Abstract
OBJECTIVE
To investigate whether a pragmatic structured education program with and without pedometer use is effective for promoting physical activity and improving glucose tolerance in those with impaired glucose tolerance (IGT).
RESEARCH DESIGN AND METHODS
Overweight and obese individuals with IGT were recruited from ongoing screening studies at the University Hospitals of Leicester, U.K. Participants were randomly assigned to one of three groups. Group 1 received a 3-h group-based structured education program designed to promote walking activity using personalized steps-per-day goals and pedometers. Group 2 received a 3-h group-based structured education program designed to promote walking activity using generic time-based goals. Group 3 received a brief information leaflet (control condition). Outcomes included an oral glucose tolerance test, standard anthropometric measures, ambulatory activity, and psychological variables. Follow-up was conducted at 3, 6, and 12 months.
RESULTS
A total of 87 individuals (66% male, mean age 65 years) were included in this study. At 12 months, significant decreases in 2-h postchallenge glucose and fasting glucose of −1.31 mmol/l (95% CI −2.20 to −0.43) and −0.32 mmol/l (−0.59 to −0.03), respectively, were seen in the pedometer group compared with the control group. No significant improvements in glucose control were seen in those given the standard education program.
CONCLUSIONS
This study suggests that a pragmatic structured education program that incorporates pedometer use is effective for improving glucose tolerance in those with IGT. This result is likely to have important implications for future primary care–based diabetes prevention initiatives.
Lifestyle intervention programs have successfully reduced the risk of type 2 diabetes in high-risk individuals in diverse settings (1). However, the diabetes prevention programs evaluated have tended to use resource-intensive behavior change strategies that may be difficult to implement in usual health care practice, given resource and infrastructure limitations (2,3). Furthermore, although physical inactivity is one of the most important lifestyle determinants contributing to the rising prevalence of type 2 diabetes, there is little direct evidence that previous diabetes prevention programs have been successful for promoting clinically significant increases in physical activity (4). Therefore, studies with the aim of developing and evaluating pragmatic physical activity interventions in at-risk individuals are needed.
The Prediabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) structured education program was developed in response to this need. The PREPARE program is designed to promote walking activity through pedometer use in those with impaired glucose tolerance (IGT) (5).
The primary aim of this study was to test the hypothesis that the PREPARE program is effective for improving glucose tolerance after 12 months in individuals with IGT. The secondary aim of the study was to test experimentally the role of the pedometer in promoting sustained behavior change. This information is important because the National Institute for Health and Clinical Excellence, U.K., guidance on methods to increase physical activity highlights the lack of evidence for pedometer use as an adjunct to existing methods of promoting behavior change (6).
RESEARCH DESIGN AND METHODS
Participants were recruited from ongoing population-based diabetes screening programs in Leicester, U.K., between September 2006 and March 2007; the study was completed in April 2008. Overweight or obese individuals (BMI ≥25 or ≥23 kg/m2 for South Asians) with screening-detected IGT (7) were contacted by letter and follow-up telephone call by a member of their screening team and invited to take part in the study. Individuals were recruited into the study within 12 months of their screening visit. As part of the screening programs, all individuals had their physical activity levels assessed by the short version of the international physical activity questionnaire (IPAQ) (8). Individuals who reported taking steroids were excluded.
All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. This study was approved by the Leicestershire, Northamptonshire, and Rutland National Health Service Research Ethics Committee in June 2006.
Treatment regimens and randomization
Participants were randomly assigned, using a block design, to receive either usual care, the PREPARE program, or the PREPARE program without pedometer use and were stratified by age and sex. Participant random assignment was conducted using opaque envelopes and a randomly generated number sequence by a member of our research team with no prior knowledge of recruited individuals other than their age and sex. Participants were informed of their allocated group by a member of our research team once their baseline measurements were completed.
PREPARE program
The theoretical underpinning, design, and content of the PREPARE program have been described in detail elsewhere (5). In brief, it is a single-session group-based education program. The program is 180 min long; 105 min are dedicated to addressing the causes, complications, timeline, and identity of IGT and 75 min are targeted to addressing the perceived effectiveness of exercise as a treatment for IGT, walking self-efficacy beliefs, barriers to walking, and self-regulatory strategies. The program has a written curriculum, modeled on the person-centered philosophy and learning techniques developed for the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) program (9–11), an established nationally available 6-h structured educational program designed to promote lifestyle change and self-management for those with newly diagnosed type 2 diabetes in the U.K.
