Hypoglycemia is the major, and most feared, complication of the pharmacological therapies for diabetes. In health, hypoglycemia sufficient to cause clinically relevant impairment of cognitive function or cardiac rhythm is prevented by highly efficient counterregulatory mechanisms that culminate in restoration of circulating glucose concentrations. In the accompanying article, McCrimmon (1) has summarized our current understanding of how these mechanisms may be initiated and coordinated by central glucose sensing, described the possible molecular mechanisms of neuronal glucose sensing, and alluded to the defective performance of these mechanisms in insulin-treated diabetes.
The principle clinical correlate of defective brain glucose sensing in diabetes is the change in the patient's awareness of the plasma glucose concentration. The clinical phenomenon of loss of awareness of hypoglycemia, and its associated increase in risk of severe hypoglycemia, is accompanied by measurable defects in the counterregulatory stress responses attributed at least in part to failure of central glucose sensing. As McCrimmon explains, the main determinant of the plasma glucose concentrations at which the brain responds actively to hypoglycemia appears to be the recent antecedent glucose exposure. Thus, people accustomed to chronic hyperglycemia may activate symptomatic stress responses as the glucose concentration falls within the physiological range—a barrier for some to tighten glucose control effectively. More extensively explored is the phenomenon of hypoglycemia unawareness, in which the body only mounts a protective counterregulatory response to falling blood glucose at glucose concentrations well below the physiologic norm. Added to the failure of glucagon responses to hypoglycemia that occurs early in type 1 diabetes, such additional counterregulatory failure may leave the patient symptom free and defenseless until plasma glucose concentrations are insufficient to support normal higher brain function. Confusion becomes the first sign of the hypoglycemia, and severe episodes (those in which the person is rendered incapable of self-treatment) ensue. In this article, we will look at the insights clinical research has provided into such hypoglycemia unawareness, how the clinical and laboratory research data have influenced our understanding and management of problematic hypoglycemia in those with diabetes, and the implications of the basic science for future diabetes management.
Hypoglycemia unawareness
Hypoglycemia unawareness and its associated increase in risk of severe hypoglycemia came to prominence with the publication of the three-fold increase in severe hypoglycemia seen in the intensively treated arm of the Diabetes Control and Complications Trial (DCCT). In that randomized controlled trial of intensive versus what was then conventional insulin therapy, severe hypoglycemia was not only more common in a curvilinear fashion with lower glycated hemoglobin, but there was also a significantly higher risk in the intensively treated group at any given achieved glycated hemoglobin (2). Such data have given rise to the perception that hypoglycemia unawareness and high risk of severe hypoglycemia are inextricably linked to tight glycemic control. In fact, this is not the case. Some people with diabetes and consistently high glycated hemoglobin can experience recurrent severe hypoglycemia. People who choose to set their glucose targets high to diminish risk of severe hypoglycemia do not always achieve their aim. Contemporaneously with the DCCT, the Düsseldorf program of structured education for people with type 1 diabetes was consistently achieving reductions in glycated hemoglobin with significantly reduced rates of severe hypoglycemia (3), perhaps the best definition of good glycemic control that we have. Although many studies find an inverse correlation between glycated hemoglobin and rate of severe hypoglycemia, this is not always the case. Table 1 lists factors associated with increased risk of severe hypoglycemia in clinical practice. One of the best predictors is an absence of C-peptide; another even stronger predictor is having a history of severe hypoglycemia (4). In those with type 2 diabetes, increasing age and the presence of comorbidities are proven additional risk factors, which may be relevant to those with all types of diabetes (5).
Table 1.
Contributors to increased risk of severe hypoglycemia
|
Loss of endogenous insulin and hypoglycemia risk
Lack of C-peptide indicates a complete deficiency of endogenous insulin. Its association with increased risk of severe hypoglycemia may relate to the loss of capacity for even vestigial reduction of an endogenous insulin response to hypoglycemia. In support of this, islet transplantation can restore protection from severe hypoglycemia even when the patient remains insulin requiring and on exogenous insulin, despite apparently not restoring glucagon responses to the falling glucose (6). Persistence of insulin effect has long been known to impair counterregulation and was considered to be the mechanism of increased risk of severe hypoglycemia posed by high levels of insulin antibodies in the days before monocomponent and human insulins (7). Loss of the cessation of endogenous insulin secretion as a paracrine signal to the α-cell to release glucagon may be another important contributory factor that makes total loss of all endogenous insulin secretion a high risk for severe hypoglycemia. Certainly, maintaining intraislet insulin with a sulfonylurea during hypoglycemia markedly attenuates the glucagon response (8). Amylin, which in healthy subjects is cosecreted with insulin, does not appear to affect counterregulation (9), although it may smooth out glucose profiles in subjects with type 1 diabetes (10). The evidence suggests that glucagon responses are lost in those with type 1 diabetes within the first 5 years and that at least in some people, catecholamine responses are also diminished over a longer diabetes duration (7). In those with type 2 diabetes, the glucagon deficit appears to develop in parallel with defective responses of endogenous insulin (11). Whereas no therapeutic maneuvers short of whole pancreas transplantation can restore glucagon responses to hypoglycemia in those with type 1 diabetes, some restoration of other defects can be made based on our current understanding of how they arise.
