Abstract
OBJECTIVE
To investigate the influence of primary graft function (PGF) on graft survival and metabolic control after islet transplantation with the Edmonton protocol.
RESEARCH DESIGN AND METHODS
A total of 14 consecutive patients with brittle type 1 diabetes were enrolled in this phase 2 study and received median 12,479 islet equivalents per kilogram of body weight (interquartile range 11,072–15,755) in two or three sequential infusions within 67 days (44–95). PGF was estimated 1 month after the last infusion by the β-score, a previously validated index (range 0–8) based on insulin or oral treatment requirements, plasma C-peptide, blood glucose, and A1C. Primary outcome was graft survival, defined as insulin independence with A1C ≤6.5%.
RESULTS
All patients gained insulin independence within 12 days (6–23) after the last infusion. PGF was optimal (β-score ≥7) in nine patients and suboptimal (β-score ≤6) in five. At last follow-up, 3.3 years (2.8–4.0) after islet transplantation, eight patients (57%) remained insulin independent with A1C ≤6.5%, including seven patients with optimal PGF (78%) and one with suboptimal PGF (20%) (P = 0.01, log-rank test). Graft survival was not significantly influenced by HLA mismatches or by preexisting islet autoantibodies. A1C, mean glucose, glucose variability (assessed with continuous glucose monitoring system), and glucose tolerance (using an oral glucose tolerance test) were markedly improved when compared with baseline values and were significantly lower in patients with optimal PGF than in those with suboptimal PGF.
CONCLUSIONS
Optimal PGF was associated with prolonged graft survival and better metabolic control after islet transplantation. This early outcome may represent a valuable end point in future clinical trials.
The short-term effectiveness of islet transplantation for alleviating hypoglycemia and controlling glucose homeostasis while limiting or even avoiding the need for exogenous insulin has been established (1). Despite protocol modifications in donor selection, islet preparation, or recipient treatment, insulin independence with adequate metabolic control was, however, rarely prolonged beyond 2 years (1–5). The most frequently proposed explanations include chronic allogenic rejection, recurrence of autoimmunity, and β-cell toxicity from administered immunosuppressive drugs (6). It is, however, unclear why similar drawbacks do not preclude the more durable results of pancreas transplantation (7). Alternatively, a slightly impaired glucose control associated with suboptimal graft function, even in patients with apparently successful islet transplantation, could contribute to progressive islet exhaustion and precipitate graft decline (6). In this prospective longitudinal study, we explored this hypothesis by analyzing the influence of primary graft function (PGF) on graft survival and metabolic control in a consecutive cohort of 14 patients followed up for 2 years and beyond after islet transplantation with the Edmonton protocol.
RESEARCH DESIGN AND METHODS
A total of 14 patients were enrolled in this single-center phase 2 trial initiated in 2003 after approval by an institutional review board and the French health agency (Agence Française de Securite Sanitaire des Produits de Santé). Eligible patients were male or female aged 18–65 years, with type 1 diabetes documented for more than 5 years, arginine-stimulated C-peptide <0.2 ng/ml, and hypoglycemia unawareness or documented metabolic lability. Exclusion criteria included a BMI >28 kg/m2, unstable arteriopathy or heart disease, active infection, previous transplantation, insulin daily requirements >1.2 units/kg, creatinine clearance <60 ml/min per 1.73 m2 or urinary albumin excretion >300 mg/day, malignancy, smoking, desire for pregnancy, psychiatric disorders, and lack of compliance. The study primary efficacy end point was graft survival defined as insulin independence and A1C ≤6.5%. Secondary outcomes were graft function and metabolic control.
