Abstract
Oxidative stress and mitochondrial dysfunction have been linked to dopaminergic neuron degeneration in Parkinson’s disease. We have previously shown that dopamine oxidation leads to selective dopaminergic terminal degeneration in vivo and alters mitochondrial function in vitro. In this study, we utilized 2-D difference in-gel electrophoresis to assess changes in the mitochondrial proteome following in vitro exposure to reactive dopamine quinone. A subset of proteins exhibit decreased fluorescence labeling following dopamine oxidation, suggesting a rapid loss of specific proteins. Amongst these proteins are mitochondrial creatine kinase, mitofilin, mortalin, the 75kDa subunit of NADH dehydrogenase, and superoxide dismutase 2. Western blot analyses for mitochondrial creatine kinase and mitofilin confirmed significant losses in isolated brain mitochondria exposed to dopamine quinone and PC12 cells exposed to dopamine. These results suggest that specific mitochondrial proteins are uniquely susceptible to changes in abundance following dopamine oxidation, and carry implications for mitochondrial stability in Parkinson’s disease neurodegeneration.
Keywords: Parkinson’s disease, mitochondria, PC12 cells, dopamine, dopamine quinone, proteomics, 2-D DIGE, mitofilin, mitochondrial creatine kinase
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra (SN) pars compacta and the formation of Lewy bodies (Samii et al., 2004). Most PD cases are considered sporadic, and provide us with limited clues to causes of disease pathogenesis. However, increasing evidence implicates mitochondrial dysfunction and oxidative stress in PD (Betarbet et al., 2002; Dauer and Przedborski, 2003; Jenner, 2003; Pallanck and Greenamyre, 2006).
Decreased mitochondrial Complex I (NADH dehydrogenase) activity has been observed in both the SN (Janetzky et al., 1994; Orth and Schapira, 2002; Schapira et al., 1990) and periphery (Blandini et al., 1998; Shoffner et al., 1991) of PD patients. Deficiencies and inhibition of the mitochondrial electron transport chain (ETC), a known source of reactive oxygen species (ROS), can lead to increased mitochondria-generated free radicals and oxidative stress (Beal, 2003; Lenaz et al., 2002). Increased ROS may cause damage to macromolecules, such as increased oxidation of mitochondrial proteins, making them susceptible to accumulation or proteolytic degradation (Bota and Davies, 2001; Bota and Davies, 2002; Bulteau et al., 2006).
Although multiple brain regions are involved in PD, the degeneration of dopaminergic neurons under conditions of oxidative stress suggests dopamine (DA) may be contributing to PD pathogenesis (Greenamyre and Hastings, 2004; Ogawa et al., 2005; Stokes et al., 1999). Normal DA metabolism leads to the production of ROS, and DA not adequately stored in vesicles is prone to oxidation, forming the reactive DA quinone (DAQ) (Graham et al., 1978). Dopamine-induced toxicity, demonstrated both in cell culture (Jones et al., 2000; Koshimura et al., 2000) and in vivo (Hastings et al., 1996; Rabinovic et al., 2000), is dependent on DA oxidation and the formation of reactive DA metabolites. Post-mortem studies have found increased levels of cysteinyl-DA, the covalent modification of cysteine by DAQ, in SN of PD patients (Fornstedt et al., 1989; Spencer et al., 1998). Dopamine and DAQ exposure also alter mitochondrial respiration (Berman and Hastings, 1999; Cohen et al., 1997; Gluck et al., 2002) and trigger permeability transition (Berman and Hastings, 1999) in isolated rat brain mitochondria, suggesting modification of critical mitochondrial proteins, though specific proteins have yet to be identified. As previous proteomic studies have identified alterations in mitochondrial proteins in animal models of PD (Jin et al., 2005; Palacino et al., 2004; Periquet et al., 2005; Poon et al., 2005), it is of interest to identify and characterize the mitochondrial protein targets of DA oxidation. Such proteins could become therapeutic targets in PD.
In this study, we utilized 2-D DIGE techniques in combination with cysteine- and lysine-reactive fluorescent dyes as a non-biased approach to evaluate protein alterations in rat brain mitochondria immediately following in vitro exposure to DAQ. Differential fluorescent labeling indicated a significant loss following DAQ exposure of a subset of potentially critical proteins that were identified by subsequent mass spectrometric studies. Western blot analyses confirmed decreases in two of these proteins, mitochondrial creatine kinase and mitofilin, in isolated brain mitochondria exposed to DAQ and PC12 cells exposed to DA. These findings suggest that specific mitochondrial proteins are uniquely susceptible to oxidation-induced changes in abundance, and may have implications for PD pathogenesis.
EXPERIMENTAL PROCEDURES
Materials
Cysteine-reactive maleimide-conjugated Cy3/5 cyanine Ettan DIGE Saturation Labeling dyes (Cys-CyDyes) and lysine-reactive N-hydroxysuccinimide (NHS) ester-conjugated Cy3/5 cyanine Ettan DIGE Minimal Labeling dyes (Lys-CyDyes) were purchased from GE Healthcare (Piscataway, NJ). Sequencing Grade Modified Trypsin and Gold Mass Spectrometry Grade Modified Trypsin were purchased from Promega (Madison, WI). Solutions and stocks for in-gel trypsin digest and mass spectrometry procedures were prepared using HPLC-grade ddH2O from Fisher Biotech (Pittsburgh, PA) and HPLC-grade MeOH and acetonitrile from Sigma-Aldrich (St. Louis, MO). Protease inhibitor cocktail (cat#P2714), DA, mushroom tyrosinase, and most general chemicals for SDS-PAGE, buffers, and solutions were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted. Rabbit-anti-mitochondrial creatine kinase (MtCK) and rabbit-anti-mitofilin polyclonal antibodies were generated for our laboratory by GeneMed Synthesis, Inc. (South San Francisco, CA). All other general solutions and stocks were prepared using doubly distilled water (ddH2O) from a Milli-Q system (Millipore Corp., Bedford, MA).
Mitochondrial Isolation and Respiration
All animal procedures were approved by the Animal Care and Use Committee at the University of Pittsburgh and are in accordance with guidelines put forth by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals. Mitochondria-enriched fractions were isolated from the brain tissue of adult male Sprague-Dawley rats via differential centrifugation by the method of Rosenthal et al. (1987) as previously described (Berman and Hastings, 1999; Berman et al., 2000), with elimination of the protease Nagarse. Final mitochondrial pellets were resuspended in mitochondrial isolation buffer (225 mM mannitol, 75 mM sucrose, 5 mM HEPES, 1 mg/ml FA-free BSA, and 1 mM EGTA, pH 7.4) and kept on ice. Mitochondrial protein content was determined by the Bradford method (1976). Prior to experimental use, respiration rates based on oxygen consumption were measured in the mitochondrial preparations to ensure mitochondrial health, as previously described (Berman and Hastings, 1999). Mitochondrial health was determined by the ratio of respiration active state 3, induced by the addition of ADP, to resting state 4, induced by the addition of oligomycin. Only mitochondria with a coupled state 3/state 4 ratio above 6 were used for this study.
Exposure of Isolated Mitochondria to Dopamine Quinone
Mitochondria (2 mg total protein) were exposed to DA (150 µM) and tyrosinase (150 U) in modified mitochondrial isolation buffer with 25 mM HEPES minus BSA, pH 7.4 (reaction buffer) plus protease inhibitor cocktail (2.5 µl/mg protein; Sigma) for 15 min at room temperature (RT). Following incubation, mitochondria were placed on ice and immediately pelleted by centrifugation at 15,000 × g for 15 min at 3°C. Control mitochondria underwent the same procedure in the absence of DA. Pelleted mitochondria were lysed by rigorous pipetting in denaturing 2-D DIGE lysis buffer (9 M urea, 2% w/v CHAPS, and 30 mM Tris-base, pH 8.5) in a ratio of 100 µL buffer to 1 mg protein. Insoluble material was pelleted by centrifugation (16,000 × g for 1–2 min at RT) and discarded. Protein concentrations of lysed control and DAQ-exposed samples were determined by Bradford (1976). Thiol reducing agents were excluded from the lysis buffer to maintain proteins in a non-reduced state.
Cys- and Lys-CyDye Labeling
For cysteine-dye minimal labeling 2-D DIGE, migration-matched Cy3 and Cy5 Cys-CyDyes (GE Healthcare) were rehydrated in dimethylformamide (DMF) to a concentration of 0.5 mM, aliquoted, and stored at −20°C with desiccation until use. Prior to use, an aliquot of dye was thawed to RT and diluted in DMF to a working concentration of 62.5 µM. Control and DAQ-exposed protein sample lysates were reacted with the indicated Cys-CyDye under non-reducing conditions at a ratio of 1 pmol dye per 2 µg protein. We used low concentrations of Cys-CyDyes to achieve a minimal labeling effect on non-reduced protein samples. Preliminary experiments using various dye concentrations identified 1pmol dye per 2 µg protein, which is 0.125% of the ratio utilized for saturation labeling, as optimal for minimal Cys-CyDye labeling. This concentration provided sufficient labeling for detection and imaging while maintaining reproducible results across gels (data not shown).