The role of antecedent hypoglycemia
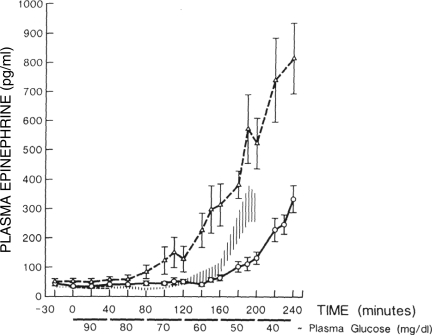
The link between defective glucose counterregulation and high risk of severe hypoglycemia is not only intuitive, it has been well demonstrated experimentally. Ryder et al. (12) showed that failure to arrest a glucose fall during an unopposed insulin infusion was associated with failure of the counterregulatory hormone response to the hypoglycemia (and incidentally had no causal relationship with diabetic autonomic neuropathy) and also with clinical problems with severe hypoglycemia. White et al. (13) used failure to counterregulate during insulin infusion as a successful predictor of future problematic hypoglycemia in subsequent application of intensified insulin therapy. Although it was initially hoped that improved glycemic control might enhance counterregulation, perhaps restoring defective glucagon responses to hypoglycemia, this did not occur. Indeed, the early methods of insulin intensification, such as those used in DCCT, were associated with increasing counterregulatory failure. Defective counterregulation appeared to be induced by the tightened glucose control (Fig. 1) (14,15).
Figure 1.
Epinephrine responses to controlled induced hypoglycemia before (△) and after (○) intensification of diabetes control. The hatched area shows the response of a group of healthy individuals who were slightly older than the diabetic subjects. Reprinted with permission from Amiel et al. (14).
A seminal study showed that the glucose concentration required to induce secretion of catecholamines in hypoglycemia could be lowered in healthy volunteers by prior exposure to two episodes of experimental hypoglycemia the preceding day (16). This work has since been repeated in people with type 1 and type 2 diabetes and appears consistent. There is some dispute about the degree of antecedent hypoglycemia required to induce the problem. Experimental reduction of arterial plasma glucose to only 3.9 mmol/l induces defects primarily in the glucagon responses to subsequent hypoglycemia, but this response is of little relevance to the insulin-deficient patient, although increases in epinephrine and muscle sympathetic nerve activity were also reduced (17). Reductions in plasma glucose to just below the physiological range (3.3 mmol/l) have more extreme effects on the catecholamine, pancreatic polypeptide, growth hormone, endogenous glucose production, and lipolytic responses to hypoglycemia, although at least 30 min of exposure to lower glucose concentrations is required to affect subjective awareness, at least in experimental models (18). In type 2 diabetes, a recent study has shown reduction of exaggerated counterregulatory hormone responses by improved glycemic control, with a further defect inducible by a single experimental exposure to a plasma glucose of 3.3 mmol/l (19).
Perhaps of greater clinical relevance has been the demonstration that awareness could be restored to the hypoglycemia unaware by avoidance of all exposure to a plasma glucose of <3 mmol/l. This was demonstrated not just in people with short duration diabetes and tight control but also in people with long duration diabetes, both using and far from using intensified therapy regimens (20). It is of interest to note that whereas most studies showed at least some restoration of both counterregulatory hormone responses to hypoglycemia as well as restoration of subjective awareness, one study showed recovery of awareness alone without any impact on defective catecholamine responses (21). This dissociation between the counterregulatory hormone responses and subjective awareness is explored below.
Cortical activation in hypoglycemia perception
McCrimmon rightly focuses his paper on current research on the molecular mechanisms of glucose sensing in the brain centers that respond to a change in glucose supply with a change in action potential brain regions such as the nuclei of the hypothalamus (1). These neurons are critical sensors and coordinators of the counterregulatory response. However, subjective awareness of hypoglycemia and the ability to respond to such awareness by taking on carbohydrates is the diabetic patient's best defense against severe hypoglycemia. Whereas the mechanisms that drive symptomatic counterregulatory responses may have origins in many brain regions, the perception of symptoms is clearly a cortical activity.
Neuroimaging studies in human subjects have consistently failed to show an increase in brain glucose uptake at euglycemia or hypoglycemia in those accustomed to hypoglycemia who are hypoglycemia unaware. This is in contrast to studies showing upregulation of glucose transporters in animal models of antecedent hypoglycemia experience and two human studies estimating global brain glucose uptake from cross-brain arteriovenous difference measurements after prolonged or recurrent hypoglycemia. Importantly, human global brain imaging does show cortical as well as brain stem activation during hypoglycemia. Teves et al. (22) showed an increase in regional brain perfusion using [15O]-H2O water positron emission tomography (PET) in anterior cingulate cortex and thalamus during hypoglycemia in healthy volunteers. Although primary changes in cerebral blood flow do not directly alter rates of cerebral metabolism, changes in regional brain perfusion detected by water PET are thought to be driven by regional changes in neuronal activation and their associated regional changes in cerebral metabolism and thus form a surrogate marker of neuronal activation. Bingham et al. (23), using [11C]-3-O-methyl-d-glucose PET, also showed an increase in glucose tracer uptake in the prefrontal cortex and anterior cingulate cortex during hypoglycemia sufficient to lower global brain glucose content in subjects with type 1 diabetes. Importantly, in the latter study, cerebral metabolic rate for glucose in the brain showed a relative rise in aware subjects versus a relative fall in unaware subjects. This is explained by understanding that the tracer uptake and metabolism are reflecting neuronal activity rather than insulin-stimulated glucose uptake, with the increased glucose uptake reflecting the involvement of cortical brain regions in the generation or detection of symptoms. The anterior cingulate cortex is an important brain region for interoception, monitoring the body's internal state, and is activated during increase in sympathetic nervous system activation. In the study by Bingham et al., the relative fall in the surrogate marker of neuronal activation was associated with failure of subjective awareness and not with complete failure of hormonal stress response.
