Abstract
OBJECTIVE
Fibroblast growth factor (FGF)-21 is highly expressed in the liver and regulates hepatic glucose production and lipid metabolism in rodents. However, its role in the pathogenesis of type 2 diabetes in humans remains to be defined. The aim of this study was to quantitate circulating plasma FGF-21 levels and examine their relationship with insulin sensitivity in subjects with varying degrees of obesity and glucose tolerance.
RESEARCH DESIGN AND METHODS
Forty-one subjects (8 lean with normal glucose tolerance [NGT], 9 obese with NGT, 12 with impaired fasting glucose [IFG]/impaired glucose tolerance [IGT], and 12 type 2 diabetic subjects) received an oral glucose tolerance test (OGTT) and a hyperinsulinemic-euglycemic clamp (80 mU/m2 per min) combined with 3-[3H] glucose infusion.
RESULTS
Subjects with type 2 diabetes, subjects with IGT, and obese subjects with NGT were insulin resistant compared with lean subjects with NGT. Plasma FGF-21 levels progressively increased from 3.9 ± 0.3 ng/ml in lean subjects with NGT to 4.9 ± 0.2 in obese subjects with NGT to 5.2 ± 0.2 in subjects with IGT and to 5.3 ± 0.2 in type 2 diabetic subjects. FGF-21 levels correlated inversely with whole-body (primarily reflects muscle) insulin sensitivity (r = −0.421, P = 0.007) and directly with the hepatic insulin resistance index (r = 0.344, P = 0.034). FGF-21 levels also correlated with measures of glycemia (fasting plasma glucose [r = 0.312, P = 0.05], 2-h plasma glucose [r = 0.414, P = 0.01], and A1C [r = 0.325, P = 0.04]).
CONCLUSIONS
Plasma FGF-21 levels are increased in insulin-resistant states and correlate with hepatic and whole-body (muscle) insulin resistance. FGF-21 may play a role in pathogenesis of hepatic and whole-body insulin resistance in type 2 diabetes.
Fibroblast growth factors (FGFs) represent a group of peptides that regulate diverse biological functions, including cell differentiation, cell growth, and angiogenesis (1,2). Recently, a subfamily of FGFs that interact with nuclear receptors has been identified that plays an important role in liver, bone, and adipose tissue metabolism (3,4). This subfamily contains FGF-19, which regulates energy expenditure (5,6); FGF-23, which regulates phosphate metabolism and excretion (7); and the recently described FGF-21, which regulates glucose homeostasis (8,9).
FGF-21 is a novel protein that has been implicated in the regulation of lipid and glucose metabolism under fasting and ketotic conditions (9,10). In murine models, FGF-21 was reported to be expressed predominantly in liver (11), but its expression has also been reported in adipose tissue and pancreatic β-cells (12). In a primate model of diabetes, Kharitonenkov et al. (9) reported a reduction in plasma glucose, insulin, triglycerides, LDL cholesterol, and HDL cholesterol levels following 6 weeks of recombinant FGF-21 administration. In diet-induced obese mice, FGF-21 reversed hepatic steatosis and improved insulin sensitivity (13). In adipose tissue, FGF-21 was shown to increase glucose uptake (9). Based on these observations, FGF-21 has been proposed as a potential therapeutic agent for type 2 diabetes in humans (14). However, few studies in humans have examined the relationship between FGF-21 and glucose/lipid metabolism. Chen et al. (15) reported that patients with newly diagnosed type 2 diabetes had significantly higher plasma FGF-21 concentrations than nondiabetic control subjects, and FGF-21 negatively correlated with fasting plasma glucose. More recently, Zhang et al. (16) found that FGF-21 concentrations are elevated in obese nondiabetic individuals compared with lean healthy control subjects and that the circulating levels correlated positively with adiposity and fasting insulin and negatively with HDL cholesterol. Conversely, in patients with anorexia nervosa, plasma FGF-21 concentrations are decreased and increased following weight gain (17). In the present study, we examined the relationship between plasma FGF-21 concentrations and direct measurements of peripheral and hepatic insulin sensitivity in subjects with varying degrees of obesity and glucose tolerance.
