Abstract
OBJECTIVE
Increased serum ferritin levels and iron stores may be involved in the development of abnormal glucose tolerance in women presenting with obesity and/or polycystic ovary syndrome (PCOS). We aimed to study the determinants of serum ferritin levels in premenopausal women among indexes of insulin resistance, adiposity, hyperandrogenism, and genotypes pertaining to inflammation, oxidative stress, and iron metabolism.
RESEARCH DESIGN AND METHODS
A total of 257 premenopausal women, classified depending on the presence or absence of PCOS, obesity, and/or abnormal glucose tolerance, underwent a complete metabolic evaluation, serum ferritin, haptoglobin, and C-reactive protein (CRP) measurements, and genotyping for proinflammatory and prooxidant variants and mutations in the HFE gene.
RESULTS
Serum ferritin concentrations were increased in women presenting with PCOS and/or abnormal glucose tolerance, independent of obesity. A stepwise multivariate linear regression analysis (R2 = 0.18, P < 0.0001) retained menstrual dysfunction (β = 0.14, P = 0.035), free testosterone (β = 0.14, P = 0.052), insulin sensitivity index (β = −0.12, P = 0.012), the His63Asp variant in HFE (β = 0.16, P = 0.008), and abnormal glucose tolerance (β = 0.15, P = 0.015) as significant predictors of the logarithm of ferritin levels, whereas CRP, haptoglobin, waist-to-hip ratio, or variants in the TNFα, TNFRSF1B, IL6, IL6ST, IL6Rα, PON1, and HFE Cys282Tyr mutation exerted no influence.
CONCLUSIONS
Androgen excess (partly because of hyperandrogenemia and partly because of menstrual dysfunction), insulin resistance, abnormal glucose tolerance, and the HFE His63Asp variant correlate with ferritin levels in premenopausal women.
Mounting evidence suggests that increased body iron stores are involved in the pathogenesis of insulin-resistant disorders such as the metabolic syndrome and type 2 diabetes in the general population (1). This involvement appears to be bidirectional, because not only does iron accumulation favor insulin resistance and may contribute to pancreatic β-cell dysfunction and diabetes, but also insulin resistance may in turn facilitate iron accumulation within the body (2,3). Interestingly, oxidative stress and inflammation are involved in the interplay between iron overload and insulin resistance (2,4).
Although most of the evidence linking iron metabolism and disorders of glucose metabolism has been provided from the study of middle-aged or older men and of postmenopausal women (2,5), body iron stores also influence insulin resistance and glucose metabolism in premenopausal women (6,7).
Ferritin is the cellular storage protein for iron. We have reported that serum ferritin levels are increased in women presenting either with obesity or polycystic ovary syndrome (PCOS) and especially when both conditions are present in the same patient (6). Therefore, both androgen excess and insulin resistance may underlie this finding. In addition, because these changes occurred independent of changes in serum inflammatory markers, the increased ferritin level indicates that body iron stores are actually increased in these women and do not result from the secondary role of ferritin as an acute-phase marker (6).
Of note, serum ferritin levels were clearly increased in the small subset of these women presenting with abnormal glucose tolerance, both in the PCOS and nonhyperandrogenic subgroups (6). This finding suggests that increased body iron stores could be related to the development of abnormalities in glucose metabolism in these patients, because progressive iron accumulation in the pancreas is a recognized pathogenic mechanism of disorders of glucose tolerance in patients with iron overload (8).
We hypothesized that the reduced menstrual losses due to the oligomenorrhea present in most women with PCOS could contribute to their increased iron stores, yet recent data from our group suggest that insulin resistance is actually one of the major players explaining their increased serum ferritin levels: whereas serum ferritin levels did not change after restoring regular menses by using an oral contraceptive for 6 months, these levels decreased markedly after insulin sensitization with metformin (3).