Islet transplantation
Islet transplantation consisted of up to three sequential fresh islet infusions within 3 months, with the aim of reaching adequate metabolic control without exogenous insulin. Islets were isolated from ABO-compatible deceased donor pancreata with a negative cross-match and evaluated as previously described (8). The access to the portal vein was gained under general anesthesia by percutaneous catheterization of a peripheral portal branch under ultrasound guidance or by surgical catheterization of a small mesenteric vein. In all cases, heparin (35 units/kg) was added to the final product, gently infused by gravity with portal pressure monitoring. Immunosupression consisted of tacrolimus (Prograf) (Astellas, Fujisawa, Japan), target trough levels at 3–6 ng/ml, and sirolimus (Rapamune) (Wyeth Pharmaceuticals France, Paris, France), target trough levels at 12–15 ng/ml for 3 months and at 7–10 ng/ml thereafter. A five-dose induction course of dacluzimab (Zenapax) (1 mg/kg) (Roche, Welwyn Garden City, U.K.) was administered biweekly beginning 1 h before the first infusion.
Follow-up
Patients were seen weekly during 1 month after each islet infusion, monthly during the 1st year, and every 6 months thereafter for routine clinical and biological evaluation, including fasting blood glucose, basal and postprandial plasma C-peptide, A1C, and sirolimus and tacrolimus trough levels. Quarterly, glucose variability was assessed over 3 consecutive days with a continuous glucose monitoring system (CGMS) (Medtronic MiniMed, Northridge, CA). At each annual visit, an oral glucose tolerance test (OGTT) (75g) was performed in patients who remained insulin independent to assess glucose tolerance as proposed by the American Diabetes Association (9). The presence and type of autoantibodies (insulinoma-associated protein 2 [IA2], GAD, and islet cell antibody [ICA]) and panel-reactive anti-HLA (A, B, and DR) antibodies were evaluated before transplantation, after each islet infusion, yearly during follow-up, and, in case of graft loss, 3 months after discontinuation of immunosuppression. Exogenous insulin was reintroduced when A1C increased >6.5% on two consecutive measurements.
Outcomes
Graft function was estimated with the β-score, a previously validated composite index ranging from 0 (no graft function) to 8 (excellent graft function) (2). The β-score was calculated as initially described by Ryan et al. (2), giving two points for normal fasting glucose (≤5.5 mmol/l), A1C (≤6.1%), stimulated and/or basal C-peptide (>0.3 nmol/l), and absence of insulin or oral hypoglycemic agent use. No point was awarded if fasting glucose was in the diabetic range (>7 mmol/l), A1C was >6.9%, C-peptide secretion was undetectable on stimulation, or daily insulin use was >0.24 units/kg. One point was given for intermediate values. Graft function was considered optimal when the β-score was 7 or 8, suboptimal when the β-score was 4 to 6, or poor when the β-score was 3 or less. The primary graft function was defined for each patient as the β-score calculated at the first monthly evaluation after the last infusion. Metabolic control outcomes were basal C-peptide, A1C, mean glucose, glucose SD, and low glucose excursions (time with glucose <3.85 mmo/l) monitored by CGMS. Graft survival outcome was the loss of insulin independence and/or adequate metabolic control (A1C >6.5%). Adverse events were classified according to the National Cancer Institute common terminology criteria for adverse events (version 3.0); grade 3–5 events were monitored.
Data analysis
Continuous variables were expressed as median (interquartile range) or mean ± SD when indicated and were compared using the Mann-Whitney U test, Wilcoxon's signed-rank test, or ANOVA and post hoc Fisher's protected least significant differences test, when appropriate. Categorical variables were compared with the Fisher's exact test. The Kaplan-Meier estimates for graft survival outcomes were made for the overall data and strata-defined variables and compared using the Mantel-Cox log-rank test. Correlations were assessed by linear regression. All calculations were computed with Statview (Abacus Concepts, Berkeley, CA). A P level <0.05 was considered significant.