As part of the program, participants were provided with a pedometer (SW-200; Yamax, Tokyo, Japan) and encouraged to set personalized steps-per-day goals based on their baseline ambulatory activity level. Sedentary participants were encouraged to increase their activity levels by at least 3,000 steps per day, equivalent to ∼30 min of walking (12). Those achieving >6,000 steps per day were encouraged to try to reach at least 9,000 steps per day, an amount that is likely to include 30 min of walking activity in addition to usual daily activity (12). Those achieving >9,000 steps per day were encouraged to at least maintain their current activity levels and were informed that health benefits could be achieved by increasing their activity levels further. Goal attainment was encouraged through the use of proximal objectives, such as increasing ambulatory activity by 500 steps per day every 2 weeks. Participants were enabled to set an action plan detailing where, when, and how their first proximal goal would be reached and encouraged to repeat this process for each new proximal goal. They were also encouraged to wear their pedometer on a daily basis and to self-monitor their ambulatory activity using a steps-per-day log.
PREPARE program without pedometer use
This group received the PREPARE program, but instead of receiving pedometers, participants were encouraged to set time-based goals designed to match the advice given to the pedometer group. Sedentary individuals were encouraged to try to achieve at least 30 min of moderate-intensity physical activity per day. Those already achieving 30 min of moderate-intensity physical activity were encouraged to at least maintain their current activity levels and were informed that health benefits could be achieved by increasing their activity levels further. As with the pedometer group, participants were encouraged to set proximal goals, such as increasing moderate-intensity activity by 5 min/day every 2 weeks, form action plans, and record their daily activity levels. Individuals wishing to set vigorous-intensity activity goals were advised that they should consult their general practitioner before commencing the program.
Intervention delivery and follow-up
Both versions of the PREPARE program were delivered by two educators trained through the DESMOND program. Programs were delivered at the Diabetes Research Unit, Leicester Royal Infirmary, U.K., within 1 month of baseline measurements. Individuals in both intervention groups also received a brief (10-min) review of progress during their 3- and 6-month clinical measurement session delivered by the same educators who had delivered the initial educational program.
Usual care
Participants randomly assigned to the control group were sent a brief information sheet in the mail, detailing the likely causes, consequences, symptoms, and timeline associated with IGT, along with information about how physical activity can be used to treat/control the condition.
Measures
All outcomes were measured at baseline and at 3, 6, and 12 months; 2-h glucose was the primary outcome, and all other outcomes were secondary.
Biochemical analysis
At their baseline appointments, participants underwent an oral glucose tolerance test (fasting and 2-h glucose). Participants arrived at their appointment after a 12-h fast and 24 h of avoiding vigorous exercise. Those with a diagnosis type 2 diabetes at baseline were excluded from the study (7). All biochemical analyses were conducted blinded to treatment group.
Plasma glucose was measured using a glucose oxidase method on the Beckman AutoAnalyzer (Beckman, High Wycombe, U.K.). Serum cholesterol was analyzed using a cholesterol enzymatic assay (Abbott Clinical Chemistry, Chicago, IL). HDL cholesterol was analyzed using the ultra-HDL assay (Abbott Clinical Chemistry). Serum triglyceride was analyzed using the triglyceride glycerol phosphate oxidase assay (Abbott Clinical Chemistry). Biochemical measurements were performed in the same laboratory located within Leicester Royal Infirmary using stable methodology standardized to external quality assurance reference values.
Physical activity
Physical activity was measured objectively by pedometer and subjectively by questionnaire. Sealed piezoelectric pedometers with a 7-day memory (NL-800; NEW-LIFESTYLES, Lee's Summit, MO) were used to measure ambulatory activity. These pedometers were different from the motivational instruments used in the primary intervention condition and have been shown to be more accurate than traditional spring-levered pedometers in overweight and obese individuals (13). For the purposes of this study at least 3 valid days of data were required; a valid day constituted at least 12 h of wear time.