The present study was undertaken with the aim of identifying, in a large series of premenopausal women, the determinants of increased serum ferritin levels (an index of body iron stores and a risk factor for the development of abnormalities of glucose tolerance) among markers of hyperandrogenism, adiposity, insulin resistance, and genomic variants related to chronic inflammation, oxidative stress, and iron metabolism.
RESEARCH DESIGN AND METHODS
A total of 257 premenopausal women were included. The group was composed of consecutive patients reporting to the Department of Endocrinology because of PCOS and/or weight excess and of healthy nonhyperandrogenic nonobese volunteers recruited from the staff of Hospital Universitario Ramón y Cajal. Women were classified according to their BMI into obese (BMI ≥30 kg/m2, n = 128) and nonobese (BMI <30 kg/m2, n = 129) subgroups. PCOS was diagnosed in 149 women presenting with clinical and/or biochemical hyperandrogenism in addition to oligo-ovulation as reported previously (9), thereby fulfilling all of the current definitions of the syndrome (10–12), whereas 108 women showed no sign of hyperandrogenism, had normal androgen levels, and had regular ovulatory menstrual cycles.
Menstrual and ovulatory dysfunction were defined by the presence of oligomenorrhea (cycles longer than 35 days) or amenorrhea (absence of menstrual bleeding for at least three usual cycle lengths) or, in women presenting with regular menstrual cycles (cycles between 26 and 35 days), by lack of ovulation according to body temperature charts and/or serum progesterone levels < 12.7 nmol/l during the luteal phase of the menstrual cycle (9).
None of the women had a personal history of hypertension, diabetes, or cardiovascular events. Women who took oral contraceptives, antiandrogens, insulin sensitizers, iron supplements, or drugs that might interfere with blood pressure regulation, lipid profile, or carbohydrate metabolism within the previous 6 months or who were referred for any medical reason aside from androgen and/or weight excess were automatically excluded. Written informed consent was obtained from all of the participants, and the study was approved by the ethics committee of Hospital Universitario Ramón y Cajal.
Clinical and anthropometric variables, including the hirsutism score, BMI, waist circumference, and waist-to-hip ratio (WHR) were determined. WHR was calculated by dividing the minimal waist circumference by the hip circumference at the level of the greater trochanters, using a nonstretchable measuring tape.
Whole blood, serum, and plasma samples were obtained between days 5 and 10 of the menstrual cycle, or during amenorrhea after pregnancy was excluded. After a 3-day 300-g carbohydrate diet and 12-h overnight fasting, samples were obtained early in the morning for the measurement of total testosterone, sex hormone–binding globulin, 17-hydroxyprogesterone, androstenedione, dehydroepiandrosterone sulfate, luteinizing hormone, follicle-stimulating hormone, estradiol, thyrotropin, and prolactin. A complete hemogram and serum biochemistry and lipid profiles were also obtained. Then, a 75-g oral glucose tolerance test was performed, and samples were obtained for measurement of serum insulin and plasma glucose at 0, 30, 60, 90, and 120 min. Samples were immediately centrifuged, and serum and plasma were separated and frozen at −20°C until assayed.
The assays used for these measurements have been described in detail elsewhere (9). Free testosterone concentrations were calculated from total testosterone and sex hormone–binding globulin levels (9). Serum ferritin and C-reactive protein (CRP) concentrations were measured by automated immunochemiluminescence (Immulite 2000 Ferritin and High Sensitivity CRP; Diagnostic Products Corporation, Los Angeles, CA) with lower limits of detection of 0.88 pmol/l and 0.1 mg/l, respectively, and intra- and interassay coefficients of variation <10%. The circulating concentrations of haptoglobin were assayed by a commercial immunonephelometry method (Dade Behring, Marburg, Germany), calibrated against the international CRM 470 reference material. The composite insulin sensitivity index was calculated from circulating glucose and insulin concentrations during the oral glucose tolerance test as described by Matsuda and DeFronzo (13). Disorders of glucose tolerance were diagnosed following the recommendations of the American Diabetes Association (14).