RESULTS
Primary graft function
A total of 14 patients (supplementary Table A1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc08-1685/DC1) received a total of 38 islet preparations (supplementary Table A2) in two (n = 4) or three (n = 10) infusions within a total period of 67 days (44–95). The last infusion had to be postponed until day 140 in one patient who experienced a 1-cm hepatic arteriovenous aneurysm during the second infusion and until day 186 in another patient with previous history of blood transfusion and HLA sensitization waiting for a compatible donor. All the patients gained insulin independence 12 days (6–23) after their last infusion. PGF estimated at the first posttransplant monthly evaluation, 36 days (27–45) following last infusion, was optimal (β-score 7 or 8) in nine patients (64%) and suboptimal (β-score 6 or 5) in five (36%) (Table 1 and supplementary Table A3). Patients who experienced optimal PGF had slightly lower baseline insulin requirements than patients with suboptimal PGF (P < 0.04), but the preexistence of islet autoantibodies and the overall graft characteristics were not statistically different between the two groups (Table 2).
Table 1.
Patient and graft characteristics
| All patients | Suboptimal PGF | Optimal PGF | P* | |
|---|---|---|---|---|
| n | 14 | 5 | 9 | |
| Sex (male/female) | 7/7 | 4/1 | 3/6 | 0.09 |
| Age (years) | 42 (36–51) | 42 (38–49) | 42 (36–51) | 0.99 |
| Diabetes duration (years) | 27 (17–31) | 17 (15–27) | 28 (26–34) | 0.12 |
| Weight (kg) | 71 (61–78) | 75 (69–79) | 67 (60–76) | 0.31 |
| Baseline A1C (%) | 8.5 (7.3–8.9) | 8.7 (8.0–9.2) | 8.3 (7.3–8.6) | 0.35 |
| Basal insulin requirements (units/kg per day) | 0.53 (0.41–0.68) | 0.68 (0.54–0.83) | 0.42 (0.38–0.57) | 0.04 |
| Islet autoantibodies (yes/no) | 9/5 | 4/1 | 5/4 | 0.18 |
| Islet infusions (n) | 3 (2–3) | 3 (2.7–3) | 3 (2–3) | 0.61 |
| First to last infusion (days) | 67 (44–95) | 60 (42–91) | 73 (46–113) | 0.74 |
| Total islet mass (103 IEQ/kg) | 12.5 (11.1–15.8) | 12.6 (12.0–16.8) | 11.5 (10.5–15.7) | 0.46 |
| Total islet number (103/kg) | 14.4 (11.4–17.5) | 15.2 (13.7–20.6) | 13.4 (11.0–16.1) | 0.59 |
| Total β-cell number (106/kg) | 5.7 (5.0–12.9) | 9.4 (2.6–16.3) | 5.7 (5.1–10.1) | 0.21 |
| Total HLA mismatch (n) | 6 (3–9) | 9 (3–10) | 5 (3–8) | 0.63 |
| Last infusion to insulin independence (days) | 12 (6–23) | 23 (0–61) | 10 (6–22) | 0.23 |
| Initial A1C (%)† | 5.6 (5.2–6.1) | 6.3 (5.5–6.4) | 5.3 (5.2–5.7) | 0.38 |
| Initial fasting blood glucose (mmol/l)† | 5.7 (5.2–6.1) | 6.5 (6.1–6.9) | 5.2 (5.1–5.7) | 0.38 |
| Initial basal C-peptide (nmol/l)† | 0.5 (0.41–0.53) | 0.53 (0.5–0.53) | 0.46 (0.4–0.5) | 0.95 |
| β-Score (0–8)† | 7.0 (6.0–8.0) | 6.0 (5.7–6.0) | 8.0 (7.0–8.0) | <0.01 |
Data are median (interquartile range) unless otherwise indicated.
*Suboptimal versus optimal;
†measured at the first monthly evaluation following last islet infusion. IEQ, islet equivalent.
Table 2.
