Abstract
OBJECTIVE
Older patients with type 2 diabetes are at a particularly high risk for severe hypoglycemic episodes, and experimental studies in healthy subjects hint at a reduced awareness of hypoglycemia in aged humans. However, subjective responses to hypoglycemia have rarely been assessed in older type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
We tested hormonal, subjective, and cognitive responses (reaction time) to 30-min steady-state hypoglycemia at a level of 2.8 mmol/l in 13 older (≥65 years) and 13 middle-aged (39–64 years) type 2 diabetic patients.
RESULTS
Hormonal counterregulatory responses to hypoglycemia did not differ between older and middle-aged patients. In contrast, middle-aged patients showed a pronounced increase in autonomic and neuroglycopenic symptom scores at the end of the hypoglycemic plateau that was not observed in older patients (both P < 0.01). Also, seven middle-aged patients, but only one older participant, correctly estimated their blood glucose concentration to be <3.3 mmol/l during hypoglycemia (P = 0.011). A profound prolongation of reaction times induced by hypoglycemia in both groups persisted even after 30 min of subsequent euglycemia.
CONCLUSIONS
Our data indicate marked subjective unawareness of hypoglycemia in older type 2 diabetic patients that does not depend on altered neuroendocrine counterregulation and may contribute to the increased probability of severe hypoglycemia frequently reported in these patients. The joint occurrence of hypoglycemia unawareness and deteriorated cognitive function is a critical factor to be carefully considered in the treatment of older patients.
Hypoglycemia is the limiting factor in the glycemic management of diabetes (1). For a long time hypoglycemia was assumed a major problem only in patients suffering from type 1 diabetes (2); however, there is increasing evidence that hypoglycemic episodes are a critical factor also in type 2 diabetes (3,4). Older subjects aged >65 years, who represent the majority of type 2 diabetic patients, appear at a particularly high risk of experiencing severe hypoglycemia (3,4). Previous studies (5–7) have shown weakened perception of hypoglycemia-related symptoms in healthy older (i.e., nondiabetic older subjects, aged 65–80 years) as compared with younger subjects (aged 24–49 years). Of note, in aged humans, the perception of hypoglycemic symptoms was found to simultaneously occur with the impairment of cognitive functions during a stepwise reduction of blood glucose levels (7), contrasting the well-known hierarchical succession of central nervous responses to hypoglycemia in younger healthy adults who normally perceive hypoglycemic symptoms at higher glucose levels than cognitive dysfunction (4). The concurrence of glycemic thresholds for the onset of symptoms and of cognitive dysfunction may be expected to increase the risk for severe hypoglycemic episodes since it likely prevents behavioral counteractions (e.g., the intake of carbohydrates) (3).
To date only one study (8) has assessed subjective responses to standardized hypoglycemia in older type 2 diabetic patients (aged 72 ± 1 years), revealing an impairment in the perception of hypoglycemic symptoms that was comparable to that of age-matched healthy control subjects. Although this finding points to a decrease in hypoglycemia awareness that develops in the course of aging also in type 2 diabetic patients, this assumption has not yet been experimentally elucidated. Moreover, in the previous studies in healthy subjects (5–7), the age gap between experimental groups was rather large, raising the question as to the perception of hypoglycemia in middle-aged subjects. On this background, we examined whether older (aged ≥65 years) as compared with middle-aged (aged 39–64 years) type 2 diabetic patients differ in their subjective response to hypoglycemia and how hypoglycemia awareness in these age-groups relates to hormonal and cognitive effects of hypoglycemia.
RESEARCH DESIGN AND METHODS
We examined 13 older (aged ≥65 years) and 13 middle-aged (aged 39–64 years) type 2 diabetic patients matched for BMI, A1C, and sex in a single-step hypoglycemic clamp experiment (Table 1 for subjects' characteristics). While type 2 diabetes therapy was comparable between groups, the older patients, as expected, displayed a longer disease duration than the middle-aged subjects. However, none of the patients displayed any clinical evidence of diabetes complications, such as neuropathy, overt nephropathy (macroproteinuria), coronary heart disease, or a history of stroke. Also, none of the patients had experienced a severe hypoglycemic episode that required help from another person during the last year before the experiments. All patients gave written informed consent, and the study was approved by the local ethics committee.
