β-Cell replacement therapy's great promise is that it can safely and effectively restore insulin-independent euglycemia to individuals with diabetes. Although difficult and expensive, modern insulin-based treatment for type 1 diabetes has lead to remarkable improvements in disease prognosis (1–3). Based on recent population-based epidemiological studies, we have estimated that an individual diagnosed with type 1 diabetes today faces an excess mortality over the next 20 years of ∼2% or ∼0.1% per year (4). Even individuals with long-standing type 1 diabetes sufficiently problematic to be listed for a pancreas transplant have an annual mortality <2.0% per year (5). The perceived weaknesses associated with intensive insulin therapy are its cost estimated in 1996 to be 116,000 USD over a lifetime (6) and its inconvenience requiring meticulous attention to diet, exercise, frequent daily blood glucose measurements, and multiple daily injections. In addition, insulin therapy carries with it an increased risk of serious hypoglycemia (1). We attempt to address whether current β-cell replacement therapies overcome the shortcomings associated with medical management. Certainly, β-cell replacement therapy should not increase the subjects' risk above that associated with standard clinical care.
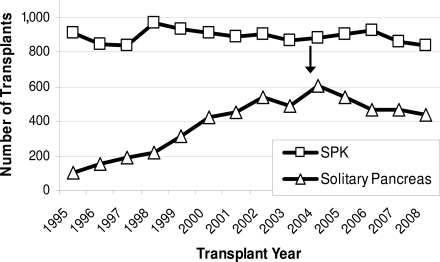
For patients with type 1 diabetes and end-stage kidney disease, simultaneous pancreas-kidney (SPK) transplantation has appropriately achieved standard of care status. SPK recipients can reasonably expect improved and sustained insulin-independent metabolic control, and the surgery demonstrably improves survival rates compared with those medically treated (5). However, the options are much less clear for subjects with long-standing diabetes and preserved kidney function defined as a serum creatinine <2.0 mg/dl. (And with modern therapies, an ever-decreasing minority will develop renal failure.) For such patients, pancreas transplant alone (PTA) or pancreas-after-kidney (PAK) transplantation, despite reasonable insulin-independence rates, does not improve survival and may even increase mortality (5). The underlying cause of the apparent excess mortality post–pancreas transplantation is not known but is likely caused by the chronic immunosuppression required to prevent allograft rejection and the nephrotoxicity associated with that therapy. Impaired kidney function is, after all, a known major risk factor for cardiovascular disease and excess mortality in diabetes (7–10). The accompanying editorial by Mineo et al. (11) cites a study by Gruessner et al. (12) that reports an ∼13% mortality in subjects awaiting PTA. Our analysis using the same national data showed that the 4-year mortality for such patients was much less (∼7.9%) (5). One plausible explanation for the difference between the two studies, both of which used the same United Network for Organ Sharing (UNOS) data, is that our analysis excluded patients with impaired renal function (serum creatinine >2.0 mg/dl) while the report from Gruessner et al. did not, and renal insufficiency is known to increase subject mortality. We suspect that our observation suggesting that solitary whole pancreas transplantation may actually increase mortality risk has resulted in a consistent decline in such procedures (PTA plus PAK) performed in the U.S. in the past 5 years (Fig. 1 ).
Figure 1.
UNOS data showing that the number of SPK transplantation procedures performed every year in the U.S. has remained stable. The number of solitary pancreas transplants (PTA and PAK) performed has declined since the published report by Venstrom et al. (5) in 2003 showing increased mortality in this group.
Islet transplantation
Isolated islet transplantation has been proposed as an alternative for these patients because it has at least two potential advantages. First, by transplanting only the islets required for insulin secretion, one avoids many surgical and postoperative complications associated with whole pancreas transplantation. Second, at least theoretically, islets can be isolated from organs otherwise deemed unsuitable for whole organ transplantation.
Unfortunately, subsequent experience has considerably dampened the tremendous enthusiasm that accompanied the “Edmonton Protocol” in 2000 (13) that reported that seven out of seven isolated islet transplant recipients maintained insulin-independent euglycemia for at least 1 year. More extensive experience from several centers worldwide (14–16) and a more prolonged follow-up from the Edmonton group have exposed several problems (17).
