Abstract
OBJECTIVE
To evaluate the efficacy and safety of actovegin in patients with diabetic polyneuropathy.
RESEARCH DESIGN AND METHODS
In this multicenter, randomized, double-blind trial, 567 patients with type 2 diabetes received 20 intravenous infusions of actovegin (2,000 mg/day) (n = 281) or placebo (n = 286) once daily followed by three tablets of actovegin (1,800 mg/day) or placebo three times daily for 140 days. Total symptom score (TSS) of the lower limbs and vibration perception threshold (VPT) were used as coprimary outcome measures, computed as the area under the curve (AUC) from repeated scores and divided by duration of exposure. Secondary end points included individual TSS symptoms, neuropathy impairment score of the lower limbs (NIS-LL), and quality of life (short form [SF]-36).
RESULTS
TSS was significantly improved during actovegin treatment compared with placebo, as assessed by AUC (−0.56 points [95% CI −0.85 to −0.27]; P = 0.0003), and from baseline to 160 days (−0.86 points [−1.22 to −0.50]; P < 0.0001). VPT (five sites per foot) decreased by 3% (95% CI 0–6; P = 0.084) with actovegin than placebo, as assessed by AUC, and by 5% (1–9; P = 0.017) after 160 days. NIS-LL sensory function, as assessed by AUC, was significantly improved with actovegin versus placebo (−0.25 [95% CI −0.46 to −0.04]; P = 0.021), as was the SF-36 mental health domain. There were no differences in the incidence of adverse events between the groups.
CONCLUSIONS
Sequential intravenous and oral actovegin treatment over 160 days improved neuropathic symptoms, VPT, sensory function, and quality of life in type 2 diabetic patients with symptomatic polyneuropathy.
Diabetic distal symmetric polyneuropathy (DPN) affects approximately one-third of patients with diabetes (1) and is responsible for substantial morbidity, being associated with excruciating neuropathic pain and foot ulcers leading to amputation (2). Neuropathic pain may affect up to 26% of the diabetic population (3) and can exert a substantial impact on quality of life, particularly through the impairment of sleep and reduced enjoyment of life (4). Several classes of analgesics are effective in the treatment of neuropathic pain, but no more than 40–60% of patients show adequate pain relief on monotherapy (5). Moreover, these drugs are frequently associated with central nervous system side effects and do not slow the progression of the underlying neuropathy (2). Based on the pathogenetic mechanisms of DPN (6), several therapeutic approaches have been developed (2,7,8). These drugs have been designed to favorably influence the pathophysiology of the disorder rather than simply relieve pain. However, despite apparent recent progress, the pharmacologic treatment of chronic symptomatic DPN remains a challenge for the physician (5).
Actovegin is a deproteinized hemoderivative produced from calf blood by ultrafiltration that contains low–molecular weight compounds of up to 5,000 Da. Oxygen absorption, oxygen utilization, and cellular energy metabolism are stimulated by actovegin (9). Furthermore, actovegin exerts insulin-like activity, such as stimulation of glucose transport, pyruvate dehydrogenase, and glucose oxidation (10,11). Because of these properties, actovegin has previously been used for treatment of cerebral vascular and degenerative disorders (12,13). In a previous small trial (14), actovegin was shown to improve nerve conduction velocity, allodynia, and subjective well-being after 24 weeks in patients with DPN.
Evidence has emerged to suggest that nerve ischemia and hypoxia appear to play a paramount role in the pathogenesis of DPN. Reduced nerve blood flow in experimental DPN may be prevented and corrected by several disease-modifying drugs (6). Against this background, we conducted a randomized, controlled trial to evaluate the efficacy and safety of sequential treatment using 20 intravenous infusions of actovegin (2,000 mg) once daily followed by oral administration (1,800 mg/day) for 140 days.
