Abstract
OBJECTIVE
Most diabetic patients with impaired renal function have a urinary albumin excretion rate in the normal range. In these patients, the etiology of renal impairment is unclear, and it is also unclear whether this nonalbumunuric renal impairment is unique to diabetes.
RESEARCH DESIGN AND METHODS
In this study, we examined the frequency and predictors of nonalbumunuric renal impairment (estimated glomerular filtration rate [eGFR] <60 ml/min per 1.73 m2) in a nationally representative cohort of 3,893 patients with type 2 diabetes and compared our findings with rates observed in the general population from the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) survey (n = 11,247).
RESULTS
Of the 23.1% of individuals with type 2 diabetes who had eGFR <60 ml/min per 1.73 m2 (95% CI 21.8–24.5%), more than half (55%) had a urinary albumin excretion rate that was persistently in the normal range. This rate of renal impairment was predictably higher than that observed in the general population (adjusted odds ratio 1.3, 95% CI 1.1–1.5, P < 0.01) but was solely due to chronic kidney disease associated with albuminuria. In contrast, renal impairment in the absence of albuminuria was less common in those with diabetes than in the general population, independent of sex, ethnicity, and duration of diabetes (0.6, 0.5–0.7, P < 0.001).
CONCLUSIONS
Nonalbuminuric renal impairment is not more common in those with diabetes. However, its impact may be more significant. New studies are required to address the pathogenesis, prevention, and treatment of nonalbuminuric renal disease.
Screening for renal impairment is now recommended as part of the annual cycle of care for individuals with type 2 diabetes (1,2). Most of those identified as having an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 have a urinary albumin excretion rate in the normal range (3,4). It has been suggested that the common finding of nonalbuminuric renal impairment is diabetic kidney disease masked by blockers of the renin-angiotensin system as well as aggressive antihypertensive and lipid-lowering therapies (5). Alternative explanations include selective parenchymal damage associated with diabetes (6), superimposed nondiabetic kidney disease, intrarenal vascular disease (7), or accelerated aging of the kidney, either alone or in combination (5). In this study, we examine the frequency and predictors of nonalbumunuric renal impairment in a large nationally representative cohort of patients with type 2 diabetes (3,8) and compare them with data observed in the age-, sex-, and ethnicity-matched general population (9).
RESEARCH DESIGN AND METHODS
The National Evaluation of the Frequency of Renal Impairment cO-existing with NIDDM (NEFRON) study was an incident-driven survey of patients with type 2 diabetes in the primary care setting. Investigator selection and its representation of practitioners in Australia is described elsewhere (3,8). In brief, 500 randomly selected investigators were requested to provide data on 10–15 consecutively presenting adult patients with established type 2 diabetes. This dataset cross-sectionally captured demographic information, including age, sex, ethnicity, history of diabetes complications, medication usage, and smoking and relevant family history, together with details of physical examination and results from the most recent blood tests and laboratory urinalysis. No attempt was made to standardize data or assessment methodologies but rather the intention was to reflect the raw results on which practitioners based their assessment and decisions about management.
The AusDiab Study
The Australian Diabetes, Obesity and Lifestyle (AusDiab) Study was a national population-based cross-sectional survey undertaken to determine the prevalence of diabetes, obesity, and other cardiovascular disease risk factors in Australian adults. The details of this study and sample selection have been described previously (9). In brief, a representative sample of 11,247 adults was recruited from 42 randomly selected urban and nonurban areas across Australia. All subjects attended a local screening and completed a series of questionnaires, physical examinations, and specific laboratory tests. Information on indicators of kidney function was available for 97.4% (n = 10,949 of 11,247) of participants from the general population (AusDiab survey). Plasma and urine creatinine concentrations were determined centrally by the modified kinetic Jaffe reaction using an Olympus AU600 autoanalyzer. Spot urine albumin concentrations were assessed centrally by immunonephelometry with the Beckman array (Beckman/Coulter, Sydney, Australia). A diagnosis of diabetes was based on self-reported physician diagnosis of diabetes confirmed either by self-reported use of hypoglycemic drugs or results from a 75-g oral glucose tolerance test. Of the population-based AusDiab cohort, 8% had diabetes, and in approximately half of these individuals, diabetes was detected for the first time at baseline screening (designated “newly diagnosed diabetes”).
