Abstract
In this communication, we report a synthesis of anisotropic colloidal superparticles from CdSe/CdS semiconductor nanorods. These anisotropic superparticles are cylindrical disks or stacked-disk arrays. We attribute the major driving forces for controlling superparticle shape to the interparticle interactions between nanorods and solvophobic interactions between a superparticle and its surrounding solvents. According to their sizes (or volumes), the superparticles adopt either single- or multi-layered structures. In addition, these SPs exhibit linearly polarized emissions, demonstrating their potential role as useful components in devices such as polarized light emitting diodes and electrooptical modulators.
The ability to control the formation of nanoparticle superlattices may allow for the synthesis of new materials with desired chemical and physical properties.1–3 In the past decades, efforts have been focused on the preparation of nanoparticle superlattices with different compositions and/or supercrystalline structures,1,2 from which several nanoparticle collective properties have been discovered.3 Recently, several approaches have been reported for making nanoparticle superlattices in the form of colloidal particles (called superparticles, SPs).4 These SPs are spheres with well-controlled sizes ranging from tens to hundreds of nanometers, and their properties can be tailored by varying the size and composition of nanoparticle building blocks or doping with functional organic molecules.4 In addition, these spherical SPs can be assembled into close-packed solid structures, demonstrating their potential as a new class of building blocks in nanoscience.4
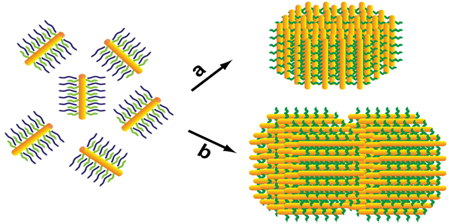
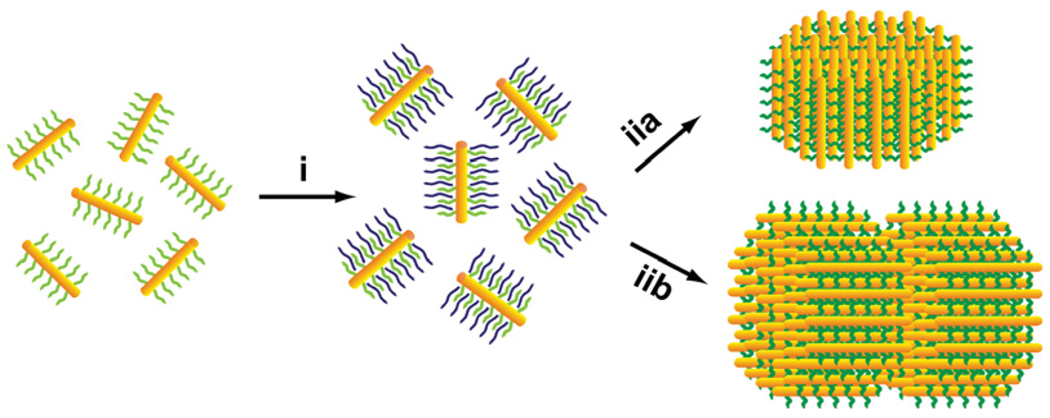
Herein, we report a synthesis of anisotropic SPs from CdSe/CdS semiconductor nanorods. The resulting SPs are cylindrical disks or stacked-disk arrays, and they exhibit photoluminescence (PL), linearly polarized along their axial direction. In this work, CdSe/CdS nanorod building blocks were prepared using a literature method.5 The anisotropic SPs were synthesized by using the approach of controlling solvophobic interactions. With this approach, we have previously synthesized spherical SPs from spherical nanoparticles of various sizes and compositions.4a,4b This SP synthesis includes two major steps (Scheme 1): (i) preparation of water-soluble nanoparticle-micelles, and (ii) growth of SPs by using nanoparticle-micelles in ethylene glycol, in which an annealing treatment (at 80 °C) is required to improve the supercrystallinity of the resulting SPs.4a,4b
Scheme 1.
The synthesis of anisotropic SPs: (i) preparation of nanorod-micelles, and (ii) the formation of cylindrical disk (a), and a bilayer stacked-disk array (b).
