Abstract
OBJECTIVE
To investigate changes in the foot muscle energy reserves in diabetic non-neuropathic and neuropathic patients.
RESEARCH DESIGN AND METHODS
We measured the phosphocreatinine (PCr)/inorganic phosphate (Pi) ratio, total 31P concentration, and the lipid/water ratio in the muscles in the metatarsal head region using MRI spectroscopy in healthy control subjects and non-neuropathic and neuropathic diabetic patients.
RESULTS
The PCr/Pi ratio was higher in the control subjects (3.23 ± 0.43) followed by the non-neuropathic group (2.61 ± 0.36), whereas it was lowest in the neuropathic group (0.60 ± 1.02) (P < 0.0001). There were no differences in total 31P concentration and lipid/water ratio between the control and non-neuropathic groups, but both measurements were different in the neuropathic group (P < 0.0001).
CONCLUSIONS
Resting foot muscle energy reserves are affected before the development of peripheral diabetic neuropathy and are associated with the endothelial dysfunction and inflammation.
Foot muscles undergo atrophy in the presence of diabetic neuropathy leading to prominent metatarsal heads and the development of the so-called “minor foot” (1). However, little information is available regarding the function of the foot muscles, especially in the absence of peripheral neuropathy. Magnetic resonance imaging (MRI) measurements of phosphorus (31P) metabolites can evaluate the muscle energy reserves and provide valuable information regarding the muscle function (2). Phosphocreatinine (PCr) is the energy reservoir that maintains the concentration of adenosine triphosphate (ATP) at levels required for cellular function and maintenance, whereas inorganic phosphate (Pi) is a metabolite produced by the hydrolysis of ATP to adenosine diphosphate ADP (3). In the situation when the oxygen supply to the muscles is insufficient, such as intensive exercise in healthy subjects or tissue ischemia, the PCr/Pi ratio decreases. In the present study, we examined the effect of diabetes and peripheral neuropathy in the foot muscle energy reserves by using a clinical 3-Tesla (3T) MR scanner with multinuclear imaging capability and the MRI technique known as rapid acquisition with relaxation enhancement (RARE), which allows the quantification of the 31P cellular metabolites (4). We have also investigated the association of these changes to changes in the endothelial function and levels of serum cytokines that related to the development of inflammation and vascular disease.
RESEARCH DESIGN AND METHODS
We studied 64 subjects divided into three groups matched for age and sex: 24 healthy nondiabetic subjects, 22 diabetic subjects with no clinical peripheral neuropathy, and 18 neuropathic diabetic subjects. The study protocol was approved by our institutional review board. All participants gave written informed consent.
Procedures
Presence of diabetic peripheral neuropathy was determined according to standard clinical techniques as previously described (5). Endothelium-dependent vasodilation in the macro- and microcirculation was measured as previously described (6).
MRI measurements of phosphorus (31P) metabolites
Phosphorus-31 MRI data were acquired on a GE 3T whole-body MR scanner (General Electric Medical Systems, Milwaukee, WI). A transaxial imaging plane was prescribed through the metatarsal head region of the foot. Separate images of PCr and Pi cellular metabolites were acquired using a rapid acquisition with relaxation enhancement (RARE) pulse sequence with chemical selectivity capabilities (7). The PCr and Pi images were acquired simultaneously in a single 6-min scan. After the 31P imaging, standard T2-weighted (T2-W) proton (1H) spin-echo MRI was performed to acquire detailed images of the anatomy. The scan time for the T2-W 1H images was 6 min and 24 s. The spatial resolution of the PCr and Pi images was 0.47 × 0.47 × 2.5 cm (voxel volume = 0.55 cm3) and the T2-W images 0.06 × 0.06 × 0.25 cm (voxel volume = 0.0009 cm3).
For the measurements of the intramuscular lipid and water content, single-voxel 1H spectra were acquired using the point resolved spectral selection (PRESS) technique without water suppression. For each subject, the PCr and Pi concentrations in the metatarsal head region were calculated as previously described (8). A PCr/Pi map was generated by dividing the PCr image by the Pi image. The resulting PCr/Pi map was registered to the T2-W anatomical images to identify the muscle beds that had abnormal PCr/Pi ratios.
Statistical analysis
Statistical analysis was performed in collaboration with a biostatistician (C.G.). For parametrically distributed data, the ANOVA test was used. The contribution of cytokines and growth factors in the variation of MRI measurements was assessed by univariate and multivariate stepwise regression analysis.
RESULTS
The clinical characteristic of the three studied groups are shown in Table A1 in the online appendix (available at http://care.diabetesjournals.org/cgi/content/full/dc09-0536/DC1). The results of biochemical measurements are shown in Table A2 in the online appendix. MRI T1-weighted and PCr/Pi images from a healthy control subject, a diabetic non-neuropathic patient, and a neuropathic patient are shown in the figure in the online appendix.