RESEARCH DESIGN AND METHODS
Forty-one subjects participated in the study (8 lean subjects with normal glucose tolerance [NGT], 9 obese with NGT, 12 with impaired glucose tolerance (IGT), and 12 with type 2 diabetes). All subjects were in good general health based on medical history, physical examination, screening blood chemistry and hematologic tests, urinalysis, and electrocardiogram. Weight was stable in all subjects (±2 lb) for at least 3 months before the study. None of the subjects participated in any heavy exercise, and they were instructed not to engage in any vigorous exercise for at least 3 days before the study. None of the nondiabetic subjects were taking medications known to affect lipid and glucose metabolism. Subjects who had ever received insulin or thiazolidinediones were excluded. Each study volunteer received 1) an oral glucose tolerance test (OGTT) and 2) a hyperinsulinemic-euglycemic insulin clamp with 3-[3H]glucose to examine both hepatic and peripheral (primarily reflects muscle) insulin sensitivity. The purpose, nature, and potential risks of the study were explained to all subjects, and written voluntary consent was obtained before their participation. All research procedures were approved by the institutional review board of the University of Texas Health Science Center at San Antonio.
OGTT
Baseline blood samples for determination of plasma glucose, free fatty acids (FFAs), insulin, and C-peptide concentrations were drawn at −30, −15, and 0 min. At time zero, subjects ingested 75 g of glucose in 300 ml orange-flavored water, and plasma glucose, FFAs, and insulin were measured at 15-min intervals for 2 h.
Hyperinsulinemic-euglycemic clamp
All studies were conducted in the general clinical research center of the University of Texas Health Science Center at San Antonio and began at 0700 h after a 12-h overnight fast. A prime (25 μCi)-continuous (0.25 μCi/min) infusion of 3-[3H]glucose was started, and 2 h (3 h for diabetic subjects) were allowed for isotopic equilibration. In type 2 diabetes, the priming dose of tritiated glucose was increased in proportion to the increase in fasting plasma glucose concentration. At the end of the tracer equilibration period, a primed-continuous insulin infusion (80 mU/m2 per min) was started, and plasma glucose was measured every 5 min. Based on the negative feedback principle, a variable infusion of 20% glucose was adjusted to maintain plasma glucose concentration constant at each subject's fasting glucose level in the control group (18). In diabetic subjects, plasma glucose concentration was allowed to decrease to 100 mg/dl, at which level it was maintained.
Analytical determinations
Plasma glucose was measured at bedside with the glucose oxidase method (Beckman Instruments, Fullerton, CA). Plasma insulin concentration was measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA). Tritiated glucose specific activity was determined on deproteinized plasma samples as previously described (19). Plasma FFA concentration was determined by an enzymatic calorimetric quantification method (Wako Chemicals, Nuess, Germany). Plasma FGF-21 concentrations were measured by radioimmunoassay (Phoenix Pharmaceuticals, Burlingam, CA) at baseline in duplicate from plasma collected prior to start of the euglycemic insulin clamp. This assay has been reported to cross-react specifically with human FGF-21 (100%). The intra- and interassay coefficients of variation were 3.6 and 3.9%, respectively. Serum creatinine was measured with an automated enzymatic assay, and glomerular filtration rate was estimated with the Cockcroft-Gault formula (20).
