Abstract
Due to the shortage of organs, living donor acceptance criteria are becoming less stringent. An accurate determination of the glomerular filtration rate (GFR) is critical in the evaluation of living kidney donors and a value exceeding 80ml/min per 1.73m2 is usually considered suitable. To improve strategies for kidney donor screening, an understanding of factors that affect GFR is needed. Here we studied the relationships between donor GFR measured by 125I-iothalamate clearances (mGFR) and age, gender, race, and decade of care in living kidney donors evaluated at the Cleveland Clinic from 1972 to 2005. We report the normal reference ranges for 1057 prospective donors (56% female, 11% African American). Females had slightly higher mGFR than males after adjustment for body surface area, but there were no differences due to race. The lower limit of normal for donors (5th percentile) was less than 80 ml/min per 1.73m2 for females over age 45 and for males over age 40. We found a significant doubling in the rate of GFR decline in donors over age 45 as compared to younger donors. The age of the donors and body mass index increased over time, but their mGFR, adjusted for body surface area, significantly declined by 1.49±0.61 ml/min per 1.73m2 per decade of testing. Our study shows that age and gender are important factors determining normal GFR in living kidney donors.
Keywords: age, gender, GFR, living donor, MDRD, race
Living donor kidney transplantation is currently considered the best treatment for end-stage renal disease (ESRD) patients.1,2 Owing to the unfortunate combination of a high incidence of patients with ESRD and a concomitant increased waiting time for suitable deceased donors, there is increasing pressure for the acceptance criteria for living kidney donors to become more liberal and variable.3 Current recommendations suggest that living kidney donors should have a glomerular filtration rate (GFR) of at least 80 ml/min per 1.73m2 regardless of age, gender, or race to proceed with donation.4–8 Consistent with this approach, the National Kidney Foundation (NKF) advocates the use of a fixed cutoff value of estimated GFR (eGFR) to define chronic kidney disease (CKD).9 In fact, the current Kidney Disease Outcomes Quality Initiative recommendations allow the diagnosis of CKD when a subject demonstrates an eGFR below 60 ml/min per 1.73m2 independent of underlying clinical status or other evidence of kidney damage such as proteinuria. Because of the increasing demand for organs as well as the constantly changing demographics of the American population, the suitability of the evaluated kidney donor, including renal function, has also evolved into less strict clinical criteria of acceptance.3,4,10 Thus, characterizing the current reference ranges of GFR will provide extremely important knowledge to improve classification between normal kidneys and kidneys with disease.
Unique to the practice of medicine, potential living kidney donors undergo extensive evaluation with the central goal of confirming suspected health instead of suspected disease. A crucial component of the evaluation involves the assessment of renal function. Most centers perform this critical step using either eGFR or a timed creatinine clearance, despite known inaccuracies with these methods.11–14 Other aspects of the medical evaluation process include ruling out hypertension, diabetes, and preexistent kidney disease, because unilateral nephrectomy with any of these conditions may compromise needed kidney function. As such, kidney donors who pass the medical evaluation may be regarded as being ‘healthy’ or ‘normal.’ Indeed, long-term follow up studies have shown that kidney donors may even live longer15 with minimal risk of developing ESRD.16 However, the living donor population is rapidly changing. Data from the Organ Procurement and Transplantation Network show that the proportion of living kidney donors who are at least 50 years old has almost doubled over the past 20 years.17 In fact, some transplant programs have even begun to allow selected hypertensive individuals to donate with encouraging short-term follow-ups.18
The investigation of GFR in ‘health’ has been a subject of research for the past 70 years.19,20 Most prior studies have not included African-American (AA) subjects and in several studies, a complete and thorough evaluation to rule out occult co-morbidities or disease was not carried out or sample size was small. On the basis of previous reports, GFR is known to decline with aging,19,21–23 and may vary by sex and race.24–26 Thus, the lower limit of normal GFR in subjects medically cleared to donate a kidney may be expected to vary depending on these demographic factors. With the changing characteristics of the general population, there may also be secular trends in GFR for the potential donor population. The extensive research database of 125I-iothalamate GFR studies performed at the Cleveland Clinic over more than 30 years provides a unique opportunity to characterize normal GFR in Caucasians, but more importantly in AA subjects, a hitherto understudied population. The main objectives of our current study are (1) to analyze and characterize GFR as measured by 125I-iothalamate urinary clearances in a healthy adult population, including AA subjects, and compare it with creatinine-based eGFR, (2) to study the relationships between donor GFR and age, gender, race, and body size, and (3) to quantify any variation in GFR over time as a consequence of temporal changes in the population characteristics.