Insufficient safety
Immunosuppression-related risks.
All islet transplant recipients must be immunosuppressed to prevent graft rejection, and such therapy is associated with significant toxicity. Calcineurin phosphatase inhibitors remain the mainstay of many antirejection regimens, and the agents are well known to progressively impair renal function. Indeed, depending on the organ transplanted, Ojo et al. (18) calculated that 7–21% will develop renal failure, and early studies testing cyclosporine in individuals with recent-onset type 1 diabetes were also marred by drug-induced declines in renal function (19). Moreover, tacrolimus and sirolimus (the antirejection agents originally used in the Edmonton Protocol) are known to impair insulin sensitivity (20) and islet vascularization (21), and in vitro studies suggest direct β-cell toxicity (22). Tacrolimus is also associated with hypertension and hypercholesterolemia such that islet transplant recipients more often require antihypertensive and cholesterol-lowering medications following the transplantation (14,17). Non–antigen-specific immunosuppression is also known to increase an individual's risk for malignancy. Of 325 islet allograft recipients entered into the Clinical Islet Transplant Registry (CITR) as of April 2008, 14 (4%) developed various neoplasms and, of these, four were classified as possibly or definitely related to immunosuppression (14). Most important, seven deaths have been reported among the 325 CITR islet allograft recipients (one because of viral meningitis while on immunosuppression, five from strokes [within 2–3 years postinfusion], and three from unknown causes).
Some have argued that islet transplantation represents an ideal clinical model to test new immunosuppressive regimens. We question that thinking. Many factors can lead to islet allograft failure including islet graft quality, alloimmune rejection, recurrent anti–β-cell autoimmunity, and glucose or lipotoxicity associated with the intrahepatic location following their intraportal infusion (rev. in (23)). And yet, clinical investigators lack assays for reliably predicting islet quality or to adequately monitor anti-islet immune responses. We are unable to diagnose acute rejection (unlike kidney allograft rejection) until the islet grafts have failed, and rescue therapies do not exist. With so many factors possibly contributing to graft failure, and considering our inability to precisely measure the various contributing factors, we argue it is difficult or impossible to critically assess a new immunosuppressive regimen in the islet transplant recipient. Further, we argue that immunosuppressive agents shown to be effective and safe for one indication generally transfer well to another. Our point is that newer immunomodulatory strategies can be more effectively tested in other transplant settings (in particular, kidney transplantation); should safe and effective strategies be identified, then testing that approach in islet transplantation makes great sense.
Procedure-related complications.
Although isolated islet transplantation has fewer acute complications than whole organ transplantation, it is not free of serious complications. Procedure-related events include hemorrhage, infections, anemia, portal vein thrombosis, cholecystitis, and lymphopenia (0.06–0.36 events/person-year) (14). We acknowledge that the rate of these acute complications has declined in recent years.
Insufficient efficacy
Following the initial enthusiasm regarding the Edmonton protocol, it has become quite clear that long-term insulin independence is infrequently attained. Only ∼10% of the recipients from the Edmonton trial were still insulin independent 5 years following their transplant, while only 14% of the islet allograft recipients from the Immune Tolerance Network (ITN) trial remained insulin independent by 2 years following their procedure (15,24,25). Twenty-three percent of the islet allograft recipients reported to the CITR were insulin independent at 2 years; however, most had multiple islet infusions (14). These results stand in stark contrast to those obtained in solid pancreas allograft recipients in whom the insulin intendance rate at 5 years is nearly threefold higher; thus, the glycemic control is better and more sustained and requires only one donor pancreas (26,27), whereas most islet allograft recipients require islets from two or more donors. Furthermore, in studies such as the ITN trial, insulin independence was defined rather loosely (i.e., “FBS not to exceed 140 mg/dl more than three times/week and 2-h postprandial BG [blood glucose] not to exceed 180 mg/dl more than four times/week”). This begs the question of why glycemic thresholds for islet transplant recipients should be any different than for the general population. A key challenge facing islet transplantation is this inexorable decline in islet function that is presumably secondary to auto- and alloimmune attack, with perhaps other factors at play such as immediate islet loss postinfusion and poor revascularization postengraftment (28,29). Although developments such as anticoagulation to minimize the immediate islet loss that occurs during the infusion (30), alternate implantation sites, or islet encapsulation may improve islet survival, such potential advances have not been validated in clinical trials. A recent small series of five consecutive subjects given a supplemental islet infusion, then combination therapy with exenatide and etanercept, reported sustained insulin independence for up to 18 months (31). These results are encouraging; however, the numbers are small and the follow-up is short.
