Abstract
Objectives
(1) To define a set of health state descriptions related to screening, diagnosis, prognosis, and toxicities relevant to ovarian cancer; (2) To derive a set of quality of life-related utilities to be used for cost-effectiveness analyses.
Methods
A comprehensive list of health states was developed to represent the experiences of diagnostic testing for ovarian cancer, natural history of ovarian cancer (e.g., newly diagnosed early stage ovarian cancer, recurrent progressive ovarian cancer) and the most common chemotherapy-related toxicities (e.g. alopecia, peripheral neuropathy, pain, neutropenia, fatigue). Valuation of each health state was obtained through individual interviews of 13 ovarian cancer patients and 37 female members of the general public. Interviews employed visual analog score (VAS) and time trade off (TTO) methods of health state valuation.
Results
Mean TTO-derived utilities were higher than VAS-derived utilities by 0.118 units (p<0.0001). Mean VAS-derived utilities for screening tests were 0.83 and 0.81 for true negative blood test and ultrasound; 0.79 and 0.78 for false negative blood test and ultrasound, respectively. Patients and volunteers generally agreed in their preference ranking of chemotherapy-associated states, with lowest rankings being given to febrile neutropenia, grade 3–4 fatigue, and grade 3–4 nausea/vomiting. For 55% of chemotherapy-associated health states the average utility assigned was higher for patients than for volunteers.
Conclusions
This study establishes societal preferences for a number of health states related to screening, diagnosis and treatment of ovarian cancer that can be used for assessing the cost-effectiveness of different ovarian cancer screening and treatment regimens.
Introduction
Ovarian cancer is usually diagnosed at an advanced stage when cure is unlikely. Most patients with advanced disease achieve clinical remission following surgery and chemotherapy but eventually relapse; those who experience recurrence often receive multiple salvage chemotherapy regimens. The practice of treating patients whose recurrent disease is incurable with toxicity-inducing regimens has sometimes been questioned; supportive care is often considered a reasonable alternative to costly and toxic salvage chemotherapy regimens that may have no effect on overall survival [1–3]. It is therefore imperative that the quality of life associated with any management decision related to ovarian cancer be closely examined.
Cost-effectiveness models offer one way to examine options in the management of a disease. For ovarian cancer, treatment choices often center around the cost of a specific chemotherapy treatment, the survival expected to result from its use, and its toxicities [2]. These models are used to determine the costs and outcomes associated with the different management strategies, which in turn can be used to determine whether one treatment option should be preferred over another. Strategies are usually compared using cost per year of life saved, which quantifies improvements in survival, or cost per quality adjusted life year (QALY), which quantifies improvements in survival and disease-related morbidity. Of the two, QALYs are the recommended outcome for cost-effectiveness analyses[4]. In order to report results in QALYs, a utility must be assigned to the health state of interest. A utility is a number between 0 and 1, with 1 representing perfect health and 0 representing death. Accepted methods for calculating a utility include the time trade off (TTO) and standard gamble (SG) methods, which are usually performed via an extensive interview designed to determine an individual’s preferences for one health state (e.g., progressive metastatic ovarian cancer) compared to another (e.g., perfect health)[5]. Members of the general public are the recommended group from which to derive health state-specific valuations for performing cost-effectiveness analyses from a societal perspective[4].
There is a paucity of validated health state-specific utilities related to the diagnosis of ovarian cancer. Prior studies that have derived health state-related utilities for ovarian cancer have addressed specific clinical scenarios[6–10]. Due to differences between studies in the methodology used to evaluate health state preferences, as well the combination in some studies of multiple different symptoms within each health state, utilities derived from prior studies are not always appropriate for use in decision models evaluating screening or treatment decisions for ovarian cancer[6–10]. We created a set of health states that correspond to ovarian cancer screening, diagnosis, progression, and the most frequent toxicities encountered by patients receiving standard chemotherapy regimens for ovarian cancer. We interviewed individuals from two populations: (1) patients with a current or prior diagnosis of ovarian cancer, and (2) female members of the general public without a diagnosis of ovarian cancer. Interviews were conducted in order to derive, using an established methodology, a set of health state-related utilities. Such scores can then be used to determine the cost per QALY of prevention, screening, and treatment strategies for women with ovarian cancer.