Samples were labeled Cys-Cy5 control and Cys-Cy3 DAQ, or the reciprocal to control for differential dye affinity. Samples were gently vortexed and incubated in the dark for 45 min at RT. The reaction was quenched by adding an equal volume amount of 2-D DIGE sample buffer (9 M urea, 2% w/v CHAPS, 2% v/v 3–10 IPG ampholyte buffer, 130 mM dithiothreitol (DTT), and a trace of bromophenol blue in ddH2O). Final DIGE samples were prepared by combining equal amounts of Cys-CyDye labeled control protein and Cys-CyDye labeled DAQ-exposed protein.
Lysine-dye minimal-labeling 2-D DIGE analysis was utilized to control for changes in protein abundance between control and DAQ-exposure groups in comparison to Cys-CyDye DIGE. Migration-matched Cy3 and Cy5 Lys-CyDyes were rehydrated in DMF to a concentration of 0.5 mM and stored at −20°C with desiccation until use. Prior to use, dyes were thawed to RT and diluted 1:1 in DMF. Control and DAQ-exposed protein sample lysates were reacted with the indicated Lys-CyDye (Lys-Cy5 control and Lys-Cy3 DAQ, or the reciprocal) under non-reducing conditions at a ratio of 2 pmol dye per 1µg protein in the dark for 30 min on ice. The reaction was quenched by the addition of free lysine to a final concentration of 385 µM and incubated 15 min on ice. Labeled samples were diluted 1:1 with 2-D DIGE sample buffer. Equal protein amounts of the Lys-CyDye labeled control and the DAQ-exposed samples were combined to generate a final DIGE sample for 2-D gel electrophoresis. Each DIGE gel experiment and its associated parallel gels (Cys- and Lys-CyDye DIGE gels and reciprocals) were generated from independent mitochondrial isolation and DAQ exposure experiments.
2-Dimension Difference In-Gel Electrophoresis
Samples (250 µg) were isoelectrically focused via sample cup loading on rehydrated 18 cm linear 3–10 pH Immobiline DryStrips (GE Healthcare), using a Multiphor II system with a 3501XL power supply (GE Healthcare), and using a 4-phase program with a total run of 75 kVhr. Focused strips were stored at −80°C until the second dimension run. Prior to second dimension electrophoresis, DryStrips were equilibrated at RT for 10 min in an equilibration buffer (75 mM Tris-HCl pH 6.8, 6 M urea, 30% v/v glycerol, 1% w/v SDS) supplemented with 30 mM DTT, followed by 10 min at RT in equilibration buffer supplemented with 240 mM iodoacetamide. Equilibrated DryStrips were trimmed to 13.5–15 cm and run in second dimension 12% SDS-PAGE (1.5mm thick gels, Hoefer SE600 Ruby Electrophoresis Unit).
Fluorescence Detection and Spot Picking
Immediately following the second dimension run, gels were scanned on a Typhoon 9400 scanner using ImageQuant software (GE Healthcare) to obtain a 200 µm resolution image of the gel. Following imaging, gels were fixed overnight in a 40% MeOH, 1% acetic acid solution at 4°C. The gels were scanned a second time using a fluorescent scanning automated spot picker, designed by Dr. Jonathan Minden of Carnegie Mellon University (instrumentation housed in the University of Pittsburgh Genomics and Proteomics Core Laboratories). The digital scans of the Cy3 and Cy5 dyes within each gel were compared visually with the aid of Image J imaging software (NIH). Protein spots that exhibited a noticeable change in fluorescence, and several that exhibited no change, were then picked utilizing the automated picker.
In-gel Trypsin Digest and Protein Identification
Immediately following excision from 2-D DIGE gels, gel plugs were washed with 50:50 MeOH:50 mM ammonium bicarbonate followed by dehydration in acetonitrile (ACN) and drying by speed-vacuum. The dried plugs were rehydrated with 10 µl of 20 µg/ml trypsin in 20 mM ammonium bicarbonate and then incubated for 4 hr at 42°C. Samples were extracted by repeated washing in 1% trifluoroacetic acid in 50:50 ACN:H2O extraction buffer, dried completely via speed-vacuum, and stored for up to 2 weeks with desiccation at 4°C.
Protein identification was completed using MALDI-TOF mass spectrometry (MS) with peptide mass fingerprinting. For MS analysis, dried samples were rehydrated (2–3 µl of 0.3% trifluoroacetic acid, 1 mM ammonium citrate in 50:50 ACN:H2O; plus an equal volume of saturated α-cyano-4-hydroxycinnamic acid matrix solution), spotted onto a target, and mass spectra obtained using an Applied Biosystems 4700 MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Foster City, CA). Spectra were calibrated via external standard and internal trypsin calibration. Mass spectra retrieved from MALDI-TOF MS were processed by GPS Explorer™ (ver. 3) MS data analysis software (Applied Biosystems, Foster City, CA) coupled with Mascot™ search engine (Matrix Science) for peak list generation and database search. Resulting peak lists were searched against the National Center for Biotechnology Information non-redundant (NCBInr) database, specifying “All Entries” or “Rattus” species. See Supplementary Data S2 for annotated spectra, peak lists, and search criteria associated with top ranked hits. A positive protein identification for a given spot was accepted when a top ranked hit yielded a statistically significant probability-based MOWSE protein score and protein score confidence interval > 90% with a peptide count ≥ 6, protein coverage >20%, a predicted molecular weight that was relative to the protein spot position on the gel, and could be replicated across two or more separate 2-D DIGE gel experiments.
Fluorescence Imaging and Quantitative Image Analysis
For quantitative image analysis, 2-D DIGE gels were scanned for fluorescence imaging on a Typhoon 9400 laser scanner using ImageQuant software (GE Healthcare) at 100 µm resolution using photomultiplier tube (PMT) voltage settings below saturation for each dye (Cy3/5). Settings were determined for the first set of gels, both Cys- and Lys-CyDye labeled, then all further gels were scanned using the same PMT voltage settings, or ratio as necessary, to obtain non-saturation images. In-gel quantitative comparisons of fluorescence were completed using the Difference In-Gel Analysis (DIA) module of DeCyder Differential Analysis software (GE Healthcare). Fold change ratios, based on volume ratios of the individual spots and internally normalized by DeCyder, were determined and recorded for DeCyder-defined spots that corresponded to proteins previously identified by MS analysis. For each selected spot within a gel, the fold change was converted to percent DAQ-exposed mitochondrial protein fluorescence of control and averaged across all analyzed Cys-CyDye DIGE gels (n=6 total gels from 5 separate mitochondrial experiments) or Lys-CyDye DIGE gels (n=7 total gels from 5 separate mitochondrial experiments) using Excel (Microsoft Corp.). Images obtained from ImageQuant were prepared for presentation using Adobe Photoshop (Adobe).
PC12 Cell Culture and Mitochondrial Isolation
PC12 cells were maintained in DMEM supplemented with 7% horse serum (HS) and 7% fetal bovine serum (FBS). For differentiation, cells were subcultured on rat-tail collagen coated 100 mm plates at 1.5 × 106 cells/plate in DMEM supplemented with 1% HS, 1% FBS, and 0.1 µg/ml NGF for 6 days. Media was then replaced with fresh differentiating media with or without 150 µM DA and cells were incubated for 16 hrs. Cells were collected by force pipetting, rinsed with PBS, and isolated by centrifugation. Mitochondrial enriched fractions were prepared from 10 confluent plates in each group using methods similar to those for isolating rat brain mitochondria, with the modification of protease inhibitor cocktail (2 µl/ml) being present in the mitochondrial isolation buffer throughout the isolation process. Mitochondria were lysed in 2-D DIGE lysis buffer and final protein concentrations were determined by Bradford (1976).
SDS-PAGE and Western Blot Immunodetection of Select Proteins
Lysed rat brain and PC12 cell mitochondrial protein samples (50 µg/lane) were run on 5–20% gradient SDS-PAGE (Hoefer ® Mighty Small II apparatus) and transferred to nitrocellulose (BioRad) for Western blot analysis via a BioRad Trans-Blot ® Semi-Dry Electrophoretic Transfer system. The membrane was removed, washed briefly in Tris-buffered saline (TBS), blocked in 0.2% w/v fat-free dry milk for 30min, rinsed briefly in TBS plus 0.1% Tween-20 (tTBS), and placed in a 1:1000 dilution of rabbit anti-MtCK or 1:5000 dilution of rabbit anti-mitofilin primary antibody in tTBS overnight at 4°C. Immunoreactive bands were visualized using the BioRad Immune-Star ® goat-anti-rabbit (dil 1:10,000) chemiluminescence detection kit and exposed to Biomax MR Film (Kodak). Mouse-anti-COXIV (dil 1:37,000; AbCam) and rabbit-anti-voltage-dependent anion channel 1 (VDAC1) (dil 1:2000; AbCam) were used as loading controls for rat brain mitochondria and for PC12 mitochondria, respectively. VDAC1 was selected because in parallel 2-D DIGE studies with PC12 cell mitochondria the protein did not significantly change following DA exposure (unpublished data). Films were digitally scanned and the densities of immunoreactive bands were determined using UN-SCAN-IT Gel (ver. 5.1) densitometry software (Silk Scientific; Orem, Utah).