RESULTS
Population characteristics
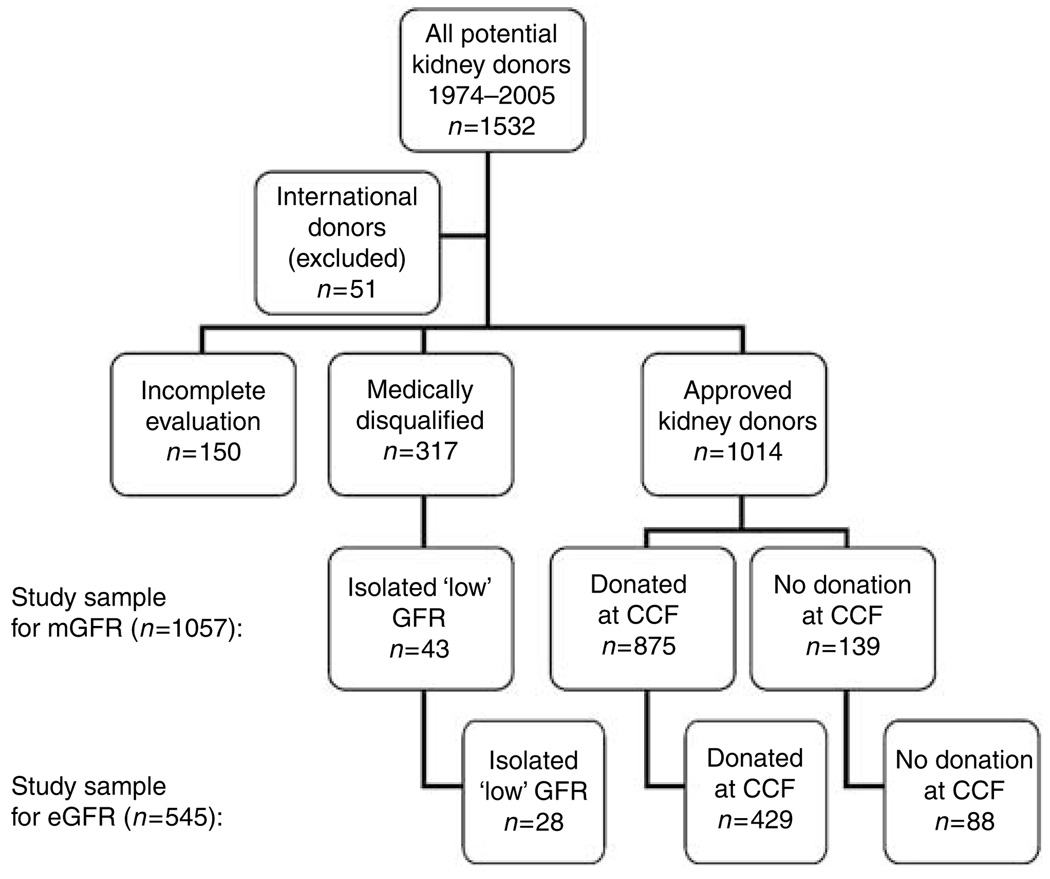
Figure 1 shows how the study sample was identified. After exclusion of international donors (n=51), a total of 1481 subjects were screened for analyses. Of these, 317 subjects completed the donor evaluation but were deemed not suitable donor candidates due to medical abnormalities discovered during the evaluation. The causes for medical exclusion were: hypertension (n=69, 22%), obesity (body mass index (BMI) greater than 35) (n=33, 10%), proteinuria, hematuria or abnormal renal anatomy identified by imaging studies (n=49, 15%), disorders of glucose metabolism or diabetes mellitus (n=20, 6%), bilateral kidney stones (n=18, 6%), malignancy or high risk for malignancy (n=9, 3%), chronic infections (n=14, 4%), familial or genetic diseases (n=6, 2%), isolated low GFR (n=43, 14%), and others (n=56, 18%). Another 150 potential living kidney donors completed the medical evaluation but did not donate (mostly because of alternative donors becoming available, recipients becoming not transplantable, or positive cross-match). Importantly, no medical abnormalities were discovered in this subgroup. The other 1014 donors underwent unilateral donor nephrectomy, either at the Cleveland Clinic (n=875) or at a different institution (n=139). As mentioned above, 43 prospective donors passed the medical evaluation but were not approved for donation because the transplant committee’s arbitrary opinion was that their measured GFR (mGFR) was too low. To define reference ranges in a study sample that was not selected on GFR, they were included in the final study sample for mGFR (n=1057) and eGFR (n=545).
Figure 1. Breakdown of prospective living kidney donors.
Note: Excluded for serum creatinine prior to 1996 (n=512). CCF, Cleveland Clinic Foundation.
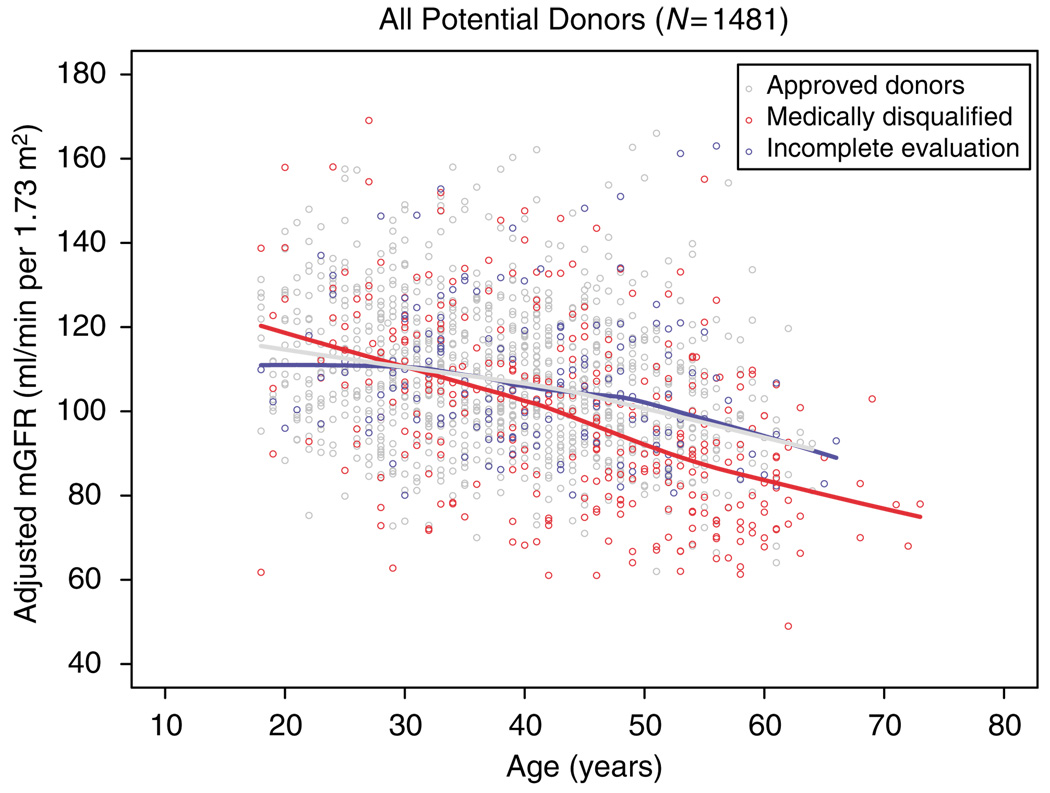
The characteristics of all screened subjects are shown in Table 1. The mean age of the approved donors (n=1014) was 38.5±10.4 years, 56% were women, and 11.1% were AA. The mean adjusted mGFR was 107.6±16.8 ml/min per 1.73m2. Those subjects who were found to have a medical condition were older (43.9±12.0 years, P ≤0.001), had a higher BMI (28.0±5.0, P≤0.001), and had a lower mGFR (98.8 ml/min per 1.73m2, P<0.001) (Figure 2) than the approved donors. The mGFR of those subjects in whom the evaluation was not completed was similar, suggesting that most of these subjects would have actually been approved for donation.
Table 1.