Methods
Health states: first draft
Following Institutional Review Board approval, we developed a set of health state descriptions corresponding to the experiences of undergoing screening and diagnostic tests for ovarian cancer (Group A, states 1–4), various phases in the natural history of ovarian cancer (Group B, states 5–14), and the toxicities associated with its treatment with chemotherapy (Group C, states 15–25). These descriptions capture physical and emotional aspects of each health state, as well as the time involved for treatment or diagnosis (Appendix 1). With regard to chemotherapy-related toxicities, we identified those which are both most common and of sufficient severity to potentially affect QoL, including: peripheral neuropathy, nausea and vomiting, alopecia, myalgia/arthralgia, neutropenia, fatigue, and stomatitis. For most toxicities we developed a description of “mild to moderate” effects which generally correspond to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grades 1–2 (usually managed as an outpatient) and “severe” effects incorporating CTCAE grades 3–4 (usually requiring hospitalization or major intervention)[11]. We also included a number of descriptive health states that comprise frequently experienced combinations of disease status and common toxicities (e.g., newly diagnosed advanced ovarian cancer with grade 1–2 neurotoxicity, grade 1 nausea/vomiting, and grade 2 alopecia). We performed exploratory interviews with two practicing gynecologic oncologists, one gynecologic oncology nurse clinician, and two women with a history of ovarian cancer and chemotherapy treatment in constructing health state descriptions.
Health states focus group
A focus group composed of two gynecologic oncology nurse clinicians, one gynecologic oncologist, one gynecologic oncology physician’s assistant and one clinical social worker reviewed the drafted health state descriptions in detail and made suggestions regarding the severity or appropriateness of symptoms described as well as their emotional and social consequences. This process resulted in the final version of the 25 descriptive health states (Appendix 1).
Health state valuation
Subjects
Thirty-seven female members of the public without a personal history of ovarian cancer (“volunteers”) and thirteen women with a prior diagnosis of ovarian cancer (either currently being treated or previously treated; “patients”) were recruited to valuate the 25 health states. Volunteers were recruited using flyers on public blackboards at Duke University Medical Center. Eligibility criteria for volunteers consisted of no personal history of ovarian cancer. Patients were recruited using flyers placed in examination rooms at Duke University Gynecologic Oncology Clinic. Eligibility criteria for patients consisted of a confirmed personal history of ovarian cancer. A nominal monetary incentive was offered for participation. Volunteers were asked to evaluate a random sample of ten of the 25 descriptive health states (Groups A–C). Patients evaluated only Group C (chemotherapy-associated states) due to concern regarding the impact of detailed descriptions of prognosis and survival on the psychological well-being of women with a diagnosis of ovarian cancer. All recruited subjects were able to complete the study.
Interview method
Subjects were interviewed by a single trained research nurse using the visual analog score (VAS) and time trade off (TTO) methods[12]. The subject was first asked to read a health state description and then asked to listen while it was read aloud. She was then asked to rate the health state by placing a mark on a continuum from zero to 100, with 100 representing perfect health and zero representing death. The scale contained no gradations other than 0 and 100. The VAS was calculated as the distance from zero to the mark placed by the subject, divided by the measured distance from zero to 100 on the VAS. The TTO interview was then administered as follows (Appendix 2): The subject was asked to choose between (a) 30 years in the health state described and (b) a lower number of years in a state of perfect health. The utility of the health state, a ratio between 0 and 1, was calculated as the minimum number of years of perfect health the patient would prefer in exchange for 30 years in the health state described, divided by 30.
Statistical analysis
The mean, standard deviation, median, and range of values for each health state was calculated for patients and volunteers separately. Comparison between patient and volunteer responses was performed using the Wilcoxon Rank-Sum test. The Spearman correlation coefficient was calculated to compare scores obtained using the two valuation methods (VAS and TTO). The student t-test was used to determine whether mean TTO-and VAS-derived utilities were significantly different.
Results
Of 50 participating subjects, 13 were patients and 37 were volunteers. The mean age of patients was 58 (range 41–81) and volunteers, 41 (range 20–59). Subject characteristics are listed in Table 1. The median time from diagnosis of patients was 19 months. Twelve of 13 patients had a disease stage of IIIC or IV; four had experienced disease recurrence or persistence, and six patients had received two prior cytotoxic chemotherapy regimens. Six patients had been treated on clinical trials of consolidation therapy, receiving taxanes, biologic agents or placebo. Each patient performed valuation of 11 health states, while each volunteer rated an average of 10 health states. One volunteer participated twice; an average of the valuations was included in the analysis for health states evaluated twice by this subject. VAS and TTO-derived utilities were significantly positively correlated in 14/36 (39%) of valuations. TTO-derived utilities were on average higher than VAS-derived utilities by 0.118 units (p<0.0001). Twenty-four of 25 (96%) health states had a higher average TTO- than VAS-derived utility.