Physical activity was also measured using the long last-7-days self-administered format of the IPAQ. This questionnaire provides a measure of walking and other moderate- to vigorous-intensity activities carried out for ≥10 continuous minutes at work, in the home, as transport, and during leisure time. The IPAQ has been shown to have reasonable validity compared with accelerometer data (ρ ∼0.4) and test-retest reliability (ρ ∼0.7) in the U.K. (8).
Psychological analysis
Perceptions and perceived knowledge of IGT.
Perceptions and perceived knowledge of IGT were measured with the validated brief illness perceptions questionnaire (14). This instrument was used to measure five cognitive illness representations (consequences, timeline, personal control, treatment, and symptom load attributed to IGT), two emotional representations (concern and negative emotion affect attributed to having IGT), and perceived knowledge of IGT. Each item was answered using an 11-point Likert scale.
Self-efficacy.
Participants' confidence in their ability to exercise in the face of five commonly identified barriers (tired, bad mood, bad weather, lack of time, and holiday) was measured using an 11-point Likert scale (15).
Demographic and anthropometric data
Arterial blood pressure was measured in the sitting position (Omron Healthcare, Henfield, U.K.); three measurements were obtained and the average of the last two measurements was used. Body weight (Tanita TBE 611 scale; Tanita, West Drayton, U.K.), waist circumference (midpoint between the lower costal margin and iliac crest), and height were also measured to the nearest 0.1 kg and 0.5 cm, respectively. Information on current smoking status, medication history, and ethnicity were obtained by self-report.
Data analysis
Based on a power of 80%, a significance of 0.05, and a SD of 1 mmol/l and allowing for a 50% dropout rate (noncompleters and those diagnosed with type 2 diabetes at baseline), 34 participants were required per group to detect a 1 mmol/l difference in 2-h glucose levels between each intervention group and control group at 12 months. Those not attending the final 12-month follow-up measurement session and those diagnosed with type 2 diabetes at baseline were excluded from the analysis. Those diagnosed with type 2 diabetes at 3 and 6 months (n = 4) were treated according to the screening studies from which they were recruited and had subsequent follow-up data imputed using their last observation carried forward. To have numeric parity across time points, missing biochemical, pedometer, and anthropometric data at 3 months (n = 2) and 6 months (n = 2), resulting from those not attending all intermediary follow-up sessions, were imputed using the next observation carried backward. All individuals included in the analysis were analyzed in the group to which they were assigned.
Between-group comparisons of change in measured outcomes at 3, 6, and 12 months were conducted using ANCOVA procedures; baseline data were included as a covariate. Each intervention group was compared with the control group using simple a priori contrasts; because this study had one primary hypothesis, adjustment was not made for multiple group, time, or outcome comparisons. Nonetheless, secondary outcomes were interpreted with caution and in relation to the overall pattern of results. All variables were checked for normality using the Kolmogorov-Smirnov test and visual inspection after the removal of extreme outliers (a value at least 4 SD from the mean). Tests were two-sided; P < 0.05 was considered significant. All analysis was carried out with SPSS 14.0 for Windows (SPSS, Chicago, IL).
RESULTS
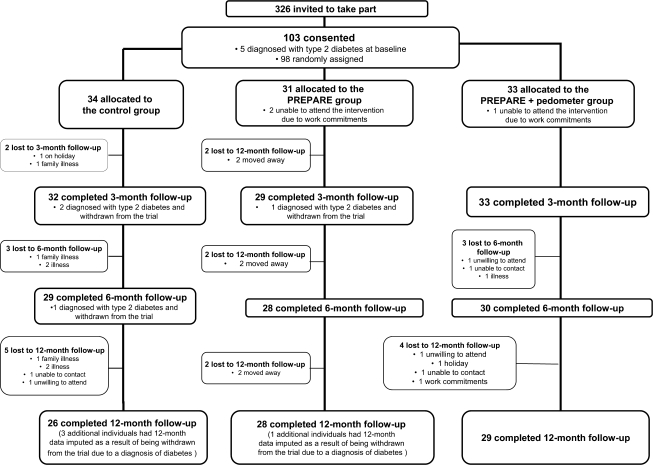
The trial profile is shown in Fig. 1. Of 326 individuals invited to take part in the study, 103 (32%) consented. Those who consented to take part had similar levels of self-reported walking and overall physical activity and were of an age and ethnicity similar to those who declined the invitation; however, more men than women agreed to take part (63% of study participants were male compared with 55% of those invited to take part; P = 0.03). Five individuals were excluded from the study at baseline because of a diagnosis of type 2 diabetes. Over the course of the trial diabetes was diagnosed in three individuals in the control group and one individual in the education group without pedometer use; no cases of diabetes were observed in those given the pedometer version of the PREPARE program. Eleven participants were lost to 12-month follow-up. There were no significant demographic, biochemical, or lifestyle differences at baseline between attendees and nonattendees at 12 months.