Living kidney donor characteristics
| Medically disqualified for donation | ||||
|---|---|---|---|---|
| Approved donors | All | Isolated ‘low GFR’ | Incomplete donor evaluation | |
| n (%) | 1014 (68.4) | 317 (21.4) | 43 (2.9) | 150 (10.1) |
| Age (years) | 38.5 ± 10.4 | 43.9 ± 12.0* | 51.6 ± 10.4* | 40.8 ± 10.5* |
| Age >45 year old, n (%) | 284 (28.0) | 155 (48.9)* | 32 (74.4)* | 51 (34.0) |
| Female gender | 569 (56.1) | 194 (61.2) | 27 (62.8) | 88 (58.7) |
| African American | 113 (11.1) | 48 (15.1) | 4 (9.3) | 18 (12.0) |
| Evaluations prior to 1990 | 300 (29.6) | 84 (26.5) | 1 (2.3)* | 17 (11.3)* |
| Body surface area | 1.86 ± 0.23 | 1.88 ± 0.20 | 1.92 ± 0.23 | 1.89 ± 0.23 |
| Body mass index (kg/m2) | 26.2 ± 4.2 | 28.0 ± 5.0* | 28.4 ± 5.0* | 27.5 ± 4.8* |
| mGFR/BSA (ml/min per 1.73m2) | 107.6 ± 16.8 | 98.8 ± 22.0* | 73.4 ± 4.9* | 107.6 ± 17.1 |
| mGFR (ml/min) | 115.4 ± 21.8 | 107.4 ± 26.0* | 81.5 ± 11.7* | 116.9±20.4 |
BSA, body surface area; mGFR, measured glomerular filtration rate.
P<0.05 compared with approved donors.
Figure 2. Renal function in prospective kidney donors.
Plot depicting measured radiolabeled iothalamate glomerular filtration rates against donor age for each of the groups.
AA donors (n=113) were younger than non-AA (n=901) (36.1±9.1 vs 38.8±10.5 years old, P=0.003), had a higher BMI (27.6±4.2 vs 26.1±4.2, P≤0.001) and had a higher serum creatinine level (0.96±0.22 vs 0.90±0.14, P=0.002). Female donors (n=569) were older than male donors (n=445) (39.6±10.3 vs 37.2±10.5, P<0.001), less heavy (25.5±4.4 vs 27.2±3.8, P<0.001), and had a lower serum creatinine (0.80±0.14 vs 1.04±0.17, P<0.001). Donors older than 45 years of age (n=284) were heavier (BMI 26.8±3.9 vs 26.0±4.3, P=0.009) and had lower creatinine values than younger ones (n=730) (creatinine 0.87±0.20 vs 0.92±0.19, P=0.003).
Relationship between age, gender, and race with mGFR
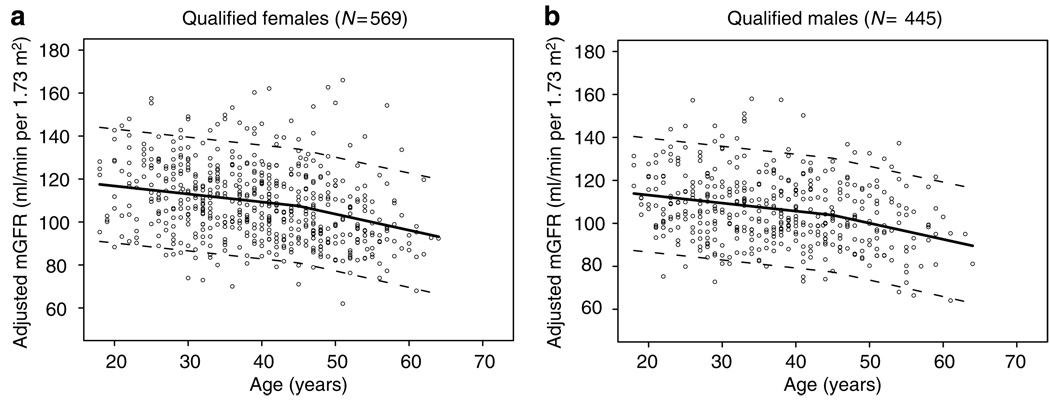
By univariate analysis, there were statistically significant differences in adjusted mGFR between women and men (108.7±17.5 vs 106.1±15.8 ml/min per 1.73m2, P=0.015) but not AA compared with non-AA (108.5±14.8 vs 107.4±17.1 ml/min per 1.73m2, P=0.47). Donors older than 45 years of age had a lower mGFR than younger ones (101.5±16.6 vs 109.9±16.4 ml/min per 1.73m2, respectively, P<0.001). By multivariate linear regression analysis, donor gender (women had 3.61 ml/min per 1.73m2 higher mGFR than men, P<0.001) and donor age (mGFR declined at a rate of −3.73 ml/min per 1.73m2 per decade of life up to the age of 45 years, P<0.001, and at a rate of −7.53 ml/min per 1.73m2 thereafter, P<0.001) (Figure 3), but not donor race (−0.35 ml/min per 1.73m2 in AA vs non-AA, P=0.83) were found to be factors associated with mGFR.
Figure 3. Effects of age on renal function.
Influence of donor age on the slope of glomerular filtration rate in (a) women and (b) men.
Secular trends with mGFR
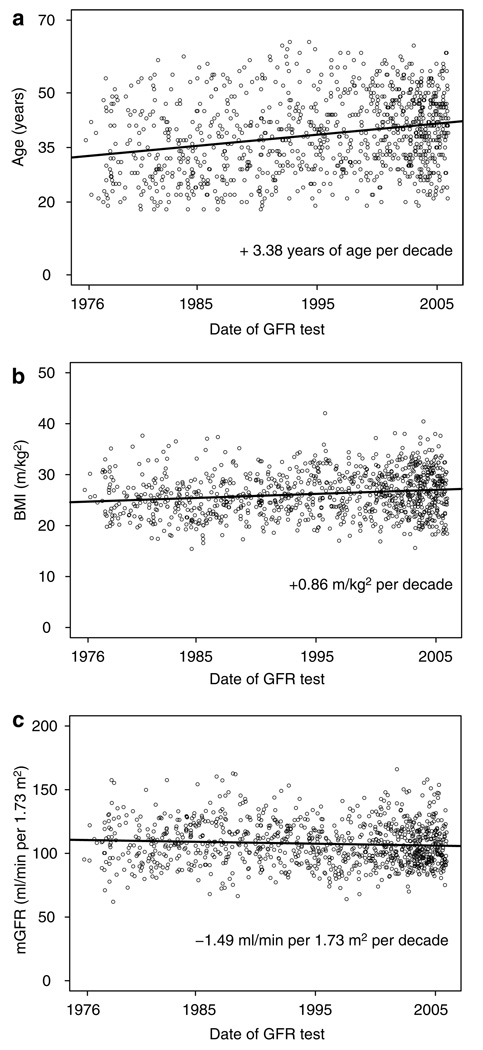
We then studied whether mGFR has changed over three decades from changes in donor acceptance criteria and changes in the general population as a whole. The age and BMI of the accepted donors significantly increased over the past three decades (3.38 years of age and 0.86 units of BMI for every 10-year increase in calendar year of testing, P<0.001 for both), whereas the uncorrected mGFR remained stable over time (−0.14 ml/min for every 10-year increase in calendar year of testing, P=0.86). Consequently, the mGFR adjusted for body surface area (BSA) of living donors has decreased by −1.49±0.61 ml/min per 1.73m2 per decade (P=0.015) (Figure 4).