RESEARCH DESIGN AND METHODS
This was a multicenter (26 centers, three countries), randomized, double-blind, placebo-controlled, parallel-group clinical trial (AV-007-IM). Patients were followed for ∼6 months from the screening visit to the end of the oral treatment period, with efficacy assessments at screening, at every fifth infusion visit, and every 4 weeks during the oral treatment period. Adverse events (AEs) were assessed at all visits. Approval was obtained from local ethics committees, and all patients provided written informed consent. After a maximum screening period of 5 days, a total of 569 type 2 diabetic patients with symptomatic diabetic peripheral polyneuropathy were randomly assigned via an interactive voice response service to treatment with either actovegin (Nycomed Austria) or placebo. To homogenize the study population, the randomization procedure was stratified according to site and the presence or absence of insulin treatment.
Treatment consisted of 20 once-daily intravenous infusions (actovegin 20% with 8 mg/ml or placebo in 250 ml sodium chloride 0.9%; infusion rate: 2 ml/min) for 20–36 days, followed by three tablets (200 mg actovegin per coated tablet or placebo) three times daily for 140 days, with a permitted variation of 125–155 days. In the intention-to-treat (ITT) population, the median (range) periods of intravenous and oral treatment were 25 days (1–38) and 146 days (17–169) for actovegin and 25 days (1–37) and 146 days (10–169) for placebo, respectively.
All bottles containing solution for infusion (active and placebo) were identical and had a nontransparent plastic cover, while tubes for infusion were manufactured in colored plastic material. Before blinding (i.e., before application of the plastic cover), the bottles were stored for ≥3 months and the solution was checked visually for foreign bodies. The coated tablets (active and placebo) were identical in size and appearance.
Inclusion criteria were age between 18 and 65 years; type 2 diabetes according to the American Diabetes Association criteria (15); evidence of symptomatic DPN (i.e., total symptom score [TSS] ≥6 and neuropathy impairment score of the lower limbs [NIS-LL] ≥2, vibration perception threshold [VPT] ≤30 volts, and palpable pulses of posterior tibial artery and dorsal artery of the foot); A1C <10%; patient able to meet the center visits over the trial period; stable dose of tricyclic antidepressants, anticonvulsants, mexiletine, or neuroleptics in patients receiving these drugs for neuropathic pain within the last month; acceptable contraceptive method (hormonal pills, patches, implants, injections, or intrauterine device) in female patients of childbearing potential; and a negative pregnancy test before the first dose of trial medication.
Exclusion criteria included known allergy to actovegin or similar preparations; asymmetrical neuropathy of the trunk or proximal lower limbs; foot ulcer or infection; severe cardiac failure, pulmonary edema, oliguria, anuria, or generalized edema; polyneuropathy due to causes other than diabetes; hospitalization due to DPN within the last month; prior use of medications such as isoniazid, nitrofurantoin, vincristine, and phenytoin; use of cerebrolysin, α-lipoic acid, opiates, transcutaneous electrial nerve stimulation, or acupuncture within the last month; mental, psychiatric, or other conditions that may compromise data collection and understanding of written and verbal information given in the trial; present and/or previous chronic alcohol abuse; and serum creatinine >120 μmol/l.
Primary outcome measures
The two coprimary end points were the TSS and VPT. TSS is a bidimensional summation of the severity and frequency of the four main positive neuropathic sensory symptoms: pain, burning, paresthesia, and numbness (8). VPT was measured using a biothesiometer (Bio-Medical Instrument Company, Newbury, OH) on both feet at five sites: the medial malleolus, medial head of the first metatarsal bone, pulp of the great toe, lateral head of the fifth metatarsal bone, and tuberosity of the fifth metatarsal bone. Scores of the five measurements were averaged for each foot. The two scores were treated as repeat measurements in the statistical model. TSS and VPT were assessed at screening, after 5, 10, 15, and 20 infusions, and every 4 weeks (±5 days) during the oral treatment period.