Definitions used and statistical methods
eGFR was determined using the standard four-variable Modification of Diet in Renal Disease formula (10),
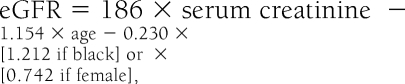 |
which has been shown to be a reliable tool for the determination of impaired kidney function in Australian patients with type 2 diabetes (11) and is automatically reported to practitioners whenever a serum creatinine test is requested (12). For the purposes of analysis, patients were stratified according to standard Kidney Disease Outcomes Quality Initiative guidelines, such that individuals with an eGFR <60 ml/min per 1.73 m2 were said to have renal impairment (13). Albuminuria was stratified according to International Diabetes Federation guidelines, such that men with a urinary albumin-to-creatinine ratio <2.5 mg/mmol and women with a ratio <3.5 mg/mmol were considered to have normoalbuminuria (14). Risks for an eGFR <60 ml/min per 1.73 m2 with and without normoalbuminuria were determined by multivariate logistic regression analysis and expressed as odds ratios (ORs), adjusting for age, sex, ethnicity, and body surface area. The association between age and the frequency of renal impairment was characterized using a regression spline, adjusting for sex, ethnicity, and body surface area.
RESULTS
NEFRON patient characteristics
The NEFRON survey collected data from 3,893 individuals with type 2 diabetes presenting consecutively to their general practitioner. The clinical characteristics of these patients were described previously (3,8). In brief, half of all patients were male (52%), with a median age of 66 years and a median duration of diagnosed diabetes of 6 years; 82.5% of patients were white, 10.2% were Asian, and 3.7% were identified as Indigenous Australians by their practitioners.
Frequency of renal impairment
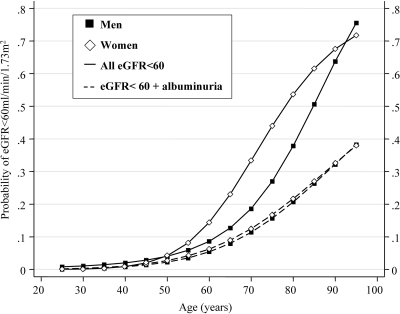
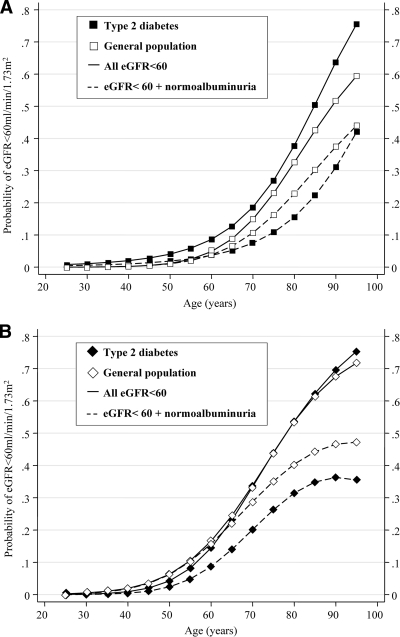
In patients with type 2 diabetes presenting to their practitioner, 23.1% had an eGFR <60 ml/min per 1.73 m2 (95% CI 21.8–24.5%). The finding of an eGFR <60 ml/min per 1.73 m2 was more common in diabetic women than in men (adjusted OR 1.8, 95% CI 1.5–2.3, P < 0.001) (Fig. 1). Men with diabetes were more likely to have an eGFR <60 ml/min per 1.73 m2 (1.5, 1.2–1.9, P < 0.001), compared with nondiabetic men from the general population, after adjusting for age, sex, ethnicity, and body surface area (Fig. 2 ). In contrast, women with type 2 diabetes were as likely to have an eGFR <60 ml/min per 1.73 m2 as women from the general population (0.9, 0.8–1.1, P = 0.3). This finding was the same across all age-groups (Fig. 2).
Figure 1.
Age-associated increase in the probability of any renal impairment (——) and renal impairment with albuminuria (– – –) in men (■) and women (♢) with type 2 diabetes from the NEFRON study.
Figure 2.