Figure 4. Secular trends of renal function over 3 decades.
Influence of testing era on (a) living donor age, (b) body mass index, and (c) glomerular filtration rate adjusted for body surface area.
Comparison of mGFR with eGFR
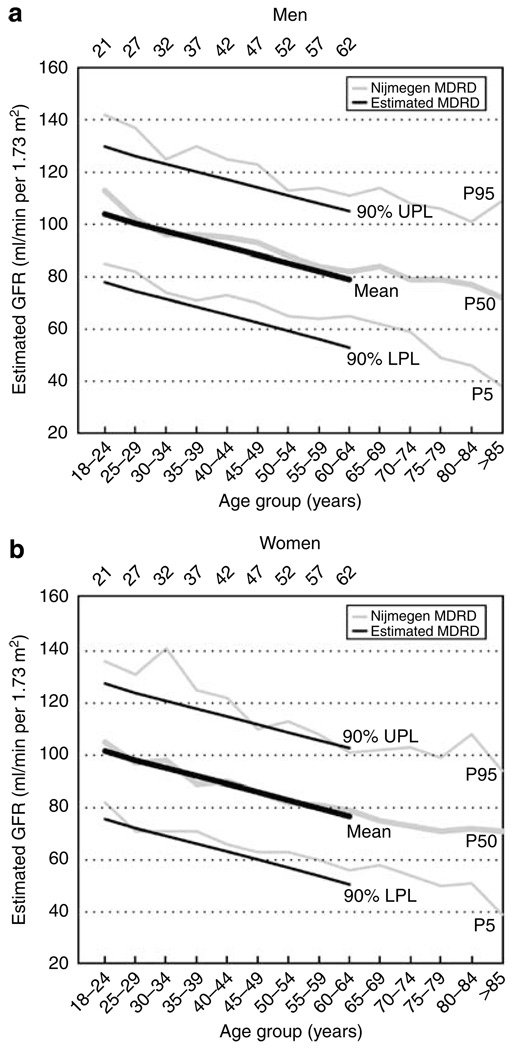
Table 2 shows the lower and upper limits of normal values for mGFR corrected for BSA and how GFR varies based on demographic variables. eGFR by the re-expressed Modification of Diet in Renal Disease (MDRD) study equation is also shown. The mGFR is consistently higher than the eGFR in non-AA subjects. A difference between mGFR and eGFR in AA subjects was less evident, though it should be noted that the MDRD equation factors AA with a 21.2% higher eGFR than in non-AA at the same serum creatinine level. We then calculated the mean expected mGFR and eGFR for male and female living donors (with the estimated 5th and 95th percentile reference range) (Table 3). This analysis was not stratified based on race, because unlike age and sex, there was no difference in mean mGFR by race groups. Estimates of eGFR during 1996–2005 (n=545) by the re-expressed MDRD equation as stratified by gender and age are also shown for comparison. Figure 5 shows how the expected eGFR using data from Table 3 compares to previously reported eGFR derived from a predominantly Caucasian European healthy population.27,28
Table 2.
Observed or actual adjusted GFR by 125I-iothalamate urinary clearances (and eGFR by re-expressed MDRD study equation) in prospective living kidney donorsa
| 125I-Iothalamate GFR (eGFR) (ml/min per 1.73m2) | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Age subgroups (years) |
n mGFR (n eGFR) |
5th percentile | 25th percentile | Median | 75th percentile | 95th percentile |
| All patients | Overall | 1057 (545) | 78 (66) | 94 (79) | 105 (88) | 118 (101) | 136 (120) |
| Non-AA men | Overall | 411 (189) | 76 (68) | 94 (81) | 104 (89) | 116 (102) | 133 (117) |
| 18–25 | 58 (11) | 90 (80) | 101 (85) | 110 (98) | 122 (106) | 136 (138) | |
| 26–35 | 120 (57) | 85 (79) | 97 (88) | 110 (99) | 119 (111) | 143 (117) | |
| 36–45 | 123 (55) | 82 (71) | 96 (84) | 103 (93) | 115 (105) | 129 (121) | |
| 46–55 | 85 (50) | 72 (66) | 87 (73) | 100 (81) | 114 (90) | 130 (104) | |
| ≥56 | 25 (16) | 66 (64) | 75 (70) | 92 (83) | 99 (94) | 117 (100) | |
| AA men | Overall | 50 (26) | 80 (76) | 93 (87) | 100 (100) | 111 (106) | 129 (121) |
| 18–25 | 9 (1) | 109 | 122 (144) | 128 | |||
| 26–35 | 17 (10) | 92 (98) | 100 (103) | 110 (110) | |||
| 36–45 | 16 (7) | 93 (91) | 97 (103) | 106 (105) | |||
| 46–55 | 7 (7) | 80 (80) | 96 (89) | 100 (100) | |||
| ≥56 | 1 (1) | 80 (87) | |||||
| Non-AA women | Overall | 529 (290) | 77 (64) | 93 (76) | 105 (87) | 119 (96) | 140 (120) |
| 18–25 | 46 (13) | 91 (77) | 103 (102) | 122 (103) | 129 (117) | 148 (146) | |
| 26–35 | 138 (62) | 83 (72) | 99 (82) | 111 (95) | 124 (98) | 140 (115) | |
| 36–45 | 165 (97) | 79 (63) | 95 (78) | 106 (89) | 119 (92) | 140 (125) | |
| 46–55 | 137 (94) | 80 (67) | 90 (76) | 97 (86) | 111 (88) | 132 (120) | |
| ≥56 | 43 (24) | 68 (53) | 76 (65) | 91 (74) | 98 (85) | 122 (98) | |
| AA women | Overall | 67 (40) | 84 (78) | 103 (86) | 112 (98) | 121 (106) | 136 (124) |
| 18–25 | 5 (2) | 109 (84) | 117 (106) | 119 (128) | |||
| 26–35 | 25 (11) | 106 (99) | 115 (101) | 123 (118) | |||
| 36–45 | 21 (14) | 105 (86) | 113 (96) | 117 (108) | |||
| 46–55 | 14 (11) | 87 (81) | 108 (93) | 112 (106) | |||
| ≥56 | 2 (2) | 84 (78) | 87 (79) | 91 (80) | |||
AA, African American; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; mGFR, measured glomerular filtration rate.