Secondary outcome measures
The NIS-LL was assessed on the same days as the primary end points and was computed as the sum score of a standard group of examinations of muscle strength (0 = normal to 4 = paralyzed), reflexes (0 = normal to 2 = absent with reinforcement), and touch pressure, vibration, joint position and motion, and pinprick sensation (0 = normal to 2 = absent for each modality) of the great toe and was scored for both sides of the body (16). All participating centers were trained by a senior neurologist (I.S.) to adequately perform the NIS-LL. Quality of life was assessed by the short-form (SF)-36 questionnaire (second version) validated in local languages (17) and was completed by patients at randomization and after the intravenous and oral treatment periods. Additional exploratory analyses included the scores of the four individual TSS symptoms and three individual components of the NIS-LL. In addition, the effects of alcohol use (categorized as never, monthly or less, two to four times a month, two to three times a week, and four or more times a week) and smoking habits on treatment were assessed.
Safety parameters
Physical examination and assessment of vital signs and safety laboratory parameters were performed at screening and after the intravenous and oral treatment periods. Fasting blood glucose was measured at the same time intervals as the TSS. A1C was measured at screening, after the infusion period, and after 2 and 5 months of oral treatment.
Statistical analysis
The two primary outcome measures (TSS and VPT) were computed as the area under the curve (AUC) averaged over the time of exposure. The AUC calculations were performed by the trapezoidal method. Intermediate missing values were interpolated linearly in the calculations. VPT was log transformed. If a patient dropped out prematurely, the average was calculated for the exposure period. The primary analysis included the ITT population. To support the primary analysis, a comparison of the mean change in the individual outcome measures from baseline to end of trial in the two treatment groups was calculated. An ANCOVA with treatment, center, and insulin treatment stratum as fixed effects (VPT additionally adjusted for age) and the baseline outcome measure as a covariate was used. Based on the linear model, an F test was used to test the effect of treatment. The mean difference between treatments was estimated with a 95% CI based on the model. Since there were two primary end points, the Hochberg procedure was applied for multiplicity adjustment (18), ensuring an overall significance level of ≤5%. As a consequence of the multiplicity adjustment, a significant result (after adjustment) for either of the primary end points indicated a positive study outcome for the given end point. Possible center interaction was explored by including an interaction term in the ANCOVA model as a sensitivity analysis. Additional supportive analyses included smoking and alcohol use as separate covariates in the ANCOVA.
The power of the trial was required to be 90%. The sample size consideration was based on a two-sample t test of the hypothesis of no mean difference between treatments. The sample size calculation was based on the TSS, for which a mean of one point is considered as the minimum clinically meaningful treatment difference (19). The sample size based on the TSS was set to 480 patients, with an assumed SD of ±3.1. To compensate for possible dropouts, the final sample size required was 550 patients. Throughout the statistical analyses, two-sided tests at a significance level of α = 0.05 were used.
RESULTS
A total of 661 patients were screened and 569 patients were randomized, 567 of whom were exposed to the study medication (ITT population) and 513 of whom completed all assessments in the study, giving a dropout rate of 9.8%. The per-protocol population consisted of 506 patients. The flow of the patients through the trial is shown in the online appendix (available at http://care.diabetesjournals.org/cgi/content/full/dc09-0545/DC1). The demographic and clinical characteristics of the patients are shown in Table 1. As a sign of homogeneity, no clinically relevant baseline differences between the groups were noted for any of the listed parameters.
Table 1.