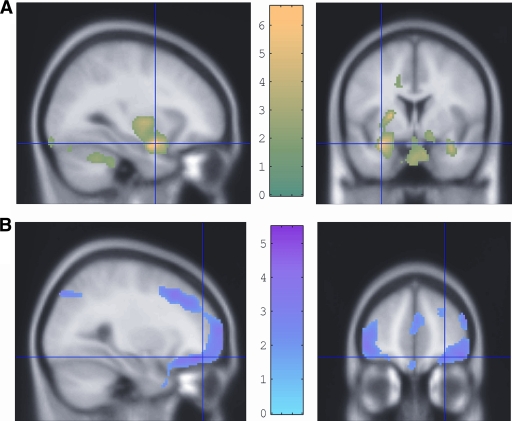
A possible additional clinical significance of changes in the cortical response to acute hypoglycemia was illustrated in a recent analysis of 18-fluoro-deoxy-glucose uptake during hypoglycemia that differentiated between the aware and unaware (24). Engagement of appetite control and reward-seeking networks involved in food seeking was seen, but the responses of these networks were measurably reduced in hypoglycemia unawareness, with failure of amygdala and orbifrontal cortex responses in particular (Fig. 2). This suggests habituation of higher behavioral responses to hypoglycemia, akin to stress desensitization, being a basis for unawareness. If confirmed, this would argue that the hypoglycemia-unaware person is unaware both of the hypoglycemia itself but also of its dangers and unpleasantness. This may have important therapeutic implications.
Figure 2.
Regions of enhanced 18-fluoro-deoxy-glucose uptake during hypoglycemia displayed on magnetic resonance imaging brain slices. A: Relatively greater 18-fluoro-deoxy-glucose uptake in amygdala, cerebellum, and brainstem in people with type 1 diabetes and hypoglycemia awareness than in people with type 1 diabetes and hypoglycemia unawareness, consistent with a greater anxiety and vigilance response in the former. B: Relatively reduced 18-fluoro-deoxy-glucose uptake in the right lateral orbitolfrontal cortex in people with good awareness of hypoglycemia compared with those who are unaware. The reduced activation of this brain region in those with awareness suggested by these data is compatible with the recognition of the unpleasantness or danger of the stimulus encouraging behavior to avoid hypoglycemia in future. This seems to be significantly less effective in those who are unaware. Reprinted with permission from Mason et al. (28).
Role of nonglucose substrates for brain metabolism in hypoglycemia unawareness
McCrimmon describes the evidence showing the ability of nonglucose fuels, particularly ketones and lactate, to sustain cerebral function during hypoglycemia, and such information is used clinically in treating some forms of epilepsy with ketogenic diets. Infusions of lactate and ketones can sustain cortical function and delay counterregulation during induced hypoglycemia (25,26), and lactate infusion reduced brain glucose uptake in a neuroimaging study (27). Neuroimaging data shows an upregulation of transporters for monocarboxylic acid transporters in hypoglycemia unawareness, the concept being that because the neurons are better able to use lactate during hypoglycemia, there is less neuronal drive for counterregulation (28). As yet, neuroimaging data have compared diabetic subjects with hypoglycemia unawareness only with nondiabetic control subjects, so the effect of diabetes alone remains uncertain. If, however, the effect is associated with reduced awareness and delayed counterregulation, it is likely that there are regional differences in its importance across the brain because of the absence of protection from severe hypoglycemia in those who are hypoglycemia unaware. Clinically, it is difficult to see how these data may be exploited. It is difficult to raise lactate levels with current agents such as metformin in healthy subjects.
Alanine has also been shown to support some aspects of cognitive function during hypoglycemia (29). It may appear paradoxical that it also enhances the counterregulatory responses to hypoglycemia, but this is restricted to the glucagon responses, which are exaggerated (30). A similar potentially beneficial effect is seen with oral amino acid ingestion. The increase in blood glucose concentration from a bedtime snack is transient (the main function of the snack being to counteract the tail action of the pre–evening meal insulin), but including protein has been suggested to enhance the defenses against nocturnal hypoglycemia by providing amino acid. In contrast to alanine, raising circulating fatty acids with intralipid impacts only on the centrally mediated hormonal responses to hypoglycemia with no effect on cognition, a combination unlikely to be exploitable therapeutically (31).
The risk of repeat hypoglycemia
Anecdotally, many patients experiencing hypoglycemia will describe how episodes tend to occur in clusters with great variation in the weekly frequency. This may of course be caused by a subacute change in insulin requirement related to other factors such as lifestyle events. Cox et al. (32) have identified that greater changes in glucose excursions are seen in the 48 h before severe hypoglycemia in patients at high risk and that a given episode of severe hypoglycemia is preceded by a significant increase in the frequency of low blood glucose measurements converted into a low blood glucose index. The increased risk of a second hypoglycemic episode occurring within 24 h of a first episode is also likely to be enhanced by the downregulation of symptomatic stress responses induced by the first episode. George et al. (33) showed that only norepinephrine responses were still impaired 48 h after an index hypoglycemia exposure in type 1 diabetic patients, although generalized reduction in response is seen within 24 h. If, as some authorities suggest, brain glycogen is important in the defenses against severe hypoglycemia and it takes 24 h to restore them after hypoglycemia (34), this could be another contributory factor.
