Abstract
Fluorination has gained an increasingly important role in drug discovery and development. Here we describe a versatile strategy that combines cytochrome P450-catalyzed oxygenation with deoxofluorination to achieve mono- and polyfluorination of non-reactive sites in a variety of organic scaffolds. This procedure was applied for the rapid identification of fluorinated drug derivatives with enhanced membrane permeability.
Fluorination has become an increasingly important tool for fine-tuning the pharmacokinetic and pharmacological properties of drugs and lead compounds, leading to a growing number of fluorine-containing pharmaceuticals on the market.1 Benefits of hydrogen-to-fluorine substitutions arise principally from their effects on membrane permeability, metabolic stability, and/or receptor-binding properties of bioactive molecules.1–3 In many cases, fluorination of much less active precursors yielded potent drugs, with enhanced bioavailability, reduced toxicity, or improved affinity for the target receptor.3 A number of methods have been developed for synthesis of fluorinated compounds,4,5 including asymmetric fluorination strategies6,7 and chemo-enzymatic approaches8,9. Despite this progress, selective incorporation of fluorine at non-activated or weakly-reactive sites of a target scaffold remains difficult and may require several synthetic steps.
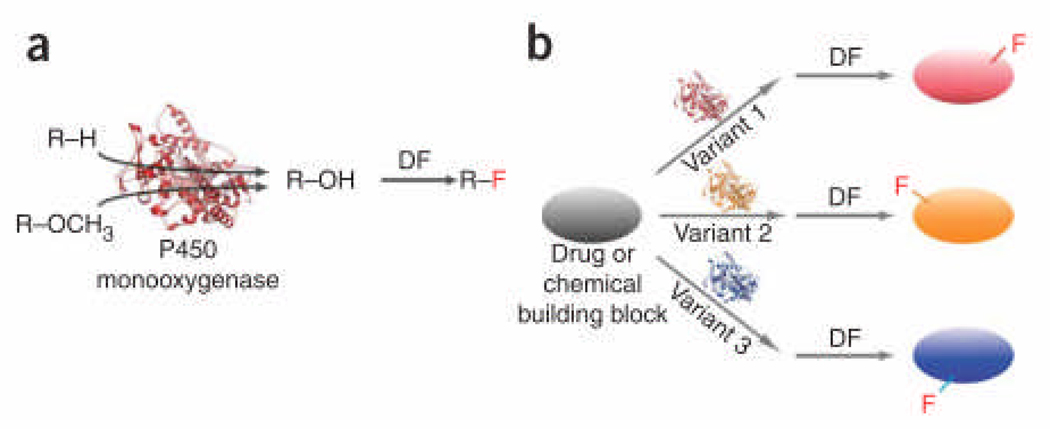
Here we describe a facile, two-step procedure for the selective fluorination of one or more non-activated sites in an organic molecule. This approach couples the exceptional ability of cytochrome P450 monooxygenases to selectively insert oxygen into non-reactive C-H bonds with a deoxofluorination (DF) reaction in which the newly generated hydroxyl group is substituted by fluorine by means of a nucleophilic fluorinating reagent (Fig. 1). To test the validity of this approach, we targeted various classes of small molecules, including marketed pharmaceuticals (Supplementary Figure 1 online). For the enzymatic step, we used variants of the bacterial long-chain fatty acid hydroxylase P450BM3 from Bacillus megaterium. Catalytic self-sufficiency, high monooxygenase activity, and high expression level in E. coli render P450BM3 an attractive catalyst for in vitro and in vivo applications.10 For this work, we assembled a panel of 96 P450s derived from a catalytically-promiscuous P450BM3 variant identified in the early stages of the directed evolution of a proficient alkane monoxygenase.11 These variants were found to exhibit good activity and various degrees of selectivity on alkanes and non-alkane substrates.11
Figure 1. Cytochrome P450-based approach for selective fluorination of organic molecules.