Table 1.
Subject Characteristics
| Patients | Volunteers | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | ||
| Age | 58 | 55 | 41–81 | 41 | 42 | 20–59 | |
|
| |||||||
| Race | |||||||
| Caucasian | 13 | 18 | |||||
| African American | 0 | 11 | |||||
| Hispanic | 0 | 1 | |||||
| Unknown/not reported | 0 | 7 | |||||
|
| |||||||
| Stage | |||||||
| IIB | 1 | ||||||
| IIIC | 8 | ||||||
| IV | 4 | ||||||
|
| |||||||
| Recurrent/persistent disease | |||||||
| yes | 4 | ||||||
| no | 9 | ||||||
|
| |||||||
| Prior chemotherapy regimens* | |||||||
| 1 | 7 | ||||||
| 2 | 6 | ||||||
|
| |||||||
| Active chemotherapy treatment* | |||||||
| yes | 5 | ||||||
| no | 8 | ||||||
| If not active, months since last chemotherapy treatment* | 10.5 | 1–84 | |||||
Excluding biologic versus placebo treatments given on blinded clinical trials of consolidation therapy
Utilities for ovarian cancer screening-related health states are listed in Table 2. The mean VAS-derived utilities for screening-related states were slightly lower in false positive scenarios: 0.83 for a screening blood test versus 0.81 for false positive blood test requiring an additional ultrasound; 0.79 for screening transvaginal ultrasound versus 0.78 for a false positive ultrasound resulting in laparoscopy. TTO-derived utilities were similar for all the screening-related states (median 0.97 for each).
Table 2.
Utilities for ovarian cancer screening-related health states, visual analog score (VAS) and time trade off (TTO) methods
| Health State | n | Visual Analog Score | Time Trade Off Score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Mean | SD | Median | Range | Mean | SD | ||
| Screening blood test | 15 | 0.90 | 0.39–1 | 0.83 | 0.17 | 0.97 | 0.33–0.97 | 0.90 | 0.18 |
| Screening transvaginal ultrasound | 15 | 0.92 | 0.21–1 | 0.79 | 0.23 | 0.97 | 0.03–0.97 | 0.83 | 0.27 |
| Screening blood test with false positive result | 16 | 0.89 | 0.49–1 | 0.81 | 0.17 | 0.97 | 0.03–0.97 | 0.88 | 0.26 |
| Screening transvaginal ultrasound with false positive result | 15 | 0.85 | 0.31–1 | 0.78 | 0.21 | 0.97 | 0.5–0.97 | 0.90 | 0.14 |
Utilities for ovarian cancer diagnosis-related health states are listed in Table 3. Newly diagnosed early stage ovarian cancer and ovarian cancer remission were the two highest ranked states using both valuation methods. The three lowest ranked states using both methods were end stage ovarian cancer, recurrent progressive ovarian cancer with grade 3–4 toxicity, and recurrent progressive ovarian cancer with grade 1–2 toxicity.
Table 3.