Demographic, laboratory, and efficacy parameters in the ITT population at baseline
| Actovegin | Placebo | |
|---|---|---|
| n | 281 | 286 |
| Age (years) | 55.7 ± 6.4 | 55.6 ± 6.3 |
| Sex (% male) | 31 | 27 |
| Race (Caucasian/Mongolian) (%) | 95/5 | 93/7 |
| BMI (kg/m2) | 30.6 ± 5.5 | 30.7 ± 4.8 |
| Systolic blood pressure (mmHg) | 134.6 ± 12.5 | 135.2 ± 12.7 |
| Diastolic blood pressure (mmHg) | 81.1 ± 7.3 | 81.6 ± 7.4 |
| Heart rate (bpm) | 74.1 ± 6.4 | 74.8 ± 6.0 |
| Smoker (%) | 10 | 15 |
| Alcohol drinker (%) | 58 | 53 |
| Insulin treatment (%) | 41 | 41 |
| Duration of diabetes (years) | 8.4 ± 6.4 | 7.9 ± 6.7 |
| Duration of neuropathy (years) | 2.9 ± 3.0 | 2.5 ± 2.8 |
| Retinopathy (%) | 26 | 19 |
| Nephropathy (%) | 5 | 4 |
| Cardiac disorders (%) | 41 | 33 |
| Peripheral artery disease (%) | 11 | 10 |
| Hypertension (%) | 79 | 81 |
| A1C (%) | 7.9 ± 1.5 | 7.7 ± 1.5 |
| Fasting blood glucose (mmol/l) | 8.4 ± 2.2 | 8.3 ± 2.3 |
| TSS | 8.3 ± 1.7 | 8.4 ± 1.6 |
| VPT (volts) | 19.7 ± 6.3 | 20.0 ± 5.8 |
| NIS-LL | 8.4 ± 6.5 | 8.8 ± 7.3 |
| SF-36, physical health | 39.8 ± 7.7 | 39.9 ± 7.5 |
| SF-36, mental health | 39.8 ± 11.9 | 39.9 ± 10.3 |
Data are means ± SD, unless otherwise indicated.
The TSS, averaged over the time course of the trial, was 0.56 points lower among patients in the actovegin group compared with the placebo group (95% CI 0.27–0.85; P = 0.0003). When analyzed from baseline to 160 days, TSS improved by 0.86 points on actovegin compared with placebo (0.50–1.22; P < 0.001). VPT decreased by 3% in the actovegin group compared with the placebo group (95% CI 0–6; P = 0.08) when averaged over the course of the trial and by 5% after 160 days (1–9; P = 0.017).
The mean effect of actovegin upon TSS scores varied across centers, from −2.93 points (95% CI −4.27 to −1.60) to 1.19 points (−0.67 to 3.04), with evidence of a treatment-by-center interaction (P < 0.001). The effect of actovegin on VPT scores also varied across centers, from a reduction of 21% (95% CI 9–31) to an increase of 11% (−9 to 35), with evidence of a treatment-by-center interaction (P = 0.02). No differences in the primary end points were noted between patients with and without insulin treatment. Furthermore, there was no statistically significant interaction of the treatment effect with smoking or drinking habits (data not shown).
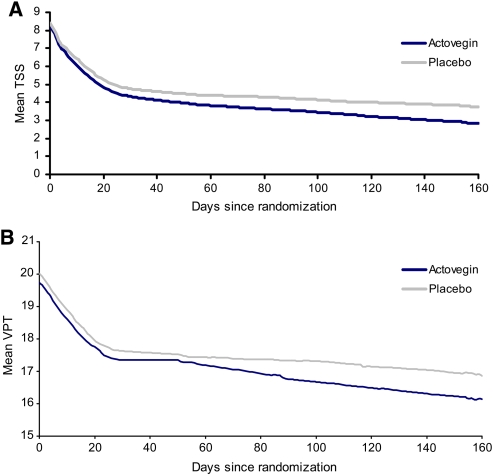
The mean values of TSS and VPT during the intravenous and oral treatment phases of the trial are illustrated in Fig. 1. A relatively high placebo effect in TSS was observed. The response rate after 160 days, if defined as a clinically meaningful reduction in TSS of ≥50%, was 73% in the actovegin group and 61% in the placebo group. The changes in the individual outcome measures from baseline to end of treatment in the ITT population are shown in Table 2. The TSS and its individual neuropathic symptoms, as well as VPT, were reduced significantly after 160 days with actovegin treatment than with placebo (all P < 0.05). NIS-LL tended to improve with actovegin compared with placebo after 160 days (P = 0.08) because of significantly improved sensory nerve function (P = 0.005) but not muscle strength (P = 0.731) or muscle reflexes (P = 0.571). The mental health domain of SF-36 was significantly improved after 160 days of actovegin treatment compared with placebo (P = 0.027), whereas changes in the physical health domain were not significantly different between groups (P = 0.101).