Metabolic control
| Patients with suboptimal PGF (n = 5) |
Patients with optimal PGF (n = 9) |
|||||
|---|---|---|---|---|---|---|
| Inclusion | 2 years | Last follow-up | Inclusion | 2 years | Last follow-up | |
| Insulin independence | 0 (0) | 1 (20) | 1 (20) | 0 (0) | 8 (89)*† | 7 (78)*† |
| A1C (%) | 8.7 (8.0–9.2) | 7.8 (7.4–8.7) | 8.3 (7.4–9.4) | 8.3 (7.3–8.6) | 5.8 (5.4–6.5)*† | 6.2 (5.6–6.7)*† |
| Mean glucose (mmol/l) | 9.4 (7.5–12.4) | 8.7 (6.3–10.6) | 7.4 (6.3–10.6) | 8.1 (6.5–11.6) | 5.7 (5.4–6.1)*† | 5.3 (5.1–5.6)*† |
| Glucose SD (mmol/l) | 3.9 (3.0–4.9) | 1.8 (1.4–4.4) | 2.3 (1.6–4.4) | 3.1 (2.2–4.4) | 0.8 (0.7–1.0)* | 1.0 (0.6–1.4)*† |
| Low glucose excursion (%) | 9 (1–18) | 0 (0–4) | 3 (0–5) | 16 (3–22) | 0 (0–1)* | 0 (–0)* |
| Basal C-peptide (nmol/l) | 0 (–0) | 0.17 (0–0.53) | 0 (0–0.43) | 0 (–0) | 0.5 (0.4–0.6)* | 0.5 (0.43–0.6)*† |
| β-Score (0–8) | 0 (–0) | 1 (0–3.8) | 2 (0–4.5) | 0 (0) | 7 (4.8–8)*† | 7 (5.8–8)*† |
| Functioning graft | 0 (0) | 2 (40) | 2 (40) | 0 (0) | 9 (100)*† | 9 (100)*† |
| ADA criteria‡ | ||||||
| Normal | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 5 (56)* | 5 (56)*† |
| Glucose intolerance | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (22) | 4 (44) |
| Diabetes | 5 (100) | 4 (80) | 5 (100) | 9 (100) | 2 (22) | 0 (0) |
Data are median (interquartile range) or n (%).
*P < 0.05 versus inclusion (Wilcoxon's test);
†P < 0.05 versus suboptimal PGF (Mann-Whitney U test).
‡Defined by the American Diabetes Association (ref. 9); normal when fasting blood glucose was <5.6 mmol/l and 2-h postload glucose during OGTT was <7.8 mmol/l; diabetes when fasting blood glucose was ≥7 mmol/l or 2-h postload glucose during OGTT was ≥11.1 mmol/l; and glucose intolerance when intermediate values.
Graft survival
At the time of this report, all patients were alive and none of the patients were lost to follow-up, 39 months (32–48) after islet transplantation. At 1 year, 10 patients (71%) met the study primary end point. At last follow-up, eight patients (57%) remained insulin independent with adequate metabolic control (A1C ≤6.5%) 2–5 years after islet transplantation. Six patients had to return to small doses of insulin and/or antidiabetic oral agents to maintain optimal metabolic control 6–36 months after islet transplantation. Three of them eventually lost graft function 1, 2, and 12 months, respectively, after insulin reintroduction and discontinued immunosuppression. Figure 1 illustrates the Kaplan-Meier estimates (95% CI) of the proportion of patients with insulin independence and adequate metabolic control and with persisting C-peptide secretion (≥0.5 ng/ml) during follow-up. Graft survival was significantly influenced by PGF (P = 0.01, log-rank test). Conversely the presence (n = 8) or absence (n = 6) of islet autoantibodies before transplantation had no significant influence on graft survival (P = 0.40, log-rank test).
Figure 1.
The Kaplan-Meier estimates of the proportions of patients with insulin independence and A1C ≤6.5% (A and B) and with persisting graft function (basal C-peptide ≥0.5 ng/ml) (C and D). Left panels represent these proportions among the entire cohort (number at risk: 14 at baseline and at 2 years, 7 at 3 years, and 3 at 4 years) and right panels in patients with optimal initial graft function (solid line) (n = 9) and in those with suboptimal initial graft function (interrupted line) (n = 5). Circles indicate censored data. *Mantel-Cox log-rank test, optimal PGF vs. suboptimal PGF.