5th and 95th percentiles were not calculated for subgroups with fewer than 20 subjects.
Table 3.
‘Expected’ adjusted GFR by 125I-iothalamate urinary clearances (and re-expressed MDRD equation) in prospective living kidney donors derived from the actual or observed GFR values presented in Table 2a
|
125I-Iothalamate GFR (re-expressed MDRD study equation) (ml/min per 1.73m2) |
|||
|---|---|---|---|
| Age (years) |
Expected 5th percentile |
Mean expected value |
Expected 95th percentile |
| Women | |||
| 20 | 89 (76) | 116 (102) | 144 (128) |
| 25 | 87 (73) | 114 (99) | 142 (125) |
| 30 | 85 (70) | 113 (96) | 140 (122) |
| 35 | 83 (67) | 111 (93) | 138 (119) |
| 40 | 81 (64) | 109 (90) | 136 (116) |
| 45 | 79 (61) | 107 (87) | 134 (113) |
| 50 | 74 (58) | 101 (84) | 129 (110) |
| 55 | 69 (55) | 96 (81) | 124 (107) |
| 60 | 64 (52) | 91 (78) | 119 (104) |
| Men | |||
| 20 | 86 (79) | 113 (105) | 141 (131) |
| 25 | 84 (76) | 111 (102) | 139 (127) |
| 30 | 82 (73) | 109 (99) | 137 (124) |
| 35 | 80 (70) | 107 (96) | 135 (121) |
| 40 | 78 (67) | 105 (93) | 133 (118) |
| 45 | 76 (64) | 103 (90) | 131 (115) |
| 50 | 71 (61) | 98 (86) | 126 (112) |
| 55 | 66 (57) | 93 (83) | 121 (109) |
| 60 | 60 (54) | 88 (80) | 116 (106) |
GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
For mGFR, all living kidney donors (n=1057), for MDRD all living kidney donors from 1996 to 2005 with measured serum creatinine (n=545).
Figure 5. Comparison of estimated GFR in two different cohorts.
Mean, 5th, and 95th percentiles for expected eGFR by the re-expressed MDRD equation in living kidney donors (black lines) and eGFR by the re-expressed MDRD equation in subjects participating in the Nijmegen study28 (gray lines) among different age groups for (a) men and (b) women.
Comparison of a low GFR by classification systems
During the study period, individuals were excluded from donation by a committee opinion for an isolated low mGFR. Table 4 compares the number and percentage of potential donors who would have been excluded from donation with different classification systems and how these donors would have differed with respect to GFR and demographics. Consistent with the underestimation of GFR in kidney donors by the MDRD equation, substantially more donors would have been excluded by eGFR than by donor committee (27.1 vs 4.1%, P<0.001). Assuming an mGFR cutoff value of greater than 80 ml/min per 1.73m2 to allow donation, from 545 subjects with both mGFR and eGFR, 12 subjects had an eGFR above that value out of 38 subjects with an mGFR below that cutoff (false-positive rate of 31.5%), and of 507 subjects with an mGFR above the cutoff, 122 subjects would have been rejected by eGFR (false-negative rate of 24.1%). The specificity and sensitivity of eGFR to accept or reject subjects based on an mGFR value of 80 ml/min per 1.73m2 was 68.4 and 75.9%, respectively. The donor committee exclusions were more consistent with exclusion by mGFR <80 ml/min per 1.73m2 (kappa=0.73) than age- and sex-specific thresholds (kappa=0.44–0.45). Persons who would be excluded from donation based on Cleveland Clinic age–sex-specific 5th percentile and Mayo Clinic age-specific 5th percentile showed some consistency (kappa=0.75). Switching from a donor committee or an mGFR <80 ml/ min per 1.73m2 to age–sex-specific thresholds would lead to a similar number of overall exclusions, but an overall younger age and higher GFR among persons who are excluded.
Table 4.
Comparison of persons who would be excluded for low mGFR among otherwise healthy kidney donors as determined by different classification systems
| What actually happened |
What could have happened instead | ||||
|---|---|---|---|---|---|
| Characteristics | Excluded by committee |
mGFR <80 ml/min per 1.73m2a |
eGFR <80 ml/min per 1.73m2a |
Mayo Clinic age-specific mGFR 5th percentile22 |
Cleveland Clinic age–sex-specific mGFR 5th percenile |
| n (%) | 43 (4.1) | 67 (6.3) | 148 (27.1) | 67 (6.3) | 45 (4.2)b |
| Kappa (agreement) | Reference | 0.73 | 0.16 | 0.44 | 0.41 |
| mGFR, mean±s.d., n | 73.4±4.9, 43 | 73.2±4.6, 67 | 94.1±15.4, 148 | 76.1±7.3, 67 | 75.4±6.5, 45 |
| eGFR, mean±s.d., n | 71.4±10.5, 28 | 72.5±11.4, 38 | 71.9±6.6, 148 | 77.4±15.6, 35 | 77.4±12.2, 20 |
| Age (years), mean±s.d., n | 51.6±10.4, 43 | 49±11, 67 | 46.1±9.1, 148 | 40.2±11.6, 67 | 38.7±9.6, 45 |
| Men (%) | 37.2 | 43.3 | 28.4 | 47.7 | 33.0 |
| African Americans (%) | 9 | 5.9 | 4.05 | 10.4 | 8.9 |
For mGFR, all living kidney donors (n=1057); for MDRD, all living kidney donors from 1996 to 2005 with measured serum creatinine (n=545).