DNA analysis
Genomic DNA was obtained from whole blood samples using a Nucleon BACC3 DNA isolation kit (Amersham, Buckinghamshire, U.K.) and was used to genotype several polymorphisms related to inflammation or oxidative stress that had been previously found to be associated with metabolic disorders such as PCOS, obesity, diabetes, and insulin resistance and variants involved in iron metabolism. Genotyping of the dinucleotide CA repeat in intron 8 of the interleukin (IL)-6 receptor-α (IL6Rα) locus (rs57636717) was performed by PCR using a fluorescently labeled forward primer (15). Amplified fragments were resolved by capillary electrophoresis on an ABI Prism 3100 automated genetic analyzer (Applied Biosystems, Foster City, CA), and their sizes were determined using GeneMapper 4.0 software. We detected 13 different alleles with sizes ranging from 143 to 169 bp, of which the 149-bp allele was the most frequent. For statistical analyses, subjects were genotyped as homozygous for 149-bp alleles, heterozygous for 149-bp alleles, and noncarriers of any 149-bp allele (15). Several genomic variants were analyzed by PCR–restriction fragment–length polymorphism as described previously: variants His63Asp (c.187C>G) and Cys282Tyr (c.845G>A) in exons 2 and 4 (rs1799945 and rs1800562, respectively) of the HFE gene (7); polymorphism −308G>A (rs1800629) in the promoter of the tumor necrosis factor (TNF)-α (TNFα) gene (16); variant Met196Arg (c.587T>G; rs1061622) in exon 6 of the TNF receptor 2 (TNFRSF1B) gene (17); polymorphism −174G>C (rs1800795) in the IL-6 gene promoter (18); variant Gly148Arg (c.442G>C; rs2228044) in exon 5 of the IL-6 signal transducer (IL6ST) gene (15); and polymorphism −108C>T (rs705379) in the paraoxonase (PON1) gene (19).
Statistical analysis
Data are shown as means ± SD unless otherwise stated. The Kolmogorov-Smirnov statistic was applied to continuous variables. Logarithmic or square-root transformations were applied as needed to ensure normal distribution of the variables. Univariate general linear models were used to evaluate the influence of obesity, PCOS, and glucose tolerance on clinical and biochemical variables, with introduction of age as a covariate to control for possible age differences among the groups. A multivariate linear regression analysis was used to determine the influence of clinical, biochemical, metabolic, and genetic variants on the logarithm of serum ferritin concentrations as described below. Depending on the median of the whole population of premenopausal women, serum ferritin levels were categorized into values below, or equal to or above, the median value. The associations of these two groups with categorical and continuous variables were analyzed by χ2 and Fisher's exact tests as appropriate. P < 0.05 was considered statistically significant. Analyses were performed using SPSS Statistics 17 (SPSS, Chicago, IL).
RESULTS
The influence of PCOS and of obesity on clinical and biochemical variables is summarized in Table 1. Because patients with PCOS were younger than nonhyperandrogenic women and obese women were older than nonobese women, the influence of age was controlled by introducing this variable as a covariate in the comparisons described below.
Table 1.