Calculations
Under steady-state postabsorptive conditions, the rate of endogenous glucose appearance (Ra) was calculated as the 3-[3H]glucose infusion rate (dpm/min) divided by the steady-state plasma 3-[3H]glucose–specific activity (dpm/mg). During the euglycemic insulin clamp, the rate of whole-body glucose appearance (Ra) was calculated with Steele's equation (21), using a distribution volume of 250 ml/kg. Endogenous glucose production (EGP) was calculated by subtracting the exogenous glucose infusion rate from Ra. The rate of insulin-mediated whole-body glucose disposal (Rd) was determined by adding the rate of residual EGP to the exogenous glucose infusion rate. In the postabsorptive state, fasting plasma insulin is the primary determinant of EGP (22). The hepatic insulin resistance index was calculated as the product of EGP and fasting plasma insulin concentration (23). Similarly, since fasting insulin concentration is the most important regulator of fasting plasma FFA concentration, adipocyte insulin resistance was calculated as the product of fasting plasma FFAs and fasting plasma insulin concentration (24).
Statistical analysis
Data were expressed as means ± SE, unless otherwise specified. SPSS version 15 statistical package (Chicago, IL) was used for all calculations. Pearson's or Spearman's correlations were used to examine the relationship between plasma FGF-21 levels and markers of insulin sensitivity, as well as with anthropometric parameters. ANOVA with post hoc analysis with Bonferroni correction was used to compare significant differences between groups.
RESULTS
Study population and clinical characteristics
Type 2 diabetic subjects were slightly, but not significantly, older than subjects with NGT. BMI was similar in obese subjects with NGT, IGT, and type 2 diabetes. Type 2 diabetic individuals had significantly higher fasting plasma glucose, plasma insulin and triglycerides, and A1C compared with lean subjects; NGT (Table 1 ). Type 2 diabetes had significantly lower HDL cholesterol concentration compared with NGT. Subjects with IGT and type 2 diabetes had significantly lower whole-body (primarily muscle) glucose uptake compared with lean subjects with NGT (Table 1). Hepatic insulin resistance (EGP × fasting insulin) in obese subjects with NGT was slightly, but not significantly, elevated compared with NGT subjects. However, subjects with IFG/IGT and type 2 diabetes displayed significantly greater hepatic insulin resistance (P < 0.05). Similarly, the adipocyte insulin resistance (FFA × fasting insulin) was significantly higher in subjects with IFG/IGT and type 2 diabetes.
Table 1.
Clinical characteristics
| Lean subjects with NGT | Obese subjects with NGT | Subjects with IFG/IGT | Type 2 diabetic subjects | |
|---|---|---|---|---|
| n | 8 | 9 | 12 | 12 |
| Age (years) | 40 ± 5 | 45 ± 2 | 44 ± 3 | 54 ± 2 |
| BMI (kg/m2) | 24 ± 1 | 32 ± 2* | 31 ± 2* | 34 ± 1* |
| Systolic blood pressure (mmHg) | 119 ± 3 | 129 ± 5 | 127 ± 4 | 140 ± 4* |
| Diastolic blood pressure (mmHg) | 69 ± 3 | 77 ± 3 | 76 ± 3 | 80 ± 3 |
| A1C (%) | 5.1 ± 0.2 | 5.4 ± 0.1 | 5.5 ± 0.4 | 7.4 ± 1* |
| Fasting plasma glucose (mg/dl) | 92 ± 5 | 96 ± 3 | 105 ± 2* | 152 ± 12*† |
| 2-h glucose (mg/dl) | 98 ± 4 | 102 ± 7 | 140 ± 6* | 244 ± 23*† |
| Fasting plasma insulin (μU/ml) | 2.6 ± 0.7 | 3.8 ± 1 | 9.8 ± 2*† | 11.3 ± 2*† |
| Fasting free fatty acids (μEq/l) | 477 ± 39 | 510 ± 48* | 585 ± 36* | 639 ± 27*† |
| Triglycerides (mg/dl) | 84 ± 16 | 103 ± 25 | 133 ± 17 | 202 ± 53*† |
| Total cholesterol (mg/dl) | 173 ± 11 | 198 ± 20 | 181 ± 11 | 170 ± 7 |
| HDL cholesterol (mg/dl) | 54 ± 4 | 46 ± 4 | 43 ± 4* | 35 ± 2*† |
| LDL cholesterol (mg/dl) | 103 ± 20 | 131 ± 19 | 111 ± 10 | 96 ± 6 |
| HOMA-IR | 0.6 ± 0.4 | 0.9 ± 0.3 | 2 ± 1* | 4.5 ± 2*† |
| Rd (mg/kg · min−1) | 10.7 ± 2 | 8.6 ± 0.5* | 7.5 ± 1* | 4.7 ± 0.3*† |
| Hepatic insulin resistance index | 5.6 ± 1 | 9 ± 2.5 | 18 ± 4† | 28 ± 6*† |
| Adipocyte insulin resistance index | 1.2 ± 0.3 | 2.04 ± 0.6 | 5.4 ± 1.2*† | 7.4 ± 1.3*† |
Data are means ± SE. HOMA-IR, homeostasis model assessment of insulin resistance.