The most encouraging results cited in the editorial by Mineo et al. such as from a study by Vantyghem et al. (32) raise several questions. First, Vantyghem et al. (32) infused more islets than most groups (on average, islets from 2.7 donors/recipient). The most straightforward explanation for their high insulin independence rate may be simply that better early β-cell function (conferred by transplanting more islets) predicts better late islet function. Even so, islet transplantation is limited by its expense and the insufficient islet supply. If the protocol employed by Vantyghem et al. was widely adopted (as discussed more completely in the “Cost and availability” section), the average cost for organs alone would increase by ∼50,000 USD per patient and the available organs will be spread more thinly among potential recipients. Second, the editorial by Mineo et al. states that Vantyghem et al. achieved 5-year insulin independence rates of ∼57%. However, Vantyghem et al. only estimated 5-year graft survival from limited data; of the 14 subjects in the study, only 7 had reached the 3-year postislet transplant time and only 3 reached the 4-year posttransplant time period. We argue that great care should be exercised when projecting results based on such limited data. Third, the islet transplant recipients in this study lost creatinine clearance at a rate of 1.8 ml · min−1 · year−1 per 1.73 m2. This rate of renal function loss is less than those reported by other groups using the same immunosuppressive agents and levels. In fact, therapeutic use of calcineurin phosphatase inhibitors is invariably associated with decreasing renal function for the group receiving that treatment. (Though within a treatment group, some individuals seem more resistant to the nephrotoxicity than others.) Is there any reason to suspect that individuals with type 1 diabetes who receive an islet transplant are somehow resistant to calcineurin inhibitor–induced nephrotoxicity? Indeed, even Vantyghem et al. report that of the 14 subjects in their study, 5 developed new microalbuminuria following islet transplantation.
Islet transplantation improves metabolic control, but at what cost?
Most islet transplant recipients retain some graft function, i.e., continued circulating C-peptide, for several years following infusion (14,15,17,25). This partial islet graft function serves to facilitate the individual's ability to achieve desired glycemic control targets and to decrease the frequency of severe hypoglycemic. Reports also suggest an improved quality of life (33,34). However, if this is the only clinical gain from a complicated, expensive procedure with significant risks, is it justified?
Islet transplantation and complications
Renal function.
Impaired renal function is a major determinant of cardiovascular disease and mortality in patients with diabetes (7–10). Any new alternative therapy for diabetes must reduce, and certainly not hasten, the renal disease progression. Unfortunately, with some exceptions, most published clinical islet (15,35) and whole pancreas transplantation studies have shown a progressive decline in renal function in patients following the procedure (18,36). In the 3 years following islet allograft transplantation, the participants in the Edmonton trial had declining glomerular filtration rate (GFR) (on average, 0.39 ml · min−1 · month−1) and many had new or progressive microalbuminuria (35). In the ITN trial, the GFR also declined at a rate of 0.45 ml · min−1 · month−1 (15). In comparison, the GFR declined at 0.114 ml · min−1 · month−1 in the Epidemiology of Diabetes Interventions and Complications (EDIC) medically treated subjects and at 0.183 ml · min−1 · month−1 in another study of subjects with long-standing type 1 diabetes (36).