Therapeutic strategies for improving the defenses against hypoglycemia
Avoiding hypoglycemic experience.
Based on the evidence that exposure to antecedent hypoglycemia induces and maintains counterregulatory failure and hypoglycemia unawareness and that restoration of counterregulatory responses or awareness of early hypoglycemia is achieved by hypoglycemia avoidance, helping insulin users to minimize hypoglycemia exposure is an important focus for current research. Education programs that apparently focused exclusively on this showed success in reducing hypoglycemia experience (35). However, a particularly impressive combination of improved glycated hemoglobin and large reduction in severe hypoglycemia rate sustained over at ≥3 years was achieved by the Düsseldorf program and has since been reproduced in other countries, including the U.K., where it was translated into Dose Adjustment for Normal Eating (DAFNE) (36–38). These are 5 day in- or outpatient programs delivered to small groups of patients in which principles of adult education are used to transfer skills in insulin dose adjustment to the insulin users. The courses share a common curriculum delivered by trained educators, and the best regularly undergo quality control by peer review and are audited. The insulin regimens are based on tested algorithms for dose adjustment. Patients are taught to use premeal and prebed blood glucose tests to adjust doses, correcting instantly for readings that are out of target and also prospectively by reflecting on the results of the previous few days. The regimens are now not of themselves remarkable. They stress providing meal insulin and basal insulin requirements independently, allowing flexibility of meal timing without loss of glycemic control. Patients use carbohydrate counting to determine meal doses, which are described in ratios of insulin units required per unit carbohydrate consumed at any meal. Much of the course is spent perfecting the art of accurate carbohydrate counting using food models, food plates, exercises, and real meals. Sessions also discuss other factors that influence diabetes control, provide algorithms for adjusting for these factors, and explain the rationale for the glucose targets chosen. A recent audit of the U.K.'s DAFNE program has shown that awareness of hypoglycemia can be restored to nearly half the patients entering the program with hypoglycemia unawareness at 1 year post-training (38). Although a causal link between reduced hypoglycemia experience and restored awareness is not proven by these data, it is a plausible hypothesis. It is unlikely that one single element of the Düsseldorf-based programs is responsible for their success. The explicit splitting of basal insulin replacement from meal replacement using algorithms developed by experimentation, the support of the small-group education delivery and use of adult education techniques, and the opportunity to take time to learn how insulin works with food all are likely to be important. That a clinical audit of the U.K.'s DAFNE program has recently shown a beneficial impact on depression and anxiety in participants highlights the complex nature of these interventions (38).
Technology has helped as well. Meta-analyses have failed to show a significant impact on diabetes control of the newer insulin analogues in either type 1 or type 2 diabetes (39,40). However, in studies, the flatter action profiles of the long-acting analogues, insulin glargine and detemir, and the highly peaked shorter duration of action of the rapid-acting analogues, humalog and insulin aspart, consistently show less hypoglycemia, especially at night. Clinical benefits may best be seen when they are properly used in people at increased risk of hypoglycemia problems. Continuous subcutaneous insulin infusion has long been associated in clinical observational studies with reduced frequency of severe hypoglycemia, and this is supported by a series of randomized controlled trials and a recent meta-analysis (41). No one has specifically shown that pump therapy per se has been able to restore counterregulatory failure, but it seems a likely association of the improved experience of severe hypoglycemia. Most recently, the availability of real-time glucose monitoring, by which the patient can observe the direction and rate of change of plasma glucose in real time using an electrode inserted into the interstitial fluid of the subcutaneous space, has shown an ability to improve glycated hemoglobin (with no increased risk of hypoglycemia) in adults actively practicing intensified insulin therapy, primarily with insulin infusion (42).
The evidence supports the hypothesis that all the above strategies may be diminishing the risk of severe hypoglycemia by reducing the frequency of exposure to hypoglycemia of any kind. This would be expected to improve hypoglycemia awareness and counterregulatory responses. Although it is not clear what constitutes the degree of hypoglycemia experience that creates risk, in a study by Cranston et al. (20) showing restoration of both hormonal and symptom responses to experimentally induced hypoglycemia, the investigation was not performed until the patients had demonstrated a 3-week absence of exposure (on conventional intermittent home blood glucose monitoring) to plasma glucose concentrations <3 mmol/l. Coincidentally, 2.8–3 mmol/l is the arterialised plasma glucose concentration in clamp-induced experimental hypoglycemia at which cognitive function first becomes detectable (as a deterioration in complex reaction time) (43). Perhaps for these reasons, the European Medicines Agency (EMEA), responsible for the scientific evaluation of applications for European marketing authorization for medicinal products, has chosen <3 mmol/l as its definition of hypoglycemia when examining side effects of diabetes therapies (44). This strategy is in contrast to the definition favored by an American Diabetes Association workgroup of <4 mmol/l (45) based on the evidence of activation of endogenous counterregulatory responses (increased glucagon and reduced endogenous insulin in health) and the ability of experimental exposure of 3.9 mmol/l to induce some defects in the counterregulatory response to subsequent hypoglycemia. Because 3.9 mmol/l lies within the physiological range and the pancreatic responses to hypoglycemia are impaired in insulin-deficient diabetes anyway (as far as we know irretrievably), most clinical guidelines use 3.5 mmol/l as the defining value of hypoglycemia requiring treatment. This is important because defining a patient as experiencing significant hypoglycemia has implications on their employment and leisure opportunities. In particular, labeling a person as being hypoglycemia unaware has important implications for those individuals, and defining the glucose measurement below which appearance of symptoms should be identified as having inadequate awareness is fraught with pitfalls. Practically, and given the inaccuracies of home blood glucose measurement, the definition of hypoglycemia unawareness should not be applied to people who are fully aware of hypoglycemia at 3– 4 mmol/l or even slightly less. The diagnosis is best made clinically on the basis of the person's ability to recognize and successfully treat episodes of hypoglycemia, almost irrespective of the glucose concentration measured at the time.