In this annealing treatment, suitable capping ligands are critical for stabilizing SPs. Poly(vinyl pyrrolidone) and gelatin were found to be appropriate ligands for making stable spherical SPs.4a,4b However, these ligands cannot effectively passivate the surface of CdSe/CdS nanorod SPs, often resulting in SP samples with aggregation and poor solubility (Fig. S1).6 These results arise, in part, because the ligands have a weak affinity for the (100) facets of CdSe/CdS nanorods. To overcome this difficulty, we used dual-interaction ligands (e.g., dithiol functionalized Tween-20, Tween-SH) to passivate these anisotropic SPs in the growth step.6 These dual-interaction ligands have a strong affinity for CdSe/CdS nanorods, because the ligands bind onto the surface of the nanorods through both coordinate bonding and hydrophobic van der Waals interactions. Indeed, the use of these ligands has led to the formation of stable cylindrical supercrystalline SPs (Fig. 1).
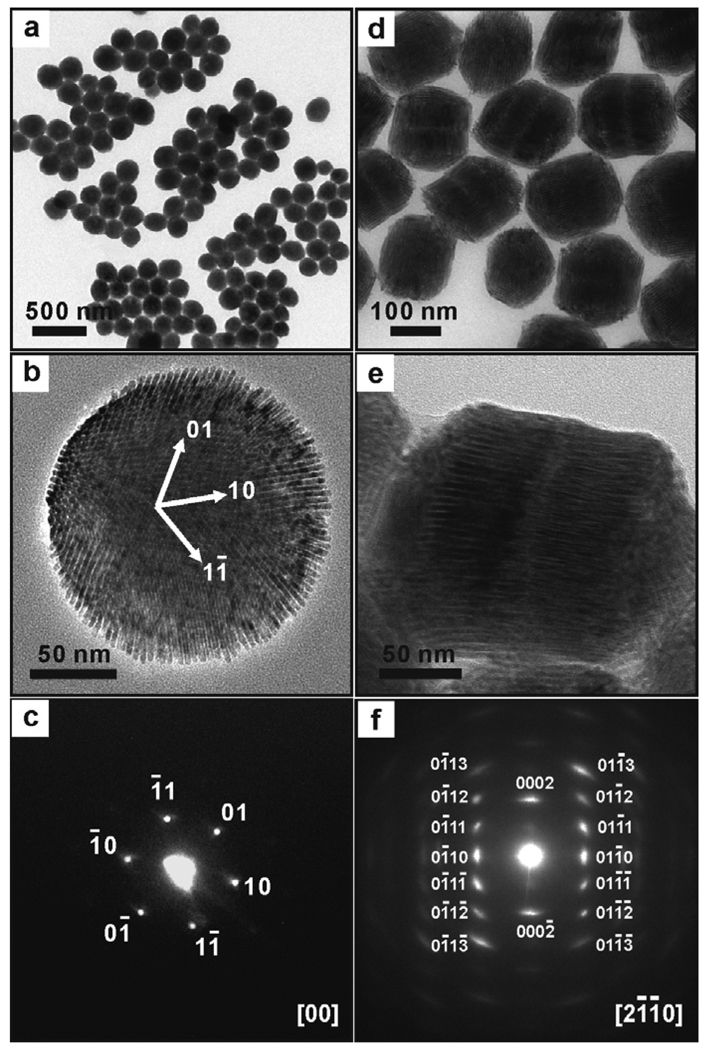
Figure 1.
TEM images of cylindrical SPs: (a) and (b): single-layer disks, (d) and (e): bilayer stacked-disk arrays, (c): a small-angle electron diffraction (ED) pattern of the SP shown in (b), and (f): a wide-angle ED pattern of the bilayer SP shown in (e).