Utilities for diagnosis-related health states, VAS and TTO methods
| Health State | n | Visual Analog Score | Time Trade Off Score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Mean | SD | Median | Range | Mean | SD | ||
| Ovarian cancer-clinical remission | 16 | 0.75 | 0.32–1 | 0.72 | 0.21 | 0.95 | 0.03–0.97 | 0.83 | 0.25 |
| Early ovarian cancer – newly diagnosed | 16 | 0.67 | 0.24–0.94 | 0.62 | 0.19 | 0.93 | 0.03–0.97 | 0.81 | 0.26 |
| Newly diagnosed ovarian cancer – chemotherapy/grade 1–2 toxicity | 16 | 0.56 | 0.22–0.86 | 0.50 | 0.21 | 0.67 | 0.03–0.97 | 0.60 | 0.31 |
| Recurrent ovarian cancer – responding to chemotherapy/grade 3–4 toxicity | 14 | 0.39 | 0.17–0.91 | 0.40 | 0.19 | 0.67 | 0.17–0.97 | 0.61 | 0.24 |
| Recurrent ovarian cancer – responding to chemotherapy/grade 1–2 toxicity | 15 | 0.43 | 0.22–0.89 | 0.44 | 0.20 | 0.50 | 0.03–0.93 | 0.50 | 0.34 |
| Advanced ovarian cancer – newly diagnosed | 14 | 0.41 | 0.15–0.85 | 0.45 | 0.23 | 0.50 | 0.03–0.93 | 0.55 | 0.29 |
| Newly diagnosed ovarian cancer – chemotherapy/grade 3–4 toxicity | 15 | 0.39 | 0.06–0.9 | 0.37 | 0.22 | 0.50 | 0.03–0.97 | 0.49 | 0.36 |
| Recurrent ovarian cancer – progressive/grade 3–4 toxicity | 15 | 0.17 | 0.05–0.92 | 0.27 | 0.23 | 0.50 | 0.03–0.93 | 0.47 | 0.34 |
| Recurrent ovarian cancer – progressive/grade 1–2 toxicity | 16 | 0.37 | 0.02–0.80 | 0.36 | 0.20 | 0.42 | 0.03–0.93 | 0.40 | 0.33 |
| End stage ovarian cancer | 15 | 0.08 | 0.01–1 | 0.16 | 0.25 | 0.03 | 0.03–0.83 | 0.16 | 0.25 |
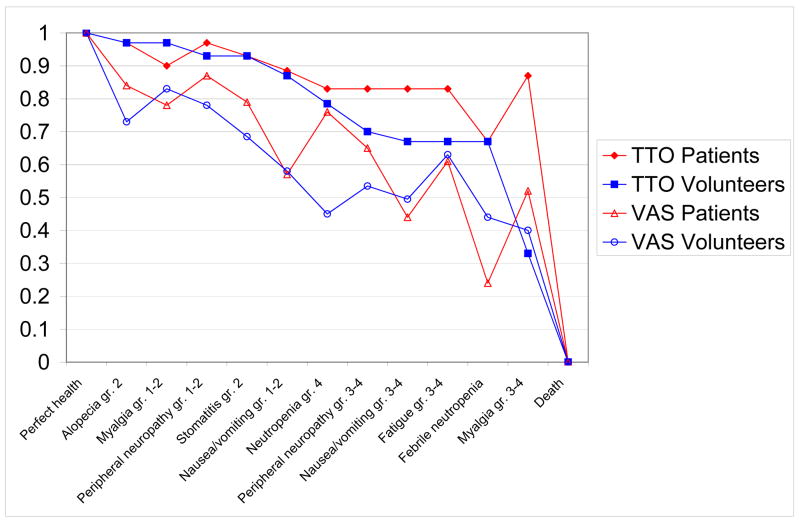
Table 4 lists TTO-derived utilities for chemotherapy-associated health states; TTO and VAS results for patients and volunteers are depicted in Figure 1. Both patients and volunteers ranked grade 2 alopecia as most preferable, followed by grade 1–2 peripheral neuropathy, grade 2 stomatitis, and grade 1–2 myalgia. Volunteers ranked grade 3–4 myalgias lowest, while both patients and volunteers ranked febrile neutropenia, grade 3–4 fatigue, and grade 3–4 nausea/vomiting at or near the bottom. Six out of 11 (55%) chemotherapy-associated health states were given higher mean utilities by patients than by volunteers using either TTO or VAS methods. Grade 1–2 peripheral neuropathy (VAS median = 0.87 for patients and 0.78 for volunteers, p=0.025) and grade 3–4 myalgia (TTO median = 0.87 for patients and 0.33 for volunteers, p=0.07) were rated significantly more highly by patients than volunteers.
Table 4.