Table 1.
Clinical characteristics of the study population
| Middle-aged patients | Older patients | P | |
|---|---|---|---|
| n | 13 | 13 | |
| Sex (female/male) | 6/7 | 5/8 | 0.69 |
| Age (years) | 51 ± 2 | 70 ± 1 | <0.001 |
| Diabetes duration (years) | 5 ± 1 | 12 ± 2 | 0.008 |
| A1C (%) | 7.4 ± 4 | 7.4 ± 2 | 0.97 |
| BMI (kg/m2) | 27 ± 1 | 27 ± 1 | 1.00 |
| Diabetes therapy | |||
| Diet alone | 3 | 2 | 0.62 |
| Metformin | 7 | 9 | 0.42 |
| Sulfonurea | 2 | 3 | 0.62 |
| Insulin | 6 | 7 | 0.70 |
| Insulin dose (units · kg−1 · day−1) | 0.20 ± 0.07 | 0.26 ± 0.07 | 0.92 |
Data are means ± SE and prevalences. P values derive from χ2 or Student's t test.
On the day of the experiment, patients reported to the medical research unit at 0730 h. The experiment took place in a sound-attenuated room with patients sitting on a bed with their trunk in an almost upright position (∼60°) and their legs in a horizontal position. For blood sampling, a cannula was inserted into a vein on the back of a hand that was placed in a heated box (50–55°C) to obtain arterialized venous blood. A second cannula was inserted into an antecubital vein of the contralateral arm. Both cannulae were connected to long, thin tubes that enabled blood sampling and adjustment of the rate of dextrose infusion from an adjacent room without being noticed by the subject. After a 30-min baseline period starting at 0800 h, a bolus of 0.08 IU human insulin per kg body wt (Insuman Rapid; Aventis, Strasbourg, France) was administered over 4 min. Thereafter, insulin was infused at a constant rate of 2.5 mU per kg body wt per min. Blood glucose concentration was measured every 5 min and was allowed to fall to a level of 2.8 mmol/l, where it was maintained for the next 30 min by appropriately adjusted infusion of 20% dextrose solution. Immediately after the 30-min hypoglycemic plateau, the insulin infusion was stopped and blood glucose levels were normalized by increasing the rate of dextrose infusion. Blood samples were drawn once during the baseline period (i.e., before the clamp) and every 15 min during the 30-min hypoglycemic plateau.
During the baseline period, at the beginning and end of the 30-min hypoglycemic plateau and 30 min thereafter, patients filled in a semiquantitative symptom questionnaire, rating 11 symptoms (i.e., dizziness, tingling, blurred vision, difficulty to concentrate, faintness, anxiety, palpitation, hunger, sweating, irritability, and tremor) from 0 (not at all) to 9 (severe). In accordance with previous investigators (9), the first five symptoms were considered neuroglycopenic symptoms and the latter six were considered autonomic symptoms. Immediately after filling in the questionnaires, patients were asked to estimate their current blood glucose level. Before the symptom questionnaire, reaction time to auditory stimuli was recorded during a standard vigilance task (oddball paradigm) as a measure of cognitive function. This task required the patient to discriminate target pips (pitch: 1,200 Hz, duration: 60 ms, intensity: 64 dB SPL, probability = 0.1) from randomly interspersed frequent standard pips of lower pitch (800 Hz) and to press a button with the thumb of the dominant hand as quickly as possible whenever he/she recognized a target pip. Each task sequence contained ∼400 pips, presented with interstimulus intervals randomly varying between 1,000 and 3,000 ms.