Figure 1.
TSS (A) and VPT (B) during treatment with actovegin (n = 281) or placebo (n = 286).
Table 2.
Changes in the individual outcome measures from baseline to end of treatment in the ITT population treated with actovegin or placebo
| Actovegin | Placebo | Difference (95% CI) | P | |
|---|---|---|---|---|
| n | 281 | 286 | ||
| TSS | −5.5 ± 2.6 | −4.7 ± 2.9 | −0.86 (−1.22 to −0.50) | <0.0001 |
| Lancinating pain | −1.2 ± 1.2 | −1.0 ± 1.2 | −0.20 (−0.32 to −0.08) | 0.0015 |
| Burning pain | −1.5 ± 1.1 | −1.3 ± 1.2 | −0.26 (−0.38 to −0.14) | <0.0001 |
| Paresthesia | −1.3 ± 1.1 | −1.2 ± 1.1 | −0.21 (−0.33 to −0.09) | 0.0007 |
| Numbness | −1.4 ± 1.1 | −1.2 ± 1.1 | −0.24 (−0.38 to −0.10) | 0.0010 |
| VPT (volts) | −3.6 ± 4.5 | −2.9 ± 4.7 | −5% (−9 to −1) | 0.017 |
| NIS-LL | −3.9 ± 4.7 | −3.7 ± 5.0 | −0.48 (−1.01 to 0.06) | 0.080 |
| NIS-LL sensory function | −2.1 ± 2.1 | −1.7 ± 2.1 | −0.38 (−0.64 to −0.12) | 0.0045 |
| NIS-LL reflexes | −0.5 ± 1.3 | −0.6 ± 1.3 | −0.05 (−0.22 to 0.12) | 0.571 |
| NIS-LL muscle strength | −1.3 ± 3.1 | −1.4 ± 3.3 | −0.06 (−0.38 to 0.26 | 0.731 |
| SF-36, physical health | 4.4 ± 7.0 | 3.6 ± 7.1 | 0.91 (−0.18 to 2.00) | 0.101 |
| SF-36, mental health | 5.5 ± 10.6 | 3.8 ± 10.1 | 1.53 (0.17 to 2.88) | 0.027 |
Data are means ± SD, unless otherwise indicated. The first two columns represent the raw within-group differences over time. The last two columns represent model-adjusted between-group differences in the development over time.
After 160 days, A1C decreased by −0.15 ± 1.48% in the actovegin group and increased by 0.10 ± 1.65% in the placebo group (P = 0.04 between groups). Fasting blood glucose decreased from baseline to end of study by −0.40 ± 2.66 mmol/l in the actovegin group and by −0.18 ± 2.51 mmol/l in the placebo group (P = 0.19 between groups).
Safety analysis did not reveal any relevant differences in treatment-emergent AEs (TEAEs) or serious AEs (SAEs) between groups. There were 186 TEAEs in 92 patients in the actovegin group, while 198 TEAEs occurred in 100 patients in the placebo group. Of these TEAEs, 41 (22%) and 35 (18%) were considered possibly or probably related to actovegin and placebo, respectively. Ten SAEs were reported in 7 patients during actovegin treatment, while 11 SAEs occurred in 10 patients treated with placebo. The most frequent SAEs were cardiac disorders (seven events in six patients) and infections (five events in five patients). No deaths occurred during the study.