Despite good evidence of restoration of hypoglycemia awareness by hypoglycemia avoidance in research studies, translating this into sustainable benefit is not universally successful. Whereas about half of patients entering a DAFNE program who have hypoglycemia unawareness were hypoglycemia aware 1 year later, about half still reported themselves as being hypoglycemia unaware (38). The adaptation of the hedonic aspects of the stress response to hypoglycemia in those who were unaware seen in the neuroimaging studies described above is consistent with the failure of any central signal that hypoglycemia is unpleasant or dangerous. This aspect of unawareness is likely to reduce motivation for making behavioral changes directed at hypoglycemia avoidance. There are some early clinic data to suggest that the ability to change insulin regimens to avoid hypoglycemia is impaired in those who are hypoglycemia unaware (46). This may explain the failure of purely educational strategies to reduce problematic hypoglycemia in everyone. It is not known whether the changes in the cortical responses to hypoglycemia are part of the hypoglycemia-induced syndrome of unawareness and counterregulatory deficit, in which case they should be reversible, or whether they reflect a predisposition to respond to stress in this manner. The potential for therapies directed at reversing behavior patterns engendered by stress desensitization to help people with intractable hypoglycemia unawareness needs urgent investigation.
Future directions for adjunctive therapies to protect against severe hypoglycemia.
Despite its lack of universal or lasting success, avoidance of hypoglycemia remains the most proven and effective strategy for reducing an individual's risk of severe hypoglycemia. Research into improving our ability to avoid exposure to subphysiological glucose concentrations remains a top priority. Current therapeutic strategies should target avoidance of exposure to low glucose concentrations and ways of achieving this including ruling out or treating predisposing comorbidities, use of structured patient education around insulin usage and hypoglycemia avoidance as well as use of pump therapy, glucose monitoring strategies, and even for intractable problems, consideration of replacement of active islet tissue by transplantation. However, complete protection against hypoglycemia remains an elusive goal, and research continues into other nonglycemic strategies that might give further protection.
Based on an understanding of the mechanisms of glucose sensing and normal counterregulation, people are beginning to test the use of pharmacological agents to defend against severe hypoglycemia. To counteract the observed defect in catecholamine responses to hypoglycemia in those with long-standing type 1 diabetes, Cryer and colleagues (47) examined the ability of the β2-adrenergic agonist terbutaline to diminish the risk of nocturnal hypoglycemia. The first study did reduce hypoglycemia rates but at the expense of a generalized elevation of the blood glucose through the night. More recently a lower dose has been tried that achieved a better balance, but the effects of elevating background catecholamine action will require careful further testing.
McCrimmon describes three molecular pathways implicated in glucose sensing and the initiation of counterregulation: the AMP-activated protein kinase (AMPK); the ATP-sensitive K+ (KATP) channels, and the corticotropin-releasing hormone receptor family (1). Some of these may be amenable to pharmacological manipulation.
The methylxanthine derivatives antagonize central adenosine A2 receptors and increase intracellular cAMP, activating AMPK. They are associated with enhanced neuronal activation, alertness, and catecholamine secretion; drive the hepatic glucose response to adrenaline and glucagon; induce vasopressor responses; and reduce cerebral blood flow. Their cerebral effects, enhanced neuronal activation, and reduced cerebral blood flow are the reverse of the effects of hypoglycemia. Nevertheless, both caffeine and theophylline have been shown to enhance symptomatic and hormonal responses to hypoglycemia, effects which some have suggested may relate to a greater degree of neuroglycopenia at any given blood glucose concentration by limiting the increase in cerebral blood flow that hypoglycemia should elicit. Both agents have been shown to increase counterregulatory responses. In the case of theophylline, 2 weeks of therapy resulted in loss of the ability of the drug to enhance the catecholamine responses to hypoglycemia but preservation of the enhancement of vascular, sweating, and symptomatic responses (48). It is not, however, known whether the therapy was associated with changes in glycemic control as a contributory mechanism. In the case of caffeine, again some of its apparently beneficial effects on subjective awareness of hypoglycemia are retained (49), but this may be at least in part because it reduces the frequency of hypoglycemia, as shown by continuous glucose monitoring (50). Caffeine may also exacerbate aspects of cortical function deficits in susceptible individuals while helping preserve others during hypoglycemia (51). Other drugs that activate AMPK directly or activate adenylate cyclase may also be worth investigating. These include metformin and prostacyclin.
The complexity of manipulation of the molecular mechanisms of glucose sensing is illustrated by examining attempts to exploit the involvement of the KATP channels in neuronal glucose sensing to enhance counterregulatory responses. Intracerebrovascular administration of the KATP channel opener diazoxide increases counterregulatory hormone responses, and sulfonylurea reduces them (1). In a human study, however, a single dose of the sulfonylurea glibenclamide had equivocal effect on normal glucose counterregulatory hormone release but diminished the cognitive dysfunction seen with the hypoglycemia, compatible with the presence of sulfonylurea receptors in the cortex (52). Further work needs to be done in this area, including apparently paradoxical studies of the effects of both ATP channel openers such as diazoxide-like agents and closers such as glibenclamide.