In a typical synthesis, a chloroform solution of CdSe/CdS nanorods (55.4 ± 2.6 nm in length and 4.4 ± 0.2 nm in diameter, 10 mg/mL, 1.0 mL) was mixed with an aqueous solution of dodecyl trimethylammonium bromide (65 mM, 1.0 mL) under stirring. Chloroform was removed from the mixture via bubbling Ar, yielding a clear nanorod-micelle aqueous solution. Under vigorous stirring, this nanorod-micelle solution was injected into a three-neck flask with ethylene glycol (5.0 mL). After 10 min of stirring, a Tween-20-SH aqueous solution (0.1 mM, 1.0 mL) was injected into the flask, and the mixture solution was heated to 80°C at a rate of 10°C/min. This temperature was maintained for 1 h, and then the reaction solution was cooled to room temperature. Colloidal SPs were precipitated from the synthesis solution by centrifugation, with a typical yield of about 70%.
The resulting colloidal SPs are dispersible in polar solvents such as ethanol and water. Transmission electron microscope (TEM) observations reveal that the SPs are cylindrical disks rather than spheres. These cylindrical disks are mainly vertically oriented on the TEM substrate (Fig. 1a), but a small amount of the disks are still laterally oriented on the substrate, giving a side-view of these SPs (Fig. S2).6 The diameter of these disks is 160 nm with a relative standard deviation (σ) of 11% (Fig. 1a). A typical side-view image of these SPs shows that the height of the disks is about 56 nm, and the disks are single-layer assemblies of CdSe/CdS nanorods.6
The supercrystalline structure of these cylindrical SPs was characterized by TEM and small-angle electron diffraction (ED) studies. Because they are single-layer nanorod assemblies, these SPs possess a 2-dimensional (2D) structure. A typical TEM image shows that the SPs exhibit a superlattice-fringe pattern, which is the [00]-zone projection of a p6 2D lattice structure with a lattice constant of 6.0 ± 0.2 nm (Fig. 1b).7 The [00] projection image displays the characteristic hexagonal cross-fringes with a spacing of 5.2 nm, which can be indexed as the (01), (10), and (11) crystal lines in the 2D superlattice (Fig. 1b). The inter-line spacing and angles obtained from the TEM image are consistent with the corresponding small-angle ED pattern (Fig. 1c). The ED pattern shows a sharp-spot array, which demonstrates the perfection of the 2D superlattice.
The formation of cylindrical disks is likely driven by the two major forces: the anisotropic interparticle interactions between CdSe/CdS nanorods, and the repulsive solvophobic interactions between an SP and ethylene glycol solvent molecules. The anisotropic interparticle interactions favor a hexagonally close packing of CdSe/CdS nanorods along their c-axis inside an SP; this packing characteristic can maximize the volume free energy of this SP. The repulsive solvophobic interactions favor an SP with a round shape, which minimizes the SP’s surface free energy.8 Therefore, SPs made from nanorods appear as cylindrical disks. In contrast, if spherical nanoparticles are used as building blocks, the interparticle interactions are isotropic, and thus resulting SPs normally adopt a spherical shape.4
An interesting question is how the shape of SPs changes with a further increase of their size (or volume). To answer this question, we have managed to grow larger sized SPs using a synthesis protocol similar to the one described above, but with a more concentrated nanorod-micelle solution (25 mg/mL, 1.0 mL). TEM measurements show that these larger sized SPs exhibit stacked-disk arrays with very clear fine lines between nanorod layers (Fig. S3 and Fig. 1d).6 Such a shape change should also be the consequence of a compromise between the anisotropic interparticle interactions and the repulsive solvophobic interactions, because stacked-disk arrays have a smaller surface area than that of single-layer disks at the same total volume. Although these larger sized SPs have a broad size distribution (σ~31%, Fig. S3),6 we have obtained a sample of bilayer stacked SPs with a narrow size distribution (σ~9%) by using a careful size-selective separation using centrifugation (Fig. 1d). These bilayer SPs have a diameter of 155 ± 13 nm and a length of 112 ± 8 nm, and nearly all of the SPs are laterally oriented on the substrate (Fig. 1d and e). In addition, the SPs exhibit a typical wide-angle ED pattern, which can be indexed corresponding to the [2 1 1 2] zone diffraction of wurtzite CdS (Fig. 1f).9 The single crystal-like diffraction spots in the pattern indicate that the alignment of nanorod building blocks in an SP is ordered at the atomic level.