CONCLUSIONS
The results of this multicenter, randomized, controlled clinical trial show that treatment of symptomatic DPN with intravenous infusions of actovegin (2,000 mg) once daily for 20 days followed by oral administration (1,800 mg/day) for 140 days improves neuropathic symptoms as scored by the TSS, VPT on both feet, the sensory nerve function component of the NIS-LL, and quality of life as evidenced by the mental health domain of the SF-36. This trial also confirms the favorable safety profile of actovegin, which has been demonstrated in previous controlled clinical trials (11–14) and during almost 50 years of postmarketing experience.
The magnitude of the treatment effect observed in this trial deserves comment. A consensus panel previously suggested that a clinically meaningful difference between active treatment and placebo for changes in positive sensory symptoms from baseline is 0.834 points on a 10-point scale and 1 point on a scale similar to the TSS, if an average of several symptom descriptors is used (19). As the mean difference for the changes in TSS between actovegin and placebo after 160 days was 0.86 points, we believe that the drug exerted a clinically meaningful effect on the main neuropathic symptoms.
However, an essential prerequisite for a disease-modifying drug treatment to be effective is a favorable impact on the natural progression of DPN, which is primarily driven by the sensory neuropathic deficits (impairments) rather than symptoms. In this trial, improvement was observed for both neuropathic symptoms (TSS, pain, paresthesia, and numbness) and sensory deficits (VPT, NIS-LL sensory component), further supporting the notion that the effect of actovegin was clinically meaningful. Elevated VPT is an independent risk factor for the development of diabetic foot ulcers. In a 1-year prospective multicenter study, the hazard of the first foot ulcer increased by 5.6% with each volt increase in VPT at baseline (20). In the present trial, the improvement in VPT from baseline to the end of the study was significantly larger in the actovegin group compared with the placebo group (between-group difference: 5% [95% CI 1–9]; P = 0.017). With a baseline mean of ∼20 volts, the implication is that VPT improved by 1 volt more in the actovegin group than the placebo group. Thus, the observed effect of actovegin on VPT appears to reflect a clinically relevant improvement.
This study has several limitations. First, perhaps not surprisingly given that a total of 26 centers from three countries participated in this trial, a center-treatment interaction effect was noted. Although intensive training was carried out in order to standardize all relevant procedures, intercenter variations were to be expected. However, it is reassuring that the observed treatment effects persisted after appropriate adjustment for center. Second, treatment with actovegin was associated with a slight improvement in A1C levels, resulting in a mean difference versus placebo of 0.25%. When adjusting for the changes in A1C, the treatment effect on the TSS decreased minimally from 0.86 to 0.83 points. Thus, although the effect of actovegin on A1C was statistically significant, as well as potentially beneficial, we consider the effect size unlikely to introduce bias toward the observed favorable effect of actovegin on TSS. Third, no objective peripheral nerve function tests, such as nerve conduction studies, were used that could have been more sensitive to a treatment effect with actovegin than VPT as a psychophysical measure. Fourth, a relatively high placebo effect on the TSS was noted. In the placebo group, the response rate, if defined as a clinically meaningful reduction in TSS of ≥50%, reached 61%. Despite such a high placebo effect, the corresponding response rate in the actovegin group of 73% may be considered clinically relevant, as the relative advantage of actovegin versus placebo is ∼20%. A recent meta-analysis (21) showed that the placebo effect does not reach a plateau even after 19 weeks of treatment but tends to continue. Thus, sustained improvement of symptoms, such as pain in the placebo group with increasing duration of the trial, renders it difficult to show superiority of the active drug over placebo.