A possible final common pathway through which glucose sensing may affect counterregulatory stress responses is through manipulating brain concentrations of the inhibitory neurotransmitter γ-aminobutyric acid (GABA), disinhibiting the stress responses. As described by McCrimmon in an animal study, localized blocade of γ-aminobutyric acid–receptor-A1 receptors in the glucose-sensing ventromedial hypothalamus can enhance glucagon and epinephrine responses to hypoglycemia, although growth hormone and cortisol responses were unaffected (1). In a single-dose healthy volunteer study, modafinil, which diminishes brain GABA levels by noradrenergic stimulation of serotonergic neurons, enhanced heart rate responses and symptom scores during hypoglycemia and supported some aspects of cognitive function (53). It had no effect on catecholamine or glucagon responses. Modafinil is used to support higher brain function in narcolepsy, and its actions in the hypoglycemia study may be exclusively cortical. In contrast, selective serotonin reuptake inhibitors, by blocking also neuronal norepinephrine transport, can enhance sympathetic outflow activity, and in experimental human studies, high-dose fluoxetine for 6 weeks prior to induced hypoglycemia enhanced epinephrine and muscle sympathetic nerve activity in hypoglycemia but had no effect on subjective awareness (54). The clinical benefit of these observations is uncertain. As yet, there has been no clinical translation of the emerging data on the corticotropin-releasing factor receptor involvement in glucose sensing.
Further implications of the research into glucose sensing and impact on other pharmacological therapies in diabetes.
The investigation of the molecular mechanisms for glucose sensing have implications for drug development in diabetes apart from the protection from hypoglycemia. In the search for better therapies for hyperglycemia, agents that are likely to affect glucose sensing are actively being explored. Current initiatives include the development of activators of glucokinase, which may be expected to lower plasma glucose by enhancement of liver glucose uptake and by enhancement of insulin secretion (55). If the glucokinase activators have access to the central nervous system, they may also alter hypoglycemia sensing. Similarly, sodium-glucose transporters are a target for therapeutic blood glucose lowering by encouraging renal excretion of glucose (56). Sodium-glucose cotransporters (SGLTs) are found in gut and in the brain, including the ventromedial hypothalamus (VMH) (1). Phlorizin, a nonselective inhibitor of SGLTs too toxic for human use, inhibits glucose-induced excitation of ventromedial hypothalamus neurons; therefore, SGLT inhibition may paradoxically stimulate a stress response that might be expected to exaggerate the counterregulatory hormone response, although this has not been tested.
SUMMARY AND CONCLUSIONS
Hypoglycemia remains a significant limitation to optimal treatment of diabetes with insulin and insulin secretagogues. Current research into the physiology of hypoglycemic counterregulation has helped us understand how to reduce risk of severe hypoglycemia, but much remains to be understood and exploited. In those with type 1 diabetes, risk for hypoglycemia is increased by the completeness of the insulin deficiency as well as the associated failure of glucagon responses to hypoglycemia and additional failure of other counterregulatory mechanisms created at least in part by repeated exposure to modest hypoglycemia itself. For those with long duration type 2 diabetes, hypoglycemia risk also increases with increasing insulin deficiency. Teaching patients to use insulin flexibly around changes in diet, exercise, alcohol ingestion, and other factors influencing insulin requirements and sensitivity can improve glycemic control while reducing hypoglycemia risk. Thereafter, increasing use of technology in both insulin delivery and, more recently, glucose sensing may be helpful. For the patient with truly intractable hypoglycemia, replacement of functional islet tissue by islet or organ transplantation is also a current therapeutic option. For the future, research into agents that may influence glucose sensing or cerebral and peripheral metabolism may offer new ways of enhancing defenses against hypoglycemia in the delivery of truly good glycemic control.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
References
- 1. McCrimmon R: Hypoglycemia: lessons from the laboratory. Diabetes Care 2009; 32: 1357– 1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The DCCT Research Group. Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care 1995; 18: 1415– 1427 [DOI] [PubMed] [Google Scholar]
- 3. Mühlhauser I, Jorgens V, Berger M, Graninger W, Gurtler W, Hornke L, Kunz A, Schernthaner G, Scholz V, Voss HE: Bicentric evaluation of a teaching and treatment programme for type 1 (insulin-dependent) diabetic patients: improvement of metabolic control and other measures of diabetes care for up to 22 months. Diabetologia 1983; 25: 470– 476 [DOI] [PubMed] [Google Scholar]
- 4. Mühlhauser I, Overmann H, Bender R, Bott U, Berger M: Risk factors of severe hypoglycaemia in adult patients with type I diabetes: a prospective population based study. Diabetologia 1998; 41: 1274– 1282 [DOI] [PubMed] [Google Scholar]