*P < 0.05 vs. lean subjects with NGT;
†P < 0.05 vs. obese subjects with NGT.
Plasma FGF-21 changes in relation to glucose tolerance
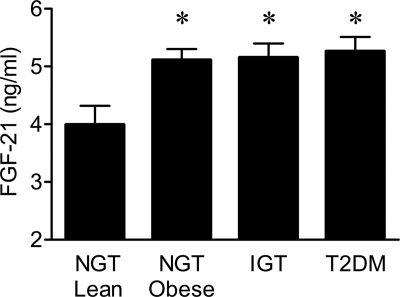
Plasma FGF-21 was higher in obese subjects with NGT versus lean subjects with NGT (4.92 ± 0.17 vs. 3.88 ± 0.30 ng/ml, P = 0.04). Subjects with IGT (5.22 ± 0.23 ng/ml, P < 0.05 vs. lean subjects with NGT) and type 2 diabetes (5.27 ± 0.23, P < 0.05 vs. lean subjects with NGT) also had increased plasma FGF-21 levels (Fig. 1). Plasma FGF-21 concentration correlated with A1C (r = 0.325, P = 0.04), fasting plasma glucose (r = 0.312, P = 0.05), and 2-h glucose (r = 0.414, P = 0.01). There was also a direct association between plasma FGF-21 and BMI (r = 0.456, P < 0.001) in the entire group. A recent report (25) demonstrated elevated plasma FGF-21 levels in patients with chronic kidney disease. We did not observe any correlation between plasma FGF-21 and either glomerular filtration rate (r = 0.089, P = NS) or serum creatinine (r = 0.277, P = 0.08).
Figure 1.
Plasma FGF-21 concentration in lean subjects with NGT and obese subjects with NGT, IGT, and type 2 diabetes (T2DM). Data are means ± SE. *P < 0.05 vs. lean NGT.
Relationship between FGF-21 and whole-body and hepatic insulin resistance
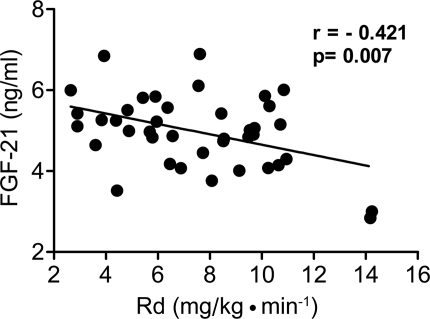
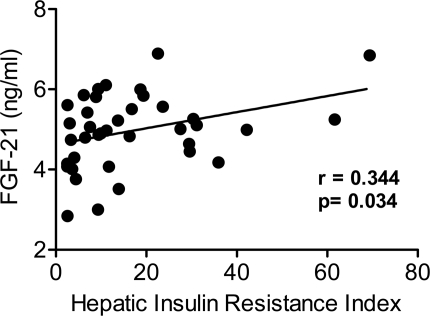
The insulin-stimulated rate of glucose disposal (Rd) correlated inversely with plasma FGF-21 concentration (−0.421, P < 0.01) (Fig. 2). A positive correlation also was observed between FGF-21 level and hepatic insulin resistance index (0.344, P = 0.034) (Fig. 3) and adipocyte insulin resistance index (0.318, P = 0.045).