Note: For the Cleveland Clinic cutoff points we are using Table 3, which splits individuals out only by age and sex.
DISCUSSION
Our study contributes to the current literature because of several novel and clinically important findings: (1) we provide information for normal reference values of both measured and eGFR in the single largest cohort of living kidney donors undergoing strict medical evaluation (who thus can be characterized as ‘healthy subjects’), (2) we characterize differences in normal GFR based on age, gender, and race, and (3) we describe secular trends of donor demographic and anthropometric changes over a 30-year period and their impact on kidney function. Importantly, knowledge of renal function in health has recently been a matter of intense scrutiny by the NKF and the medical scientific community.9,29 Therefore, the information derived from this cohort of living kidney donors is also of significant relevance to the medical community in general because it characterizes the normal range of GFR.
Gaining information about kidney function is perhaps one of the most critical elements in determining the state of health in prospective kidney donors. Current recommendations suggest that potential donors with a GFR of 80 ml/min per 1.73m2 are acceptable for donation. However, this cutoff value is weakly supported by medical evidence.4,5 Moreover, these recommendations do not clearly specify the techniques to calculate renal function. The majority of transplant centers rely on creatinine clearances, which overestimates GFR, and may be allowing donation in subjects with lower than ideal GFR for age and gender. Also, the NKF advocates a predefined cutoff value of eGFR for defining ‘health’ vs ‘disease’ irrespective of age and gender (and perhaps race).9 Concerns about this approach have recently been raised because of the possibility of misclassifying subjects with low eGFR as ‘diseased’ rather than as ‘at risk’ or simply healthy for their age and gender.30–34 The observations derived from this study provide further evidence against recommendations of using ‘fixed’ GFR cutoff levels irrespective of age, gender, and race (critical variables in the MDRD equation). In fact, in this relatively young cohort, women over the age of 50 years were at risk of being misclassified as having CKD on the primary basis of an isolated low eGFR. Moreover, assuming a physiological GFR decline of approximately 8 ml/min per 1.73m2 per decade after the age of 45 years, one could also expect that low eGFRs will be common in healthy aging subjects. Reasoning behind this approach is based on epidemiologic data that show a decreased eGFR being associated with increased mortality.35 However, in the setting of living donation, it is yet unclear whether these former donors with low normal eGFR values are at risk for a progressive decline in GFR other than what is expected with aging. In fact, most data suggest that former donors are at similar risk for developing ESRD and a higher life expectancy than the general population, despite having lower post-donation GFR than controls.15,16,36–39 The information on donor GFR presented here suggests we should reconsider the use of ‘fixed’ GFR cutoff values to make clinically important decisions. From the presented data, it is evident that an mGFR of 80 ml/min per 1.73m2 can be higher or lower than the estimated lower limits of normal GFR depending on whether the donor is 25 or 55 years of age. During the donor selection process, future recommendations should consider normal GFR reference values based on age and gender prior to nephrectomy instead of fixed cutoff values, as this approach may potentially put healthy young subjects at risk for the development of kidney dysfunction during their lifetimes, an understudied area in clinical transplantation.
The information on how gender, race, and age relate to mGFR is another important new observation derived from this large cohort. Since the early times of study of kidney function in normal humans, GFR has been normalized to BSA to account for the effects of body size on absolute GFR,20 an adjustment that more specifically permits a better comparison between genders and subjects of different sizes. Most of the published literature suggests that after adjustment for BSA, women have similar GFR levels to those of men.22 After correcting for age and race, we found a statistically higher mGFR in women than men. However, this difference was no longer present if we studied the entire cohort (n=1532) that included those subjects who were found to have a medical condition that prevented them from donation (data not shown). It was therefore unclear whether this small difference of less than 3% in a subset of the entire cohort was a truly biological difference in GFR or it was due to differences in physiological demand for GFR that is inadequately modeled by BSA estimated by the Dubois and Dubois formula published almost a century ago.40 Nevertheless, this difference is unlikely to be clinically relevant. With respect to AA race, this is the first report to study GFR in this important sub-population at higher risk for poor renal outcomes.41 Importantly, in the state of health there is no difference in normal GFR in AA subjects compared with non-AA subjects, despite a difference in creatinine levels.
Previous studies have reported on the rate of GFR decline with aging, another important issue to consider when defining normality in the context of risk vs disease. Interestingly, we found that GFR does not display a constant rate of decline by age, a very important consideration that theoretically impacts the applicability of a ‘fixed’ age factor used in current GFR estimation models. We found that the GFR declines at a rate of approximately 4 ml/min per 1.73m2 per decade in subjects younger than 45 years of age, similar to what has been previously reported.22 However, an interesting finding is that GFR decline seems to accelerate as subjects age (~8 ml/min per 1.73m2 per decade after the age 45 years). Granerus and Aurell42 published similar rates of GFR decline for subjects younger and older than 50 years of age, and more recently, Fehrman-Ekholm and Skeppholm37 reported even faster rates of decline in healthy subjects older than 70 years of age, suggesting that as human subjects age, GFR declines in an accelerated manner independent of the presence of disease. The data derived from this cohort of kidney donors, in whom an extensive evaluation ruled out significant disease, suggest that GFR decline is a physiologic process of normal aging. This observation again suggests that models to screen for kidney disease or determine normality should account for age-related physiological variations of organ function. As shown in Table 4, this approach would exclude about 5% of donors, similar to the percentage that is excluded by committee or exclusion by mGFR <80 ml/min per 1.73m2. But the excluded donors would be on average younger and have higher GFRs. It is also interesting to note that the lowest 5th percentile of expected eGFR of the studied population is similar to the one reported by Wetzels and co-workers27,28 in a mostly Caucasian community population considered healthy.
We finally looked at how the characteristics of the living donor subjects varied over three decades of GFR testing as living donation increased in popularity and criteria for acceptance became less stringent. It is not surprising to see that older and heavier donors are now being considered for donation. Although the uncorrected mGFR has remained stable over time, the mGFR adjusted for BSA has demonstrated a slight but progressive decrement. This decrease in mGFR is accounted by the increasing age and BMI of the living donor population. This is an important epidemiological observation of potential future public health considering that the American population continues to age and increase in weight.