Statistical Analysis
Cys- and Lys-CyDye MS-identified proteins whose relative DAQ-exposed fluorescence values (as percent of control) fell outside of a defined range of 83.3–120% (±1.2 fold) were selected as different from control. The range represents two standard deviations in a Cy5-labeled control versus Cy3-labeled control gel analyzed by DeCyder (data not shown), and is the recommended threshold for determining significant change in DeCyder analysis. Statistical significance was determined using a 1-sample two-tailed Z-test on the DAQ-exposed mitochondrial protein spot volume intensities expressed as percent of control, as determined from DeCyder analysis. The Z-test is optimal, as DeCyder DIA software calculates changes between corresponding control and treated protein spots within a gel as a volume ratio of the two samples, generating one value of “fold change” for each protein spot that compares both groups. The ratios are then internally normalized across the entire constellation of labeled spots. Significance for each changed DA-exposed protein from control (valued at 100% control) was determined when p<0.01. The percent control values were directly calculated from the normalized DeCyder volume ratios. For Western blot analysis, rat brain mitochondria samples were run in duplicate or triplicate for each of n=6–7 separate experiments, and PC12 mitochondria samples were run in triplicate for each of n=4 separate experiments. Significance between group means was determined by two-factor ANOVA with replication followed by post-hoc Bonferroni tests.
RESULTS
A Subset of Proteins Exhibit Decreased Cysteine-reactive CyDye Labeling Following DAQ Exposure
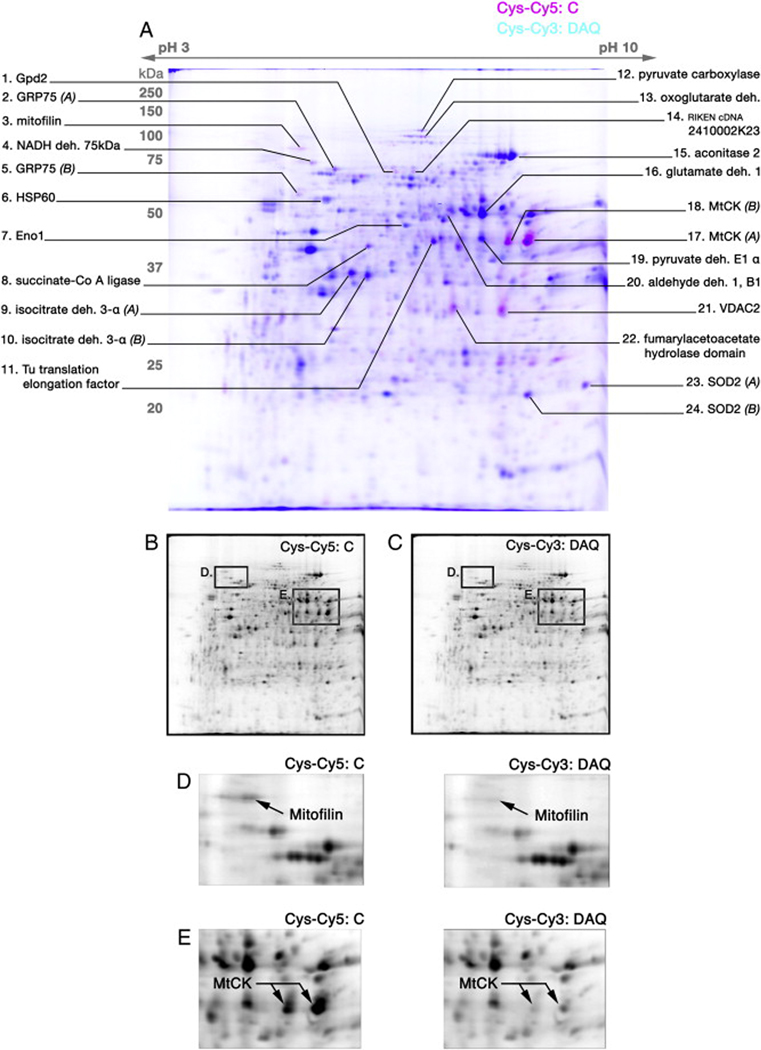
Cys-Cy5 labeled control (magenta) and Cys-Cy3 labeled DAQ-exposed (cyan) mitochondrial protein lysates were compared in equal protein amounts on the same gel, revealing a reproducible pattern of protein spots (Figure 1A; n=10), within which a subset of observed spots displayed a differential fluorescence intensity. Specifically, a subset of proteins exhibited relative decreased cysteine-reactive dye fluorescence following DAQ exposure as compared to control. Protein spots of interest, including those that exhibited an obvious change in fluorescence, were identified by peptide mass fingerprinting (Figure 1A). All identified proteins were determined to be mitochondria-associated proteins. A listing of all proteins identified and their corresponding data can be found in Supplemental Data S1 and S2.
Figure 1. 2-D DIGE using Cys-CyDye labeling.
Isolated brain mitochondria were exposed to DA (150µM)/tyrosinase (150U) for 15 min. Lysed control (C) and DAQ-exposed (DAQ) mitochondrial proteins were reacted separately with a minimal concentration of Cys-Cy5 and Cys-Cy3 CyDyes, respectively, and then analyzed by 2-D DIGE. (A) A representative Cys-CyDye DIGE gel of control (Cys-Cy5, pseudocolored magenta) and DAQ-exposed (Cys-Cy3, pseudocolored cyan) mitochondrial protein. Spots exhibiting differential labeling present as magenta (decreased following DAQ) or cyan (increased following DAQ) hue, while blue spots represent minimal or no differential labeling. Specific protein spots of interest were picked from the gel and identified via MS analysis, and identities are presented with their associated spot (deh. – dehydrogenase). (B, C) Insets of separate black & white images obtained from ImageQuant of the DIGE gel for Cy5 (C) and Cy3 (DAQ-exposed) fluorophores. The pseudocolor overlay of B and C generated the image in A. (D) Magnified views of the region containing the identified protein spot mitofilin, comparing fluorescence intensity between Cys-Cy5 labeled (C) and Cys-Cy3 labeled (DAQ-exposed) images of the gel. (E) Magnified views of the region containing the identified protein spot mitochondrial creatine kinase (MtCK), comparing fluorescence intensity between Cys-Cy5 labeled (C) and Cys-Cy3 labeled (DAQ-exposed) images of the gel. These images demonstrate a decrease in Cys-CyDye fluorescent labeling following mitochondrial DAQ exposure for both mitofilin and MtCK.
Quantitative DeCyder analyses across a set of Cys-CyDye gels (n=6) confirmed significantly decreased fluorescence labeling of a subset of proteins in DAQ-exposed mitochondria as compared to control (Table 1). Two of these proteins, mitofilin (−65±2%), associated with mitochondrial cristae organization (John et al., 2005), and ubiquitous mitochondrial creatine kinase (MtCK) (−82±3%), associated with ADP-ATP exchange and the permeability transition pore (Beutner et al., 1998; Schlattner et al., 1998; Vyssokikh and Brdiczka, 2003), participate in mitochondrial structure maintenance. Both proteins exhibited a strong decrease in fluorescence intensity following exposure of mitochondria to DAQ (Figure 1D-E), with MtCK exhibiting the largest changes in relative fluorescence in Cys-CyDye 2-D DIGE gels. Other identified proteins whose relative fluorescence intensities were significantly reduced included several proteins of the Krebs cycle, including pyruvate carboxylase (−36±6%), succinate-CoA ligase (−43±4%), and oxoglutarate dehydrogenase (−49±5%), a protein previously shown to be inhibited by reactive metabolites of 5-cysteinyl-DA (Shen et al., 2000).
Table 1.