- 5. Amiel SA, Dixon T, Mann R, Jameson K: Hypoglycaemia in type 2 diabetes. Diabet Med 2008; 25: 245– 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paty BW, Ryan EA, Shapiro AM, Lakey JR, Robertson RP: Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes; 2002; 51: 3428– 3434 [DOI] [PubMed] [Google Scholar]
- 7. Bolli G, de Feo P, Compagnucci P, Cartechini MG, Angeletti G, Santeusanio F, Brunetti P, Gerich JE: Abnormal glucose counterregulation in insulin-dependent diabetes mellitus: interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 1983; 32: 134– 141 [DOI] [PubMed] [Google Scholar]
- 8. Peacey SR, Rostami-Hodjegan A, George E, Tucker GT, Heller SR: The use of tolbutamide-induced hypoglycemia to examine the intraislet role of insulin in mediating glucagon release in normal humans. J Clin Endocrinol Metab 1997; 82: 1458– 1461 [DOI] [PubMed] [Google Scholar]
- 9. Amiel SA, Heller SR, Macdonald IA, Schwartz SL, Klaff LJ, Ruggles JA, Weyer C, Kolterman OG, Maggs DG: The effect of pramlintide on hormonal, metabolic or symptomatic responses to insulin-induced hypoglycaemia in patients with type 1 diabetes. Diabetes Obes Metab 2005; 7: 504– 516 [DOI] [PubMed] [Google Scholar]
- 10. Kovatchev BP, Crean J, McCall A: Pramlintide reduces the risks associated with glucose variability in type 1 diabetes. Diabetes Technol Ther 2008; 10: 391– 396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Israelian Z, Szoke E, Woerle J, Bokhari S, Schorr M, Schwenke DC, Cryer PE, Gerich JE, Meyer C: Multiple defects in counterregulation of hypoglycemia in modestly advanced type 2 diabetes mellitus. Metabolism 2006; 55: 593– 598 [DOI] [PubMed] [Google Scholar]
- 12. Ryder RE, Owens DR, Hayes TM, Ghatei MA, Bloom SR: Unawareness of hypoglycaemia and inadequate hypoglycaemic counterregulation: no causal relation with diabetic autonomic neuropathy. BMJ 1990; 301: 783– 787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White NH, Skor DA, Cryer PE, Levandoski LA, Bier DM, Santiago JV: Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med 1983; 308: 485– 491 [DOI] [PubMed] [Google Scholar]
- 14. Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV: Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 1988; 17: 901– 907 [DOI] [PubMed] [Google Scholar]
- 15. Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS: Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987; 316: 1376– 1383 [DOI] [PubMed] [Google Scholar]
- 16. Heller SR, Cryer PE: Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 1991; 40: 223– 226 [DOI] [PubMed] [Google Scholar]
- 17. Davis SN, Shavers C, Mosqueda-Garcia R, Costa F: Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes 1997; 46: 1328– 1335 [DOI] [PubMed] [Google Scholar]
- 18. Davis SN, Mann S, Galassetti P, Neill RA, Tate D, Ertl AC, Costa F: Effects of differing durations of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia in normal humans. Diabetes 2000; 49: 1897– 903 [DOI] [PubMed] [Google Scholar]
- 19. Davis SN, Mann S, Briscoe VJ, Ertl AC, Tate DB: Effects of intensive therapy and antecedent hypoglycemia on counterregulatory responses to hypoglycemia in type 2 diabetes. Diabetes 2009; 58: 701– 709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA: Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 1994; 344: 283– 287 [DOI] [PubMed] [Google Scholar]
- 21. Dagogo-Jack S, Rattarasarn C, Cryer PE: Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 1994; 43: 1426– 1434 [DOI] [PubMed] [Google Scholar]
- 22. Teves D, Videen TO, Cryer PE, Powers WJ: Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A 2004; 101: 6217– 6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bingham EM, Dunn JT, Smith D, Sutcliffe-Goulden J, Reed LJ, Marsden PK, Amiel SA: Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus: an [11C]-3-O-methyl-D-glucose PET study. Diabetologia 2005; 48: 2080– 2089 [DOI] [PubMed] [Google Scholar]
- 24. Dunn JT, Cranston I, Marsden PK, Amiel SA, Reed LJ: Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: a new player in hypoglycemia unawareness? Diabetes 2007; 56: 2766– 2773 [DOI] [PubMed] [Google Scholar]
- 25. Maran A, Cranston I, Lomas J, Macdonald I, Amiel SA: Protection by lactate of cerebral function during hypoglycaemia. Lancet 1994; 343: 16– 20 [DOI] [PubMed] [Google Scholar]
- 26. Amiel SA, Archibald HR, Chusney G, Williams AJ, Gale EA: Ketone infusion lowers hormonal responses to hypoglycaemia: evidence for acute cerebral utilization of a non-glucose fuel. Clin Sci (Lond) 1991; 81: 189– 194 [DOI] [PubMed] [Google Scholar]
- 27. Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA: Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab 2003; 23: 658– 664 [DOI] [PubMed] [Google Scholar]
- 28. Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI: Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 2006; 55: 929– 934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans ML, Hopkins D, Macdonald IA, Amiel SA: Alanine infusion during hypoglycaemia partly supports cognitive performance in healthy human subjects. Diabet Med 2004; 21: 440– 446 [DOI] [PubMed] [Google Scholar]
- 30. Porcellati F, Pampanelli S, Rossetti P, Busciantella Ricci N, Marzotti S, Lucidi P, Santeusanio F, Bolli GB, Fanelli CG: Effect of the amino acid alanine on glucagon secretion in non-diabetic and type 1 diabetic subjects during hyperinsulinaemic euglycaemia, hypoglycaemia and post-hypoglycaemic hyperglycaemia. Diabetologia 2007; 50: 422– 430 [DOI] [PubMed] [Google Scholar]