Influence of obesity and PCOS on clinical and biochemical variables of premenopausal women
| Nonobese women (n = 129) |
Obese women (n = 128) |
PCOS (n = 149) vs. control (n = 108) |
Obese (n = 128) vs. nonobese (n = 129) |
Interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCOS patients | Nonhyperandrogenic women | PCOS patients | Nonhyperandrogenic women | F/χ2 | P value | F/χ2 | P value | F | P value | |
| n | 82 | 47 | 67 | 61 | ||||||
| Age (years) | 24 ± 6 | 29 ± 7 | 27 ± 7 | 33 ± 8 | 43.97 | <0.001 | 11.79 | 0.001 | 0.19 | 0.664 |
| BMI (kg/m2) | 24.4 ± 3.5 | 24.4 ± 4.1 | 36.9 ± 5.5 | 36.7 ± 5.4 | 1.73 | 0.190 | 404.80 | <0.001 | 0.09 | 0.764 |
| Waist circumference (cm) | 73 ± 10 | 76 ± 11 | 97 ± 13 | 96 ± 13 | 0.94 | 0.334 | 212.62 | <0.001 | 2.17 | 0.142 |
| WHR | 0.75 ± 0.07 | 0.76 ± 0.08 | 0.82 ± 0.08 | 0.80 ± 0.08 | 8.37 | 0.004 | 26.50 | <0.001 | 2.30 | 0.131 |
| Hirsutism score | 11 ± 6 | 2 ± 2 | 10 ± 6 | 1 ± 2 | 217.83 | <0.001 | 3.36 | 0.068 | 2.27 | 0.133 |
| Free testosterone (pmol/l) | 37 ± 18 | 19 ± 8 | 54 ± 25 | 24 ± 10 | 116.10 | <0.001 | 33.60 | <0.001 | 1.82 | 0.179 |
| Estradiol (pmol/l) | 134 ± 80 | 245 ± 233 | 180 ± 129 | 231 ± 198 | 10.80 | 0.001 | 0.414 | 0.521 | 4.17 | 0.042 |
| Luteinizing hormone (units/l) | 6.6 ± 3.9 | 5.3 ± 3.1 | 6.1 ± 3.8 | 4.9 ± 3.1 | 5.20 | 0.023 | 0.902 | 0.343 | 0.01 | 0.995 |
| Follicle-stimulating hormone (units/l) | 5.7 ± 4.2 | 6.4 ± 6.1 | 6.2 ± 4.5 | 5.8 ± 1.8 | 0.361 | 0.548 | 0.01 | 0.948 | 0.59 | 0.442 |
| Fasting insulin (pmol/l) | 75 ± 58 | 58 ± 46 | 137 ± 75 | 84 ± 44 | 15.19 | <0.001 | 46.69 | <0.001 | 1.77 | 0.185 |
| Fasting glucose (mmol/l) | 4.9 ± 0.4 | 5.0 ± 0.4 | 5.3 ± 0.5 | 5.2 ± 0.5 | 0.02 | 0.890 | 20.08 | <0.001 | 3.86 | 0.051 |
| Insulin sensitivity index | 5.4 ± 3.0 | 7.0 ± 3.8 | 2.8 ± 2.1 | 4.8 ± 3.6 | 27.34 | <0.001 | 52.23 | <0.001 | 3.53 | 0.061 |
| CRP (mg/l) | 1.3 ± 1.7 | 1.3 ± 1.6 | 6.0 ± 5.6 | 5.6 ± 5.1 | 1.68 | 0.196 | 161.77 | <0.001 | 0.29 | 0.558 |
| Haptoglobin (μmol/l) | 12 ± 4 | 12 ± 4 | 17 ± 5 | 17 ± 5 | 0.01 | 0.969 | 66.13 | <0.001 | 0.38 | 0.539 |
| Regular menstruation | 16 (20) | 47 (100) | 10 (15) | 61 (100) | ||||||
| Oligomenorrhea | 47 (57) | 0 (0) | 35 (52) | 0 (0) | 171.00 | <0.001 | 2.45 | 0.294 | — | — |
| Amenorrhea | 19 (23) | 0 (0) | 22 (33) | 0 (0) | ||||||
| Normal glucose tolerance | 74 (90) | 40 (85) | 46 (70) | 43 (70) | 0.512 | 0.474 | 13.72 | <0.001 | — | — |
| Abnormal glucose tolerance | 8 (10) | 7 (15) | 21 (31) | 18 (30) | ||||||
Data are means ± SD or raw numbers (%). Continuous data were used for univariate general linear models and, because age was different between patients with PCOS and nonhyperandrogenic women and between obese and nonobese women, age was introduced as a covariate in the analysis of all the other variables. Categorical data were analyzed by χ2 tests.