(a) Hydroxyl groups are introduced (via direct oxygen insertion) or exposed (via hydroxylation/demethylation) in a target scaffold using a P450 monooxygenase and substituted for fluorine using a nucleophilic fluorinating agent. DF: deoxofluorination. (b) Different fluorinated derivatives of a molecule of interest are obtained using P450 variants with different regioselectivities.
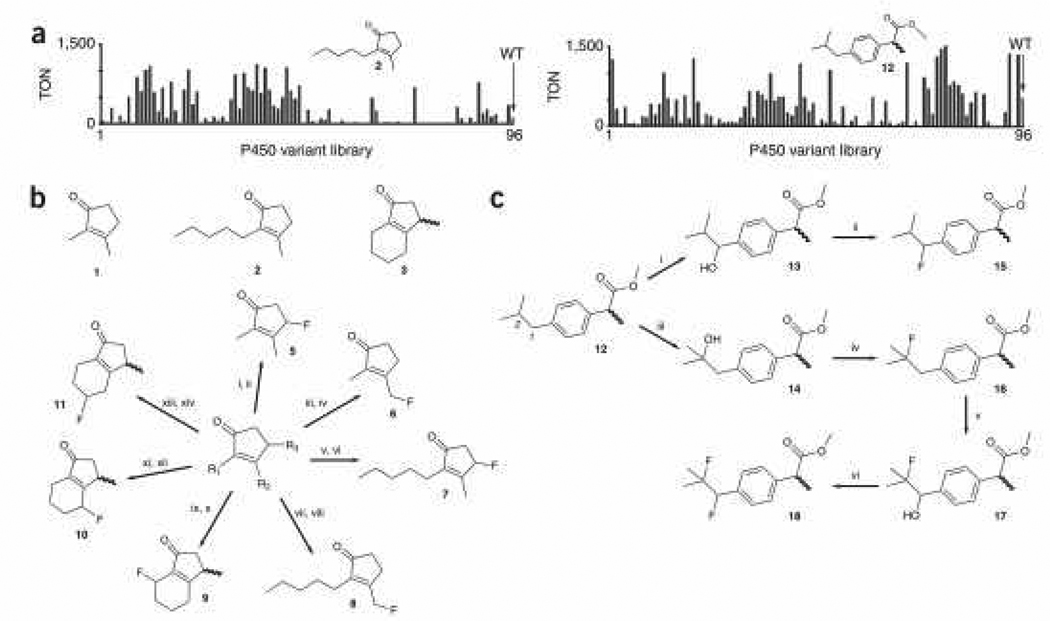
The first group of test molecules (1, 2 and 3; Fig. 2a,b) contains a cyclopentenone moiety found in several natural products (e.g. jasmonoids, cyclopentanoid antibiotic, and prostaglandins). The synthesis and functionalization of these scaffolds is not trivial.12 The activities of the enzymes towards these substrates were probed in multi-well format using GC and GC-MS (Fig. 2a). Depending on the substrate, approximately 30 to 50% of the 96 enzyme variants displayed useful activity (>800 turnovers), while 30 to 50% of this active subset showed moderate to excellent regioselectivity (50–100%). The most active and selective variants were applied in preparative scale reactions (100–300 mg) using ~ 0.05 mol% catalyst. Compared to 96-well plate reactions, three to four times higher turnover numbers could be obtained using purified enzyme and longer reaction times (24–48 hours). After flash chromatography purification, the hydroxylated products were subjected to deoxofluorination using the common nucleophilic fluorinating agent diethylaminosulfur trifluoride (DAST, 4). The identities and purities of the fluorinated products were established by GC-MS, HRMS, and 1H-, 13C- and 19F-NMR (Supplementary Methods online). Using this strategy, we were able to target two to three different sites on each substrate with good to excellent regioselectivity (55–100%), affording the fluorinated derivatives 5, 6, 7, 8, 9, 10 and 11 with yields of up to 80% over the two steps.
Figure 2. Chemo-enzymatic fluorination of organic molecules.