We recognize several limitations. Kidney donors are a selected healthy population and therefore some observations may not be applicable to the general population, which is not selected on health or disease. This may be a particular concern in the elderly since only 2/1057 donors were over the age of 70 years. It is also important to recognize that some metabolic conditions (for example, hyperuricemia, dyslipidemia, and so on) may not prevent a subject from donating but yet may influence kidney function.43 Nevertheless, the fact that kidney donors are selected on overall health is useful for defining reference ranges.44 Also, reporting values of eGFR in this study shows the systematic underestimation of mGFR by the MDRD equation across the spectrum of age and gender, particularly in non-AA groups. We also show that there is no difference in the normal range for mGFR between AA and non-AA race as others have shown with creatinine clearance.24 It is also critically important to interpret the presented data on eGFR with caution as it is not currently advocated by the NKF to use this method in this clinical setting. However, comparisons of eGFR with mGFR are used in this study to demonstrate the limitations of current approaches to make clinically important decisions. Finally, to determine the long-term clinical and public health implications of low GFR under different classification schemes, further studies assessing outcomes of living donors by their pre-donation mGFR are needed to assess donor morbidity and mortality.
In conclusion, careful attention to living donor renal function evaluation is needed. More importantly, consideration of normal ranges of GFR in health are central to the decision making process when determining health vs disease, as well as when selecting living kidney donors, so as to continue to make this source of organ donation the success that it has been for the past 50 years.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the Cleveland Clinic. A historical chart review was performed on all potential living kidney donors 18 years and older who underwent 125I-sodium iothalamate urinary clearance determinations as part of the donor evaluation process from January 1974 to December 2005. All data were retrieved from electronic or paper medical records. The list of evaluated donors was initially obtained from the Renal Transplant Program at the Cleveland Clinic, and then cross-checked with the Renal Function Laboratory registry for accuracy. After a telephone questionnaire to screen for known medical conditions that would disqualify prospective donors up-front, all donors were invited to visit the Cleveland Clinic for a full medical evaluation. Donor’s age was not generally used as a criterion to disqualify a prospective donor, though the extent to which the perception of health influences that older individuals undergo evaluations as potential donors is unknown. The race of the subjects was self-reported. Renal function as measured by 125I-iothalamate GFR during this initial visit was carried out in all subjects. The donor evaluation consisted of a full history and physical examination independently performed by a clinician as well as a surgeon, followed by an extensive laboratory testing (complete metabolic profile, blood cell count, lipid panel, glucose tolerance test, viral serologies, urine analysis and culture, and 24-hour urine collection for proteinuria), imaging of the urinary tract, chest X-ray, and electrocardiogram.
Details of 125I-sodium iothalamate GFR determination at the Cleveland Clinic have been previously described.45 Patients received a water load before the test. 125I-sodium iothalamate (25 µCu; Glofil; Questor Pharmaceuticals, Union City, CA, USA) was injected subcutaneously without epinephrine. Baseline urine and blood samples were obtained. A voluntary-voided urine sample was discarded, followed by two timed clearance urine collections. Blood samples were drawn before and after each urine collection. Isotope activity was determined by gamma counting of 0.5 ml of plasma or urine on a Packard Minaxi 5000 series counter (Perkin Elmer Life Sciences, Downers Grove, IL). The counts in each period were the average of the samples for each clearance period. The mean mGFR was calculated from two consecutive clearance values, and the results were standardized to BSA (1.73m2) using the Dubois and Dubois formula.40
GFR was also estimated using the recently re-expressed MDRD equation after standardizing the serum creatinine values measured at the Cleveland Clinic traceable to the National Institute of Standards and Technology sample with the following formula: Cleveland Clinic standardized serum creatinine=0.906×(0.099+0.981×Cleveland Clinic serum creatinine).46 We limit the analysis of eGFRs to all the living donors studied after 1996, where serum creatinine calibration bias was determined and corrected.11 All serum creatinine measurements were performed on the same day of the GFR measurement.
Statistical analysis
We compared continuous and categorical variables between approved donors and each of the other two donor categories (‘incomplete evaluation’ and ‘medically disqualified’ groups) using t-tests and χ2 tests, respectively. Among approved donors, we compared AA vs non-AA, men vs women, and subjects older than 45 years of age vs younger subjects using t-tests for continuous variables and χ2 tests for categorical variables. We used linear regression to assess the relationship between age, gender, and race with mGFR as well as the relationship between time period of testing with mGFR, BMI, and age. We also used linear regression to obtain predicted estimates and 90% prediction intervals for adjusted mGFR and eGFR at different levels of age and gender. Assuming that the criteria for living donors remain the same, we would expect future observations of mGFR from living donors to have 90% probability of falling within the presented prediction intervals (that is, the lowest and the highest estimated 5th percentiles). Observed percentiles (5, 25, 50, 75, and 95) of donor mGFR and eGFR stratified by gender, race, and age groups using data from 1996 to 2005 are also reported. The kappa methodology was used to compare various approaches to donor exclusion solely based on mGFR, including the cutoffs used by the Mayo Clinic as an external validation sample.47 A kappa of 1 implies complete agreement, whereas a kappa of 0 implies agreement no greater than expected by chance.