Protein alterations in isolated rat brain mitochondria exposed to DAQ
| Protein Spot |
Protein | Protein Identification | NCBI Accession# |
Predicted Protein MW; pI |
Protein Score; C.I.%** |
Peptide Count | % Coverage | Cys-CyDye (% of control, ± SEM)# |
Lys-CyDye (% of control, ± SEM)# |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Gpd2 | Gpd2 protein [Rattus norvegicus] (glycerol-3-phosphate dehydrogenase) | gi|54035427 | 80921.3; 6.18 | 101; 100% | 17 | 31% | 32.3±2.74* | 46.6±4.55* |
| 2 | GRP75 (A) | grp75 [Rattus sp.] | gi|1000439 | 73983.9; 5.87 | 243; 100% | 27 | 53% | 78.8±2.60* | 73.7±3.30* |
| 3 | mitofilin | similar to inner membrane protein, mitochondrial (mitofilin); motor protein [Rattus norvegicus] | gi|34855983 | 82304.9; 5.37 | 199; 100% | 26 | 42% | 35.2±2.39* | 45.8±2.51* |
| 4 | NADH deh. 75kDa | NADH dehydrogenase (ubiquinone) Fe-S protein 1, 75 kDa [Rattus norvegicus] | gi|51858651 | 79361.6; 5.65 | 245; 100% | 30 | 49% | 54.5±3.24* | 54.1±3.48* |
| 5 | GRP75 (B) | dnaK-type molecular chaperone grp75 precursor - rat | gi|2119726 | 73698.8; 5.87 | 141; 100% | 19 | 42% | 73.3±3.41* | 75.1±3.35* |
| 8 | succinate-CoA ligase | PREDICTED: similar to succinate-Coenzyme A ligase, ADP-forming, beta subunit [Rattus norvegicus] | gi|62661722 | 50274.1; 7.57 | 156; 100% | 20 | 52% | 57.0±4.48* | 57.9±2.22* |
| 9 | isocitrate deh. 3-alpha (A) | isocitrate dehydrogenase 3 (NAD+) alpha [Rattus norvegicus] | gi|16758446 | 39588; 6.47 | 89; 99.996% | 13 | 37% | 86.1±2.28 | 80.6±1.95* |
| 11 | Tu translation elongation factor | PREDICTED: similar to Tu translation elongation factor, mitochondrial [Rattus norvegicus] | gi|109462848 | 49890.1; 7.23 | 209; 100% | 21 | 65% | 72.7±2.26* | 59.1±1.43* |
| 12 | pyruvate carboxylase | Pc protein [Rattus norvegicus] | gi|55716041 | 129694.7; 6.34 | 213; 100% | 31 | 39% | 63.9±5.90* | 69.0±1.71* |
| 13 | oxoglutarate dehydrogenase | Similar to oxoglutarate dehydrogenase (lipoamide) [Rattus norvegicus] | gi|53734284 | 116221.4; 6.3 | 85; 99.99% | 18 | 21% | 50.5±4.91* | 54.8±3.77* |
| 14 | RIKEN cDNA 2410002K23 | similar to RIKEN cDNA 2410002K23 [Rattus norvegicus] | gi|34868689 | 80410.8; 6.56 | 114; 100% | 19 | 29% | 74.0±7.00* | 56.1±5.17* |
| 17 | MtCK (A) | ubiquitous mitochondrial creatine kinase [Rattus rattus] | gi|57539 | 46999.3; 8.72 | 115; 100% | 16 | 49% | 18.1±2.71* | 24.6±2.11* |
| 18 | MtCK (B) | creatine kinase, mitochondrial 1, ubiquitous [Rattus norvegicus] | gi|60678254 | 46932.2; 8.58 | 128; 100% | 17 | 54% | 26.6±3.24* | 41.4±2.93* |
| 20 | aldehyde deh. 1, B1 | Aldehyde dehydrogenase 1 family, member B1(predicted) [Rattus norvegicus] | gi|51858643 | 58101.6; 6.62 | 125; 100% | 15 | 43% | N.D.*** | 64.2±3.94* |
| 21 | VDAC2 | B-36 VDAC 36 kda voltage dependent anion channel [rats, hippocampus, Peptide, 295 aa] | gi|299036 | 31699.6; 7.44 | 56; 94.448% | 8 | 40% | 25.5±3.56* | 31.7±3.25* |
| 22 | fumarylacetoacetate hydrolase domain | PREDICTED: similar to fumarylacetoacetate hydrolase domain containing 2A [Rattus norvegicus] | gi|34858672 | 40314; 8.49 | 122; 100% | 15 | 56% | 34.9±2.61* | 43.9±2.09* |
| 23 | SOD2 (A) | unnamed protein product [Rattus norvegicus] (Superoxide dismutase 2) | gi|56691 | 24667.6; 8.96 | 126; 100% | 13 | 65% | 65.2±3.06* | 64.6±2.35* |
| 24 | SOD2 (B) | unnamed protein product [Rattus norvegicus] (Superoxide dismutase 2) | gi|56691 | 24667.6; 8.96 | 59; 96.016% | 8 | 41% | 72.3±2.67* | N.D.**** |
Significance from control (100%), p<0.01, for proteins outside of the cutoff of 83.3–120% of control (1.2 fold change)
Probability-based MOWSE score (Protein Score) and Protein Score Confidence Interval (C.I.) represent the top Protein Score and C.I. pairing obtained across all gels, Cys- and Lys-CyDye, in which the protein was confidently identified (n = 2–11)
Insufficient data for DeCyder analysis
Protein not identified via MS analysis in Lys-DIGE gels
Normalized fold change in fluorescence of DAQ sample compared to control as determined by DeCyder analysis, expressed as percent of control (100%) ± standard error of the mean (SEM)
When DAQ exposure to mitochondria was carried out in the presence of 1mM N-acetylcysteine, an antioxidant, no differentially labeled spots were observed with Cys-CyDye DIGE (data not shown), demonstrating that DA oxidation is necessary to elicit the differential labeling of proteins. However, Cys-CyDye DIGE analysis alone cannot distinguish whether the reductions in labeling intensity of a specific protein are the result of a change in the redox state of its thiols by oxidation/modification by DAQ, or a change in protein abundance. We utilized DIGE-compatible lysine-reactive fluorescent dyes to probe this distinction.
Lysine-reactive CyDye DIGE Reveals Differential Fluorescent Labeling Comparable to Cysteinereactive CyDye DIGE
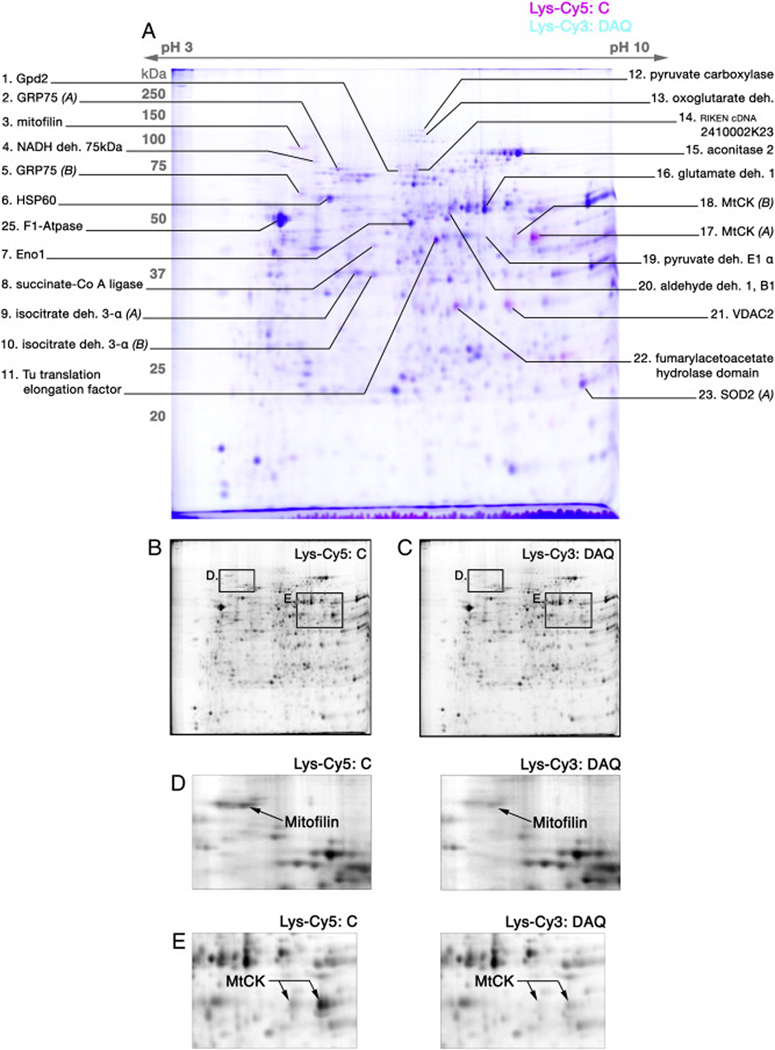
As with the Cys-CyDye DIGE experiments, Lys-CyDye DIGE analysis revealed a reproducible pattern of protein spots (Figure 2A; n=6), within which a subset of observed protein spots displayed differential fluorescence intensity. Further, Lys-CyDye labeled DIGE gels resulted in an overall protein spot pattern similar to Cys-CyDye labeled DIGE gels (Figure 1 & Figure 2), as confirmed by MS analysis and peptide mass fingerprinting of spots from Lys-CyDye gels. In addition, proteins of interest demonstrated a similar differential fluorescence with both Cys- and Lys-CyDye 2-D DIGE (Figure 2). The proteins mitofilin and MtCK, in particular, both demonstrated a noticeable decrease in Lys-CyDye fluorescence intensity following DAQ exposure as compared to control (Figure 2D-E).
Figure 2. 2-D DIGE using Lys-CyDye labeling.
Isolated brain mitochondria were exposed to DA (150µM)/tyrosinase (150U) for 15 min, lysed control (C) and DAQ-exposed (DAQ) mitochondrial proteins reacted separately with a minimal concentration of Lys-Cy5 and Lys-Cy3 CyDyes, respectively, and then analyzed by 2-D DIGE. (A) A representative Lys-CyDye DIGE gel of control (Lys-Cy5, pseudocolored magenta) and DAQ-exposed (Lys-Cy3, pseudocolored cyan) mitochondrial protein. Spots exhibiting a magenta or cyan hue represent differential labeling indicative of alterations in protein level, while blue spots represent minimal or no differential labeling. Specific protein spots of interest were picked from the gel and identified via MS analysis, and confirmed identities are presented with their associated spot (deh. – dehydrogenase). (B, C) Insets of separate black & white images obtained from ImageQuant of the DIGE gel for Cy5 (C) and Cy3 (DAQ-exposed) fluorophores. The pseudocolor overlay of B and C generated the image in A. (D) Magnified views of the region containing the identified protein spot mitofilin, comparing fluorescence intensity between Lys-Cy5 labeled (C) and Lys-Cy3 labeled (DAQ-exposed) images of the gel. (E) Magnified views of the region containing the identified protein spot mitochondrial creatine kinase (MtCK), comparing fluorescence intensity between Lys-Cy5 labeled (C) and Lys-Cy3 labeled (DAQ-exposed) images of the gel. These images demonstrate a decrease in Lys-CyDye fluorescent labeling following mitochondrial DAQ exposure for both mitofilin and MtCK.