Time trade off utilities for chemotherapy-related health states, patient and volunteer
| Health State | Patients | Volunteers | P value* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Range | Mean | S.D. | n | Median | Range | Mean | SD | ||
| Alopecia-grade 2 | 12 | 0.97 | 0.5–1 | 0.90 | 0.15 | 14 | 0.97 | 0.03–0.97 | 0.84 | 0.29 | 0.53 |
| Peripheral neuropathy- grade 1–2 | 13 | 0.97 | 0.83–1 | 0.95 | 0.04 | 15 | 0.93 | 0.03–0.97 | 0.81 | 0.29 | 0.055 |
| Stomatitis-grade 2 | 13 | 0.93 | 0.5–0.97 | 0.88 | 0.14 | 14 | 0.93 | 0.67–0.97 | 0.91 | 0.08 | 0.80 |
| Myalgia/pain-grade 1–2 | 13 | 0.90 | 0.5–1 | 0.86 | 0.15 | 15 | 0.97 | 0.67–0.97 | 0.89 | 0.12 | 0.645 |
| Nausea/vomiting-grade 1–2 | 12 | 0.88 | 0.03–0.97 | 0.65 | 0.38 | 15 | 0.87 | 0.03–0.97 | 0.76 | 0.28 | 0.80 |
| Myalgia/pain-grade 3–4 | 13 | 0.87 | 0.17–0.97 | 0.72 | 0.30 | 15 | 0.33 | 0.03–0.97 | 0.46 | 0.39 | 0.07 |
| Neutropenia-grade 4 | 13 | 0.83 | 0.03–0.97 | 0.70 | 0.30 | 16 | 0.78 | 0.03–0.97 | 0.64 | 0.36 | 0.91 |
| Peripheral neuropathy- grade 3–4 | 13 | 0.83 | 0.03–1 | 0.73 | 0.27 | 14 | 0.70 | 0.03–0.97 | 0.65 | 0.31 | 0.48 |
| Nausea/vomiting-grade 3–4 | 13 | 0.83 | 0.03–0.97 | 0.60 | 0.40 | 16 | 0.67 | 0.03–0.97 | 0.63 | 0.30 | 0.967 |
| Fatigue grade 3–4 | 13 | 0.83 | 0.03–1 | 0.66 | 0.35 | 13 | 0.67 | 0.03–0.97 | 0.58 | 0.33 | 0.45 |
| Febrile neutropenia | 13 | 0.67 | 0.03–0.93 | 0.54 | 0.33 | 15 | 0.67 | 0.03–0.97 | 0.56 | 0.34 | 0.73 |
Wilcoxon rank-sum test
Figure 1.
Median chemotherapy-related health state preferences
Discussion
The consideration of quality of life is of utmost importance to those helping women make decisions about their health care. This is further highlighted in the context of management decisions for an often incurable malignancy. This study establishes societal preferences for health states related to screening for ovarian cancer, its diagnosis, prognosis, and treatment. It also provides utilities for common clinical scenarios such as the false positive screening test, newly diagnosed cancer of early or late stage with commonly experienced levels of toxicity, and different phases in the natural history of ovarian cancer, including levels of progression and remission. In addition, this study reports valuations of chemotherapy-related states obtained from both volunteers and women with a diagnosis of ovarian cancer. These results are a resource to those who wish to construct health economic models, especially given the paucity of previously validated health state-related utilities concerning patients with ovarian cancer.
This study complements those of others who have derived health preference utilities related to ovarian cancer treatment. [6] [7–10, 13, 14] These have often addressed specific clinical questions but may not have always been appropriate for use in more general cost-effectiveness analyses conducted for informing policy decisions. For example, in performing a cost-utility analysis of two primary chemotherapy regimens for treatment of ovarian cancer[6], Ortega et al (1997) incorporated both a specific chemotherapy regimen and the patient’s disease status into each health state description, such that the resulting utilities are not easily generalizable. Sun et al (2002) interviewed 40 patients with advanced ovarian cancer during inpatient high dose chemotherapy/stem cell transplant admissions[7]. Utilities were assigned to health states associated with a number of chemotherapy toxicities. However, the study deviated from the traditional TTO method in that patients were asked to compare two different toxicity profiles (as opposed to comparing one toxicity description with perfect health). Similar to the findings of Sun et al in this and a second study using VAS methodology[8], we found that severe nausea and vomiting received one of the lowest rankings from patients, while alopecia was perceived as one of the least bothersome. In addition, as the CTC grade of a toxicity increased, the utility dropped. Also similar to the Sun study, we found that TTO-derived utilities tended to be higher than those derived using the VAS. Visual scales for comparing health state preferences are subject to inherent measurement biases and are generally less accurate than the TTO method [12].