Patients with PCOS had increased serum androgen and luteinizing hormone levels and decreased estradiol concentrations and were insulin resistant and had increased WHR compared with their nonhyperandrogenic counterparts irrespective of obesity. Obesity was characterized by increased indexes of insulin resistance, increased frequency of abnormalities in glucose tolerance, and increased serum CRP, haptoglobin, and free testosterone levels compared with those in nonobese women, both in patients with PCOS and in nonhyperandrogenic women.
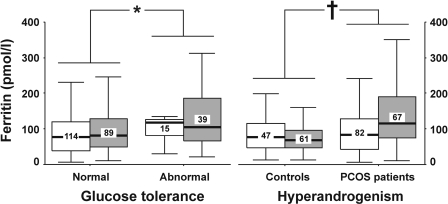
Serum ferritin levels were influenced independently by PCOS and glucose intolerance (Fig. 1). Patients with PCOS presented with increased serum ferritin levels compared with those in nonhyperandrogenic women. When considered as a whole, women presenting with abnormal glucose tolerance had higher serum ferritin concentrations compared with those showing normal glucose values during fasting and after an oral glucose tolerance test. Of note, these results occurred both in the nonobese and in the obese subgroups, and obesity did not influence serum ferritin concentrations after controlling for both PCOS and glucose tolerance (Fig. 1).
Figure 1.
Serum ferritin levels depending on the presence or absence of abnormalities of glucose tolerance and PCOS in nonobese □ and obese ▩ women. The box plot includes the median (horizontal line) and the interquartile range, and the whiskers indicate the minimum and maximum data values, unless outliers are present, in which case the whiskers extend to a maximum of 1.5 times the interquartile range. The figures inside the boxes are the numbers of women in each subgroup. The logarithm of serum ferritin levels was used in a general linear model in which glucose tolerance, PCOS, and obesity were introduced as independent variables and age was introduced as a covariate to correct for the difference in age between patients and control subjects and between obese and nonobese women. No interaction was found among independent variables. *P = 0.001 between women presenting with or without abnormalities of glucose tolerance. †P = 0.034 between patients with PCOS and nonhyperandrogenic control women.
Because serum ferritin levels were not distributed normally, their logarithm was introduced as a dependent variable in multiple linear regression models using age; BMI; glucose tolerance (codified as normal or abnormal); menstrual cycles (codified as regular menstruation, oligomenorrhea, or amenorrhea); serum free testosterone, CRP, and haptoglobin levels; the insulin sensitivity index; and the genomic variants related to iron metabolism, inflammation, and oxidative stress as independent variables. The model that considered all independent variables explained 22% of the variability in the logarithm of serum ferritin concentrations (R2 = 0.22, P < 0.0001) (Fig. 2 ).
Figure 2.
Multiple linear regression analysis of the logarithm of serum ferritin concentrations. The squares are the standardized regression coefficients (β, the change in terms of SDs in the dependent variable that results from a change of 1 SD in an independent variable), and the error bars indicate the 95% CI of β. Menstrual history and genomic variants were coded as dummy variables: regular menstruation was coded 0, 1 was used for oligomenorrhea, and 2 was used for amenorrhea. Variants in TNFα, TNFRSF1B, IL6, IL6ST, HFE, and PON1 loci were coded as 0 for homozygosity for wild-type alleles, 1 for heterozygosity, and 2 for homozygosity for mutant alleles. The IL6Rα polymorphism was coded 0 for homozygosity for 149-bp alleles, 1 for subjects carrying only one 149-bp allele, and 2 for subjects carrying two non–149-bp alleles. Finally, HFE His63Asp/Cys282Tyr double heterozygotes were coded 1 and subjects without double heterozygosity were coded 0.