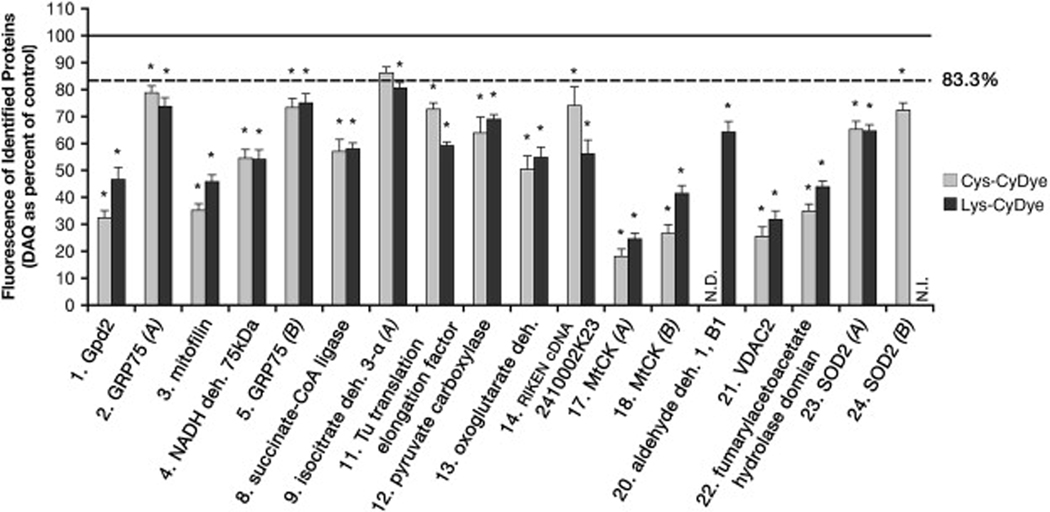
Quantitative DeCyder analysis across a set of Lys-CyDye gels (n=7) confirmed the significantly decreased fluorescence labeling of the subset of differentially labeled proteins in DAQ-exposed mitochondria as compared to control (Table 1). DeCyder analyses also demonstrate that the values of percent of control are comparable for all identified differentially labeled proteins between Cys- and Lys- CyDye 2-D DIGE (Figure 3). In particular, the proteins MtCK, mitofilin, fumarylacetoacetate hydrolase domain containing 2A, voltage-dependent anion channel 2 (VDAC2), and glycerol-3-phosphate dehydrogenase all exhibit fluorescence labeling decreased greater than 50% in both Cys- and Lys-CyDye DIGE analyses, and represent the proteins with the greatest decrease in fluorescence intensity in mitochondria exposed to DAQ as compared to control. These findings suggest that alterations demonstrated by our 2-D DIGE analyses result primarily from decreases in a specific subset of proteins, and not from oxidation of thiols.
Figure 3. Quantitative analyses of Cys-CyDye and Lys-CyDye fluorescence intensities of mitochondrial proteins following DAQ.
Cy5 and Cy3 images obtained from the Typhoon 9400 scanner for DIGE gels were analyzed by comparison using DeCyder software. Normalized values of fold change in fluorescence obtained from DeCyder were converted to fluorescence of proteins following DAQ as percent of control. The graph shows the mean percent of control value for annotated protein spots in the Cys-CyDye gel in Figure 1 (Cys-CyDye) and the Lys-CyDye gel in Figure 2 (Lys-CyDye). N.D. indicates insufficient data for analysis. N.I. indicates that MS data was insufficient to verify identification of SOD2 (B) in Lys-CyDye DIGE gels. Proteins with differential fluorescence below a cutoff of 83.3% of control (1.2 fold change) were considered changed and analyzed for significance from control. (mean ± SEM; n= 6 Cys-CyDye DIGE; n= 7 Lys-CyDye DIGE; *, significance from control (100%), p<0.01)
Comparison of Lys- and Cys-Dye DIGE
Reciprocal labeling experiments with the Cys-CyDyes (n=3) and Lys-CyDyes (n=5) confirmed that the differential labeling was not influenced by preferential binding by the dye (data not shown). Most of the subset of proteins showing altered levels of labeling has been successfully identified (Table 1), though due to limitations in detection sensitivity, we were unable to identify all proteins exhibiting differential labeling. Despite this, it was obvious that the vast majority of mitochondrial proteins do not show any change in labeling following DAQ exposure (Figure 1A & Figure 2A). As only a fraction of the total observable spots on the 2-D DIGE gel exhibited a significant decrease in fluorescence, these proteins represent a unique subset of the mitochondrial proteome that are susceptible to alterations induced by DAQ exposure and result in decreased protein levels.
These data suggest the effect of DAQ exposure quickly results in reduced levels of specific proteins, but the exact mode of DAQ-induced alterations of these proteins is unclear. In an attempt to block possible proteolytic degradation, mitochondria were exposed to DAQ in the presence of a 12.5-fold greater concentration of protease inhibitor cocktail than normally used, but this did not have any apparent effect on the observed changes in protein levels as detected with Cys- or Lys-CyDyes (data not shown).
To our knowledge, this is the first study to examine the use of Cys-CyDyes in a minimal-labeling scheme as compared to the more-typically utilized Lys-CyDyes. The maleimide moiety has a high reactivity with and selectivity for cysteine residues (and lesser reactivity with primary amines), and Cys-CyDyes are typically used under saturation-labeling conditions (Shaw et al., 2003). Although we initially anticipated that we would observe differential labeling indicative of oxidative modification of cysteinyl residues, minimal labeling using Cys-CyDyes detected only alterations in protein levels nearly identical to labeling by Lys-CyDyes. Thus, differential labeling observed in Cys- and Lys-CyDye DIGE gels represents changes in abundance of proteins.
The possibility exists that while cysteines are likely being modified by exposure to DAQ, DAQ-induced modification may be below detection limits under our experimental conditions. Data from a parallel 2-D DIGE study of mitochondria isolated from PC12 cells exposed to DA similarly indicate that using Cys-CyDyes at low concentrations primarily detects changes in protein levels (unpublished data). Although a reasonable approach in theory, labeling and detection of bulk protein thiols as conducted here and investigated by others (Chan et al., 2005; Hurd et al., 2007) is not an effective method for detecting changes in thiol redox state.
It is important to note that under manufacturer-recommended protocols, purchased quantities of both Lys-CyDyes and Cys-CyDyes are only sufficient for five to ten experiments. The Cys-CyDye minimal labeling methodology utilizes only a fraction of the recommended dye concentration, and increases the number of possible experiments that can be performed for a single purchased set of dyes by as much as 800 fold. This methodology, however, does have the drawback that it can only detect proteins containing reactive cysteine residues. Thus controls, such as Lys-CyDye DIGE or Western blot analyses, would be necessary to validate any observations or to evaluate proteins lacking cysteine residues. Nevertheless, with increased cost effectiveness, the methodology described here may make the Cys- CyDyes an ideal choice for certain DIGE experiments.
Comparable Decreases in Mitofilin and MtCK Levels Observed by Western Blot
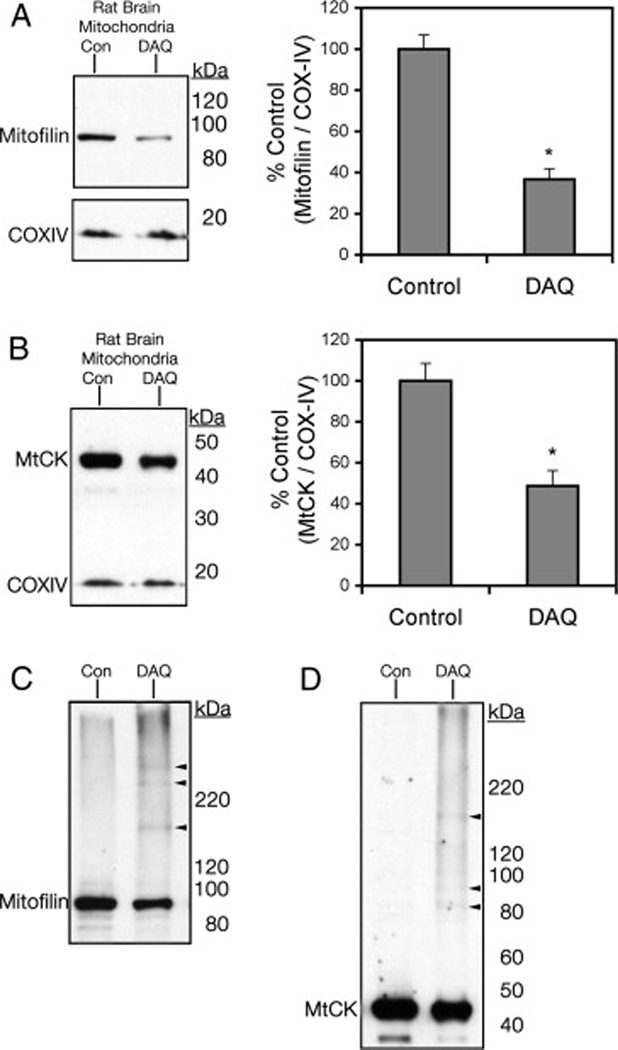
To confirm the decreases in protein levels observed in the DIGE experiments, Western blot analyses of two representative proteins, mitofilin and MtCK, were carried out on mitochondrial samples treated with the same exposure to DAQ as the DIGE experiments. Comparing lysates from control and DAQ-exposed rat brain mitochondria on Western blot, we observed significant decreases of both mitofilin and MtCK protein levels (−63.4±5.2%, n=7, and −51.4±7.5%, n=6, respectively, P<0.05) comparable to DIGE observations following exposure to DAQ (Figure 4A-B). We also observed immunoreactive bands at higher molecular weights for both MtCK and mitofilin after exposure to DAQ (Fig 4C-D), suggesting possible protein aggregation or crosslinking.