While we asked only volunteers to valuate diagnosis- and prognosis-related health states, we obtained valuations from both patients and volunteers for health states related to the experience of chemotherapy treatment. We found that patients usually assigned a higher preference to chemotherapy-associated states than did volunteers. Similarly, Calhoun et al (1998) previously evaluated physician and patient preferences for ototoxicity, nephrotoxicity, and neurotoxicity of increasing severity (5 states for each toxicity)[13] and found that physician-assigned utilities were markedly lower than those assigned by patients. This may indicate clinicians’ bias toward withholding potentially toxic treatments due to perception of a lower QoL associated with treatment. In another study, Calhoun et al (2004) administered a utilities questionnaire to women with ovarian cancer, women without ovarian cancer who were at different levels of risk, and gynecologic oncologists regarding their preferences for specific toxicities at varying levels of severity. Similar to our study, the authors found that utility scores varied significantly between groups, with patients who had experienced specific toxicities assigning more favorable utility scores than those who had not[9]. While health state valuations are traditionally performed by members of the public[4], the QoL as perceived by the patient is a critical piece of information for those making recommendations about treatment in the setting of recurrent, often incurable disease. This valuation of health states related to the treatment of ovarian cancer will allow us to further refine existing models related to the choice of treatment for various stages and phases in the natural history of this disease [15, 16].
An alternative way to derive a set of QoL-related utilities is to convert the results of a QoL questionnaire to a utility score. Such conversions are usually not mathematically simple and must be validated. Stein et al (2007) obtained EORTC-QLQ-C30 questionnaire results from 66 women with advanced ovarian cancer who were participating in clinical trials[10] and used a data clustering method to derive generic health state descriptions to represent each cluster, which were then presented to a panel of 39 members of the general public for assignment of utility scores. Because clusters of generic symptoms (e.g., fatigue, pain, shortness of breath, anxiety) which were not identifiable as ovarian cancer-related conditions, were addressed in the health state descriptions, the results of this study can not be directly used in a cost-effectiveness analysis outside the context of a specific clinical trial that uses the same QoL questionnaire.
The ability to place a value on the physical and emotional experiences of undergoing screening is important in the quality-adjustment of any survival advantage that might be afforded by cancer screening. We found that false positive screening states were rated slightly lower using the VAS, but not TTO, method compared to true negative and true positive states. While there is no currently recommended screening test for ovarian cancer, the ongoing discovery and validation of single- and multiple-biomarker assays and the performance of large clinical trials of multimodal ovarian cancer screening make evaluation of screening from a health economic perspective extremely relevant[17–21]. Given the low prevalence of ovarian cancer, even screening tests whose specificity exceeds 99% may be inadequate due to the large number of false positive results[22]. While we did not observe a significant difference in utilities between screening tests with versus without false positive results, the experience of a screening test alone appears to result in a QoL below that of perfect health. Even this slight decrement in QoL resulting from a screening test may affect its potential cost effectiveness in the setting of a disease with low prevalence such as ovarian cancer.
This study is limited by its small size, particularly with regard to the small number of women with ovarian cancer who performed health state valuations (n=13); each health state received 16 or fewer valuations. Clearly a larger study of a more diverse population of both patients and volunteers is needed to confirm this study’s findings. Future studies should consider including male volunteers and should include additional demographic information such as education and income levels, dependant family members, close contacts or family members with ovarian cancer. Other potential sources of bias include the collection of data by a single interviewer and the 30 year time frame used in the time trade off interview. In order to present a realistic life expectancy for volunteers, we chose 30 years, which may have introduced unintentional bias into patients’ valuation of some health states. Finally, due to concerns about the subjects’ emotional well being, we did not ask women with ovarian cancer to evaluate health states in groups A and B which contained prognostic information and recurrence scenarios. This limits our interpretation of the utilities obtained when considering the perspective of the patient. However, health state valuations by volunteers are considered most appropriate for use in health economic models that are designed from a societal perspective [4].
In conclusion, we have established societal preferences for a group of health states related to screening, diagnosis, and treatment for ovarian cancer. We have established patient and societal preferences for the toxicities most commonly experienced by women with ovarian cancer, with the severity of each description calibrated to mild-moderate (grade 1–2) or severe (grade 3–4) CTC criteria. This QoL-related data will be a useful resource for construction of health economic models and may be relevant to future clinical trial design.
