 1991;15:497–500. doi: 10.1016/s0149-7634(05)80138-1. [DOI] [PubMed] [Google Scholar]
- Zaharia MD, Kulczycki J, Shanks N, Meaney MJ, Anisman H. The effects of early postnatal stimulation on Morris water-maze acquisition in adult mice: Genetic and maternal factors. Psychopharmacology. 1996;128:227–239. doi: 10.1007/s002130050130. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: The first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking — A comparative approach to a human trait. Behav Brain Sci. 1984;7:413–434. [Google Scholar]