(a) Screening of P450 library in 96-well format. Reactions were carried out in the presence of the substrate, P450 enzyme from cell lysate, and a glucose-6-phosphate dehydrogenase-based NADPH regeneration system. TON: turnover number. Standard error is within 15%. WT = wild-type P450BM3. (b) Selective fluorination of cyclopentenone derivatives. Reagents and conditions: i) 1, 0.04 mol% var-H3, 88%; ii) DAST (1.2 equiv), CH2Cl2, −78°C, 12 h, 90% (20% ee) ; iii) 1, 0.04 mol% var-G6, 45%; iv) DAST (1.2 equiv), CH2Cl2, −78°C, 12 h, 85%; v) 2, 0.05 mol% var-H3, 85%; vi) DAST (1.3 equiv), CH2Cl2, −78°C, 12 h, 92% (78% ee); vii) 2, 0.05 mol% var-G4, 42%; viii) DAST (1.5 equiv), CH2Cl2, −78°C, 12 h, 89%; ix) 3, 0.05 mol% var-D10, 69%; x) DAST (1.2 equiv), CH2Cl2, −78°C, 3 h, 88% (dr 1:8.5, major: 5% ee, minor: 71% ee); xi) 3, 0.05 mol% var-G4, 62%; xii) DAST (1.2 equiv), CH2Cl2, −78°C, 5 h, 92% (dr 4:96, major: 0% ee, minor: 57% ee); xiii) 3, 0.07 mol% var-G5, 32%; xiv) DAST (1.2 equiv), CH2Cl2, −78°C, 5 h, 90% (dr not measurable). (c) Selective mono- and difluorination of prodrug ibuprofen methyl ester. Reagents and conditions: i) 0.1 mol% var-B4, 72%; ii) DAST (1.4 equiv), CH2Cl2, −78°C, 12 h, 86% (dr 1:3.2, major: 19% ee, minor: 44% ee); iii) 0.05 mol% var-G4, 88%; iv) DAST (1.4 equiv), CH2Cl2, −78°C, 12 h, 95%; v) 0.06 mol% var-B2, 93%; vi) DAST (1.2 equiv), CH2Cl2, −78°C, 12 h, 98% (dr 1:3.7, major: 9% ee, minor: 9% ee). All experimental procedures are described in detail in Supplementary Methods. The sequences of the P450 variants are described in Supplementary Table 2. Yields refer to the isolated products. Enantiomeric excess values were determined by chiral GC analysis.
Next we tested this fluorination strategy on a methyl ester pro-drug of the anti-inflammatory drug ibuprofen (12; Fig. 2a,c). While preparation of α-fluoro derivatives of this compound is straightforward,13 incorporating fluorine atoms in the poorly reactive isobutyl group is not. The general procedure described above enabled us to identify two chemo-enzymatic routes to achieve this goal in a selective (position 1: 75%; position 2: 100%) and efficient manner (yields over two steps for 15 and 16 were 62% and 84%, respectively) and at preparative scales (150–200 mg).
We then investigated whether two sequential chemo-enzymatic transformations could be used to fluorinate multiple sites of the same molecule. P450 variant B4 (var-B4)—which was used to convert 12 to 13—was found to retain comparable activity on 16, providing a possible route to the desired 17 intermediate. Re-screening of 12-active variants on 16, however, led to the identification of a more suitable candidate, var-B2, with higher activity than var-B4 towards 16 and excellent (100%) 2-regioselectivity. Using var-B2, the synthesis of fluoro-hydroxy derivative 17 was afforded in higher yields (93% vs. 72% for 12→13) and required less catalyst (0.06 mol% vs. 0.1 mol% for 12 to 13). 17 was then converted quantitatively to the desired difluoroderivative 18.
The value of the present approach as synthetic tool for asymmetric fluorination was also examined. In the absence of anchimeric group participation, the DF reaction generally preserves the enantiopurity of the enzymatic products through inversion of configuration.14 Chiral GC analysis showed appreciable stereoselectivity during preparation of 7 (78% ee), 9 (dr 1:8.5), 10 (dr 4:96), 15 (dr 1:3.2), and 18 (dr 1:3.7) (see Supplementary Fig. 2–Supplementary Fig. 6 online for gas chromatography traces). We then extended our previous investigations on 2-phenyl acetic acid esters,15 carrying out the asymmetric synthesis of the corresponding 2-fluoro-2-aryl acetic acid derivatives at 100 mg-scale (19a, 20a, 21a, 22a and 23a; Supplementary Table 1 online). In this case, up to 89% ee in up to 60% yield (two steps) were achieved.