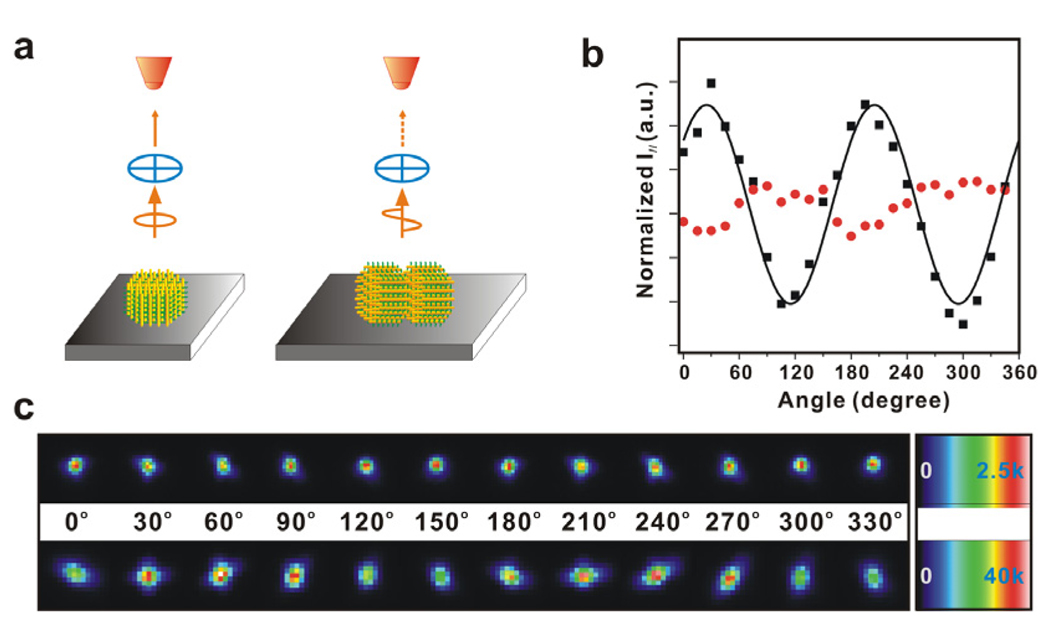
Moreover, these cylindrical SPs possess an anisotropic PL property. In a typical experiment, the PL from single-disk SPs does not have a significant linear polarization, but individual bilayer SPs exhibit much stronger linearly polarized emissions (Fig. 2). This result is consistent with the orientation and structure of the two types of SPs in the TEM observations (Fig. 1 and 2), and the linear polarized emissions of SPs should originate from those of single CdSe/CdS nanorod building blocks.5a, 10 The result also corresponds with the fact that the total PL intensity of a typical bilayer SP is more than an order of magnitude larger than that of a typical single-disk SP, while their volume difference is only about a factor of two (Fig. 1 and 2).6
Figure 2.
(a) Scheme for polarized PL measurements. (b) Normalized I// as a function of polarizer angle: a single disk in red, and a single stacked-disk array in black. I// is the PL intensity measured at the direction parallel to the optical axis of the polarizer. The values of I// are normalized with their mean, and solid line is a cos2 fit. (c) PL images of SPs as a function of polarizer angle: a single disk (top) and a single stacked-disk array (bottom).
In conclusion, we report a synthesis of cylindrical supercystalline SPs from CdSe/CdS nanorods. We demonstrate that the anisotropic interparticle interactions between nanorods as well as solvophobic interactions between an SP and its surrounding solvents play the major roles in controlling the shape of SPs. According to their sizes (or volumes), the SPs adopt either single- or multi-layered structures. In addition, these SPs, with linearly polarized emissions, would be useful as functional components in devices such as polarized light emitting diodes and electrooptical modulators.5a, 11
Supplementary Material
Supporting Information Available: Detailed synthetic procedure and TEM images. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We thank Kerry Siebein for TEM measurements. Y.C.C. acknowledges the NSF (DMR-0645520 Career Award), ONR (N00014-06-1-0911) and the American Chemical Society Petroleum Research Fund (42542-G10) for support of this research. A.D.Q.L. acknowledges the NSF (CHE-0805547) and NIH (GM065306).