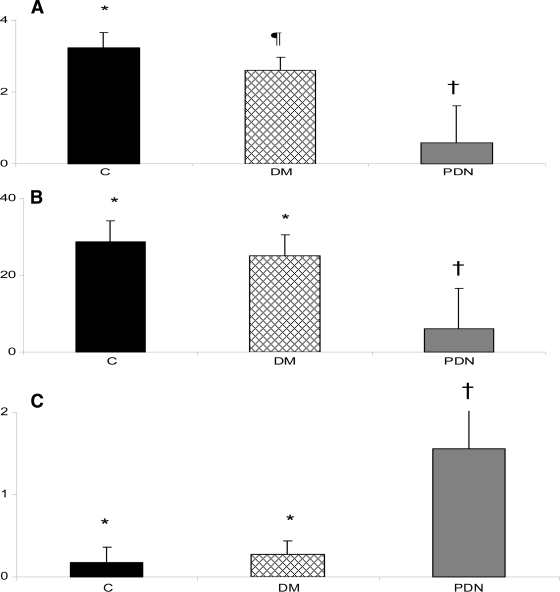
The results of the PCr/Pi ratio are shown in Fig. 1 A. There were significant differences among all groups, being higher in the control subjects (3.23 ± 0.43) followed by the non-neuropathic group (2.61 ± 0.36), whereas the PCr/Pi ratio was lowest in the neuropathic group (0.60 ± 1.02) (P < 0.0001). The results of the total 31P concentration are shown in Fig. 1B. There were no differences between the control (28.7 ± 5.4) and non-neuropathic groups (25.0 ± 5.5), but the neuropathic group had a lower concentration (6.0 ± 10.5) (P < 0.0001). The lipid/water ratio was also similar in the control (0.18 ± 0.11) and the non-neuropathic groups (0.27 ± 0.17) and higher in the neuropathic group (1.56 ± 0.52) (P < 0.0001) (Fig. 1C).
Figure 1.
A: Results of the PCr/Pi ratio. Significant differences were found among all groups. The PCr/Pi was significantly higher in the control group (C) followed by the non-neuropathic group (DM) and was lowest in the neuropathic group (PDN) (* vs. ¶ vs. †: P < 0.0001). B: Results of the total 31P concentration. The 31P concentration was similar in the control and the non-neuropathic group and lower in the neuropathic group (* vs. †: P < 0.0001). C: Results of the foot lipid/water ratio. The ratio was similar in the control and the non-neuropathic group and lower in the neuropathic group (* vs. †: P < 0.0001).
When all subjects were considered as one group, significant correlations were found between PCr/Pi ratio and flow-mediated dilation (FMD) (r = 0.51, P < 0.0001), neuropathy disability score (NDS) (r = −0.75, P < 0.0001), vibration perception threshold (r = −0.72, P < 0.0001), Semmes Weinstein monofilaments (r = −0.83, P < 0.0001), granulocyte colony–stimulating factor (r = −0.29, P < 0.05), platelet-derived growth factor AA (r = 0.29, P < 0.05), tumor necrosis factor-α (r = −0.30, P < 0.05), sE-selectin (r = −0.35, P < 0.01), soluble intracellular adhesion molecule (r = −0.36, P < 0.01), and soluble vascular cell adhesion molecule (sVCAM) (r = −0.40, P < 0.01). Borderline significances were found in the correlations between PCr/Pi ratio and the skin blood flow at the dorsum of the foot (r = −0.25, P = 0.059) and vascular endothelial growth factor (r = −0.26, P = 0.054). On stepwise multivariate analysis, NDS, FMD, and sE-selectin contributed 59, 6, and 5%, respectively, to the variation of the PCr/Pi ratio. The total 31P concentration correlated with FMD (r = −0.41, P < 0.001), NDS (r = −0.73, P < 0.0001), vibration perception threshold (r = −0.68, P < 0.0001), Semmes Weinstein monofilaments (r = −0.81, P < 0.0001), tumor necrosis factor-α (r = −0.30, P < 0.05), and soluble vascular cell adhesion molecule (r = −0.38, P < 0.05). On stepwise multivariate analysis, NDS, sVCAM, and FMD contributed 56, 4, and 2%, respectively, to the variation of the total 31P concentration.
To exclude neuropathy as a confounding factor, we performed the same analysis as above in a separate group that included the control and non-neuropathic subjects but excluded the diabetic neuropathic patients. In this analysis, the PCr/Pi ratio correlated with FMD (r = 0.42, P < 0.01) and vascular endothelial growth factor (r = −0.35, P < 0.05). The total 31P concentration inversely correlated with the skin blood flow at the dorsum of the foot (r = −0.39, P < 0.01) and vascular endothelial growth factor (r = −0.39, P < 0.05).