Metabolic control
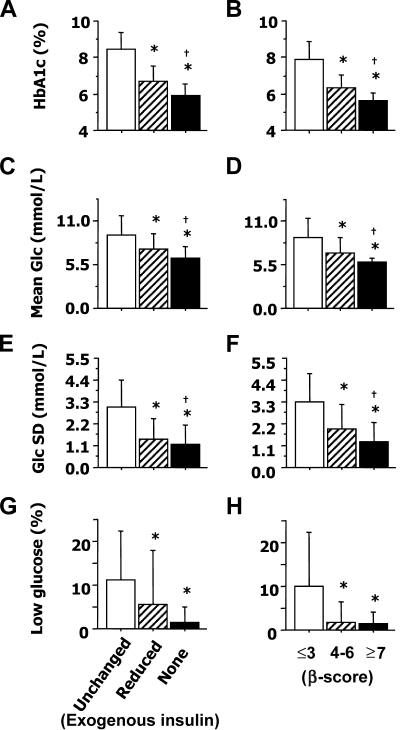
All metabolic parameters were significantly improved at 2 years and at last follow-up when compared with baseline values in the entire cohort of 14 patients (intent-to-treat analysis). With the exception of low glucose excursions, these outcomes were also markedly lower in patients with optimal PGF than in those with suboptimal PGF (Table 2). We also analyzed the correlation between the values of β-score and metabolic control outcomes measured throughout follow-up in the 14 patients before and after their transplantation (n = 92). By design, the β-score was closely linked with A1C (r = −0.88; P < 0.0001), fasting blood glucose (r = −0.88; P < 0.0001), and C-peptide (r = 0.58; P < 0.0001). This score was also significantly correlated with the values of mean glucose (r = −0.65; P < 0.0001), glucose SD (r = −0.62; P < 0.0001), and low glucose excursions (r = −0.50; P < 0.0001) measured by CGMS. Furthermore, the β-score was correlated in insulin-independent patients with 2-h postload glucose level during OGTT (n = 27) (r = −0.71; P < 0.0001). A marked improvement of metabolic control was already apparent during periods with suboptimal graft function (Fig. 2 ). A1C, mean glucose, and glucose SD were, however, significantly lower when graft function was optimal (P < 0.05 vs. suboptimal) and reached 5.9 ± 0.6%, 6.3 ± 1.7 mmol/l, and 1.3 ± 1.0 mmol/l, respectively, in patients maintaining insulin independence.
Figure 2.
Graft function and metabolic control in 14 patients before and after islet transplantation. A1C (A and B), mean blood glucose (Glc) (C and D), glucose SD (E and F), and low glucose excursion (time with blood glucose <3.8 mmol/l) (G and H) associated with poor (□), suboptimal (▨), or optimal (■) graft function as reflected by insulin requirements (A, C, E, and G) or by the β-score (B, D, F, and H). *P < 0.05 vs. poor function; †P < 0.05 vs. suboptimal function. The number of separate measurements reflected by each bar is as follows: 20 (□), 14 (▨), and 58 (■) (insulin requirements); 28 (□), 24 (▨), and 40 (■) (β-score). Data are means ± SD.
Safety
A total of 42 grade 3 or higher adverse events were observed in 11 patients (0.9 event per patient year). Grade 4 events (n = 3) included one bile leak following percutaneous infusion and one mechanical bowel obstruction after surgical infusion both requiring reintervention and one neutropenia episode requiring lenograstim treatment and discontinuation of immunosuppression. Grade 3 events were expected and related to the procedure (n = 3) and/or the immunosuppression (n = 36). The five most frequent were neutropenia (n = 11), anemia (n = 5), diarrhea (n = 4), elevated liver enzymes (n = 3), and increase of osteoarthritis pain (n = 3). At last follow-up, serum creatinine remained in the normal range in all patients and the change from baseline of creatinine clearance (estimated by the Cockcroft-Gault formula) was −0.15 ml · min−1 · month−1 per 1.73 m2 (−0.34 to 0.41) (increased in six patients and decreased in eight). Microalbuminuria (>30 mg/day) persisted in one patient and developed in five patients. At the time of this report, no donor-specific allosensitization has been detected using lymphocytotoxicity test.