Blood glucose concentration was measured using the glucose dehydrogenase method (HemoCue B-Glucose-Analyzer; Ängelholm, Sweden). Serum insulin, C-peptide, cortisol, and growth hormone concentrations were measured by commercial enzyme-linked immunoassays (all Immulite; DPC, Los Angeles, CA). Plasma ACTH and glucagon concentrations were also measured by immunoassays (ACTH: Immulite, DPC; glucagon: Adaltis, Montreal, Canada). Plasma epinephrine and norepinephrine were measured by standard high-performance liquid chromatography with electrochemical detection (Chromosystems, Munich, Germany). Data are reported as means ± SE. For statistical analyses, data were z transformed to achieve normal distribution whenever necessary. Statistical analysis was generally based on ANOVA, including the repeated-measure factor “hypo” for effects of hypoglycemia and the between-subject factor “age” for the older and middle-aged patient groups. For pairwise comparisons, unpaired Student's t tests and χ2 tests were used. A P value <0.05 was considered significant.
RESULTS
Baseline blood glucose levels did not differ between groups (7.2 ± 0.6 vs. 7.1 ± 0.4 mmol/l; P = 0.83). The hypoglycemic plateau was reached on average 39.2 ± 5.7 min after starting the insulin infusion in the middle-aged patients and 43.8 ± 4.5 min after in the older patients (P = 0.53). During steady-state hypoglycemia, blood glucose levels were comparable between the two groups (2.7 ± 0.03 vs. 2.8 ± 0.02 mmol/l; P = 0.71), as were levels during the recovery period (P = 0.25). There were also no group differences in baseline concentrations of insulin (middle-aged 113 ± 28 vs. older 304 ± 209 pmol/l; P = 0.38) and C-peptide (middle-aged 0.62 ± 0.07 vs. older 0.51 ± 0.06 nmol/l; P = 0.27). During the hypoglycemic clamp, serum insulin levels were on average 2,159 ± 160 pmol/l in the middle-aged and 1,812 ± 215 pmol/l in the older patients (P = 0.20). In response to hypoglycemia, serum C-peptide levels decreased to comparable nadir levels in both groups (0.27 ± 0.02 vs. 0.28 ± 0.04 nmol/l; P = 0.76).
Levels of counterregulatory hormones at baseline and at the end of the hypoglycemic clamp are provided in Table 2. ANOVA indicated a significant increase in epinephrine (P = 0.002 for the hypo main effect), norepinephrine (P < 0.001), ACTH (P = 0.048), cortisol (P = 0.008), and growth hormone (P = 0.002) during hypoglycemia, but there were no difference in these increases between the two patient groups (all P > 0.18 for the respective group × hypo interaction terms). Glucagon levels did not significantly change during the clamp (P = 0.07) nor did they show any difference between groups (P = 0.57).
Table 2.
Counterregulatory hormone levels at baseline and at the end of the hypoglycemic clamp
| Middle-aged patients | Older patients | P | |
|---|---|---|---|
| n | 13 | 13 | |
| Epinephrine (pmol/l) | |||
| Baseline | 233 ± 62 | 191 ± 47 | 0.59 |
| Hypoglycemia | 874 ± 176 | 786 ± 313 | 0.81 |
| Norepinephrine (μmol/l) | |||
| Baseline | 2,177 ± 324 | 2,021 ± 206 | 0.69 |
| Hypoglycemia | 2,504 ± 305 | 2,563 ± 250 | 0.88 |
| ACTH (pmol/l) | |||
| Baseline | 4.99 ± 0.890 | 4.57 ± 0.643 | 0.70 |
| Hypoglycemia | 12.72 ± 3.217 | 7.10 ± 1.920 | 0.15 |
| Cortisol (nmol/l) | |||
| Baseline | 387 ± 41 | 426 ± 35 | 0.47 |
| Hypoglycemia | 548 ± 58 | 476 ± 44 | 0.33 |
| Growth hormone (pmol/l) | |||
| Baseline | 26.9 ± 11.6 | 50.4 ± 25.2 | 0.41 |
| Hypoglycemia | 250.4 ± 59.5 | 245.6 ± 135.7 | 0.98 |
| Glucagon (pmol/l) | |||
| Baseline | 49.9 ± 9.3 | 38.2 ± 4.1 | 0.26 |
| Hypoglycemia | 41.3 ± 10.5 | 31.1 ± 2.6 | 0.36 |
Data are means ± SE. P values derive from Student's t test.