References
- 1.(a) Park SY, Lytton-Jean AKR, Lee B, Weigand S, Schatz GC, Mirkin CA. Nature. 2008;451:553. doi: 10.1038/nature06508. [DOI] [PubMed] [Google Scholar]; (b) Shevchenko EV, Talapin DV, Kotov NA, O'Brien S, Murray CB. Nature. 2006;439:55. doi: 10.1038/nature04414. [DOI] [PubMed] [Google Scholar]
- 2.(a) Sun S, Murray CB, Weller D, Folks L, Moser A. Science. 2000;287:1989. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]; (b) Murray CB, Kagan CR, Bawendi MG. Science. 1995;270:1335. [Google Scholar]; (c) Kalsin AM, Fialkowski M, Paszewski M, Smoukov SK, Bishop KJM, Grzybowski BA. Science. 2006;312:420. doi: 10.1126/science.1125124. [DOI] [PubMed] [Google Scholar]
- 3.(a) Collier CP, Saykally RJ, Shiang JJ, Henrichs SE, Heath JR. Science. 1997;277:1978. [Google Scholar]; (b) Courty A, Mermet A, Albouy PA, Duval E, Pileni MP. Nature Mater. 2005;4:395. doi: 10.1038/nmat1366. [DOI] [PubMed] [Google Scholar]
- 4.(a) Zhuang JQ, Wu HM, Yang YA, Cao YC. J. Am. Chem. Soc. 2007;129:14166. doi: 10.1021/ja076494i. [DOI] [PubMed] [Google Scholar]; (b) Zhuang JQ, Wu HM, Yang YA, Cao YC. Angew. Chem. Int. Ed. 2008;47:2208. doi: 10.1002/anie.200705049. [DOI] [PubMed] [Google Scholar]; (c) Klajn R, Gray TP, Wesson PJ, Smoukov SK, Grzybowski BA. Adv. Funct. Mater. 2008;18:2763. [Google Scholar]; (d) Wang D, Xie T, Peng Q, Li Y. J. Am. Chem. Soc. 2008;130:4016. doi: 10.1021/ja710004h. [DOI] [PubMed] [Google Scholar]; (e) Bai F, Wang D, Huo Z, Chen W, Liu L, Liang X, Chen C, Wang X, Peng Q, Li Y. Angew. Chem. Int. Ed. 2007;46:6650. doi: 10.1002/anie.200701355. [DOI] [PubMed] [Google Scholar]
- 5.(a) Carbone L, Nobile C, Giorgi MD, Sala FD, Morello G, et al. Nano lett. 2007;7:2942. doi: 10.1021/nl0717661. [DOI] [PubMed] [Google Scholar]; (b) Talapin DV, Nelson JH, Shevchenko EV, Aloni S, Sadtler B, Alivisatos AP. Nano lett. 2007;7:2951. doi: 10.1021/nl072003g. [DOI] [PubMed] [Google Scholar]
- 6.See Supporting Information.
- 7.Hahn T. International Table for Crystallography. Vol 1. Boston: D.Reidel Publishing; 1983. [Google Scholar]
- 8.Butt HJ, Graf K, Kappl M. Physics and Chemistry of Interfaces. Weinheim: Wiley; 2006. [Google Scholar]
- 9.Williams DB, Carter CB. Transmission Electron Microscopy. New York: Plenum Press; 1996. [Google Scholar]
- 10.Hu J, Li L-S, Yang W, Manna L, Wang L-W, Alivisatos AP. Science. 2001;292:2060. doi: 10.1126/science.1060810. [DOI] [PubMed] [Google Scholar]
- 11.Li L-S, Walda J, Manna L, Alivisatos AP. Nano lett. 2002;2:557. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Detailed synthetic procedure and TEM images. This material is available free of charge via the Internet at http://pubs.acs.org.