Conversely, when the independent variables were introduced using a stepwise method (probability to enter ≤0.05; probability to remove ≥0.10), the model (R2 = 0.18, P < 0.0001) retained only menstrual dysfunction (β = 0.14, P = 0.035), serum free testosterone levels (β = 0.14, P = 0.052), insulin sensitivity index (β = −0.12, P = 0.012), His63Asp variant in HFE (β = 0.16, P = 0.008), and abnormal glucose tolerance (β = 0.15, P = 0.015) as significant predictors of the logarithm of serum ferritin levels, whereas all the other clinical, biochemical, and genomic variables were excluded (Fig. 2). Finally, a similar stepwise regression method (R2 = 0.17, P < 0.0001) adjusted for age and BMI (these variables were manually entered into the model) retained only menstrual dysfunction (β = 0.18, P = 0.003), abnormal glucose tolerance (β = 0.17, P = 0.013), His63Asp variant in HFE (β = 0.17, P = 0.004), and insulin sensitivity index (β = −0.15, P = 0.031) as predictors of the logarithm of serum ferritin levels (Fig. 2).
When considering the median of the serum ferritin concentrations of the whole population (83 pmol/l) as a cutoff value, premenopausal women presenting with a serum ferritin level above the median had an odds ratio (OR) for abnormal glucose tolerance of 2.4 (95% CI 1.3–4.4, χ2 = 7.420, P = 0.009) and an OR for PCOS of 2.2 (1.3–3.7, χ2 = 9.524, P = 0.002).
CONCLUSIONS
The metabolic consequences of iron overload are exemplified by the development of abnormalities of glucose tolerance in primary or secondary hemochromatosis. However, less severe iron overload also plays an important role in the development of abnormalities in glucose tolerance (2), as demonstrated by the improvement in insulin resistance and glucose tolerance in type 2 diabetic patients after the iron depletion achieved by repeated blood letting (20) or the higher insulin sensitivity associated with reduced iron stores in frequent blood donors from the general population (21).
Our present results demonstrate that serum ferritin levels, an accurate marker of body iron stores in the absence of acute inflammatory syndromes (2), are also related to abnormal glucose tolerance in premenopausal women. Serum ferritin levels above the median were associated with a 2.4-fold OR for presenting with abnormal glucose tolerance, and ferritin concentrations were clearly higher in women in whom fasting or postload glucose levels were above the normal range. Furthermore, our present results suggest that the body iron stores of premenopausal women are associated with several factors including menstrual dysfunction, insulin resistance, and the His63Asp variant in HFE even after controlling for the difference in age among patients with PCOS and nonhyperandrogenic control subjects and between obese and nonobese women.
We have reported previously, in a much smaller series, that body iron stores are increased in premenopausal women presenting with PCOS and obesity and that these associations were independent of mutations in HFE and were not influenced by changes in markers of chronic inflammation (6,7). Our present findings confirm that serum ferritin levels are increased in patients with PCOS and that, accordingly, women presenting with serum ferritin levels above the median have a 2.2 OR of having PCOS. Yet when controlling for the presence or absence of abnormal glucose tolerance, the previously reported association with obesity disappears, suggesting that it is the increased prevalence of disordered glucose tolerance in obese women and not weight excess by itself that is responsible for increased body iron stores in these women.
According to the regression analyses presented here, menstrual dysfunction, increased androgen levels, and insulin resistance, together with the HFE His63Asp variant and abnormal glucose tolerance, are among the strongest predictors of serum ferritin levels. On the contrary, these levels are not influenced by indexes of global or abdominal adiposity or by genomic variants related to chronic inflammation and oxidative stress.
Therefore, it appears that androgen excess and insulin resistance, which are present in many patients with PCOS, collaborate in increasing body iron stores in premenopausal women. The effects of insulin resistance and hyperinsulinism on body iron stores might depend on a direct insulin stimulation of intestinal iron absorption by upregulating activity of hypoxia-inducible factor-1α and downregulating hepcidin expression (22,23) and may be counteracted in patients with PCOS by administering insulin sensitizers (3).