Supplementary Material
Acknowledgments
This study was funded by the NCI through Duke Comprehensive Cancer Center Core Support Grant #5P30-CA14236
LH is supported by a grant from the American Board of Obstetrics and Gynecology/American Association of Obstetricians and Gynecologists Foundation.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rocconi RP, Case AS, Straughn JM, Jr, Estes JM, Partridge EE. Role of chemotherapy for patients with recurrent platinum-resistant advanced epithelial ovarian cancer: A cost-effectiveness analysis. Cancer. 2006;107:536–43. doi: 10.1002/cncr.22045. [DOI] [PubMed] [Google Scholar]
- 2.Donovan KA, Greene PG, Shuster JL, Partridge EE, Tucker DC. Treatment preferences in recurrent ovarian cancer. Gynecol Oncol. 2002;86:200–11. doi: 10.1006/gyno.2002.6748. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik A, Doyle C, Oza AM. Palliative therapy in advanced ovarian cancer: balancing patient expectations, quality of life and cost. Anticancer Drugs. 1998;9:869–78. doi: 10.1097/00001813-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Gold MSJ, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. Oxford University Press; Ney York: 1996. [Google Scholar]
- 5.Brazier J, Deverill M, Green C, Harper R, Booth A. A review of the use of health status measures in economic evaluation. Health Technol Assess. 1999;3: i–iv. 1–164. [PubMed] [Google Scholar]
- 6.Ortega A, Dranitsaris G, Sturgeon J, Sutherland H, Oza A. Cost-utility analysis of paclitaxel in combination with cisplatin for patients with advanced ovarian cancer. Gynecol Oncol. 1997;66:454–63. doi: 10.1006/gyno.1997.4786. [DOI] [PubMed] [Google Scholar]
- 7.Sun CC, Bodurka DC, Donato ML, Rubenstein EB, Borden CL, Basen-Engquist K, Munsell MF, Kavanagh JJ, Gershenson DM. Patient preferences regarding side effects of chemotherapy for ovarian cancer: do they change over time? Gynecol Oncol. 2002;87:118–28. doi: 10.1006/gyno.2002.6807. [DOI] [PubMed] [Google Scholar]
- 8.Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, Smith JA, Wharton JT, Rubenstein EB. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13:219–27. doi: 10.1007/s00520-004-0710-6. [DOI] [PubMed] [Google Scholar]
- 9.Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93:164–9. doi: 10.1016/j.ygyno.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Stein K, Sugar C, Velikova G, Stark D. Putting the ‘Q’ in quality adjusted life years (QALYs) for advanced ovarian cancer - An approach using data clustering methods and the internet. Eur J Cancer. 2007;43:104–13. doi: 10.1016/j.ejca.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events. National Cancer Institute; 2006. [Google Scholar]
- 12.Drummond MFOBB, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 2. New York: Oxford University Press; 1997. [Google Scholar]
- 13.Calhoun EA, Bennett CL, Peeples PA, Lurain JR, Roland PY, Weinstein JM, Fishman DA. Perceptions of cisplatin-related toxicity among ovarian cancer patients and gynecologic oncologists. Gynecol Oncol. 1998;71:369–75. doi: 10.1006/gyno.1998.5189. [DOI] [PubMed] [Google Scholar]
- 14.Grann VR, Jacobson JS, Sundararajan V, Albert SM, Troxel AB, Neugut AI. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J Sci Am. 1999;5:283–92. [PubMed] [Google Scholar]
- 15.Havrilesky LJ, Alvarez Secord A, Darcy KM, Armstrong DK, Kulasingam S. Cost effectiveness of intraperitoneal compared with intravenous chemotherapy for women with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. Journal of Clinical Oncology. 2008:26. doi: 10.1200/JCO.2007.13.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havrilesky LJ, Secord AA, Kulasingam S, Myers E. Management of platinum-sensitive recurrent ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol. 2007;107:211–8. doi: 10.1016/j.ygyno.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, Macdonald N, Dawnay A, Jeyarajah A, Bast RC, Jr, Oram D, Jacobs IJ. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol. 2005;23:7919–26. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 18.Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, Hartge P, Fagerstrom RM, Ragard LR, Chia D, Izmirlian G, Fouad M, Johnson CC, Gohagan JK. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–9. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, Bast RC., Jr The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, Malinowski DP, Fischer TJ, Berchuck A. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008 doi: 10.1016/j.ygyno.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 21.Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, Yue L, Bray-Ward P, Ward DC. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:7677–82. doi: 10.1073/pnas.0502178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, Baggerly KA, Atkinson EN, Skates S, Zhang Z, Lokshin A, Menon U, Jacobs I, Lu K. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15 (Suppl 3):274–81. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.