Figure 2.
Correlation between plasma FGF-21 concentration and insulin-stimulated glucose disposal during the hyperinsulinemic-euglycemic clamp.
Figure 3.
Correlation between plasma FGF-21 concentration and hepatic insulin resistance index.
CONCLUSIONS
FGF-21 was discovered during a high-throughput assay for secreted proteins that increased glucose uptake in 3T3L-1 adipocytes (9). Subsequent studies (4,9,13) showed that administration of recombinant FGF-21 in rodent models of diabetes and in diabetic rhesus monkeys improved blood glucose and the lipid profile. However, in none of these studies were the plasma levels of FGF-21 compared between diabetic and nondiabetic animals.
In the present study, we demonstrate that plasma FGF-21 levels are elevated in insulin-resistant states (obesity, IGT/IFG, type 2 diabetes) and are inversely correlated with both peripheral and hepatic insulin sensitivity. This is consistent with two other reports (15,16) in humans that demonstrated elevated plasma FGF-21 concentration in obesity, IGT, and type 2 diabetes. The novelty of our study is that we demonstrate for the first time in humans that the increase in plasma FGF-21 levels are strongly correlated with the severity of whole-body (primarily reflects muscle) and hepatic insulin resistance.
Our study is in agreement with two previous studies in Asians, in which increased plasma FGF-21 levels were observed in newly diagnosed, drug-naïve diabetic subjects and in treated type 2 diabetic subjects (26). In a Chinese population, plasma FGF-21 levels correlated with markers of the insulin resistance (metabolic) syndrome (16). However, this later study did not measure either hepatic or peripheral insulin sensitivity.
In rodent models, FGF-21 stimulates glucose uptake in 3TL3 adipocytes and increases GLUT4 expression in adipocytes. Arner et al. (27) demonstrated that FGF-21 inhibits lipolysis in human adipocytes and suggested that this may contribute to the protein's insulin-sensitizing effect in humans. A synergistic interaction has been described between FGF-21 and rosiglitazone to stimulate glucose uptake (28). Contrary to these observations, in the present study plasma FGF-21 concentrations were positively correlated with adipocyte insulin resistance. With regard to the liver, in animal models FGF-21 has been shown to be expressed primarily in liver, and its glucose-lowering effects of FGF-21 have been suggested to be mediated by its actions on liver (9,11). In contrast, in the present study, we demonstrate a positive correlation between elevated FGF-21 levels and hepatic insulin resistance.
The apparently divergent results of the current study in humans and previous studies in animals could reflect a true species difference in the metabolic effects of FGF-21 in humans versus animals or may be less contradictory than they appear. Thus, the elevated plasma FGF-21 levels in insulin-resistant states may simply reflect a compensatory response to offset the peripheral and/or hepatic insulin resistance and not be a cause of the insulin resistance. Since our observations are cross-sectional in nature, it is not possible to establish a cause-and-effect relationship (i.e., what is primary and what is secondary). It also is not possible to distinguish whether the increased plasma FGF-21 levels in obese subjects and subjects with IGT/IFG and type 2 diabetes are related to insulin resistance or obesity, since all three groups had similarly elevated FGF-21 levels. Further studies will be required to further elucidate the role of FGF-21 in glucose homeostasis and whether FGF-21 will sensitize target issues (liver, adipocytes, muscle) to insulin, as has been reported in animal models of diabetes.