Figure 1.
PREPARE program study profile.
Baseline demographic, anthropometric, biochemical, and lifestyle characteristics of the study participants are shown in Table 1; the majority of participants were inactive based on pedometer counts (12). There was no significant difference between groups in any of the measured variables at baseline; however, levels of self-reported physical activity were substantially, although not significantly, higher in the pedometer group.
Table 1.
Clinical, lifestyle, and demographic characteristics of study participants overall and by group at baseline
| Total | Control | PREPARE | PREPARE with pedometer | |
|---|---|---|---|---|
| n | 87 | 29 | 29 | 29 |
| Age | 65 ± 8 | 65 ± 10 | 64 ± 7 | 66 ± 8 |
| Sex | ||||
| Male | 57 (66) | 17 (59) | 20 (69) | 20 (69) |
| Female | 30 (34) | 12 (41) | 9 (31) | 9 (31) |
| Ethnicity | ||||
| White | 65 (75) | 20 (69) | 20 (69) | 25 (86) |
| South Asian | 21 (24) | 9 (31) | 8 (31) | 4 (14) |
| Black | 1 (1) | 0 (0) | 1 (3) | 0 (0) |
| Blood pressure medication | ||||
| β-Blockers | 29 (34) | 10 (34) | 11 (38) | 8 (28) |
| ACE inhibitors | 17 (20) | 5 (19) | 4 (14) | 8 (28) |
| Statins | 47 (55) | 17 (57) | 14 (48) | 16 (55) |
| Smoking status | 8 (9) | 5 (17) | 2 (7) | 1 (3) |
| Pedometer counts (steps per day) | 6,681 ± 3,462 | 6,873 ± 3,537 | 6,560 ± 4,424 | 6,600 ± 2,402 |
| Self-reported walking activity (MET-min/week) | 990 (445–2,123) | 801 (292–2,161) | 891 (297–2079) | 1,386 (594–2,772) |
| Total self-reported energy expenditure (MET-min/week) | 2,580 (1,180–4,719) | 2,335 (923–3,921) | 2,359 (947–3,989) | 3,480 (1,524–6,339) |
| BMI (kg/m2) | 29.2 ± 4.7 | 29.8 ± 4.4 | 29.5 ± 4.9 | 28.7 ± 4.8 |
| Waist circumference (cm) | 102 ± 11 | 103 ± 9 | 103 ± 11 | 99 ± 12 |
| Weight (kg) | 80.8 ± 15.1 | 81.1 ± 15.0 | 81.9 ± 14.2 | 79.4 ± 16.4 |
| Systolic blood pressure (mmHg) | 142 ± 16 | 141 ± 15 | 144 ± 17 | 139 ± 15 |
| Diastolic blood pressure (mmHg) | 81 ± 9 | 81 ± 10 | 82 ± 8 | 79 ± 10 |
| 2-h glucose (mmol/l) | 8.4 ± 2.0 | 8.4 ± 2.1 | 8.1 ± 1.8 | 8.8 ± 2.2 |
| Fasting glucose (mmol/l) | 5.6 ± 0.6 | 5.7 ± 0.5 | 5.6 ± 0.6 | 5.6 ± 0.5 |
| Total cholesterol (mmol/l) | 4.7 ± 1.0 | 4.7 ± 0.9 | 4.8 ± 1.0 | 4.7 ± 1.1 |
| HDL cholesterol (mmol/l) | 1.3 (1.1–1.4) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 1.2 (1.1–1.4) |
| Triglycerides (mmol/l) | 1.3 (1.0–1.8) | 1.2 (1.0–1.7) | 1.3 (0.9–1.7) | 1.4 (0.8–1.9) |
Categorical data are n (column percent), parametric continuous data as mean ± SD, and nonparametric data as median (interquartile range).