The effect of androgen excess on body iron stores might result from the well-known stimulatory effect of androgens on erythropoiesis, thereby increasing intestinal iron absorption (24) but may also result from the iron-sparing effect of reduced menstrual losses due to the chronic menstrual dysfunction of PCOS. This iron-sparing mechanism may take years to result in increased iron stores, explaining why regularization of menstrual bleeding in patients with PCOS by administering antiandrogenic contraceptive pills for 6 months had no evident impact on serum ferritin levels in our previous report (3).
Our present results also suggest that the His63Asp variant in HFE influences body iron stores in premenopausal women, in conceptual agreement with the partial loss of HFE function induced by this mutation in animal models, leading to a variable degree of hepatic iron loading (25). However, more importantly, the relatively strong association of increased serum ferritin levels with abnormal glucose tolerance raises the possibility that increased iron stores played some pathogenic role in the development of such metabolic derangements, given that progressive iron accumulation in the pancreas contributes to β-cell dysfunction and abnormal glucose tolerance in syndromes of iron overload (8). However, because association does not imply causality, this hypothesis is speculative.
In summary, body iron stores, as reflected by serum ferritin concentrations, are interrelated with androgen excess, insulin resistance, and the His63Asp variant in HFE in premenopausal women and are associated with the development of abnormal glucose tolerance in this particular population.
Acknowledgments
This study was supported by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (Grants FIS PI080944). Centro de Investigaciones Biomédicas en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) is an initiative of Instituto de Salud Carlos III, Ministry of Science and Innovation.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Tuomainen TP, Nyyssonen K, Salonen R, Tervahauta A, Korpela H, Lakka T, Kaplan GA, Salonen JT: Body iron stores are associated with serum insulin and blood glucose concentrations: population study in 1,013 eastern Finnish men. Diabetes Care 1997; 20: 426– 428 [DOI] [PubMed] [Google Scholar]
- 2. Fernandez-Real JM, Lopez-Bermejo A, Ricart W: Cross-talk between iron metabolism and diabetes. Diabetes 2002; 51: 2348– 2354 [DOI] [PubMed] [Google Scholar]
- 3. Luque-Ramirez M, Alvarez-Blasco F, Botella-Carretero JI, Sanchon R, San Millan JL, Escobar-Morreale HF: The increased body iron stores of obese women with polycystic ovary syndrome are a consequence of insulin resistance and hyperinsulinism, and do not result from reduced menstrual losses. Diabetes Care 2007; 30: 2309– 2313 [DOI] [PubMed] [Google Scholar]
- 4. Hirayama M, Kohgo Y, Kondo H, Shintani N, Fujikawa K, Sasaki K, Kato J, Niitsu Y: Regulation of iron metabolism in HepG2 cells: a possible role for cytokines in the hepatic deposition of iron. Hepatology 1993; 18: 874– 880 [DOI] [PubMed] [Google Scholar]
- 5. Jehn M, Clark JM, Guallar E: Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 2004; 27: 2422– 2428 [DOI] [PubMed] [Google Scholar]
- 6. Escobar-Morreale HF, Luque-Ramirez M, Alvarez-Blasco F, Botella-Carretero JI, Sancho J, San Millan JL: Body iron stores are increased in overweight and obese women with polycystic ovary syndrome. Diabetes Care 2005; 28: 2042– 2044 [DOI] [PubMed] [Google Scholar]
- 7. Botella-Carretero JI, Luque-Ramirez M, Alvarez-Blasco F, San Millan JL, Escobar-Morreale HF: Mutations in the hereditary hemochromatosis gene are not associated with the increased body iron stores observed in overweight and obese women with polycystic ovary syndrome. Diabetes Care 2006; 29: 2556. [DOI] [PubMed] [Google Scholar]
