Abstract
OBJECTIVE
Intensive insulin therapy (IIT) reduces morbidity and mortality in patients in surgical intensive care units. The aim of this study is to assess the effect of IIT using a closed-loop system in hepatectomized patients.
RESEARCH DESIGN AND METHODS
Patients were randomly assigned to receive IIT using a closed-loop system: an artificial pancreas (AP group) or conventional insulin therapy using the sliding-scale method (SS group).
RESULTS
The incidence of surgical-site infection in the AP group was significantly lower than that in the SS group. The length of hospitalization required for patients in the AP group was significantly shorter than that in the SS group.
CONCLUSIONS
Total hospital costs for patients in the AP group were significantly lower than for patients in the SS group. IIT using a closed-loop system maintained near-normoglycemia and contributed to a reduction in the incidence of SSI and total hospital costs due to shortened hospitalization.
Large randomized trials in which the use of tight blood glucose control with intensive insulin therapy (IIT) was compared with standard blood glucose control in surgical intensive care unit (ICU) patients have demonstrated that strict control of postoperative blood glucose levels not only significantly reduced patient mortality but also reduced morbidity (1,2). These results helped initiate several short-lived multicenter randomized control studies designed to evaluate the benefit of tight glycemic control with IIT (3,4). Unfortunately, however, these clinical trials were stopped early, mainly because of the high incidence of hypoglycemia (10–17%) induced by IIT (5).
Considering the frequency of the use of IIT in patients undergoing surgical treatment in the ICU, we conducted a prospective randomized controlled trial to evaluate the postoperative condition of the patients and the effect of a closed-loop artificial pancreas (6–9) on tight glycemic control during IIT in hepatectomized patients.
RESEARCH DESIGN AND METHODS
Of the 91 people approached to take part in this prospective randomized study, 88 agreed to participate. Patients were informed of the purpose and details of the study, and written consent was obtained from them before enrollment. The study was approved by the local ethics committee at the Kochi Medical School and was carried out in accordance with the Helsinki Declaration. All studies were performed between April 2007 and June 2008. We prospectively divided patients into two groups: one for whom glucose levels were controlled using a manual injection of insulin according to the commonly used sliding scale (SS group, n = 44) (10) and a second group that received programmed infusions of insulin determined by the control algorithm of the closed-loop system (AP group, n = 44).
The primary end point of this study was to determine whether the incidence of SSI is reduced by perioperative tight glycemic control. SSI was monitored just after operation until discharge by the infection control team at our institute. The secondary end point was to evaluate the costs during the hospital stay in each patient group.
Continuous variables are presented as the mean ± SD. Dichotomous variables are presented as both number and percentage values. P < 0.05 was considered significant. Data were analyzed using the Student's t test (two tailed), with dichotomous variables analyzed by the χ2 test (two tailed) or Fisher's exact test (two tailed).
RESULTS
There was also no difference in the laboratory data, including nutritional parameters, liver function, fasting insulin concentration, and fasting blood glucose level between the two groups. Diabetes status was checked by a diabetologist, and diabetic subjects all had type 2 diabetes. The presence of a previous medical history for diabetes was equally distributed between the two groups (31.8% in the SS group and 38.6% in the AP group, respectively). There was no significant predisposition to these operative procedures between the two groups. The operation time and estimated volume of blood loss did not differ significantly between the two groups. Final liver tumor diagnoses were hepatocellular carcinoma in 29 patients and adenocarcinoma in 15 patients in both the SS and AP groups. There were no operative mortalities (0%) 30 days after hepatic resection in either group, and all patients were discharged.
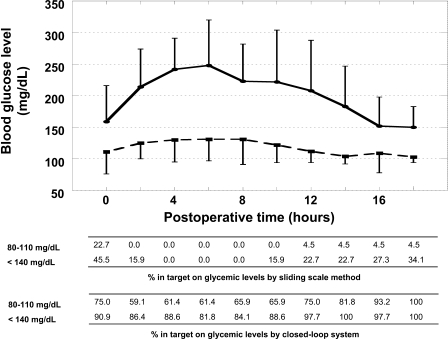
Neither group experienced hypoglycemia (<40 mg/dl). Perioperative blood glucose levels in the AP group were near 100 mg/dl, but those in the SS group were >150 mg/dl. Mean blood glucose levels were adequately controlled by a closed-loop glycemic control system (Fig. 1 ). The percentages in blood glucose target at very tight control (80–110 mg/dl) and moderately tight control (<140 mg/dl) by a closed-loop system during surgical ICU were at least >60 and 85%, respectively (Fig. 1) (5). In contrast, the postoperative glucose control according to the sliding-scale method was very poor. In all of the SS group patients, the total insulin used according to the routine sliding scale was below 24 units. In the patients in the AP group, a total amount of 175 ± 93 units of insulin was consumed for IIT managed by the STG-22 (a closed-loop glycemic control system, artificial pancreas) for 18 h after the hepatic resection. The incidence of SSI in the AP group was significantly lower than that in the SS group (P = 0.030). It is of note that patients in the AP group required a significantly shorter hospitalization than patients in the SS group (P = 0.049). The total hospital actual costs, including the costs of using the closed-loop system, for the original surgical admission of patients in the AP group ($16,407) were significantly lower than those for patients in the SS group ($21,879; P = 0.047). None of the patients in the present study required operation-related readmission.