The mechanisms by which actovegin exerts its effect on neuropathic symptoms is not clear, but it has been shown to improve the cellular energy level, enhance glucose uptake and metabolism, and to increase oxygen absorption and utilization (9–11). Dose-dependent effects on oxygen absorption have been shown to be related to an increased synthesis of high-energy phosphates (9). Actovegin promotes oxidative metabolism and shifts the redox-balance of the cells into the direction of oxidized substrates. This also leads to an increased availability of energy-rich phosphates, such as ATP and creatine phosphate. Furthermore, actovegin may protect against hypoxic cell injury (22), which also could explain its effect, since reduced endoneurial blood flow and nerve ischemia are thought to play a major pathogenetic role in DPN (6). Moreover, recent in vitro studies using freshly prepared primary rat neurons showed that actovegin increases the cell number, neurite length, and the number of synaptic connections of neurons in a dose-dependent manner and inhibits apoptosis as measured by caspase-3 activity (M. Elmlinger et al., unpublished observations).
Actovegin contains inositolphospho-oligosaccharides (IPOs) integrated in the cell membrane, which activate glucose transport (23). IPOs can contribute to up to 50% of the maximum insulin effect on glucose transport (10) and also stimulate the activity of certain enzymes including pyruvate dehydrogenase (24), the key enzyme of the citric acid cycle. IPOs are released from liver membranes upon insulin stimulation (22) and mimic a wide spectrum of insulin-like activities in various cells due to their soluble nature and widespread distribution (25). Thus, there is direct and indirect evidence to support the notion that actovegin exerts an insulin-like effect leading to enhancement of glucose utilization with a direct impact on the cellular metabolism and energy balance in distinct cellular systems.
In conclusion, treatment with actovegin intravenously for 20 days and subsequent oral treatment for 140 days was safe and effective in improving neuropathic symptoms, VPT, sensory nerve function, and mental health in type 2 diabetic patients with symptomatic polyneuropathy. The mechanisms by which actovegin exerts these favorable effects on nerve function remain to be established.
Supplementary Material
Acknowledgments
This study was supported by Nycomed, Roskilde, Denmark. D.Z. and I.G. are consultants for Nycomed and have received honoraria for speaking activities from Nycomed. L.M. is an employee of Nycomed. No other potential conflicts of interest relevant to this article were reported.
We thank the participating center staff and patients for their efforts and commitment during the trial.
Footnotes
Clinical trials reg. no. NCT00483730, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. the KORA Study Group. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008; 31: 464– 469 [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D: Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956– 962 [DOI] [PubMed] [Google Scholar]
- 3. Davies M, Brophy S, Williams R, Taylor A: The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006; 29: 1518– 1522 [DOI] [PubMed] [Google Scholar]
- 4. Galer BS, Gianas A, Jensen MP: Painful diabetic neuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 2000; 47: 123– 128 [DOI] [PubMed] [Google Scholar]
- 5. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS: Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007; 132: 237– 251 [DOI] [PubMed] [Google Scholar]
- 6. Cameron NE, Eaton SE, Cotter MA, Tesfaye S: Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001; 44: 1973– 1988 [DOI] [PubMed] [Google Scholar]
- 7. Chalk C, Benstead TJ, Moore F: Aldose reductase inhibitors for the treatment of diabetic polyneuropathy. Cochrane Database Syst Rev 2007; 4: CD004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ziegler D, Hanefeld M, Ruhnau KJ, Meißner HP, Lobisch M, Schütte K, Gries FA. the ALADIN Study Group: Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant α-lipoic acid: a 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38: 1425– 1433 [DOI] [PubMed] [Google Scholar]