Age-associated increase in the probability of any renal impairment (——) and nonalbuminuric renal impairment (– – –) in men (A) and women (B) with type 2 diabetes (■, ♦) and in the general population (□, ♢).
Frequency of nonalbuminuric renal impairment
More than half (55%) of all diabetic patients in the NEFRON survey with an eGFR <60 ml/min per 1.73 m2 had normoalbuminuria on their most recent urinalysis. Most (98%) of these patients were also reported as being persistently normoalbuminuric by their practitioner. Nonalbuminuric renal impairment was significantly more common in women with type 2 diabetes than in diabetic men and entirely explained the sex difference in the frequency of renal impairment (Fig. 1).
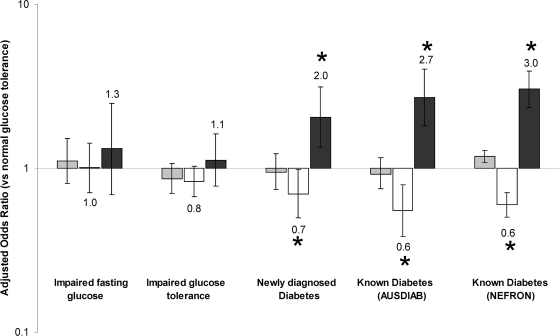
Compared with the general population, the frequency of nonalbuminuric renal impairment was less common overall in both diabetic men and diabetic women (men: adjusted OR 0.7, 95% CI 0.6–0.9, P = 0.03; women: 0.6, 0.5–0.7, P < 0.001) (Fig. 2), after adjusting for age, sex, ethnicity, and body surface area. Similar findings were observed in diabetic patients from the population-based AusDiab survey (0.7, 0.5–0.9, P < 0.001) with results comparable to those observed in the clinic-based NEFRON cohort, even in individuals with newly diagnosed diabetes (Fig. 3).
Figure 3.
Adjusted risk of eGFR <60 ml/min per 1.73 m2 (▩) and eGFR >60 ml/min per 1.73 m2 without and with albuminuria (□ and ■, respectively), stratified according to glucose tolerance and the presence of diabetes, adjusted for age, sex, ethnicity, and body surface area (*multivariate P < 0.01 vs. individuals with normal glucose tolerance).
The frequency of (any) renal impairment was positively associated with patient age (Fig. 1). However, the likelihood of that renal impairment being associated with normal urinary albumin excretion was not significantly modified in either sex by the age of the individual or in the presence or absence of diabetes (Fig. 2).
Significant ethnic differences were seen in the presentation of renal impairment in individuals with type 2 diabetes: 54% of all white patients and 54% of all Asian patients with type 2 diabetes and an eGFR <60 ml/min per 1.73 m2 had normal levels of albumin in their urine. In contrast, fewer than 17% of diabetic individuals with an Indigenous background and an eGFR <60 ml/min per 1.73 m2 had normoalbuminuria. Similarly, <20% of Polynesian patients with an eGFR <60 ml/min per 1.73 m2 had normal urinary albumin excretion.
Frequency of renal impairment associated with elevated albuminuria
Type 2 diabetes was associated with an increased frequency of albuminuric renal impairment compared with that in the nondiabetic general population (men: adjusted odds ratio 3.4, 95% CI 2.5–4.7; women: 2.6, 1.9–3.5; both P < 0.001) after adjustment for age, sex, ethnicity, and body surface area. Renal impairment associated with elevated albuminuria was also more common in the small number of diabetic patients included in the AusDiab survey (2.4, 2.0–2.7; P < 0.001), even in those with newly diagnosed diabetes (Fig. 3). The adjusted frequency of albuminuric renal impairment was not different between men and women with type 2 diabetes (P = 0.4) (Fig. 1), regardless of ethnicity. This finding was consistent with the similar frequency of albuminuria seen in men and women with an eGFR >60 ml/min per 1.73 m2 (sex difference P = 0.1).