- 8. Dymock IW, Cassar J, Pyke DA, Oakley WG, Williams R: Observations on the pathogenesis, complications and treatment of diabetes in 115 cases of haemochromatosis. Am J Med 1972; 52: 203– 210 [DOI] [PubMed] [Google Scholar]
- 9. Escobar-Morreale HF, Sanchon R, San Millan JL: A prospective study of the prevalence of nonclassical congenital adrenal hyperplasia among women presenting with hyperandrogenic symptoms and signs. J Clin Endocrinol Metab 2008; 93: 527– 533 [DOI] [PubMed] [Google Scholar]
- 10. Zawadzki JK, Dunaif A: Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In Polycystic ovary syndrome. Dunaif A, Givens JR, Haseltine FP, Merriam GR. Eds. Boston, Blackwell Scientific Publications, 1992, p. 377– 384 [Google Scholar]
- 11. The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41– 47 [DOI] [PubMed] [Google Scholar]
- 12. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF: Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006; 91: 4237– 4245 [DOI] [PubMed] [Google Scholar]
- 13. Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462– 1470 [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008; 31( Suppl. 1): S55– S60 [DOI] [PubMed] [Google Scholar]
- 15. Escobar-Morreale HF, Calvo RM, Villuendas G, Sancho J, San Millan JL: Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obes Res 2003; 11: 987– 996 [DOI] [PubMed] [Google Scholar]
- 16. Escobar-Morreale HF, Calvo RM, Sancho J, San Millán JL: TNF-α and hyperandrogenism: a clinical, biochemical and molecular genetic study. J Clin Endocrinol Metab 2001; 86: 3761– 3767 [DOI] [PubMed] [Google Scholar]
- 17. Peral B, San Millan JL, Castello R, Moghetti P, Escobar-Morreale HF: The methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. J Clin Endocrinol Metab 2002; 87: 3977– 3983 [DOI] [PubMed] [Google Scholar]
- 18. Villuendas G, San Millan JL, Sancho J, Escobar-Morreale HF: The −597 G/A and −174 G/C polymorphisms in the promoter of the interleukin 6 gene (IL6) are associated with hyperandrogenism. J Clin Endocrinol Metab 2002; 87: 1134– 1141 [DOI] [PubMed] [Google Scholar]
- 19. San Millan JL, Corton M, Villuendas G, Sancho J, Peral B, Escobar-Morreale HF: Association of the polycystic ovary syndrome with genomic variants related to insulin resistance, type 2 diabetes mellitus, and obesity. J Clin Endocrinol Metab 2004; 89: 2640– 2646 [DOI] [PubMed] [Google Scholar]
- 20. Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W: Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes 2002; 51: 1000– 1004 [DOI] [PubMed] [Google Scholar]
- 21. Fernandez-Real JM, Lopez-Bermejo A, Ricart W: Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem 2005; 51: 1201– 1205 [DOI] [PubMed] [Google Scholar]
- 22. McCarty MF: Hyperinsulinemia may boost both hematocrit and iron absorption by up-regulating activity of hypoxia-inducible factor-1α. Med Hypotheses 2003; 61: 567– 573 [DOI] [PubMed] [Google Scholar]
- 23. Le Guenno G, Chanseaume E, Ruivard M, Morio B, Mazur A: Study of iron metabolism disturbances in an animal model of insulin resistance. Diabetes Res Clin Pract 2007; 77: 363– 370 [DOI] [PubMed] [Google Scholar]
- 24. Berria R, Gastaldelli A, Lucidi S, Belfort R, De Filippis E, Easton C, Brytzki R, Cusi K, Jovanovic L, DeFronzo R: Reduction in hematocrit level after pioglitazone treatment is correlated with decreased plasma free testosterone level, not hemodilution, in women with polycystic ovary syndrome. Clin Pharmacol Ther 2006; 80: 105– 114 [DOI] [PubMed] [Google Scholar]
- 25. Tomatsu S, Orii KO, Fleming RE, Holden CC, Waheed A, Britton RS, Gutierrez MA, Velez-Castrillon S, Bacon BR, Sly WS: Contribution of the H63D mutation in HFE to murine hereditary hemochromatosis. Proc Natl Acad Sci U S A 2003; 100: 15788– 15793 [DOI] [PMC free article] [PubMed] [Google Scholar]