The principal criticism of the published studies has been the lack of appropriate control groups to compare rates of various end-organ complications. Recently, Thompson and colleagues (37) published their findings comparing islet transplantation with intensive medical therapy on the progression of type 1 diabetes–associated complications. The crossover design study included 42 subjects and was prospective but was nonrandomized and unblinded; patients enrolled in an islet transplant protocol were followed prior to and (for some) following their transplant. There was no statistical difference in rate of decline in GFR before or after transplant; however, subjects had a steeper decline in GFR while on medical therapy (0.45 ml · min−1 · month−1) than was predicted for the general population (0.083 ml · min−1 · month−1). Interestingly, the rate of GFR decline in this study's medically treated population was greater than rates previously reported for patients with long-standing diabetes. Conversely, the transplant recipients in this study experienced a much slower GFR decline (0.12 ml · min−1 · month−1) than had previously been described in other large clinical β-cell transplantation trials. The results are intriguing but confusing given that (except for the use of antithymocyte globulin during induction) the antirejection therapy was not substantially different from previous clinical islet transplantation trials.
Retinopathy.
Thomson and colleagues (37) report that islet allograft recipients appeared to have less progressive retinopathy. Although this finding is promising and consistent with results following pancreas transplantation (38,39), we point out the study's small size and state that glycemic control following islet transplant is not as persistent or physiological as that following whole organ transplantation.
Macrovascular complications.
Islet transplantation has not been shown to influence cardiovascular disease given that the islet recipient number remains relatively small and the follow-up is short. However, some of the immunosuppressive agents commonly used to maintain graft function in islet recipients are known to raise blood pressure and cause lipid abnormalities—two major risk factors for cardiovascular events. Further, solitary pancreas transplantation, with much higher rates of insulin independence, has not been shown to improve mortality, and some data suggest it may actually worsen survival (5).
HLA sensitization.
Most recipients require islets from multiple donors to achieve insulin independence. Islets are allocated based only on ABO compatibility, so recipients risk sensitization to multiple HLA antigens. Indeed, Shapiro and colleagues (40) recently reported that a third of islet transplant recipients develop anti–donor-specific HLA antibodies and that most develop antibodies against both class I and class II antigens. This group of individuals will have fewer suitable donors should, for example, renal replacement therapy be required in the future.
Cost and availability
Clinical islet transplantation is an expensive proposition. First-year expenses for a typical procedure are estimated to be >150,000 USD (41): ∼20,000 USD for hospital and follow-up visit charges, 30,000 USD for a year's immunosuppressive agents, and 100,000 USD for isolating islets because the charge for each pancreas is about 25,000 USD, each recipient requires, on average, islets from two donors, and the islet isolation effort succeeds only about half the time. (Thus, four pancreata are typically required for each recipient.) The lifetime cost is likely to be much higher because the recipients would require routine medical visits associated with immunosuppressive agent complications, laboratory monitoring of immunosuppressive drug levels, and medical/surgical care for complications. And given that most recipients continue to take insulin, these costs are added to the costs associated with routine diabetes care. Aside from costs, broadly applying islet transplantation is otherwise limited by the supply of brain-dead donor organs (currently only ∼8,000 per year in the U.S.), inefficient isolation techniques, and the need for specialized centers to perform the procedure. Indeed, Mineo et al. argue for potential benefit using pancreata from UNOS for islet transplantation; i.e., of the >8,000 donors available each year, only ∼25% were used for whole organ transplantation, leaving an additional 6,000 organs that could be used as a source for clinical islet transplantation trials. And yet, given that organ procurement for clinical purposes mandates that each organ procured pay an equal share of all costs required (∼25,000 USD for each organ procured) and if all 6,000 organs were procured for islet isolation efforts, the annual cost for those organs alone would be 6,000 times $25,000 USD or 150,000,000 USD. Clearly, such costs are difficult to justify during these fiscally constrained times.