At baseline, scores of self-rated autonomic (3.1 ± 1.1 vs. 1.8 ± 0.8; P = 0.36) and neuroglycopenic (0.8 ± 0.5 vs. 0.7 ± 0.6; P = 0.67) symptoms did not differ between the middle-aged and older patients. Likewise, at the beginning of the hypoglycemic plateau, symptom ratings were comparable between middle-aged and older patients (autonomic symptoms, 3.2 ± 1.4 vs. 1.9 ± 0.9; P = 0.42; neuroglycopenic symptoms, 2.0 ± 1.2 vs. 1.5 ± 1.0; P = 0.61), remaining essentially unchanged in comparison to baseline scores (autonomic symptoms P > 0.82; neuroglycopenic symptoms P > 0.11, for both groups). However, at the end of the hypoglycemic interval, scores of autonomic and neuroglycopenic symptoms markedly increased in middle-aged patients, whereas symptom scores in the older patients remained almost at baseline level (P = 0.009 and P = 0.007 for the respective group × hypo interaction terms) (Fig. 1). Also, at the end of the hypoglycemic clamp, 7 of 13 middle-aged patients, but only 1 of 13 older patients, correctly estimated their blood glucose level to be <3.3 mmol/l (P = 0.011).
Figure 1.
Means ± SE scores of self-rated autonomic (A) and neuroglycopenic (B) symptoms during the baseline period, at the beginning and end of the 30-min hypoglycemic plateau (indicated by gray shade), and 30 min after restoration of euglycemia in 13 middle-aged (39–64 years) (□) and 13 older (≥65 years) (■) diabetic patients. *P < 0.05; **P < 0.01.
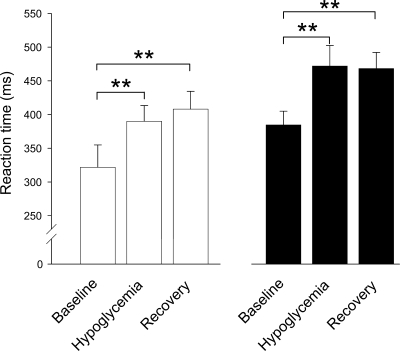
Older patients overall tended to show longer reaction time than middle-aged patients (P = 0.06 for the group main effect) (Fig. 2). The prolongation of reaction time induced by hypoglycemia (P < 0.001 for the hypo main effect) did not differ between the two patient groups (P = 0.26 for the group × hypo interaction term). Of note, reaction time remained prolonged in both groups after euglycemia had been reestablished for 30 min (57 ± 19 ms in middle-aged and 82 ± 23 in older patients vs. respective baseline values; P = 0.012 and P = 0.003, respectively).
Figure 2.
Means ± SE reaction time during an auditory vigilance task at baseline, during hypoglycemia, and after restoration of euglycemia in 13 middle-aged (39–64 years) (□) and 13 older (≥65 years) (■) diabetic patients. **P < 0.01.
CONCLUSIONS
Our data indicate that type 2 diabetic patients aged ≥65 years in contrast to middle-aged patients fail to perceive neuroglycopenic and autonomic hypoglycemic symptoms even in the presence of a comparable prolongation of reaction time induced by hypoglycemia. The age-related impairment of hypoglycemia awareness was found not to depend on alterations in neuroendocrine counterregulation because hormonal responses to hypoglycemia were similar in both age-groups. Also, the present study excludes a contribution of the quality of glycemic control as reflected by A1C levels and of diabetes medication to hypoglycemia unawareness because the two groups were comparable regarding these variables.
The markedly longer diabetes duration in the older compared with the middle-aged group may have biased our results and, in principle, may represent the critical factor determining hypoglycemia unawareness in older type 2 diabetic patients. To clarify this issue, further studies are necessary that should match type 2 diabetic patients for disease duration rather than for age. Still, from the clinical point of view, this issue appears of minor relevance because age and disease duration are highly correlated in the majority of older type 2 diabetic patients. Theoretically, asymptomatic nocturnal hypoglycemic episodes occurring in the night before the experiment, which were not systematically controlled for in our study, could have influenced our results. However, it appears rather unlikely that a possible emergence of nocturnal hypoglycemia selectively affected one of the patient groups, both of which were comparable regarding A1C levels and medication.