Impaired glucose tolerance in the general population and renal impairment
Many adults in the Australian general population have impaired glucose tolerance and/or elevated fasting glucose, particularly as they reach advanced age (15). To explore whether this factor contributed to the findings detailed above, all nondiabetic individuals within the AusDiab cohort were further stratified on the basis of an oral glucose tolerance test. However, after adjustment for age, ethnicity, body surface area, and sex, neither impaired glucose tolerance nor elevated fasting glucose was associated with any significant change in the frequency of renal impairment, with or without albuminuria (Fig. 3).
Renal impairment and comorbid disease in type 2 diabetes
Diabetic patients with an eGFR <60 ml/min per 1.73 m2 and albuminuria were more likely to have a history of hypertension, retinopathy, macrovascular disease, or a first-degree relative with chronic kidney disease than diabetic individuals with an eGFR >60 ml/min per 1.73 m2 (all P < 0.05). This difference was not observed in individuals with nonalbuminuric renal impairment, after adjustment for confounding factors (Table 1 ). However, in individuals with an eGFR <60 ml/min per 1.73 m2, both with and without albuminuria, rates of visual impairment, atrial fibrillation, and heart failure were significantly higher than those of diabetic individuals with an eGFR >60 ml/min per 1.73 m2 (Table 1).
Table 1.
Clinical characteristics of patients with type 2 diabetes from the NEFRON study stratified according to the presence and absence of renal impairment and albumin excretion rate
| eGFR ≥60 ml/min per 1.73 m2 | eGFR <60 ml/min per 1.73 m2 |
|||
|---|---|---|---|---|
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | ||
| n | 3,063 | 506 | 295 | 119 |
| Age (years) | 63 ± 1 | 73 ± 1* | 74 ± 1* | 71 ± 1* |
| Sex (% male) | 55 ± 1 | 36 ± 2* | 50 ± 4* | 57 ± 5* |
| Diabetes duration (years) | 8 ± 1 | 9 ± 1* | 11 ± 1* | 12 ± 1* |
| Caucasian (%) | 81 ± 1 | 91 ± 2* | 87 ± 2* | 74 ± 4* |
| Indigenous Australian (%) | 4 ± 1 | 1 ± 1* | 4 ± 1* | 10 ± 2* |
| Asian (%) | 11 ± 1 | 6 ± 1* | 6 ± 1* | 11 ± 3* |
| Weight (kg) | 86 ± 1 | 81 ± 1* | 81 ± 1* | 82 ± 2* |
| Height (cm) | 166 ± 1 | 164 ± 1* | 165 ± 1* | 166 ± 1* |
| Smoking (% current) | 11 ± 1 | 5 ± 1† | 8 ± 2‡ | 13 ± 3‡ |
| Smoking (% ex) | 32 ± 1 | 29 ± 2 | 35 ± 2 | 27 ± 4 |
| A1C (%) | 7.4 ± 0.1 | 7.0 ± 0.1† | 7.3 ± 0.1‡ | 7.5 ± 0.2‡ |
| Fasting plasma glucose (mmol/l) | 8.0 ± 0.1 | 7.4 ± 0.1† | 7.8 ± 0.1† | 8.5 ± 0.1†‡ |
| Lipid-lowering therapy (%) | 63 ± 1 | 70 ± 2† | 74 ± 3† | 75 ± 4† |
| LDL cholesterol (mmol/l) | 2.5 ± 0.1 | 2.4 ± 0.1† | 2.3 ± 0.1† | 2.2 ± 0.1† |
| HDL cholesterol (mmol/l) | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 |
| Triglycerides (mmol/l) | 2.0 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.2 ± 0.1 |
| Systolic blood pressure (mmHg) | 133 ± 1 | 135 ± 1† | 134 ± 1† | 137 ± 2† |
| Diastolic blood pressure (mmHg) | 77 ± 1 | 75 ± 1† | 75 ± 1† | 76 ± 1† |
| Treatment for hypertension (%) | 67 ± 1 | 80 ± 1† | 86 ± 1†‡ | 89 ± 2†‡ |
| No. of antihypertensive agents | 1.4 ± 0.1 | 1.9 ± 0.1† | 2.2 ± 0.1† | 2.5 ± 0.1†‡ |
| Duration of hypertension (years) | 10 ± 1 | 14 ± 1† | 15 ± 1† | 14 ± 2† |
| Renin-angiotensin system blockade (%) | 67 ± 1 | 80 ± 1† | 84 ± 2† | 88 ± 3† |
| Calcium channel blocker (%) | 24 ± 1 | 29 ± 2† | 36 ± 3†‡ | 44 ± 5†‡ |
| Diuretic (%) | 24 ± 1 | 42 ± 2† | 44 ± 3† | 45 ± 5† |
| Retinopathy (%) | 8 ± 1 | 10 ± 1 | 17 ± 2†‡ | 25 ± 4†‡ |
| Visual impairment (%) | 15 ± 1 | 31 ± 3† | 38 ± 4† | 31 ± 5† |