Conclusion
Type 1 diabetes is a life-changing diagnosis that requires the patient's nearly constant attention. Although insulin therapy is cumbersome, for most it can deliver good metabolic control with resulting protection from end-organ damage. Admittedly, multiple daily insulin injections are difficult for many, and over time, appropriate glycemic control can be increasingly difficult with more frequent and severe hypoglycemia. β-Cell replacement could help overcome these difficulties. Even so, islet transplantation does not (for more than a few years) negate the need for insulin and the associated inconveniences and has not convincingly been shown to favorably change the end-organ complication rate, at least with regard to the kidney, which is the most significant determinant of patient survival. Moreover, the transplantation procedure and subsequent immunosuppression have significant associated risks. Most concerning is the worsening renal function observed in islet recipients on immunosuppression, but recipients also face increased risk of infections, neoplasms, and deleterious metabolic and cardiovascular effects. Further, islet transplantation is an expensive therapy that cannot be widely applied because of limited supply of organs and poor islet survival. Thus, there is little clinical benefit to be gained from clinical islet transplantation, while there are numerous serious concerns regarding its safety and efficacy. The risks associated with islet transplantation lead us to conclude that it should only rarely, if ever, be performed to improve metabolic control or to reduce hypoglycemia risk. Rather, we suggest that improved insulin delivery systems can achieve the same goal with far less cost and minimal risk to nearly all patients, and for those failing best efforts using insulin, whole pancreas transplantation remains the best “last resort” therapy. We continue to enthusiastically support ongoing research to overcome the problems that at present shroud islet transplantation's great promise.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
References
- 1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643– 2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ: The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006; 55: 1463– 1469 [DOI] [PubMed] [Google Scholar]
- 4. Khan M, Harlan DM: Transplant-based treatments for the patient with long-standing type 1 diabetes. In Diabetes: Translating Research into Practice. Greenbaum CJ, Harrison LC. Eds. New York, Informa Healthcare USA, 2008, p. 193– 208 [Google Scholar]
- 5. Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM: Pancreas transplantation decreases survival for patients with diabetes and preserved kidney function. JAMA 2003; 290: 2817– 2823 [DOI] [PubMed] [Google Scholar]
- 6. Stern Z, Levy R: Analysis of direct cost of standard compared with intensive insulin treatment of insulin-dependent diabetes mellitus and cost of complications. Acta Diabetol 1996; 33: 48– 52 [DOI] [PubMed] [Google Scholar]
- 7. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296– 1305 [DOI] [PubMed] [Google Scholar]
- 8. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Appleyard M, Jensen JS: Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110: 32– 35 [DOI] [PubMed] [Google Scholar]
- 9. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286: 421– 426 [DOI] [PubMed] [Google Scholar]
- 10. Valmadrid CT, Klein R, Moss SE, Klein BE: The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med 2000; 160: 1093– 1100 [DOI] [PubMed] [Google Scholar]
- 11. Mineo D, Pileggi A, Alejandro R, Ricordi C: Steady progress and current challenges in clinical islet transplantation. Diabetes Care 2009; 32: 1563– 1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gruessner RW, Sutherland DE, Gruessner AC: Mortality assessment for pancreas transplants. Am J Transplant 2004; 4: 2018– 2026 [DOI] [PubMed] [Google Scholar]
- 13. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230– 238 [DOI] [PubMed] [Google Scholar]
- 14. Alejandro R, Barton FB, Hering BJ, Wease S: 2008 update from the Collaborative Islet Transplant Registry. Transplantation 2008; 86: 1783– 1788 [DOI] [PubMed] [Google Scholar]
- 15. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318– 1330 [DOI] [PubMed] [Google Scholar]
- 16. Hirshberg B, Rother KI, Digon BJ, Lee J, Gaglia JL, Hines K, Read EJ, Chang R, Wood BJ, Harlan DM: Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care 2003; 26: 3288– 3295 [DOI] [PubMed] [Google Scholar]
- 17. Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM: Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54: 2060– 2069 [DOI] [PubMed] [Google Scholar]