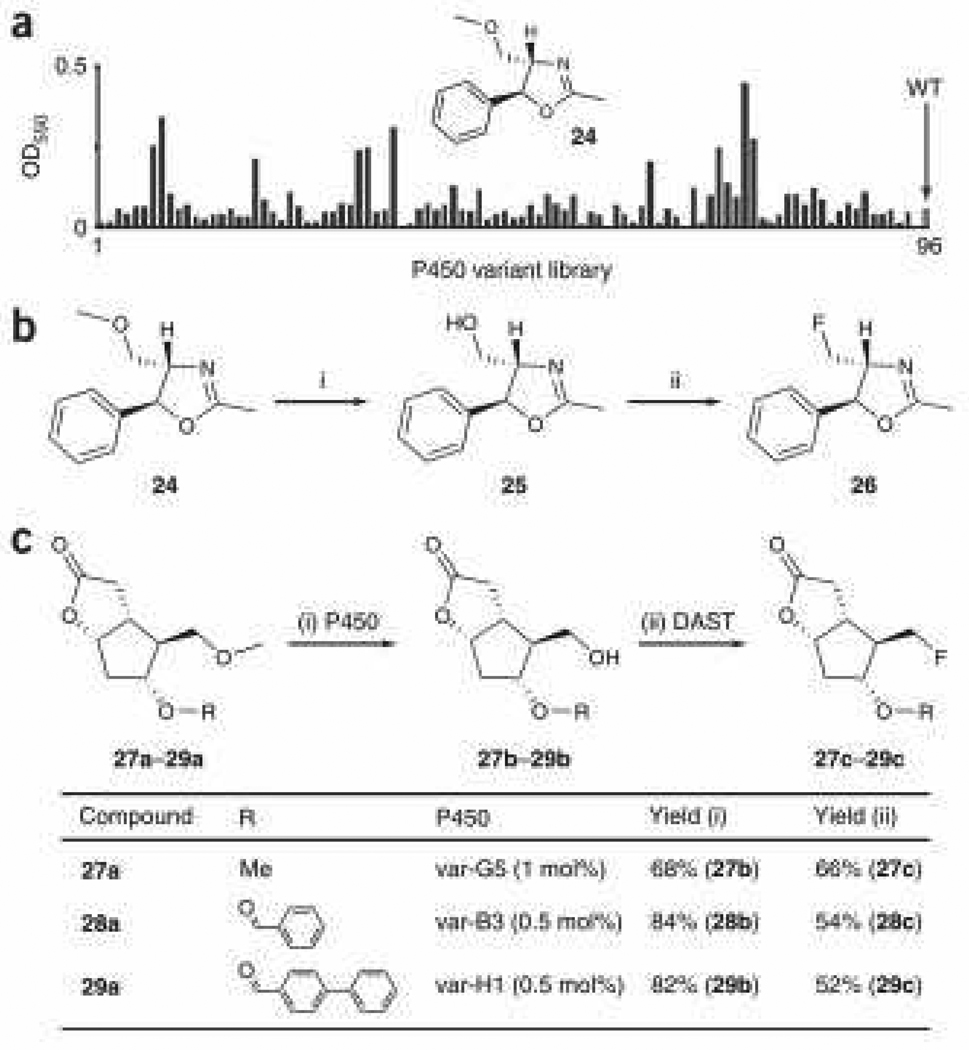
P450-catalyzed hydroxylation of methoxy groups leads to exposure of a free hydroxyl group through decomposition of the hemi-acetal produced and release of formaldehyde. We reasoned that our chemo-enzymatic strategy could be extended to substitute a methoxy group for fluorine, a challenging transformation for traditional chemical methods. This approach was first tested on the 5-phenyl oxazoline derivative 24 (Fig. 3a,b). The demethylation activities of the P450 variants could be easily assessed using a colorimetric screen (Fig. 3a). The most active variants from the screen were further analyzed with respect to the regioselectivity of oxidation using GC-MS. The highly selective P450 variant var-H1 (95%) was thus applied in combination to DF to afford the desired fluorine-containing compound (26).