Recent studies have suggested that plasma FGF-21 concentrations are affected by the glomerular filtration rate and therefore may be related to the level of renal function (25). Patients undergoing dialysis have significantly increased plasma FGF-21 levels compared with control subjects, and this is independent of the glucose/lipid metabolic status (25). Although none of the participants enrolled in our study had impaired renal function, glomerular filtration rate spanned a wide range. Nonetheless, we did not find a significant relationship between plasma FGF-21 concentration and estimated glomerular filtration rate. Thus, in our sample, FGF-21 concentrations are unlikely to be affected by this parameter.
In summary, elevated plasma FGF-21 concentrations in humans appear to be related to the presence of hepatic and peripheral insulin resistance. Whether the increase in plasma FGF-21 represents a compensatory effect to offset insulin resistance or is a causative factor in the development of insulin resistance is yet to be determined.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Bottcher RT, Niehrs C: Fibroblast growth factor signaling during early vertebrate development. Endocr Rev 2005; 26: 63– 77 [DOI] [PubMed] [Google Scholar]
- 2. Huang X, Yu C, Jin C, Yang C, Xie R, Cao D, Wang F, McKeehan WL: Forced expression of hepatocyte-specific fibroblast growth factor 21 delays initiation of chemically induced hepatocarcinogenesis. Mol Carcinog 2006; 45: 934– 942 [DOI] [PubMed] [Google Scholar]
- 3. Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M: Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 2007; 27: 3417– 3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ: The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007; 148: 774– 781 [DOI] [PubMed] [Google Scholar]
- 5. Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA: Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004; 145: 2594– 2603 [DOI] [PubMed] [Google Scholar]
- 6. Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA: Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002; 143: 1741– 1747 [DOI] [PubMed] [Google Scholar]
- 7. Quarles LD: FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab 2003; 285: E1– E9 [DOI] [PubMed] [Google Scholar]
- 8. Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB: FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol 2008; 215: 1– 7 [DOI] [PubMed] [Google Scholar]
- 9. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB: FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627– 1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E: Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007; 5: 426– 437 [DOI] [PubMed] [Google Scholar]
- 11. Nishimura T, Nakatake Y, Konishi M, Itoh N: Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000; 1492: 203– 206 [DOI] [PubMed] [Google Scholar]
- 12. Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M: Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 2007; 282: 26687– 26695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM: Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009; 58: 250– 259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kharitonenkov A, Shanafelt AB: Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs 2008; 22: 37– 44 [DOI] [PubMed] [Google Scholar]
- 15. Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G: Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2008; 116: 65– 68 [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A: Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246– 1253 [DOI] [PubMed] [Google Scholar]
- 17. Dostalova I, Kavalkova P, Haluzikova D, Lacinova Z, Mraz M, Papezova H, Haluzik M: Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab 2008; 93: 3627– 3632 [DOI] [PubMed] [Google Scholar]
- 18. DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214– E223 [DOI] [PubMed] [Google Scholar]
- 19. Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA: Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus: evidence for multiple sites of insulin resistance. J Clin Invest 1989; 84: 205– 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31– 41 [DOI] [PubMed] [Google Scholar]
- 21. Steele R, Wall JS, De Bodo RC, Altszuler N: Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 1956; 187: 15– 24 [DOI] [PubMed] [Google Scholar]
- 22. DeFronzo RA, Ferrannini E: Regulation of hepatic glucose metabolism in humans. Diabetes Metab Rev 1987; 3: 415– 459 [DOI] [PubMed] [Google Scholar]
- 23. Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462– 1470 [DOI] [PubMed] [Google Scholar]
- 24. Groop LC, Bonadonna RC, Shank M, Petrides AS, DeFronzo RA: Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest 1991; 87: 83– 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stein S, Bachmann A, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M: Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care 2009; 32: 126– 128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li L, Yang G, Ning H, Yang M, Liu H, Chen W: Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Res Clin Pract 2008; 82: 209– 213 [DOI] [PubMed] [Google Scholar]
- 27. Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Ryden M: FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett 2008; 582: 1725– 1730 [DOI] [PubMed] [Google Scholar]
- 28. Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A: Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J Cell Physiol 2007; 210: 1– 6 [DOI] [PubMed] [Google Scholar]