- 18. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM: Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 931– 940 [DOI] [PubMed] [Google Scholar]
- 19. Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention: association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 1988; 37: 1574– 1582 [PubMed] [Google Scholar]
- 20. Johnston O, Rose CL, Webster AC, Gill JS: Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol 2008; 19: 1411– 1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, Galimi F, Romagnoli R, Franchello A, Salizzoni M, Perin PC, Ricordi C, Segoloni GP, Camussi G: Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant 2006; 6: 2601– 2611 [DOI] [PubMed] [Google Scholar]
- 22. Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007; 117: 2553– 2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rother KI, Harlan DM: Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest 2004; 114: 877– 883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AM: Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 2001; 50: 710– 719 [DOI] [PubMed] [Google Scholar]
- 25. Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AM: Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 2002; 51: 2148– 2157 [DOI] [PubMed] [Google Scholar]
- 26. Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP, Gruessner AC, Najarian JS: Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233: 463– 501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sutherland DE, Gruessner AC: Long-term results after pancreas transplantation. Transplant Proc 2007; 39: 2323– 2325 [DOI] [PubMed] [Google Scholar]
- 28. Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, Lundgren T, Salmela K, Tibell A, Tufveson G: Current status of clinical islet transplantation. Transplantation 2005; 79: 1289– 1293 [DOI] [PubMed] [Google Scholar]
- 29. Korsgren O, Lundgren T, Felldin M, Foss A, Isaksson B, Permert J, Persson NH, Rafael E, Ryden M, Salmela K, Tibell A, Tufveson G, Nilsson B: Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia 2008; 51: 227– 232 [DOI] [PubMed] [Google Scholar]
- 30. Ichii H, Ricordi C: Current status of islet cell transplantation. J Hepatobiliary Pancreat Surg 2009; 16: 101– 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, Mendez AJ, Selvaggi G, Ricordi C, Alejandro R: Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation 2008; 86: 1658– 1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vantyghem M-C, Kerr-Conte J, Arnalsteen L, Sergent G, Defrance F, Gmyr V, Declerck N, Raverdy V, Vandewalle B, Pigny P, Noel C, Pattou F: Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 2009; 32: 1473– 1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poggioli R, Faradji RN, Ponte G, Betancourt A, Messinger S, Baidal DA, Froud T, Ricordi C, Alejandro R: Quality of life after islet transplantation. Am J Transplant 2006; 6: 371– 378 [DOI] [PubMed] [Google Scholar]
- 34. Barshes NR, Vanatta JM, Mote A, Lee TC, Schock AP, Balkrishnan R, Brunicardi FC, Goss JA: Health-related quality of life after pancreatic islet transplantation: a longitudinal study. Transplantation 2005; 79: 1727– 1730 [DOI] [PubMed] [Google Scholar]
- 35. Senior PA, Zeman M, Paty BW, Ryan EA, Shapiro AM: Changes in renal function after clinical islet transplantation: four-year observational study. Am J Transplant 2007; 7: 91– 98 [DOI] [PubMed] [Google Scholar]
- 36. Fioretto P, Mauer SM, Bilous RW, Goetz FC, Sutherland DE, Steffes MW: Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet 1993; 342: 1193– 1196 [DOI] [PubMed] [Google Scholar]
- 37. Warnock GL, Thompson DM, Meloche RM, Shapiro RJ, Ao Z, Keown P, Johnson JD, Verchere CB, Partovi N, Begg IS, Fung M, Kozak SE, Tong SO, Alghofaili KM, Harris C: A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation 2008; 86: 1762– 1766 [DOI] [PubMed] [Google Scholar]
- 38. Chow VC, Pai RP, Chapman JR, O'Connell PJ, Allen RD, Mitchell P, Nankivell BJ: Diabetic retinopathy after combined kidney-pancreas transplantation. Clin Transplant 1999; 13: 356– 362 [DOI] [PubMed] [Google Scholar]
- 39. Pearce IA, Ilango B, Sells RA, Wong D: Stabilisation of diabetic retinopathy following simultaneous pancreas and kidney transplant. Br J Ophthalmol 2000; 84: 736– 740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, Imes S, Halpin A, Ryan EA, Shapiro AM: High risk of sensitization after failed islet transplantation. Am J Transplant 2007; 7: 2311– 2317 [DOI] [PubMed] [Google Scholar]
- 41. Guignard AP, Oberholzer J, Benhamou PY, Touzet S, Bucher P, Penfornis A, Bayle F, Kessler L, Thivolet C, Badet L, Morel P, Colin C: Cost analysis of human islet transplantation for the treatment of type 1 diabetes in the Swiss-French Consortium GRAGIL. Diabetes Care 2004; 27: 895– 900 [DOI] [PubMed] [Google Scholar]