| Macrovascular disease (%) | 28 ± 1 | 41 ± 2† | 52 ± 3†‡ | 55 ± 5†‡ |
| Treatment for heart failure (%) | 5 ± 1 | 14 ± 2† | 19 ± 2†‡ | 19 ± 4†‡ |
| Atrial fibrillation (%) | 4 ± 1 | 7 ± 1† | 14 ± 2†‡ | 8 ± 2† |
| Anemia (%) | 13 ± 1 | 28 ± 2† | 44 ± 3†‡ | 49 ± 5†‡ |
| Family history of renal disease (%) | 6 ± 1 | 9 ± 1† | 9 ± 2† | 13 ± 3†‡ |
| History of urinary tract infection (%) | 9 ± 1 | 13 ± 1† | 16 ± 2† | 18 ± 3†‡ |
Data are means ± SEM.
*Univariate P < 0.01, included in multivariate adjustments.
†P < 0.05 vs. eGFR >60 ml/min per 1.73 m2, adjusted for age, sex, duration of diabetes, body surface area, and ethnicity.
‡P < 0.05 vs. eGFR <60 ml/min per 1.73 m2 + normoalbuminuria, adjusted for age, sex, duration of diabetes, body surface area, and ethnicity.
Practitioner perceptions of renal impairment in type 2 diabetes
For all individuals with an eGFR <60 ml/min per 1.73 m2, those with nonalbuminuric renal impairment were less likely to be identified by their general practitioner as having (any) impaired kidney function (62%) or “moderate to severe renal impairment” (26%) than those with an eGFR <60 ml/min per 1.73 m2 and elevated albuminuria (83 and 50%, respectively, P < 0.001). In addition, patients with nonalbuminuric renal impairment were less likely to have had their choice of drug type or drug dose adjusted compared with individuals with an eGFR <60 ml/min per 1.73 m2 and elevated albuminuria (both P < 0.01).
CONCLUSIONS
Recent studies have highlighted the large numbers of diabetic individuals with an eGFR <60 ml/min per 1.73 m2 without elevated urinary albumin excretion (so-called nonalbumunuric renal impairment) (3–7,16). Although many explanations have been offered for this finding, the general population has similar or greater frequency of nonalbuminuric renal impairment after adjustment for age, sex, and other factors. Because estimation of kidney function is now widely recommended as part of routine screening in all individuals with type 2 diabetes (1,2), it is possible that nonalbumunuric renal impairment is being increasingly identified as clinicians have begun routinely estimating the GFR of their patients. This does not imply that this condition should be ignored. Because the risks associated with renal impairment are additive, its impact may be more significant in those with diabetes. Indeed, in individuals with diabetes, renal impairment (in the presence or absence of albuminuria) amplifies the risk of complications, including anemia (17), heart failure (18), adverse drug reactions (18), hypertension, and premature mortality (16,19).
The cause(s) of nonalbuminuric renal impairment remain to be established. In our study many individuals with diabetes were treated with agents that are known to reduce urinary albumin excretion, including agents that block the renin-angiotensin system and aggressive antihypertensive and lipid-lowering therapies. It is therefore likely that some of those with nonalbuminuric renal impairment may have had higher levels of urinary albumin excretion if not for their treatment (i.e., masking albuminuric renal impairment). This possibility would mean that nonalbuminuric renal impairment is even less common in those with diabetes compared with the general population than suggested by our figures. It is not possible to adjust for such treatment effects due to confounding by indication, even in subjects with newly diagnosed diabetes. Moreover, such data are representative of clinical practice, where most patients receive multiple antiproteinuric therapies, even before a diagnosis of diabetes is made.