The mechanisms underlying the severe impairment of hypoglycemia awareness in our older patients cannot be derived from our data. Given that the hormonal responses were pronounced and, importantly, equally strong in both age-groups, a mediation by neuroendocrine counteregulatory failure as suggested by previous studies (6) can be excluded. Rather, it might be speculated that the aged brain displays a diminished capability of perceiving physiological and cognitive alterations due to hypoglycemia. This assumption is buttressed by our finding that older patients, while being completely unaware of the hypoglycemic state, show a marked prolongation of reaction time similar to that found in middle-aged patients. In both groups, reaction time was still prolonged 30 min after restoration of euglycemia (i.e., when self-rated symptoms in the middle-aged group had already returned to baseline levels). Considering that prolonged reaction time may affect everyday life (e.g., by increasing the risk of having accidents), failure to perceive respective warning symptoms during hypoglycemia is of high relevance for patients, which underlines the clinical implications of our findings, although they probably cannot be generalized to the effects of shorter hypoyglycemic episodes that may not elicit such prolonged deteriorating effects on reaction time. Also, reaction time is a single aspect of cognitive function, which further limits respective conclusions.
In summary, our results indicate distinct hypoglycemia unawareness in the presence of pronounced hypoglycemia-induced reaction time prolongation in older type 2 diabetic patients. This finding may, at least in part, explain why older patients are at a particularly high risk of suffering from severe hypoglycemic episodes. Given that the risk of hypoglycemia increases with the efficacy of glycemic control as reflected by low A1C levels (1,3), our results strongly support the view that glycemic targets for patients should be defined on an individual basis, thus taking into account factors such as age and probably also disease duration. This strategy appears to be of particular value considering that the recent results of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (10) have massively challenged the traditional “low-as-possible” dogma in diabetes care.
Acknowledgments
This study was supported in part by a grant from the Germany Diabetes Society (Deutsche Diabetes-Gesellschaft) to B.S.
No potential conflicts of interest relevant to this article were reported.
We thank Christiane Otten and Maria Baron for their expert and invaluable laboratory assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Cryer PE: Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002; 45: 937– 948 [DOI] [PubMed] [Google Scholar]
- 2. Heller SR: What we know about counterregulation in type 2 diabetes. Diabetes Nutr Metab 2002; 15: 372– 375 [PubMed] [Google Scholar]
- 3. Amiel SA, Dixon T, Mann R, Jameson K: Hypoglycaemia in type 2 diabetes. Diabet Med 2008; 25: 245– 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zammitt NN, Frier BM: Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care 2005; 28: 2948– 2961 [DOI] [PubMed] [Google Scholar]
- 5. Brierley EJ, Broughton DL, James OFW, Alberti KGMM: Reduced awareness of hypoglycaemia in the elderly despite an intact counter-regulatory response. QJMed 1995; 88: 439– 445 [PubMed] [Google Scholar]
- 6. Meneilly GS, Cheung E, Tuokko H: Altered responses to hypoglycemia of healthy elderly people. J Clin Endocrinol Metab 1994; 78: 1341– 1348 [DOI] [PubMed] [Google Scholar]
- 7. Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA: Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care 1997; 20: 135– 141 [DOI] [PubMed] [Google Scholar]
- 8. Meneilly GS, Cheung E, Tuokko H: Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes 1994; 43: 403– 410 [DOI] [PubMed] [Google Scholar]
- 9. Veneman T, Mitrakou A, Mokan M, Cryer PE, Gerich J: Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes 1993; 42: 1233– 1237 [DOI] [PubMed] [Google Scholar]
- 10. Dluhy RG, McMahon GT: Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med 2008; 358: 2630– 2633 [DOI] [PubMed] [Google Scholar]