Figure 1.
Postoperative blood glucose levels in the SS and AP groups during the first 18 h following surgery.
CONCLUSIONS
The risk of severe hypoglycemia (glucose <40 mg/dl) with IIT has been shown to increase from 5.1 to 18.7% in ICU studies (2,5,11). In the present study, there were no occurrences of hypoglycemia. Clearly, we support a recent report that suggests the development of accurate continuous blood glucose–monitoring devices and preferably closed-loop systems for computer-assisted blood glucose control in the ICU will help avoid hypoglycemia (12).
A limited number of studies describing SSI after hepatic resection have reported a wide-ranging incident rate of 10.8–26.0% (13–15). The total caloric requirement was calculated according to the Harris-Benedict equation. The results of our study proposed that controlling postoperative glucose levels using insulin therapy and maintaining an adequate calorie level contributed to a reduction in the incidence of SSI (2.3 vs. 18.2%). In the current study, tight glycemic control was performed for 18 h in patients with liver resection at surgical ICU and excellent glucose control was successfully observed without hypoglycemia by using a closed-loop system. We strongly believe that perioperative tight glycemic control for an abbreviated period (at least for 18 h) from the postoperative early stage had a prevention of postoperative infectious morbidities.
When the overall costs during hospitalization were calculated, patients with SSI had a crude median cost of $28,681 compared with $16,352 for uninfected patients (P < 0.001). This is the first report that demonstrates that perioperative tight glycemic control might reduce the incidence of postoperative SSI and decrease the total costs associated with hospitalization for patients undergoing hepatic surgery for liver neoplasm.
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology; the Pancreas Research Foundation of Japan; and The Kochi University President's Discretionary Grant.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P: Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med 2003; 31: 359– 366 [DOI] [PubMed] [Google Scholar]
- 2. Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345: 1359– 1367 [DOI] [PubMed] [Google Scholar]
- 3. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K: German Competence Network Sepsis (SepNet): intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358: 125– 139 [DOI] [PubMed] [Google Scholar]
- 4. University Hospital of Liege. Glucontrol study: comparing the effects of two glucose control regimens by insulin in intensive care unit patients. 5 April 2006. Available at http://www.clinicaltrials.gov/ct/show/NCT00107601. Accessed 22 December 2006
- 5. Wiener RS, Wiener DC, Larson RJ: Benefits and risks of tight glucose control in critically ill adults. JAMA 2008; 300: 933– 944 [DOI] [PubMed] [Google Scholar]
- 6. Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K: The accuracy of a continuous blood glucose monitor during surgery. Anesth Analg 2008; 106: 160– 163 [DOI] [PubMed] [Google Scholar]
- 7. Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K: Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand 2009; 53: 66– 71 [DOI] [PubMed] [Google Scholar]
- 8. Hanazaki K, Nose Y, Brunicardi FC: Artificial endocrine pancreas. J Am Coll Surg 2001; 193: 310– 322 [DOI] [PubMed] [Google Scholar]
- 9. Okabayashi T, Hnazaki K, Nishimori I, Sugimoto T, Maeda H, Yatabe T, Dabanaka K, Kobayashi M, Yamashita K: Continuous post-operative blood glucose monitoring and control using a closed-loop system in patients undergoing hepatic resection. Dig Dis Sci 2008; 53: 1405– 1410 [DOI] [PubMed] [Google Scholar]
- 10. Alfonso A, Koops MK, Mong DP, Vigersky RA: Glycemic control with regular versus lispro insulin sliding scales in hospitalized type 2 diabetics. J Diabetes Complications 2006; 20: 153– 157 [DOI] [PubMed] [Google Scholar]
- 11. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R: Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354: 449– 461 [DOI] [PubMed] [Google Scholar]
- 12. Van den Berghe G: Insulin therapy in the intensive care unit should be targeted to maintain blood glucose between 4.4 mmol/l and 6.1 mmol/l. Diabetologia 2008; 51: 911– 915 [DOI] [PubMed] [Google Scholar]
- 13. Ambiru S, Kato A, Kimura F, Shimizu H, Yoshidome H, Otsuka M, Miyazaki M: Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect 2008; 68: 230– 233 [DOI] [PubMed] [Google Scholar]
- 14. Togo S, Matsuo K, Tanaka K, Matsumoto C, Shimizu T, Ueda M, Morioka D, Nagano Y, Endo I, Shimada H: Perioperative infection control and its effectiveness in hepatectomy patients. J Gastroenterol Hepatol 2007; 22: 1942– 1948 [DOI] [PubMed] [Google Scholar]
- 15. Hayashibe A, Sakamoto K, Shinbo M, Makimoto S, Nakamoto T: New method for prevention of bile leakage after hepatic resection. J Surg Oncol 2006; 94: 57– 60 [DOI] [PubMed] [Google Scholar]