Seven diabetic patients (three non-neuropathic and four neuropathic, four males, mean age 55 ± 7 years) had a second MRI spectroscopy test after an average 23-month period from the first one. There was a significant decline in the PCr/Pi ratio (−0.18 ± 0.18, P < 0.05) but no difference in the total 31P concentration levels (−1.1 ± 2.32, NS).
CONCLUSIONS
The main findings of the present study are that there are significant differences in the muscle energy reserves among all three studied groups. Thus, the PCr/Pi ratio was highest in the control group, followed by the non-neuropathic group, and lowest in the neuropathic group. In addition, the total 31P concentration, an indication of the muscle volume, was decreased in the neuropathic group but was comparable in the control and non-neuropathic groups. Similarly, the lipid/water ratio, an indication of muscle atrophy, was increased in the neuropathic group, whereas no differences existed between the control and non-neuropathic groups. Finally, muscle changes were associated with changes in the nerve function, endothelial function of the large vessels, and serum cytokines.
There are limited data available regarding the effects of diabetes and peripheral neuropathy on muscle energy reserves. Previous studies have shown reduced energy reserves in diabetic patients at skeletal muscles during resting and exercise and the myocardium (8–11). The results of the present study indicate that compared with healthy control subjects, there is a stepwise reduction in the foot muscle energy reserves in diabetic patients without peripheral neuropathy and a more severe reduction in diabetic patients with clinical neuropathy. The main reason for identifying this difference is the technology we used, which has a higher spatial resolution than previous methods, resulting in more accurate measurements.
Our findings also suggest that diabetes and the associated endothelial dysfunction and inflammation have an adverse effect on the foot muscle energy reserves even before the development of clinical neuropathy. However, the development of neuropathy has a much higher impact and leads to a considerable deterioration of these abnormalities. Further studies will be required to examine the effect of diabetes in muscles that are not affected so severely by peripheral neuropathy, such as the forearm or leg muscles.
In the present study, we have also examined the changes in MRI spectroscopic measurements over a 23-month period on average. It is of interest that we noted a deterioration of the PCr/Pi ratio but no changes in the total 31P concentration. These results indicate that functional changes can progress even in the absence of atrophy progression over a 23-month period. Confirmation of these finding by future studies may lead to the use of these MRI spectroscopic measurements as surrogate end points that will evaluate the effect of new therapeutic approaches on the muscle function of diabetic patients with or without peripheral neuropathy and/or ischemia.
Supplementary Material
Acknowledgments
This work was partly supported by National Institutes of Health Grants R01-HL075678 and R01 DK076937 to A.V.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG: A prospective study of risk factors for diabetic foot ulcer: the Seattle Diabetic Foot Study. Diabetes Care 1999; 22: 1036– 1042 [DOI] [PubMed] [Google Scholar]
- 2. Chao H, Bowers JL, Holtzman D, Mulkern RV: RARE imaging of PCr in human forearm muscles. J Magn Reson Imaging 1997; 7: 1048– 1055 [DOI] [PubMed] [Google Scholar]
- 3. Greenman RL, Elliott MA, Vandenborne K, Schnall MD, Lenkinski RE: Fast imaging of phosphocreatine using a RARE pulse sequence. Magn Reson Med 1998; 39: 851– 854 [DOI] [PubMed] [Google Scholar]
- 4. Greenman RL: Quantification of the 31P metabolite concentration in human skeletal muscle from RARE signal intensity. Magn Reson Med 2004; 52: 1036– 1042 [DOI] [PubMed] [Google Scholar]
- 5. Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A: Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000; 23: 606– 611 [DOI] [PubMed] [Google Scholar]
- 6. Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A: Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 1999; 48: 1856– 1862 [DOI] [PubMed] [Google Scholar]
- 7. Greenman R, Axel L, Ferrari V, Lenkinski R: Fast imaging of phosphocreatine in the normal human myocardium using a three-dimensional RARE pulse sequence at 4 Tesla. J Magn Reson Imaging 2002; 15: 467– 472 [DOI] [PubMed] [Google Scholar]
- 8. Greenman RL, Panasyuk S, Wang X, Lyons TE, Dinh T, Longoria L, Giurini JM, Freeman J, Khaodhiar L, Veves A: Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet 2005; 366: 1711– 1717 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki E, Kashiwag A, Hidaka H, Maegawa H, Nishio Y, Kojima H, Haneda M, Yasuda H, Morikawa S, Inubushi T, Kikkawa R: 1H and 31P-magnetic resonance spectroscopy and imaging as a new diagnostic tool to evaluate neuropathic foot ulcers in type II diabetes. Diabetologia 2000; 43: 165– 172 [DOI] [PubMed] [Google Scholar]
- 10. Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K: Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003; 107: 3040– 3046 [DOI] [PubMed] [Google Scholar]
- 11. Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK: Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol 2003; 42: 328– 335 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.