- 9. Kuninaka T, Senga Y, Senga H, Weiner M: Nature of enhanced mitochondrial oxidative metabolism by a calf blood extract. J Cell Physiol 1991; 146: 148– 155 [DOI] [PubMed] [Google Scholar]
- 10. Obermaier-Kusser B, Muhlbacher C, Mushack J, Seffer E, Ermel B, Machicao F: Further evidence for a two-step model of glucose-transport regulation: inositol phosphate oligosaccharides regulate glucose-carrier activity. Biochem J 1989; 261: 699– 705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacob S, Dietze GJ, Machicao F, Kuntz G, Augustin HJ: Improvement of glucose metabolism in patients with type II diabetes after treatment with a hemodialysate. Arzneimittelforschung 1996; 46: 269– 272 [PubMed] [Google Scholar]
- 12. Kanowski S, Kinzler E, Lehmann E, Schweizer A, Kuntz G: Confirmed clinical efficacy of Actovegin® in elderly patients with organic brain syndrome. Pharmacopsychiatry 1995; 28: 125– 133 [DOI] [PubMed] [Google Scholar]
- 13. Herrmann WM, Bohn-Olszewsky WJ, Kuntz G: Infusionstherapie mit Actovegin bei Patienten mit primärer degenerativer Demenz vom Alzheimer-Type und Multiinfarkt-Demenz. Ergebnisse einer prospektiven, Placebokontrollierten Doppelblindstudie bei stationären Patienten. Z Geriatrie 1992; 5: 46– 55 [ article in German] [Google Scholar]
- 14. Jensen W, Beck E: Treatment of the diabetic polyneuropathy: a controlled double blind study. Med Welt 1987; 38: 838– 841 [Google Scholar]
- 15. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32 ( Suppl. 1): S62– S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dyck PJ, Karnes JL, O'Brien PC, Litchy WJ, Low PA, Melton LJ, 3rd: The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology 1992; 42: 1164– 1170 [DOI] [PubMed] [Google Scholar]
- 17. McHorney CA, Ware JE, Lu JF, Sherbourne CD: The MOS 36-Item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994; 32: 40– 66 [DOI] [PubMed] [Google Scholar]
- 18. Hochberg Y: A sharper Bonferroni procedure for multiple tests for significance. Biometrika 1988; 75: 800– 802 [Google Scholar]
- 19. Apfel SC, Asbury AK, Bril V, Burns TM, Campbell JN, Chalk CH, Dyck PJ, Dyck PJ, Feldman EL, Fields HL, Grant IA, Griffin JW, Klein CJ, Lindblom U, Litchy WJ, Low PA, Melanson M, Mendell JR, Merren MD, O'Brien PC, Rendell M, Rizza RA, Service FJ, Thomas PK, Walk D, Wang AK, Wessel K, Windebank AJ, Ziegler D, Zochodne DW. the Ad Hoc Panel on Endpoints for Diabetic Neuropathy Trials. Positive neuropathic sensory symptoms as endpoints in diabetic neuropathy trials. J Neurol Sci 2001; 189: 3– 5 [DOI] [PubMed] [Google Scholar]
- 20. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ: Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998; 21: 1071– 1075 [DOI] [PubMed] [Google Scholar]
- 21. Quessy SN, Rowbotham MC: Placebo response in neuropathic pain trials. Pain 2008; 138: 479– 483 [DOI] [PubMed] [Google Scholar]
- 22. De Groot, Brecht M, Machicao F: Evidence for a factor protective against hypoxic liver parenchymal cell injury in a protein-free blood extract. Res Comm Chem Path Pharm 1990; 68: 125– 128 [PubMed] [Google Scholar]
- 23. Machicao F, Mushack J, Seffer E, Ermel B, Häring HU: Mannose, glucosamine and inositol monophosphate inhibit the effect of insulin on lipogenesis. Biochem J 1990; 266: 909– 916 [PMC free article] [PubMed] [Google Scholar]
- 24. Gottschalk WK, Jarret L: The insulinomimetic effects of the polar head group of an insulin-sensitive glycophospholipid on piruvate dehydrogenase in both subcellular and whole cell assay. Arch Biochem Biophys 1988; 261: 175– 185 [DOI] [PubMed] [Google Scholar]
- 25. Fox JA, Soliz NM, Saltiel AR: Purification of a phosphatidylinositol-glycan-specific phospholipase C from liver plasma membranes: a possible target of insulin action. Proc Natl Acad Sci U S A 1987; 84: 2663– 2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.