Figure 4. Confirmation of protein level alteration following mitochondrial DAQ exposure.
Western blot analysis was utilized to confirm DIGE analysis results for mitochondrial creatine kinase (MtCK) and mitofilin. Isolated mitochondria were exposed to DAQ, then lysed and subjected to SDS-PAGE and Western blot analysis. Representative Western blots of (A) mitofilin (n=7) and (B) MtCK (n=6) from intact rat brain mitochondria, Control (Con) versus DAQ (DAQ), are presented with densitometry analysis representing DAQ-exposed band density as percent of Control band density. Data is represented as a ratio of detection of the loading control, COXIV (mean ± SEM; *, significant from control, p<0.05). Extended film exposure revealed higher molecular weight detection (arrowheads) of (C) mitofilin and (D) MtCK in isolated mitochondria following DAQ.
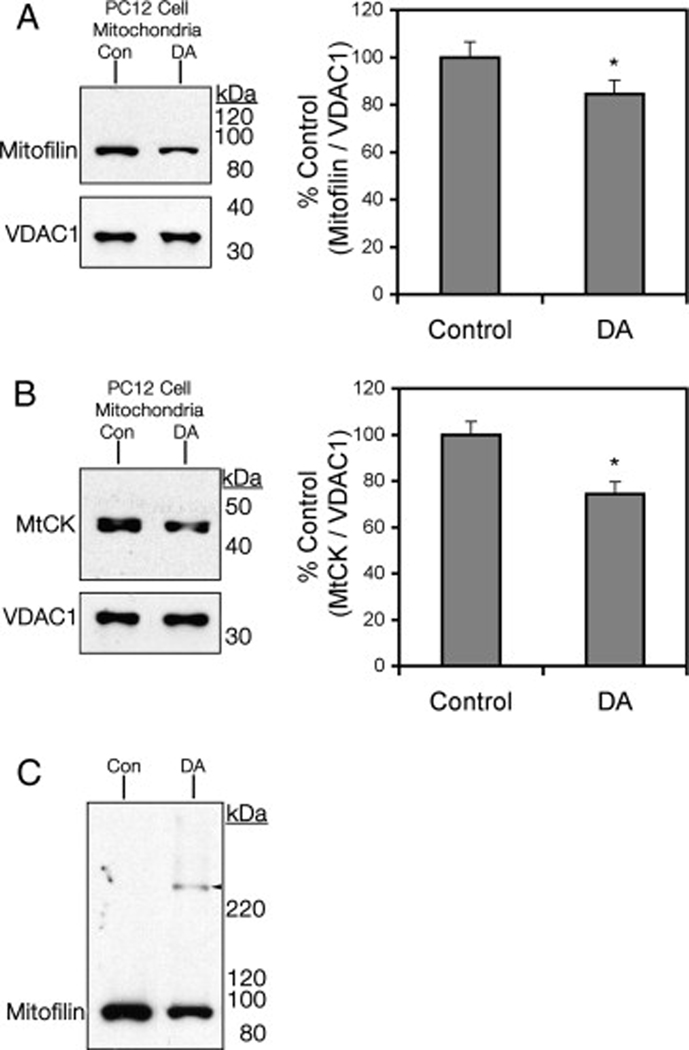
Decreased Mitochondrial Levels of Mitofilin and MtCK in PC12 Cells Exposed to DA
To examine whether the DA-induced decrease in mitofilin and MtCK translates to the cellular environment, we utilized an established model of DA-induced PC12 cell toxicity. Mitochondria were isolated from differentiated PC12 cells following exposure to control media or media containing 150 µM DA for 16 hr. This concentration and time point were previously demonstrated in our laboratory to result in covalent modification of cellular proteins by DAQ (indicative of DA oxidation) prior to significant cell death at 24 hrs (unpublished data). Lysates of mitochondria isolated from both treatment groups were subjected to SDS-PAGE and Western blot analyses. Data showed a significant decrease in both mitofilin and MtCK protein levels (−15.5±5.8% and −25.7±5.4%, respectively, n=4, P<0.05) in mitochondria of DA-exposed PC12 cells as compared to controls (Figure 5A-B). Also, as observed in isolated rat brain mitochondria exposed to DAQ, higher molecular weight bands were detected for mitofilin in mitochondria from DA-exposed PC12 cells (Fig 5C), indicative of potential protein crosslinking and/or aggregation.
Figure 5. Detection of mitofilin and MtCK in PC12 cell mitochondria following DA exposure.
Control and DA-exposed PC12 cell mitochondria were collected, lysed, and subjected to SDS-PAGE and Western blot analysis using VDAC1 as a protein loading control. Representative Western blots of (A) mitofilin and (B) MtCK in mitochondria from PC12 cells, Control (Con) versus DA are presented with densitometry analysis representing band density of DA-exposed as percent of Control band density. Data is represented as a ratio of detection of the loading control, VDAC1 (mean ± SEM, n=4; *, significant from control, p<0.05). (C) Extended film exposure revealed higher molecular weight detection (arrowhead) of mitofilin in PC12 cell mitochondria following DA.
DISCUSSION
Summary
Oxidation of DA to reactive metabolites, ROS, and DAQ is thought to contribute to the oxidative stress, mitochondrial dysfunction, and dopaminergic neuron degeneration in PD. In this study, we sought to identify mitochondrial proteins susceptible to DA oxidation using an unbiased proteomics approach. We found that exposure of isolated brain mitochondria to reactive DAQ resulted in a rapid loss of specific proteins. The altered proteins identified in this study (listed in Table 1) encompass a range of mitochondrial functions including structural maintenance, transport, and metabolism, suggesting that DA oxidation may have detrimental effects on mitochondrial function. Indeed, loss or altered function of many of the proteins we have identified in this study have been previously associated with oxidative stress, mitochondrial dysfunction, and neurodegenerative diseases including PD (Beutner et al., 1998; Jin et al., 2006a; Kim et al., 2001; Myung et al., 2003; Scott, 2006; Suh et al., 2004; Vyssokikh and Brdiczka, 2003).
Two of the proteins whose abundance are most decreased following DAQ exposure as determined by 2-D DIGE analysis are MtCK and mitofilin, and their respective decreases in abundance were confirmed by Western blot analyses. Levels of both proteins were also significantly decreased in the mitochondria of PC12 cells exposed to DA, suggesting susceptibility of MtCK and mitofilin to DA oxidation. Western blot analyses also revealed the presence of higher molecular weight species immunoreactive for MtCK in rat brain mitochondria and for mitofilin in both rat brain and PC12 cell mitochondria following DA oxidation. These results suggest increased protein aggregation and/or crosslinking as a potential consequence of oxidation by DAQ, though the nature of these interactions has yet to be examined.
Mitochondrial Creatine Kinase and Mitofilin Levels Are Altered Following Dopamine Oxidation
The protein whose relative fluorescence intensity was most decreased in our study, MtCK (−75% Lys-CyDye), is important in regulating ATP equilibrium in cells by generating phosphocreatine to help buffer against rapidly fluctuating energy usage (Schlattner et al., 1998). MtCK, an octameric protein, also plays a key role in mitochondrial morphology through formation and stabilization of contact sites between the inner and outer mitochondrial membranes (Lenz et al., 2007; Speer et al., 2005). The cysteine-dependent activity of MtCK is known to be highly sensitive to oxidative modification (Dolder et al., 2001). Oxidative stress may also result in dissociation of MtCK’s octameric structure into dimers (Dolder et al., 2001; Wendt et al., 2003), disrupting contact sites and potentially facilitating opening of the mitochondrial permeability transition pore (PTP) (Brdiczka et al., 2006; Vyssokikh and Brdiczka, 2003). Thus, DA oxidation-induced modifications to MtCK activity or stability may have impacts on mitochondrial structural integrity and energy metabolism.
Another protein with significantly decreased abundance is the inner mitochondrial membrane protein mitofilin (−54% Lys-CyDye). The presence of mitofilin has been shown to be critical for maintenance of mitochondrial cristae structure (John et al., 2005), though the specific role of mitofilin in the mitochondria is unknown. A recent study suggested mitofilin forms a complex with mitochondrial proteins Sam50 and metaxins 1 and 2, integral in mitochondrial protein import (Xie et al., 2007), though the exact relationship mitofilin shares with these proteins has not been evaluated. Various studies have shown that mitofilin is susceptible to oxidative stress, demonstrating oxidatively-modified cysteine and tryptophan residues (Suh et al., 2004; Taylor et al., 2003) and ROS-induced reduction of protein levels (Magi et al., 2004). A proteomic study on fetal Down syndrome brain tissue demonstrated a nearly 50% decrease in mitofilin, one of three proteins found to be significantly decreased in association with the disorder (Myung et al., 2003). Our study shows, using a non-biased approach, that mitofilin is susceptible to oxidative stress, resulting in decreased mitofilin protein levels. Such loss may impact mitochondrial structure and function, both of which are relevant to PD pathogenesis. Though the major function and interacting proteins of mitofilin are only beginning to be defined, a lot more remains to be understood about the important role of this protein within the mitochondria.