The number of diabetic individuals with nonalbuminuric renal impairment may also be lower because those individuals predisposed to (any) renal disease develop albuminuric disease when diabetes is superimposed and nonalbuminuric disease in its absence. Certainly, the inherited susceptibility to diabetic nephropathy is not specific to diabetic renal disease, such that family members of those with end-stage renal disease due to diabetes have higher rates of nondiabetic as well as diabetic renal disease (20). Some of this association may reflect common risk factors such as hypertension, dyslipidemia, impaired glucose tolerance, and obesity.
The standard immunochemical urinary albumin assays used in this study detected immunoreactive albumin, whereas high-performance liquid chromatography (HPLC) detects both immunoreactive and immunounreactive albumin. We have previously demonstrated in the AusDiab cohort that HPLC-based assays result in 17.4% of patients classified as normoalbuminuric by means of standard assays being reclassified as microalbuminuric, especially those with diabetes (OR 3.7) (21). Although HPLC-based tests were not performed on the NEFRON cohort, if consistent, these data would again suggest that there are even fewer diabetic individuals with normoalbuminuric renal impairment than observed in the general population.
This study has a number of strengths including a large unselected nationally representative cohort of patients with type 2 diabetes presenting to their general practitioner as well as those in the general population. This large cohort has facilitated appropriate adjustment for age, sex, ethnicity, and body size, factors that have the potential to confound interpretation of the relative prevalence of renal disease. However, as a clinic-based, incident-driven study, NEFRON also has a number of limitations, being inherently (and deliberately) biased toward the kind of patients with diabetes who regularly see their practitioner and the nonstandardized laboratory results practitioners use to make their clinical decisions. Initial recruitment of investigators was based on interest in undertaking the study, although every effort was made to ensure a representative distribution of general practices according to state, regional area, and metropolitan versus rural practice. Selection bias in relation to participating investigators and subsequently enrolled diabetic patients also cannot be ruled out. Nonetheless, the consistency of the clinic-based NEFRON findings with those observed in the smaller number of diabetic individuals in the population-based AusDiab survey that used standardized data suggests that our findings may be generalizable to those with type 2 diabetes. However, it should also be noted that our findings may also be confounded by survival bias, such that high-risk individuals will be increasingly underrepresented due to drop out as levels of renal function decline.
Record numbers of individuals with type 2 diabetes are seeing doctors. From our surveys, we can anticipate that more than half of these individuals will also present with chronic kidney disease (3). Many will have an eGFR <60 ml/min per 1.73 m2, at least half of whom will not have albuminuria. Nonetheless, a reduced eGFR identifies individuals at increased risk of adverse events, and much can be done to prevent these complications once identified. Although most recent studies have justifiably focused on the prevention of albuminuric kidney disease in diabetes, our data raise the possibility that the lesion leading to nonalbuminuric renal impairment is partly preventable by multifactorial interventions that comprise standard diabetes care. Moreover, because nonalbuminuric disease is the most common presentation of renal impairment, this effect may be a major benefit of such therapies and one that may be overlooked by albuminuria-centric studies. New studies are urgently required to address the pathogenesis, prevention, and treatment of nonalbuminuric renal impairment, which is, in reality, the most common cause of renal impairment in the western world.
Acknowledgments
The NEFRON study was conducted as a collaboration between the Baker Heart Research Institute, Kidney Health Australia, and Servier Australia. It was unconditionally funded by Servier Australia. The AusDiab study was supported by the Commonwealth Department of Health and Aged Care; State Governments of Queensland, South Australia, Tasmania, Western Australia, and Victoria and Territory Health Services; the Australian Kidney Foundation; Diabetes Australia (Northern Territory); and the International Diabetes Institute. M.C.T. is supported by the Bootle Award from Kidney Health Australia and funding from the National Health and Medical Research Council and the Juvenile Diabetes Research Foundation.