CONCLUSIONS
This prospective cohort study showed that insulin independence with adequate metabolic control can be maintained beyond 2 years after islet transplantation in most recipients, when optimal graft function is initially achieved. Our results also suggest that PGF plays a preeminent role in the long-term outcome of islet transplantation. To assess graft function, we used the β-score, a composite index that was previously correlated with postprandial glucose (10) and arginine-induced insulin secretion (11) after islet transplantation. In addition, we found herein a significant reciprocal relationship between the β-score and OGTT outcome as well as with mean glucose and glucose variability, two independent predictors of secondary complications in type 1 diabetic patients (12). Most importantly, this simple index also allowed us to quantify PGF after sequential islet transplantation. With insulin independence and adequate metabolic control in seven out of nine cases (78%) and normal glucose tolerance in five (56%), the results observed after islet transplantation in patients with optimal PGF matched beyond 2 years those of pancreas transplantation (7). The small number of subjects enrolled is an obvious limitation of our findings, but the simplicity of the β-score will allow their validation in other centers.
Noteworthy, we did not find any significant influence of immunological markers on PGF or graft survival. For its limited power, this study does not exclude the likely role also played by alloimmunity (13) and autoimmunity (14) in islet graft decline. Our results suggest, however, that under the profound immunosuppression induced by the Edmonton protocol, the long-term outcome of islet transplantation depends above all on PGF. A similar long-term influence of PGF is already established for kidney allotransplantation (15). Besides just delaying graft loss, optimal kidney graft function is associated with a decrease in alloimmune graft impairment (16). Even marginal hyperglycemia is deleterious for β-cells (17). The impaired metabolic control associated with suboptimal graft function (Fig. 2) may have therefore directly contributed to islet decline in patients with lower PGF. Two independent autopsy reports further support this nonimmunological mechanism of chronic islet impairment (18,19).
The risks associated with islet transplantation appear limited compared with those of pancreas transplantation (7). They remain, however, markedly superior to the risks associated with exogenous insulin delivery, even with implantable delivery systems (20). Adopting a conservative approach for portal vein access and strictly limiting the volume of infused tissue, we avoided herein portal thrombosis and significant bleeding. However, we did not eliminate adverse events related to the procedure that required reintervention after 2 of the 38 islet infusions (5%). Other complications were expected (5) and mainly related to chronic immunosuppression. In line with Fung et al. (21), we did not observe the early renal decline reported elsewhere with the Edmonton protocol (22). Renal impairment-like donor-specific sensitization may, however, emerge after a longer follow-up or when assessed with more sensitive methods. It cannot be fully excluded at this time in our patients.
In summary, this study showed that islet graft survival and adequate metabolic control can be markedly prolonged when optimal graft function is initially achieved and suggests that PGF has a preeminent influence on long-term islet transplantation outcome. Whether this improved metabolic control is sufficient to overcome the risks associated with chronic immunosuppression will only be answered by ongoing (21) and future controlled studies comparing this procedure with more established therapies. Our results suggest, however, that besides developing new immunosuppressive strategies with better safety profiles, research efforts in islet transplantation should now focus on optimizing PGF. In addition to increasing the mass of transplanted islet cells (23), significant progress remains necessary to improve islet potency (24) and limit the early loss of islets after transplantation (25). PGF may serve as a valuable early end point to facilitate the comparison of alternative strategies.
Supplementary Material
Acknowledgments
This study was supported by the French Ministry of Health (PHRC 2001), the European Community (Fond Européen de Développement Regional), Conseil Régional Nord Pas de Calais (EGID, European Genomic Institute for Diabetes), and Groupement Inter Hospitalier G4 (Amiens, Caen, Lille, Rouen). Our team is also part of the European (ECIT) and French (DIACELL) consortia for islet transplantation supported by the Juvenile Diabetes Research Foundation and the Association de Langue Française pour l'Etude du Diabète et des Maladies Métaboliques, respectively.