Proteins Associated With Mitochondrial Dysfunction and Neurodegeneration Are Altered Following Dopamine Quinone Exposure
The altered proteins we have identified cover a wide range of critical mitochondrial functions, and the inhibition of any one could be detrimental to mitochondrial function. Previous proteomic studies utilizing animal models of PD have also demonstrated alterations in mitochondrial proteins, including oxidation of key metabolism proteins (Poon et al., 2005) and changes in expression and/or abundance of critical proteins (Jin et al., 2005; Palacino et al., 2004; Periquet et al., 2005) Several of the proteins described here, such as the Complex I 75 kDa subunit, VDAC2, and mortalin, have also displayed altered abundance in previous animal models of PD (Jin et al., 2005; Periquet et al., 2005).
The level of the NADH-ubiquinone oxidoreductase 75 kDa Fe-S protein, a key subunit of mitochondrial Complex I, was significantly decreased (−46% Lys-CyDye) in isolated rat brain mitochondria following exposure to DAQ. Mutation and deletion of the 75 kDa subunit are linked to mitochondrial encephalopathy (Benit et al., 2001; Bulgiani et al., 2004); and led to reduced levels of Complex I, reduced Complex I activity, and increased mitochondrial ROS accumulation in fibroblasts (Iuso et al., 2006). As previously discussed, Complex I dysfunction has been directly linked to PD, and inhibition of Complex I and the ETC are known to lead to further ROS production by the mitochondria (Beal, 2003). In the 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP)-treated mouse model of PD, proteomic analysis demonstrated a significant loss of the 75 kDa subunit in SN pars compacta tissue (Jin et al., 2005). Protein levels of the 75 kDa subunit were also significantly reduced in various brain regions of Down syndrome and Alzheimer’s disease patients (Kim et al., 2001). Thus, loss of the 75 kDa subunit could be detrimental to cellular viability.
The voltage-dependent anion channel 2 (VDAC2) was greatly decreased (−68% Lys-CyDye) in fluorescence labeling following DAQ exposure in rat brain mitochondria. The VDAC proteins, like MtCK, have a role in the PTP. VDAC is also believed to be a key player in mitochondrial-mediated apoptosis (Shoshan-Barmatz et al., 2006). VDAC2, in particular, has been shown to regulate the proapoptotic molecule BAK (Cheng et al., 2003), a function that may be compromised if DA oxidation reduces protein levels. A previous study also observed a significant decrease of VDAC2 in the SN of MPTP-treated mice (Jin et al., 2005). While we found a decreased relative abundance of the VDAC2 isoform upon DAQ exposure, other isoforms of VDAC (VDAC1 and 3) were not detected. Thus, further analyses will be necessary to evaluate whether these isoforms are equally susceptible to DAQ-induced changes.
The mitochondrial protein chaperone, heat shock protein 70/glucose regulated protein75/mortalin (mortalin), another protein whose levels were demonstrably decreased (−26% Lys-CyDye) in this study, has also been linked to PD. Mortalin serves as an important chaperone in mitochondria, with a major role in mitochondrial protein import and folding (Geissler et al., 2001). A recent proteomic study found mortalin abundance to be decreased in SNpc of PD patients and in MES cells exposed to the mitochondrial Complex I inhibitor rotenone (Jin et al., 2006a). Mortalin was also shown to interact with PD-related proteins DJ-1 and α-synuclein in cultured cells (Jin et al., 2006b), with enhanced DJ-1 interaction following oxidative stress (Li et al., 2005). These studies combined with our present findings suggest that mortalin protein interactions and abundance are susceptible to oxidative stress. Thus, it is possible that loss of this protein would have a major impact on importation and incorporation of key mitochondrial proteins, particularly at times of stress. The exact role of mortalin in PD pathogenesis, however, remains to be elucidated.
The cause of the loss of specific proteins identified in this study has yet to be determined. One source may be rapid aggregation of oxidatively modified proteins. The Western blot analyses of mitofilin and MtCK indicate that protein aggregation or covalent protein-protein interactions do occur rapidly following in vitro DAQ exposure (15 min). Another possibility may be the rapid proteolytic degradation of oxidatively modified proteins. It is well established that mitochondria contain proteases dedicated to degradation of misfolded, denatured, and oxidatively modified proteins (Bota and Davies, 2001; Bota and Davies, 2002). While preliminary experiments using increased levels of protease inhibitors in the DAQ-exposure reaction did not appear to affect protein loss, protection by protease inhibitors may be confounded by the presence of intact mitochondrial membranes in our preparations. More work will be necessary to elucidate the nature of the observed protein loss.
Conclusions
Our results demonstrate that DA oxidation results in the loss of a select subset of mitochondrial proteins. Such an event, if not countered promptly, could lead to severely decreased mitochondrial function and stability. A major significance of the MtCK and mitofilin alterations observed may be in the roles of these proteins in maintaining mitochondrial morphology (John et al., 2005; Lenz et al., 2007; Speer et al., 2005). Dopamine oxidation-induced alterations in MtCK and mitofilin may lead to disruption of key protein-protein interactions and to mitochondrial structure reorganization.
Given the functions of proteins such as MtCK, mitofilin, and mortalin, and their responses to oxidative stress, it is possible that mitochondrial protein alterations resulting from DA oxidation may lead to impaired mitochondrial protein import, cristae reorganization, and PTP formation. The mitochondrial state may, in turn, deteriorate further due to alterations in key proteins involved in the Krebs cycle and ETC function, including the 75 kDa subunit of Complex I, leading to increased ROS formation. This could create a vicious cycle of oxidative damage, resulting in amplified mitochondrial dysfunction and, ultimately, neuronal degeneration and disease progression. Further study will be necessary to evaluate this hypothesis and the specific roles for the proteins identified in this study within mitochondrial dysfunction. Characterizing the susceptibility of mitochondrial proteins to DA oxidation may be key to understanding the contribution of DA to the progression of PD pathogenesis, and for developing novel therapeutic strategies for PD treatment.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jiyan An, Dr. Ashraf Elamin, proteomics manager Dr. Manimalha Balasubramani, and proteomics director Dr. Billy Day of the University of Pittsburgh Genomics and Proteomics Core Laboratories for their excellent technical expertise, advice, and assistance. We also thank Michael Fischer of the Biostatistics Consulting Service in the Dept. of Biostatistics, University of Pittsburgh Graduate School of Public Health for his assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by grants from the National Institutes of Health, NS44076, DA09601, and AG20899.