Figure 3. Chemo-enzymatic methoxy-to-fluorine transformation.
(a) Screening of P450 demethylation activities using a Purpald-based assay for detection of formaldehyde formation in 96-well plate format. Standard error is within 15%. WT = wild-type P450BM3. (b) Methoxy-to-fluorine transformation in a 5-phenyl oxazoline derivative. Reagents and conditions: i) 0.1 mol% var-H1, KPi pH 8.0, room temperature, 48 h, 92%; ii) DAST (1.0 equiv), CH2Cl2, 0°C, 12 h, 40%. (c) Methoxy-to-fluorine transformation in Corey lactone derivatives. All experimental procedures are described in detail in Supplementary Methods. The sequences of the P450 variants are described in Supplementary Table 2. Yields refer to the isolated products.
The same approach was tested on a set of derivatives of the synthetically important building block Corey lactone (Fig. 3c). The use of various Corey lactones (27a, 28a and 29a) enabled us to investigate the tolerance of the enzymatic transformation to structural variations within the target scaffold. Based on the colorimetric screen, 30 variants displayed activity on at least one Corey lactone (12 on 27a, 17 on 28a, 5 on 29a). Twelve variants were found to accept both 27a and 28a, five 28a and 29a, and five 27a and 29a. Notably, four variants (~10% of the Corey lactone-active variants) could be used to activate the substrate for subsequent fluorination, regardless of the size of the variable substituent. Using the most active and selective enzymes towards each of the Corey lactones, the desired fluorine-containing compounds 27c, 28c, and 29c were synthesized and isolated.
Ibuprofen has recently shown promising activity against amyloidogenic diseases.16 Anti-amyloidogenic drugs with high brain permeability are intensively sought.16 We tested 15, 16 and 18 in a membrane permeability assay that mimicks the composition of the blood-brain barrier (BBB). While 12 has only modest BBB-crossing potential, monofluorinated 15 and 16 and difluorinated 18 exhibit, respectively, very good and excellent membrane permeability properties (effective permeability value > 10 × 10−6 cm s−1, Supplementary Fig. 7 online) which demonstrates how this procedure could be applied to rapidly screen various hydrogen to fluorine substitutions in a target molecule for improvement in chemo-physical or biological properties.
This chemo-enzymatic approach has proven useful for fluorinating 13 of the 16 molecules tested (see Supplementary Fig. 1). The molecular weight of these compounds ranges between 110 and 450 Da. About 75% of commercial drugs fall within this window.17 Much larger molecules may have restricted access to the enzyme’s active site, representing suboptimal targets for this chemo-enzymatic approach. As the P450BM3 active site is largely hydrophobic, highly polar compounds may also be poor substrates. Difficulties were mostly associated with solubilisation of the substrate in aqueous media (30) or with the occurrence of side reactions—in particular elimination—during the deoxofluorination transformation (hydroxylated 31 and 32), which prevented isolation of the enzymatic and fluorinated products, respectively, in satisfactory yields. These issues could be addressed, however, by using P450BM3 variants with increased activity in the presence of organic co-solvent18 and applying milder nucleophilic fluorination methods.
Overall, the described methodology extends the range of available tools for selective fluorination. Importantly, it provides a concise solution to selective incorporation of fluorine atoms at relatively unreactive sites in a variety of organic molecules and at a preparative scale. The regio- and stereoselectivity of this chemical transformation can be altered by engineering the protein catalyst. This strategy is versatile, as the same chemo-enzymatic route can be applied to various structurally-related scaffolds, and more chemo-enzymatic transformations can be combined for selective fluorination of multiple sites of the same molecule. A common strategy for improving drugs’ in vivo half-life involves blocking the sites in the molecule that are susceptible to attack by human P450s with fluorine substituents.2,3 It is worth noting that fluorination of position 2 in 15 and 18 would prevent one of the major P450-dependent routes of ibuprofen metabolism in humans.19 An added advantage of this approach is thus to enable fluorination of metabolically vulnerable sites in the target molecule. We expect this procedure to find utility in the rapid identification of drug or lead compound derivatives with improved chemo-physical/biological properties as well as in the preparation of fluorinated synthons for chemical synthesis or fragment-based drug discovery programs.