Glucose regulation and physical activity
Table 2 shows changes in measures of physical activity and glucose regulation at 3, 6, and 12 months; the intervention effect for each intervention group is also provided. Two-hour glucose decreased significantly in the pedometer group compared with that in the control group at 3 months (−1.46 mmol/l, 95% CI −2.36 to −0.56) and 12 months (−1.31 mmol/l, −2.20 to −0.43). Fasting glucose also decreased significantly in the pedometer group compared with that in the control group at 3 months (−0.37 mmol/l, −0.63 to −0.11), 6 months (−0.30 mmol/l, −0.57 to −0.03), and 12 months (−0.32 mmol/l, −0.59 to −0.03). There was no difference in either fasting or 2-h glucose in those given the education program without pedometer use compared with that in the control group at any follow-up time point.
Table 2.
Change from baseline and the associated intervention effect for glucose and physical activity measurements at 3, 6, and 12 months
| Control | n * | Intervention 1 (PREPARE) | n * | Intervention 2 (PREPARE with pedometer) | n * | Adjusted intervention effect (intervention 1 vs. control) | P | Adjusted intervention effect (intervention 2 vs. control) | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2-h glucose (mmol/l) | ||||||||||
| 3 months | 0.09 (−0.67 to 0.86) | 29 | 0.29 (−0.40 to 0.98) | 29 | −1.50 (−2.24 to −0.76) | 29 | 0.05 (−0.85 to 0.94) | 0.681 | −1.46 (−2.36 to −0.56) | 0.002 |
| 6 month | −0.58 (−1.23 to 0.06) | 29 | 0.09 (−0.58 to 0.75) | 29 | −1.40 (−2.26 to −0.54) | 29 | 0.52 (−0.37 to 1.42) | 0.252 | −0.67 (−1.57 to 0.22) | 0.139 |
| 12 months | −0.30 (−1.04 to 0.45) | 29 | 0.19 (−0.41 to 0.80) | 29 | −1.75 (−2.57 to −0.94) | 29 | 0.34 (−0.55 to 1.22) | 0.450 | −1.31 (−2.20 to −0.43) | 0.004 |
| Fasting glucose (mmol/l) | ||||||||||
| 3 months | 0.11 (−0.10 to 0.32) | 29 | 0.04 (−0.12 to 0.21) | 29 | −0.25 (−0.43 to −0.07) | 28 | −0.07 (−0.32 to 0.18) | 0.600 | −0.37 (−0.63 to −0.11) | 0.006 |
| 6 months | −0.08 (−0.30 to 0.15) | 29 | −0.19 (−0.33 to −0.05) | 29 | −0.35 (−0.57 to −0.13) | 28 | −0.12 (−0.39 to 0.14) | 0.353 | −0.30 (−0.57 to −0.03) | 0.028 |
| 12 months | 0.10 (−0.15 to 0.34) | 29 | −0.03 (−0.18 to 0.12) | 29 | −0.20 (−0.40 to −0.01) | 28 | −0.13 (−0.41 to 0.14) | 0.336 | −0.32 (−0.59 to −0.03) | 0.028 |
| Ambulatory activity (steps/day) | ||||||||||
| 3 months | 552 (−290 to 1,393) | 28 | 1,362 (502 to 2,221) | 25 | 2,395 (1,285 to 3,505) | 29 | 794 (−542 to 2,131) | 0.240 | 1,863 (576 to 3,150) | 0.005 |
| 6 months | −152 (−778 to 573) | 28 | 870 (−54 to 1,793) | 25 | 2,093 (944 to 3,242) | 29 | 968 (−297 to 2,234) | 0.132 | 2,207 (989 to 3,426) | 0.001 |
| 12 months | −940 (−1,574 to −307) | 28 | 549 (−290 to 1,390) | 25 | 1,039 (135 to 1,943) | 29 | 1,401 (417 to 2,385) | 0.006 | 1,902 (954 to 2,859) | <0.001 |
| Self-reported walking activity (MET-min/week) | ||||||||||
| 3 months | 275 (−377 to 927) | 23 | 475 (−112 to 1,064) | 23 | 1,605 (712 to 2,498) | 28 | 269 (−766 to 1,304) | 0.606 | 1,516 (530 to 2,501) | 0.003 |
| 6 months | 123 (−619 to 864) | 24 | 154 (−582 to 889) | 24 | 1,083 (517 to 1,649) | 28 | −23 (−889 to 842) | 0.957 | 1,031 (206 to 1,755) | 0.015 |
| 12 months | −361 (−849 to 127) | 26 | 421 (−224 to 1,067) | 23 | 708 (72 to 1,344) | 27 | 764 (14 to 1,515) | 0.046 | 1,150 (428 to 1,872) | 0.002 |