- 31. Evans ML, Matyka K, Lomas J, Pernet A, Cranston IC, Macdonald I, Amiel SA: Reduced counterregulation during hypoglycemia with raised circulating nonglucose lipid substrates: evidence for regional differences in metabolic capacity in the human brain? J Clin Endocrinol Metab 1998; 83: 2952– 2959 [DOI] [PubMed] [Google Scholar]
- 32. Cox DJ, Gonder-Frederick L, Ritterband L, Clarke W, Kovatchev BP: Prediction of severe hypoglycemia. Diabetes Care 2007; 30: 1370– 1373 [DOI] [PubMed] [Google Scholar]
- 33. George E, Marques JL, Harris ND, Macdonald IA, Hardisty CA, Heller SR: Preservation of physiological responses to hypoglycemia 2 days after antecedent hypoglycemia in patients with IDDM. Diabetes Care 1997; 20: 1293– 1298 [DOI] [PubMed] [Google Scholar]
- 34. Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER: Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res 2005; 79: 42– 47 [DOI] [PubMed] [Google Scholar]
- 35. Cox DJ, Gonder-Frederick L, Polonsky W, Schlundt D, Kovatchev B, Clarke W: Blood glucose awareness training (BGAT-2): long-term benefits. Diabetes Care 2001; 24: 637– 642 [DOI] [PubMed] [Google Scholar]
- 36. Sämann A, Mühlhauser I, Bender R, Kloos Ch, Müller UA: Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia 2005; 48: 1965– 1970 [DOI] [PubMed] [Google Scholar]
- 37. The DAFNE Study Group. Training in flexible intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomized controlled trial. BMJ 2002; 325: 746– 752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hopkins D, Laurence I, Mansell P, Thompson G, Amiel SA, Heller S: Routine structured education reduces HbA1c & hypoglycemia and improves psychological health in patients with type 1 diabetes mellitus (Abstract). Diabetes 2008; 57( Suppl. 1): A37 [Google Scholar]
- 39. Siebenhofer A, Plank J, Berghold A, Jeitler K, Horvath K, Narath M, Gfrerer R, Pieber TR: Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2007; 2: CD003287. [DOI] [PubMed] [Google Scholar]
- 40. Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Kaiser T, Pieber TR, Siebenhofer A: Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007; 2: CD005613. [DOI] [PubMed] [Google Scholar]
- 41. Pickup JC, Sutton AJ: Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008; 25: 765– 774 [DOI] [PubMed] [Google Scholar]
- 42. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464– 1476 [DOI] [PubMed] [Google Scholar]
- 43. Maran A, Lomas J, Macdonald IA, Amiel SA: Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia 1995; 38: 1412– 1418 [DOI] [PubMed] [Google Scholar]
- 44. European Agency for Evaluation of Medicinal Products (EMEA): Note for guidance on clinical investigation of medicinal products in the treatment of diabetes mellitus [article online], 2002. Available at http://www.emea.europa.eu/pdfs/human/ewp/108000en.pdf. Accessed 1 September 2007
- 45. American Diabetes Association (ADA) Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245– 1249 [DOI] [PubMed] [Google Scholar]
- 46. Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA: Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Diabetes Care 2009; 32: 1196– 1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooperberg BA, Breckenridge SM, Arbelaez AM, Cryer PE: Terbutaline and the prevention of nocturnal hypoglycemia in type 1 diabetes. Diabetes Care 2008; 31: 2271– 2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Galan BE, Tack CJ, Lenders JW, Lutterman JA, Smits P: Effect of 2 weeks of theophylline on glucose counterregulation in patients with type 1 diabetes and unawareness of hypoglycemia. Clin Pharmacol Ther 2003; 741: 77– 84 [DOI] [PubMed] [Google Scholar]
- 49. Watson JM, Sherwin RS, Deary IJ, Scott L, Kerr D: Dissociation of augmented physiological, hormonal and cognitive responses to hypoglycaemia with sustained caffeine use Clin Sci (Lond) 2003; 104: 447– 454 [DOI] [PubMed] [Google Scholar]
- 50. Richardson T, Thomas P, Ryder J, Kerr D: Influence of caffeine on frequency of hypoglycemia detected by continuous interstitial glucose monitoring system in patients with long-standing type 1 diabetes. Diabetes Care 2005; 28: 1316– 1320 [DOI] [PubMed] [Google Scholar]
- 51. Owen G, Watson J, McGown A, Sharma S, Deary I, Kerr D, Barrett G: Influence of hypoglycaemia, with or without caffeine ingestion, on visual sensation and performance. Clin Sci (Lond) 2001; 100: 619– 626 [PubMed] [Google Scholar]
- 52. Bingham E, Hopkins D, Pernet A, Reid H, Macdonald IA, Amiel SA: The effects of KATP channel modulators on counterregulatory responses and cognitive function during acute controlled hypoglycaemia in healthy men: a pilot study. Diabet Med 2003; 20: 231– 237 [DOI] [PubMed] [Google Scholar]
- 53. Smith D, Pernet A, Rosenthal JM, Bingham EM, Reid H, Macdonald IA, Amiel SA: The effect of modafinil on counter-regulatory and cognitive responses to hypoglycaemia. Diabetologia 2004; 47: 1704– 1711 [DOI] [PubMed] [Google Scholar]
- 54. Briscoe VJ, Ertl AC, Tate DB, Davis SN: Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes 2008; 57: 3315– 3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leighton B, Atkinson A, Coghlan MP: Small molecule glucokinase activators as novel anti-diabetic agents. Biochem Soc Trans 2005; 33: 371– 374 [DOI] [PubMed] [Google Scholar]
- 56. Jabbour SA, Goldstein BJ: Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract 2008; 62: 1279– 1284 [DOI] [PubMed] [Google Scholar]