No potential conflicts of interest relevant to this article were reported.
We are indebted to Francoise Dufosse (immunological monitoring); Isabelle Martinache (quality assurance); Patrick Devos (study design); Rimed Ezzouaoui and Licia Touzet (graft coordination); Robert Caiazzo, Thomas Hubert, Thomas Jany, and Catherine Latteux (pancreas procurement); Isanga Aluka, Sandrine Belaïch, Nathalie Delalleau, and Nina Mikkola (islet isolation); Bruno Lukowiak and Ericka Moerman (islet assessment); Pierrette Perimenis and Sophie Marcelli-Tourvieille (patient management); Helene Verstavel and Anne Sophie Vanceulebroek (metabolic evaluation); the clinical staff of the Department of Endocrine Surgery and the Department of Endocrinology and Metabolism; the Delegation à la Recherche Clinique; the coordination teams of Agence de Biomedicine; and Jean Lefebvre and Charles Proye for their seminal support.
Footnotes
Clinical trial reg. no. NCT00446264, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318– 1330 [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM: Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54: 2060– 2069 [DOI] [PubMed] [Google Scholar]
- 3.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, Ferreira JV, Pugliese A, Esquenazi VV, Kenyon NS, Alejandro R: Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 2005; 5: 2037– 2046 [DOI] [PubMed] [Google Scholar]
- 4.Gerber PA, Pavlicek V, Demartines N, Zuellig R, Pfammatter T, Wuthrich R, Weber M, Spinas GA, Lehmann R: Simultaneous islet-kidney vs pancreas-kidney transplantation in type 1 diabetes mellitus: a 5 year single centre follow-up. Diabetologia 2008; 51: 110– 119 [DOI] [PubMed] [Google Scholar]
- 5.Collaborative Islet Transplantation Registry Annual Report Rockville, Maryland, The EMMES Corporation, 2007 [Google Scholar]
- 6.Bertuzzi F, Ricordi C: Prediction of clinical outcome in islet allotransplantation. Diabetes Care 2007; 30: 410– 417 [DOI] [PubMed] [Google Scholar]
- 7.Larsen JL: Pancreas transplantation: indications and consequences. Endocr Rev 2004; 25: 919– 946 [DOI] [PubMed] [Google Scholar]
- 8.Hubert T, Strecker G, Gmyr V, Arnalsteen L, Garrigue D, Ezzouaoui R, Caiazzo R, Dezfoulian G, Averland B, Vandewalle B, Vantyghem MC, Kerr-Conte J, Pattou F: Acute insulin response to arginine in deceased donors predicts the outcome of human islet isolation. Am J Transplant 2008; 8: 872– 876 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Diagnosis and classification of diabetes mellitus (Position Statement). Diabetes Care 2007; 30( Suppl. 1): S42– S47 [DOI] [PubMed] [Google Scholar]
- 10.Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AM: β-Score: an assessment of β-cell function after islet transplantation. Diabetes Care 2005; 28: 343– 347 [DOI] [PubMed] [Google Scholar]
- 11.Caumo A, Maffi P, Nano R, Bertuzzi F, Luzi L, Secchi A, Bonifacio E, Piemonti L: Transplant estimated function: a simple index to evaluate β-cell secretion after islet transplantation. Diabetes Care 2008; 31: 301– 305 [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick ES, Rigby AS, Atkin SL: Mean blood glucose compared with HbA(1c) in the prediction of cardiovascular disease in patients with type 1 diabetes. Diabetologia 2008; 51: 365– 371 [DOI] [PubMed] [Google Scholar]