| Total moderate- to vigorous-intensity physical activity (MET-min/week) | ||||||||||
| 3 months | 21 (−2031 to 2074) | 23 | 1,031 (−826 to 2,888) | 23 | 3,403 (1,214 to 5,991) | 28 | 975 (−1720 to 3,672) | 0.473 | 3,930 (1,340 to 6,520) | 0.003 |
| 6 months | 340 (−1048 to 1,729) | 24 | 1,533 (−254 to 3,320) | 24 | 3,830 (1,637 to 6,024) | 28 | 928 (−2008 to 3,242) | 0.468 | 3,557 (1,126 to 5,987) | 0.005 |
| 12 months | −1,377 (−2852 to 98) | 26 | 1,459 (327 to 2,571) | 23 | 1,589 (48 to 3,130) | 27 | 2,364 (513 to 4,214) | 0.013 | 3,060 (1,301 to 4,819) | 0.001 |
Data are means (95% CI). All reported intervention effects were adjusted for baseline value.
*Number of available datasets after excluding missing or invalid data and extreme outliers.
Objectively measured ambulatory activity and self-reported walking and overall moderate- to vigorous-intensity physical activity increased significantly in the pedometer group compared with those in the control group at 3, 6, and 12 months. In the group without pedometer use, ambulatory activity and self-reported walking and moderate- to vigorous-intensity physical activity increased significantly compared with those in the control group at 12 months; however, there was no significant increase in any measure of physical activity in this group compared with that in the control group at 3 or 6 months.
Lipids, blood pressure, and body weight
There was no difference in measured blood lipids, body weight, waist circumference, or blood pressure in either of the intervention groups compared with that in the control group at any time point (supplementary Table A1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-0130/DC1).
Psychological factors
Compared with the control group, both intervention groups achieved significant increases in perceived knowledge of IGT, perceived effectiveness of exercise as a treatment for IGT, and self-efficacy beliefs at 12 months (supplementary Table A2, available in an online appendix). No significant differences were seen in other measured illness perception or emotional representations.
CONCLUSIONS
This study showed that group-based structured education designed to promote increased walking activity through pedometer use is effective for improving glucose tolerance in those with screening-detected IGT, whereas the same program without pedometer use did not result in improved glucose tolerance. The decrease in 2-h glucose seen in the pedometer group at 12 months of −1.31 mmol/l (95% CI −2.20 to −0.43) is greater than that reported in previous lifestyle diabetes prevention programs. For example a meta-analysis of eight studies reported an overall intervention effect in 2-h glucose of −0.84 mmol/l (−1.29 to −0.39) at 12 months and a reduction in relative risk of developing type 2 diabetes of 0.55 (0.44–0.69) (16). Those in the pedometer group also achieved a significant decrease in fasting glucose of −0.32 mmol/l (−0.59 to −0.03) compared with that in the control group; this is in contrast to previous diabetes prevention programs in which lifestyle change had consistently failed to reduce fasting plasma glucose levels (3). However, a recent study in individuals with a family history of type 2 diabetes reported that change in physical activity, as measured by accelerometers, was associated with change in fasting glucose at 12 months (17).