The AusDiab study was also supported by Eli Lilly (Australia); Janssen-Cilag (Australia); Abbott (formally Knoll) (Australia); Merck Lipha s.a. Alphapharm; Merck Sharp & Dohme (Australia); Pharmacia and Upjohn; Roche Diagnostics; GlaxoSmithKline; Bio-Rad Laboratories; HITECH Pathology; and Qantas Airways. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Standards of medical care in diabetes—2008. Diabetes Care 2008; 31( Suppl.1): S12– S54 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008; 32( Suppl.1): S1– S201 [DOI] [PubMed] [Google Scholar]
- 3.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH: The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study). Med J Aust 2006; 185: 140– 144 [DOI] [PubMed] [Google Scholar]
- 4.Thomas MC, MacIsaac RJ, Tsalamandris C, Molyneaux L, Goubina I, Fulcher G, Yue D, Jerums G: The burden of anaemia in type 2 diabetes and the role of nephropathy: a cross-sectional audit. Nephrol Dial Transplant 2004; 19: 1792– 1797 [DOI] [PubMed] [Google Scholar]
- 5.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G: Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004; 27: 195– 200 [DOI] [PubMed] [Google Scholar]
- 6.Kramer HJ, Nguyen QD, Curhan G, Hsu CY: Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 289: 3273– 3277 [DOI] [PubMed] [Google Scholar]
- 7.MacIsaac RJ, Jerums G: Albuminuric and non-albuminuric pathways to renal impairment in diabetes. Minerva Endocrinol 2005; 30: 161– 177 [PubMed] [Google Scholar]
- 8.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME: The assessment of kidney function by general practitioners in Australian patients with type 2 diabetes (NEFRON-2). Med J Aust 2006; 185: 259– 262 [DOI] [PubMed] [Google Scholar]
- 9.Dunstan DW, Zimmet PZ, Welborn TA, Cameron AJ, Shaw J, de Courten M, Jolley D, McCarty DJ: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)—methods and response rates. Diabetes Res Clin Pract 2002; 57: 119– 129 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461– 470 [DOI] [PubMed] [Google Scholar]
- 11.MacIsaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, Poon A, Jenkins MA, Ratnåike SI, Power DA, Jerums G: Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C– and creatine-based methods. Diabetologia 2006; 49: 1686– 1689 [DOI] [PubMed] [Google Scholar]
- 12.Mathew TH: Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust 2005; 183: 138– 141 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139: 137– 147 [DOI] [PubMed] [Google Scholar]
- 14.International Diabetes Federation Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes. Brussels, International Diabetes Federation, 2005 [Google Scholar]
- 15.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM, Wong TY, McNeil J, Shaw JE: Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007; 116: 151– 157 [DOI] [PubMed] [Google Scholar]
- 16.So WY, Kong AP, Ma RC, Ozaki R, Szeto CC, Chan NN, Ng V, Ho CS, Lam CW, Chow CC, Cockram CS, Chan JC, Tong PC: Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care 2006; 29: 2046– 2052 [DOI] [PubMed] [Google Scholar]
- 17.Thomas MC: Anemia in diabetes: marker or mediator of microvascular disease? Nat Clin Pract Nephrol 2007; 3: 20– 30 [DOI] [PubMed] [Google Scholar]
- 18.Ekundayo OJ, Muchimba M, Aban IB, Ritchie C, Campbell RC, Ahmed A: Multimorbidity due to diabetes mellitus and chronic kidney disease and outcomes in chronic heart failure. Am J Cardiol 2009; 103: 88– 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough PA, Li S, Jurkovitz CT, Stevens L, Collins AJ, Chen SC, Norris KC, McFarlane S, Johnson B, Shlipak MG, Obialo CI, Brown WW, Vassalotti J, Whaley-Connell AT, Brenner RM, Bakris GL: Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J 2008; 156: 277– 283 [DOI] [PubMed] [Google Scholar]
- 20.Thompson CF, Simmons D, Collins JF, Cecil A: Predisposition to nephropathy in Polynesians is associated with family history of renal disease, not diabetes mellitus. Diabet Med 2001; 18: 40– 46 [DOI] [PubMed] [Google Scholar]
- 21.Polkinghorne KR, Su Q, Chadban SJ, Shaw JE, Zimmet PZ, Atkins RC: Population prevalence of albuminuria in the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study: immunonephelometry compared with high-performance liquid chromatography. Am J Kidney Dis 2006; 47: 604– 613 [DOI] [PubMed] [Google Scholar]