Supplementary Material
Note: Supplementary information is available on the Nature Chemical Biology website.
ACKNOWLEDGEMENTS
We are grateful to Mona Shahgholi for assistance with the LC-MS and HRMS analyses. This work was supported by US National Institutes of Health grant GM068664 and US Department of agriculture grant 2006–35505–16660 to F.H.A., and by the Jacobs Institute (Caltech). A.R. acknowledges the Deutsche Forschungsgemeinschaft (DFG) for financial support.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturechemicalbiology/.
REFERENCES
- 1.Muller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 2.Park BK, Kitteringham NR, O'Neill PM. Annu. Rev. Pharmacol. Toicol. 2001;41:443–470. doi: 10.1146/annurev.pharmtox.41.1.443. [DOI] [PubMed] [Google Scholar]
- 3.Bohm HJ, et al. Chembiochem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch P. Modern fluoroorganic chemistry. Weinheim: Wiler-VCH; 2004. [Google Scholar]
- 5.Shimizu M, Hiyama T. Angew. Chem. Int. Ed. Eng. 2005;44:214–231. doi: 10.1002/anie.200460441. [DOI] [PubMed] [Google Scholar]
- 6.Ma JA, Cahard D. Chem. Rev. 2004;104:6119–6146. doi: 10.1021/cr030143e. [DOI] [PubMed] [Google Scholar]
- 7.Bobbio C, Gouverneur V. Org. Biomol. Chem. 2006;4:2065–2075. doi: 10.1039/b603163c. [DOI] [PubMed] [Google Scholar]
- 8.Günter H. J Fluorine Chem. 2004;125:875–894. [Google Scholar]
- 9.Iacazio G, Réglier M. Tetrahedron: Asymmetry. 2005;16:3633–3639. [Google Scholar]
- 10.Warman AJ, et al. Biochem. Soc. Trans. 2005;33:747–753. doi: 10.1042/BST0330747. [DOI] [PubMed] [Google Scholar]
- 11.Fasan R, Meharenna YT, Snow CD, Poulos TL, Arnold FH. J. Mol. Biol. 2008;383:1069–1080. doi: 10.1016/j.jmb.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikolajczyk M, Mikina M, Zurawinski R. Pure Appl. Chem. 1999;71:473–480. [Google Scholar]
- 13.Fujisawa H, Fujiwara T, Takeuchi Y, Omata K. Chem. Pharm. Bull. 2005;53:524–528. doi: 10.1248/cpb.53.524. [DOI] [PubMed] [Google Scholar]
- 14.Singh RP, Shreeve JM. Synthesis. 2002;17:2561–2578. [Google Scholar]
- 15.Landwehr M, et al. J. Am. Chem. Soc. 2006;128:6058–6059. doi: 10.1021/ja061261x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuchtenberger S, Beher D, Weggen S. Curr. Pharm. Des. 2006;12:4337–4355. doi: 10.2174/138161206778793029. [DOI] [PubMed] [Google Scholar]
- 17.Feher M, Schmidt JM. J Chem. Inf. Comput. Sci. 2003;43:218–227. doi: 10.1021/ci0200467. [DOI] [PubMed] [Google Scholar]
- 18.Wong TS, Arnold FH, Schwaneberg U. Biotechnol. Bioeng. 2004;85:351–358. doi: 10.1002/bit.10896. [DOI] [PubMed] [Google Scholar]
- 19.Hamman MA, Thompson GA, Hall SD. Biochem. Pharmacol. 1997;54:33–41. doi: 10.1016/s0006-2952(97)00143-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Chemical Biology website.