It has been suggested that weight loss was the primary determinant of the reduced risk of developing type 2 diabetes observed in previous lifestyle diabetes prevention programs (4,18), in part because these programs have only demonstrated small increases in physical activity (4). In contrast, although no changes to body weight or waist circumference were observed in this study, substantial increases in ambulatory activity of ∼2,000 steps per day, equivalent to ∼140 min of moderate-intensity walking activity per week (12), were seen in the pedometer group compared with the control group at each follow-up time point. The results of this study are consistent with numerous mechanisms linking physical activity directly to reduced insulin resistance and improved glucose control (19). In the group given the education program without pedometer use, the relatively low level of ambulatory activity at 12 months and/or the lack of sustained increases in physical activity across the study period may explain why glucose levels were unchanged.
The lack of an intervention effect on cholesterol and blood pressure levels is consistent with a study in individuals with a family history of type 2 diabetes, which showed that change in overall physical activity, as measured by accelerometers, was significantly associated with reductions in fasting insulin and glucose, but not with blood pressure or HDL cholesterol, after adjustment for markers of adiposity (17). Another prospective study using objectively measured physical activity energy expenditure reported similar findings (20). The PREPARE program study adds to this evidence by suggesting that in individuals with IGT, of whom many were taking antihypertensive and/or statin medication, glucose regulation is more sensitive to change than blood pressure or cholesterol with increased physical activity.
Both versions of the PREPARE program positively influenced several of the key psychological determinants on which the program was grounded. This finding suggests that the pedometer was crucial in promoting the self-regulatory strategies needed to convert the motivational impact of the education program into sustained behavior change and improved glucose tolerance. This study adds to evidence from other intervention studies that have consistently shown that pedometer-based programs are successful at initiating increased ambulatory activity in those with type 2 diabetes and the general population over the short-term (<6 months) (21).
This study has several important limitations. First, the small sample size precluded meaningful subgroup analysis, which is important, given the heterogeneity of the study sample. Second, the study was conducted in a single center by a dedicated research team; this limits the generalizability of the findings. However, the intervention used in this trial was robustly developed according to established criteria for developing and evaluating complex interventions which included a theory, modeling, and exploratory trial phase; therefore, this study is reproducible (22). Third, the methodology of this study did not allow us to determine whether the success of the pedometer version of the PREPARE program was solely or partly due to the reactivity of wearing a pedometer and keeping a daily step count log. However, studies have shown that the reactivity of wearing an open pedometer in adults is minimal and likely to be temporary (23,24), suggesting that some form of additional support is required to facilitate sustained behavior change.
These limitations notwithstanding, this study is the first randomized controlled trial to show behavior change and improved glucose tolerance in individuals with IGT after a pragmatic structured education program. Traditional diabetes prevention programs have used intensive counseling strategies that would be difficult to deliver in a “real world” health care setting; for example, the Finnish Diabetes Prevention Study reported a median of 20 counseling sessions per patient over the 4-year intervention period (25). Therefore, pragmatic interventions that are compatible with the infrastructure and resources available to national health services are required. The PREPARE program study suggests one possible approach in the U.K., not least because it could use existing national and regional educator training and the quality assurance and development infrastructure that have been developed for delivering type 2 diabetes self-management programs, such as the DESMOND program (8–10). However, larger multicentered randomized controlled trials are needed to confirm the efficacy and cost-effectiveness of this approach for preventing type 2 diabetes in at-risk populations.
Supplementary Material
Acknowledgments
This trial was funded by a grant from Diabetes UK.
No potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT00566319, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K: Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. Br Med J 2007; 334: 299– 308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sherwin RS, Anderson RM, Buse JB, Chin MH, Eddy D, Fradkin J, Ganiats TG, Ginsberg HN, Kahn R, Nwankwo R, Rewers M, Schlessinger L, Stern M, Vinicor F, Zinman B: Prevention or delay of type 2 diabetes. Diabetes Care 2004; 27( Suppl. 1): S47– S54 [DOI] [PubMed] [Google Scholar]
- 3. Yates T, Khunti K, Davies MJ: Prevention of diabetes: a reality in primary care? Primary Care Diabetes 2007; 1: 119– 121 [DOI] [PubMed] [Google Scholar]
- 4. Yates T, Khunti K, Bull F, Gorely T, Davies MJ: The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia 2007; 50: 1116– 1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yates T, Davies M, Gorely T, Bull F, Khuni K: Rationale, design and baseline data from the PREPARE (Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement) programme study: a randomized controlled trial. Patient Educ Couns 2008; 73: 264– 271 [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Clinical Excellence. Four Commonly Used Methods to Increase Physical Activity: Brief Interventions in Primary Care, Exercise Referral Schemes, Pedometers and Community-Based Exercise Programmes for Walking and Cycling. London, NICE, 2006 [Google Scholar]
- 7. World Health Organization Diabetes Guideline Development Committee. Definition and Diagnoses of Diabetes Mellitus and Intermediate Hyperglycemia. Geneva, World Health Org., 2006 [Google Scholar]
- 8. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P: International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381– 1395 [DOI] [PubMed] [Google Scholar]
- 9. Skinner TC, Carey M, Cradock S, Daly H, Davies M, Doherty Y, Heller S, Khunti K, Oliver L: Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND): process modelling of pilot study, Patient Educ Couns 2006; 64: 369– 377 [DOI] [PubMed] [Google Scholar]
- 10. Davies MJ, Heller S, Campbell MJ, Carey ME, Dallosso HM, Daly H, Doherty Y, Eaton S, Fox C, Oliver L, Rantell K, Rayman G, Khunti K: Effectiveness of a structured education programme on individuals newly diagnosed with type 2 diabetes: a cluster randomised controlled trial of the DESMOND programme. BMJ 2008; 336: 491– 495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skinner T, Carey M, Cradock S, Dallosso H, Daly H, Davies M, Doherty Y, Heller S, Khunti K, Oliver L: ‘Educator talk’ and patient change: some insights from the DESMOND (Diabetes Education and Self Management for Ongoing and Newly Diagnosed) randomized controlled trial. Diabet Med 2008; 25: 1117– 1120 [DOI] [PubMed] [Google Scholar]
- 12. Tudor-Locke C, Bassett DR, Jr: How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004; 34: 1– 8 [DOI] [PubMed] [Google Scholar]
- 13. Crouter SE, Schneider PL, Bassett DR, Jr: Spring-levered versus piezo-electric pedometer accuracy in overweight and obese adults. Med Sci Sports Exerc 2005; 37: 1673– 1679 [DOI] [PubMed] [Google Scholar]
- 14. Broadbent E, Petrie KJ, Main J, Weinman J: The brief illness perception questionnaire. J Psychosom Res 2006; 60: 631– 637 [DOI] [PubMed] [Google Scholar]
- 15. Marcus BH, Selby VC, Niaura RS, Rossi JS: Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992; 63: 60– 66 [DOI] [PubMed] [Google Scholar]
- 16. Yamaoka K, Tango T: Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2005; 28: 2780– 2786 [DOI] [PubMed] [Google Scholar]
- 17. Simmons RK, Griffin SJ, Steele R, Wareham NJ, Ekelund U: Increasing overall physical activity and aerobic fitness is associated with improvements in metabolic risk: cohort analysis of the ProActive trial. Diabetologia 2008; 51: 787– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J: Effect of weight loss with lifestyle intervention on risk of diabetes, Diabetes Care 2006; 29: 2102– 2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawley JA: Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab Res Rev 2004; 20: 383– 393 [DOI] [PubMed] [Google Scholar]
- 20. Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ: Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care 2007; 30: 2101– 2106 [DOI] [PubMed] [Google Scholar]
- 21. Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR: Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007; 298: 2296– 2304 [DOI] [PubMed] [Google Scholar]
- 22. Framework for development and evaluation of RCTs for complex intervention to improve health [article online], 2000. Available from http://www.mrc.ac.uk/utilities/documentrecord/index.htm?d=mrc003372. Accessed 1 May 2009
- 23. Eastep E, Beveridge S, Eisenman P, Ransdell L, Shultz B: Does augmented feedback from pedometers increase adults' walking behavior? Percept Mot Skills 2004; 99: 392– 402 [DOI] [PubMed] [Google Scholar]
- 24. Matevey C, Rogers LQ, Dawson E, Tudor-Locke C: Lack of reactivity during pedometer self-monitoring in adults. Meas Phys Educ Exerc Sci 2006; 10: 1– 11 [Google Scholar]
- 25. Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen-Kiukaanniemi S, Laakso M: Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006; 368: 1673– 1679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.