- 13.Rickels MR, Kamoun M, Kearns J, Markmann JF, Naji A: Evidence for allograft rejection in an islet transplant recipient and effect on beta-cell secretory capacity. J Clin Endocrinol Metab 2007; 92: 2410– 2414 [DOI] [PubMed] [Google Scholar]
- 14.The Worcester Human Islet Transplantation Group Autoimmunity after islet-cell allotransplantation. N Engl J Med 2006; 355: 1397– 1399 [DOI] [PubMed] [Google Scholar]
- 15.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S: High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 1995; 333: 333– 336 [DOI] [PubMed] [Google Scholar]
- 16.Terasaki PI, Ozawa M: Predictive value of HLA antibodies and serum creatinine in chronic rejection: results of a 2-year prospective trial. Transplantation 2005; 80: 1194– 1197 [DOI] [PubMed] [Google Scholar]
- 17.Szoke E, Shrayyef MZ, Messing S, Woerle HJ, van Haeften TW, Meyer C, Mitrakou A, Pimenta W, Gerich JE: Effect of aging on glucose homeostasis: accelerated deterioration of β-cell function in individuals with impaired glucose tolerance. Diabetes Care 2008; 31: 539– 543 [DOI] [PubMed] [Google Scholar]
- 18.Davalli AM, Maffi P, Socci C, Sanvito F, Freschi M, Bertuzzi F, Falqui L, Di Carlo V, Pozza G, Secchi A: Insights from a successful case of intrahepatic islet transplantation into a type 1 diabetic patient. J Clin Endocrinol Metab 2000; 85: 3847– 3852 [DOI] [PubMed] [Google Scholar]
- 19.Smith RN, Kent SC, Nagle J, Selig M, Iafrate AJ, Najafian N, Hafler DA, Auchincloss H, Orban T, Cagliero E: Pathology of an islet transplant 2 years after transplantation: evidence for a nonimmunological loss. Transplantation 2008; 86: 54– 62 [DOI] [PubMed] [Google Scholar]
- 20.Vantyghem MC, Marcelli-Tourvieille S, Fermon C, Duhamel A, Raverdy V, Arnalsteen L, Kerr-Conte J, Noel C, Fontaine P, Pattou F: Intraperitoneal insulin infusion versus islet transplantation: comparative study in patients with type 1 diabetes. Transplantation 2009; 87: 66– 71 [DOI] [PubMed] [Google Scholar]
- 21.Fung MA, Warnock GL, Ao Z, Keown P, Meloche M, Shapiro RJ, Ho S, Worsley D, Meneilly GS, Al Ghofaili K, Kozak SE, Tong SO, Trinh M, Blackburn L, Kozak RM, Fensom BA, Thompson DM: The effect of medical therapy and islet cell transplantation on diabetic nephropathy: an interim report. Transplantation 2007; 84: 17– 22 [DOI] [PubMed] [Google Scholar]
- 22.Maffi P, Bertuzzi F, De Taddeo F, Magistretti P, Nano R, Fiorina P, Caumo A, Pozzi P, Socci C, Venturini M, del Maschio A, Secchi A: Kidney function after islet transplant alone in type 1 diabetes: impact of immunosuppressive therapy on progression of diabetic nephropathy. Diabetes Care 2007; 30: 1150– 1155 [DOI] [PubMed] [Google Scholar]
- 23.Keymeulen B, Gillard P, Mathieu C, Movahedi B, Maleux G, Delvaux G, Ysebaert D, Roep B, Vandemeulebroucke E, Marichal M, In 't Veld P, Bogdani M, Hendrieckx C, Gorus F, Ling Z, van Rood J, Pipeleers D: Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci U S A 2006; 103: 17444– 17449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caiazzo R, Gmyr V, Kremer B, Hubert T, Soudan B, Lukowiak B, Vandewalle B, Vantyghem MC, Pattou F, Kerr-Conte J: Quantitative in vivo islet potency assay in normoglycemic nude mice correlates with primary graft function after clinical transplantation. Transplantation 2008; 86: 360– 363 [DOI] [PubMed] [Google Scholar]
- 25.Eich T, Eriksson O, Lundgren T: Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med 2007; 356: 2754– 2755 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.