ACKNOWLEDGMENTS
We thank Hank Rolin for his technical expertise in GFR measurements and Dr Phillip Hall for his Renal Laboratory leadership. We also thank Sandra Bronoff for the editorial assistance. Dr Emilio D. Poggio has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Only internal departmental funds were used to complete this study. There is no conflict of interest or financial disclosure related to this study reported by any of the authors. Dr Poggio is supported by a grant from the NIH, NIAID (K23-AI068824-02).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988–1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 2.Leichtman AB, Cohen D, Keith D, et al. Kidney and pancreas transplantation in the United States, 1997–2006: the HRSA Breakthrough Collaboratives and the 58 DSA Challenge. Am J Transplant. 2008;8:946–957. doi: 10.1111/j.1600-6143.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- 3.Reese PP, Feldman HI, McBride MA, et al. Substantial variation in the acceptance of medically complex live kidney donors across US renal transplant centers. Am J Transplant. 2008;8:2062–2070. doi: 10.1111/j.1600-6143.2008.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis CL, Delmonico FL. Living-donor kidney transplantation: a review of the current practices for the live donor. J Am Soc Nephrol. 2005;16:2098–2110. doi: 10.1681/ASN.2004100824. [DOI] [PubMed] [Google Scholar]
- 5.Delmonico F. A report of the Amsterdam forum on the care of the live kidney donor: data and medical guidelines. Transplantation. 2005;79:S53–S66. [PubMed] [Google Scholar]
- 6.Kasiske BL, Ravenscraft M, Ramos EL, et al. The evaluation of living renal transplant donors: clinical practice guidelines. Ad Hoc Clinical Practice Guidelines Subcommittee of the Patient Care and Education Committee of the American Society of Transplant Physicians. J Am Soc Nephrol. 1996;7:2288–2313. doi: 10.1681/ASN.V7112288. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, Bia MJ. The evaluation and selection of living kidney donors. Am J Kidney Dis. 1995;26:387–398. doi: 10.1016/0272-6386(95)90664-9. [DOI] [PubMed] [Google Scholar]
- 8.Davis C. Evaluation of the living kidney donor: current perspectives. Am J Kidney Dis. 2004;43:508–530. doi: 10.1053/j.ajkd.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Matas AJ, Bartlett ST, Leichtman AB, et al. Morbidity and mortality after living kidney donation, 1999–2001: survey of United States transplant centers. Am J Transplant. 2003;3:830–834. [PubMed] [Google Scholar]
- 11.Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft–Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 12.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 13.Rule AD, Torres VE, Chapman AB, et al. Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: the consortium of radiologic imaging studies of polycystic kidney disease cohort. J Am Soc Nephrol. 2006;17:854–862. doi: 10.1681/ASN.2005070697. [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 15.Fehrman-Ekholm I, Elinder CG, Stenbeck M, et al. Kidney donors live longer. Transplantation. 1997;64:976–978. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Fehrman-Ekholm I, Norden G, Lennerling A, et al. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82:1646–1648. doi: 10.1097/01.tp.0000250728.73268.e3. [DOI] [PubMed] [Google Scholar]
- 17.Living Donors Recovered in the US http://www.optn.org/latestData/rptData.asp. (vol 2007) The Organ Procurement and Transplantation Network. 2007
- 18.Textor SC, Taler SJ, Driscoll N, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2004;78:276–282. doi: 10.1097/01.tp.0000128168.97735.b3. [DOI] [PubMed] [Google Scholar]
- 19.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950;29:496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith H. Comparative physiology of the kidney. In: Smith HW, editor. The Kidney: Structure and Function in Health and Disease. New York, NY: Oxford University Press; 1951. pp. 520–574. [Google Scholar]
- 21.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 22.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 24.James GD, Sealey JE, Alderman M, et al. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens. 1988;1:124–131. doi: 10.1093/ajh/1.2.124. [DOI] [PubMed] [Google Scholar]
- 25.Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 26.Foley RN, Wang C, Ishani A, et al. NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol. 2007;18:2575–2582. doi: 10.1681/ASN.2006121411. [DOI] [PubMed] [Google Scholar]
- 27.Wetzels J, Kiemeney B, Swinkels D, et al. Age and gender specific reference values of estimated GFR in Caucasians: results of the Nijmegen Biomedical Study. Kidney Int. 2007;72:632–637. doi: 10.1038/sj.ki.5002374. [DOI] [PubMed] [Google Scholar]
- 28.Wetzels JF, Willems HL, den Heijer M. Age- and gender-specific reference values of estimated glomerular filtration rate in a Caucasian population: results of the Nijmegen Biomedical Study. Kidney Int. 2008;73:657–658. doi: 10.1038/sj.ki.5002755. [DOI] [PubMed] [Google Scholar]
- 29.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 30.Couser WG. Chronic kidney disease the promise and the perils. J Am Soc Nephrol. 2007;18:2803–2805. doi: 10.1681/ASN.2007080964. [DOI] [PubMed] [Google Scholar]
- 31.Poggio ED, Rule AD. Can we do better than a single estimated GFR threshold when screening for chronic kidney disease? Kidney Int. 2007;72:534–536. doi: 10.1038/sj.ki.5002452. [DOI] [PubMed] [Google Scholar]
- 32.Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol. 2008;19:844–846. doi: 10.1681/ASN.2008010110. [DOI] [PubMed] [Google Scholar]
- 33.de Jong PE, Gansevoort RT. Fact or fiction of the epidemic of chronic kidney disease – let us not squabble about estimated GFR only, but also focus on albuminuria. Nephrol Dial Transplant. 2008;23:1092–1095. doi: 10.1093/ndt/gfn028. [DOI] [PubMed] [Google Scholar]
- 34.Glassock RJ, Winearls C. An epidemic of chronic kidney disease: fact or fiction? Nephrol Dial Transplant. 2008;23:1117–1121. doi: 10.1093/ndt/gfn086. [DOI] [PubMed] [Google Scholar]
- 35.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 36.Fehrman-Ekholm I, Duner F, Brink B, et al. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 2001;72:444–449. doi: 10.1097/00007890-200108150-00015. [DOI] [PubMed] [Google Scholar]
- 37.Fehrman-Ekholm I, Skeppholm L. Renal function in the elderly (<70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand J Urol Nephrol. 2004;38:73–77. doi: 10.1080/00365590310015750. [DOI] [PubMed] [Google Scholar]
- 38.Garg AX, Muirhead N, Knoll G, et al. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]
- 39.Goldfarb DA, Matin SF, Braun WE, et al. Renal outcome 25 years after donor nephrectomy. J Urol. 2001;166:2043–2047. [PubMed] [Google Scholar]
- 40.DuBois D, DuBois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 41.McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 42.Granerus G, Aurell M. Reference values for 51Cr-EDTA clearance as a measure of glomerular filtration rate. Scand J Clin Lab Invest. 1981;41:611–616. doi: 10.3109/00365518109090505. [DOI] [PubMed] [Google Scholar]
- 43.Issa N, Stephany B, Fatica R, et al. Donor factors influencing graft outcomes in live donor kidney transplantation. Transplantation. 2007;83:593–599. doi: 10.1097/01.tp.0000256284.78721.ba. [DOI] [PubMed] [Google Scholar]
- 44.NCCLS. How to Define and Determine Reference Intervals in the Clinical Laboratory; Approved Guideline. 2nd edn. Wayne, PA: NCCLS document C28-A2[ISBN 1-56238-406-6] NCCLS; 2000. pp. 19087–1898. [Google Scholar]
- 45.Israelit AH, Long DL, White MG, et al. Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate. Kidney Int. 1973;4:346–349. doi: 10.1038/ki.1973.127. [DOI] [PubMed] [Google Scholar]
- 46.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors [erratum appears in Am J Kidney Dis 2004; 44: 1126] Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]