REFERENCES
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J Neurochem. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- Berman SB, Watkins SC, Hastings TG. Quantitative biochemical and ultrastructural comparison of mitochondrial permeability transition in isolated brain and liver mitochondria: evidence for reduced sensitivity of brain mitochondria. Exp Neurol. 2000;164:415–425. doi: 10.1006/exnr.2000.7438. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson's disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta. 1998;1368:7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Greenamyre JT. Quantitative study of mitochondrial complex I in platelets of parkinsonian patients. Mov Disord. 1998;13:11–15. doi: 10.1002/mds.870130106. [DOI] [PubMed] [Google Scholar]
- Bota DA, Davies KJ. Protein degradation in mitochondria: implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion. 2001;1:33–49. doi: 10.1016/s1567-7249(01)00005-8. [DOI] [PubMed] [Google Scholar]
- Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brdiczka DG, Zorov DB, Sheu SS. Mitochondrial contact sites: their role in energy metabolism and apoptosis. Biochim Biophys Acta. 2006;1762:148–163. doi: 10.1016/j.bbadis.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Chan HL, Gharbi S, Gaffney PR, Cramer R, Waterfield MD, Timms JF. Proteomic analysis of redox- and ErbB2-dependent changes in mammary luminal epithelial cells using cysteine- and lysine-labelling two-dimensional difference gel electrophoresis. Proteomics. 2005;5:2908–2926. doi: 10.1002/pmic.200401300. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosisc. Science. 2003:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Cohen G, Farooqui R, Kesler N. Parkinson disease: a new link between monoamine oxidase and mitochondrial electron flow. Proc Natl Acad Sci U S A. 1997;94:4890–4894. doi: 10.1073/pnas.94.10.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dolder M, Wendt S, Wallimann T. Mitochondrial creatine kinase in contact sites: interaction with porin and adenine nucleotide translocase, role in permeability transition and sensitivity to oxidative damage. Biol Signals Recept. 2001;10:93–111. doi: 10.1159/000046878. [DOI] [PubMed] [Google Scholar]
- Fornstedt B, Brun A, Rosengren E, Carlsson A. The apparent autoxidation rate of catechols in dopamine-rich regions of human brains increases with the degree of depigmentation of substantia nigra. J Neural Transm Park Dis Dement Sect. 1989;1:279–295. doi: 10.1007/BF02263482. [DOI] [PubMed] [Google Scholar]
- Geissler A, Rassow J, Pfanner N, Voos W. Mitochondrial import driving forces: enhanced trapping by matrix Hsp70 stimulates translocation and reduces the membrane potential dependence of loosely folded preproteins. Mol Cell Biol. 2001;21:7097–7104. doi: 10.1128/MCB.21.20.7097-7104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck M, Ehrhart J, Jayatilleke E, Zeevalk GD. Inhibition of brain mitochondrial respiration by dopamine: involvement of H(2)O(2) and hydroxyl radicals but not glutathione-protein-mixed disulfides. J Neurochem. 2002;82:66–74. doi: 10.1046/j.1471-4159.2002.00938.x. [DOI] [PubMed] [Google Scholar]
- Graham DG, Tiffany SM, Bell WR, Jr., Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978;14:644–653. [PubMed] [Google Scholar]
- Greenamyre JT, Hastings TG. Biomedicine. Parkinson's--divergent causes, convergent mechanisms. Science. 2004;304:1120–1122. doi: 10.1126/science.1098966. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TR, Prime TA, Harbour ME, Lilley KS, Murphy MP. Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis: implications for mitochondrial redox signaling. J Biol Chem. 2007;282:22040–22051. doi: 10.1074/jbc.M703591200. [DOI] [PubMed] [Google Scholar]
- Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zochling R, Boissl KW, Reichmann H. Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson's disease. Neurosci Lett. 1994;169:126–128. doi: 10.1016/0304-3940(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53 Suppl 3:S26–S36. doi: 10.1002/ana.10483. discussion S36-8. [DOI] [PubMed] [Google Scholar]
- Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol Cell Proteomics. 2006a;5:1193–1204. doi: 10.1074/mcp.M500382-MCP200. [DOI] [PubMed] [Google Scholar]
- Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J. Identification of novel proteins interacting with both a-synuclein and DJ-1. Mol Cell Proteomics. 2006b doi: 10.1074/mcp.M600182-MCP200. In Press. [DOI] [PubMed] [Google Scholar]
- Jin J, Meredith GE, Chen L, Zhou Y, Xu J, Shie FS, Lockhart P, Zhang J. Quantitative proteomic analysis of mitochondrial proteins: relevance to Lewy body formation and Parkinson's disease. Brain Res Mol Brain Res. 2005;134:119–138. doi: 10.1016/j.molbrainres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JM, Rangell L, Bennett MJ, Zha J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell. 2005;16:1543–1554. doi: 10.1091/mbc.E04-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Gunasekar PG, Borowitz JL, Isom GE. Dopamine-induced apoptosis is mediated by oxidative stress and Is enhanced by cyanide in differentiated PC12 cells. J Neurochem. 2000;74:2296–2304. doi: 10.1046/j.1471-4159.2000.0742296.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Vlkolinsky R, Cairns N, Fountoulakis M, Lubec G. The reduction of NADH ubiquinone oxidoreductase 24- and 75-kDa subunits in brains of patients with Down syndrome and Alzheimer's disease. Life Sci. 2001;68:2741–2750. doi: 10.1016/s0024-3205(01)01074-8. [DOI] [PubMed] [Google Scholar]
- Koshimura K, Tanaka J, Murakami Y, Kato Y. Effects of dopamine and L-DOPA on survival of PC12 cells. J Neurosci Res. 2000;62:112–119. doi: 10.1002/1097-4547(20001001)62:1<112::AID-JNR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Bovina C, D'Aurelio M, Fato R, Formiggini G, Genova ML, Giuliano G, Merlo Pich M, Paolucci U, Parenti Castelli G, Ventura B. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Lenz H, Schmidt M, Welge V, Kueper T, Schlattner U, Wallimann T, Elsasser HP, Wittern KP, Wenck H, Staeb F, Blatt T. Inhibition of cytosolic and mitochondrial creatine kinase by siRNA in HaCaT- and HeLaS3-cells affects cell viability and mitochondrial morphology. Mol Cell Biochem. 2007 doi: 10.1007/s11010-007-9565-8. [DOI] [PubMed] [Google Scholar]
- Li HM, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. Association of DJ-1 with chaperones and enhanced association and colocalization with mitochondrial Hsp70 by oxidative stress. Free Radic Res. 2005;39:1091–1099. doi: 10.1080/10715760500260348. [DOI] [PubMed] [Google Scholar]
- Magi B, Ettorre A, Liberatori S, Bini L, Andreassi M, Frosali S, Neri P, Pallini V, Di Stefano A. Selectivity of protein carbonylation in the apoptotic response to oxidative stress associated with photodynamic therapy: a cell biochemical and proteomic investigation. Cell Death Differ. 2004;11:842–852. doi: 10.1038/sj.cdd.4401427. [DOI] [PubMed] [Google Scholar]
- Myung JK, Gulesserian T, Fountoulakis M, Lubec G. Deranged hypothetical proteins Rik protein, Nit protein 2 and mitochondrial inner membrane protein, Mitofilin, in fetal Down syndrome brain. Cell Mol Biol (Noisy-le-grand) 2003;49:739–746. [PubMed] [Google Scholar]
- Ogawa N, Asanuma M, Miyazaki I, Diaz-Corrales FJ, Miyoshi K. L-DOPA treatment from the viewpoint of neuroprotection. Possible mechanism of specific and progressive dopaminergic neuronal death in Parkinson's disease. J Neurol. 2005;252 Suppl 4:IV23–IV31. doi: 10.1007/s00415-005-4006-7. [DOI] [PubMed] [Google Scholar]
- Orth M, Schapira AH. Mitochondrial involvement in Parkinson's disease. Neurochem Int. 2002;40:533–541. doi: 10.1016/s0197-0186(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Pallanck L, Greenamyre JT. Neurodegenerative disease: pink, parkin and the brain. Nature. 2006;441:1058. doi: 10.1038/4411058a. [DOI] [PubMed] [Google Scholar]
- Periquet M, Corti O, Jacquier S, Brice A. Proteomic analysis of parkin knockout mice: alterations in energy metabolism, protein handling and synaptic function. J Neurochem. 2005;95:1259–1276. doi: 10.1111/j.1471-4159.2005.03442.x. [DOI] [PubMed] [Google Scholar]
- Poon HF, Frasier M, Shreve N, Calabrese V, Wolozin B, Butterfield DA. Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice--a model of familial Parkinson's disease. Neurobiol Dis. 2005;18:492–498. doi: 10.1016/j.nbd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Rabinovic AD, Lewis DA, Hastings TG. Role of oxidative changes in the degeneration of dopamine terminals after injection of neurotoxic levels of dopamine. Neuroscience. 2000;101:67–76. doi: 10.1016/s0306-4522(00)00293-1. [DOI] [PubMed] [Google Scholar]
- Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab. 1987;7:752–758. doi: 10.1038/jcbfm.1987.130. [DOI] [PubMed] [Google Scholar]
- Samii A, Nutt JG, Ransom BR. Parkinson#s disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Forstner M, Eder M, Stachowiak O, Fritz-Wolf K, Wallimann T. Functional aspects of the X-ray structure of mitochondrial creatine kinase: a molecular physiology approach. Mol Cell Biochem. 1998;184:125–140. [PubMed] [Google Scholar]
- Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin Med Genet. 2006;142:121–126. doi: 10.1002/ajmg.c.30092. [DOI] [PubMed] [Google Scholar]
- Shaw J, Rowlinson R, Nickson J, Stone T, Sweet A, Williams K, Tonge R. Evaluation of saturation labelling two-dimensional difference gel electrophoresis fluorescent dyes. Proteomics. 2003;3:1181–1195. doi: 10.1002/pmic.200300439. [DOI] [PubMed] [Google Scholar]
- Shen XM, Li H, Dryhurst G. Oxidative metabolites of 5-S-cysteinyldopamine inhibit the alpha-ketoglutarate dehydrogenase complex: possible relevance to the pathogenesis of Parkinson's disease. J Neural Transm. 2000;107:959–978. doi: 10.1007/s007020070045. [DOI] [PubMed] [Google Scholar]
- Shoffner JM, Watts RL, Juncos JL, Torroni A, Wallace DC. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991;30:332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- Speer O, Back N, Buerklen T, Brdiczka D, Koretsky A, Wallimann T, Eriksson O. Octameric mitochondrial creatine kinase induces and stabilizes contact sites between the inner and outer membrane. Biochem J. 2005;385:445–450. doi: 10.1042/BJ20040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem. 1998;71:2112–2122. doi: 10.1046/j.1471-4159.1998.71052112.x. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Suh SK, Hood BL, Kim BJ, Conrads TP, Veenstra TD, Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- Taylor SW, Fahy E, Murray J, Capaldi RA, Ghosh SS. Oxidative post-translational modification of tryptophan residues in cardiac mitochondrial proteins. J Biol Chem. 2003;278:19587–19590. doi: 10.1074/jbc.C300135200. [DOI] [PubMed] [Google Scholar]
- Vyssokikh MY, Brdiczka D. The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim Pol. 2003;50:389–404. [PubMed] [Google Scholar]
- Wendt S, Schlattner U, Wallimann T. Differential effects of peroxynitrite on human mitochondrial creatine kinase isoenzymes. Inactivation, octamer destabilization, and identification of involved residues. J Biol Chem. 2003;278:1125–1130. doi: 10.1074/jbc.M208572200. [DOI] [PubMed] [Google Scholar]
- Xie J, Marusich MF, Souda P, Whitelegge J, Capaldi RA. The mitochondrial inner membrane protein Mitofilin exists as a complex with SAM50, metaxins 1 and 2, coiled-coil-helix coiled-coil-helix domain-containing protein 3 and 6 and DnaJC11. FEBS Lett. 2007;581:3545–3549. doi: 10.1016/j.febslet.2007.06.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.